Abstract
The spectacular marine-like diversity of the endemic fauna of Lake Tanganyika, the oldest of the African Great Lakes, led early researchers to suggest that the lake must have once been connected to the ocean. Recent geophysical reconstructions clearly indicate that Lake Tanganyika formed by rifting in the African subcontinent and was never directly linked to the sea. Although the Lake has a high proportion of specialized endemics, the absence of close relatives outside Tanganyika has complicated phylogeographic reconstructions of the timing of lake colonization and intralacustrine diversification. The freshwater herring of Lake Tanganyika are members of a large group of pellonuline herring found in western and southern Africa, offering one of the best opportunities to trace the evolutionary history of members of Tanganyika's biota. Molecular phylogenetic reconstructions indicate that herring colonized West Africa 25–50MYA, at the end of a major marine incursion in the region. Pellonuline herring subsequently experienced an evolutionary radiation in West Africa, spreading across the continent and reaching East Africa's Lake Tanganyika during its early formation. While Lake Tanganyika has never been directly connected with the sea, the endemic freshwater herring of the lake are the descendents of an ancient marine incursion, a scenario which may also explain the origin of other Tanganyikan endemics.
Introduction
Ancient lakes are home to disproportionate levels of freshwater biodiversity. As standing bodies of water which have existed for at least 100,000 years [1], these habitats have been remarkably stable when compared to more typically transitory freshwater environments. As a consequence, lakes such as Lake Baikal (25–30 MY) and the African Great Lakes Malawi (1–2 MY) and Tanganyika (9–12 MY) all contain exceptionally high numbers of freshwater taxa, of which up to 99% are endemics [2].
Habitat stability is thought to promote niche partitioning and resource specialization, providing an important engine for speciation [3]. Due to the relative stability of ancient lakes, these habitats have long been recognized as centers of spectacular adaptive radiation, exemplified by the highly specialized cichlid fishes of the African Great Lakes [4], [5]. This pattern of often rapid in situ adaptive radiation has also been documented in other groups of fishes [6] and invertebrates [7]–[9]. At the same time, there is a growing appreciation that in addition to their role as centers of diversification, ancient lakes have also played an important role as evolutionary reservoirs, maintaining diverse groups of organisms that have been extirpated outside their borders [10]–[13].
While each of the African Great Lakes is home to high levels of endemic biodiversity, Lake Tanganyika is distinguished from Lakes Malawi and Victoria by the taxonomic breadth and morphological diversity of its endemic fauna. Freshwater lineages of crustaceans and gastropods found in the lake exhibit striking morphological similarities to marine species and cnidarians and clupeiform fishes, groups typically restricted to marine environments, are also found within the lake [14]. Due to strong morphological affinities between Lake Tanganyika's fauna and marine organisms, early investigators proposed that the lake must have been directly connected to the ocean at some point in its history [15], [16]. Subsequent investigations of the geology of the region however indicate that the African Great Lakes were formed by rifting in the African subcontinent and were thus never in direct contact with the ocean [17], [18]. While the hypothesis of a direct marine connection [16] appears invalid, the enigma of the “Tanganyika Problem” remains unanswered: namely, how did such a specialized and unique freshwater biota come to be found within the Lake?
Unfortunately, attempts to elucidate the evolutionary origins of Tanganyikan endemics have been hampered by the absence of close relatives outside the lake. While Tanganyika appears to have been colonized by at least four ancient lineages of gastropods [12] and eight seeding lineages of cichlid fishes [11], the colonization history of these groups cannot be easily traced due to the absence of close extant and/or fossil relatives in the African subcontinent.
While clupeid fishes dominate marine fish communities and anadromous populations inhabit brackish waters, freshwater diversity of this group is typically low. West Africa is home to the largest evolutionary radiation of freshwater clupeid fishes [19], including at least twenty species of the subfamily Pellonulinae. The pellonuline herring of West Africa exhibit striking adaptations for life in freshwater, including carnivorous forms with large canine teeth (Cyanothrissa and Odaxothrissa), species almost completely lacking scales (Thrattidion) and a general tendency towards reduced size, exemplified by species that attain sexual maturity at less than 20 mm SL (Thrattidion and Sierrathrissa) [20]. Pellonuline herring are also found in southern and central Africa and Madagascar as well as Australia, India and eastern Asia [21]. Two pellonuline species are endemic to Lake Tanganyika, where they are dominant members of the pelagic zone [22].
A comprehensive treatment of fossil and recent clupeomorph fishes has questioned the monophyly of clupeid subfamilies, including the pellonulines [21]. The pellonuline herring of Africa appear to fall into two major groups, the Pellonulini, a tribe containing taxa from western and central Africa, and the Ehiravini, a tribe of herring from southern Africa and India [21]. A more recent morphological investigation of African pellonuline herring supported this hypothesis and suggested that the herring of Lake Tanganyika are closely allied with those of West Africa [19]. A clear understanding of the historical biogeography of pellonuline herring may be one of our best opportunities to reconstruct the evolutionary history of members of the unique fauna of Lake Tanganyika.
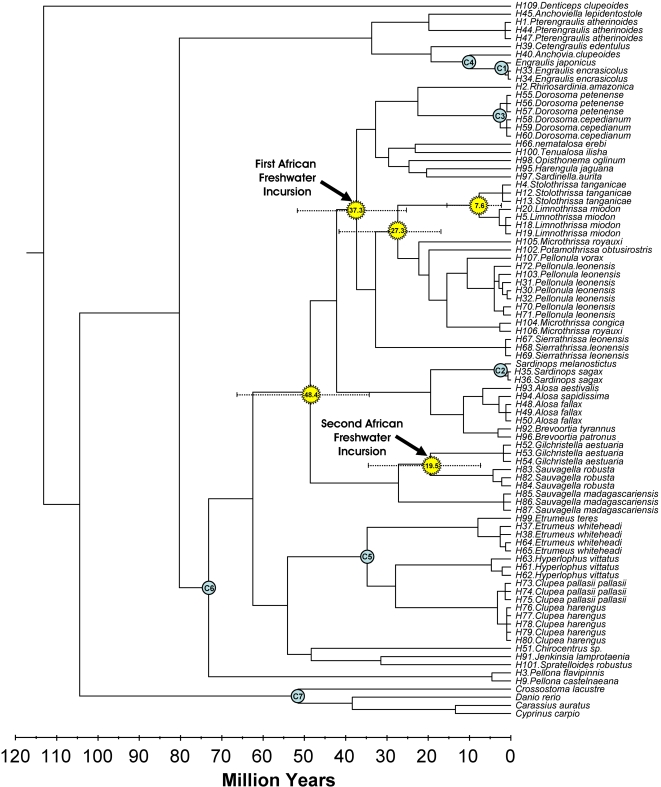
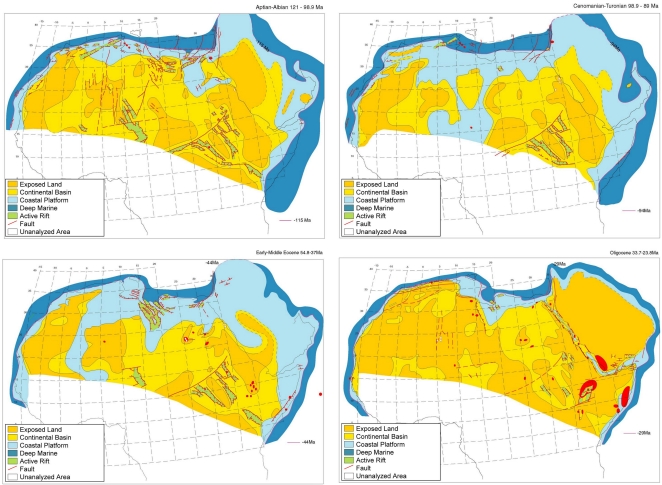
Here, we construct a phylogeny of clupeiform fishes based on three mitochondrial DNA genes and use a multipoint fossil calibration to determine both the timing of freshwater colonization of Africa by pellonuline herring and the timing of the colonization and diversification of herring within Lake Tanganyika. Molecular phylogenetic reconstructions reject the monophyly of pellonuline herring and support strong affinities between the endemic herring of Tanganyika and freshwater pellonulines found in western Africa. Molecular clock analyses indicate that the colonization of African freshwater by marine herring occurred during the Eocene (25–50 MYA), at the end of a period of major marine incursion in West Africa [Fig. 1; 23]. Herring subsequently spread across central Africa, colonizing Lake Tanganyika and diversifying into the two present-day endemics 2–16 MYA.
Results
Preliminary Sequence Analyses
Mitochondrial sequences of 12S rDNA, 16S rDNA and cytochrome b (Cytb) were collected, collated and aligned for 49 species (90 specimens), resulting in a total sequence length of between 1,360 and 2,510 bp per specimen. Despite repeated attempts to amplify Cytb from Spratelloides robustus (H101), this individual failed to yield any PCR product for this gene. While a ML homogeneity test rejected congruency of sequences from the three target loci (p<0.003 for all topologies), sequence data were concatenated in accordance with a total evidence approach [24]. Analyses of Cytb sequence data revealed saturation of third codon transitions for Kimura-2-parameter distances greater than 0.40, a pattern confirmed by a statistical test [25] which indicated substantial saturation at third codon positions (Iss<Iss.sym; p = 0.388). Third codon positions of Cytb were consequently eliminated from further analyses, resulting in a concatenated dataset of between 1,049 and 1,811 bp of sequence data per individual.
Phylogenetic Relationships among Clupeiform Fishes
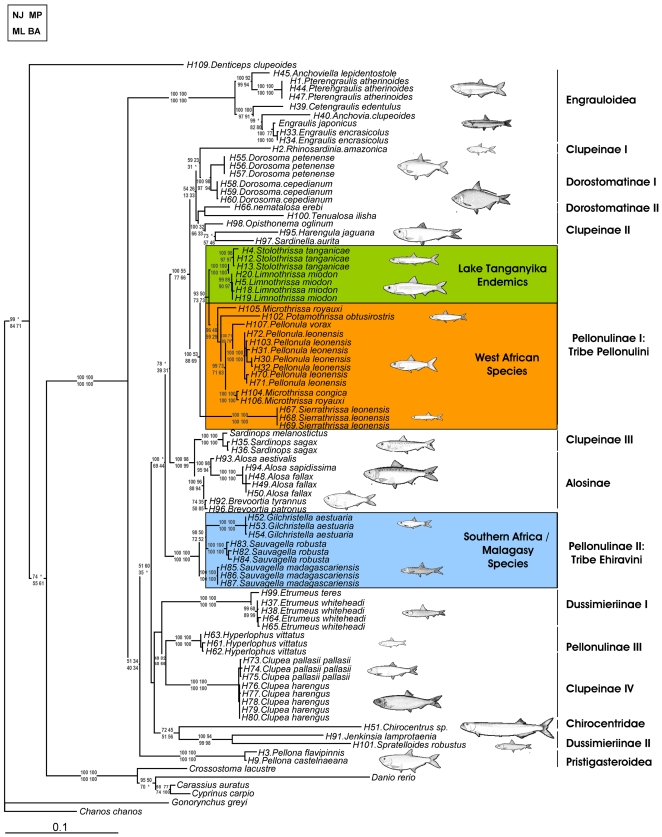
While molecular phylogenetic reconstruction provided strong support for most subfamily groups of clupeiforms, resolution was weaker at deeper levels of the phylogeny (Fig. 2). Nonetheless, several major patterns were clear. Molecular phylogenetic analyses uncovered most traditional groupings of clupeiform fishes (Fig. 2) and identified several major incongruencies with previous morphological-based phylogenies [reviewed in 21]. Although the maximum likelihood phylogeny placed Denticeps clupeoides, the sole living member of the Denticipitoidei, outside the Clupeiformes (Fig. 2), a Shimodaira-Hasegawa (SH) test did not reject a monophyletic clupeiform assemblage (Table 1; LRT: P = 0.8963). Phylogenetic reconstruction supported the monophyly of the Engrauloidea and Pristigasteroidea, along with the Chirocentridae and Alosinae. In contrast, the Clupeinae, Pellonulinae, Dorostomatinae and Dussimierinae all formed polyphyletic assemblages (Fig. 2) and monophyly could be statistically rejected for both the Clupeinae and Pellonulinae (Table 1; SH LRT test: p<0.001 for both subfamilies).
Figure 2. Maximum likelihood tree topology based on the combined dataset of 1,811bp of 12S, 16S and Cytochrome b.
Numbers on branches represent bootstrap support for Distance, Maximum Parsimony and Maximum Likelihood analyses and posterior probabilities from Bayesian analysis. Traditional Clupeoid groups and major African freshwater lineages indicated. Clupeid diagrams reprinted with the permission of the Food and Agriculture Organization of the United Nations [34], [75].
Table 1. Shimodiara-Hasegawa [63] test of alternative topologies.
| Topology | Likelihood | Lmax-Lα | P |
| ML Topology (Fig. 2) | 14940.81 | 0.0 | |
| Monophyletic Clupeiformes (Denticipitoidei, Clupeoididei) | 14941.77 | 0.96 | 0.8963 |
| Monophyletic Clupeidae (Dussumieriinae, Pellonulinae, Dorosomatinae, Clupeinae, Alosinae) | 14945.02 | 4.21 | 0.8049 |
| Monophyletic Dorostomatinae | 14946.51 | 5.70 | 0.7103 |
| Monophyletic Dussumieriinae | 14950.11 | 9.30 | 0.6529 |
| Monophyletic Clupeinae | 15085.89 | 145.08 | <0.001 |
| Monophyletic Pellonulinae | 15021.73 | 80.92 | <0.001 |
Tree topology, estimated likelihood, log-likelihood differences and P-values for alternative topologies tested (χ2-test). Lmax: Maximum likelihood topology; Lα: Likelihood of topology α.
Polyphyly of African Pellonuline Herring
The pellonuline herring of Africa fall into two major lineages, consistent with Grande's [21] suggested taxonomic groupings (Fig. 2). The first group (tribe Ehiravini) includes Sauvagella spp. and Gilchristella aestuaria, riverine herring from southern Africa and Madagascar [26]. The second group (tribe Pellonulini) contains Limnothrissa miodon and Stolothrissa tanganicae, the two species restricted to Lake Tanganyika, and a large group of West African herring. Hyperlophus vittatus, an Australian pellonuline, forms part of a third cluster of non-pellonuline herring (Fig. 2). As highlighted above, a SH test rejected the monophyly of the Pellonulinae (Table 1; LRT: P<0.001). The herring of Tanganyika are nested within a larger group of West African herring (Fig. 2) and are most parsimoniously derived from this group (West Africa→Tanganyika: 1 Step; Tanganyika→West Africa: 2 Steps).
Local and Relaxed Molecular Clocks
A global molecular clock was rejected in favor of lineage-specific rates of molecular evolution (LRT: unconstrained model –ln L = 14940.81; constrained model –ln L = 15209.60; χ2 88 = 537.58; p<0.001). A Bayesian-based approach, incorporating multiple fossil calibration points, was used to estimate divergence times for critical nodes in the phylogeny.
Three independent runs of the relaxed clock generated consistent results (Fig. 3). Molecular clock calibrations indicate that pellonuline herring reached Madagascar 48 MYA (95% reliability interval: 34.0–66.2). Mainland Africa was subsequently colonized twice by pellonulids: (1) an independent colonization of western Africa by the Pellonulini approximately 37 MYA (95% reliability interval: 25.0–53.3 MYA) and (2) the southern Africa colonization of Gilchristella aestuaria from Malagasy ancestors which took place 20 MYA (95% reliability interval: 7.5–34.4 MYA) (Fig. 3). The pellonuline herring of Lake Tanganyika diverged from a large group of West African species approximately 27 MYA (95% reliability interval: 25.0–53.3 MYA), diverging into the two present-day Tanganyikan endemics 8 MYA (95% reliability interval: 2.1–15.9 MYA). While the reliability intervals of these divergence time estimates are large, they are consistent with major geophysical changes on the African continent. The colonization of West Africa 37 MYA is consistent with the end of a major marine incursion in the region (Fig. 1) and the split between the two Tanganyikan endemics suggests divergence during the early stages of lake formation approximately 9–12 MYA.
Figure 3. Linearized phylogenetic tree with node ages calculated with Multidivtime [64] using 12S, 16S and Cytochrome b gene partitions.
Fossil calibration points (C1–C7), key divergence times and inferred freshwater colonization events are indicated on the phylogeny along with 95% reliability intervals.
Figure 1. Paleotectonic reconstruction of the African subcontinent illustrating a major marine incursion in the African subcontinent which lasted from the late Cretaceous (Cenomanian-Turonian) through the end of the Eocene (Early-Middle Eocene).
Figures adapted with permission from the author [74].
Discussion
The endemic herring of Lake Tanganyika are the descendants of a group of herring that colonized the African continent during a major marine incursion that occurred in West Africa from 100-35 MYA. Pellonuline herring subsequently diversified in West Africa, spreading across the continent and reaching Lake Tanganyika during the early stages of its formation. While Lake Tanganyika was never in direct contact with the ocean, the herring of the lake are the first group whose ancestry can be traced back to a marine environment, indirectly supporting Moore's [16] thesis on the marine affinities of Tanganyika's biota. The herring of Lake Tanganyika have not diverged significantly from their West African relatives in morphology [19], indicating that the exceptional stability of the Lake has not prompted dramatic morphological innovation in this group, a hypothesis which has been put forward to explain the diversity of other Lake inhabitants [14].
In the absence of close relatives for most of the thalassoid taxa of Lake Tanganyika, it remains difficult to determine what proportion of the morphological diversity found in the Lake is due to in situ diversification and how much of this diversity reflects characteristics already present in the Lake's colonizers. Recent work suggests that the gastropods of Lake Tanganyika may be an interesting candidate for research in this respect [27]. While early researchers including Moore [16] were unable to identify close relatives of Tanganyika's gastropods outside the lake, a recent study has suggested that at least four extant genera of snails may be close relatives of the major lineages of Tanganyikan gastropods [12], [27]. Three of four of these genera are restricted to the Congo basin and West Africa, while the fourth is widespread in Africa, Madagascar and the Middle East. Molecular analyses confirm a close genetic relationship between this widespread lineage (Cleopatra spp.) and a group of Tanganyikan endemics [12], [28], but the remaining three genera have not yet been the focus of a phylogenetic study. The characterization of these outgroup gastropod taxa would allow the determination of the timing of the colonization and diversification of this group in Lake Tanganyika and would help to clarify whether the pattern of freshwater colonization and spread exhibited by pellonuline herring is relevant for other taxonomic groups.
Geological Evidence Suggests Repeated Marine Incursions Into Central Africa
Moore [16] identified vast marine deposits in the African interior and used this finding as one of his strongest arguments for a historical connection between Lake Tanganyika and the sea. More recent geophysical surveys indicate that major marine incursions into Africa have occurred repeatedly in geological time. Fossils of marine fishes have been found in limestone beds of the Congo basin, providing conclusive evidence of a major marine incursion during the late Jurassic (150 MYA) [29]. Analyses of the sedimentology of Central and West Africa indicate that sea level increases caused by climate fluctuations continued to spur marine incursions after the Jurassic and a large marine seaway is thought to have extended from Libya to West Africa from the late Cretaceous through to the Eocene [Fig. 1; 23]. Given the frequency and extent of marine incursions into the African continent over the past 150 MY, it is somewhat surprising that so little attention has been paid to the possibility that freshwater capture of marine organisms has contributed to the present-day aquatic biodiversity of Africa.
While the frequency of marine incursions into the Congo basin is thought to have slowed after the Mesozoic due to changes in the geology of the region [29], Beadle [30] indicates that the Congo basin was dominated by a large inland sea during the Pliocene (2–5 MYA). If this is indeed the case, the presence of a large stable water body in central Africa at this time may have facilitated the dispersal of freshwater organisms between western and eastern Africa. The presence of this palaeolake may have also fostered increased rates of speciation during the Pliocene, a pattern recently suggested for the cichlid fishes of palaeolake Makgadikgadi in southern Africa [31].
Lake Tanganyikan Herring: Evolutionary Stasis Despite Early Colonization
Molecular clock estimates indicate that the herring of Lake Tanganyika have been present in the lake for at least 2 MY and likely much longer (7.6MY; 95% reliability interval: 2.1–15.9MY). However, despite an extended tenure in the lake, this group has only diversified into two species, an extremely modest diversity when compared to the more than 200 species of cichlid fishes found in Lake Tanganyika [11]. Cichlid fishes have radiated repeatedly, both in the neotropics and in Africa, most notably in the African Great Lakes, where lineages of at least 200, 700 and 500 cichlid fishes are found in Lakes Tanganyika, Malawi and Victoria respectively [32]. Several recent reviews of evolutionary radiations have identified three key stages which characterize species-rich radiations [3], [33]. The first stage, diversification in habitat, occurs in the early stages of evolutionary radiations, when resource competition promotes the use of different habitats. The second phase of evolutionary radiation involves further niche partitioning, as morphological changes allow species to exploit underutilized resources. Streelman and Danley [3] suggest that species diversity can only be fully realized after a third phase of radiation, diversification in secondary sexual characteristics associated with reproduction. While the order and importance of these three stages may vary among radiations, most species-rich radiations appear to have involved some form of all three of these stages. While there is some degree of habitat partitioning between the more onshore (Limnothrissa miodon) and offshore (Stolothrissa tanganicae) herring species of Lake Tanganyika [22], these species have not significantly diverged in their resource utilization [34] and there is no indication of secondary sexual characteristics associated with assortative mating, suggesting that any radiation of this group is still in its initial phase. The lack of major radiation of the herring of Tanganyika may be due to intrinsic differences between herring and cichlid fishes which influence their speciation potential [33] or may be related to the different habitats inhabited by these species. Alternatively, the potential for an adaptive radiation of herring may have been limited by the presence of an already diverse cichlid fauna in the lake soon after its formation (see above).
As essentially pelagic fishes, the possibility for allopatric divergence in the herring of Lake Tanganyika may be reduced when compared to the nearshore cichlids of the Lake. Several recent studies have highlighted the importance of lake level fluctuations in the diversification of its endemic cichlids [35], [36]. These authors suggest that allopatric speciation likely played an important role in the initial stages of diversification among littoral cichlids. The sole population genetic study of Tanganyika's herring revealed no significant population structure in populations of L. miodon from the lake [37], indicating little evidence of intralacustrine divergence in this species. The species-level diversity of pelagic cichlids of Lake Tanganyika is also lower than that of littoral groups [38], though modest radiations have occurred in several tribes of pelagic and deep-water cichlids [39]–[41].
Clupeiform Fishes: Weak Support for Traditional Subfamily Relationships
While the taxonomic sampling here is the most comprehensive of any molecular study of clupeomorph fishes, several groups are nonetheless only poorly represented (<20% of Pristigasteridae, Engraulidae, Clupeinae and Dorostomatinae; Table 2). Grande [21] suggested that the Clupeinae, Alosinae and Dorostomatinae were likely all artificial groupings that would be further subdivided following further investigations. This hypothesis is supported for the Clupeinae (4 distinct lineages and statistical rejection of subfamily monophyly), but monophyly of both the Alosinae and Dorostomatinae cannot be statistically rejected. As only a subset of species from each of these subfamilies were included here (Table 2), future studies should aim to exhaustively sample species at the subfamily level to rigorously test Grande's morphological hypotheses.
Table 2. Taxonomic sampling of the present study.
| Order | Suborder | Superfamily | Family | Subfamily | Extant Species | Included Species |
| Clupeiformes | ||||||
| Denticipitoidei | 1 | 1 | ||||
| Clupeoidei | ||||||
| Pristigasteroidea | Pristigasteridae | Pristigasterinae | 30 | 2 | ||
| Engrauloidea | Engraulidae | 130 | 6 | |||
| Clupeoidea | Chirocentridae | 2 | 1 | |||
| Clupeidae | Pellonulinae | 41 | 12 | |||
| Dussumieriinae | 11 | 4 | ||||
| Dorostomatinae | 22 | 3 | ||||
| Alosinae | 19 | 5 | ||||
| Clupeinae | 61 | 9 |
Clupeiform taxonomic groupings follow Grande [21]. The endemic herring of Lake Tanganyika (Stolothrissa tanganicae and Limnothrissa miodon) are members of the Pellonulinae.
Although almost 2000bp of sequence data were analyzed for the taxa included here, phylogenetic relationships at deeper branches in the phylogeny remain only poorly resolved. These results are consistent with two recent investigations of clupeiform fishes which, despite similar taxonomic sampling, yielded conflicting results concerning several of the intraorder relationships. Li and Orti [42] employed a combination of mitochondrial and nuclear genes to investigate relationships among the Clupeiformes. Li and Orti statistically rejected the monophyly of the Clupeinae and found that Denticeps clupeoides clustered together with the cyprinid outgroups included in their study, a pattern that they suggested might be due to the high GC content of this species relative to other clupeomorphs.
A second recent study used complete mitochondrial genome sequences to investigate the clupeiform question [43]. In contrast with the results of Li and Orti, Lavoue et al. found that Denticeps clupeoides clustered together with the other Clupeomorphs. This study also statistically rejected the monophyly of the Clupeidae as well as the subfamilies Alosinae, Clupeinae and Dorostomatinae, in line with Grande's [21] hypothesis of polyphyly of these groups on the basis of morphological data. Both Lavoue et al and Li and Orti supported a sister-group relationship between the Engrauloidea and Clupeoidea [44], a pattern also found here, but the two analyses conflict in their placement of the Pristigasteridae and Chirocentridae, two groups whose placement is also only weakly supported in this study. While the results of the Li and Orti [42] and Lavoue et al. [43] studies suggest that additional molecular data might help to better resolve relationships among the clupeiform fishes, more extensive taxonomic sampling will be essential before undertaking a major revision of this group.
Of particular interest in light of the marine incursion scenario put forth here is the grouping of Ethmalosa fimbriata, an estuarine species widespread along the coasts of West Africa, with the freshwater pellonuline herring found in the region [43]. This species has been the focus of a recent phylogeographic study [45], which suggests that the historical population structure of the species has been strongly influenced by Pleistocene sea level fluctuations in the region, when local populations of the species were isolated in freshwater refuges. As this euryhaline species may be the closest living marine relative of the freshwater pellonulines of West Africa, future comparisons between the morphology and physiology of Ethmalosa and its pellonuline relatives may help to identify key innovations that allowed the ancestor of these groups to successfully colonize freshwater.
Materials and Methods
Sample Collection, PCR Amplification and DNA Sequencing
Specimens were collected by the authors or provided by colleagues between 1999–2003 (Tables 2 & 3). All specimens were preserved in 70% ethanol and total genomic DNA was extracted by proteinase K/SDS digestion and purified by phenol-chloroform extraction and ethanol precipitation [46]. Several recent investigations have supported a close phylogenetic relationship between clupeiform fishes and the Ostariophysi [47], [48]. Published sequences for Carassius auratus, Crossostoma lacustre, Cyprinus carpio, and Danio rerio (Cypriniformes) as well as Gonorhynchus greyi and Chanos chanos (Gonorhynchiformes) were included as outgroups.
Table 3. Specimen collection information.
| Sample # | Species | Collection Locality (Country) (Date) | Collector/Reference |
| H1 | Pterengraulis atherinoides | Braganca Paulista (Brazil) (16/07/00) | AM |
| H2 | Rhinosardinia amazonica | Braganca Paulista (Brazil) (16/07/00) | AM |
| H3 | Pellona flavipinnis | (Brazil) | AM |
| H4 | Stolothrissa tanganicae | Lake Tanganyika (Zambia) (03/11/99) | ABW |
| H5 | Limnothrissa miodon | Lake Tanganyika (Zambia) (03/11/99) | ABW |
| H9 | Pellona castelnaeana | (Brazil) | IF |
| H12 | Stolothrissa tanganicae | Lake Tanganyika (Zambia) (25/12/00) | ABW |
| H13 | Stolothrissa tanganicae | Lake Tanganyika (Zambia) (25/12/00) | ABW |
| H18 | Limnothrissa miodon | Malagarasi River (Tanzania) (12/12/00) | ABW |
| H19 | Limnothrissa miodon | Malagarasi River (Tanzania) (12/12/00) | ABW |
| H20 | Limnothrissa miodon | Malagarasi River (Tanzania) (12/12/00) | ABW |
| H30 | Pellonula leonensis | Tano Basin (Ivory Coast) (XX/04/00) | GTT |
| H31 | Pellonula leonensis | Tano Basin (Ivory Coast) (XX/04/00) | GTT |
| H32 | Pellonula leonensis | Tano Basin (Ivory Coast) (XX/04/00) | GTT |
| H33 | Engraulis encrasicolus | Hout Bay (South Africa) (08/08/01) | CVL |
| H34 | Engraulis encrasicolus | Hout Bay (South Africa) (08/08/01) | CVL |
| H35 | Sardinops sagax ocellatus | Hout Bay (South Africa) (07/08/01) | CVL |
| H36 | Sardinops sagax ocellatus | Hout Bay (South Africa) (07/08/01) | CVL |
| H37 | Etrumeus whiteheadi | Hout Bay (South Africa) (07/08/01) | CVL |
| H38 | Etrumeus whiteheadi | Hout Bay (South Africa) (07/08/01) | CVL |
| H39 | Cetengraulis edentulus | Braganca (Brazil) (1999) | UK |
| H40 | Anchovia clupeoides | Braganca (Brazil) (1999) | UK |
| H44 | Pterengraulis atherinoides | Braganca (Brazil) (1999) | UK |
| H45 | Anchoviella lepidentostole | Braganca (Brazil) (1999) | UK |
| H47 | Pterengraulis atherinoides | Braganca (Brazil) (1999) | UK |
| H48 | Alosa fallax (Severn33) | Severn (England) (June 1–6/00) | MA |
| H49 | Alosa fallax (Severn40) | Severn (England) (June 1–6/00) | MA |
| H50 | Alosa fallax (Severn44) | Severn (England) (June 1–6/00) | MA |
| H51 | Chirocentrus sp. | (Singapore) (XX/XX/98) | BV |
| H52 | Gilchristella aestuaria | Eastern Cape (South Africa) (09/11/01) | RB |
| H53 | Gilchristella aestuaria | Orange River (Nigeria) (02/05/01) | RB |
| H54 | Gilchristella aestuaria | Lake Piti (Mozambique) (29/09/01) | RB |
| H55 | Dorosoma petenense | Brazos River (Texas) (04/02/02) | RL |
| H56 | Dorosoma petenense | Brazos River (Texas) (04/02/02) | RL |
| H57 | Dorosoma petenense | Brazos River (Texas) (04/02/02) | RL |
| H58 | Dorosoma cepedianum | Lake Wauberg (Florida) (22/01/02) | KT |
| H59 | Dorosoma cepedianum | Lake Wauberg (Florida) (22/01/02) | KT |
| H60 | Dorosoma cepedianum | Lake Wauberg (Florida) (22/01/02) | KT |
| H61 | Hyperlophus vittatus | Bunbury, Western Australia (Australia) (XX/01/02) | DG |
| H62 | Hyperlophus vittatus | Bunbury, Western Australia (Australia) (XX/01/02) | DG |
| H63 | Hyperlophus vittatus | Bunbury, Western Australia) (Australia) (XX/01/02) | DG |
| H64 | Etrumeus whiteheadi | Hout Bay (South Africa) (07/08/01) | CVL |
| H65 | Etrumeus whiteheadi | Hout Bay (South Africa) (07/08/01) | CVL |
| H66 | Nematalosa erebi | Fish River, Darwin (Australia) (05/09/01) | HL |
| H67 | Sierrathrissa leonensis | Volta Basin (Ghana) (23/01/01) | GTT |
| H68 | Sierrathrissa leonensis | Volta Basin (Ghana) (23/01/01) | CVL |
| H69 | Sierrathrissa leonensis | Volta Basin (Ghana) (23/01/01) | CVL |
| H70 | Pellonula leonensis | Volta Basin (Ghana) (23/01/01) | CVL |
| H71 | Pellonula leonensis | Volta Basin (Ghana) (23/01/01) | CVL |
| H72 | Pellonula leonensis | Volta Basin (Ghana) (23/01/01) | CVL |
| H73 | Clupea pallasii pallasii | Cape Flattery, Washington (USA) (XX/09/02) | LW |
| H74 | Clupea pallasii pallasii | Cape Flattery, Washington (USA) (XX/09/02) | LW |
| H75 | Clupea pallasii pallasii | Cape Flattery, Washington (USA) (XX/09/02) | LW |
| H76 | Clupea harengus | Sept Iles, Quebec (Canada) (17/06/02) | IM |
| H77 | Clupea harengus | Sept Iles, Quebec (Canada) (17/06/02) | IM |
| H78 | Clupea harengus | Sept Iles, Quebec (Canada) (17/06/02) | IM |
| H79 | Clupea harengus | La Romaine, Quebec (Canada) (07/06/02) | IM |
| H80 | Clupea harengus | La Romaine, Quebec (Canada) (07/06/02) | IM |
| H82 | Sauvagella robusta | Ambomboa River (Madagascar) (XX/XX/96) | JS |
| H83 | Sauvagella robusta | Ambomboa River (Madagascar) (XX/XX/96) | JS |
| H84 | Sauvagella robusta | Ambomboa River (Madagascar) (XX/XX/96) | JS |
| H85 | Sauvagella madagascariensis | Onive River (Madagascar) (XX/02/94) | JS |
| H86 | Sauvagella madagascariensis | Onive River (Madagascar) (XX/02/94) | JS |
| H87 | Sauvagella madagascariensis | Onive River (Madagascar) (XX/02/94) | JS |
| H91 | Jenkinsia lamprotaenia | Carrie Bow Bay (Belize) (07/19/91) | EW |
| H92 | Brevoortia tyrannus | mid Atlantic Bight (USA) (03/09/95) | KS |
| H93 | Alosa aestivalis | mid Atlantic Bight (USA) (03/09/95) | KS |
| H94 | Alosa sapidissima | mid Atlantic Bight (USA) (03/09/95) | KS |
| H95 | Harengula jaguana | Brownsville, Texas (USA) (06/19/02) | KM |
| H96 | Brevoortia patronus | Brownsville, Texas (USA) (06/19/02) | KM |
| H97 | Sardinella aurita | Brownsville, Texas (USA) (06/19/02) | KM |
| H98 | Opisthonema oglinum | Brownsville, Texas (USA) (06/19/02) | KM |
| H99 | Etrumeus teres | Brownsville, Texas (USA) (06/19/02) | KM |
| H100 | Tenualosa ilisha | Padma River (Bangladesh) (15/01/04) | HK |
| H101 | Spratelloides robustus | Myponga, Gulf St. Vincent (Australia) (29/04/01) | PR |
| H102 | Potamothrissa obtusirostris | Congo River, Brazzaville (16/01/03) | VM |
| H103 | Pellonula leonensis | Gamba Lagoon, Brazzavlle (Congo-Brazzaville) (10/02/03) | VM |
| H104 | Microthrissa congica | Congo River, Brazzaville (Congo-Brazzaville) (16/01/03) | VM |
| H105 | Microthrissa royauxi | Congo River, Brazzaville (Congo-Brazzaville) (16/01/03) | VM |
| H106 | Microthrissa congica | Congo River, Malebo (Congo-Brazzaville) (28/05/03) | VM |
| H107 | Pellonula vorax | Ndogo Lagoon (Congo) (10/02/03) | VM |
| H109 | Denticeps clupeoides | AM | |
| NC_003097 | Engraulis japonicus | [76] | |
| NC_002616 | Sardinops melanostictus | (Japan) | [77] |
| NC_004702 | Gonorhynchus greyi | (Australia) | [78] |
| NC_004693 | Chanos chanos | Sulawesi (Indonesia) | [78] |
| NC_002079 | Carassius auratus | [79] | |
| NC_001727 | Crossostoma lacustre | Dahu River, Taiwan (China) | [80] |
| NC_001606 | Cyprinus carpio | [81] | |
| NC_002333 | Danio rerio | [82] |
Collectors: ABW (Tony Wilson); AM (Axel Meyer); BV (Byrappa Venkatesh); CVL (Carl van der Lingen/Megan Terry); DG (Daniel Gaughan); EW (Ed Wiley); GTT (Guy Teugels); HL (Helen Larson); HK (Haseena Khan); IF (Izeni Farias); IM (Ian McQuinn); JS (John Sparks/Melanie Stiassny); KM (Kris McNyset); KS (Kate Shaw); KT (Kim Tugend/Mike Allen); LW (Laurie Weitcamp/Mike Ford); MA (Miran Aprahamian); PR (Paul Rodgers); RB (Roger Bills/Sally Terry); RL (Raymond Li/Fran Gelwick); UK (Uwe Krumme), VM (Victor Mamonekene/Melanie Stiassny).
The polymerase chain reaction (PCR) was used to amplify a total of 2,608 bp from three fragments of mitochondrial DNA. A 548 bp segment of the large subunit (16S) mitochondrial ribosomal gene was amplified using primers L2510 and H3058 [49], while primers L1090 [46] and H2001 [50] were used to amplify 911 bp of the small subunit (12S) mitochondrial ribosomal gene, tRNA-Valine and 16S. 1,149 bp of the cytochrome b (Cytb) gene were amplified with L14725 [51] and H15926 [52]. Reaction conditions are described in Wilson et al. [52]. Sequencing reactions were prepared as in Wilson et al. [52] and visualized on an ABI 3100 automated sequencer. DNA sequences have been submitted to GenBank (Accession numbers: EU552549-EU552793).
Sequence Alignment and Phylogenetic Reconstruction
The orthologous DNA sequences obtained were aligned, using default settings, by CLUSTALW [53] and optimized by eye. Optimization of rDNA gene fragment alignments was facilitated through the use of secondary structure models for teleost long and short subunit RNAs [54], [55]. Regions of the optimized alignment which could not be reliably aligned were eliminated from analysis (data alignment available upon request), resulting in an alignment of 525 bp for 16S, 520 bp for 12S and 1,149 bp for the Cytb dataset, for a total of 2,194 bp. Data partitions were tested for substitution saturation using a non-parametric statistical test implemented by DAMBE 4.5.47 [56]. Prior to concatenating the three sequence alignments, the congruency of data partitions was tested with a likelihood-based congruency test (α = 0.05; 10000 RELL bootstrap replicates) [57], using maximum likelihood (ML) topologies generated from individual gene analyses as well as the overall ML tree (see below).
Neighbor-joining distance and maximum parsimony analyses were performed with PAUPV4b10 [58], with indels coded as missing data. Parsimony minimal analyses included a full heuristic search with random addition (50 replicates), the TBR branch swapping algorithm and the MULPARS option. For parsimony analyses, a transversion/transition weighting of three was used. Neighbor-joining analyses applied a GTR+I+G model of substitution [59], with transition rate matrix (1.9150 9.8250 3.6271 0.8214 17.2997), gamma shape parameter (0.5214), proportion of invariable sites (0.4838) and nucleotide frequencies (A: 0.2764; C: 0.2780; G: 0.2168; T: 0.2288) estimated from the dataset using Modeltest V3.7 [60]. Reliability of phylogenetic signal was tested using 500 bootstrap replicates for both parsimony and NJ distance analyses. A single random addition of taxa was used for each replicate of the parsimony bootstrap.
The overall ML tree topology for each gene and the concatenated dataset was determined using GarliV0.951 [61] with model parameters as estimated by Modeltest. The initial tree topology was constructed by random addition, the stopgen and stoptime parameters were both set to 10,000,000 and search termination settings were set at default values. Four independent runs of each tree search produced final likelihood values that varied by less than 3.5. The tree was the highest likelihood value was used for subsequent analyses. Phylogenetic reliability of the overall ML tree was tested using 500 bootstrap replicates.
Phylogenetic relationships were also estimated according to a Bayesian method of phylogenetic inference implemented by MrBayes v3.1.2 [62]. Posterior probabilities of phylogenetic trees were approximated by a 1,000,000-generation Metropolis-coupled Markov chain Monte Carlo simulation (MCMCMC; four chains, chain temperature = 0.2), under a GTR+I+G model of sequence evolution, with simultaneous estimation of parameters, sampling every 1,000th generation. A 50% majority-rule consensus tree was constructed following a 100,000-generation burn-in to allow chains to reach stationarity. Three separate runs of MrBayes v3.1.2 under these parameter settings generated qualitatively similar results.
To test morphological-based hypotheses on the taxonomic relationships among clupeiform fishes, the ML topology and branch lengths were recalculated as above, with major groupings constrained to be monophyletic. The deviation between these alternative topologies and the unconstrained ML topology was tested using a Shimodaira-Hasegawa (SH) test [63] with 10000 RELL bootstrap replicates.
Fossil Calibration and Molecular Clock
To investigate whether rates of molecular evolution fit with a strict molecular clock model, the likelihood of the ML phylogeny was recalculated with the constraint of global molecular clock using the Rambaut parameterization for clock optimization implemented in PAUP4b10 [58]. The likelihood of the clock-based tree was compared with that of the unconstrained topology using a likelihood ratio test (LRT).
A relaxed molecular clock method allowing autocorrelated rates of evolution along branches [64] was also implemented here. This Bayesian-based method allows for uncertainty in fossil calibration points and permits variation in rates of molecular evolution among genes. Molecular clock calibration followed the protocols outlined in Rutschmann [65]. Briefly, model parameters were estimated for each gene partition using PAML V3.14 [66] under a model of evolution incorporating variable nucleotide frequencies, a transition:transversion parameter and nucleotide variation across sites [F84+G model; described in 67]. Branch lengths of the ML tree were optimized for each gene partition and the variance-covariance matrix of evolutionary rates was estimated using Estbranches. Finally, divergence time estimates were calculated using the Bayesian MCMCMC approach implemented in Multidivtime [64], which simultaneously considers branch length estimates and variance-covariance matrices from each data partition. Posterior probabilities of divergence time estimates were determined following a 100,000 cycle burn-in. The MCMCMC chain was sampled every 100th cycle for a total of 2,000,000 cycles. Rates of genetic change were set to vary freely among gene partitions and a prior root-to-tip divergence time estimate was set at 146 MY. Three runs of this program from different starting points yielded consistent estimates of divergence times.
A suite of seven fossil calibration points for clupeoid and cyprinid fishes were included for calibration of the molecular clock used here: C1–Earliest fossil of Engaulis japonicus: 0–2 MY (Kokubu Group, Japan; Yabumoto [68]), C2–Earliest fossil of Sardinops melanostictus: 0–2 MY (Kokobu Group, Japan; Yabumoto [68]), C3–Earliest fossil of Dorosoma petenense: 2–3 MY (Gatuna Formation, New Mexico; Miller [69]), C4–Earliest engraulid fossil: Engraulis tethensis: 6–12 MY (Mesaoria Group, Cyprus; Grande and Nelson [70]), C5–Earliest Etrumeus sp. fossil: Etrumeus hafizi: 23–38 MY (Estabanhat, Iran; Arambourg [71], Grande [21]), C6–Earliest pristigasterid fossil: Gastroclupea branisai: 66–94 MY (El Molino Formation, Bolivia; Branisa [72], Grande [21]) and C7–Earliest cyprinid fossil: Parabarbus sp.: 49–55 MY [Sytchevskaya (1986) in 73].
Acknowledgments
We thank M. Allen (U. Florida), M. Aprahamiani (Environment Agency, United Kingdom), R. Bills (SAIAB), I. Farias (U. Federal do Amazonas, M. Ford (NWFSC, Seattle), D. Gaughan (Department of Fisheries, Western Australia), F. Gelwick (Texas A&M), H. Larson (Museum of the Northern Territory, Darwin), R. Li (Texas A&M), H. Khan (U. Dhaka), U. Krumme (Center for Marine Tropical Ecology, Bremen), V. Mamonekene (Congo-Brazzaville), K. McNyset (Kansas U.), I. McQuinn (DFO Quebec), P. Rodgers (SARDI Australia), K. Shaw (Kansas U.), J. Sparks (AMNH), M. Stiassny (AMNH), M. Terry (Marine and Coastal Management, South Africa), S. Terry (SAIAB), K. Tugend (U. Florida), L. Weitcamp (NWFSC, Seattle), E. Wiley (Kansas U.), C. van der Lingen (Marine and Coastal Management, South Africa) and B. Venkatesh (IMCB, Singapore) for assistance with collections. Guy Teugels passed away before the completion of this project–his contributions to African ichthyology will be sorely missed.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was funded by research grants from the National Research Council and Swiss National Science Foundation to ABW and a grant from the Deutsche Forschungsgemeinschaft to AM. The funding agencies played no role in the analysis or interpretation of the data presented here.
References
- 1.Gorthner A. What is an ancient lake? In: Martens K, Goddeeris B, Coulter G, editors. Archiv. Hydrobiologie Beih. Ergebn. Limnologie. Speciation in Ancient Lakes. Stuttgart: E. Schweizerbart'sche Verlagsbuchhandlung; 1994. pp. 97–100. [Google Scholar]
- 2.Martens K, Goddeeris B, Coulter G. Stuttgart, Germany: E. Schweizerbart'sche Verlagsbuchhandlung; 1994. Speciation in Ancient Lakes. p. 508. [Google Scholar]
- 3.Streelman JT, Danley PD. The stages of vertebrate evolutionary radiation. Trends Ecol Evol. 2003;18:126–131. [Google Scholar]
- 4.Stiassny MLJ, Meyer A. Cichlids of the Rift Lakes. Sci Am. 1999;280:64–69. [Google Scholar]
- 5.Sturmbauer C. Explosive speciation in cichlid fishes of the African Great Lakes: a dynamic model of adaptive radiation. J Fish Biol. 1998;53:18–36. [Google Scholar]
- 6.Kontula T, Kirilchik SV, Vainola R. Endemic diversification of the monophyletic cottoid fish species flock in Lake Baikal explored with mtDNA sequencing. Mol Phylo Evol. 2003;27:143–155. doi: 10.1016/s1055-7903(02)00376-7. [DOI] [PubMed] [Google Scholar]
- 7.von Rintelen T, Wilson AB, Meyer A, Glaubrecht M. Escalation and trophic specialization drive adaptive radiation of freshwater gastropods in ancient lakes on Sulawesi, Indonesia. Proc R Soc Lond B Biol Sci. 2004;271:2541–2549. doi: 10.1098/rspb.2004.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albrecht C, Trajanovski S, Kuhn K, Streit B, Wilke T. Rapid evolution of an ancient lake species flock: Freshwater limpets (Gastropoda : Ancylidae) in the Balkan Lake Ohrid. Org Divers Evol. 2006;6:294–307. [Google Scholar]
- 9.Marijnissen SAE, Michel E, Daniels SR, Erpenbeck D, Menken SBJ, et al. Molecular evidence for recent divergence of Lake Tanganyika endemic crabs (Decapoda : Platythelphusidae). Mol Phylo Evol. 2006;40:628–634. doi: 10.1016/j.ympev.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 10.Nishida M. Lake Tanganyika as an evolutionary reservoir of old lineages of East African cichlid fishes: Inferences from allozyme data. Experientia (Basel) 1991;47:974–979. [Google Scholar]
- 11.Salzburger W, Meyer A, Baric S, Verheyen E, Sturmbauer C. Phylogeny of the Lake Tanganyika cichlid species flock and its relationship to the Central and East African Haplochromine cichlid fish faunas. Syst Biol. 2002;51:113–135. doi: 10.1080/106351502753475907. [DOI] [PubMed] [Google Scholar]
- 12.Wilson AB, Glaubrecht M, Meyer A. Ancient lakes as evolutionary reservoirs: evidence from the thalassoid gastropods of Lake Tanganyika. Proc R Soc Lond B Biol Sci. 2004;271:529–536. doi: 10.1098/rspb.2003.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salzburger W, Mack T, Verheyen E, Meyer A. Out of Tanganyika: Genesis, explosive speciation, key-innovations and phylogeography of the haplochromine cichlid fishes. BMC Evol Biol. 2005;5 doi: 10.1186/1471-2148-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coulter GW. London, England: Natural History Museum; 1991. Lake Tanganyika and Its Life. p. 354. [Google Scholar]
- 15.Moore JES. The fresh-water fauna of Lake Tanganyika. Nature. 1897;56:198–200. [Google Scholar]
- 16.Moore JES. London, England: Hurst and Blackett; 1903. The Tanganyika Problem. p. 371. [Google Scholar]
- 17.Tiercelin JJ, Mondeguer A. The geology of the Tanganyika trough. In: Coulter GW, editor. Lake Tanganyika and its life. London, England: Natural History Museum; 1991. pp. 7–48. [Google Scholar]
- 18.Cohen AS, Lezzar KE, Tiercelin JJ, Soreghan M. New palaeogeographic and lake-level reconstructions of Lake Tanganyika: Implications for tectonic, climatic and biological evolution in a rift lake. Basin Res. 1997;9:107–132. [Google Scholar]
- 19.Gourene G, Teugels G. Synopsis de la classification et phylogenie des Pellonulinae de l'Afrique Occidentale et Centrale (Teleostei; Clupeidae). J Afr Zool. 1994;108:77–91. [Google Scholar]
- 20.Whitehead PJP, Teugels G. The West African pygmy herring Sierrathrissa leonensis: General features, visceral anatomy and osteology. Amer Mus Novit. 1985;2835:1–44. [Google Scholar]
- 21.Grande L. Recent and fossil clupeomorph fishes with materials for revision of the subgroups of clupeoids. Bull Am Mus Nat Hist. 1985:235–372. [Google Scholar]
- 22.Phiri H, Shirakihara K. Distribution and seasonal movement of pelagic fish in southern Lake Tanganyika. Fish Res. 1999;41:63–71. [Google Scholar]
- 23.Guiraud R, Bosworth W, Thierry J, Delplanque A. Phanerozoic geological evolution of Northern and Central Africa: An overview. J Afr Earth Sci. 2005;43:83–143. [Google Scholar]
- 24.Barker FK, Lutzoni FM. The utility of the incongruence length difference test. Syst Biol. 2002;51:625–637. doi: 10.1080/10635150290102302. [DOI] [PubMed] [Google Scholar]
- 25.Xia XH, Xie Z, Salemi M, Chen L, Wang Y. An index of substitution saturation and its application. Mol Phylo Evol. 2003;26:1–7. doi: 10.1016/s1055-7903(02)00326-3. [DOI] [PubMed] [Google Scholar]
- 26.Stiassny MLJ. Revision of Sauvagella Bertin (Clupeidae; Pellonulinae; Ehiravini) with a description of a new species from the freshwaters of Madagascar and diagnosis of the Ehiravini. Copeia. 2002:67–76. [Google Scholar]
- 27.Van Damme D, Pickford M. The late Cenozoic Thiaridae (Mollusca, Gastropoda, Cerithioidea) of the Albertine Rift Valley (Uganda-Congo) and their bearing on the origin and evolution of the Tanganyikan thalassoid malacofauna. Hydrobiologia. 2003;498:1–83. [Google Scholar]
- 28.Michel E. Phylogeny of a gastropod species flock: Exploring speciation in Lake Tanganyika in a molecular framework. In: Rossiter A, Kawanabe H, editors. Ancient lakes: Biodiversity, ecology and evolution. New York, New York: Academic Press; 2000. pp. 275–302. [Google Scholar]
- 29.Giresse P. Mesozoic-Cenozoic history of the Congo Basin. J Afr Earth Sci. 2005;43:301–315. [Google Scholar]
- 30.Beadle LC. London: Longman; 1974. The inland waters of Africa. p. 365. [Google Scholar]
- 31.Joyce DA, Lunt DH, Bills R, Turner GF, Katongo C, et al. An extant cichlid fish radiation emerged in an extinct Pleistocene lake. Nature. 2005;435:90–95. doi: 10.1038/nature03489. [DOI] [PubMed] [Google Scholar]
- 32.Turner GF, Seehausen O, Knight ME, Allender CJ, Robinson RL. How many species of cichlid fishes are there in African lakes? Mol Ecol. 2001;10:793–806. doi: 10.1046/j.1365-294x.2001.01200.x. [DOI] [PubMed] [Google Scholar]
- 33.Kocher TD. Adaptive evolution and explosive speciation: The cichlid fish model. Nat Rev Genet. 2004;5:288–298. doi: 10.1038/nrg1316. [DOI] [PubMed] [Google Scholar]
- 34.Whitehead PJP. FAO species catalogue. Vol. 7: Clupeoid fishes of the world. An annotated and illustrated catalogue of the herrings, sardines, pilchards, sprats, shads, anchovies and wolf-herrings. Part 1-Chirocentridae, Clupeidae and Pristigasteridae. FAO Fish Synop. 1985;125:1–303. [Google Scholar]
- 35.Verheyen E, Rüber L, Snoeks J, Meyer A. Mitochondrial phylogeography of rock-dwelling cichlid fishes reveals evolutionary influence of historical lake level fluctuations of Lake Tanganyika, Africa. Philos Trans R Soc Lond B Biol Sci. 1996;351:797–805. doi: 10.1098/rstb.1996.0074. [DOI] [PubMed] [Google Scholar]
- 36.Sturmbauer C, Baric S, Salzburger W, Rüber L, Verheyen E. Lake Level Fluctuations Synchronize Genetic Divergences of Cichlid Fishes in African Lakes. Mol Biol Evol. 2001;18:144–154. doi: 10.1093/oxfordjournals.molbev.a003788. [DOI] [PubMed] [Google Scholar]
- 37.Hauser L, Carvalho GR, Pitcher TJ. Genetic population structure in the Lake Tanganyika sardine Limnothrissa miodon. J Fish Biol. 1998;53:413–429. [Google Scholar]
- 38.Snoeks J, Rüber L, Verheyen E. The Tanganyika problem: Comments on the taxonomy and distribution patterns of its cichlid fauna. In: Martens K, Goddeeris B, Coulter G, editors. Archiv. Hydrobiologie Beih. Ergebn. Limnologie. Speciation in Ancient Lakes. Stuttgart: E. Schweizerbart'sche Verlagsbuchhandlung; 1994. pp. 355–372. [Google Scholar]
- 39.Brandstatter A, Salzburger W, Sturmbauer C. Mitochondrial phylogeny of the Cyprichromini, a lineage of open-water cichlid fishes endemic to Lake Tanganyika, East Africa. Mol Phylo Evol. 2005;34:382–391. doi: 10.1016/j.ympev.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 40.Duftner N, Koblmuller S, Sturmbauer C. Evolutionary relationships of the Limnochromini, a tribe of benthic deepwater Cichlid fish endemic to Lake Tanganyika, East Africa. J Mol Evol. 2005;60:277–289. doi: 10.1007/s00239-004-0017-8. [DOI] [PubMed] [Google Scholar]
- 41.Koblmuller S, Duftner N, Katongo C, Phiri H, Sturmbauer C. Ancient divergence in bathypelagic Lake Tanganyika deepwater cichlids: Mitochondrial phylogeny of the tribe Bathybatini. J Mol Evol. 2005;60:297–314. doi: 10.1007/s00239-004-0033-8. [DOI] [PubMed] [Google Scholar]
- 42.Li CH, Orti G. Molecular phylogeny of Clupeiformes (Actinopterygii) inferred from nuclear and mitochondrial DNA sequences. Mol Phylo Evol. 2007;44:386–398. doi: 10.1016/j.ympev.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 43.Lavoue S, Miya M, Saitoh K, Ishiguro NB, Nishida M. Phylogenetic relationships among anchovies, sardines, herrings and their relatives (Clupeiformes), inferred from whole mitogenome sequences. Mol Phylo Evol. 2007;43:1096–1105. doi: 10.1016/j.ympev.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 44.di Dario F. Evidence supporting a sister-group relationship between Clupeoidea and Engrauloidea (Clupeomorpha). Copeia. 2002;2002:496–503. [Google Scholar]
- 45.Durand JD, Tine M, Panfili J, Thiaw OT, Lae R. Impact of glaciations and geographic distance on the genetic structure of a tropical estuarine fish, Ethmalosa fimbriata (Clupeidae, S. Bowdich, 1825). Mol Phylo Evol. 2005;36:277–287. doi: 10.1016/j.ympev.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 46.Kocher TD, Thomas WK, Meyer A, Edwards SV, Pääbo S, et al. Dynamics of mitochondrial DNA evolution in animals: Amplification and sequencing with conserved primers. Proc Natl Acad Sci U S A. 1989;86:6196–6200. doi: 10.1073/pnas.86.16.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Briggs JC. The biogeography of otophysan fishes (Ostariophysi : Otophysi): a new appraisal. J Biogeog. 2005;32:287–294. [Google Scholar]
- 48.Lavoue S, Miya M, Inoue JG, Saitoh K, Ishiguro NB, et al. Molecular systematics of the gonorynchiform fishes (Teleostei) based on whole mitogenome sequences: Implications for higher-level relationships within the Otocephala. Mol Phylo Evol. 2005;37:165–177. doi: 10.1016/j.ympev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 49.Palumbi SR, Martin AP, Romano SL, McMillan WOD, Stice L, et al. Honolulu, Hawaii: University of Hawaii; 1991. The simple fool's guide to PCR. [Google Scholar]
- 50.Hrbek T, Larson A. The evolution of diapause in the killifish family Rivulidae (Atherinomorpha, Cyprinodontiformes): A molecular phylogenetic and biogeographic perspective. Evolution. 1999;53:1200–1216. doi: 10.1111/j.1558-5646.1999.tb04533.x. [DOI] [PubMed] [Google Scholar]
- 51.Pääbo S, Thomas WK, Whitfield KM, Kumazawa Y, Wilson AC. Rearrangements of mitochondrial transfer RNA genes in marsupials. J Mol Evol. 1991;33:426–430. doi: 10.1007/BF02103134. [DOI] [PubMed] [Google Scholar]
- 52.Wilson AB, Vincent A, Ahnesjö I, Meyer A. Male pregnancy in seahorses and pipefishes (Family Syngnathidae): Rapid diversification of paternal brood pouch morphology inferred from a molecular phylogeny. J Hered. 2001;92:159–166. doi: 10.1093/jhered/92.2.159. [DOI] [PubMed] [Google Scholar]
- 53.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucl Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waters JM, Lopez JA, Wallis GP. Molecular phylogenetics and biogeography of galaxiid fishes (Osteichthyes : Galaxiidae): Dispersal, vicariance, and the position of Lepidogalaxias salamandroides. Syst Biol. 2000;49:777–795. doi: 10.1080/106351500750049824. [DOI] [PubMed] [Google Scholar]
- 55.Wang HY, Lee SC. Secondary structure of mitochondrial 12S rRNA among fish and its phylogenetic applications. Mol Biol Evol. 2002;19:138–148. doi: 10.1093/oxfordjournals.molbev.a004066. [DOI] [PubMed] [Google Scholar]
- 56.Xia X, Xie Z. Dambe: software package for data analysis in molecular biology and evolution. J Hered. 2001;92:371–373. doi: 10.1093/jhered/92.4.371. [DOI] [PubMed] [Google Scholar]
- 57.Waddell PJ, Kishino H, Ota R. Rapid evaluation of the phylogenetic congruence of sequence data using likelihood ratio tests. Mol Biol Evol. 2000;17:1988–1992. doi: 10.1093/oxfordjournals.molbev.a026300. [DOI] [PubMed] [Google Scholar]
- 58.Swofford D. Sunderland, Massachusetts: Sinauer Associates; 2000. PAUP*. Phylogenetic analysis using parsimony (*and other methods). [Google Scholar]
- 59.Hasegawa M, Kishino H, Yano T. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J Mol Evol. 1985;22:160–174. doi: 10.1007/BF02101694. [DOI] [PubMed] [Google Scholar]
- 60.Posada D, Crandall KA. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- 61.Zwickl DJ. University of Texas at Austin; 2006. Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion [dissertation]. p. 115. [Google Scholar]
- 62.Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 63.Shimodaira H, Hasegawa M. Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Mol Biol Evol. 1999;16:1114–1116. [Google Scholar]
- 64.Thorne JL, Kishino H. Divergence time and evolutionary rate estimation with multilocus data. Syst Biol. 2002;51:689–702. doi: 10.1080/10635150290102456. [DOI] [PubMed] [Google Scholar]
- 65.Rutschmann F. Bayesian molecular dating using PAML/Multidivtime. A step-by-step manual. 2005 [Google Scholar]
- 66.Yang Z. London, England: University College London; 2000. Phylogenetic Analysis by Maximum Likelihood (PAML), version 3.14 [computer program]. [Google Scholar]
- 67.Kishino H, Hasegawa M. Evaluation of the maximum likelihood estimate of the evolutionary tree topologies from DNA sequence data, and the branching order in hominoidea. J Mol Evol. 1989;29:170–179. doi: 10.1007/BF02100115. [DOI] [PubMed] [Google Scholar]
- 68.Yabumoto Y. Pleistocene clupeid and engraulidid fishes from the Kokubu group in Kagoshima Prefecture, Japan. Bull Kitakyushu Mus Nat Hist. 1988;8:55–74. [Google Scholar]
- 69.Miller RR. First Fossil Record (Plio Pleistocene) of Threadfin Shad, Dorosoma petenense, from the Gatuna Formation of Southeastern New Mexico. J Paleont. 1982;56:423–425. [Google Scholar]
- 70.Grande L, Nelson G. Interrelationships of fossil and recent anchovies (Teleostei: Engrauloidea) and description of a new species from the Miocene of Cyprus. Amer Mus Novit. 1985:1–16. [Google Scholar]
- 71.Arambourg C. Note préliminaire sur quelques poissons fossiles nouveaux. Bull Soc Geol France. 1943;8:281–288. [Google Scholar]
- 72.Branisa L, Hoffstetter R, Signeux J. Additions a la fauna ichthyologique du crétacé supérieur de Bolivie. Bull Mus Nat Hist Nat. 1964;36:279–297. [Google Scholar]
- 73.Cavender TM. The fossil record of the Cyprinidae. In: Winfield, Ian J, Nelson, Joseph S, editors. Cyprinid Fishes: Systematics, Biology and Exploitation. London: Chapman & Hall; 1991. pp. 34–54. [Google Scholar]
- 74.Guiraud R. Stampfli G, Borel G, Cavazza W, Mosar J, Ziegler PA, editors. Northern Africa. The Palaeotectonic Atlas of the PeriTethyan Domain. European Geophysical Society. 2001.
- 75.Whitehead PJP. FAO species catalogue. Vol. 7: Clupeoid fishes of the world. An annotated and illustrated catalogue of the herrings, sardines, pilchards, sprats, shads, anchovies and wolf-herrings. Part 2-Engraulidae. FAO Fish Synop. 1988;125:1–579. [Google Scholar]
- 76.Inoue JG, Miya M, Tsukamoto K, Nishida M. Complete mitochondrial DNA sequence of the Japanese anchovy Engraulis japonicus. Fish Sci. 2001;67:828–835. [Google Scholar]
- 77.Inoue JG, Miya M, Tsukamoto K, Nishida M. Complete mitochondrial DNA sequence of the Japanese sardine Sardinops melanostictus. Fish Sci. 2000;66:924–932. [Google Scholar]
- 78.Saitoh K, Miya M, Inoue JG, Ishiguro NB, Nishida M. Mitochondrial genomics of ostariophysan fishes: Perspectives on phylogeny and biogeography. J Mol Evol. 2003;56:464–472. doi: 10.1007/s00239-002-2417-y. [DOI] [PubMed] [Google Scholar]
- 79.Tzeng CS, Hui CF, Shen SC, Huang PC. The complete nucleotide-sequence of the Crossostoma lacustre mitochondrial genome-Conservation and variations among vertebrates. Nucl Acids Res. 1992;20:4853–4858. doi: 10.1093/nar/20.18.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tzeng CS, Hui CF, Shen SC, Huang PC. The Complete Nucleotide-Sequence of the Crossostoma Lacustre Mitochondrial Genome-Conservation and Variations Among Vertebrates. Nucl Acids Res. 1992;20:4853–4858. doi: 10.1093/nar/20.18.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chang YS, Huang FL, Lo TB. The complete nucleotide sequence and gene organization of carp (Cyprinus carpio) mitochondrial genome. J Mol Evol. 1994;38:138–155. doi: 10.1007/BF00166161. [DOI] [PubMed] [Google Scholar]
- 82.Broughton RE, Milam JE, Roe BA. The complete sequence of the zebrafish (Danio rerio) mitochondrial genome and evolutionary patterns in vertebrate mitochondrial DNA. Genome Res. 2001;11:1958–1967. doi: 10.1101/gr.156801. [DOI] [PMC free article] [PubMed] [Google Scholar]