Abstract
Antigenic variation is a subtle process of fundamental importance to the survival of a microbial pathogen. In Plasmodium falciparum malaria, PfEMP1 is the major variable antigen and adhesin expressed at the surface of the infected erythrocyte, which is encoded for by members of a family of 60 var-genes. Peri-nuclear repositioning and epigenetic mechanisms control their mono-allelic expression. The switching of PfEMP1 depends in part on variable transition rates and short-lived immune responses to shared minor epitopes. Here we show var-genes to switch to a common gene that is highly transcribed, but sparsely translated into PfEMP1 and not expressed at the erythrocyte surface. Highly clonal and adhesive P. falciparum, which expressed distinct var-genes and the corresponding PfEMP1s at onset, were propagated without enrichment or panning. The parasites successively and spontaneously switched to transcribe a shared var-gene (var2csa) matched by the loss of PfEMP1 surface expression and host cell-binding. The var2csa gene repositioned in the peri-nuclear area upon activation, away from the telomeric clusters and heterochromatin to transcribe spliced, full-length RNA. Despite abundant transcripts, the level of intracellular PfEMP1 was low suggesting post-transcriptional mechanisms to partake in protein expression. In vivo, off-switching and translational repression may constitute one pathway, among others, coordinating PfEMP1 expression.
Introduction
Pathogens constrained to survive within a mammalian host are under evolutionary pressure to acquire mechanisms that favor a chronic infection. Asexual Plasmodium falciparum endure by means of antigenic variation. Manifestations of this success are the recrudescence of parasites, the occurrence of super-infections, the establishment of chronic asymptomatic infections, and the paucity of sterile immunity. The maintenance of antigen expression is coordinated by the spleen, given that parasites of splenectomized human and animal hosts do not sequester and do not express PfEMP1 on the infected erythrocyte surface [1]–[3]. How P. falciparum manages to coordinate the expression of its variable antigens to subsist in the host is intriguing and in part unexplained.
Immune evasion of Plasmodium falciparum infected erythrocytes (IE) is predominately mediated by the antigenically variable and highly polymorphic cell surface antigen Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1). PfEMP1 is a major adhesin that mediates binding to a variety of host-receptors causing sequestration of IEs in the microvasculature of various organs and severe disease in children and pregnant women. Pregnancy-associated malaria (PAM) constitutes one of the malaria syndromes where the involved PfEMP1 species and host receptors have been characterized. VAR2CSA, which is also of particular interest for the present study, has previously been identified as the major PfEMP1 implicated in PAM and shown to interact with chondroitin sulphate A (CSA), non-immune immunoglobulins and possibly other host-receptors, engendering placental sequestration and pathogenesis in PAM [4]–[9]. Understanding the mechanisms that regulate the expression of a particular PfEMP1 is an important piece of the puzzle of understanding disease pathogenesis and may help to identify additional targets for combating the disease. Yet, how the parasite switches on and off PfEMP1 expression is at present only partly understood.
PfEMP1 is encoded for by members of the multi-gene family var. Each parasite genome harbors approximately 60 var-genes of high sequence diversity [10] but at any given time, only a single PfEMP1 species is expressed in each parasite. It has been demonstrated that this mutually exclusive expression of PfEMP1 is regulated at the level of var-gene transcription initiation [11]–[13] unlike mammalian systems where regulation occurs through negative feedback at the level of protein production [14]. Epigenetic mechanisms involving chromatin modification, activation or silencing by sterile genetic elements and repositioning of var loci in sub-nuclear compartments also play important roles in the switching and exclusive expression pattern of var-genes [15]–[21]. Further, the parasite carries an epigenetic memory at the level of the chromatin involving the methylation of the histone H3 [22].
It has until recently been controversial whether a single full-length var-gene is being exclusively transcribed or multiple var-genes are simultaneously present [23]–[25]. Using microarrays specifically designed to detect var-genes, with multiple probes covering the entire length of every var-gene in the genome, it was recently found that several full-length var-genes are being transcribed simultaneously but also that short spurious transcripts are present [26]. The untranslated full-length var-genes in fact share the same group of upstream promoter sequences as the translated, implying that “loose” transcription [12], [13] might be attributable to cross var-gene transcriptional activation within the same group [26]. Still there is one var transcript that is dominant both in ring- and trophozoite stages which is later translated into the corresponding PfEMP1 [23], [25].
The expression dynamics of var-genes and their switching rates have previously been studied using different approaches including in vitro and in vivo assays and mathematical modeling [27]–[30]. To gain a better understanding of the succession of var switching we have here monitored two highly clonal parasites during in vitro growth without enrichment or panning for ≈200 generations (>1 year). The var-genes were studied using a comprehensive set of tools to monitor var- RNA and DNA (microarray, qRT-PCR, northern blot, fluorescent in situ hybridization (FISH)) and to follow the expression of PfEMP1 (cell-assays, immunoblotting, FACS). The results of the study suggest that var-gene switching, at least for a subset of genes, involves a programmed process where a large majority of parasites switch to transcribe a single member of the var-gene family, PFL0030c or var2csa, which however seems to be translationally repressed. The implications of our findings for the pathogenesis of disease and survival of the parasite in vivo are discussed.
Results
Var-gene expression and phenotypic changes in separate 3D7 clones over 200 generations
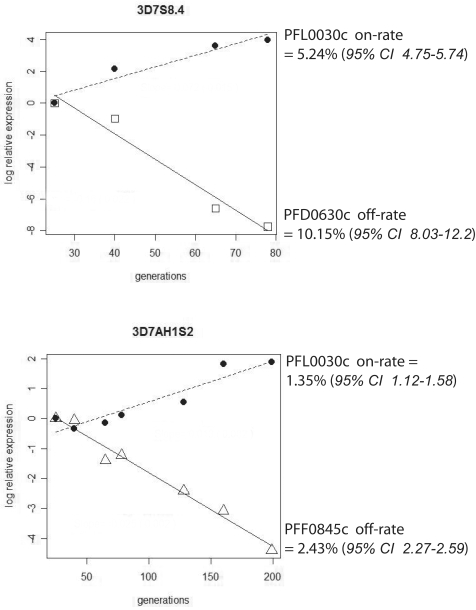
To follow var-gene switching over time, two parasite clones were monitored for ≈200 generations during which, both RNA expression and phenotypic changes were investigated at least once a month. The parasites were 3D7S8.4 and 3D7AH1S2, which had been serially selected for either rosette formation or adhesion to CD36 and then cloned by micromanipulation. The rosetting clone 3D7S8.4 transcribed the var-gene PFD0630c at onset whilst the 3D7AH1S2 clone selected for CD36 adhesion transcribed PFF0845c. Both var-genes are internally located on chromosomes 4 and 6, respectively. The global transcriptional profiles of these early generation clones have previously been established during multiple array experiments and the results have been confirmed using northern blot and RT-PCR [26]. When following the RNA expression with qPCR over time we found that the transcription of both initially identified var-genes (PFD0630c/PFF0845c) were gradually switched off, with an off-rate of 10.15 and 2.43% per generation, respectively. A total loss of PFD0630c transcription was observed after 80–130 generations in 3D7S8.4, while it took 175–200 generations for 3D7AH1S2 to completely loose transcription of PFF0845c. Instead, transcripts of var2csa (PFL0030c) began to appear in both clones. The loss of the dominant var-genes was intimately linked to an increase of var2csa (PFL0030c) transcription both in 3D7S8.4 and 3D7AH1S2, with on-rates of 5.24% and 1.35% per generation, respectively (Figure 1 & 2).
Figure 1. “On” and “Off” switching rate of dominant var and var2csa in 3D7S8.4 and 3D7AH1S2.
Regression models were created by using the log of comparative expression level, log(2ΔΔCt) versus generations. In the case of 3D7S8.4, a linear range of regression was observed up to ∼100 generations. Thus, the var on/off rates for 3D7S8.4 were determined up to 100 generations, whilst for the 3D7AH1S2, the estimations were made up to 200 generations, as the linear regression was maintained for a longer time period. The var on/off rates for 3D7S8.4 and 3D7AH1S2 were determined by the 2−ΔΔCt method, where ΔΔCt = (Ctvar−Cthouse-keeping)tn−(Ctvar−Cthouse-keeping)t0. The value for the first data collection (t0) was set as the baseline level (i.e. 15 and 25 generations for 3D7S8.4 and 3D7AH1S2, respectively.) The fold-change in the transcription compared to the baseline level was plotted, and the on/off rates of particular var-genes were estimated by the exponential function of the slope of the regression.
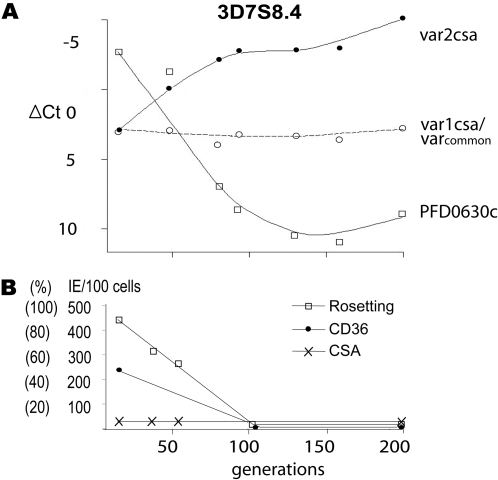
Figure 2. Changes in var-gene expression and adhesive phenotypes in clonal P. falciparum 3D7S8.4 over 200 generations of in-vitro growth.
The transcription levels were determined by qPCR and are presented as ΔCt values being the difference in cycle threshold between the var-gene and the seryl-tRNA synthetase housekeeping gene (control). The transcription levels of varcommon and of var-genes that were dominantly expressed at particular time points are shown. The adhesive phenotypes of the IE of the two parasites were followed for rosetting, CSA- and CD36-binding as described in Table 1 and the methods section.
Down-regulation of the initial var transcription was strictly paralleled by off-switching of corresponding adhesive phenotypes. Rosetting and CD36 binding began to decline already after 25 generations of growth and had disappeared at 80–100 generations in 3D7S8.4, and no CSA binding was detected in this parasite (Figure 2). CD36 binding was one third of the original level in 3D7AH1S2 after 100 generations of growth and was not seen in the late generation parasites (Table 1 and data not shown).
Table 1. Adhesion profile of 3D7AH1S2 and 3D7S8.4 at early and late generations.
| Clones Generations | 3D7S8.4 | 3D7AH1S2 | ||
| 16 | >190 | 16 | >190 | |
| Rosetting | ≈90% | - | ≈10% | - |
| Autoagglutination | - | - | - | - |
| CHO-CD36a | ≤250 | ≤10 | ≈500 | ≤10 |
| CHO-ICAMa | ≤50 | ≤10 | ≤50 | ≤10 |
| CHOa | ≤50 | ≤10 | ≤50 | ≤10 |
| Soluble Heparinb | - | - | - | - |
| Soluble CD31b | - | - | - | - |
| CSAcd | - | - | - | - |
| TSPc | - | - | ≈175 | - |
| Placentae | ≈500 | - | - | - |
a: Number of IEs bound per 100 cells; b: % of IEs showing surface fluorescence when incubated with Alexa-labelled antigens; c: CSA or thrombospondin (TSP) -coated plastic (50 µg/ml); d: A postive control parasite FCR3CSA bound at ≈235±45 IEs/mm2; e: Number of IEs bound to 1 mm2 of placental tissue; -: Lack of detectable adhesion
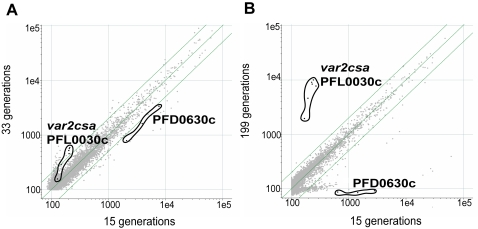
Having found that both 3D7S8.4 and 3D7AH1S2 converge to var2csa transcription, matched by the loss the binding ability, we decided to explore the underlying mechanisms in some detail. 3D7S8.4, which rapidly lost its binding phenotype and had a faster off- and on- switching rate of the dominant var-genes, was therefore chosen for further analyses. To confirm the data generated by qRT-PCR we regularly followed the parasite using microarrays (Figure 3). As can be seen from Figure 3, the var-gene PFD0630c was dominantly transcribed in the 15 generation parasites (Fig. 3A), while expressed to a lesser extent in 33 generation parasites and completely absent in those grown for ≈200 generations. The var2csa gene appeared to be dominantly transcribed in the long-term cultured non-selected parasites. The oligonucleotide coverage of var2csa was high, with four detecting the DBL-1x, DBL-4ε, DBL-5ε domains and the upstream open reading frame (uORF; chr12_glimmerm_22). The remaining two oligonucleotides targeted the DBL-6ε domain at the 3′ end. Significantly higher signals from all six oligonucleotides were observed in the long-term cultured 3D7S8.4 parasites (199 generations) as compared to the recently cloned one (15 generations; Figure 3B), implying that the var2csa transcripts were full length from the 5′ to the 3′ end of the exon-I of the gene.
Figure 3. Comparisons of global transcriptomes of 3D7S8.4 as studied by microarray analysis.
RNA was obtained from 15 vs. 33 generations and 15 vs. 199 generations. (A) off-switching of the originally dominant var PFD0630c and on-switching of PFL0030c (var2csa) was already seen at 33 generations; (B) var2csa (PFL0030c) was highly up-regulated in long-term cultured parasites and almost no PFD0630c was detected (background signal level) at 199 generations.
Transcription of full-length var2csa mRNA in sub-clones
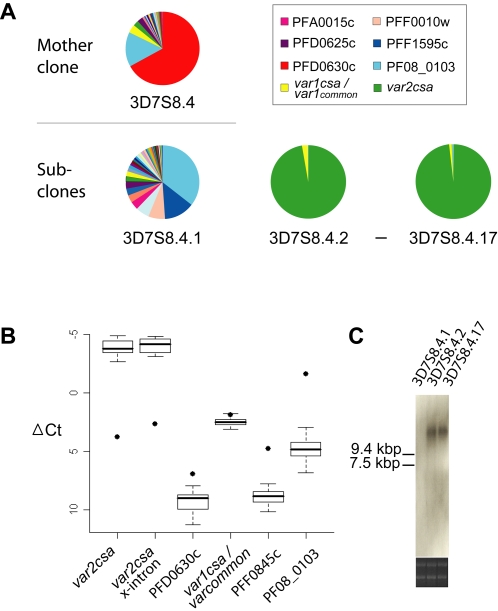
To further investigate the expression of var2csa and other var-genes, 3D7S8.4 was sub-cloned at 200 generations by micromanipulation, thereby generating a set of 17 new clones (3D7S8.4.1 to 3D7S8.4.17). Transcription of all clones was investigated using a set of qPCR primers covering all var-genes (Figure 4A & B). 16 out of 17 clones showed identical profiles with high levels of var2csa transcription. An exception was clone 3D7S8.4.1, which dominantly expressed another var-gene (PF08_0103). The pseudogene var1csa/var common (PFE1640w) was constitutively expressed in all parasite samples, though at a fairly low level (Figure 4B), in line with previous observations on the ubiquitous nature of constitutive transcription of this var species across parasite isolates [31], [32].
Figure 4. Expression of var2csa and other var-genes after long-term cultivation of P. falciparum in vitro.
(A) The pie charts show the relative transcription level of each var-gene in the different clones. The original 3D7S8.4 clone (15 generations) dominantly expressed the PFD0630c, but sub-populations of other var-genes were also detected. In contrast, the var2csa gene was found to be highly expressed in 16 out of 17 sub-clones (3D7S8.4.2-3D7S8.4.17) but in one (3D7S8.4.1) generated from the long-term cultured ∼200 generations 3D7S8.4. (B) Expression levels of different var-genes in the 3D7S8.4 sub-clones, 3D7S8.4.1-17. var2csa was highly expressed in all the subclones except for 3D7S8.4.1 (•), which dominantly transcribed PF08_0103. varcommon was expressed in all the parasites though at a relatively low level. (C) Northern-blot analysis of var2csa expression in total RNA extracted from the sub-clones 3D7S8.4.1, 3D7S8.4.2 and 3D7S8.4.17. The membrane was hybridized with a var2csa DBL1-2x probe. Comparable amounts of total RNA from each sample were loaded as shown in the ethidium bromide-stained gel (lower panel).
To investigate whether the var2csa expressed in the sub-clones was a correctly spliced transcript, we examined the RNA using qRT-PCR with primers mapping to opposite sides of the intron region (var2csa x-intron; Figure 4A). No difference in amplification was observed between the var2csa exon specific primers and the var2csa x-intron primers suggesting the presence of a spliced transcript. Northern blot was further used to verify the full-length feature of the var2csa transcript. Abundant var2csa transcripts of approximately 13 kb were present in the sub-clones, a molecular size corresponding to that of var-gene mRNA in CSA selected parasites (Figure 4C and not shown). We thus conclude that the samples do not contain any DNA contaminants or unspliced transcripts implying functionality of the var2csa mRNA detected.
Sparse PfEMP1 production and lack of PfEMP1 surface expression albeit high level of var2csa transcripts
No specific binding of the IE of long-term cultured parasites (≈200 generations) was seen to either cells or different host receptors albeit the high level of var2csa transcription detected. Rosetting was absent as was binding to CD31, CD36, ICAM-1, HS, CSA, TSP, CHO-cells and placental tissue (Table 1).
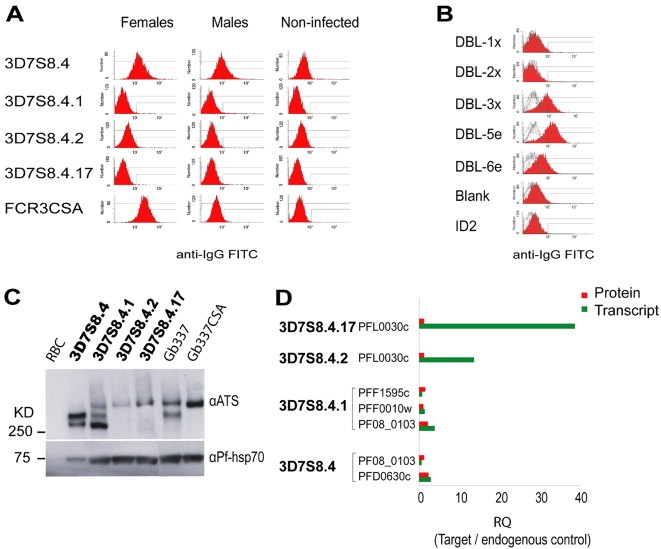
To confirm the lack of VAR2CSA surface expression, IEs were further studied by flow cytometry using different sera and IgG preparations. IgG specific for DBL1x, DBL2x, DBL3x, DBL5e and DBL6e of VAR2CSA raised in rabbits as well as pooled plasma from P. falciparum-exposed men or multi-gravid women from Ghana were used. The IEs of the original rosetting clone 3D7S8.4 studied at 15 generations was recognized to a similar degree by the plasma pools from men and pregnant women, but not by the different anti-VAR2CSA IgG. However, the non-adhesive IEs of 3D7S8.4 grown for ≈200 generations in vitro was neither recognized by the pooled patient sera of either sex, nor by the anti-VAR2CSA IgGs. The same was true for IEs of the sub-clones 3D7S8.4.1, 3D7S8.4.2 and 3D7S8.4.17 (Figure 5A & B). In contrast, IE of the control parasite FCR3CSA and Gb337CSA that had been repeatedly selected for adhesion to CSA showed good differential reactivity with the plasma pool obtained from pregnant women versus those of men and was well-recognized by IgG raised to the DBL3x, the DBL5e and the DBL6e domain of VAR2CSA (Figure 5A & B and data not shown).
Figure 5. Detection of PfEMP1 in different P. falciparum clones by flow cytometry- and immunoblotting.
The IEs were labeled (A) with IgG from plasma pools of P. falciparum exposed males (Males), females (Females) or non-infected Danes, and (B) IEs used in (A) were labeled with IgG from rabbit-sera raised against different domains of VAR2CSA (DBL-1x; DBL-2x, DBL-3x, DBL-5e, DBL-6e) or with a serum raised to an irrelevant control antigen (ID2), positive control (FCR3CSA) is shown in red. (C) The blot illustrates the PfEMP1 expression in whole cell-lysates of late trophozoite infected RBCs of the 3D7S8.4 early and late generation clones. Parasites with bolded lettering are described in greater detail in D. The blot was incubated with a PfEMP1 antibody raised against the conserved part of the acidic terminal segment (ATS). Anti-PfHsp-70 reactivity was used as loading control. Uninfected RBCs were included as negative control. Gb337 and Gb337CSA are included as positive controls of VAR2CSA expressing parasites. Whilst Gb337 is heterogeneous in its PfEMP1 expression, upon repeated panning on CSA, only the higher molecular weight protein is expressed. D) The graph illustrates the inter-relationship of the transcript versus protein levels of the dominant var-gene(s) in the 3D7S8.4 early and late generation clones.
Having found that there was no serum- or IgG reactivity with the IE surface of the long-term cultivated parasites, we subsequently performed immunoblot analysis to investigate whether the var2csa transcripts were translated into protein, using an anti-PfEMP1 monoclonal antibody preparation (anti-ATS). In the mother clone 3D7S8.4, a ∼290 kDa band was dominantly labeled, corresponding well to the size of the major var transcript identified in this parasite (PFD0630c); in the control parasite Gb337CSA it appeared as a band of ∼340–360 kDa whilst in the switch clone 3D7S8.4.1 which dominantly transcribed var-gene PF08_0103 it appeared as a ∼250 kDa band. In concordance with the estimated size of the dominant var2csa transcript (∼355 kDa), a faint high molecular weight band was also observed in the long-term cultured sub-clones 3D7S8.4.2 and 3D7S8.4.17. However, despite the high level of var2csa transcripts in these parasites, the relative abundance of the translational products was sparse, hence contrasting the inter-relationship of transcript versus protein levels of the major PfEMP1s in the non-var2csa expressing clones (3D7S8.4 & 3D7S8.4.1) (Figure 5 C & D). This finding implies that the translational products of var2csa in the long-term cultured parasites are significantly controlled through post-transcriptional mechanisms [33].
Activation and silencing of the var2csa gene involve sub-nuclear repositioning
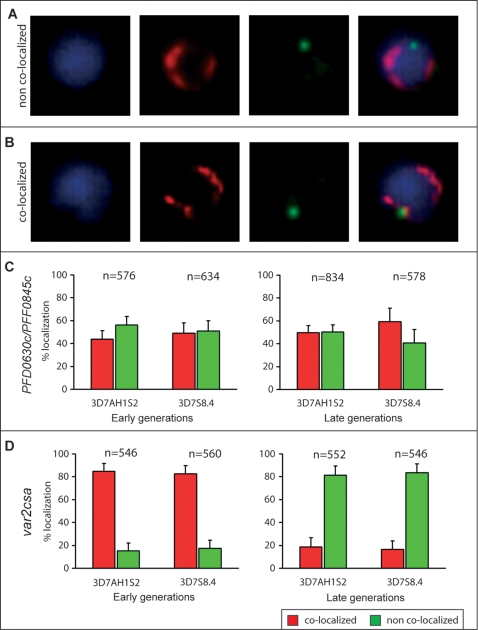
Repositioning of var loci within the sub-nucleus has been found associated with the expression of subtelomerically located var-genes [17], [19]. We consequently studied the var-genes expressed in the cloned parasites (PFD0630c; PFF0845c) and the location of the telomere-repeats (rep20) using two-color fluorescent in situ hybridization (FISH; Figure 6). The great majority (>90%) of PFD0630c and PFF0845c were located at the rim of the nuclei (referred to as zone A by Ralph et al. [19]). Neither of the genes, which were of the upsC type and located internally on chromosomes 4 and 6, did however reposition in respect to telomere-repeats/heterochromatin as their transcriptional states changed. Their peri-nuclear gene locations (Figure 6C) were the same in parasites obtained immediately after cloning and in those obtained after long-term growth. Approximately half of the genes were located within the telomeric clusters/heterochromatin and approximately half outside of it (∼600 nuclei /gene counted). In contrast the var2csa gene was found to be associated with the telomeric clusters (rep20) at onset of the experiment while after long-term cultivation (194–206 generations), the activated var2csa gene dissociated away from the telomeric clusters and heterochromatin in more than 80% of the parasites (Figure 6D). As for the upsC var-genes, var2csa was located at the rim of the nuclei.
Figure 6. Repositioning of var-genes in the peri-nuclear area as seen by FISH in P. falciparum 3D7S8.4 and 3D7AH1S2 ring-stage parasites at onset and after 200 generations of in vitro growth.
(A & B) Representative pictures illustrate different localization patterns of telomeric clusters (rep20, red probe) compared with that of a var-gene (green probe) in nuclei stained with DAPI (blue). Telomeric clusters appear as horseshoe-like bundles where condensed heterochromatins are located. (C) The var-genes expressed at the onset of the experiment (PFD0630c/PFF0845c) did not reposition and did not show any correlation between their transcriptional states and peri-nuclear positions in the same parasites. (D) In contrast, in early generation parasites (15–21 generations) var2csa is in a silenced state and co-localizes with the telomeric clusters. After 194-206 generations var2csa transcription is active in both parasites (3D7SS8.4, 3D7AH1S2) with a concordant repositioning from telomeric clusters to a zone without condensed genetic material.
Switching to var2csa is independent of genomic rearrangement
Using the microarray we monitored the parasite for global transcriptional changes throughout the study. Some of the genes were found down-regulated (Table S1) upon long-term in vitro propagation. Still, the skeleton-binding protein 1 (SBP1), previously shown to be required for PfEMP1 trafficking [34], was found transcribed at similar levels throughout the experiment and normal levels of RNA were present for genes encoding KAHRP and PfEMP3 when the parasites at early generations switched to transcribe var2csa (Table S1, Figure S1) suggesting that the lack of binding of the IE and surface expression was not due to deficient PfEMP1 transport at least in the <50–80 generation parasites (Figure 1). However, the transcription of the knob-associated histidine rich protein (KAHRP) and PfEMP3 was found absent in the 199 generation parasites due to a possible late spontaneous deletion of the left arm of chromosome 2 ([35]). Complementary comparative genomic hybridizations between 3D7S8.4 of 19 and 56 generations revealed only four genes (PFC1000w, PFE0635c, PF08_0138, PF10_0229) to have undergone either deletion or sequence polymorphisms in the later generations (not shown). This suggests that switching to var2csa happened before any major genomic rearrangements occurred, involving genes encoding proteins such as KAHRP or PfEMP3.
Discussion
If it were so that a microbe would express antigenic variants in an un-coordinated manner the host would shortly raise an immune response to each and every variant and clear the infection. Parasites have consequently developed means to handle this host challenge. P falciparum, for example, successfully evades immune recognition of PfEMP1 by combining allelic exclusion of the var-genes with switching, yet the exact mechanisms by which this occurs are not known. To increase our understanding on var-gene switching we performed a systematic analysis of the transcriptional profile of var-genes over time in phenotypically distinct 3D7 parasite clones. In the absence of selective pressure, 95% of the parasites derived from a single clone eventually switched to transcribe a single member of the var-gene family, PFL0030c or var2csa. However, albeit the high levels of var2csa transcripts, the relative abundance of translated product was sparse and no PfEMP1 surface expression was observed, implying that var2csa may be a target for post-transcriptional regulation.
Sequence analysis of the 3D7 genome has revealed the var-gene repertoire to fall into different classes depending on chromosomal location, the orientation of the gene as well as on the upstream flanking sequence, the latter known as upsA, -B, -C, -D and -E. The two var-genes expressed in our parasites at onset belonged to the chromosomal internal cluster upsC var-genes (PFD0630c and PFF0845c). A recent study demonstrated that different var-genes have different intrinsic switching rates and that var-genes in centromeric location have higher “on” rates and lower “off” rates [36]. We found that this phenomenon does not apply to all central var-genes, at least not in the case of PFD0630c. In the present study, off-switching in 3D7S8.4 parasites was observed already at 33 generations of growth (<10 weeks). Furthermore, although the same var-genes (PFF0845c/MAL6p1.252) and the same genotypic parasites (here 3D7AH1S2) were monitored in both studies, the “off” rates were considerably dissimilar. In the present study the parasites had switched from the dominant var-gene (PFF0845c) already at 48 generations (<14 weeks), whereas in a study by Frank et al [36] the expression of the same dominant var-gene remained unchanged up to 66 generations (19 weeks). These findings may indicate that the switching rate is not inherited on genomic level but is rather under epigenetic control.
An inter-relationship between the “off” and the “on” rates was found where “off“ rates of the original var-genes were about twice those of the var2csa “on” rates, arguing that switching to other intermediate var-genes also potentially occurred. Still, var2csa expression was dominant in the late generation parasites, which may in part be due to differences in proliferation rates in-between the var2csa- and non-var2csa expressing clones (Figure 7). It is possible that the higher multiplication rates in the var2csa-expressing clones endowed these parasites with a selective growth advantage over other switch variants. Further, we do not know whether the var2csa locus in the late generation parasites (3D7S8.4.2-17 sub-clones) would remain activated until an episode of exogenous challenge or whether they spontaneously would switch at a slow rate to another var variant. Upon panning of some of the sub-clones on placental sections or on CD36 expressing cells we could not retrieve any major binding IE populations (data not shown). This may suggest that this group of parasites does not revert back to PfEMP1 expression or that other types of stimuli may be required. It has to be noted that compared to other strains, such as HB3 and FCR3, it is difficult to retrieve 3D7 parasites panned on CSA [37]. On the other hand, up-regulation of var2csa transcription in 3D7 without selection is readily observed [36], [38].
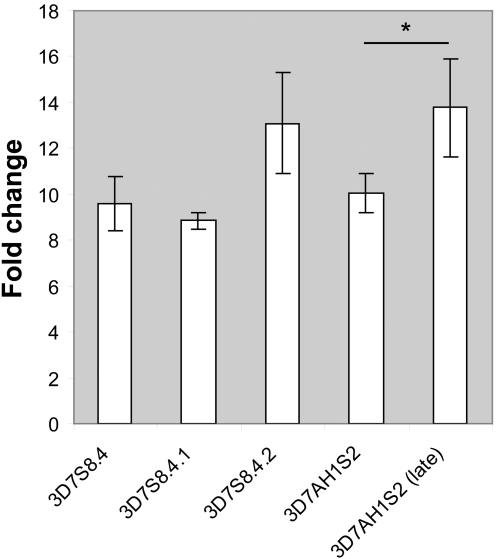
Figure 7. Quantitative differences in complete cycle multiplication rates between different parasites.
Mean of fold change per erythrocytic cycle for at least three consecutive 48 hour cycles were calculated from blood smear parasitemias. Significant differences between “early” versus ”late” generation clones (3D7S8.4 vs 3D7S8.4.1; 3D7S8.4 vs 3D7S8.4.2 and 3D7AH1S2 vs 3D7AH1S2 late) were determined using an unpaired Student́s t-test at a 5% significance level. Analysis was performed in SPSS version 12.0. *P<0.05; Significantly higher multiplication rate was observed in the late S2-clone. The same trend was also observed in the other VAR2CSA expressing late generation clone S8.4.2, the difference here however was not statistically significant (P = 0.067).
As both parasite clones studied herein expressed upsC var-genes at onset and both eventually converged to var2csa transcription, and given that the var switching history of a particular parasite has been shown to result in a particular switching rate [30], it is possible that switching to var2csa is favored in parasites previously transcribing a particular group of var-genes (upsC). This, however, remains to be confirmed with a larger set of parasites both ex vivo and in vitro. Recent data suggest that transcription of in particular upsC var-genes predominate in children with asymptomatic infections [39]. Interestingly var2csa transcription, although mainly associated with pregnancy isolates, has occasionally been observed in samples from children with acute malaria [40], [41] and in peripheral-blood parasites of experimentally infected humans [24]. Moreover, performing a pilot study on other in vitro adapted parasites (FCR3, TM180 and 7G8), we found trace amounts of var2csa in FCR3 after 50 generations of growth without any selective pressure (data not shown).
Repositioning of var loci within the peri-nuclear area has been found to be associated with the expression of subtelomerically located var-genes. Similarly, the var2csa gene was here discovered to reposition from the telomeric clusters and the condensed heterochromatin to occupy a suggested site of transcription, supporting the notion that translocation of the var2csa gene into a suggested transcription site may in certain cases silence the transcription of other var-genes. However, although var2csa repositioned and was highly transcribed, the sparse presence of translational products and absence of surface expression imply an additional layer of control, which remains to be further investigated. The exploitation of transcriptional repression as a post-transcriptional regulatory mechanism has previously been described only for transcripts of P. berghei gametocyte origin [42].
There is today good evidence to suggest that the var2csa gene encodes an important PfEMP1-species, a ligand associated with the sequestration of IEs in PAM [4]–[9]. This gene is however atypical to other var-genes and apart from having a different domain-architecture and an uncommon upstream flanking region it is remarkably conserved across different plasmodium species. A var2csa ortholog is for example present in P. reichenowi, a parasite which has been predicted to have diverged from P. falciparum several million years ago [43]. The expression of var2csa transcripts in isolates of children and adults during malaria infections and the obvious lack of antibody responses to the corresponding encoded protein in these groups of patients suggest the presence of var2csa off-switching in vivo. Yet, under what circumstances would a functional transcript avoid to produce a translational product, and is it compatible with parasite growth in vivo during an infection that an IE does not express a surface PfEMP1? One might speculate that in vivo a temporal expansion of parasites expressing a post-transcriptionally regulated gene may coordinate PfEMP1 expression by permitting splenic clearance of the off-switch IEs thus giving only the remaining minute populations of other variants a possibility to survive. Such a pathway, among others (see Figure 8), would protect against the rapid exhaustion of the variant-antigen repertoire.
Figure 8. Suggested switching pathways of var-genes and their PfEMP1 in P. falciparum.
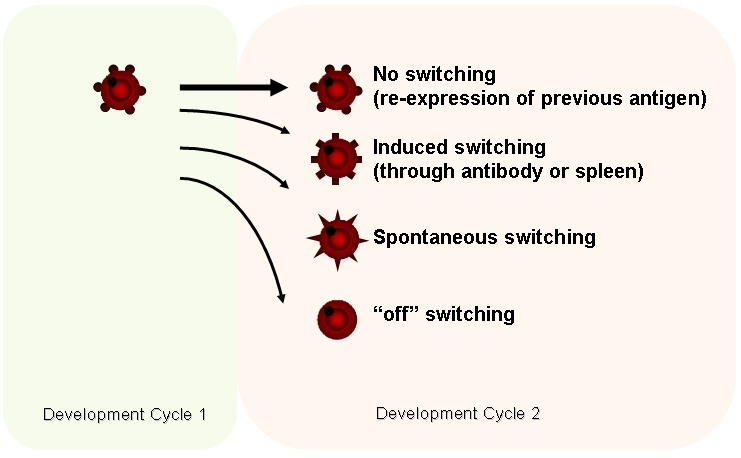
It is possible that among the var-genes solely var2csa can be translationally repressed to avoid exposure of the corresponding PfEMP1 to the host immune system prior to pregnancy. The suppression may be released upon pregnancy by specific factors or conditions in the placenta allowing for expansion of parasites translating the PfEMP1var2CSA and expressing it at the IE surface. In the future, it will be interesting to study the prevalence of off-switching in different isolates including those obtained from splenectomized donors, and further assess what role post-transcriptional regulation has for different genes across the life cycle stages of P.falciparum.
Materials and Methods
Parasites and RNA preparation
The parasite 3D7AH1S2 was generated by multiple panning of 3D7AH1 parasites on CD36-CHO transfectants, followed by micro-manipulation cloning while 3D7S8.4 was selected twice from 3D7 parasites for the rosetting phenotype by micro-manipulation cloning. FCR3CSA was selected by repeated panning of FCR3 IE on CSA-coated Petri dishes [26]. 17 sub-clones (3D7S8.4.1-17) were randomly picked by micromanipulation from 3D7S8.4 after 200 generations of in vitro growth. Phenotypic characterization of the parasites was performed as described elsewhere [23] using trophozoite IE at 5–8% parasitemia. For RNA preparation, parasites were first tightly synchronized during three consecutive rounds of 5% D-sorbitol treatment. Parasites were then collected as trophozoite-IE (24±4 hours) and total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA) and stored at −80°C.
Microarray
The P. falciparum genome microarray was used [26]. 20 µg of total RNA was labeled following an amino-allyl dye coupling protocol. Hybridization and washing were performed using a Lucidea automated slide processor (Amersham Biosciences) and slides were scanned with a GenePix 4000 B scanner (Axon Instruments). At least one dye-swap experiment per generation post-cloning was performed. Signals and local background intensities from each spot were retrieved using GenePix Pro 5.1 software (Axon Instruments). Spots that passed the quality controls (default settings), together with visual inspection, were analyzed using GeneSpring 6.1 (Silicon Genetics). Local background filtering was applied and LOWESS was adopted to normalize the data [44]. Protocols and microarray data are publicly available at www.ebi.ac.uk/arrayexpress: A-MEXP-75 and E-MEXP-1199.
Quantitative real-time RT-PCR
Total RNA was treated with Deoxyribonuclease I (Ambion) to remove contaminating DNA. cDNA synthesis was performed with 1 µg of total RNA and reverse transcribed using Superscript III Reverse Transcriptase (Invitrogen) with random primers and OligoDT (Invitrogen) as described by the manufacturer. For qRT-PCR, we employed the primer set of Salanti et al. and conducted the experiment as previously described [7]. An additional primer pair targeting the flanking regions of var2csa (var2csa x-intron: forward 5′AATAATACCAGTGACATTCTGCAAAA3′ and reverse 5′ACACGTAAAAGGTCCACAGGTG 3′) was added for evaluation of splicing and DNA contamination. All experiments included primers for two housekeeping genes, seryl-tRNA synthetase and fructose-bisphosphate aldolase, as endogenous controls. Levels of var transcription were calculated by the ΔCt method, in which the Ct (cycle threshold) for each var-gene was compared with that for the endogenous controls.
Northern Blot
3D7 var2csa (PFL0030c) was PCR amplified from 3D7 genomic DNA with primers PFL0030c/F (AAATGGAAATCCGAATGGG) and PFL0030c/R (TGAGTCAAGGGTGTGTTCTTGGGGGTAAACC) and then ligated into a T7 3′ element employing the TOPO-Tools system (Invitrogen) as recommended by the manufacturer. Digitonin-labeled anti-sense RNA was produced using the Dig RNA labeling kit (Roche). Total RNA (2 µg) from trophozoite stage parasites was transferred to nylon membranes (Roche). Blots were pre-hybridized in Dig Easy Hyb buffer and then probed with 100ng/ml Digitonin-labeled anti-sense RNA in Dig Easy Hyb buffer (Roche) at 65°C overnight. After hybridization membranes were washed twice with 2x SSC, 0.1% SDS at RT and twice with 0.5×SSC, 0.1% SDS at 65°C. Hybridized RNA was detected with the Dig Luminescent detection kit (Roche) as described by the supplier.
SDS-PAGE and immunoblot analysis
Pigmented IEs were harvested using a MACS magnetic cell sorter (Miltenyi BioTec) and solubilized in reducing SDS sample buffer. The total parasite extracts were separated on 3–8% tris-acetate gradient gels (Invitrogen) and transferred to nitrocellulose membranes. Membranes were blocked in 5% non-fat milk powder and probed with a mouse monoclonal raised against the conserved C-terminal acidic-terminal-sequence (ATS) of PfEMP1s (1∶250) (a kind gift from A. Cowman). The membranes were stripped and re-probed with a mouse anti-Pfhsp70 (1∶2000) (a kind gift from C. Fernandez). The antibody was raised against the C-terminal part of hsp70 and differentially recognizes P. falciparum and not human Hsp70 [45]. Detection by enhanced chemiluminescence (ECL; Amersham Biosciences) was performed after secondary detection with sheep anti–mouse Ig-HRP conjugate (1∶5000, Amersham Biosciences).
Flow cytometry
Variant surface antigen expression was tested by flow cytometry using plasma pools from P. falciparum-exposed women or men, or non-infected human serum. Specific VAR2CSA surface expression was assessed using antisera to VAR2CSA DBL1-3x, ID2 (inter-domain between DBL2x and DBL3x) and DBL5-6e. In brief, 2×105 late-stage IEs were purified (to >75% parasitemia) by exposure to a strong magnetic field (Miltenyi BioTec), stained with ethidium bromide (Sigma) and sequentially incubated with 5 µl of plasma or antisera, 0.4 µl of goat anti-human IgG (Dako) and 4 µl of fluorescein isothiocyanate (FITC)-conjugated rabbit anti-goat IgG (Dako) in a total volume of 100 µl. Samples were washed 2 times in PBS with 2% fetal calf serum between each antibody incubation step as described elsewhere [46]. All plasma samples were tested simultaneously with each parasite isolate.
Fluorescent in situ hybridization (FISH)
FISH was conducted according to previously described methodology with minor modifications [47]. The dsDNA probes targeting PFD0630c, PFF0845c and PFL0030c/var2csa were amplified using the specific primers PFD0630c/F (5′-GAT GAC GAC AAG CCA AAT ACC-3′), PFD0630c/R (5′-ACA TAA TCC GCC TCC AGT TC-3′), PFF0845c/F (5′-CGT TGG ATG ACT GAA TGG TCC G-3′), PFF0845c/R (5′-TCA CCG AGG TCT ATG CTG AAC TGG-3′), PFL0030c/F (5′-AAG GAT AGA ATG GAA TGG AAT GAG C-3′) and PFL0030c/R (5′-CAC CAA TCG TCA ACT TTT TCG G-3′). PCR products were cloned into pCRII-TOPO vector and transformed into One Shot TOP10 Chemically Competent Cells (Invitrogen) according to the supplier's recommendations. Plasmids containing var-gene fragments were labeled using the Fluorescein-High Prime and Rep20 containing pUC9 plasmids (a kind gift from A. Scherf) with Biotin-High Prime kit (Roche Applied Science). Highly synchronous parasites (18±2 hours post invasion) were isolated from their host erythrocytes using saponin (0.05% w/v) and deposited as monolayers on Poly-L-lysine coated microscope slides (Menzel-Gläser). Air-dried monolayers were fixed with 4% paraformaldehyde (PFA) for 15 minutes at room temperature and treated with RNase (20 mg/ml in 2×SSC) for 30 minutes at 37°C. 100 ng of labeled and heat denatured probe (in 50% deionized formamide, 10% dextran sulphate, 1×SSC and 250 mg/ml herring sperm DNA) were added to parasite preparations before hybridization at 95°C for 3 minutes and at 37°C for 12 hours. After hybridization, parasites were washed twice in 50% deionized formamide/2×SSC at 45°C, twice in 2×SSC first at 45°C then 50°C and once in 4×SSC at room temperature. Blocking with 1% BSA in PBS was followed by incubation with Avidin-Rhodamine (Roche Applied Science) in 1% BSA in PBS for detection of the biotinylated Rep20 probes. Parasites were finally washed once in 1% BSA in PBS and twice in a solution of 100 mM Tris-HCl/150 mM NaCl/0.5% (v/v) Tween 20 at room temperature and mounted in Vectashield (Vector Laboratories). Preparations were visualized using a Leica DMRE microscope and imaged with a Hamamatsu C4880 cooled CCD camera.
Multiplication rate determination
All parasite clones were initially synchronized with 5% D-sorbitol treatment. Each clone was diluted to a low level of parasitemia (geomean = 0.3%) at schizont stage and thin blood smears were prepared. Cultures were subsequently incubated for 12–16 hours to allow invasion of RBCs and blood smears of the ring-stage parasites were prepared. The smears were stained with Giemsa and the number of IEs with schizonts (parasitemiastart) or rings (parasitemianew) were assessed visually by light microscopy. A minimum of 1000 IEs were counted per smear (geomean = 1500). Data from a minimum of three biological replicates were generated for each clone. The multiplication rate for each clone was obtained by calculating the fold change in parasitemia (parasitemianew/parasitemiastart) for each 48 hour cycle.
Relative estimation of transcript versus protein abundance
The plot in Figure 5D was generated using the quantitative real-time PCR data to calculate the relative transcript copy number of the dominant transcripts in each parasite clone. Protein expression levels of the corresponding transcripts were assessed using a semi-quantitative approach based on measurements of the signal intensities of the PfEMP1 bands on western blots. Relative quantity (RQ) was obtained by dividing the target quantity with the quantity of the endogenous control in each sample. Seryl t-RNA synthetase and Pfhsp70 were used as endogenous controls for transcript versus protein data respectively.
Supporting Information
(0.54 MB PNG)
(0.01 MB PDF)
Acknowledgments
We thank A. Salanti and M. Nielsen at University of Copenhagen, Denmark for technical support with qPCR and FACS. We are grateful to Prof. A. Cowman at the Walter and Eliza Hall Institute of Medical Research, Australia for providing the PfEMP1 monoclonal antibody, Prof. C. Fernandez at Stockholm University, Sweden for providing the Pfhsp70 antibody and Prof. A. Scherf at Institut Pasteur, France for providing the Rep20 plasmid. We thank A. Waldén at KTH, Sweden for technical assistance and B. Yip at MEB, Karolinska Institute for help.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The work was funded by grants from the Swedish Institute for Infectious Disease Control (SMI), the European Union (BioMalPar), the Swedish Research Council and the Swedish International Development Cooperation Agency (Sida/ SAREC).
References
- 1.Barnwell JW, Howard RJ, Miller LH. Influence of the spleen on the expression of surface antigens on parasitized erythrocytes. Ciba Found Symp. 1983;94:117–136. doi: 10.1002/9780470715444.ch8. [DOI] [PubMed] [Google Scholar]
- 2.David PH, Hommel M, Miller LH, Udeinya IJ, Oligino LD. Parasite sequestration in Plasmodium falciparum malaria: spleen and antibody modulation of cytoadherence of infected erythrocytes. Proc Natl Acad Sci U S A. 1983;80:5075–5079. doi: 10.1073/pnas.80.16.5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hommel M, David PH, Oligino LD. Surface alterations of erythrocytes in Plasmodium falciparum malaria. Antigenic variation, antigenic diversity, and the role of the spleen. J Exp Med. 1983;157:1137–1148. doi: 10.1084/jem.157.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avril M, Gamain B, Lepolard C, Viaud N, Scherf A, et al. Characterization of anti-var2CSA-PfEMP1 cytoadhesion inhibitory mouse monoclonal antibodies. Microbes Infect. 2006;8:2863–2871. doi: 10.1016/j.micinf.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Gamain B, Trimnell AR, Scheidig C, Scherf A, Miller LH, et al. Identification of multiple chondroitin sulfate A (CSA)-binding domains in the var2CSA gene transcribed in CSA-binding parasites. J Infect Dis. 2005;191:1010–1013. doi: 10.1086/428137. [DOI] [PubMed] [Google Scholar]
- 6.Salanti A, Dahlback M, Turner L, Nielsen MA, Barfod L, et al. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J Exp Med. 2004;200:1197–1203. doi: 10.1084/jem.20041579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salanti A, Staalsoe T, Lavstsen T, Jensen AT, Sowa MP, et al. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol Microbiol. 2003;49:179–191. doi: 10.1046/j.1365-2958.2003.03570.x. [DOI] [PubMed] [Google Scholar]
- 8.Rasti N, Namusoke F, Chene A, Chen Q, Staalsoe T, et al. Nonimmune immunoglobulin binding and multiple adhesion characterize Plasmodium falciparum-infected erythrocytes of placental origin. Proc Natl Acad Sci U S A. 2006;103:13795–13800. doi: 10.1073/pnas.0601519103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viebig NK, Gamain B, Scheidig C, Lepolard C, Przyborski J, et al. A single member of the Plasmodium falciparum var multigene family determines cytoadhesion to the placental receptor chondroitin sulphate A. EMBO Rep. 2005;6:775–781. doi: 10.1038/sj.embor.7400466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardner MJ, Hall N, Fung E, White O, Berriman M, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kyes S, Christodoulou Z, Pinches R, Kriek N, Horrocks P, et al. Plasmodium falciparum var gene expression is developmentally controlled at the level of RNA polymerase II-mediated transcription initiation. Mol Microbiol. 2007;63:1237–1247. doi: 10.1111/j.1365-2958.2007.05587.x. [DOI] [PubMed] [Google Scholar]
- 12.Scherf A, Hernandez-Rivas R, Buffet P, Bottius E, Benatar C, et al. Antigenic variation in malaria: in situ switching, relaxed and mutually exclusive transcription of var genes during intra-erythrocytic development in Plasmodium falciparum. Embo J. 1998;17:5418–5426. doi: 10.1093/emboj/17.18.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Q, Fernandez V, Sundstrom A, Schlichtherle M, Datta S, et al. Developmental selection of var gene expression in Plasmodium falciparum. Nature. 1998;394:392–395. doi: 10.1038/28660. [DOI] [PubMed] [Google Scholar]
- 14.Dzikowski R, Frank M, Deitsch K. Mutually exclusive expression of virulence genes by malaria parasites is regulated independently of antigen production. PLoS Pathog. 2006;2:e22. doi: 10.1371/journal.ppat.0020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voss TS, Kaestli M, Vogel D, Bopp S, Beck HP. Identification of nuclear proteins that interact differentially with Plasmodium falciparum var gene promoters. Mol Microbiol. 2003;48:1593–1607. doi: 10.1046/j.1365-2958.2003.03528.x. [DOI] [PubMed] [Google Scholar]
- 16.Voss TS, Healer J, Marty AJ, Duffy MF, Thompson JK, et al. A var gene promoter controls allelic exclusion of virulence genes in Plasmodium falciparum malaria. Nature. 2006;439:1004–1008. doi: 10.1038/nature04407. [DOI] [PubMed] [Google Scholar]
- 17.Duraisingh MT, Voss TS, Marty AJ, Duffy MF, Good RT, et al. Heterochromatin silencing and locus repositioning linked to regulation of virulence genes in Plasmodium falciparum. Cell. 2005;121:13–24. doi: 10.1016/j.cell.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 18.Frank M, Dzikowski R, Costantini D, Amulic B, Berdougo E, et al. Strict pairing of var promoters and introns is required for var gene silencing in the malaria parasite Plasmodium falciparum. J Biol Chem. 2006;281:9942–9952. doi: 10.1074/jbc.M513067200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ralph SA, Scheidig-Benatar C, Scherf A. Antigenic variation in Plasmodium falciparum is associated with movement of var loci between subnuclear locations. Proc Natl Acad Sci U S A. 2005;102:5414–5419. doi: 10.1073/pnas.0408883102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freitas-Junior LH, Hernandez-Rivas R, Ralph SA, Montiel-Condado D, Ruvalcaba-Salazar OK, et al. Telomeric heterochromatin propagation and histone acetylation control mutually exclusive expression of antigenic variation genes in malaria parasites. Cell. 2005;121:25–36. doi: 10.1016/j.cell.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 21.Calderwood MS, Gannoun-Zaki L, Wellems TE, Deitsch KW. Plasmodium falciparum var genes are regulated by two regions with separate promoters, one upstream of the coding region and a second within the intron. J Biol Chem. 2003;278:34125–34132. doi: 10.1074/jbc.M213065200. [DOI] [PubMed] [Google Scholar]
- 22.Chookajorn T, Dzikowski R, Frank M, Li F, Jiwani AZ, et al. Epigenetic memory at malaria virulence genes. Proc Natl Acad Sci U S A. 2007;104:899–902. doi: 10.1073/pnas.0609084103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duffy MF, Brown GV, Basuki W, Krejany EO, Noviyanti R, et al. Transcription of multiple var genes by individual, trophozoite-stage Plasmodium falciparum cells expressing a chondroitin sulphate A binding phenotype. Mol Microbiol. 2002;43:1285–1293. doi: 10.1046/j.1365-2958.2002.02822.x. [DOI] [PubMed] [Google Scholar]
- 24.Lavstsen T, Magistrado P, Hermsen CC, Salanti A, Jensen AT, et al. Expression of Plasmodium falciparum erythrocyte membrane protein 1 in experimentally infected humans. Malar J. 2005;4:21. doi: 10.1186/1475-2875-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noviyanti R, Brown GV, Wickham ME, Duffy MF, Cowman AF, et al. Multiple var gene transcripts are expressed in Plasmodium falciparum infected erythrocytes selected for adhesion. Mol Biochem Parasitol. 2001;114:227–237. doi: 10.1016/s0166-6851(01)00266-3. [DOI] [PubMed] [Google Scholar]
- 26.Mok BW, Ribacke U, Winter G, Yip BH, Tan CS, et al. Comparative transcriptomal analysis of isogenic Plasmodium falciparum clones of distinct antigenic and adhesive phenotypes. Mol Biochem Parasitol. 2007;151:184–192. doi: 10.1016/j.molbiopara.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Gatton ML, Peters JM, Fowler EV, Cheng Q. Switching rates of Plasmodium falciparum var genes: faster than we thought? Trends Parasitol. 2003;19:202–208. doi: 10.1016/s1471-4922(03)00067-9. [DOI] [PubMed] [Google Scholar]
- 28.Roberts DJ, Craig AG, Berendt AR, Pinches R, Nash G, et al. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature. 1992;357:689–692. doi: 10.1038/357689a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaestli M, Cortes A, Lagog M, Ott M, Beck HP. Longitudinal assessment of Plasmodium falciparum var gene transcription in naturally infected asymptomatic children in Papua New Guinea. J Infect Dis. 2004;189:1942–1951. doi: 10.1086/383250. [DOI] [PubMed] [Google Scholar]
- 30.Horrocks P, Pinches R, Christodoulou Z, Kyes SA, Newbold CI. Variable var transition rates underlie antigenic variation in malaria. Proc Natl Acad Sci U S A. 2004;101:11129–11134. doi: 10.1073/pnas.0402347101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kyes SA, Christodoulou Z, Raza A, Horrocks P, Pinches R, et al. A well-conserved Plasmodium falciparum var gene shows an unusual stage-specific transcript pattern. Mol Microbiol. 2003;48:1339–1348. doi: 10.1046/j.1365-2958.2003.03505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winter G, Chen Q, Flick K, Kremsner P, Fernandez V, et al. The 3D7var5.2 (var COMMON) type var gene family is commonly expressed in non-placental Plasmodium falciparum malaria. Mol Biochem Parasitol. 2003;127:179–191. doi: 10.1016/s0166-6851(03)00004-5. [DOI] [PubMed] [Google Scholar]
- 33.Coulson RM, Hall N, Ouzounis CA. Comparative genomics of transcriptional control in the human malaria parasite Plasmodium falciparum. Genome Res. 2004;14:1548–1554. doi: 10.1101/gr.2218604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maier AG, Rug M, O'Neill MT, Beeson JG, Marti M, et al. Skeleton-binding protein 1 functions at the parasitophorous vacuole membrane to traffic PfEMP1 to the Plasmodium falciparum-infected erythrocyte surface. Blood. 2007;109:1289–1297. doi: 10.1182/blood-2006-08-043364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biggs BA, Kemp DJ, Brown GV. Subtelomeric chromosome deletions in field isolates of Plasmodium falciparum and their relationship to loss of cytoadherence in vitro. Proc Natl Acad Sci U S A. 1989;86:2428–2432. doi: 10.1073/pnas.86.7.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frank M, Dzikowski R, Amulic B, Deitsch K. Variable switching rates of malaria virulence genes are associated with chromosomal position. Mol Microbiol. 2007;64:1486–1498. doi: 10.1111/j.1365-2958.2007.05736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haase RN, Megnekou R, Lundquist M, Ofori MF, Hviid L, et al. Plasmodium falciparum parasites expressing pregnancy-specific variant surface antigens adhere strongly to the choriocarcinoma cell line BeWo. Infect Immun. 2006;74:3035–3038. doi: 10.1128/IAI.74.5.3035-3038.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duffy MF, Byrne TJ, Elliott SR, Wilson DW, Rogerson SJ, et al. Broad analysis reveals a consistent pattern of var gene transcription in Plasmodium falciparum repeatedly selected for a defined adhesion phenotype. Mol Microbiol. 2005;56:774–788. doi: 10.1111/j.1365-2958.2005.04577.x. [DOI] [PubMed] [Google Scholar]
- 39.Kaestli M, Cockburn IA, Cortes A, Baea K, Rowe JA, et al. Virulence of malaria is associated with differential expression of Plasmodium falciparum var gene subgroups in a case-control study. J Infect Dis. 2006;193:1567–1574. doi: 10.1086/503776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duffy MF, Caragounis A, Noviyanti R, Kyriacou HM, Choong EK, et al. Transcribed var genes associated with placental malaria in Malawian women. Infect Immun. 2006;74:4875–4883. doi: 10.1128/IAI.01978-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rottmann M, Lavstsen T, Mugasa JP, Kaestli M, Jensen AT, et al. Differential expression of var gene groups is associated with morbidity caused by Plasmodium falciparum infection in Tanzanian children. Infect Immun. 2006;74:3904–3911. doi: 10.1128/IAI.02073-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mair GR, Braks JA, Garver LS, Wiegant JC, Hall N, et al. Regulation of sexual development of Plasmodium by translational repression. Science. 2006;313:667–669. doi: 10.1126/science.1125129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trimnell AR, Kraemer SM, Mukherjee S, Phippard DJ, Janes JH, et al. Global genetic diversity and evolution of var genes associated with placental and severe childhood malaria. Mol Biochem Parasitol. 2006;148:169–180. doi: 10.1016/j.molbiopara.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 44.Yang YH, Dudoit S, Luu P, Lin DM, Peng V, et al. Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res. 2002;30:e15. doi: 10.1093/nar/30.4.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qazi KR, Wikman M, Vasconcelos NM, Berzins K, Stahl S, et al. Enhancement of DNA vaccine potency by linkage of Plasmodium falciparum malarial antigen gene fused with a fragment of HSP70 gene. Vaccine. 2005;23:1114–1125. doi: 10.1016/j.vaccine.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 46.Nielsen MA, Grevstad B, TM AE, Kurtzhals JA, Giha H, et al. Differential induction of immunoglobulin G to Plasmodium falciparum variant surface antigens during the transmission season in Daraweesh, Sudan. J Infect Dis. 2005;192:520–527. doi: 10.1086/431678. [DOI] [PubMed] [Google Scholar]
- 47.Ribacke U, Mok BW, Wirta V, Normark J, Lundeberg J, et al. Genome wide gene amplifications and deletions in Plasmodium falciparum. Mol Biochem Parasitol. 2007;155:33–44. doi: 10.1016/j.molbiopara.2007.05.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(0.54 MB PNG)
(0.01 MB PDF)