Abstract
Pulmonary venous stenosis should be considered in patients presenting with respiratory symptoms after atrial fibrillation ablation
Case reports
Case 1
A 70 year old woman was referred by her general physician to the respiratory clinic with a few days’ history of haemoptysis without any associated chest pain, fever, or dyspnoea. The only medical history of note was a successful pulmonary venous isolation procedure for paroxysmal atrial fibrillation in the previous week. She was a lifelong non-smoker and was previously fit and well. Physical examination and routine blood tests were unremarkable. The electrocardiogram showed sinus rhythm. A small (2 cm) opacity was seen on the chest radiography in the left mid-zone. Computed tomography of the thorax and abdomen showed only numerous ill-defined patchy lesions with ground-glass shadowing in the left upper lobe, without any evidence of malignancy. Bronchoscopy showed altered blood in the left upper lobe bronchus, and lavage specimens were negative for malignancy and infection, including tuberculosis. She was treated empirically for an atypical pneumonia.
Haemoptysis recurred six weeks later. Further tests including autoantibody screen, aspergillus precipitins, and complement status had negative results. On a repeat scan of the thorax, patchy ground-glass shadowing persisted in the left upper lobe. Bronchoscopy was repeated, with transbronchial biopsies taken from the left upper lobe. Histological examination showed focal occlusion of blood vessels, with recanalisation and presence of haemosiderin-laden macrophages suggestive of alveolar haemorrhage. The overall appearances were characteristic of veno-occlusive pulmonary disease. Review of the original thoracic scan showed significant impairment of contrast opacification at the left upper pulmonary venous ostium, suggestive of severe pulmonary venous stenosis (fig 1). The stenosis was confirmed by pulmonary venous angiography and relieved by percutaneous balloon venoplasty. The patient was symptom free after 12 months of follow-up.
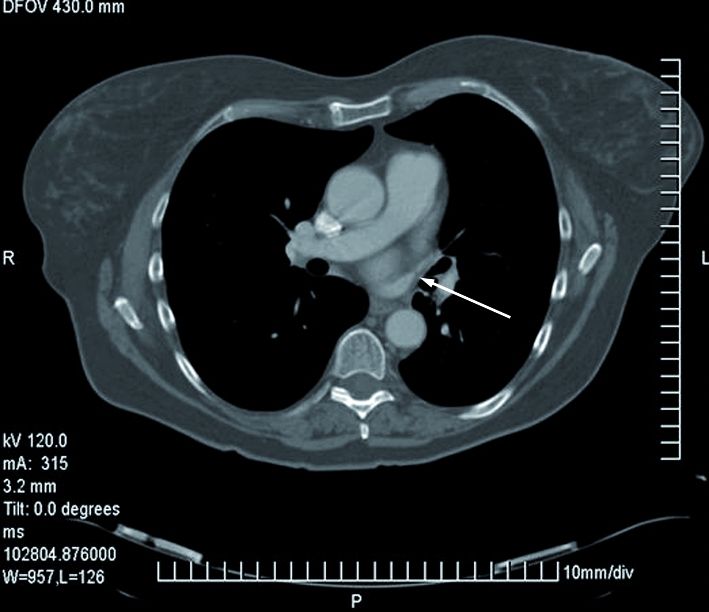
Fig 1 Computed tomography of chest, showing left upper pulmonary venous stenosis (arrow)
Case 2
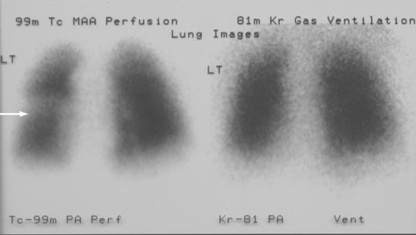
A 55 year old man presented at his local accident and emergency department with chest pain. His only medical history was persistent lone atrial fibrillation that had been treated with pulmonary venous isolation four months earlier. He continued taking warfarin after his procedure and at the time of admission had responded well (international normalised ratio 3.2). Physical examination was unremarkable, as were his cardiac enzymes, arterial blood gases, inflammatory markers, electrocardiogram, routine biochemistry, amylase concentration, and liver function tests. Despite a low clinical probability of embolism, a ventilation-perfusion scan was performed; it showed a left lung ventilation-perfusion mismatch and was reported to be consistent with a high probability of a pulmonary embolus (fig 2). A diagnosis of pulmonary embolism was given to the patient, with an anticoagulation target range of 3.5-4.0. During a routine review at our institution, he reported infrequent but similar episodes of discomfort in the left side of the chest. Computed tomography pulmonary venography showed a 90% stenosis in the left upper pulmonary vein. As his symptoms had been gradually improving, he was treated conservatively.
Fig 2 Ventilation-perfusion scan showing mismatch of patient in case 2 with left upper pulmonary venous stenosis. Left panel shows a clear pulmonary perfusion defect in the middle of the left lung (arrow); right panel shows the homogeneous uptake of the ventilated tracer.
Case 3
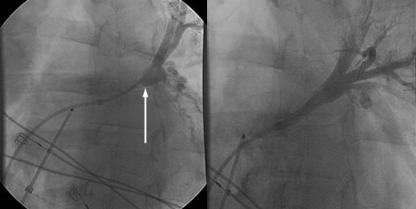
A 41 year old man with idiopathic dilated cardiomyopathy and persistent atrial fibrillation presented to the general cardiology clinic with breathlessness and pleuritic chest pain six weeks after his second pulmonary venous isolation. As he was a former smoker, coronary angiography, chest x ray, and lung function tests were done to exclude coronary artery disease and pulmonary pathologies. No abnormalities were detected. Six months after onset of symptoms, cardiac magnetic resonance imaging showed severe left upper pulmonary venous stenosis, which was treated successfully with balloon venoplasty (figs 3 and 4).
Fig 3 Fluoroscopic left upper pulmonary venography in case 3. Left: severe stenosis at pulmonary vein ostium, resulting in severe hold-up of contrast when injected via a catheter (arrow); right: post-angioplasty venography showing less contrast hold-up and reduction of stenosis to less than 50%
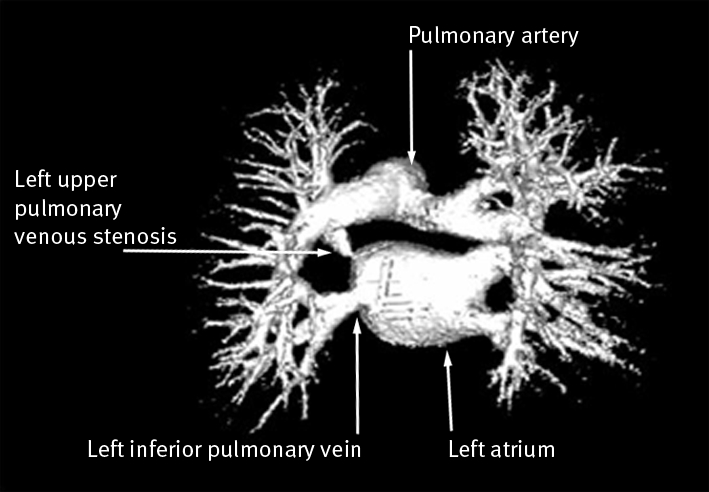
Fig 4 Virtual reconstruction of cardiac magnetic resonance imaging from case 3 showing left upper pulmonary venous stenosis. Narrowing of the LUPV ostium contrasts with normal calibre of left inferior pulmonary vein ostium
Discussion
All three patients presented with the well recognised complication of pulmonary venous stenosis following pulmonary venous isolation, a widespread and increasingly used treatment for atrial fibrillation. In all cases, this differential diagnosis was not considered at the time of presentation, which led to numerous unnecessary investigations and potentially dangerous errors in patient management.
Pulmonary venous isolation is an effective treatment for patients with symptoms and atrial fibrillation refractory to treatment with antiarrhythmic drugs.1 About 70% of patients with paroxysmal atrial fibrillation remain free from atrial fibrillation without the use of antiarrhythmic agents one year after the procedure.1 2 This technique stemmed from the discovery that premature atrial ectopic beats that initiate atrial fibrillation originate predominantly from the atrial myocardial sleeves that extend into the pulmonary veins.2 By delivering radiofrequency energy at the junctions between the four pulmonary veins and the left atrium (also known as the pulmonary venous ostia), it is possible to electrically disconnect and thus isolate these arrhythmogenic sources of ectopy from the atria.
Despite modifications to the technique for pulmonary venous isolation, pulmonary venous stenosis remains a recognised complication of such ablation, with 2-5% of patients affected by pulmonary venous stenosis.3 4 The thermal injury caused by radiofrequency ablation produces tissue necrosis, and although the resulting scar electrically disconnects the pulmonary venous musculature from the atria, it can also thicken and contract, leading to luminal narrowing or obstruction.5 The ease of recognising pulmonary venous stenosis varies according to the imaging modality and intensity of surveillance. Furthermore, the degree of luminal narrowing may progress up to six months after the procedure.6 Clinically, almost all patients with mild (<50%) or moderate (50-69%) stenosis do not have symptoms.6 Development of respiratory symptoms (cough, haemoptysis, atypical non-pleuritic chest pain, and dyspnoea) is associated with severe pulmonary venous stenosis or the involvement of multiple pulmonary veins. Ventilation-perfusion scans can be useful in determining the functional importance of the stenosis, as perfusion defects of the affected lung segments indicate severe stenosis.4 Previous reports have commented that radiological findings on perfusion scans may suggest a high probability of pulmonary emboli, as in case 2.
Before the advent of pulmonary venous isolation, pulmonary veno-occlusive disease was extremely rare. In most cases of naturally occurring pulmonary veno-occlusive disease, the cause is unknown. It may occur as a complication of conditions such as lupus, leukaemia, lymphoma, or chemotherapy and is associated with poor survival rates of a few weeks in infants and a few years in adults. It is also an extremely rare cause of primary pulmonary hypertension.
Although angioplasty has been effective in short term relief of symptoms in patients with severe pulmonary venous stenosis after pulmonary venous isolation, there is a substantial risk of restenosis.7 However, long term freedom from such symptoms does not seem to depend on patency of the vein. After angioplasty, 90% of patients reported that their symptoms had improved, despite restenosis.8 This may be due to collateralisation or other beneficial physiological adaptations. The long term risk of developing pulmonary hypertension in patients with pulmonary venous stenosis remains unknown, and it remains unclear whether intervention is needed in asymptomatic patients with severe stenosis.
To date, more than 20 000 atrial fibrillation ablation procedures have been carried out in more than 80 European centres.9 This figure is set to rise further in the United Kingdom after the recent publication of clinical guidelines from the National Institute for Health and Clinical Excellence supporting the use of percutaneous radiofrequency ablation for atrial fibrillation.10 In view of increasing numbers of atrial fibrillation patients having catheter ablation treatment, general physicians, radiologists, and emergency healthcare professionals should be aware of this “new” iatrogenic condition which can mimic more common pulmonary diseases such as pneumonia and pulmonary embolism. Failure to consider pulmonary venous stenosis as a differential diagnosis in these patients could lead to unnecessary investigations and delay in starting effective treatment.
Contributors: PK performed the literature search and wrote the article; TW had the idea for the article; ARW was responsible for reporting all cardiac computed tomography scans of patients who underwent atrial fibrillation ablation; OMK and WO were responsible for identifying and managing case 1; PK was responsible for identifying and managing case 2 and was involved in the clinical management of cases 1 and 3; DWD and NSP were involved in the management of all cases. All authors were also involved in the interpretation of the clinical data, revising the article critically for important intellectual content and its final approval of the version to be published. NSP is guarantor.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Sauer WH, McKernan ML, Lin D, Gerstenfeld EP, Callans DJ, Marchlinski FE. Clinical predictors and outcomes associated with acute return of pulmonary vein conduction during pulmonary vein isolation for treatment of atrial fibrillation. Heart Rhythm 2006;3:1024-8. [DOI] [PubMed] [Google Scholar]
- 2.Hocini M, Sanders P, Jais P, Hsu LF, Takahashi Y, Rotter M, et al. Techniques for curative treatment of atrial fibrillation. J Cardiovasc Electrophysiol 2004;15:1467-71. [DOI] [PubMed] [Google Scholar]
- 3.Purerfellner H, Cihal R, Aichinger J, Martinek M, Nesser HJ. Pulmonary vein stenosis by ostial irrigated-tip ablation: incidence, time course, and prediction. J Cardiovasc Electrophysiol 2003;14:158-64. [DOI] [PubMed] [Google Scholar]
- 4.Saad EB, Marrouche NF, Saad CP, Ha E, Bash D, White RD, et al. Pulmonary vein stenosis after catheter ablation of atrial fibrillation: emergence of a new clinical syndrome. Ann Intern Med 2003;138:634-8. [DOI] [PubMed] [Google Scholar]
- 5.Taylor GW, Kay GN, Zheng X, Bishop S, Ideker RE. Pathological effects of extensive radiofrequency energy applications in the pulmonary veins in dogs. Circulation 2000;101(14):1736-42. [DOI] [PubMed] [Google Scholar]
- 6.Saad EB, Rossillo A, Saad CP, Martin DO, Bhargava M, Erciyes D, et al. Pulmonary vein stenosis after radiofrequency ablation of atrial fibrillation: functional characterization, evolution, and influence of the ablation strategy. Circulation 2003;108:3102-7. [DOI] [PubMed] [Google Scholar]
- 7.Qureshi AM, Prieto LR, Latson LA, Lane GK, Mesia CI, Radvansky P, et al. Transcatheter angioplasty for acquired pulmonary vein stenosis after radiofrequency ablation. Circulation 2003;108:1336-42. [DOI] [PubMed] [Google Scholar]
- 8.Mahapatra S, Peterson LA, Monihan KM, Munger TM, Packer DL. Long-term symptom improvement in patients with pulmonary vein stenosis from atrial fibrillation ablation despite restenosis. Heart Rhythm 2004;1(suppl):S88 [Google Scholar]
- 9.Nieuwlaat R, Capucci A, Camm AJ, Olsson SB, Andresen D, Davies DW, et al. Atrial fibrillation management: a prospective survey in ESC member countries: the Euro Heart Survey on Atrial Fibrillation. Eur Heart J 2005;26:2422-34. [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Health and Clinical Excellence. Percutaneous radiofrequency catheter ablation for atrial fibrillation. 2006. www.nice.org.uk/guidance/IPG168