Abstract
Background
Atypical nevi are a common risk factor for melanoma.
Objective
To determine the utility of monitoring dermoscopic photographs of atypical nevi in a high-risk population.
Methods
Over a 4.5-year period, digital dermoscopic photographs were taken of clinically atypical nevi at initial and follow-up visits, such that side-by-side comparisons could be made.
Results
A total of 5945 lesions were monitored in 297 patients over 3–52 months (median 22 months) and 324 lesions were biopsied. Photographic changes were noted in 96/5945 (1.6%) lesions, which included 64 dysplastic nevi (67%), 25 common nevi (26%), and one melanoma (1.0%). Of six melanomas biopsied during the follow-up period, only one was detected by dermoscopic photographic change at follow-up.
Conclusions
Most clinically atypical melanocytic nevi are stable over time, and lesions exhibiting dermoscopic changes are most likely to be dysplastic nevi. While dermoscopy is a useful tool for clinical examination, the sensitivity of dermoscopic monitoring is limited by melanomas that may arise in normal skin or in clinically benign nevi that were not initially photographed.
INTRODUCTION
Various strategies have been employed for early melanoma detection, but there is no consensus as to the most effective and practical approach for screening high-risk patients. Clinical examination alone, even by trained dermatologists, has limited sensitivity for melanoma detection.1 Dermoscopy, which provides enhanced detail of pigmentation patterns and allows visualization of deeper structures, increases diagnostic accuracy for experienced users2 and reduces overall biopsy rates.3 However, various dermoscopic algorithms generally have a sensitivity for melanoma detection of only 80%,4 possibly because they are based on morphologic structures that may not be present in early lesions. Although melanoma is often discovered by patients on self-examination, applying the ABCD criteria5 may not be helpful for those with multiple atypical nevi already exhibiting these features. Since the risk of transformation of an individual nevus to melanoma, however, is estimated to be only 1 in 200,000 in younger patients,6 prophylactic removal of all melanocytic nevi is unlikely to enhance patient survival. Rather, most agree that careful observation with particular attention to evolving changes in appearance should direct which lesions are biopsied or removed.5
Changes in melanocytic nevi over time can best be appreciated through side-by-side comparisons, and two general approaches have been employed. First, in several studies,7–10 baseline regional photographs were taken to provide a comparative reference point for subsequent follow-up examinations. Such clinical photographs facilitate detection of new lesions, which is important since melanoma often develops de novo in clinically normal skin rather than from pre-existing nevi,11, 12 but may not provide sufficient detail to detect clinically significant changes in a specific nevus. Alternatively, multiple studies13–16 have described monitoring nevi by comparison of serially-taken digital dermoscopic photographs. While this approach is highly sensitive for detection of morphologic changes over time, it is not useful for detection of new lesions.
Here we report our experience using this latter approach in which baseline dermoscopic images of atypical nevi were archived, then serial dermoscopic photographs were repeated at each follow-up visit.
METHODS
Patients
This study was approved by the Institutional Review Board at the University of Utah (IRB# 16799). During the period from November 1999 to July 2004, patients with atypical nevi were seen and followed in the Mole Mapping Clinic at the Huntsman Cancer Institute. All patients undergoing dermoscopic monitoring had clinically atypical nevi, and in addition approximately 25% had a personal history of melanoma and 10% had a positive family history of melanoma. All patients were counseled regarding the importance of sun protection, regular self-skin examinations, the ABCD criteria for detecting melanoma, and were asked to return every 6–12 months. Patients were also provided hard copies of regional and macro photographs taken on the first visit to facilitate detection both of new nevi and of gross changes in existing nevi during self-skin examinations at home.
Photography
All patients at the initial visit had baseline digital dermoscopic photographs taken of their clinically atypical nevi (> 2 mm in diameter) using an imaging and archiving system (MoleMaxII; Derma Instruments, San Diego, CA). Inclusion criteria for photography were lesions with one or more of the following characteristics: asymmetry, irregular or fuzzy borders, non-uniform pigmentation (color variation), and size greater than 1 cm. Digital dermoscopic images were taken at 30-fold magnification, and stored as jpeg files with a resolution of approximately 600 × 400 pixels. At each follow-up visit, dermoscopic images were taken of all previously photographed nevi, and new and prior dermoscopic images were compared side-by-side on a split screen by the dermatologist (G.M.B. or D.G.). We did not employ rigid objective criteria for determining whether a lesion had changed, but rather dermoscopic photographs exhibiting interval asymmetric changes in size or pigmentation or the appearance of recognized dermoscopic structural changes4 were noted as having changed. Lesions demonstrating small symmetric changes in size or uniform changes in pigmentation were generally due to one or more of the following: expected lesion growth in proportion to skin area in young patients, seasonal sun exposure, variable intensity of the light source, or variable pressure applied to the lesion during photography, and were not considered to have changed. In some cases, dermoscopic photographs were not helpful due to either poor photo quality or the presence of uniform dark pigmentation which precluded assessment of potential changes. Determination of the dermatologist’s assessment of particular photographs was made by review of the chart notes.
Biopsies and excisions
Biopsies were performed using either standard shave or punch technique, such that the entire clinical lesion was removed. With few exceptions, partial biopsies were not done. In some cases, excisional biopsy or re-excision for definitive lesion removal was performed. Standard histologic evaluation of hematoxylin- and eosin-stained sections was performed by a trained dermatopathologist. All melanomas were re-reviewed (by S.R.F.) to confirm diagnosis and assess nevus origin. Diagnosis of dysplastic and common nevi, and melanoma, was based on well-established architectural and cytologic criteria. Specimens revealing non-melanocytic pathology such as carcinoma, and re-excisions of recently biopsied or recurrent melanocytic lesions including melanoma and cutaneous melanoma metastases, were not considered relevant for the study and excluded. In addition, 31 biopsies (in 16 patients) were excluded because the photographic equipment was not available on the date of biopsy, or the physician’s assessment of the photographs could not be ascertained by chart review.
RESULTS
Biopsies of monitored lesions
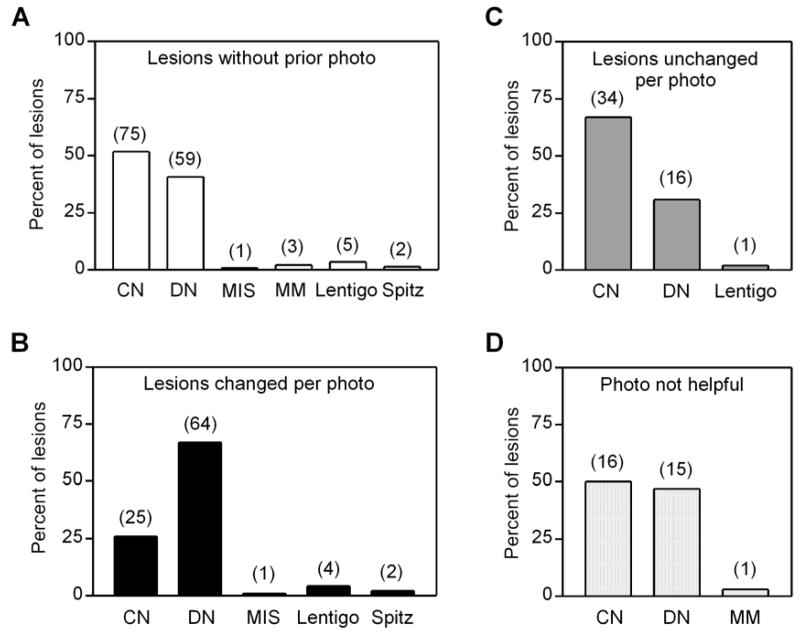
In 297 patients with atypical nevi, 5,945 lesions (range 1–71, median 19 per patient) were monitored over 3–52 months (median 22 months). In 136 patients, 324 relevant biopsies (see Methods) were performed. Most of the monitored lesions were stable, and did not exhibit change during the observation period (stable dysplastic nevus, Figure 1A). By contrast only 96 lesions, or 1.6% of those monitored, were noted to have changed by comparison to a previous photograph. All lesions that demonstrated significant change – most commonly increased focal pigmentation and/or changes in lesion border – were biopsied. The remaining biopsies were categorized into three additional groups as depicted in Figure 2. The most common category was represented by 145 lesions (45% of biopsies) that had not been previously photographed, either because they represented a new lesion or were not selected for dermoscopic photography on the initial clinical exam (Figure 2). In 51 cases (16% of biopsies), lesions were biopsied despite lack of photographic change due to patient concern or request (i.e. new onset itching or bleeding) or physician concern (i.e. to rule out melanoma) (Figure 2). Finally, in 32 cases (9.9% of biopsies), prior photographs were not helpful; most commonly this was due to uniform dark pigmentation of the lesion which precluded determination as to whether the lesion had changed, but occasionally prior photographs were not of sufficient resolution to appreciate interval changes.
Figure 1.
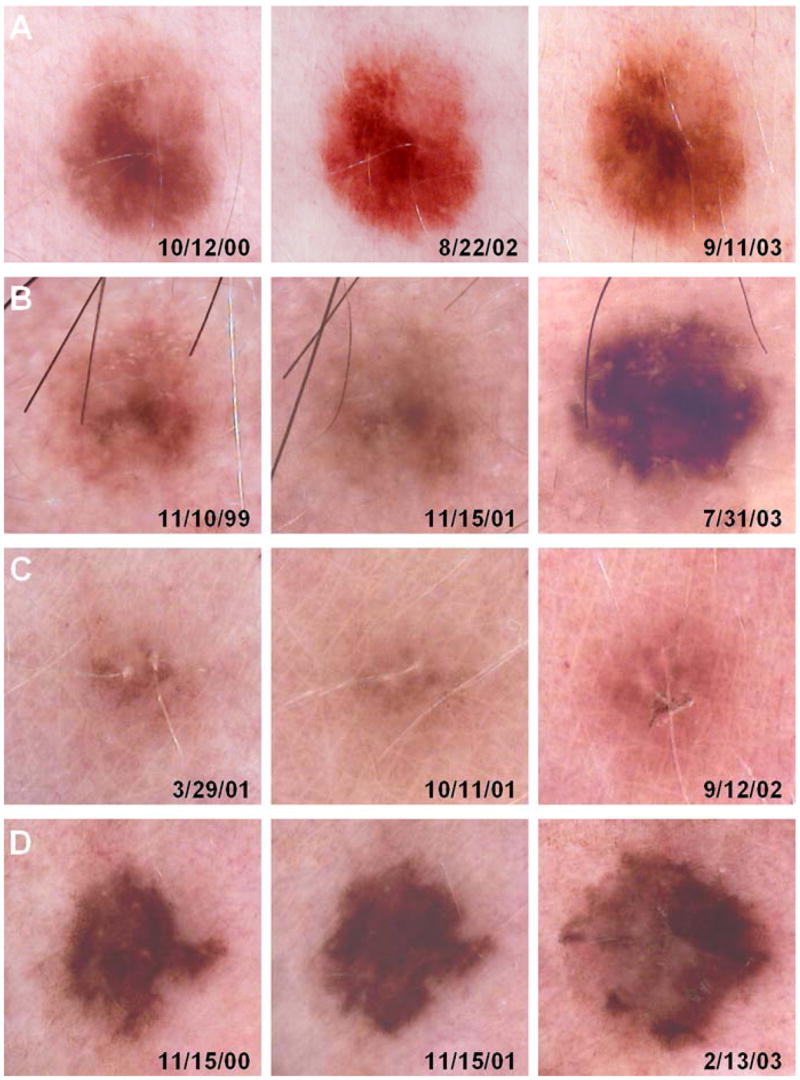
Serial dermoscopic images of nevi. (A) Photos of stable clinically atypical nevus on upper back of a 40-year-old male, taken 10/12/00, 8/22/02, and 9/11/03 when biopsy demonstrated dysplastic nevus with mild atypia. (B) Photos of changing lesion on scalp of 53–year-old male, taken on 11/10/99, 11/15/01, and 7/31/03 when biopsy revealed dysplastic nevus with moderate atypia. (C) Photos of changing lesion on thigh of 41-year-old female, taken on 3/29/01, 10/11/01, and 9/12/02 when biopsy demonstrated nevus without dysplastic features. (D) Photos of changing lesion on the right breast of a 39-year-old female, taken 11/15/00, 11/15/01, and 2/13/03 when excision demonstrated melanoma-in-situ.
Figure 2.
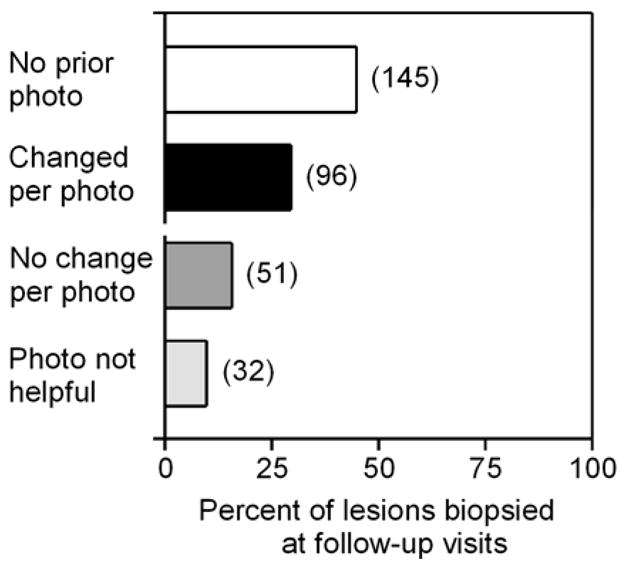
Role of photography in lesions biopsied at follow-up visits. The 324 lesions biopsied at follow-up visits were categorized as indicated. Number of lesions indicated in parentheses adjacent to bars.
A histologic breakdown of these categories of biopsies of monitored lesions is presented in Figure 3. Of the 145 lesions that were not photographed, the majority (92%) proved to be nevi, represented by a greater percentage of common than dysplastic nevi (52% vs. 41%) (Figure 3A). Four melanomas (out of 6 diagnosed in follow-up) were included in this category, as well as five lentigines and two Spitz nevi (Figure 3A). Of the 96 lesions that had changed per prior photograph, almost all (93%) were nevi, with dysplastic nevi predominating over common nevi (67% vs. 26%), and the remaining lesions were Spitz nevi, lentigines, and one melanoma (Figure 3B). Thus although photographic change was more commonly associated with dysplastic nevi (Figure 1B), many lesions in this category proved to be common nevi without dysplasia (Figure 1C, 3C) and only one lesion was melanoma (Figure 3D). Changes in lesions that proved to be common nevi were usually the development of dark dots or eccentric pigmentation. Of 51 lesions that were biopsied despite lack of photographic change, almost all (98%) were nevi, with common nevi predominating over dysplastic nevi (67% vs. 31%), and none was melanoma (Figure 3C). Finally, of 32 biopsies where photographs were not helpful in assessing change, almost all were nevi (97%) with roughly equal representation of common and dysplastic nevi (Figure 3D).
Figure 3.
Histologic diagnoses of lesions biopsied at follow-up visits. Lesions were categorized as (A) not previously photographed, (B) changed per prior photograph, (C) unchanged per prior photograph, and (D) unclear if changed. Number of lesions indicated in parentheses above bars. CN, common nevus; DN, dysplastic nevus; MIS, melanoma in situ; MM, invasive melanoma.
Of the six melanomas diagnosed on follow-up visits, two were lentigo maligna or melanoma-in-situ, two were lentigo maligna melanoma, and two were superficial spreading melanoma. The depth for invasive lesions ranged from 0.23–0.35 mm. Of the two lesions of superficial spreading melanoma, one showed histologic evidence of a pre-existing (dysplastic) nevus.
Role of photography
Of the 324 biopsied lesions, a prior photograph was available in 179 (55%) cases (Figure 2). When available, a photographic comparison made a difference in the clinician’s decision to biopsy in 96 of 179 (54%) cases, while in the remaining 83 (46%) cases either the prior photograph was not helpful or was irrelevant because either patient or physician concern motivated the biopsy (Figure 2). Of the six new melanomas biopsied at follow-up, 4/6 (67%) had not been previously photographed (Figure 3A), and in one case, the prior photograph was not helpful (Figure 3D). Finally, one melanoma (melanoma-in-situ) was detected by photographic change (Figure 1D, 3B). Among photographed lesions, no melanomas were detected in lesions that had not demonstrated photographic change. Although all patients were provided hard copy photographs of regional and macro photographs, their compliance in reviewing them and performing self-skin examinations was highly variable. No melanomas, nor dysplastic nevi requiring definitive excision, were detected by patients using these photographs. Photographic monitoring was associated with an increase in the proportion of dysplastic to common nevi, as most lesions demonstrating photographic change were dysplastic nevi (Figure 3B) while most stable (Figure 3C) or unphotographed (Figure 3A) lesions were more likely to be common nevi. Finally, the biopsy rate was extremely low during follow-up visits as only 324 biopsies of melanocytic lesions were performed in 297 patients, corresponding to a rate of only 1.1 biopsy per patient over the 4.5-year study period.
DISCUSSION
The two primary goals of early melanoma detection are clear: first, biopsy melanomas while monitoring nevi; and second, avoid unnecessary biopsies/excisions. However, there is currently no consensus as to the best screening approach – that is both sensitive and practical – to meet these goals in high-risk patients. Clinical evaluation with the naked eye, dermoscopy, and photographic comparison represent increasingly complex levels of examination that may be applied to individual melanocytic lesions. Of these, only photographic comparison allows assessment of change – now considered to be the most important clinical characteristic of developing or growing melanoma.5
Photographic comparison has been incorporated into two general screening approaches as described in the literature. First, Lucas et al7 and Banky et al10 used baseline regional photographs to detect new lesions and changes in pre-existing lesions at follow-up visits. Dermoscopy was then applied to selected lesions to guide biopsy decisions. In these two studies, the fraction of melanomas that were in-situ was 11/167 and 2/18,10 respectively. While this approach appears effective in detecting melanomas, it is unclear if the invasive melanomas presenting as changing lesions could have been detected earlier with higher resolution photographs. A second approach, involving comparison of sequentially taken digital dermoscopic photographs, has been more frequently described.13–16 In these studies, the fraction of in-situ melanomas was 5/8,13 5/7,14 4/4,15 and 9/18.16 Haenssle et al.16 reported that monitoring of dermoscopic photographs increased the sensitivity of melanoma detection over that associated with dermoscopy alone. While this approach appears more sensitive for early melanoma detection, in that a relatively higher percentage of melanomas were diagnosed as in-situ, it is unclear in most of these studies whether any melanomas were missed because they either presented as new lesions or arose from nevi that were not monitored by dermoscopic photographs since total melanomas that developed in these patients was not reported.
It was reassuring to find that the vast majority of atypical nevi in our patients were quite stable over time, as only 1.6% of monitored lesions were noted to have changed by photographic comparison. This rate of change compares with rates of 4–6.4% reported in other studies13, 15, 17 in which dermoscopic photographs were monitored. While some nevi did exhibit change, in the majority of our cases these changes were not histologically concerning, revealing only common or dysplastic nevi usually not requiring further excision. The availability of photographic comparison was associated with an extremely low biopsy rate of 1.1 nevi per patient during the 4-year monitoring period. By contrast, it is common for some dermatologists to remove several atypical nevi at each visit, and one study of patients with atypical nevi by Cohen et al.18 documented an average of 17.7 nevi removed per patient over a 4-year period. In that study, photographic comparison was not used, and removal of 3361 atypical nevi yielded only 15 melanomas (0.4% of biopsies); in the subset of patients without prior history of melanoma, melanoma was detected in only 0.17% of biopsies.18 In their university-based pigmented lesion clinic, Carli et al.3 reported excision and melanoma rates of 15.6% (9% when dermoscopy was used) and 1%, respectively, without photographic monitoring. Thus using photographic change as one criteria for biopsy in our experience was quite effective in minimizing unnecessary biopsies.
We diagnosed six melanomas on follow-up visits in 324 (1.9%) biopsies. If the 51 lesions that were biopsied without photographic change are excluded, the melanoma detection rate during follow-up increases to 2.2% (6/273). This rate is comparable to rates of 1–4% reported in prior studies.13, 15, 17 It would be predicted that biopsy of lesions demonstrating photographic changes would be associated with reduction in the melanoma rate by removal of nevi that are in transition to melanoma, and we did observe multiple lesions that proved to be dysplastic nevi demonstrated initial stability followed by subsequent photographic changes. However, it is notable that of the six melanomas we biopsied, five did not arise from a pre-existing nevus and in only one case did we detect a melanoma by photographic change at follow-up. Our findings are in accordance with those of Lucas et al.7 in which none of the melanomas detected arose from clinically atypical nevi. Thus a general melanoma screening strategy focused solely on atypical nevi will likely miss melanomas presenting as new lesions or arising from nevi that are not clinically atypical.
Thus while dermoscopic photographic comparison was effective at minimizing biopsies of benign lesions (particularly common nevi), its efficacy for the early detection of melanoma appears limited by melanomas presenting as new lesions or those not arising from pre-existing atypical nevi. Most clinically atypical nevi were stable over time, and lesions exhibiting dermoscopic changes were most likely to be dysplastic nevi rather than melanoma. Others have reported7–10 that regional photography is highly effective in detecting new nevi. Photographic comparison simply needs to be able to answer the question – is a given nevus new or changing? We suspect that regional photography, which is far less cumbersome than monitoring serially dermoscopic photographs, is a practical approach that may be sufficient (if the photographs are of sufficiently high resolution) for detecting clinically important changes in nevi. In light of our experience described here, we are currently performing total body photography and using regional (rather than dermoscopic) photographs for monitoring atypical nevi in our patients at risk for melanoma.
Acknowledgments
We thank Gena Gough, Allison Schneider, and Misty Viera for their help with patient photography.
Footnotes
Stanley R. Fuller, BS, Glen M. Bowen, MD, Ben Tanner, BS, Scott R. Florell, MD, and Douglas Grossman, MD, PhD have no significant financial conflicts of interest.
References
- 1.Brochez L, Verhaeghe E, Bleyen L, Naeyaert JM. Diagnostic ability of general practitioners and dermatologists in discriminating pigmented skin lesions. J Am Acad Dermatol. 2001;44:979–86. doi: 10.1067/mjd.2001.113442. [DOI] [PubMed] [Google Scholar]
- 2.Binder M, Schwarz M, Winkler A, Steiner A, Kaider A, Wolff K, Pehamberger H. Epiluminescence microscopy. A useful tool for the diagnosis of pigmented skin lesions for formally trained dermatologists. Arch Dermatol. 1995;131:286–91. doi: 10.1001/archderm.131.3.286. [DOI] [PubMed] [Google Scholar]
- 3.Carli P, de Giorgi V, Chiarugi A, Nardini P, Weinstock MA, Crocetti E, Stante M, Giannotti B. Addition of dermoscopy to conventional naked-eye examination in melanoma screening: a randomized study. J Am Acad Dermatol. 2004;50:683–9. doi: 10.1016/j.jaad.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Dolianitis C, Kelly J, Wolfe R, Simpson P. Comparative performance of 4 dermoscopic algorithms by nonexperts for the diagnosis of melanocytic lesions. Arch Dermatol. 2005;141:1008–14. doi: 10.1001/archderm.141.8.1008. [DOI] [PubMed] [Google Scholar]
- 5.Rigel DS, Friedman RJ, Kopf AW, Polsky D. ABCDE--an evolving concept in the early detection of melanoma. Arch Dermatol. 2005;141:1032–4. doi: 10.1001/archderm.141.8.1032. [DOI] [PubMed] [Google Scholar]
- 6.Tsao H, Bevona C, Goggins W, Quinn T. The transformation rate of moles (melanocytic nevi) into cutaneous melanoma: a population-based estimate. Arch Dermatol. 2003;139:282–8. doi: 10.1001/archderm.139.3.282. [DOI] [PubMed] [Google Scholar]
- 7.Lucas CR, Sanders LL, Murray JC, Myers SA, Hall RP, Grichnik JM. Early melanoma detection: nonuniform dermoscopic features and growth. J Am Acad Dermatol. 2003;48:663–71. doi: 10.1067/mjd.2003.283. [DOI] [PubMed] [Google Scholar]
- 8.Wang SQ, Kopf AW, Koenig K, Polsky D, Nudel K, Bart RS. Detection of melanomas in patients followed up with total cutaneous examinations, total cutaneous photography, and dermoscopy. J Am Acad Dermatol. 2004;50:15–20. doi: 10.1016/s0190-9622(03)02794-4. [DOI] [PubMed] [Google Scholar]
- 9.Feit NE, Dusza SW, Marghoob AA. Melanomas detected with the aid of total cutaneous photography. Br J Dermatol. 2004;150:706–14. doi: 10.1111/j.0007-0963.2004.05892.x. [DOI] [PubMed] [Google Scholar]
- 10.Banky JP, Kelly JW, English DR, Yeatman JM, Dowling JP. Incidence of new and changed nevi and melanomas detected using baseline images and dermoscopy in patients at high risk for melanoma. Arch Dermatol. 2005;141:998–1006. doi: 10.1001/archderm.141.8.998. [DOI] [PubMed] [Google Scholar]
- 11.Marks R, Dorevitch AP, Mason G. Do all melanomas come from “moles”? A study of the histological association between melanocytic naevi and melanoma. Australas J Dermatol. 1990;31:77–80. doi: 10.1111/j.1440-0960.1990.tb00656.x. [DOI] [PubMed] [Google Scholar]
- 12.Thomas NE, Groben P. Invasive superficial spreading melanomas arising from clinically normal skin. J Am Acad Dermatol. 2004;51:466–70. doi: 10.1016/j.jaad.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 13.Kittler H, Pehamberger H, Wolff K, Binder M. Follow-up of melanocytic skin lesions with digital epiluminescence microscopy: patterns of modifications observed in early melanoma, atypical nevi, and common nevi. J Am Acad Dermatol. 2000;43:467–76. doi: 10.1067/mjd.2000.107504. [DOI] [PubMed] [Google Scholar]
- 14.Menzies SW, Gutenev A, Avramidis M, Batrac A, McCarthy WH. Short-term digital surface microscopic monitoring of atypical or changing melanocytic lesions. Arch Dermatol. 2001;137:1583–9. doi: 10.1001/archderm.137.12.1583. [DOI] [PubMed] [Google Scholar]
- 15.Robinson JK, Nickoloff BJ. Digital epiluminescence microscopy monitoring of high-risk patients. Arch Dermatol. 2004;140:49–56. doi: 10.1001/archderm.140.1.49. [DOI] [PubMed] [Google Scholar]
- 16.Haenssle HA, Krueger U, Vente C, Thoms KM, Bertsch HP, Zutt M, Rosenberger A, Neumann C, Emmert S. Results from an observational trial: digital epiluminescence microscopy follow-up of atypical nevi increases the sensitivity and the chance of success of conventional dermoscopy in detecting melanoma. J Invest Dermatol. 2006;126:980–5. doi: 10.1038/sj.jid.5700119. [DOI] [PubMed] [Google Scholar]
- 17.Bauer J, Blum A, Strohhacker U, Garbe C. Surveillance of patients at high risk for cutaneous malignant melanoma using digital dermoscopy. Br J Dermatol. 2005;152:87–92. doi: 10.1111/j.1365-2133.2005.06370.x. [DOI] [PubMed] [Google Scholar]
- 18.Cohen MH, Cohen BJ, Shotkin JD, Morrison PT. Surgical prophylaxis of malignant melanoma. Ann Surg. 1991;213:308–14. doi: 10.1097/00000658-199104000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]