To the Editor
Cellular senescence is an irreversible withdrawal from the cell cycle without cell death, and may represent a barrier to malignancy. Replicative senescence results from critical telomere shortening, which may be overcome in tumors by expression of the hTERT telomerase (Bodnar et al., 1998). Senescence may also occur independent of telomere shortening, through expression of single oncogenes in otherwise genetically intact cells (Serrano et al., 1997). In fact, mutational activation (BRAFV600E, HRAS, NRAS) or amplification (HRAS, NRAS) of oncogenes is a relatively common finding in a variety of melanocytic nevi (Pollock et al., 2003), including dysplastic (Pollock et al., 2003), congenital (Pollock et al., 2003; Bauer et al., 2007) and Spitz (Bastian et al., 2000) nevi. These findings, combined with the capacity of these individual oncogenes to induce a senescent phenotype when introduced into normal melanocytes in vitro (Michaloglou et al., 2005; Denoyelle et al., 2006), suggest that oncogene-induced senescence represents a barrier to transformation from nevus to melanoma (Mooi et al., 2006). In support of this notion, two groups (Michaloglou et al., 2005; Gray-Schopfer et al., 2006) recently reported expression of senescence-associated beta-galactosidase (SA-β-gal) in 100% of congenital nevi examined.
We investigated expression of SA-β-gal in a panel of nevi, including congenital, common, and dysplastic subtypes. We hypothesized that if SA-β-gal was a true marker of senescence in vivo, and if failure of (or escape from) senescence in nevus cells contributes to melanoma formation, then levels of SA-β-gal might inversely correlate with histologic nevus subtypes associated with increasing risk of transformation (i.e. congenital or dysplastic > compound > intradermal). First, we validated the routinely used SA-β-gal (pH 6) staining protocol (Dimri et al., 1995) using both early and late-passage normal human foreskin melanocytes, which were prepared and propagated as described previously (Bowen et al., 2003). As expected, β-gal activity at pH 6 was detected in late (Figure 1c) but not early (Figure 1a) passage cells. Interestingly, β-gal activity was not only detectable in late passage cells demonstrating (senescence-like) spread-out morphology, but also in those bearing the spindled dendritic morphology of earlier passage cells (Figure 1c). As a control, staining was also performed at pH 4 which optimally detects lysosomal β-gal (Morreau et al., 1989), and both early and late passage cells demonstrated robust pH 4 β-gal staining (Figure 1b,d). We then applied this staining technique to a panel of 17 freshly frozen adult nevi, collected in accordance with the Declaration of Helsinki Principles and as approved by the Institutional Review Board at the University of Utah (#19304). Specimens were trisected at the time of biopsy, with the central section placed in formalin and sent for histopathologic analysis by a dermatopathologist (S.R.F.). The flanking pieces were oriented and embedded in OCT, and stored at −20 °C. After confirming the central section was adequate for diagnostic purposes, we proceeded with β-gal staining at pH 4, 5, and 6 on 8-μm frozen sections. The data are summarized in Table I. While every specimen evaluated showed varying degrees of positivity at the optimal pH 4 (Figure 1f), none of the specimens showed staining at pH 6 (i.e. SA-β-gal) (Figure 1e) (Table I). Staining at pH 5 was present only in specimens that showed strong staining at pH 4, and when present, was generally intermediate to staining seen at pH 4 and 6 (Table I). Given the somewhat surprising absence of β-gal staining at pH 6 in all 17 nevi examined (including several congenital and intradermal subtypes), we performed an additional procedural control by staining late and early passage melanocytes in frozen sections which had been prepared similarly (as the nevi) in OCT. These cells showed the same staining pattern (Figure 1g–j) as seen when stained in culture (Figure 1a–d), thus confirming that the absence of pH 6 β-gal staining in nevi was not due to an artifact of specimen processing.
Figure 1. Detection of β-gal activity in vitro and in vivo.
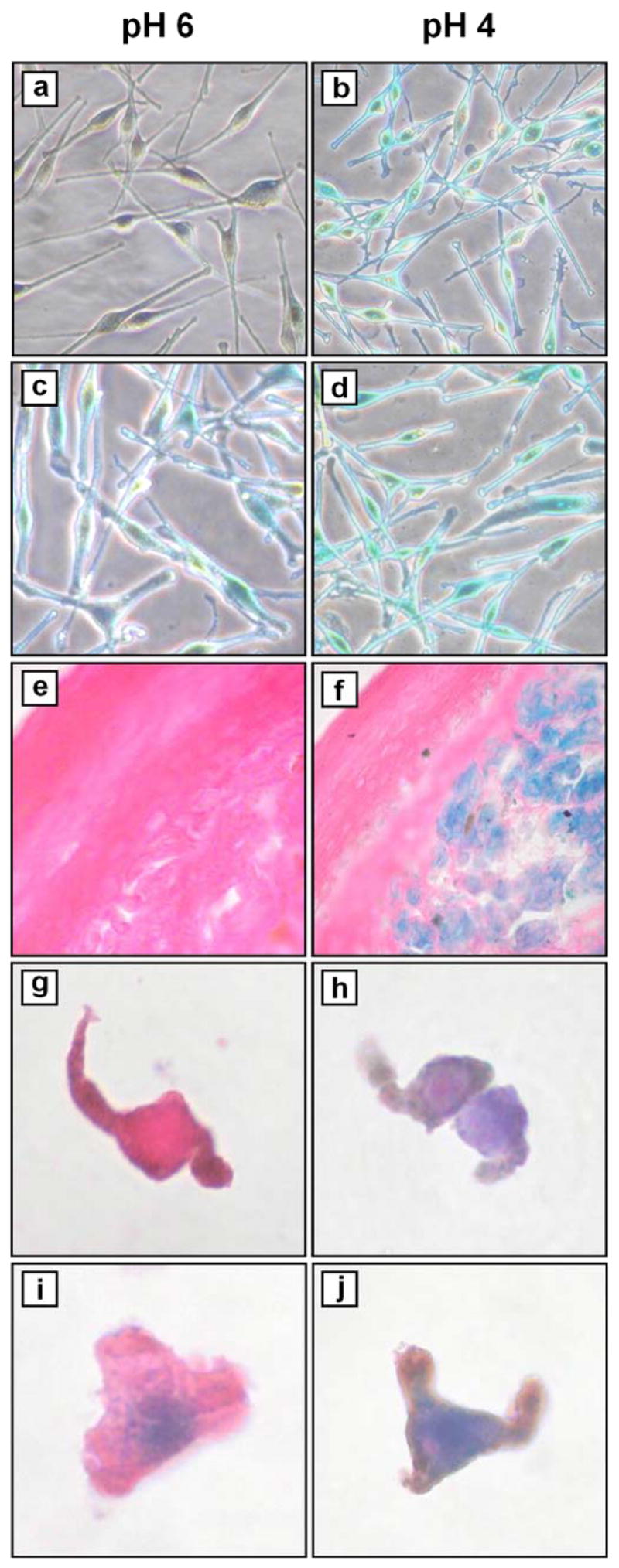
Melanocytes from (a, b) passage 2 (early) and (c, d) passage 12 (late) cultures were stained as described (Dimri et al., 1995). Briefly, cells were washed with PBS and fixed with 1% paraformaldehyde for 3 min at room temperature, then washed three times with PBS for 5 min each at room temperature. Staining was performed overnight in a non-CO2 enriched incubator at 37 °C using a solution (pH 4 or pH 6 as indicated) containing 40 mM sodium phosphate (dibasic), 40 mM citric acid, 150 mM NaCl, 2 mM MgCl2, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 1 mg/ml X-gal (5-bromo-4-chloro-3-indolyl-b-D-galactoside, Pierce Chemical Co., Rockford, IL). Cyanide salts and X-gal were added from freshly-made 100× stocks in PBS and dimethylformamide, respectively. Cells were then washed three times with PBS for 5 min each at room temperature, prior to microscopic examination and photography (×200). (e, f) nevus #5 (Table I). Frozen sections were fixed and stained as above (with additional staining at pH 5), followed by counterstaining with eosin, alcohol dehydration and mounting prior to microscopic examination and photography (×400). (g, h) Passage 3 melanocytes and (i, j) passage 15 melanocytes were trypsinized, washed with PBS, and resuspended in OCT prior to frozen sectioning. Sections were then fixed and stained in parallel with nevi, as above.
Table I.
Descriptions and β-gal staining patterns of nevi examined.
| # | Age | Sex | Site | Diagnosis | β-gal pH 4 | β-gal pH 5 | β-gal pH 6 |
|---|---|---|---|---|---|---|---|
| 1 | 48 | F | chest | CMN, congenital and dysplastic features | 1+ | nd | neg |
| 2 | 48 | M | arm | IDN, congenital features | 2+ | 1+ | neg |
| 3 | 29 | M | back | CMN, congenital and dysplastic features | 1+ | 1+ | neg |
| 4 | 27 | F | back | Dysplastic nevus, mild atypia | 1+ | neg | neg |
| 5 | 48 | F | groin | CMN | 3+ | 1+ | neg |
| 6 | 36 | M | back lower | CMN, congenital and dysplastic features | 2+ | neg | neg |
| 7 | 61 | F | leg | IDN, congenital features | 3+ | 2+ | neg |
| 8 | 23 | M | back | CMN, congenital and dysplastic features | 2+ | 1+ | neg |
| 9 | 33 | F | back | CMN, congenital features | 3+ | 2+ | neg |
| 10 | 23 | F | groin | CMN | 1+ | neg | neg |
| 11 | 44 | F | hand | CMN, congenital features | 3+ | 3+ | neg |
| 12 | 73 | F | chin | IDN | 1+ | 1+ | neg |
| 13 | 28 | M | back upper | CMN, congenital features | 2+ | 2+ | neg |
| 14 | 34 | F | arm | Blue nevus | focal/1+ | neg | neg |
| 15 | 30 | F | shoulder | Dysplastic nevus, moderate atypia | 1+ | neg | neg |
| 16 | 56 | F | back | IDN | 1+ | 1+ | neg |
| 17 | 57 | M | back | IDN | 2+ | 2+ | neg |
CMN, compound melanocytic nevus; IDN, intradermal melanocytic nevus.
Scoring: neg, negative staining; 1+, <25% cells positive; 2+, 25–75% cells positive; 3+, 75–100% cells positive; nd, not done.
Campisi and colleagues (Dimri et al., 1995) first proposed SA-β-gal as a marker for senescence based on selective staining (at pH 6) of late but not early passage epidermal cells, and the skin of older individuals (> 69 yrs). The authors noted, however, that early passage adult melanocytes growing in culture (>90%) expressed β-gal activity at pH 6 (Dimri et al., 1995). It is also curious that in aged skin, the reported pH 6 β-gal staining appears localized to a narrow band within the epidermis likely to contain mitotically active cells and was negative in the upper layers likely to contain terminally-differentiated cells (Dimri et al., 1995). Recent studies have in fact called into question the validity of pH 6 β-gal activity as a marker for senescence, and suggest that it more likely represents a non-specific marker of lysosomes. Pharmacologic alkalinization of the lysosomal compartment eliminates β-gal activity in both early and late passage human umbilical vein endothelial cells, and lysosomal content increased with replicative age in parallel with increasing β-gal activity (Kurz et al., 2000), suggesting that acquisition of pH 6 β-gal activity reflects expansion of the lysosomal compartment known to occur through serial rounds of cell division (Robbins et al., 1970; Brunk et al., 1973). Moreover, human fibroblasts either deficient in the single human β-gal-encoding gene GLB1 or treated with GLB-interfering RNA underwent replicative senescence (Lee et al., 2006). Thus pH 6 β-gal is neither necessary nor sufficient for the induction of senescence, and the intermediate level of β-gal staining we observed at pH 5 supports the notion that staining at pH 6, when present, detects an increase of the same enzyme activity seen optimally at pH 4.
Our demonstration of uniformly negative pH 6 β-gal staining in a panel of nevi stands in stark contrast with recent reports of positive staining in 23/23 congenital nevi from patients less than one year of age (Michaloglou et al., 2005) and 7/7 congenital nevi greater than 1 cm (donor age unspecified) (Gray-Schopfer et al., 2006). We only studied nevi from adults, and did not have access to congenital nevi from infants. While it is possible that only congenital nevi from the very young express this marker and it is gradually lost over time, it is somewhat counterintuitive that such nevi are senescent since they grow during this period and carry some risk of transformation. We also considered the possibility that previous data may have been generated under conditions of incompletely controlled pH resulting in detection of β-gal activity at lower pHs than intended. In our experiments, the pH of the staining solution was confirmed after overnight incubation at 37 °C in a non-CO2 enriched environment.
Although oncogene-induced senescence can be induced in cultured melanocytes (Michaloglou et al., 2005; Denoyelle et al., 2006), it seems premature to conclude that nevi are senescent due to oncogene activation. After all, nevomelanocytes in vivo are not synonymous with melanocytes in vitro. Given the fact that pH 6 β-gal staining simply detects the lysosomal expansion associated with cellular passages and is not required for senescence development in vitro, we suggest the need for more specific and biologically relevant markers of senescence in vivo. Only then can we better interrogate the attractive hypothesis that melanoma may arise in part from either an escape from or failure to develop a senescent phenotype in nevomelanocytes.
Acknowledgments
We thank Constance McManus for help with preparation of frozen tissue sections. This work was supported by NIH grants AR050102 (D.G.) and RR17525 (S.R.F.), and the Huntsman Cancer Foundation. M.A.C. is supported by the T32 training grant CA093247.
Abbreviations
- SA-β-gal
senescence-associated beta-galactosidase
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Bastian BC, LeBoit PE, Pinkel D. Mutations and copy number increase of HRAS in Spitz nevi with distinctive histopathological features. Am J Pathol. 2000;157:967–972. doi: 10.1016/S0002-9440(10)64609-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J, Curtin JA, Pinkel D, Bastian BC. Congenital melanocytic nevi frequently harbor NRAS mutations but no BRAF mutations. J Invest Dermatol. 2007;127:179–182. doi: 10.1038/sj.jid.5700490. [DOI] [PubMed] [Google Scholar]
- Bodnar AG, Ouellette M, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- Bowen AR, Hanks AN, et al. Apoptosis regulators and responses in human melanocytic and keratinocytic cells. J Invest Dermatol. 2003;120:48–55. doi: 10.1046/j.1523-1747.2003.12010.x. [DOI] [PubMed] [Google Scholar]
- Brunk U, Ericsson JL, Ponten J, Westermark B. Residual bodies and “aging” in cultured human glia cells. Effect of entrance into phase 3 and prolonged periods of confluence. Exp Cell Res. 1973;79:1–27. [PubMed] [Google Scholar]
- Denoyelle C, Abou-Rjaily G, et al. Anti-oncogenic role of the endoplasmic reticulum differentially activated by mutations in the MAPK pathway. Nat Cell Biol. 2006;8:1053–1063. doi: 10.1038/ncb1471. [DOI] [PubMed] [Google Scholar]
- Dimri GP, Lee X, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Schopfer VC, Cheong SC, et al. Cellular senescence in naevi and immortalisation in melanoma: a role for p16? Br J Cancer. 2006;95:496–505. doi: 10.1038/sj.bjc.6603283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz DJ, Decary S, Hong Y, Erusalimsky JD. Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J Cell Sci. 2000;113(Pt 20):3613–3622. doi: 10.1242/jcs.113.20.3613. [DOI] [PubMed] [Google Scholar]
- Lee BY, Han JA, et al. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell. 2006;5:187–195. doi: 10.1111/j.1474-9726.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- Michaloglou C, Vredeveld LC, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- Mooi WJ, Peeper DS. Oncogene-induced cell senescence--halting on the road to cancer. N Engl J Med. 2006;355:1037–1046. doi: 10.1056/NEJMra062285. [DOI] [PubMed] [Google Scholar]
- Morreau H, Galjart NJ, et al. Alternative splicing of beta-galactosidase mRNA generates the classic lysosomal enzyme and a beta-galactosidase-related protein. J Biol Chem. 1989;264:20655–20663. [PubMed] [Google Scholar]
- Pollock PM, Harper UL, et al. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- Robbins E, Levine EM, Eagle H. Morphologic changes accompanying senescence of cultured human diploid cells. J Exp Med. 1970;131:1211–1222. doi: 10.1084/jem.131.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]


