Abstract
The dysregulation of apoptosis occurs in many cutaneous disease states. Several apoptosis inhibitors have been shown elevated in neoplasms and in some inflammatory conditions, but their relation to proliferative and apoptotic states has not been defined. We examined the expression of the apoptosis inhibitor survivin in a panel of keratinocytic neoplasms and hyperproliferative skin lesions using both immunohistochemistry and a newly developed in situ hybridization technique. Proliferation and apoptotic indices were also assessed by immunohistochemical staining for proliferating cell nuclear antigen and TUNEL, respectively. We found the highest rate of proliferation in verrucae and psoriasis followed by actinic keratosis, squamous and basal cell carcinoma, lichen simplex chronicus, and seborrheic keratosis; all were significantly (P < 0.05) higher than normal skin. Apoptotic rate was increased in squamous (P = 0.05) and basal cell carcinoma (P = 0.03), but not significantly different from normal skin in the other lesions tested. Survivin expression was seen in most neoplasms and hyperproliferative lesions, but not normal skin. Survivin expression was often restricted to the upper third of the epidermis in psoriasis and lichen simplex chronicus, whereas all the other lesions stained diffusely. Survivin expression appears to be a consistent feature of keratinocytic neoplasms and hyperproliferative lesions and may contribute to the formation of epidermal hyperplasia seen in all of these disease states.
Keywords: Apoptosis, Keratinocyte, Proliferating cell nuclear antigen, TUNEL, Survivin
Maintenance of homeostasis in the skin requires a delicate balance among proliferation, differentiation, and apoptosis. Disruption of the regulatory mechanisms governing these normal processes likely occurs in neoplastic and hyperproliferative disease states. The expression of various inhibitors of apoptosis, primarily of the Bcl-2 family, has been examined in multiple epidermal tumors and inflammatory conditions. For example, the apoptosis inhibitors bcl-2 and bcl-XL are expressed in melanoma1–4 and nonmelanoma skin cancer.5–9 In basal and squamous cell carcinoma (SCC), bcl-2 expression was detectable in the malignant keratinocytes but not in adjacent nonmalignant cells.6 Weak bcl-2 expression was consistently found in keratinocytes comprising lesions of contact dermatitis, psoriasis, and seborrheic keratosis.6 Dummer et al10 examined bcl-2 expression in both benign and malignant cutaneous T-cell infiltrates and found significantly higher bcl-2 expression in the infiltrating T cells of mycosis fungoides, lymphomatoid papulosis, and T-cell rich cutaneous lymphoid hyperplasia, compared with normal epidermal keratinocytes. Finally, Chrysomali et al11 documented weak bcl-2 expression in the keratinocytes of oral lesions of erythema multiforme, comparable with that of control mucosa. By contrast, little is known regarding expression of the other major class of apoptosis inhibitors, Inhibitor of Apoptosis proteins, in epidermal neoplasms and hyperproliferative states.
Keratinocyte proliferation has been assessed in a number of benign and malignant skin conditions by immunohistochemical staining for proliferating cell nuclear antigen (PCNA) or Ki-67. Increased proliferation compared with normal skin was shown in psoriasis,12,13 ichthyosis,12 chronic dermatitis,12,13 and verruca vulgaris.13 Malignant diseases such as SCC,13 mycosis fungoides,10,13 and melanoma14,15 have an even higher proliferation index than these benign conditions. Distinct PCNA staining patterns may also be seen in different disease states. For example, proliferating cells tend to be diffusely spread through poorly differentiated SCC, but are localized to the periphery in well differentiated SCC and keratoacanthoma.16 The relation between proliferation rate and apoptosis in these skin diseases has not been well studied.
The apoptosis inhibitor survivin is a recently described member of the Inhibitor of Apoptosis family that is expressed in most human cancers17 and also known to be a regulator of mitosis.18 In malignant cells, survivin expression is upregulated during the G2M phase of the cell cycle and peaks during mitosis.18 It associates with the mitotic spindle apparatus and is thought to represent an important mitotic checkpoint by inhibiting apoptosis in dividing cells as they proceed through mitosis.18 We have previously reported its absence in normal skin,19,20 but high expression in melanoma14 and nonmelanoma19 skin cancers. Interestingly, survivin expression was also seen in in situ lesions and actinic keratosis,19 suggesting it may represent an early step in skin cancer development. Recently, we reported that transgenic expression of survivin in keratinocytes prevented papilloma regression and promoted conversion to SCC in a mouse chemical carcinogenesis model.21 Survivin expression in benign hyperproliferative states, however, has not been investigated. Here, we characterize survivin expression and its potential relation to proliferative and apoptotic states in a panel of keratinocytic neoplasms and hyperproliferative skin disorders.
MATERIALS AND METHODS
Specimens
The University of Utah dermatopathology database was searched for specimens with relevant diagnoses, and hematoxylin and eosin stained slides for all cases were reviewed by a dermatopathologist (S.R.F.) to confirm diagnosis. The first 20 specimens that could be obtained of each diagnosis were examined for adequate tissue remaining in the block, and those without sufficient tissue remaining were not studied. The following cases were assembled: normal skin (8), lichen simplex chronicus (LSC) (9), psoriasis (8), verruca vulgaris (11), seborrheic keratosis (11), actinic keratosis (6), invasive SCC (8), and basal cell carcinoma (BCC) (9). Fully developed lesions of psoriasis and LSC demonstrating acanthosis and hyperkeratosis, as well as typical neoplasms, were selected. None of the verrucae had the histologic pattern of a “wart-like SCC” or verrucous carcinoma. All seborrheic keratoses were of the acanthotic type. All BCC lesions were of the nodular type. Normal skin specimens were taken from the edge of large cyst excisions. Serial 5 μM sections were prepared from each paraffin block.
Immunohistochemistry
Sections were subjected to immunohistochemical studies using peroxidase-based techniques for PCNA, TUNEL, and survivin as described elsewhere.14 For PCNA staining, a 1:100 dilution of antibody (BD Biosciences, San Diego, Calif) was used. Approximately 100 cells were counted in 4 representative fields (including the basal and spinous layers) of each section. The proliferation index (PI) was calculated as the percentage of PCNA-positive cells averaged for each lesion type. The apoptotic index (AI) was defined as the number of TUNEL-positive cells per 200 × field averaged for each lesion type. TUNEL-positive cells in the granular layer and stratum corneum were not counted. Statistical significance for AI and PI, defined as P ≤ 0.05, was determined for each lesion type compared with normal skin using an unpaired 2-tailed t test (Prism, Graphpad Software, San Diego, Calif). For Survivin staining, an antibody14 concentration of 0.5–1 μg/ml was used.
In Situ Hybridization
In situ hybridization for survivin was performed in approximately half the specimens to confirm immunohistochemical results. A full-length human survivin antisense riboprobe was prepared by in vitro transcription from a linearized SP6-based plasmid using a biotin RNA-labeling kit (Roche Molecular Biochemicals, Indianapolis, Ind). After precipitation, the riboprobe was resuspended in diethylpyrocarbonate-treated deionized distilled water and stored at −20°C until needed. We did not employ a sense riboprobe as a control, given the possibility of reactivity with effector cell protease receptor 1, which is transcribed from the same locus but in the opposite direction of the survivin gene.22 Normal skin, which does not express survivin,19 served as a negative control.
After dewaxing in xylenes, rehydration by passing through a standard series of graded alcohols and rinsing in diethylpyrocarbonate-treated deionized distilled water, the sections were immersed in 4% paraformaldehyde for 20 minutes on ice, rinsed in TBS Wash Buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl), treated with 200 mM HCl for 10 minutes at room temperature, and then placed in an acetylation buffer (0.5% acetic anhydride, 100 mM Tris-HCl, pH 8.0) for 10 minutes at room temperature. The slides were rinsed 3 times in TBS Wash Buffer, then dehydrated through graded alcohols (70–95–100%), dipped briefly in CHCl3, and air-dried for 5 minutes. Riboprobe was diluted to a concentration of 500 ng/ml in a hybridization buffer (50% formamide, 2X SSC, 10% dextran sulfate, 0.01% sheared herring sperm DNA, 0.02% SDS), and 50 μL was applied to each section, which was coverslipped and then incubated at 95°C for 4 minutes. The slides were then placed in a humidified chamber at 55°C for 4 to 6 hours for hybridization. All reagents used prior to the hybridization step were RNase-free.
After hybridization, the slides were placed in 2X SSC overnight to remove the coverslips. They were then washed 3 times in 1X SSC containing 50% formamide at 55°C over 1 hour, twice in 1X SSC over 40 minutes, and then rinsed 3 times in TBS Wash Buffer. After applying 5% normal sheep serum in blocking buffer (0.03% Triton X-100, 150 mM NaCl, 100 mM Tris-HCl, pH 7.5) to each section for 30 minutes at room temperature, slides were incubated overnight at 4°C with alkaline phosphatase–conjugated mouse anti-biotin (Jackson ImmunoResearch, West Grove, Penn) diluted 1:250 in blocking buffer containing 10% normal sheep serum. After washing, BCIP/NBT substrate solution (Dako, Carpinteria, Calif) was applied to the sections for 2 hours and 40 minutes. The sections were then counterstained with Nuclear Fast Red (Vector Laboratories, Burlingame, Calif), dehydrated in a series of alcohols and xylenes, and finally mounted with TBS ShurMount (Ted Pella, Redding, Calif).
RESULTS
Keratinocyte Proliferation
Keratinocyte proliferation in the lesions was assessed by PCNA staining. The pattern of staining for all samples tended to be stronger in basal layers and diminished toward the granular layer (Fig. 1A). We calculated the PI based on the average percentage of PCNA-positive cells in each lesion type studied, and the results are summarized in Figure 2. The PI was highest in verrucae (72.1%) and psoriasis (45%), followed by SCC (28%), actinic keratosis (23%), and BCC (21.4%); seborrheic keratosis (13.9%) and LSC (12.3%) demonstrated a PI slightly higher than that seen in normal skin (6.4%) (Fig. 2). For all lesion types, the PI was statistically higher (P < 0.05) than that of normal skin.
FIGURE 1.
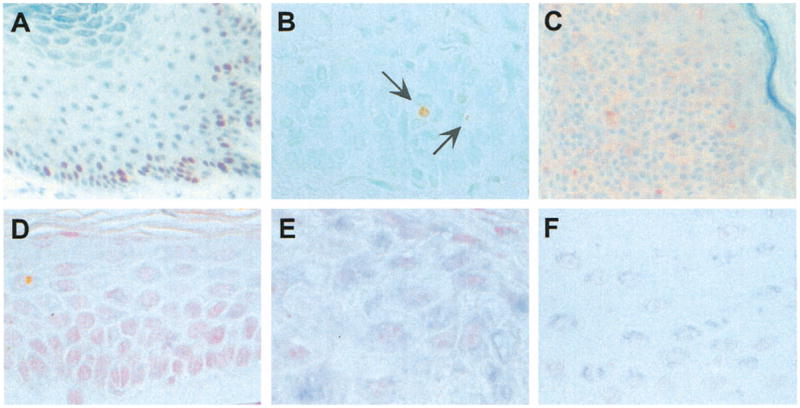
Representative proliferating cell nuclear antigen (PCNA), TUNEL, and survivin staining. (A) PCNA staining of verruca vulgaris, demonstrating increased nuclear (dark brown) reactivity at the base of the lesion. (B) TUNEL staining of basal cell carcinoma, revealing isolated positive (light brown) cells, indicated by arrows. (C) Survivin immunohistochemistry of seborrheic keratosis, showing diffuse cytoplasmic (pink) staining. (D) Survivin in situ hybridization of normal skin, demonstrating absence of expression. (E) Survivin in situ hybridization of squamous cell carcinoma, showing diffuse granular cytoplasmic (dark blue) staining. (F) Survivin in situ hybridization of psoriasis, revealing superficial and perinuclear (light blue) staining pattern.
FIGURE 2.
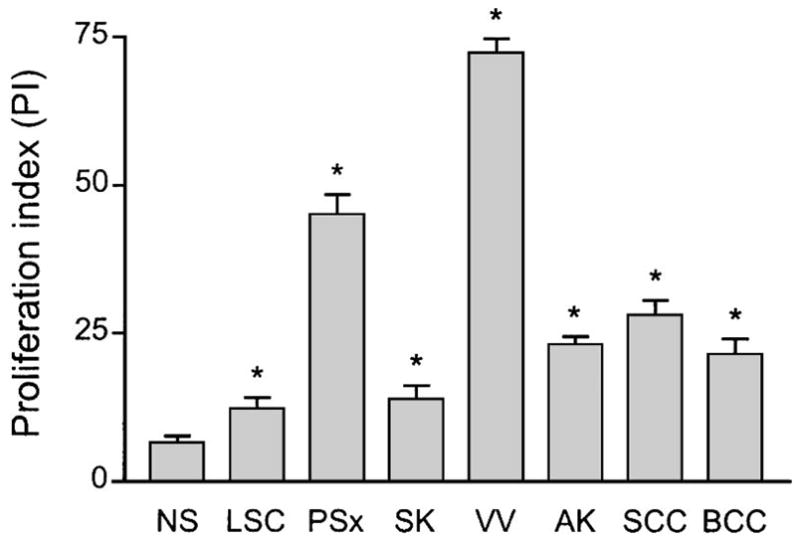
Proliferation index (PI) as determined by proliferating cell nuclear antigen (PCNA) immunohistochemistry. Bar graphs depict average percent PCNA-positive cells for normal skin (NS), lichen simplex chronicus (LSC), psoriasis (PSx), seborrheic keratosis (SK), verruca vulgaris (VV), actinic keratosis (AK), squamous cell carcinoma (SCC), and basal cell carcinoma (BCC). Error bars indicate standard error of mean, and asterisks connote statistically significant (P ≤ 0.05) difference for given lesion type compared with NS.
Keratinocyte Apoptosis
Apoptotic cells were visualized by TUNEL staining, which identified rare positive cells (Fig. 1B) in most lesions. The AI was derived from the average number of TUNEL-positive cells in each lesion type, and the results are summarized in Figure 3. The AI for SCC (1.38) and BCC (1.25) was significantly higher (P = 0.05, 0.03) than that for normal skin (0.33). Relatively lower AI values were obtained for seborrheic keratosis, verruca vulgaris, and LSC, none of which were significantly different (P > 0.05) from that of normal skin(Fig. 3). Apoptotic cells were not detected in any of the psoriasis lesions (Fig. 3).
FIGURE 3.
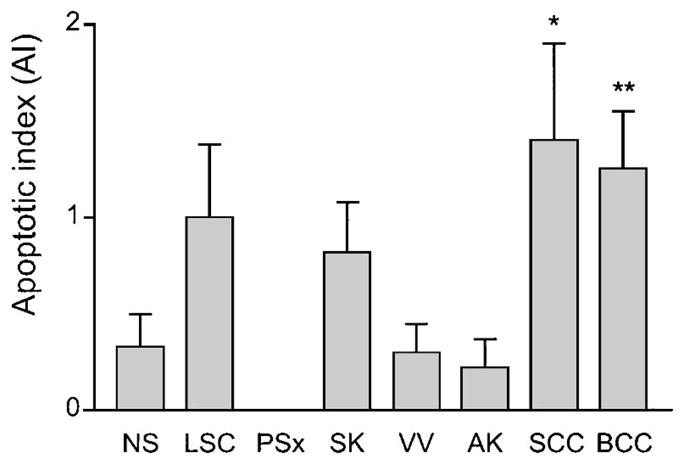
Apoptotic index (AI) as determined by TUNEL staining. Bar graphs indicate number of TUNEL-positive cells per 200×field for normal skin (NS), lichen simplex chronicus (LSC), psoriasis (PSx), seborrheic keratosis (SK), verruca vulgaris (VV), actinic keratosis (AK), squamous cell carcinoma (SCC), and basal cell carcinoma (BCC). Error bars indicate standard error of mean, and asterisks connote statistically significant (*P = 0.05, **P = 0.03) difference compared with NS.
Survivin Expression in Lesions
The presence of survivin was determined by immunohistochemistry (Fig. 1C) and by in situ hybridization (Fig. 1D–F), and the results are summarized in Table 1. Survivin was not detected in normal skin (Fig. 1D), but was expressed in most cases of actinic keratosis (83%), SCC (100%), verruca vulgaris(91%), seborrheic keratosis (100%), and psoriasis (88%). Survivin expression was seen in lower fractions of BCC (44%) and LSC (44%) (Table 1). Benign and malignant neoplasms tended to show diffuse cytoplasmic staining (Fig. 1C,E), while staining in LSC and psoriasis (Fig. 1F) tended to be weaker and, in some cases, localized to the superficial (upper one third of the epidermis) component of the lesions (Table 1). In some cases of psoriasis, the intracellular pattern of survivin staining appeared to be perinuclear (Fig. 1F).
TABLE 1.
Survivin Expression in Keratinocytic Hyperplasias and Neoplasms
| Lesion | Survivin Positive* (%) | Survivin Staining Pattern† |
|---|---|---|
| Normal skin | 0/8 (%) | — (2) |
| Lichen simplex chronicus | 4/9 (44%) | superficial (2), diffuse (2) |
| Psoriasis | 7/8 (88%) | superficial (6), diffuse (1) |
| Verrucae vulgaris | 10/11 (91%) | diffuse (3) |
| Seborreic keratosis | 11/11 (100%) | diffuse (5) |
| Actinic keratosis | 5/6 (83%) | diffuse (3), basilar (2) |
| Squamous cell carcinoma | 8/8 (100%) | diffuse (3) |
| Basal cell carcinoma | 4/9 (44%) | basilar (2), diffuse (1) |
Determined by immunohistochemistry and in situ hybridization.
Determined by in situ hybridization. In parentheses are numbers of cases exhibiting given staining pattern.
DISCUSSION
In this study, we correlated expression of the apoptosis inhibitor survivin with proliferative and apoptotic rates in a panel of keratinocytic neoplasms and hyperproliferative conditions. All the skin lesions examined demonstrated an increased proliferative rate compared with normal skin, while the differences in apoptotic rate were relatively small. Survivin was expressed in most of the lesions, but despite its association with mitotic progression18 and antiapoptotic activity,20,22 its expression did not appear to correlate with either proliferative or apoptotic status. None of the parameters examined proved useful in distinguishing benign from malignant keratinocytic lesions.
All the skin lesions exhibited significantly increased proliferation by PCNA staining compared with normal skin. In most cases we observed, as in normal skin, a gradient of increased proliferation toward the basal layer. We found the highest PI in verrucae, in contrast to a previous study by Kawahira et al13 that reported higher rates in SCC compared with verrucae and psoriasis. Interestingly, the lowest proliferation rates were found in LSC and seborrheic keratosis, although considerable epidermal hyperplasia is a characteristic feature of these lesions. Thus, while increased keratinocyte proliferation is a consistent feature of all these lesions and is probably requisite for their growth and maintenance, it is likely that other factors are also involved in the development of a hyperplastic epidermis.
Apoptosis represents a counterbalance to proliferation, and decreased apoptosis is generally thought to be associated with epidermal hyperproliferation. This notion is substantiated by mouse models with transgenic epidermal expression of apoptotic inhibitors bcl-223 and the p53 inhibitor MDM224 that displayed increased epidermal thickness, although other models—including mice transgenic for bcl-XL 25 and survivin20—had a normal-appearing epidermis. In all these mouse strains, however, transgenic expression of the apoptotic inhibitor did increase apoptotic resistance in the epidermis, as demonstrated by decreased generation of apoptotic keratinocytes (sunburn cells) in response to ultraviolet light or DNA-damaging agent. Lack of apoptotic cells in seborrheic keratosis and verrucae has been previously reported by Mori et al,26 and Laporte et al27 demonstrated a lower AI in psoriasis compared with normal skin. We found that the lesions with highest proliferative capacity, verrucae and psoriasis, had a lower AI than normal skin, although in our study, generally only 1 or 2 apoptotic cells per field were noted, and we were not able to demonstrate statistically significant differences with normal skin for most lesion types. We found that only BCC and SCC had an AI significantly different than normal skin, and in both cases, it was elevated rather than decreased. Direct detection of apoptotic cells in the epidermis by TUNEL staining, as we performed here, is inherently difficult because apoptotic cells are cleared rapidly from tissues.28 Thus, it seems that the apoptotic state of the epidermis may be best assessed by measuring responses to apoptotic stimuli in vivo, as has been done for mouse skin in studies cited above, rather than by trying to detect apoptotic cells in untreated fixed tissue. Although far more cumbersome and beyond the scope of this study, it would be interesting to measure ultraviolet-induced apoptosis in these various lesion types and normal human skin.
Survivin was expressed in most cases of each lesion type, with the exception of LSC and BCC in which only half of cases were positive. In our previous study,19 81% of BCC were positive, but this difference is likely due to the small number of cases examined in both studies and perhaps sampling error. Survivin expression was often restricted to the upper third of the epidermis in psoriasis and LSC, whereas all the other lesions stained diffusely. Past studies of survivin expression in paraffin-embedded tissues have employed immunohistochemistry and usually involved pressure cooking for antigen retrieval.14,19,29 We have found that while this procedure is excellent for solid tumors, it is quite harsh on skin tissues and often prone to nonspecific staining of the epidermis. This is the most likely explanation for one report29 describing survivin expression in the epidermis and adnexae of normal skin. Here, we used in situ hybridization with a full-length riboprobe that under high stringency conditions yielded reproducible specific staining. We confirmed our prior results14,19,20 that survivin is not expressed in normal skin. Previous studies have highlighted the expression of survivin in cancer and its absence in nonmalignant tissues.17,30 We have shown previously that survivin is expressed in skin cancer precursors including melanocytic nevi,14 actinic keratosis,19 and papillomas.21 Our demonstration here of survivin expression in verrucae is consistent with these observations since these lesions are caused by human papillomas virus infection that in some cases may develop into SCC. The E6 protein encoded by human papilloma virus may lead to survivin expression in these lesions through down-regulation of p53,31 which is a negative regulator of the survivin gene.32,33
Our demonstration of survivin expression in psoriasis, seborrheic keratosis, and LSC lesions—clearly benign processes without malignant potential—challenges the paradigm17 of survivin expression limited to premalignant and malignant tissues. The broad expression of survivin in these lesions and other skin tumors suggests that changes in apoptosis regulation likely play a role in all keratinocytic hyperproliferative states.
Acknowledgments
This work was funded in part by NIH grants K23RR17525 (S.R.F.), KO8AR48618 (D.G.), R03AR048953 (D.G.), the Huntsman Cancer Foundation (D.G.), and a Fellowship-to-Faculty Transition Award from the University of Utah funded in part by the Howard Hughes Medical Institute (D.G.).
References
- 1.Cerroni L, Soyer HP, Kerl H. bcl-2 protein expression in cutaneous malignant melanoma and benign melanocytic nevi. Am J Dermatopathol. 1995;17:7–11. doi: 10.1097/00000372-199502000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Collins KA, White WL. Intercellular adhesion molecule 1 (ICAM-1) and bcl-2 are differentially expressed in early evolving malignant melanoma. Am J Dermatopathol. 1995;17:429–438. doi: 10.1097/00000372-199510000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Selzer E, Schlagbauer-Wadl H, Okamoto I, et al. Expression of Bcl-2 family members in human melanocytes, in melanoma metastases and in melanoma cell lines. Melanoma Res. 1998;8:197–203. doi: 10.1097/00008390-199806000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Leiter U, Schmid RM, Kaskel P, et al. Antiapoptotic bcl-2 and bcl-xL in advanced malignant melanoma. Arch Dermatol Res. 2000;292:225–232. doi: 10.1007/s004030050479. [DOI] [PubMed] [Google Scholar]
- 5.Smoller BR, Van de Rijn M, Lebrun D, et al. bcl-2 expression reliably distinguishes trichoepitheliomas from basal cell carcinomas. Br J Dermatol. 1994;131:28–31. doi: 10.1111/j.1365-2133.1994.tb08453.x. [DOI] [PubMed] [Google Scholar]
- 6.Nakagawa K, Yamamura K, Maeda S, et al. bcl-2 expression in epidermal keratinocytic diseases. Cancer. 1994;74:1720–1724. doi: 10.1002/1097-0142(19940915)74:6<1720::aid-cncr2820740613>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 7.Morales-Ducret CR, van de Rijn M, LeBrun DP, et al. bcl-2 expression in primary malignancies of the skin. Arch Dermatol. 1995;131:909–912. [PubMed] [Google Scholar]
- 8.Delehedde M, Cho SH, Sarkiss M, et al. Altered expression of bcl-2 family member proteins in nonmelanoma skin cancer. Cancer. 1999;85:1514–1522. [PubMed] [Google Scholar]
- 9.Wrone-Smith T, Bergstrom J, Quevedo ME, et al. Differential expression of cell survival and cell cycle regulatory proteins in cutaneous squamo-proliferative lesions. J Dermatol Sci. 1999;19:53–67. doi: 10.1016/s0923-1811(98)00052-8. [DOI] [PubMed] [Google Scholar]
- 10.Dummer R, Michie SA, Kell D, et al. Expression of bcl-2 protein and Ki-67 nuclear proliferation antigen in benign and malignant cutaneous T-cell infiltrates. J Cutan Pathol. 1995;22:11–17. doi: 10.1111/j.1600-0560.1995.tb00733.x. [DOI] [PubMed] [Google Scholar]
- 11.Chrysomali E, Lozada-Nur F, Dekker NP, et al. Apoptosis in oral erythema multiforme. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:272–280. doi: 10.1016/s1079-2104(97)90016-0. [DOI] [PubMed] [Google Scholar]
- 12.Kanitakis J, Hoyo E, Chouvet B, et al. Keratinocyte proliferation in epidermal keratinocyte disorders evaluated through PCNA/cyclin immuno-labelling and AgNOR counting. Acta Derm Venereol. 1993;73:370–375. doi: 10.2340/0001555573370375. [DOI] [PubMed] [Google Scholar]
- 13.Kawahira K. Immunohistochemical staining of proliferating cell nuclear antigen (PCNA) in malignant and nonmalignant skin diseases. Arch Dermatol Res. 1999;291:413–418. doi: 10.1007/s004030050431. [DOI] [PubMed] [Google Scholar]
- 14.Grossman D, McNiff JM, Li F, et al. Expression and targeting of the apoptosis inhibitor, survivin, in human melanoma. J Invest Dermatol. 1999;113:1076–1081. doi: 10.1046/j.1523-1747.1999.00776.x. [DOI] [PubMed] [Google Scholar]
- 15.Florell SR, Boucher KM, Holden JA, et al. Failure to detect differences in proliferation status of nevi from CDKN2A mutation carriers and non-carriers. J Invest Dermatol. 2002;118:386–387. doi: 10.1046/j.1523-1747.2002.01659.x. [DOI] [PubMed] [Google Scholar]
- 16.Phillips P, Helm KF. Proliferating cell nuclear antigen distribution in keratoacanthoma and squamous cell carcinoma. J Cutan Pathol. 1993;20:424–428. doi: 10.1111/j.1600-0560.1993.tb00665.x. [DOI] [PubMed] [Google Scholar]
- 17.Velculescu VE, Madden SL, Zhang L, et al. Analysis of human transcriptomes. Nat Genet. 1999;23:387–388. doi: 10.1038/70487. [DOI] [PubMed] [Google Scholar]
- 18.Li F, Ambrosini G, Chu EY, et al. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature. 1998;396:580–584. doi: 10.1038/25141. [DOI] [PubMed] [Google Scholar]
- 19.Grossman D, McNiff JM, Li F, et al. Expression of the apoptosis inhibitor, survivin, in nonmelanoma skin cancer and gene targeting in a keratinocyte cell line. Lab Invest. 1999;79:1121–1126. [PubMed] [Google Scholar]
- 20.Grossman D, Kim PJ, Blanc-Brude OP, et al. Transgenic expression of survivin in keratinocytes counteracts UVB- induced apoptosis and cooperates with loss of p53. J Clin Invest. 2001;108:991–999. doi: 10.1172/JCI13345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allen SM, Florell SR, Hanks AN, et al. Survivin expression in mouse skin prevents papilloma regression and promotes chemical-induced tumor progression. Cancer Res. 2003;63:567–572. [PubMed] [Google Scholar]
- 22.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Villanueva J, Greenhalgh D, Wang XJ, et al. Human keratin-1.bcl-2 transgenic mice aberrantly express keratin 6, exhibit reduced sensitivity to keratinocyte cell death induction, and are susceptible to skin tumor formation. Oncogene. 1998;16:853–863. doi: 10.1038/sj.onc.1201610. [DOI] [PubMed] [Google Scholar]
- 24.Ganguli G, Abecassis J, Wasylyk B. MDM2 induces hyperplasia and premalignant lesions when expressed in the basal layer of the epidermis. EMBO J. 2000;19:5135–5147. doi: 10.1093/emboj/19.19.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pena JC, Fuchs E, Thompson CB. Bcl-x expression influences keratinocyte cell survival but not terminal differentiation. Cell Growth Differ. 1997;8:619–629. [PubMed] [Google Scholar]
- 26.Mori O, Hachisuka H, Kiyokawa C, et al. Apoptosis identified by DNA fragmentation in epidermal neoplasms. J Dermatol. 1995;22:917–920. doi: 10.1111/j.1346-8138.1995.tb03945.x. [DOI] [PubMed] [Google Scholar]
- 27.Laporte M, Galand P, Fokan D, et al. Apoptosis in established and healing psoriasis. Dermatology. 2000;200:314–316. doi: 10.1159/000018394. [DOI] [PubMed] [Google Scholar]
- 28.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiodino C, Cesinaro AM, Ottani D, et al. Communication: expression of the novel inhibitor of apoptosis survivin in normal and neoplastic skin. J Invest Dermatol. 1999;113:415–418. doi: 10.1046/j.1523-1747.1999.00711.x. [DOI] [PubMed] [Google Scholar]
- 30.Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- 31.Thomas M, Pim D, Banks L. The role of the E6-p53 interaction in the molecular pathogenesis of HPV. Oncogene. 1999;18:7690–7700. doi: 10.1038/sj.onc.1202953. [DOI] [PubMed] [Google Scholar]
- 32.Mirza A, McGuirk M, Hockenberry TN, et al. Human survivin is negatively regulated by wild-type p53 and participates in p53-dependent apoptotic pathway. Oncogene. 2002;21:2613–2622. doi: 10.1038/sj.onc.1205353. [DOI] [PubMed] [Google Scholar]
- 33.Hoffman WH, Biade S, Zilfou JT, et al. Transcriptional repression of the anti- apoptotic survivin gene by wild type p53. J Biol Chem. 2002;277:3247–3257. doi: 10.1074/jbc.M106643200. [DOI] [PubMed] [Google Scholar]


