Abstract
Aims/hypothesis
Our aim was to examine the association between change in physical activity energy expenditure (PAEE), total body movement (counts per day) and aerobic fitness (maximum oxygen consumption [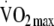 ]) over 1 year and metabolic risk among individuals with a family history of diabetes.
]) over 1 year and metabolic risk among individuals with a family history of diabetes.
Methods
Three hundred and sixty-five offspring of people with type 2 diabetes underwent measurement of energy expenditure (PAEE measured using the flex heart rate method), total body movement (daily activity counts from accelerometry data), 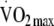 predicted from a submaximal graded treadmill exercise test and anthropometric and metabolic status at baseline and 1 year (n = 321) in the ProActive trial. Clustered metabolic risk was calculated by summing standardised values for waist circumference, fasting triacylglycerol, insulin and glucose, blood pressure and the inverse of HDL-cholesterol. Linear regression was used to quantify the association between changes in PAEE, total body movement and fitness and clustered metabolic risk at follow-up.
predicted from a submaximal graded treadmill exercise test and anthropometric and metabolic status at baseline and 1 year (n = 321) in the ProActive trial. Clustered metabolic risk was calculated by summing standardised values for waist circumference, fasting triacylglycerol, insulin and glucose, blood pressure and the inverse of HDL-cholesterol. Linear regression was used to quantify the association between changes in PAEE, total body movement and fitness and clustered metabolic risk at follow-up.
Results
Participants increased their activity by 0.01 units PAEE kJ kg−1 day−1 over 1 year. Total body movement increased by an average of 9,848 counts per day. Change in total body movement (β = −0.066, p = 0.004) and fitness (β = −0.056, p = 0.003) was associated with clustered metabolic risk at follow-up, independently of age, sex, smoking status, socioeconomic status and baseline metabolic score.
Conclusions/interpretation
Small increases in activity and fitness were associated with a reduction in clustered metabolic risk in this cohort of carefully characterised at-risk individuals. Further research to quantify the reduction in risk of type 2 diabetes associated with feasible changes in these variables should inform preventive interventions.
Clinical trial registration number: ISRCTN61323766.
Keywords: Cardio-respiratory fitness, Fitness, Metabolic risk, Metabolic syndrome, Physical activity, Syndrome X
Introduction
The metabolic syndrome has been defined as a cluster of closely related cardiovascular risk factors including visceral obesity, insulin resistance, hyperglycaemia, hypertension, hypertriacylglycerolaemia and low HDL-cholesterol [1]. The combination of these risk factors has been shown to predict type 2 diabetes, cardiovascular disease and all-cause mortality [2], with physical inactivity identified as a major underlying risk factor. Given the rising prevalence of obesity and type 2 diabetes, the metabolic syndrome represents a potentially large public health burden [3, 4]. Previous research has shown that low levels of physical activity are associated with the metabolic syndrome [5, 6], and that objectively measured physical activity energy expenditure (PAEE) predicts progression towards the metabolic syndrome independently of obesity [7], with some evidence of interaction by cardiorespiratory fitness [8, 9]. Additionally, lifestyle interventions targeting physical activity as a modifiable risk factor have shown positive effects upon various metabolic components [10]. However, physical activity remains poorly measured in most epidemiological studies, with many relying on self-reporting [11]. Furthermore, it is unclear whether metabolic risk is most effectively reduced by increases in overall energy expenditure (EE), fitness or total body movement. A better understanding of these relationships would inform the development and targeting of individual and population-based interventions to reduce risk of diabetes and related metabolic disorders.
The ProActive (UK) trial is an explanatory trial of a theory-based intervention to promote physical activity among individuals reporting low levels of activity and who are at increased risk of the consequences of a sedentary lifestyle due to their family history of type 2 diabetes. The main trial results are presented elsewhere [12]. We recently observed a cross-sectional association between total body movement and clustered metabolic risk in this cohort [13] and are now extending these observations to examine the association between change in objectively measured PAEE (measured by individually calibrated heart rate [HR] monitoring), physical activity (total body movement measured by accelerometry) and aerobic fitness and clustered metabolic risk in the ProActive trial cohort over a period of 1 year.
Methods
The ProActive trial Full details of the study have been reported elsewhere [14]. In brief, ProActive aimed to evaluate the efficacy of a theoretical, evidence- and family-based intervention programme to increase physical activity among individuals defined as high-risk through having a parental history of type 2 diabetes. Potential participants were identified via diabetes registers and medical records of family history in 20 general practices in the East Anglia region of the UK. Three hundred and sixty-five individuals aged 30–50 years and reporting low levels of activity were randomly assigned to one of three interventions: brief written advice (control group), or a behavioural-change programme at two levels of intensity delivered either by telephone (distance) or face-to-face in the family home. The theory-based intervention programme [15] was delivered by trained facilitators and aimed to support increases in physical activity through the introduction and facilitation of a range of self-regulatory skills. Main trial results indicated no significant difference in the 1 year change in objectively measured daytime physical activity expressed as a ratio to resting EE between the three trial arms [12]. Consequently, the three trial arms were pooled and a cohort analysis conducted. Complete data on PAEE, aerobic fitness, anthropometry and biochemistry were available in 365 participants at baseline and 321 participants at follow-up, in addition to socio-demographic information. Total body movement measured by accelerometry was also assessed in a subsample of participants (n = 192) at baseline and follow-up and the data were included in these analyses. The measure of socioeconomic status (SES) was based on age at finishing full-time education (above or below 16 years). Ethics approval was obtained from the Eastern England Multi-centre Research Committee (MREC) and all participants gave written informed consent.
Anthropometric and metabolic tests Participants attended the study centre after an overnight fast and a sample of venous blood was taken. Fasting plasma glucose, serum insulin levels and lipid profiles (cholesterol, triacylglycerol, HDL- and LDL-cholesterol) were measured using the hexokinase method and standard enzymatic methods, as described previously [14]. Weight was measured on standard calibrated scales and height was measured using a rigid stadiometer. BMI was calculated as weight (kg) divided by height (m) squared. Waist circumference (cm) was measured over light indoor clothing as the mid-point between the lower costal margin and the level of the anterior superior iliac crests. Body fat percentage was measured by bio-electrical impedance (Bodystat, Isle of Man, UK), and systolic and diastolic blood pressures were measured using an automated Accutorr sphygmomanometer (Accutorr, Cambridge, UK).
Assessment of aerobic fitness, PAEE and total body movement Aerobic fitness (maximum oxygen consumption [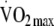 ]) was predicted as oxygen uptake at maximal HR (220 minus age [years]) by extrapolation of the regression line established during the individual calibration for the relationship between oxygen consumption and HR during a submaximal graded treadmill exercise test. O2 uptake and CO2 production was measured continuously by indirect calorimetry throughout the test (Vista XT metabolic system; Vacumed, Ventura, CA, USA).Resting EE (REE) was calculated by the Weir formula [16] using
]) was predicted as oxygen uptake at maximal HR (220 minus age [years]) by extrapolation of the regression line established during the individual calibration for the relationship between oxygen consumption and HR during a submaximal graded treadmill exercise test. O2 uptake and CO2 production was measured continuously by indirect calorimetry throughout the test (Vista XT metabolic system; Vacumed, Ventura, CA, USA).Resting EE (REE) was calculated by the Weir formula [16] using 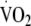 and
and 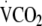 measurements obtained in the fasting state, after approximately 10 min of supine rest using the same indirect calorimetry system as previously described. PAEE was measured using the flex HR method [8]. Flex HR was calculated as the mean of the highest resting HR and the lowest HR while exercising. This point was used in the analysis of free-living minute-by-minute HR data to discriminate between rest and exercise. Below the flex HR point, EE was assumed to be equivalent to REE. EE above the flex point was predicted from the individual HR–EE regression line. Participants wore HR monitors (Polar Electro, Kemple, Finland) continuously during the waking hours over the following 4 days. PAEE was calculated by subtracting REE from the estimated average daily EE, and thereafter averaged over the 4 day period. PAEE is body size dependent, e.g. it requires more energy to move a heavier body. Similarly, aerobic fitness is highly correlated with body size or mass, e.g. larger individuals have a higher absolute aerobic capacity. Therefore, body size is a confounding factor that needs to be normalised for when analysing associations between EE variables and metabolic outcomes [17]. As such, both baseline and follow-up measures of aerobic fitness and PAEE were expressed relative to fat-free mass (FFM; kJ kg−1 FFM min−1) to adjust for between-individual differences in body size [17, 18].Free-living total body movement was assessed with an MTI Actigraph (formerly known as the CSA activity monitor) model WAM7164 (Manufacturing Technology, Fort Walton Beach, FL, USA). The accelerometer was worn over the same 4 day period as the HR monitor. Participants who did not manage to record at least 500 min/day of activity for at least 3 days were excluded from analyses. A customised program was used for data reduction and further analyses (MAHUffe; http://www.mrc-epid.cam.ac.uk). The outcome variable derived from accelerometry presented in this study is total body movement (counts per day), adjusted for monitored time, which is an indicator of the overall level of physical activity.
measurements obtained in the fasting state, after approximately 10 min of supine rest using the same indirect calorimetry system as previously described. PAEE was measured using the flex HR method [8]. Flex HR was calculated as the mean of the highest resting HR and the lowest HR while exercising. This point was used in the analysis of free-living minute-by-minute HR data to discriminate between rest and exercise. Below the flex HR point, EE was assumed to be equivalent to REE. EE above the flex point was predicted from the individual HR–EE regression line. Participants wore HR monitors (Polar Electro, Kemple, Finland) continuously during the waking hours over the following 4 days. PAEE was calculated by subtracting REE from the estimated average daily EE, and thereafter averaged over the 4 day period. PAEE is body size dependent, e.g. it requires more energy to move a heavier body. Similarly, aerobic fitness is highly correlated with body size or mass, e.g. larger individuals have a higher absolute aerobic capacity. Therefore, body size is a confounding factor that needs to be normalised for when analysing associations between EE variables and metabolic outcomes [17]. As such, both baseline and follow-up measures of aerobic fitness and PAEE were expressed relative to fat-free mass (FFM; kJ kg−1 FFM min−1) to adjust for between-individual differences in body size [17, 18].Free-living total body movement was assessed with an MTI Actigraph (formerly known as the CSA activity monitor) model WAM7164 (Manufacturing Technology, Fort Walton Beach, FL, USA). The accelerometer was worn over the same 4 day period as the HR monitor. Participants who did not manage to record at least 500 min/day of activity for at least 3 days were excluded from analyses. A customised program was used for data reduction and further analyses (MAHUffe; http://www.mrc-epid.cam.ac.uk). The outcome variable derived from accelerometry presented in this study is total body movement (counts per day), adjusted for monitored time, which is an indicator of the overall level of physical activity.
Calculation of the metabolic syndrome z score A summary score of clustered metabolic risk based on WHO criteria [1] was calculated by summing standardised values for waist circumference, triacylglycerol, fasting insulin and glucose, systolic blood pressure and the inverse of HDL-cholesterol [7–9]. Variables were standardised by subtracting the sample mean from the individual mean and dividing by the SD. Baseline and follow-up z scores were computed with the same transformation, e.g. using the mean and SD of baseline values. This continuously distributed metabolic risk variable (zMS) was also calculated without the adiposity component (i.e. waist circumference, zMS-ob). This clustered metabolic risk score increases statistical power as variables were not dichotomised, and includes a continuous measure of glycaemia (unlike some other measures of vascular risk [19]). Additionally, the International Diabetes Federation criteria were used to define metabolic syndrome as a dichotomous variable [1]. This score predicts hard clinical endpoints [20].
Statistical analyses Descriptive summary statistics were calculated separately for men and women using means and SDs at baseline and follow-up. t tests were used to examine whether there were any differences in baseline characteristics between those with and without follow-up data, as well as those with missing accelerometer data. Fasting insulin and triacylglycerol values were log transformed (ln) due to their non-normal distribution. In order to characterise change in physical activity and fitness in the ProActive cohort, we described the association between exposure variables using correlation coefficients. We then examined the proportion of metabolic syndrome and the mean clustered metabolic risk score among participants who increased and decreased their PAEE, fitness and total body movement over 1 year. We used linear regression to model change in PAEE and fitness (both per unit FFM), and total body movement ([total counts per day]/1,000, adjusted for monitored time), against individual subcomponents of the clustered metabolic risk score at follow-up (waist circumference, blood pressure, fasting triacylglycerol, insulin and glucose, and the inverse of HDL-cholesterol). These models were adjusted for age, sex, waist circumference (except when waist circumference was modelled as the outcome), smoking status, SES (assessed by self-reporting) and baseline phenotype, and were presented as standardised β-coefficients. We then tested whether change in PAEE, fitness and total body movement was associated with the clustered metabolic risk score at follow-up. The first model (obesity dependent) included all metabolic subcomponents (zMS) and was adjusted for age, sex, smoking status, SES and baseline zMS, while the second model excluded waist circumference from the zMS (obesity independent zMS-ob) and was adjusted for age, sex, smoking status, SES, baseline zMS-ob and waist circumference. Finally, we examined whether there was evidence of interaction between change in PAEE, total body movement and aerobic fitness and clustered metabolic risk, as well as interaction by age and sex. All data were analysed in continuous form, although some data were dichotomised for illustrative purposes. All analyses were completed using Stata Version 8.0. (STATA, College Station, TX, USA).
Results
Table 1 shows the anthropometric and metabolic characteristics of participants with complete data for baseline and follow-up (n = 321) stratified by sex. More women met the inclusion criteria and agreed to take part in the study than men. Participants with missing data at follow-up were slightly shorter than those with complete data (difference = 3.1 cm, p < 0.05) but not significantly different for all other baseline variables. There were also no significant differences in baseline characteristics between participants with and without accelerometer data (data not shown). In general, men were significantly taller and heavier than women but had a lower percentage body fat at baseline. In terms of the six metabolic syndrome subcomponents, waist circumference, systolic blood pressure, triacylglycerol, fasting glucose and insulin were all significantly higher in men, while HDL-cholesterol was significantly higher in women. Statistically significant differences were also observed for PAEE (kJ kg−1 FFM min−1) and 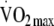 (ml kg−1 FFM min−1), with men achieving higher levels of physical activity and fitness even after adjustment for FFM. There were no significant differences in total body movement between men and women at baseline.
(ml kg−1 FFM min−1), with men achieving higher levels of physical activity and fitness even after adjustment for FFM. There were no significant differences in total body movement between men and women at baseline.
Table 1.
Anthropometric and metabolic characteristics of ProActive participants with complete baseline and follow-up data (n = 321), stratified by sex
| Men | Women | |||
|---|---|---|---|---|
| Baseline (n = 129) | Follow-up (n = 129) | Baseline (n = 192) | Follow-up (n = 192) | |
| Age (years) | 40.2 (5.8) | N/A | 40.8 (6.1) | N/A |
| Height (cm) | 177.8 (6.8) | 177.8 (7.0) | 163.4 (6.1)a | 163.2 (5.9)c |
| Weight (kg) | 89.3 (15.5) | 89.5 (15.6) | 73.7 (14.6)a | 74.0 (15.2) |
| BMI (kg/m2) | 28.2 (4.3) | 28.3 (4.4) | 27.6 (5.2) | 27.8 (5.4)d |
| Body fat (%) | 25.6 (5.3) | 25.8 (5.2) | 34.6 (6.9)a | 34.8 (7.1) |
| Waist circumference (cm) | 100.3 (11.1) | 100.9 (11.5) | 87.9 (11.8)a | 89.2 (12.5)c |
| Systolic blood pressure (mmHg) | 127.1 (11.3) | 124.5 (11.3)d | 120.6 (13.8)a | 116.8 (13.3)c |
| Diastolic blood pressure (mmHg) | 81.4 (8.8) | 79.6 (9.7)d | 76.2 (9.3)a | 73.9 (9.9)c |
| Triacylglycerol (mmol/l) | 1.8 (1.6) | 1.7 (1.3) | 1.2 (0.6)a | 1.1 (0.7) |
| Total cholesterol (mmol/l) | 5.2 (1.0) | 5.3 (1.0) | 5.1 (0.9) | 5.2 (1.0) |
| HDL-cholesterol (mmol/l) | 1.2 (0.3) | 1.2 (0.3) | 1.6 (0.4)a | 1.6 (0.4) |
| Fasting plasma insulin (mmol/l) | 70.2 (71.6) | 72.2 (52.3) | 55.0 (35.6)b | 56.3 (35.4) |
| Fasting plasma glucose (mmol/l) | 5.1 (0.9) | 5.2 (1.0) | 4.7 (0.5)a | 4.8 (0.5)d |
| PAEE (kJ kg−1 FFM min−1) | 0.13 (0.07) | 0.14 (0.08)d | 0.10 (0.08)b | 0.11 (0.07) |
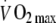 (ml kg−1 FFM min−1) (ml kg−1 FFM min−1) |
60.7 (10.54) | 61.2 (11.42) | 57.10 (10.72)b | 58.06 (10.38) |
| Daily physical activity (total count)e | 265,000 (79,000) | 275,000 (106,000) | 269,000 (109,00) | 280,000 (110,000) |
Data are means (SD)
ap < 0.001, bp < 0.05 for women vs men at baseline; cp < 0.001, dp < 0.05 for baseline vs follow-up (separately in men and women)
eThese values are based on data from n = 192 ProActive participants for whom accelerometer data were available at baseline and follow-up
For men, there were no significant differences in mean height, weight, BMI, body fat, waist circumference, triacylglycerol, HDL-cholesterol, fasting insulin and glucose from baseline to follow-up. The only metabolic subcomponent that changed significantly over time was systolic blood pressure, which reduced from 127.1 to 124.5 mmHg. In terms of the activity variables, men significantly increased their physical activity (PAEE) from 0.125 to 0.139 kJ kg−1 FFM min−1, but increases in fitness and total body movement did not achieve statistical significance. For women, there were no significant differences in mean weight, body fat, triacylglycerol, total cholesterol, HDL-cholesterol and fasting insulin from baseline to follow-up. Mean height decreased very slightly, while BMI increased from 27.6 to 27.8 kg/m2 and waist circumference increased from 87.9 to 89.2 cm. Values for both diastolic and systolic pressure decreased significantly, while fasting plasma glucose increased slightly from 4.7 to 4.8 mmol/l. Increases over the year in PAEE, total body movement and fitness did not reach statistical significance. There was no significant change in the use of cardiovascular therapies during follow-up (data not shown).
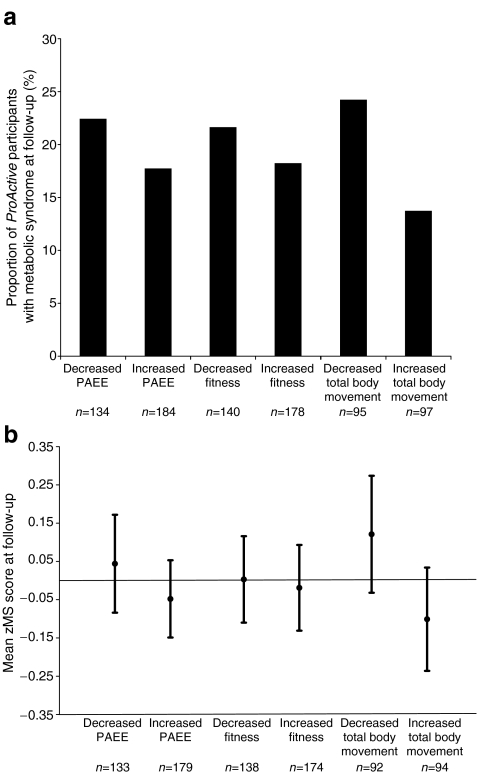
There was no evidence for a correlation between change in fitness and change in PAEE (r = 0.1) or total body movement (r = 0.0). There was limited evidence for a positive correlation between change in PAEE and total body movement (r = 0.3). The overall prevalence of metabolic syndrome was 20% at both baseline and follow-up, although there was some movement between groups. The proportion of metabolic syndrome at follow-up was higher among participants who failed to increase their PAEE, fitness and total body movement over 1 year (Fig. 1a). Similarly, clustered metabolic risk scores were lower among individuals who managed to increase their PAEE, fitness and total body movement during follow-up (Fig. 1b). None of the differences between these groups reached statistical significance but were in the expected direction of effect.
Fig. 1.
a Proportion of ProActive participants with metabolic syndrome at 1 year follow-up by those who increased and decreased their PAEE, fitness and total body movement. b Mean metabolic summary score for ProActive participants at 1 year follow-up by those who increased and decreased their PAEE, fitness and total body movement (bars represent 95% CIs)
Table 2 shows the crude and adjusted associations between change in PAEE (kJ kg−1 FFM min−1), 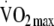 (ml kg−1 FFM min−1) and total body movement ([counts per day]/1,000) with standardised subcomponents of the clustered metabolic risk score and the whole score (zMS) at follow-up. As all outcomes are expressed in the same unit (SD), it is possible to directly compare the magnitude of associations between the different components of activity with each of the outcomes, assuming they are measured with the same degree of measurement error. In adjusted analyses, fasting glucose, insulin and HDL-cholesterol at follow-up were significantly associated with a change in fitness over time; on average, individuals who increased their fitness had reduced levels of glucose and insulin, and increased levels of HDL-cholesterol at follow-up. Similarly, fasting glucose and insulin were associated with change in total body movement over time. All other subcomponents of the clustered metabolic risk score failed to show an association with change in either physical activity, fitness or total body movement. In adjusted analyses, change in fitness, but not change in PAEE, was associated with clustered metabolic risk at follow-up with (zMS; p = 0.034) and without the obesity component (zMS-ob; p = 0.04). In addition, change in total body movement from baseline to follow-up was associated with clustered metabolic risk in both the overall (zMS; p = 0.004) and obesity-independent score (zMS-ob; p = 0.01).
(ml kg−1 FFM min−1) and total body movement ([counts per day]/1,000) with standardised subcomponents of the clustered metabolic risk score and the whole score (zMS) at follow-up. As all outcomes are expressed in the same unit (SD), it is possible to directly compare the magnitude of associations between the different components of activity with each of the outcomes, assuming they are measured with the same degree of measurement error. In adjusted analyses, fasting glucose, insulin and HDL-cholesterol at follow-up were significantly associated with a change in fitness over time; on average, individuals who increased their fitness had reduced levels of glucose and insulin, and increased levels of HDL-cholesterol at follow-up. Similarly, fasting glucose and insulin were associated with change in total body movement over time. All other subcomponents of the clustered metabolic risk score failed to show an association with change in either physical activity, fitness or total body movement. In adjusted analyses, change in fitness, but not change in PAEE, was associated with clustered metabolic risk at follow-up with (zMS; p = 0.034) and without the obesity component (zMS-ob; p = 0.04). In addition, change in total body movement from baseline to follow-up was associated with clustered metabolic risk in both the overall (zMS; p = 0.004) and obesity-independent score (zMS-ob; p = 0.01).
Table 2.
Crude and adjusted associations (standardised β-coefficients) between change in PAEE, 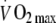 and total body movement with standardised subcomponents of the metabolic summary score and the whole score (zMS) at follow-up in the ProActive cohort over 1 year
and total body movement with standardised subcomponents of the metabolic summary score and the whole score (zMS) at follow-up in the ProActive cohort over 1 year
| Outcome (SD) | Standardised β-coefficient (95% CI) | |||||
|---|---|---|---|---|---|---|
| Change in PAEE (n = 318) | Change in 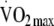 (n = 318) (n = 318) |
Change in total body movement (n = 191) | ||||
| Crude | Adjusteda | Crude | Adjusteda | Crude | Adjusteda | |
| Waist circumference | −0.061 (−0.172, 0.051) | −0.025 (−0.065, 0.016) | −0.074 (−0.185, 0.037) | −0.017 (−0.058, 0.024) | −0.117 (−0.261, 0.027) | −0.019 (−0.071, 0.032) |
| Systolic blood pressure | 0.062 (−0.047, 0.171) | 0.074 (−0.004, 0.152) | −0.017 (−0.126, 0.093) | −0.035 (−0.113, 0.044) | −0.017 (−0.158, 0.125) | 0.012 (−0.094, 0.118) |
| Logged triacylglycerol | −0.008 (−0.127, 0.110) | −0.024 (−0.100, 0.051) | −0.059 (−0.178, 0.060) | −0.009 (−0.084, 0.067) | −0.026 (−0.180, 0.128) | −0.054 (−0.149, 0.041) |
| Logged fasting insulin | −0.064 (−0.175, 0.046) | −0.024 (−0.093, 0.045) | −0.094 (−0.203, 0.016) | −0.069 (−0.137, −0.002)b | −0.097 (−0.239, 0.045) | −0.101 (−0.186, −0.017)b |
| Fasting plasma glucose | −0.005 (−0.133, 0.123) | −0.024 (−0.114, 0.067) | −0.139 (−0.266, −0.012)b | −0.090 (−0.180, 0.000)b | −0.163 (−0.328, 0.002) | −0.147 (−0.259, −0.035)b |
| HDL-cholesterol (inverse) | −0.053 (−0.165, 0.059) | −0.056 (−0.125, 0.014) | −0.115 (−0.227, −0.004)b | −0.097 (−0.166, −0.029)b | −0.027 (−0.167, 0.113) | −0.026 (−0.121, 0.069) |
| zMS | −0.031 (−0.112, 0.050) | −0.017 (−0.054, 0.020) | −0.081 (−0.160, −0.001)b | −0.056 (−0.093, −0.020)b | −0.074 (−0.176, 0.028) | −0.066 (−0.111, −0.021)b |
| zMS-ob | −0.023 (−0.103, 0.058) | −0.012 (−0.053, 0.029) | −0.083 (−0.163, −0.004)b | −0.062 (−0.102, −0.022)b | −0.065 (−0.166, 0.037) | −0.068 (−0.119, −0.016)b |
aAll coefficients adjusted for age, sex, smoking status, SES, waist circumference and baseline phenotype, except for waist circumference, which was adjusted for age, sex, smoking status, SES and baseline phenotype
bp < 0.05
There was no evidence of interaction between change in EE and aerobic fitness (p = 0.28), EE and total body movement (p = 0.13) and aerobic fitness and total body movement (p = 0.13) with clustered metabolic risk at follow-up. While there was no evidence of a significant interaction between age or sex and change in the activity variables, the β-coefficients for change in PAEE appeared stronger in men than in women, and also in older rather than younger individuals.
Discussion
Our results suggest that small increases in physical activity, measured using accelerometry and aerobic fitness, were associated with improvement in clustered metabolic risk over a 1 year period in middle-aged individuals at high risk of developing type 2 diabetes. Our results were independent of age, sex, smoking status, socioeconomic group and baseline phenotype. This confirms our earlier cross-sectional result [13] in a prospective analysis, which provides stronger inferential evidence for the association between total body movement and metabolic risk. Further, this result is supported by our finding that the proportion of metabolic syndrome at follow-up was higher, albeit non-significantly, among groups who failed to increase their PAEE, fitness and total body movement over 1 year.
Although PAEE was not significantly associated with clustered metabolic risk in our analyses, we were able to demonstrate an association between overall body movement (i.e. physical activity) assessed by accelerometry and clustered metabolic risk. This is a novel finding given that previous prospective associations between objectively measured physical activity and metabolic risk were observed in a population of healthy middle-aged Europids [7], rather than the overweight, sedentary younger adults with a high risk of developing type 2 diabetes described in this cohort. We also observed that the benefits of increased fitness and total body movement acted primarily through changes in serum glucose, insulin and HDL-cholesterol, which may suggest that blood pressure is less sensitive to changes in activity and fitness. In a comparable study, Ekelund et al. [21] observed that an increase in activity was associated with fasting insulin, triacylglycerol, 2 h glucose and clustered metabolic risk but not with systolic or diastolic blood pressure. Contrary to our findings, previous literature has shown an association between PAEE and clustered metabolic risk, both cross-sectionally [8] and prospectively over a period of 5.6 years, independently of fitness [7]. This apparent discrepancy might be explained in a number of ways. First, while the trial was powered to detect a difference in physical activity-related EE between trial arms equivalent to 2 metabolic energy equivalent (MET) h/day or approximately 30 min of brisk walking, the sample size was relatively small for a cohort analysis and may not have been large enough to detect smaller but still biologically important differences. Nonetheless, all the PAEE β-coefficients were in the same direction of effect as those for the accelerometry data but they did not reach statistical significance.
Second, the precision in the methods we used to assess PAEE and body movement may affect the observed associations. PAEE from individually calibrated HR monitoring is an integrated measure of EE above rest calculated from free-living HR data. In sedentary populations, much of the daytime is spent in the region around the flex HR, which is used to discriminate between rest and physical activity. The association between HR and EE is less precise in this region, which may influence the accuracy of predicted PAEE on an individual level. Furthermore, the flex HR method may be sensitive to the fitness level of the people under investigation [22]. In contrast, accelerometry measures the vertical accelerations of the body, i.e. physical work, and is likely to be less sensitive to the characteristics of the population in terms of a sedentary lifestyle and fitness level. If most of the activities performed are locomotor activities, such as walking, measurement of body movement by accelerometry may be superior to HR monitoring when examining associations with disease risk. Ideally, the two different measurement techniques should be combined into one single piece instrument [23].
Finally, by standardising the clustered metabolic risk score, we assumed each component made an equal contribution towards defining metabolic risk. It is unlikely that each component is equally strongly associated with metabolic risk and some variables will be more important that others in different populations. It would therefore be ideal to weight each component of the score, but data are currently unavailable.
Our results suggest that small, feasible changes in physical activity (total body movement) and fitness in an at-risk population may prevent progression towards the metabolic syndrome. This supports previous literature, which has demonstrated an inverse relationship between fitness, physical activity and risk of developing metabolic syndrome in a number of settings [5, 24–26]. At a population level, our findings have important implications. This group of middle-aged, slightly overweight individuals, identified through primary care registers via their first degree family history of diabetes, represents an accessible population who might benefit from increased physical activity and fitness levels. Primary care or public health practitioners might target this group for preventive action. Findings can also be extrapolated to inform the characteristics of public health initiatives. Increasing total body movement can be achieved through small changes in lifestyle activity, such as taking the stairs and parking the car further away from work, which may be more palatable to sedentary and at-risk populations than targeting changes in moderate to vigorous intensity activities. Regular physical activity participation also has multiple positive effects upon other diseases in addition to metabolic syndrome, such as stroke, coronary heart disease and cancer [27, 28]. However, currently there is limited evidence regarding the effectiveness of lifestyle interventions for individuals, at-risk groups or populations in the treatment and prevention of metabolic syndrome and this is an area of research that warrants further investigation. It is important to clarify whether metabolic risk is most effectively reduced by increases in overall EE, fitness or total body movement to inform the development and nature of preventive interventions.
This study had several methodological strengths. The ProActive trial allowed objective measurement of several components of physical activity and fitness over a period of 12 months in a well-defined group of individuals accessed through primary care who were at risk of diabetes. There was a high follow-up rate (88%) and standardised measures were used throughout. We used objective measures of physical activity, which reduces the error and bias commonly associated with self-report measures and our objective measures of EE, physical activity (PAEE, total body movement) and fitness have been extensively validated in the laboratory and during free-living conditions [18].
In conclusion, small increases in physical activity assessed by accelerometry and in aerobic fitness were associated with improved metabolic risk over 1 year. Further research is needed to clarify the relationship between physical activity, EE, fitness and metabolic risk to inform advice and intervention development. We may need even more precise methods of measurement to capture the true dose effect of such relationships.
Acknowledgements
The UK Medical Research Council (ref. no. ISRCTN 61323766), UK NHS R&D, the UK Royal College of General Practitioners Scientific Foundation, and Diabetes UK (ref. no. RG35259) funded the development and execution of the ProActive trial. We thank the study participants and practice teams for their collaboration and work in helping with recruitment. Also thanks to S. Sharp, J. Luan and T. Fanshawe for statistical advice, to M. Hennings for helping develop the MAHUffe software and to A. L. Kinmonth for her helpful comments. The ProActive research team includes, besides the authors, K. Williams, J. Grant, A. T. Prevost, W. Hollingworth, D. Spiegelhalter (principal investigator), W. Hardeman (principal investigator), S. Sutton (principal investigator), and A. L. Kinmonth (principal investigator).
Duality of interest The authors declare that there is no duality of interest associated with this manuscript.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- EE
energy expenditure
- FFM
fat-free mass
- HR
heart rate
- PAEE
physical activity energy expenditure
- REE
resting energy expenditure
- SES
socioeconomic status
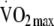
maximum oxygen consumption
- zMS
continuously distributed metabolic risk variable
- zMS-ob
continuously distributed metabolic risk variable without the adiposity component (waist circumference)
Footnotes
The other members of the ProActive research team, besides the authors, are listed in Acknowledgements.
References
- 1.Alberti KG, Zimmet P, Shaw J (2005) The metabolic syndrome—a new worldwide definition. Lancet 366:1059–1062 [DOI] [PubMed]
- 2.Ford ES (2005) Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care 28:1769–1778 [DOI] [PubMed]
- 3.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP (2001) The continuing epidemics of obesity and diabetes in the United States. JAMA 286:1195–1200 [DOI] [PubMed]
- 4.Zimmet P, Alberti KG, Shaw J (2001) Global and societal implications of the diabetes epidemic. Nature 414:782–787 [DOI] [PubMed]
- 5.Rennie KL, McCarthy N, Yazdgerdi S, Marmot M, Brunner E (2003) Association of the metabolic syndrome with both vigorous and moderate physical activity. Int J Epidemiol 32:600–606 [DOI] [PubMed]
- 6.Laaksonen DE, Lakka HM, Salonen JT, Niskanen LK, Rauramaa R, Lakka TA (2002) Low levels of leisure-time physical activity and cardiorespiratory fitness predict development of the metabolic syndrome. Diabetes Care 25:1612–1618 [DOI] [PubMed]
- 7.Ekelund U, Brage S, Franks PW, Hennings S, Emms S, Wareham NJ (2005) Physical activity energy expenditure predicts progression toward the metabolic syndrome independently of aerobic fitness in middle-aged healthy Caucasians: the Medical Research Council Ely Study. Diabetes Care 28:1195–1200 [DOI] [PubMed]
- 8.Franks PW, Ekelund U, Brage S, Wong MY, Wareham NJ (2004) Does the association of habitual physical activity with the metabolic syndrome differ by level of cardiorespiratory fitness? Diabetes Care 27:1187–1193 [DOI] [PubMed]
- 9.Brage S, Wedderkopp N, Ekelund U et al (2004) Features of the metabolic syndrome are associated with objectively measured physical activity and fitness in Danish children: the European Youth Heart Study (EYHS). Diabetes Care 27:2141–2148 [DOI] [PubMed]
- 10.Orchard TJ, Temprosa M, Goldberg R et al (2005) The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med 142:611–619 [DOI] [PMC free article] [PubMed]
- 11.Wareham NJ, Rennie KL (1998) The assessment of physical activity in individuals and populations: why try to be more precise about how physical activity is assessed? Int J Obes Relat Metab Disord 22(Suppl 2):S30–S38 [PubMed]
- 12.Kinmonth AL, Wareham NJ, Hardeman W et al (2008) Efficacy of a theory-based behavioural intervention to increase physical activity in an at-risk group in primary care (ProActive UK): a randomised trial. Lancet 371:41–48 [DOI] [PubMed]
- 13.Ekelund U, Griffin SJ, Wareham NJ (2007) Physical activity and metabolic risk in individuals with a family history of type 2 diabetes. Diabetes Care 30:337–342 [DOI] [PubMed]
- 14.Williams K, Prevost AT, Griffin S et al (2004) The proactive trial protocol—a randomised controlled trial of the efficacy of a family-based, domiciliary intervention programme to increase physical activity among individuals at high risk of diabetes [ISRCTN61323766]. BMC Public Health 4:48 [DOI] [PMC free article] [PubMed]
- 15.Hardeman W, Sutton S, Griffin S et al (2005) A causal modelling approach to the development of theory-based behaviour change programmes for trial evaluation. Health Educ Res 20:676–687 [DOI] [PubMed]
- 16.Weir JB (1949) New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109:1–9 [DOI] [PMC free article] [PubMed]
- 17.Toth MJ (2001) Comparing energy expenditure data among individuals differing in body size and composition: statistical and physiological considerations. Curr Opin Clin Nutr Metab Care 4:391–397 [DOI] [PubMed]
- 18.Ekelund U, Yngve A, Brage S, Westerterp K, Sjostrom M (2004) Body movement and physical activity energy expenditure in children and adolescents: how to adjust for differences in body size and age. Am J Clin Nutr 79:851–856 [DOI] [PubMed]
- 19.Anderson KM, Wilson PW, Odell PM, Kannel WB (1991) An updated coronary risk profile. A statement for health professionals. Circulation 83:356–362 [DOI] [PubMed]
- 20.Lawlor DA, Smith GD, Ebrahim S (2006) Does the new International Diabetes Federation definition of the metabolic syndrome predict CHD any more strongly than older definitions? Findings from the British Women’s Heart and Health Study. Diabetologia 49:41–48 [DOI] [PubMed]
- 21.Ekelund U, Franks PW, Sharp S, Brage S, Wareham NJ (2007) Increase in physical activity energy expenditure is associated with reduced metabolic risk independent of change in fatness and fitness. Diabetes Care 30:2101–2106 [DOI] [PubMed]
- 22.Ekelund U, Yngve A, Westerterp K, Sjostrom M (2002) Energy expenditure assessed by heart rate and doubly labeled water in young athletes. Med Sci Sports Exerc 34:1360–1366 [DOI] [PubMed]
- 23.Corder K, Brage S, Wareham NJ, Ekelund U (2005) Comparison of PAEE from combined and separate heart rate and movement models in children. Med Sci Sports Exerc 37:1761–1767 [DOI] [PubMed]
- 24.LaMonte MJ, Barlow CE, Jurca R, Kampert JB, Church TS, Blair SN (2005) Cardiorespiratory fitness is inversely associated with the incidence of metabolic syndrome: a prospective study of men and women. Circulation 112:505–512 [DOI] [PubMed]
- 25.Ford ES, Li C (2006) Physical activity or fitness and the metabolic syndrome. Expert Rev Cardiovasc Ther 4:897–915 [DOI] [PubMed]
- 26.Lakka TA, Laaksonen DE, Lakka HM et al (2003) Sedentary lifestyle, poor cardiorespiratory fitness, and the metabolic syndrome. Med Sci Sports Exerc 35:1279–1286 [DOI] [PubMed]
- 27.Myint PK, Luben RN, Wareham NJ et al (2006) Combined work and leisure physical activity and risk of stroke in men and women in the European Prospective Investigation into Cancer–Norfolk Prospective Population Study. Neuroepidemiology 27:122–129 [DOI] [PubMed]
- 28.Hu G, Tuomilehto J, Silventoinen K, Barengo NC, Peltonen M, Jousilahti P (2005) The effects of physical activity and body mass index on cardiovascular, cancer and all-cause mortality among 47,212 middle-aged Finnish men and women. Int J Obes Relat Metab Disord 29:894–902 [DOI] [PubMed]