Abstract
We used functional magnetic resonance imaging (fMRI) to investigate the neural correlates of self‐regulatory control across development in healthy individuals performing the Stroop interference task. Proper performance of the task requires the engagement of self‐regulatory control to inhibit an automatized response (reading) in favor of another, less automatic response (color naming). Functional MRI scans were acquired from a sample of 70 healthy individuals ranging in age from 7 to 57 years. We measured task‐related regional signal changes across the entire cerebrum and conducted correlation analyses to assess the associations of signal activation with age and with behavioral performance. The magnitude of fMRI signal change increased with age in the right inferolateral prefrontal cortex (Brodmann area [BA] 44/45) and right lenticular nucleus. Greater activation of the right inferolateral prefrontal cortex also accompanied better performance. Activity in the right frontostriatal systems increased with age and with better response inhibition, consistent with the known functions of frontostriatal circuits in self‐regulatory control. Age‐related deactivations in the mesial prefrontal cortex (BA 10), subgenual anterior cingulate cortex (BA 24), and posterior cingulate cortex (BA 31) likely represented the greater engagement of adults in self‐monitoring and free associative thought processes during the easier baseline task, consistent with the improved performance on this task in adults compared with children. Although we cannot exclude the possibility that age‐related changes in reading ability or in the strategies used to optimize task performance were responsible for our findings, the correlations of brain activation with performance suggest that changes in frontostriatal activity with age underlie the improvement in self‐regulatory control that characterizes normal human development. Hum Brain Mapp, 2006. © 2006 Wiley‐Liss, Inc.
Keywords: development, children, self‐regulation, fMRI, Stroop task
INTRODUCTION
Learning to control or inhibit behaviors that violate personal or societal norms is one of the centrally defining characteristics of normal child development. Disturbances in the maturation of self‐regulatory control processes likely contribute to the development of a variety of neuropsychiatric disorders in which children have difficulty controlling cognitive, affective, and motoric impulses. Understanding the origins of these disturbances is necessary for the rational development of improved treatment and prevention strategies, and an improved understanding of the disturbances requires a better understanding of the normal maturation at the level of brain function and neural circuits that mediate self‐regulatory control.
The Stroop task [Stroop,1935] engages self‐regulatory control processes by requiring subjects to inhibit a more automatic behavior (reading a word) in favor of a less automatic one (naming the color of the letters). When the color that a written word denotes matches the color of the ink in which the letters are printed (e.g., “R‐E‐D” written in red ink), subjects perform the task easily, as indexed by their rapid responses and infrequent errors. When the color that a word denotes does not match the color of the printed letters (e.g., “R‐E‐D” written in blue ink), however, the task is more difficult, as indicated by their slower responses and more frequent errors. Inhibiting the prepotent reading response in this condition requires the mobilization of attentional resources, the resolution of cognitive conflict, modulation of the automatic response tendency and, ultimately, self‐regulatory control.
Imaging studies of brain activity during color naming of the mismatching compared with the matching stimuli have demonstrated activation in large expanses of anterior cingulate and prefrontal cortices and the striatum in both adults [Carter et al.,2000; Leung et al.,2000; MacDonald et al.,2000; Milham et al.,2002; Pardo et al.,1990; Peterson et al.,1999b] and children [Adleman et al.,2002; Blumberg et al.,2003b]. Because numerous behavioral studies have demonstrated that performance on the Stroop task improves with age once reading skill is automatized [Comalli et al.,1962; Dash and Dash,1982; MacLeod,1991; Schiller,1966], we hypothesized that differential brain activity during performance of the Stroop task would correlate with both age and age‐related improvement in task performance, particularly within frontostriatal systems. Given the questionable assumption that coregistering voxels across the brains of children and adults accurately coregisters their corresponding anatomical and functional cytoarchitecture, we tested our hypotheses using region‐of‐interest (ROI)–based analyses, which should minimize the possible deleterious effects of tissue misregistration across age groups [Peterson,2003]. In addition, exploratory voxel‐wise analyses were employed to detect associations of age with signal changes that may not be captured with the use of larger, predefined ROIs.
PATIENTS AND METHODS
Subject Recruitment and Characterization
Subjects were recruited from randomly selected names on a telemarketing list of approximately 10,000 families in the local community. These families received introductory letters, which were then followed by screening and recruitment telephone calls. Approximately 10% of the families who were contacted ultimately participated. The Schedule for Affective Disorders and Schizophrenia was administered to all adult subjects [Endicott and Spitzer,1978; Kaufman et al.,1997], and the Kiddie‐Schedule for Affective Disorders and Schizophrenia Present and Lifetime Version was administered to all subjects under 18 years of age [Kaufman et al.,1997]. We excluded from participation any individual who met the Diagnostic and Statistical Manual of Mental Disorders (DSM)‐IV criteria for current Axis I disorder [American Psychiatric Association, 1994] or who had a lifetime history of neurological illness, past seizures or history of head trauma with loss of consciousness, mental retardation, pervasive developmental disorder, psychosis, major depression, or anxiety disorder. Written informed consent was obtained from adult subjects and the parents of participating children, and assent was obtained from the children. Subjects were paid for their participation. A total of 76 subjects were scanned, but 3 children were noted to have moved excessively during the procedure, and three additional subjects had ghosting artifact that precluded inclusion in the analyses.
Seventy subjects remained for statistical analyses. The sample included 34 men and 36 women ranging in age from 7 to 57 years (mean age, 26.65 years, standard deviation [SD], 12.8 years; quartiles: 90th percentile = 49.0 years, 75th percentile = 33.6 years; median = 23.6 years; 25th percentile = 17.0 years; and 10th percentile = 12.1 years). We confined our sample to children ages 7 and older because, unlike younger children, those above this age typically have reading skills that are sufficiently developed to experience interference on the Stroop task [Adleman et al.,2002; Comalli et al.,1962; Schiller,1966]. All subjects were predominantly right‐handed [Oldfield,1971]. Estimates of full‐scale IQs were made using the Wechsler Abbreviated Scale of Intelligence [Wechsler,1981], and socioeconomic status was quantified using the Hollingshead Four‐Factor Index of Social Status [Hollingshead,1975]. Sample characteristics are given in Table I.
Table I.
Demographic and Clinical Characteristics
| Characteristic | Subjects | |
|---|---|---|
| Children (n = 20) | Adults (n = 50) | |
| Mean age, yr | 13.52 (3.2) | 31.91 (11.4) |
| Median age, yr | 13.86 | 28.93 |
| Sex (M:F) | 11:9 | 23:27 |
| SES | 47.53 (12.0) | 52.00 (10.9) |
| WASI IQ score | ||
| Full | 117.5 (12.3) | 123.2 (14.9) |
| Verbal | 115.49 (11.6) | 123.7 (17.2) |
| Performance | 116.4 (16.2) | 119.1 (15.1) |
| Ethnicity [n (%) Caucasian] | 20 (100) | 43 (86) |
WASI, Weschler Abbreviated Scale of Intelligence. Values are mean (SD) unless otherwise specified.
Stimulus Presentation
The stimulus presentation and scanning procedures for the Stroop are presented in greater detail elsewhere [Leung et al.,2000; Peterson et al.,1999a,2002; Potenza et al.,in press]. Briefly, four color words (red, green, yellow, and blue) were presented randomly against a black background and back‐projected onto a screen that the subjects viewed through a mirror located on the scanner's head coil. The words were situated directly above a gaze‐fixation white cross‐hair, and subtended about 2.2 vertical and 3.75 horizontal degrees of the visual field. Colored word stimulus and interstimulus durations were 1,300 and 350 msec, respectively. After a 1‐second presentation of the cross‐hair, colored word stimuli were presented in “congruent” and “incongruent” blocks of 16 trials each. Congruent trial blocks consisted of words written in the corresponding color (e.g., the word red displayed in red ink), and incongruent blocks consisted of words written in a randomly selected mismatching color (e.g., the word red written in either blue, green, or yellow ink), with the constraint that no word or color was the same as the preceding word or color. The frequencies of presentation of colors and words were balanced between congruent and incongruent blocks. Four blocks each of congruent and incongruent stimuli were presented in each of two runs, and each run was 3 minutes 44 seconds in duration. Consistent with previous methods, subjects were instructed to name the color of the stimulus on each presentation, regardless of the meaning of the written word, quietly and with as little mouth movement as possible [Peterson et al.,1999a].
The presentation of stimuli within the scanner was strictly paced so that the number of stimuli would be equal across conditions (congruent and incongruent trial blocks). This method of stimulus presentation did not permit us to measure the response time that is typically used to assess the degree of interference experienced by subjects performing the task. We therefore measured behavioral interference outside of the scanner using the standard format of the Stroop [Lezak,1995]. In Task A (color naming), subjects were asked to name as quickly as possible the color (red, green, or blue) of 126 dots, 5.6 mm in diameter, arrayed randomly in 9 columns and 14 rows on an 8.5 × 11‐inch sheet of white paper, scanned left to right and then top to bottom. In Task B (word reading), they were asked to read as quickly as possible an equal number of similarly arrayed words (red, green, or blue) printed in black ink. In Task C (color‐word naming) they were asked to name as quickly as possible a similar array of words written in incongruent colors. The time to completion of each task was recorded (A, B, and C, respectively). Stroop interference was calculated as C − [(A × B)/(A + C)] [Golden,1978]. These behavioral measures were conducted after the scanning session so as not to introduce practice or habituation effects into the functional imaging data. They were then used for correlation analyses with the magnitudes of brain activation detected inside the scanner.
Image Acquisition and Processing
Acquisition.
Head positioning in the magnet was standardized using the canthometal line. A T1‐weighted sagittal localizing scan was used to position the axial images. Images were acquired on a GE Signa 1.5 Tesla LX scanner (Milwaukee, WI) and a standard quadrature head coil. The functional images were obtained using a T2*‐sensitive gradient‐recalled, single‐shot, echo‐planar pulse sequence having a repetition time (TR) = 1,750 msec, echo time (TE) = 45 msec, 60‐degree flip angle, single excitation per image, 20 × 40 cm field of view (FOV), and 64 × 128 matrix, providing a 3.1 × 3.1 mm in‐plane resolution. In all 70 subjects, 6 axial slices were acquired to correspond with axial slices in the z‐direction of Talairach space [Talairach and Tournoux,1988] beginning at the anterior commissure–posterior commissure (AC–PC) line and extending dorsally six ninths of the distance to the vertex. After hardware upgrades to the scanner console over the years of data collection, which increased our capacity for data collection, an additional 2 slices ventral to the AC–PC line were acquired in 18 subjects and an additional 4 slices—2 ventral and 2 dorsal to the AC–PC line—were acquired in 32 subjects. The 10 slices for these subjects were positioned with 2 slices below, 7 slices above, and 1 slice containing the AC–PC line. Only the 6 slices acquired in all subjects were used for image analysis. Slice thickness was a constant 7 mm, whereas the skip between slices varied between 0.5 and 2 mm to maintain a strict correspondence to the Talairach coordinate system.
Image preprocessing.
Studies were visually inspected during preprocessing. Images were discarded if artifacts such as ghosting occurred or if the subject moved >0.5 pixels in any direction. Images were then motion‐corrected using SPM99 software for three translational directions and three possible rotations [Friston et al.,1995]. Corrected images were spatially filtered using a Gaussian filter with a full‐width half maximum of 6.3 mm. Drift of baseline image intensity was removed using an eighth‐order high‐pass Butterworth filter with a frequency cut‐off equal to three fourths of the task frequency. The T1‐weighted axial anatomical images (acquired using a sagittal spoiled gradient recall sequence) and corresponding echo‐planar functional images for each subject were transformed into a common stereotactic space using a piece‐wise linear warping to a common bounding box [Talairach and Tournoux,1988].
Behavioral Analysis
Regression analyses were carried out to assess the predictive value of age on Stroop performance. Interference scores for each subject were entered as dependent variables in a linear mixed model with age, age squared (age2), full‐scale IQ, and sex included as covariates. The effect of age and the quadratic effect of age on behavioral performance were tested by assessing the statistical significance of the main effect of age and age2, respectively. Regression analyses were also carried out using the times to completion for each of the Stroop subtests, tasks A, B and C, with the same covariates included in each model. Associations of behavioral performance with age and with age2 were assessed in correlational analyses. We calculated Pearson's correlation coefficients of interference scores with age and age2, and of the times to completion for each of the Stroop subtests with age and age2.
ROI‐Based Image Analysis
A major limitation of voxel‐based approaches to image analysis is the unproven assumption that the anatomy and corresponding localization of function are the same across individuals who are of differing ages and who have differing brain sizes. In voxel‐wise analyses, individuals are compared on a single voxel‐by‐voxel basis, after individual brains are warped into the Talairach coordinate system and then superimposed one on top of the other. After this warping procedure, the location of a voxel in a brain area of one person is assumed to be in precisely the same location as the voxel identified in that brain area of another person. Children's brains, when warped into the Talairach coordinate system, cannot be assumed without justification to superimpose perfectly onto those of adults, although spatial smoothing of individual fMRI data mitigates this problem to some extent. In contrast, ROI‐based approaches to fMRI analyses are much less dependent on the assumption of a close correspondence between imaging voxels and underlying brain cytoarchitecture across individuals.
Our ROI‐based image analytic strategy has been described previously in detail [Peterson et al.,1999a]. Briefly, anatomical regions were first defined on each individual's unwarped images. The mean percent change in fMRI signal acquired during blocks of congruent and incongruent stimuli was calculated at each pixel for every subject. We chose to use these percentage signal changes in statistical analyses because they are less prone to systematic influence by subject motion than are t‐statistics; the latter can be systematically reduced in children because they may move more than adults do, thus producing larger signal variances that could systematically bias t‐statistics across groups. These mean percent signal changes for each voxel within each ROI were then averaged to provide a descriptive statistic representing the overall task‐dependent change in fMRI signal within the entire ROI. This measure of ROI‐based signal change for each individual was then used to compare activation in that ROI across different individuals. For our analyses, 53 ROIs were defined using standard stereotactic coordinates [Talairach and Tournoux,1988], with the exception of one ROI for the thalamus and three for the basal ganglia (caudate, putamen, and globus pallidus), which were hand‐circumscribed on the T1‐weighted axial anatomical images. Grids corresponding to these ROIs were then overlaid on the fMRI activation maps. Signal changes associated with the presentation of the incongruent minus congruent stimuli were averaged across all pixels within each defined ROI for each subject. These region‐wise mean signal differences thus served as measures of the differential signal change associated with task performance.
A priori hypothesis testing.
The following statistical analyses were carried out in SAS v8.0 (SAS Institute Inc., Cary, NC) using a mixed model analysis. Our a priori hypothesis was tested using hierarchical, well‐formulated mixed models of repeated measures over the entire cerebrum [Blumberg et al.,2003b; Peterson et al.,1999b] to account for the intercorrelation of fMRI signal change across ROIs. Fifty‐three ROIs were employed as dependent measures in a linear mixed model that included age, age squared, hemisphere (right, left), IQ, and sex as covariates. The regionally specific effects of age on Stroop‐related signal change were assessed by testing the statistical significance of the ROI‐by‐age interaction. In addition, the quadratic effect of age was explored by testing the statistical significance of the ROI‐by‐age squared interaction. All two‐ and three‐way interactions involving region‐wise mean signal change (ROI) were considered for inclusion in the models. Terms that were not significant (P = 0.05) were eliminated via backward stepwise regression, with the constraint that the models had to be hierarchically well‐formulated at each step (i.e., all possible lower‐order terms had to be included in the model, regardless of their statistical significance) [Morrell et al.,1997]. To identify components that contributed most to the significance of the interactions used for testing our hypotheses, we conducted post‐hoc analyses in which we examined parameter estimates and component terms in the analyses of fixed effects for the final model, followed by analyses of least square means and standard errors to assist in the interpretation of significant findings. Regions that exhibited significant differences at P < 0.01 are reported. The post‐hoc probability values were assigned to the number of activations found in each region without correction for multiple comparisons because the overall study‐wide experimental error of <0.05 had already been established in the omnibus test. Similar strategies designed to address the issue of multiple comparisons are invoked in standard multivariate tests of significance [Hair et al.,1992].
Behavioral correlates.
Pearson's partial correlation coefficients were calculated between interference scores obtained outside of the scanner and the average regional signal changes for each ROI. These analyses were intended to identify correlates that were independent of age, so that we could assess whether brain areas where age‐related changes in activation during the task corresponded with brain areas that contributed to improved performance on the task.
Voxel‐Wise Exploratory Analyses
We considered the following voxel‐wise analyses as exploratory and hypothesis‐generating. We report voxels that were identified on maps of correlations with age using a P‐value threshold <0.05 together with the requirement that the activation occurred in a spatial cluster of at least 25 adjacent pixels. The combined application of a statistical threshold and cluster filter has previously been shown to reduce substantially the false‐positive identification of activated pixels at any given threshold [Forman et al.,1995]. Based on an approximation formula (equation #11 in Friston et al.,1994], our estimation of the effective P‐value for this conjoint requirement was <0.000005.
Age correlates.
Exploratory assessment of age correlates to supplement the ROI‐based analyses was conducted with a voxel‐wise regression analysis using the general linear model [Worsley et al.,1996]. The mean percent change in fMRI signal acquired during blocks of congruent and incongruent stimuli was calculated at each pixel for every subject [Peterson et al.,1999b]. To determine in which voxels age was correlated with Stroop‐related signal change for all subjects, these mean percent changes were entered as dependent variables in a model with subject age as the independent variable.
Quadratic and sex effects.
We included age, age2, and sex as covariates in another voxel‐wise regression analysis using the general linear model. The quadratic effect of age was assessed by the statistical significance of the age2 term. We then assessed the statistical significance of the age‐by‐sex interaction at each voxel and examined the nature of sex differences in age correlates in voxels where this interaction was significant by viewing separate maps of age correlations for the male and female subjects.
Behavioral correlates.
We conducted a voxel‐wise regression analysis correlating Stroop interference scores with task‐related fMRI signal change to identify brain regions where the magnitude of activation accounted for a significant portion of variance in behavioral performance.
RESULTS
Behavioral Performance
Regression analyses were carried out to assess the predictive value of age, full‐scale IQ (FSIQ), and sex on Stroop performance. The quadratic effect of age explained a significant proportion of the variance in Stroop interference scores (F [1,59] = 9.92, β = 0.75, P = 0.002; Fig. 1A) and in the Stroop task subtest scores (color naming: F [1,59] = 11.91, β = 0.03, P = 0.001; word reading: F [1,59] = 18.57, β = 0.03, P < 0.0001; and color‐word naming: F [1,59] = 12.34, β = 0.08, P < 0.001; Fig. 1B). Performance was poor among the youngest participants but improved (i.e., interference lessened) in the adolescents and remained stable in the younger and older adults. FSIQ and sex did not account for significant proportions of the variance in the interference scores (FSIQ: P = 0.95; sex: P = 0.08) or the scores from the three subtests (FSIQ: P > 0.1; sex: P > 0.1).
Figure 1.
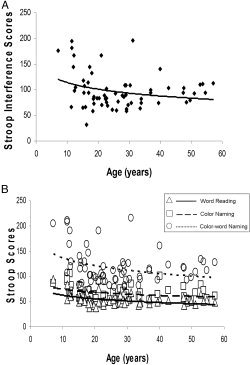
A: Interference scores plotted against age showing the curvilinear association with behavioral performance. B: Plots of correlations with subtest scores show that much of the variance in interference scores derives from the color‐word naming scores. Triangles, word reading; squares, color naming; circles, color‐word naming.
ROI Analyses
A priori hypothesis testing.
Omnibus testing with all ROIs revealed a significant ROI × age interaction (F [52,6918] = 1.47; P = 0.01). Because no significant quadratic effect of age was detected (ROI × age2 interaction; P = 0.80), the quadratic term was eliminated from the final model. Post‐hoc analyses to determine which brain regions contributed most to the ROI × age interaction identified three regions: right inferolateral prefrontal cortex (Brodmann area [BA] 46, P = 0.06), superior temporal gyrus bilaterally (BA 22, P < 0.01), and left subgenual anterior cingulate (BA 24/32, P < 0.01). A positive correlation of age with regional signal changes in the right inferolateral prefrontal cortex (BA 46; r = 0.30; P = 0.01) indicated that the magnitude of signal change increased with age in this region (Fig. 2A). Inverse linear correlations of age with regional signal changes in the left subgenual anterior cingulate (BA 24; r = −0.31; P < 0.01) and right superior temporal gyrus (BA 22, r = −0.29; P = 0.01) demonstrated Stroop‐related deactivations with age in these regions (Fig. 2B–C).
Figure 2.
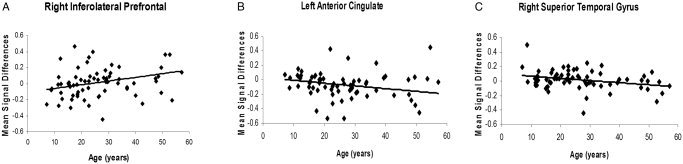
The region‐wise mean signal differences plotted against age in the ROIs corresponding to right inferolateral prefrontal cortex (BA 46; A), left anterior cingulate cortex (BA 24/32; B), and right superior temporal gyrus (BA 22; C).
A significant ROI × age × sex interaction (F [53,7021] = 2.64; P < 0.0001) indicated that the effect of age on Stroop‐related activation differed between males and females. Post‐hoc analyses of fixed effects identified the subgenual anterior cingulate cortex (BA 24/32) as the region contributing most to this interaction (P < 0.0001), suggesting that males, relative to females, demonstrated progressively more prominent deactivations with age in this brain region.
Behavioral correlates.
A positive association of regional signal change and interference scores in the posterior cingulate cortex ROI (BA 31, r = 0.49; P < 0.001) indicated that more activation in this region accompanied poorer performance.
Voxel‐Wise Analyses
Age correlates.
A map of age correlations is presented in Figure 3 (centers of activation are listed in Table II). Positive correlations of age with fMRI signal change were seen in the right inferolateral prefrontal cortex (BA 44/45, ztal = 0 and ztal = +9) and the right lenticular nucleus (ztal = +9), indicating that the magnitude of signal change in these areas increased with age. Inverse associations of age with signal change were detected in the right superior temporal gyrus (BA 22, ztal = 0, +9, and +18), left inferior frontal gyrus (BA 47, ztal = 0), mesial frontal cortex (BA 10/46, ztal = 0), posterior cingulate cortex (BA 31, ztal = +36), and the subgenual anterior cingulate cortex (BA 24, ztal = +9). These inverse correlations and examination of their scatterplots (Figs. 4 and 5) indicated that deactivation in these clusters of voxels strengthened with age.
Figure 3.
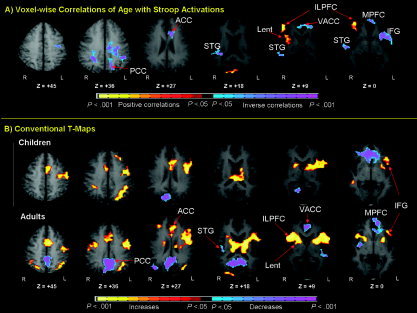
A: Voxel‐wise correlations of age with Stroop activations. These are transaxial slices positioned superiorly to inferiorly (left to right). Our estimation of the effective P‐value for the conjoint requirement of a statistical threshold and cluster filter was <0.000005 [Forman et al.,1995]. B: Group composite t‐maps for the percent fMRI signal change associated with the naming of colors in incongruent compared to congruent stimuli for children and adults. Increases in signal during the incongruent relative to congruent stimuli are coded in yellow, and decreases are coded in purple or blue. Right frontostriatal (inferolateral prefrontal cortex and lenticular nucleus) increases in activity associated with incongruent stimuli came on line progressively with age. The deactivations in mesial prefrontal cortex (MPFC), anterior cingulate cortex (ACC), and posterior cingulate cortex (PCC) indicate that greater activity in these regions was associated with processing the congruent stimuli in adults. ILPFC, inferolateral prefrontal cortex VACC, ventral anterior cingulate cortex; STG, superior temporal gyrus; Lent, lenticular nucleus; IFG, inferior frontal gyrus.
Table II.
Voxel‐Wise Correlations of Age with fMRI Activation
| ROI | Side | Slice | Talairach coordinates | BA | Correlation | P | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Mesial frontal cortex | L/R | 1 | 7 | 46 | 0 | 10 | −0.27 | 0.02 |
| Lateral frontal cortex | R | 1 | −36 | 39 | 0 | 44/45 | 0.33 | 0.006 |
| R | 2 | −36 | 39 | 9 | 46 | 0.37 | 0.001 | |
| Anterior cingulate | L/R | 2 | 10 | 19 | 9 | 24 | −0.24 | 0.04 |
| L | 4 | 10 | 19 | 27 | 32 | −0.29 | 0.01 | |
| Posterior cingulate | L | 5 | 21 | −16 | 36 | 31 | −0.27 | 0.02 |
| Inferior frontal gyrus | L | 1 | 36 | 13 | 0 | 47 | −0.32 | 0.007 |
| Superior temporal gyrus | R | 1 | −53 | −12 | 0 | 22 | −0.32 | 0.007 |
| R | 2 | −38 | −29 | 9 | 22/42 | −0.32 | 0.007 | |
| R | 3 | −38 | −29 | 18 | 22/42 | −0.32 | 0.007 | |
| Lenticular nucleus | R | 2 | −31 | 6 | 9 | NA | 0.27 | 0.02 |
Figure 4.
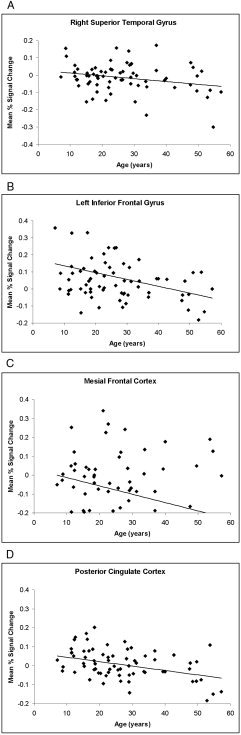
The mean percent signal change averaged across voxels in the right superior temporal gyrus (A; BA 22, Tal x, y, z = 36, 13, 0 mm), left inferior frontal gyrus (B; BA 47, Tal = 36,13,0), mesial frontal cortex (C; BA 10, Tal = 7, 46, 0), and posterior cingulate cortex (D; BA 31, Tal = 21, −16, 36) plotted against age showing that deactivation in these clusters of voxels increased progressively with age.
Figure 5.
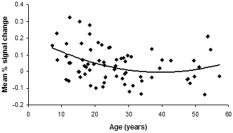
The mean percent signal change averaged across voxels in the subgenual anterior cingulate (BA 24, Tal x, y, z = 10, 19, 9 mm) plotted against age showing the curvilinear developmental trajectory of Stroop‐related activation.
Quadratic and sex effects.
Evidence of a quadratic effect for age was seen only in the subgenual anterior cingulate (P = 0.04, BA 24, ztal = +9), indicating that deactivation in this cluster of voxels increased through childhood and adolescence, but remained relatively stable thereafter through adulthood (Fig. 5). No evidence of a quadratic effect was seen in other clusters of voxels (P > 0.10). Maps viewed separately in male and female subjects revealed that, overall, males and females demonstrated similar patterns of age correlations with Stroop‐related activations, with the exception of the lenticular nucleus (Tal ztal = +9, maps not shown). As evidenced by a significant age × sex interaction (F [1,66]4 = 7.69; P = 0.007), the positive association of age with activation was carried predominantly by more signal change in these voxels with increasing age in female subjects (Males: r = −0.13; P = 0.45, females: r = 0.44; P = 0.007). This sex difference, however, was not detected in our ROI analyses, perhaps because the signal changes were distributed over a larger expanse within the ROI corresponding to the lenticular nucleus, thereby influencing the average task‐dependent signal change for that ROI. Similarly, our regional finding of a sex difference in deactivations with age in the anterior cingulate cortex (ACC) likely was not detected in these analyses because the deactivations were averaged over many voxels in our ROI analyses, thereby increasing the significance of the interaction.
Behavioral correlates.
Inverse associations of signal change with Stroop interference scores were observed in the inferolateral prefrontal cortex (BA 44/45, ztal = +18) and the right superior temporal gyrus (BA 22, ztal = +9; Fig. 6, Table III). An examination of scatterplots indicated that deactivation in these clusters of voxels accompanied poorer performance (Fig. 7). Positive correlations of signal change with interference scores, indicating more activation accompanying poorer performance, were detected in the mesial frontal (BA 10/46, ztal = 0) and the posterior cingulate (BA 23/31, ztal = +18 and ztal = +27) cortices.
Figure 6.
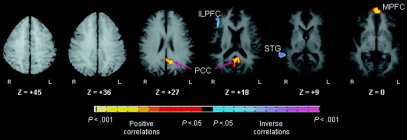
Voxel‐wise correlations of behavioral‐performance with Stroop‐related activations. These are axial slices positioned superiorly to inferiorly (left to right). PCC, posterior cingulate cortex; STG, superior temporal gyrus; ILPFC, inferolateral prefrontal cortex; MPFC, mesial prefrontal cortex
Table III.
Voxel‐Wise Correlations of Interference Scores with fMRI Activation
| ROI | Side | Slice | Talairach coordinates | BA | Correlation | P | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Mesial frontal cortex | L/R | 1 | 0.4 | 58 | 0 | 10/32 | 0.32 | 0.007 |
| Lateral frontal cortex | R | 3 | −39 | 34 | 18 | 46 | −0.27 | 0.02 |
| Posterior cingulate | L | 3 | 3 | −52 | 18 | 23/31 | 0.27 | 0.02 |
| L | 4 | 3 | −52 | 27 | 31 | 0.29 | 0.01 | |
| Superior temporal gyrus | R | 2 | −53 | −38 | 9 | 22/42 | −0.29 | 0.01 |
Figure 7.
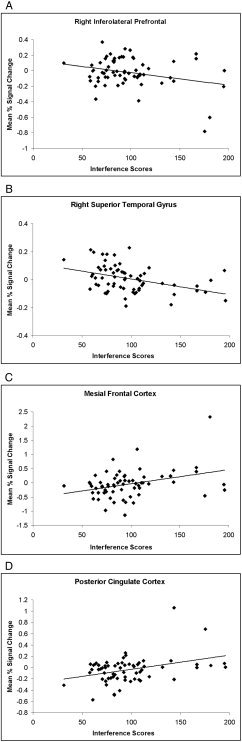
The mean percent signal change averaged across voxels in the right inferolateral prefrontal cortex (A; BA 46, Tal x, y, z = −39, 34, 18 mm) and right superior temporal gyrus (B; BA 22, Tal = −53, −38, 9) plotted against interference scores showing that deactivation in these clusters of voxels increased with poorer performance. The mean percent signal change averaged across voxels in mesial frontal cortex (C; BA 10, Tal = 4, 58, 0) and posterior cingulate cortex (D; BA 31, Tal = 3, −52, 27) plotted against interference scores showing that activation in these clusters of voxels increased with poorer performance.
DISCUSSION
Both Stroop‐related brain activations and behavioral performance correlated significantly with age. Imaging results revealed both positive and inverse age‐related signal changes in brain areas that mediate the cognitive resources necessary for optimal performance on the Stroop task. Increasing recruitment of frontostriatal systems in the right hemisphere with age (the right inferolateral prefrontal cortex [BA 44/45, ztal = +18] and right lenticular nucleus) generally corresponded with the regions that correlated with behavioral performance, indicating that the increasing engagement of frontostriatal systems with age supports an enhanced ability to engage self‐regulatory control in the service of improved task performance. Age‐related increases in deactivations in a system comprising the subgenual anterior cingulate (BA 24, ztal = +9), mesial prefrontal (BA 10/46, ztal = 0), and posterior cingulate (BA 23/31, ztal = +36) cortices were consistent with the observed improvement in task performance and presumably with the reduction of task difficulty in older subjects. These age‐related deactivations represented more activity in this system during the baseline task in adults, which we interpret as reflecting the freeing up of attentional resources and more active monitoring of internal states during the easier baseline task. Voxel‐wise findings of associations with age were in general consistent with findings of age‐related signal changes from regional significance testing. Taken together, our findings indicate the presence of normative changes in the maturation of neural circuits that subserve self‐regulatory control, a major feature of the Stroop Interference task.
Behavioral Performance
Consistent with findings from numerous prior studies, Stroop performance improved with age, likely reflecting the maturation of response inhibition [Comalli et al.,1962; Dash and Dash,1982; Schiller,1966]. Moreover, speed of performance on all three Stroop subtests (word reading, color naming, and color‐word naming) improved with age, primarily in the first two decades, indicating that the component cognitive processes required by the task mature with age as well. The improvement in word reading with age, particularly at earlier ages, likely reflects the development of reading automatism [Schiller,1966]; however, our findings do not suggest that more automatized reading skill was responsible for greater Stroop interference. Rather, much of the improvement in interference scores seemed to derive from performance on the color‐word naming subtest, which, compared with performance on the word reading and color naming subtests, improved less with age and exhibited more intersubject variability after childhood. In addition, performance improvement was not driven by outliers in age (Fig. 1). The curvilinear associations of age with performance on the Stroop subtests differ somewhat from the behavioral findings of a previous developmental study in children that showed a linear developmental trajectory on the Stroop task [Adleman et al.,2002]. Our sample, however, was considerably larger (n = 70 vs. n = 30) and included older subjects (oldest age: 57 vs. 23 years), well beyond the age in adulthood when performance scores stabilize (Fig. 1) and thus likely accounting for the curvilinear age effects that we detected in performance.
Age Correlates of Stroop‐Related Activations
Frontostriatal systems.
Both regional significance testing and voxel‐wise analyses revealed a positive association of age with Stroop activations in the right inferolateral prefrontal cortex, indicating that this finding was bona fide and not attributable to the misregistration of anatomical structures across individuals of differing ages in the voxel‐based analyses. Voxel‐wise analyses also revealed a positive association with age in the right lenticular nucleus. Scatterplots and activation maps furthermore indicated that frontostriatal activation was negligible in children and progressively came on‐line with increasing age in adolescents and adults (Fig. 3B). An inverse association of signal change with Stroop interference scores indicated that greater activation of the inferolateral cortex accompanied better performance (Fig. 6). Taken together, these findings suggest that frontostriatal activity increases both with age and with better self‐regulatory control, consistent with the known functions of frontostriatal circuits in subserving behavioral control in both health [Diamond,1988; Goldman‐Rakic,1987b] and illness [Peterson and Klein,1997; Peterson,2000; Rosenberg and Keshavan,1998]. Furthermore, these findings agree well with those of previous developmental imaging studies of response inhibition [Adleman et al.,2002; Casey et al.,1997a,c; Luna and Sweeney,2004; Luna et al.,2001; Tamm et al.,2002].
The Stroop task is known to require the recruitment of the prefrontal cortex for cognitive control in the face of interference from a competing prepotent, more salient, or more habitual behavioral response [Miller and Cohen,2001]. Patients with prefrontal impairments perform poorly on the task [Vendrell et al.,1995], suggesting that they have trouble engaging the cognitive control necessary to adhere to the task instructions and thereby to inhibit the task‐irrelevant (reading) response. In addition, the inferior prefrontal cortex has been consistently implicated in response inhibition on the go/no‐go task in several other studies [Aron et al.,2004; Booth et al.,2003; Garavan et al.,2002; Rubia et al.,2003; Tamm et al.,2002]. Our findings, however, indicate that age‐related improvements in Stroop performance may be attributable specifically to increasing activation with age in the inferolateral portion of the prefrontal cortex. Both children and adults recruited prefrontal regions, but only adults recruited the lateral prefrontal cortex, perhaps accounting for the observed improvement in self‐regulatory control with more advanced CNS maturation.
Evidence from studies of nonhuman primates suggests that inhibitory functions are hierarchically distributed across differing regions of the prefrontal cortex, with the lateral prefrontal portion mediating a higher‐order cognitive process that shifts attention across multiple perceptual dimensions [Dias et al.,1997; Wallis et al.,2001]. Damage to lateral prefrontal cortex thus causes a loss of inhibitory control in selective attention [Dias et al.,1997], producing behavioral disorganization and difficulties in implementing or flexibly switching between rules and response sets [Milner,1995; Milner and Petrides,1984]. The orbital frontal cortex, in contrast, subserves the lower‐order rule learning of simple associations within a single perceptual domain and the learning of associations between a stimulus and its reward [Dias et al.,1997; Wallis et al.,2001]. Indeed, the abnormal processing of stimulus‐reward associations is thought to mediate the effects of orbitofrontal damage in producing emotional disturbances and behavioral dysregulation [Blumberg et al.,2003a; Rolls et al.,1994]. Differing portions of the prefrontal cortex thus subserve differing inhibitory control processes [Fuster,1985,1997; Goldman‐Rakic,1987a; Iverson and Mishkin,1970; Owen et al.,1990]. Because the Stroop task presents information in two different perceptual dimensions (words and colors) and requires inhibiting information from one of those dimensions to attend to the other, correct responding (naming the color) necessitates the specific engagement of lateral prefrontal cortices. This suggestion is consistent with findings from a previous Stroop study that supported a specific role of the dorsal lateral prefrontal cortex in the representation and active maintenance of the attentional demands of the task [MacDonald et al.,2000]. Perhaps younger individuals are less able to disengage from one perceptual dimension to attend to the other, as indicated by their poorer performance on the task and failure to activate this region. This interpretation is consistent with our finding that this region comes on line during development, with evidence that the lateral prefrontal region develops later than other prefrontal regions, and with the later development of the higher‐order, integrative cognitive functions that this region subserves [Fuster,2002].
Our finding of increased activation with age in the lateral prefrontal cortex was confined statistically to the right hemisphere. A previous developmental study of the Stroop task also detected increased activation with age in the lateral prefrontal cortex, but that finding was restricted to the left hemisphere [Adleman et al.,2002]. Nevertheless, our finding of right‐hemisphere dominance of inhibitory control is consistent with a study of response inhibition on the go/no‐go task [Garavan et al.,1999] as well as with studies supporting the existence of a right hemisphere attentional system for vigilance [Pardo et al.,1991; Peterson et al.,1999a; Posner and Petersen,1990]. Vigilance alerts the subject and focuses sustained attention to subtle sensory signals within a sensory modality; thus, vigilance is required to respond rapidly while minimizing errors on incongruent trials of the Stroop task [Peterson et al.,1999b]. In addition, lesions in the right lateral prefrontal cortex have been correlated with increased errors on the Stroop, whereas patients with left frontal lesions exhibit normal performance [Vendrell et al.,1995]. Converging evidence thus suggests that the inhibitory control and attentional processes required by the Stroop task may be mediated more by the right than by the left prefrontal cortex.
In the left hemisphere, we found decreasing engagement of inferior prefrontal systems with age. A comparison of t‐maps for children and adults showed the presence of more activation in the left inferior frontal gyrus (BA 46/47) during the incongruent task in children (Fig. 2A, z = 0). This finding may reflect a developmental change in the expressive language‐based strategy used in the task, given that the inferior frontal gyrus has been implicated as a classical language area: its anterior portion (Broca's area) is a speech motor area involved in speech motor planning, speech production, and articulation [Nishitani et al.,2005]. Because children are relatively inexperienced readers compared with adults, perhaps they were parsing the word stimuli more prominently during the active task and preparing to articulate, contributing to their weaker ability to inhibit word reading and poorer performance on the task. Likewise, the decreased engagement of the left inferior frontal gyrus in adults may indicate that, in contrast to children, they were not as effortfully attempting to parse or preparing to produce the written words, contributing to their stronger ability to inhibit word reading and better task performance. The minimal variance in word reading speed across ages, however, suggests that the reading inexperience in the children was not responsible for their poorer performance.
Some studies that have employed the go/no‐go task to measure response inhibition have reported more diffuse task‐related activation of prefrontal regions in children than in adults [Booth et al.,2003; Casey et al.,1997b,c; Durston et al.,2002]. Examination of separate group activation maps for the children and adults in our sample, however, did not suggest a greater diffusiveness in activation of prefrontal cortex in children, or in any other brain region, for that matter (Fig. 3B). Moreover, examination of individual activation maps confirmed the absence of a more diffuse activation of prefrontal cortices in children (maps not shown). Although comparisons of findings from Stroop and go/no‐go studies are useful because both paradigms assess response inhibition, such comparisons are limited by the fundamental differences between the tasks. In the go/no‐go studies, for example, simple visual stimuli are presented (e.g., letters, cartoon characters, simple shapes) and subjects are instructed to respond on most trials (the “go” trials) but to inhibit response to the appearance of a rare non‐target (e.g., the letter “X,” a specific cartoon character or shape) in the “no‐go” trials. The Stroop task, in contrast, requires subjects to inhibit on incongruent trials the reading of written words that have a clear semantic content, a very different response to be inhibited. Comparisons across studies may also be limited by differences in task design (e.g., block or event‐related), differences in the control tasks, to the performance measures used in the analyses (e.g., the number of errors or misses on go or no/go trials), or to the different strategies that may be invoked by adults and children to perform differing tasks. These considerations likely contribute to discrepancies between our findings and those of certain developmental go/no‐go studies [Casey et al.,1997c; Durston et al.,2002].
Subgenual anterior cingulate, mesial frontal, and posterior cingulate cortices.
Regional significance testing and voxel‐based analyses revealed that age was inversely associated with activations in the subgenual anterior cingulate (BA 24, ztal = +9). Voxel‐based analyses also showed inverse associations with age in the inferior mesial prefrontal frontal (BA 10/46, ztal = 0) and posterior cingulate (BA 31, ztal = +36) cortices (Fig. 3). These general patterns of activation are consistent with findings from previous fMRI Stroop studies in adults [Leung et al.,2000; Mead et al.,2002; Peterson et al.,1999a]. Graphical representation indicated that the subgenual anterior cingulate (BA 24, ztal = +9), inferior mesial prefrontal (BA 10/46, ztal = 0) and posterior cingulate (BA 31, ztal = +36) cortices deactivated progressively with age (Figs. 4 and 5). In other words, neural activity associated with performance of the baseline task (the congruent stimuli) was greater than the activity associated with performance of the active task (the incongruent stimuli) in adults, but activity across the two task conditions was relatively similar in children. The greater activity of these regions during performance of the baseline task in adults can be understood in terms of their greater ease in responding to congruent stimuli. Better performance increased with age on each subtest of the Stroop task (word reading, color naming, and color‐word naming), indicating that all of these individual tasks are easier and more automatic for adults. The more automatized baseline task (the naming of colors in congruent colored word stimuli) thus was likely easier for adults as well. Their greater ease may have freed up sufficient attentional resources to permit the adult subjects to attend more to task‐irrelevant thoughts during scanning or freedom to monitor internal state, which has been shown to activate these regions most reliably [Greicius et al.,2003; Greicius and Menon,2004; Gusnard and Raichle,2001; McGuire et al.,1996; Shulman et al.,1997].
The mesial prefrontal, anterior cingulate, and posterior cingulate cortices are all anatomically and functionally closely interrelated. Histological evidence suggests that the mesial areas within frontal lobe cytoarchitecture are extensively interconnected [Barbas and Pandya,1989; Goldman‐Rakic,1987b], making continuous demarcation of the mesial prefrontal (MPFC) and subgenual anterior cingulate cortices (ACC) difficult. The continuity between these frontal areas may have contributed to the increased spatial extent of deactivations in this system in older individuals. The deactivations extending to the MPFC accompanied better performance and were only evident in adults (Figs. 7C and 3B, ztal = 0). In addition to their histological continuity, the MPFC and subgenual ACC also seem to be functionally continuous, with both areas implicated in the monitoring of emotional state. Limbic connections explain the role of the subgenual ACC in affective regulation and expression [Carmichael and Price,1995; Devinsky et al.,1995], and studies have shown that this specific area activates when subjects attend to their emotions [Lane et al.,1997,1998]. Similarly, studies using mentalizing tasks have shown that MPFC activation is associated with attention to emotional states of the self and of others [Frith and Frith,2003; Schultz et al.,2003].
The adults in our study likely activated these interconnected mesial frontal regions during the easier baseline task because their attentional capacities were freed up compared with children, giving them ample opportunity to monitor their thoughts and emotions during the scan. Children, in contrast, likely had to employ attentional resources to handle the demands of both the baseline and active tasks, contributing in children to the similarity in ACC activity across these conditions (Fig. 3B). The similarity in activity may also reflect a similarity in emotional arousal that the children experienced during cognitive processing of each of the tasks, which in turn may be a consequence of their relative immaturity in capacity for self‐regulatory control of emotions [Calkins,1994; Levesque et al.,2004; Posner and Rothbart,2000].
As in the MPFC and subgenual ACC, deactivations in the posterior cingulate cortex (PCC, BA 31) indicated that activity associated with performance of the baseline task was greater than the activity associated with performance of the active task. These deactivations were present in both children and adults, but were more prominent in the adults (Fig. 3, ztal = +36 and ztal = +27) and covered a larger spatial expanse. In addition, reduced deactivation in the PCC accompanied poorer performance, consistent with the age‐related improvements on the task. The PCC has been associated with a variety of brain functions, including evaluative functions for spatial orientation and memory [Vogt et al.,1992], successful episodic memory retrieval [Cabeza and Nyberg,2000], emotion [Maddock et al.,2003], and activity in the baseline task related to free associative thinking during the “resting” brain state [Shulman et al.,1997; Binder et al., 1999; Mazoyer et al., 2001; Raichle et al., 2001]. In addition, a previous neuroimaging study of the Stroop task in adults attributed deactivations in the PCC during the incongruent task to the undemanding nature of the control tasks, similar to that of a resting state [Mead et al.,2002]. Likewise, the greater deactivations in the PCC in the adults of our study presumably reflected their relatively greater ease in performing the baseline task, consistent with our finding that greater deactivations accompanied better task performance (Fig. 7D). Taken together, our findings indicate that the MPFC, subgenual ACC, and PCC comprise a neural system that is activated in a “default mode” by adults when they are unchallenged, likely reflecting their ongoing and unconstrained, free associative processing of thoughts and emotions [Greicius et al.,2003; Greicius and Menon,2004; Gusnard et al.,2001].
Superior temporal gyrus.
Both regional significance testing and voxel‐wise analyses demonstrated that Stroop‐related deactivations in the superior temporal gyrus (BA 22) came on line progressively in older individuals (Figs. 2C and 4A). Activity associated with performance of the baseline task (the naming of congruent word‐color stimuli) thus was greater than the activity associated with performance of the active task (the naming of incongruent colored stimuli) in adults, but activity was similar across these same task conditions in children. The superior temporal gyrus is a component of posterior language and higher order visual processing systems [Kandel et al.,1991]. Perhaps older individuals were mobilizing the higher‐order processing resources subserved by this area during the baseline task in an attempt to encode visually or verbally the colors of the congruent color‐word stimuli. Alternatively, their engagement of these areas may represent anticipation or gating out of the irrelevant written word stimuli on subsequent incongruent trials. The adults in our study were likely better than children at anticipating upcoming trials and preparing to inhibit the task‐irrelevant reading response, consistent with their better behavioral performance on the Stroop task outside of the scanner.
Strengths and Limitations
Our study of 70 subjects is to our knowledge the largest developmental fMRI study of self‐regulatory control to date. All previous developmental fMRI investigations of the Stroop Interference task [Adleman et al.,2002], go/no‐go tasks [Booth et al.,2003,2004; Bunge et al.,2002; Casey et al.,1997b; Durston et al.,2002], and the Stop paradigm [Rubia et al.,2001] have studied considerably smaller samples (<32). Our findings therefore contribute statistical robustness in a large and rigorously characterized sample to a growing literature on the normative development of self‐regulatory processes. Age correlates in task‐related brain activation that are reported in previous studies and ours, however, may reflect the use of differing strategies across ages rather than the maturation of self‐regulatory processes. This is a common limitation of developmental studies of cognitive functions, presumably addressed through use of performance matching and manipulation of task difficulty on go/no‐go tasks [Casey et al.,1997c; Diamond,2002; Durston et al.,2002]. Task performance improves with age, however, and we do not know whether the use of differing strategies contributes to this improvement or by extension, to age‐related differences in task‐related activation. Moreover, we did not assess or compare the differential use of strategies in our child and adult subjects. We additionally note that because the behavioral measures were conducted after the scanning session, we cannot exclude the possibility that practice and habituation effects from the scanning session may have differentially affected measures of behavioral performance across subjects of differing ages.
One strength of our study was the use of ROI‐based analyses to test our a priori hypotheses with the inclusion of exploratory voxel‐wise analyses. Voxel‐wise approaches are limited by the unproven assumption that the anatomy and corresponding localization of function is the same across individuals of differing ages [Peterson,2003]. Structural brain imaging studies, however, show that gray and white matter density change throughout the human lifespan [Paus et al.,1999; Sowell et al.,2003,2004], and postmortem studies have demonstrated that changes in myelination and synaptic density are prominent through child and adolescent development [Huttenlocher et al.,1982; Yakovlev and Lecours,1967]. These known age‐specific maturational processes and differences in associated cytoarchitecture of the CNS may cause errors in the registration of tissues across individuals of differing ages during the spatial normalization procedures required in voxel‐wise analyses, even in the presence of spatial smoothing. ROI‐based analyses are thought to be less vulnerable to this kind of artifact [Peterson,2003].
ROI‐based analyses, however, assume that anatomical and functional ROIs can be defined precisely, when in fact a single anatomically defined region may contain many cytoarchitectonic and functional units [Huettel et al.,2003]. Because each ROI combines data from multiple voxels, thus the signal‐to‐noise ratio will increase if the ROI is functionally homogeneous, and it will decrease if the ROI is functionally heterogeneous (e.g., if a given ROI includes both active and inactive cytoarchitectonic units). The predefined ROIs used in our ROI‐based analyses were larger and comprised many more voxels than did the corresponding brain areas in which we observed voxel‐wise associations with Stroop‐related activations. Averaging signal changes across all voxels in a given ROI, including voxels that did not activate, undoubtedly influenced the overall average of task‐dependent signal change for that ROI. Our use of larger, pre‐defined ROIs may explain why associations that were captured in exploratory voxel‐wise analyses either were not detected in regional significance testing (e.g., the inverse association of age with signal change in posterior cingulate cortices [BA 31, ztal = +36]) or were only marginally significant (e.g., the positive association of interference scores with signal change in the lateral prefrontal cortex [BA 45/46, ztal = +18]). Moreover, the averaging of signal across voxels presumably contributed to some regional findings that were not detected in our voxel‐wise analyses, e.g., the sex difference in age correlations in the subgenual ACC (BA 24/32). This sex difference was not detected in our voxel‐wise analyses, perhaps because the deactivations were distributed over a considerable expanse within the ROI, thereby increasing signal to noise characteristics within the ROI compared with individual voxels. The effect of signal averaging should therefore be taken into account when interpreting the results of our ROI‐based analyses. In addition, although we regard the findings from our exploratory voxel‐wise analyses as hypothesis‐generating and therefore as preliminary and in need of replication, we believe that our interpretations are much aided by their inclusion.
An additional limitation of this study was the use of a subvocal response to the Stroop stimuli. We chose to use subvocalization because overt speech can cause significant fMRI signal artifacts [Barch et al.,1999]. A subvocal response, however, precluded the online measurement of task performance and task compliance during scanning. Nonetheless, Stroop studies that have used overt and covert verbal responses have demonstrated similar measures of cognitive load and patterns of fMRI activation when they have been compared directly with one another [Brown et al.,1999; Laird et al.,2005; Peterson et al.,2002]. In addition, a subvocal response has been used in numerous other fMRI studies of the Stroop task in both adults [Blumberg et al.,2003a,2003c; Mead et al.,2002; Peterson et al.,1999a, 1999b,2002] and children [Adleman et al.,2002]. Finally, use of a manual response such as a button press would introduce a complex mapping function of color names to a pattern of finger response that would fundamentally change the nature of the task, and in a way that could interact profoundly with age. Given these various considerations, we regarded the subvocal response to be the most appropriate for this developmental fMRI study of the Stroop effect.
Conclusions
Our behavioral and neuroimaging findings suggest that the normative maturation of brain function, particularly within frontostriatal circuits, drives the development of self‐regulatory capacities that are required for optimal performance on the Stroop task. Our findings indicate that age‐related improvements in task performance were, in part, attributable to increasing activation in frontostriatal systems, including the lateral prefrontal cortex. This interpretation is consistent with the later development of the lateral prefrontal cortex and the higher‐order cognitive processes it subserves. In addition, age‐related deactivations in a system comprising the MPFC, subgenual ACC, and PCC likely represent the greater ease of adults in performing the baseline control task, thus contributing to the activation of this system in adults when they are unchallenged and the reduced attentional demands permit greater ongoing and unconstrained free associative processing of thoughts and emotions. Children, in contrast, presumably needed to mobilize considerable attentional resources during both the baseline and active tasks, thereby allowing them less freedom to process thoughts and emotions that are not central to the task. Deactivations in superior temporal cortices likely represent optimization in adults of the resources needed for processing of the visual and verbal aspects of the task stimuli that is mediated by these areas, and are consistent with the reduction of task difficulty in older individuals.
Self‐regulatory control of impulses that lead to socially or environmentally inappropriate actions is fundamental for successfully navigating everyday life. Maturation of self‐regulatory control may in fact be regarded as defining the ontogeny of human development, and disturbances in the maturation of these functions may contribute to the development of disordered impulse control in various childhood‐onset neuropsychiatric disorders [Spessot et al.,2004]. Future investigations should compare the developmental trajectory of brain activity during performance of the Stroop task across these various diagnostic conditions to further our understanding of this maturational process itself, the neuroanatomical systems that subserve it, and the ways in which these systems can be derailed during development.
REFERENCES
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, Reiss AL (2002): A developmental fMRI study of the Stroop color‐word task. Neuroimage 16: 61–75. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA (2004): Inhibition and the right inferior frontal cortex. Trends Cogn Sci 8: 170–177. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN (1989): Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol 286: 353–375. [DOI] [PubMed] [Google Scholar]
- Barch DM, Sabb FW, Carter CS, Braver TS, Noll DC, Cohen JD (1999): Overt verbal responding during fMRI scanning: empirical investigations of problems and potential solutions. Neuroimage 10: 642–657. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Leung HC, Skudlarski P, Lacadie CM, Fredericks CA, Harris BC, Charney DS, Gore JC, Krystal JH, Peterson BS (2003a): A functional magnetic resonance imaging study of bipolar disorder: state‐ and trait‐related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry 60: 601–609. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Martin A, Kaufman J, Leung HC, Skudlarski P, Lacadie C, Fulbright RK, Gore JC, Charney DS, Krystal JH and others (2003b): Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations from functional MRI. Am J Psychiatry 160: 1345–1347. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Martin A, Kaufman J, Leung HC, Skudlarski P, Lacadie C, Fulbright RK, Gore JC, Charney DS, Krystal JH and others (2003c): Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations from functional MRI. Am J Psychiatry 160: 1345–1347. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, Li W, Parrish TB, Gitelman DR, Mesulam MM (2003): Neural development of selective attention and response inhibition. Neuroimage 20: 737–751. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Trommer BL, Davenport ND, Parrish TB, Gitelman DR, Mesulam MM (2004): Brain–behavior correlation in children depends on the neurocognitive network. Hum Brain Mapp 23: 99–s24 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GG, Kindermann SS, Siegle GJ, Granholm E, Wong EC, Buxton RB (1999): Brain activation and pupil response during covert performance of the Stroop Color Word task. J Int Neuropsychol Soc 5: 308–319. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD (2002): Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron 33: 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L (2000): Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12: 1–47. [DOI] [PubMed] [Google Scholar]
- Calkins SD. 1994. Origins and outcomes of individual differences in emotion regulation In: Fox NA, editor. The development of emotion regulation (Monographs of the Society for Research in Child Development). p 53–72. [PubMed] [Google Scholar]
- Carmichael ST, Price JL (1995): Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol 363: 615–641. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD (2000): Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci USA 97: 1944–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Mintun M, Cohen JD (1995): Interference and facilitation effects during selective attention: an H2 15O PET study of Stroop task performance. Neuroimage 2: 264–272. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, Vauss YC, Vaituzis AC, Dickstein DP, Sarfatti SE, Rapoport JL (1997a): Implication of right frontostriatal circuitry in response inhibition and attention‐deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 36: 374–383. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor R, Giedd J, Vauss Y, Vaituzis CK, Hamburger S, Kozuch P, Rapoport JL (1997b): The role of the anterior cingulate in automatic and controlled processes: a developmental neuroanatomical study. Dev Psychobiol 30: 61–69. [PubMed] [Google Scholar]
- Casey BJ, Trainor R, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, Castellanos FX, Haxby JN, Noll DC, Cohen JN and others (1997c): A developmental functional MRI study of prefrontal activation during performance of a go/no‐go task. J Cogn Neurosci 9: 835–847. [DOI] [PubMed] [Google Scholar]
- Comalli PE Jr, Wapner S, Werner H (1962): Interference effects of Stroop color‐word test in childhood, adulthood, and aging. J Genet Psychol 100: 47–53. [DOI] [PubMed] [Google Scholar]
- Dash X, Dash AS (1982): Cognitive developmental studies of the Stroop phenomena: cross‐sectional and longitudinal data. Ind Psychol 1 24–33. [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA (1995): Contributions of anterior cingulate cortex to behavior. Brain 118: 279–306. [DOI] [PubMed] [Google Scholar]
- Diamond A (1988): Abilities and neural mechanisms underlying AB performance. Child Dev 59: 523–527. [PubMed] [Google Scholar]
- Diamond A. 2002. Normal development of prefrontal cortex from birth to young adulthood: Cognitive functions, anatomy, and biochemistry In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. London, UK: Oxford University Press. [Google Scholar]
- Dias R, Robbins TW, Roberts AC (1997): Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort Test: restriction to novel situations and independence from “on‐line” processing. J Neurosci 17: 9285–9297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Yang Y, Ulug AZ, Zimmerman RD, Casey BJ (2002): A neural basis for the development of inhibitory control. Dev Sci 5: 9–16. [Google Scholar]
- Endicott J, Spitzer RL (1978): A diagnostic interview: the Schedule for Affective Disorders and Schizophrenia. Arch Gen Psychiatry 35: 837–844. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC (1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster‐size threshold. Magn Reson Med 33: 636–647. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ (1995): Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC (1994): Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp 1: 214–220. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD (2003): Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci 358: 459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM (1985): The prefrontal cortex, mediator of cross‐temporal contingencies. Hum Neurobiol 4: 169–179. [PubMed] [Google Scholar]
- Fuster JM. 1997. The prefrontal cortex: anatomy, physiology, and neuropsychology of the frontal lobe. Philadelphia: Lippincott‐Raven. [Google Scholar]
- Fuster JM (2002): Frontal lobe and cognitive development. J Neurocytol 31: 373–385. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA (2002): Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage 17: 1820–18209. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA (1999): Right hemispheric dominance of inhibitory control: an event‐related functional MRI study. Proc Natl Acad Sci USA 96: 8301–8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden CJ. 1978. Stroop color and word test: a manual for clinical and experimental uses. Wood Dale, IL: Stoelting. [Google Scholar]
- Goldman‐Rakic P. 1987a. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory In: Geiger S, editor. Handbook of physiology. The nervous system. Bethesda, Maryland: American Physiological Society; p 373–416. [Google Scholar]
- Goldman‐Rakic P. 1987b. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory In: Mountcastle V, Plum F, Geiger S, editors. Handbook of physiology. The nervous system. Bethesda, Maryland: American Physiological Society; p 373–416. [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V (2003): Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Menon V (2004): Default‐mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J Cogn Neurosci 16: 1484–1492. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME (2001): Medial prefrontal cortex and self‐referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA 98: 4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME (2001): Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2: 685–694. [DOI] [PubMed] [Google Scholar]
- Hair JFJ, Anderson RE, Tatham RL, Black WC. 1992. Multivariate analysis of variance In: Easter F, editor. Multivariate data analysis. Ontario: Macmillan Publishing Co. p 153–181. [Google Scholar]
- Hollingshead AB. 1975. Four‐factor index of social status. New Haven, CT: Yale University Press. [Google Scholar]
- Huettel S, Song AW, McCarthy G. 2003. Functional magnetic resonance imaging. Sutherland, MA: Sinauer Associates, Inc. [Google Scholar]
- Huttenlocher PR, De Courten C, Garey LJ, Van der Loos H (1982): Synaptic development in human cerebral cortex. Int J Neurol 16–17: 144–154. [PubMed] [Google Scholar]
- Iverson S, Mishkin M (1970): Perseverative interference in monkeys following selective lesions of the inferior prefrontal cortex. Exp Brain Res. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM, editors. 1991. Principles of neural science (3rd ed.). East Norwalk, CT: Appleton and Lange. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N (1997): The Schedule for Affective Disorders and Schizophrenia for School Aged Children: Present and Lifetime Version (K‐SADS‐PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36: 980–988. [DOI] [PubMed] [Google Scholar]
- Laird AR, McMillan KM, Lancaster JL, Kochunov P, Turkeltaub PE, Pardo JV, Fox PE (2005): A comparison of label‐based review and ALE meta‐analysis in the Stroop task. Hum Brain Mapp 25: 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD, Fink GR, Chau PM, Dolan RJ (1997): Neural activation during selective attention to subjective emotional responses. Neuroreport 8: 3969–3972. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Axelrod B, Yun LS, Holmes A, Schwartz GE (1998): Neural correlates of levels of emotional awareness. Evidence of an interaction between emotion and attention in the anterior cingulate cortex. J Cogn Neurosci 10: 525–535. [DOI] [PubMed] [Google Scholar]
- Leung HC, Skudlarski P, Gatenby JC, Peterson BS, Gore JC (2000): An event‐related functional MRI study of the stroop color word interference task. Cereb Cortex 10: 552–560. [DOI] [PubMed] [Google Scholar]
- Levesque J, Joanette Y, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, Beauregard M (2004): Neural basis of emotional self‐regulation in childhood. Neuroscience 129: 361–369. [DOI] [PubMed] [Google Scholar]
- Lezak MD. 1995. Neuropsychological assessment (3rd ed.). New York: Oxford University Press. [Google Scholar]
- Luna B, Sweeney JA (2004): The emergence of collaborative brain function: FMRI studies of the development of response inhibition. Ann N Y Acad Sci 1021: 296–309. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA (2001): Maturation of widely distributed brain function subserves cognitive development. Neuroimage 13: 786–793. [DOI] [PubMed] [Google Scholar]
- MacDonald AW 3rd, Cohen JD, Stenger VA, Carter CS (2000): Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288: 1835–1838. [DOI] [PubMed] [Google Scholar]
- MacLeod CM (1991): Half a century of research on the Stroop effect: an integrative review. Psychol Bull 109: 163–203. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH (2003): Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum Brain Mapp 18: 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire PK, Paulesu E, Frackowiak RS, Frith CD (1996): Brain activity during stimulus independent thought. Neuroreport 7: 2095–2099. [PubMed] [Google Scholar]
- Mead LA, Mayer AR, Bobholz JA, Woodley SJ, Cunningham JM, Hammeke TA, Rao SM (2002): Neural basis of the Stroop interference task: response competition or selective attention? J Int Neuropsychol Soc 8: 735–742. [DOI] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, Cohen NJ (2002): Attentional control in the aging brain: insights from an fMRI study of the stroop task. Brain Cogn 49: 277–296. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD (2001): An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202. [DOI] [PubMed] [Google Scholar]
- Milner B. 1995. Aspects of human frontal lobe function In: Goldman‐Rakic P, editor. Epilepsy and the functional anatomy of the frontal lobe. New York: Raven Press; p 67–84. [Google Scholar]
- Milner B, Petrides M (1984): Behavioral effects of frontal‐lobe lesions in man. Trends Neurosci November: 403–407. [Google Scholar]
- Morrell CH, Pearson JD, Brant LJ (1997): Linear transformations of linear mixed‐effects models. Am Statistician 51: 338–343. [Google Scholar]
- Nishitani N, Schurmann M, Amunts K, Hari R (2005): Broca's region: from action to language. Physiology (Bethesda) 20: 60–69. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW (1990): Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia 28: 1021–1034. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Fox PT, Raichle ME (1991): Localization of a human system for sustained attention by positron emission tomography. Nature 349: 61–64. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Pardo PJ, Janer KW, Raichle ME (1990): The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc Natl Acad Sci USA 87: 256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC (1999): Structural maturation of neural pathways in children and adolescents: in vivo study. Science 283: 1908–1911. [DOI] [PubMed] [Google Scholar]
- Peterson B, Klein J. 1997. Neuroimaging of Tourette's syndrome neurobiologic substrate In: Peterson BS, editor. Child psychiatry clinics of North America: Neuroimaging. Philadelphia, PN: W.B. Saunders; p 343–364. [Google Scholar]
- Peterson BS. 2000. Neuroimaging studies of Tourette Syndrome. A decade of progress In: Cohen DJ, Goetz CG, Jankovic J, editors. Advances in neurology. Tourette Syndrome and Associated Disorders. Philadelphia: Lippincott Williams & Wilkins; p 179–196. [PubMed] [Google Scholar]
- Peterson BS (2003): Conceptual, methodological, and statistical challenges in brain imaging studies of developmentally based psychopathologies. Dev Psychopathol 15: 811–832. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Kane MJ, Alexander GM, Lacadie C, Skudlarski P, Leung HC, May J, Gore JC (2002): An event‐related functional MRI study comparing interference effects in the Simon and Stroop tasks. Brain Res Cogn Brain Res 13: 427–s24 440. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Gatenby JC, Zhang H, Anderson AW, Gore JC (1999a): An fMRI study of Stroop word‐color interference: evidence for cingulate subregions subserving multiple distributed attentional systems. Biol Psychiatry 45: 1237–1258. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Zhang H, Gatenby JC, Anderson AW, Gore JC (1999b): An fMRI study of Stroop Word‐Color Interference: evidence for cingulate subregions subserving multiple distributed attentional systems. Biol Psychiatry 45: 1237–1258. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE (1990): The attention system of the human brain. Annu Rev Neurosci 13: 25–42. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK (2000): Developing mechanisms of self‐regulation. Dev Psychopathol 12: 427–41. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Leung H‐C, Blumberg HP, Peterson BS, Fulbright RK, Lacadie CM, Gore JC (in press): An fMRI Stroop study of pathological gamblers. Am J Psychiatry. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J (1994): Emotion‐related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatry 57: 1518–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg DR, Keshavan MS (1998): Toward a neurodevelopmental model of obsessive‐compulsive disorder. Biol Psychiatry 43: 623–640. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, Simmons A, Williams SC, Giampietro V, Andrew CM, Taylor E (2001): Mapping motor inhibition: conjunctive brain activations across different versions of go/no‐go and stop tasks. Neuroimage 13: 250–261. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E (2003): Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage 20: 351–358. [DOI] [PubMed] [Google Scholar]
- Schiller PH (1966): Developmental study of color‐word interference. J Exp Psychol 72: 105–108. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Grelotti DJ, Klin A, Kleinman J, Van der Gaag C, Marois R, Skudlarski P (2003): The role of the fusiform face area in social cognition: implications for the pathobiology of autism. Philos Trans R Soc Lond B Biol Sci 358: 415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE (1997): Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci 9: 648–663. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW (2003): Mapping cortical change across the human life span. Nat Neurosci 6: 309–315. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Toga AW (2004): Mapping changes in the human cortex throughout the span of life. Neuroscientist 10: 372–392. [DOI] [PubMed] [Google Scholar]
- Spessot AL, Plessen KJ, Peterson BS (2004): Neuroimaging of developmental psychopathologies: the importance of self‐regulatory and neuroplastic processes in adolescence. Ann N Y Acad Sci 1021: 86–104. [DOI] [PubMed] [Google Scholar]
- Stroop JR (1935): Studies of interference in serial verbal reactions. J Exp Psychology 18: 643–662. [Google Scholar]
- Talairach J, Tournoux P. 1988. Co‐planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers. [Google Scholar]
- Tamm L, Menon V, Reiss AL (2002): Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry 41: 1231–1238. [DOI] [PubMed] [Google Scholar]
- Vendrell P, Junque C, Pujol J, Jurado MA, Molet J, Grafman J (1995): The role of prefrontal regions in the Stroop task. Neuropsychologia 33: 341–352. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR (1992): Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex 2: 435–443. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Dias R, Robbins TW, Roberts AC (2001): Dissociable contributions of the orbitofrontal and lateral prefrontal cortex of the marmoset to performance on a detour reaching task. Eur J Neurosci 13: 1797–1808. [DOI] [PubMed] [Google Scholar]
- Wechsler D. 1981. WAIS‐R Manual. Wechsler Adult Intelligence Scale‐Revised. San Antonio, TX: The Psychological Corporation. Harcourt Brace Jovanovich, Inc. [Google Scholar]
- Worsley K, Marret S, Neelin P, Vandal K, Friston K, Evans A (1996): A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 4: 58–73. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. 1967. The myelogenetic cycles of regional maturation of the brain In: Minkowski A, editor. Regional development of the brain in early life. Oxford: Blackwell Scientific Publications; p 3–70.4 [Google Scholar]


