Introduction
Exaggerated cardiovascular response during exposure to acute laboratory stressors has been postulated to be a risk factor for the development of hypertension (Manuck et al., 1990; Pickering & Gerin, 1990). Historically, research has focused on cardiovascular reactivity, defined as the magnitude of cardiovascular arousal occurring during acute stress exposure (Linden et al., 1997). Repeated elevations in blood pressures (BP) are believed to favor pathogenic adaptations, such as left ventricular remodeling and reduction in endothelial integrity (Lovallo & Gerin, 2003; Manuck et al., 1990; Obrist, 1981; Schwartz, 2003). However, recent commentaries have criticized reactivity research as being conceptually and methodologically narrow, with limited predictive ability (Carroll et al., 2001; Kamarck & Lovallo, 2003; Linden, et al., 2003; Schwartz et al., 2003; Treiber et al., 2003).
An aspect of the stress response that is receiving increasing attention is the stress recovery phase (Christenfeld et al., 2000; Haynes et al., 1991; Stewart & France, 2001; Stewart, et al., 2006). BP recovery may be defined as the extent to which elevations in BP persist following the cessation of a stressor (Linden et al., 1997). In other words, recovery emphasizes the persistence of the stress response rather than its acute magnitude. The measurement of recovery BP centers on the notion that persistently elevated BP can lead to sustained cardiovascular burden, which has been linked with end-organ damage (Devereux & Pickering, 1991). The available evidence regarding BP recovery supports its potential pathophysiological significance. Hypertensive individuals have been characterized by abnormally persistent BP responses following acute stressors (Borghi et al, 1986; Falkner & Kushner, 1989; Frederikson & Engel, 1985; Light et al., 1987; Schuler & O’Brien, 1997; Seibt et al., 1998). Prospective studies in normotensive populations have demonstrated poorer systolic BP recovery following laboratory stressors (Stewart & France, 2001; Stewart et al., 2006). Risk factors for hypertension, including African American ethnicity, male gender, and family history of hypertension also have been related to poor BP recovery (Gerin & Pickering, 1995; Gillin et al., 1996; Jackson et al., 1999).
However, the utility of evaluating BP recovery has been questioned due to its potential redundancy after considering reactivity (Linden et al., 1997). Although recovery and reactivity are unlikely to be independent characteristics, their relative importance has received little systematic scrutiny to date. Recent investigations point towards the independent utility, and perhaps superiority, of recovery compared to reactivity in predicting future BP (Rutledge et al., 2000; Stewart & France, 2001; Stewart et al., 2006).
A key criticism of the laboratory-based reactivity/recovery literature is its questionable generalizability to psychophysiological responses to real-world stressors, such as job stress or marital discord (Ming et al., 2004; Verdecchia at al., 1990). This criticism has been addressed methodologically by using laboratory BP to predict ambulatory measures of BP (ABP), typically taken over a 24 hour period outside of the laboratory. Twenty-four hour ABP measures are considered superior to laboratory measures in predicting preclinical and clinical disease states as it allows for aggregating across multiple real-world stimuli (Mancia et al., 1997; Perloff et al., 1983; Staessen et al., 1999). Earlier studies examining the predictive power of laboratory based stress reactivity have demonstrated mixed results. Morales-Ballejo (1988) reported strong correlations between systolic and diastolic BP reactivity with systolic and diastolic ABP at work, when the stressor response was aggregated. Cornish, Blanchard & Jaccard (1994) found laboratory BP to be consistent predictors of ABP, although resting BP was superior to BP measured during the stress response. In contrast, Ironson et al. (1989) found that although work ABP was correlated with laboratory-based resting BP, reactivity BP did not correlate significantly with work ABP. Similarly, Langewitz et al. (1989) did not find correlations between laboratory reactivity BP and ABP, nor did they find variability in the laboratory BP comparable to the variability in ABP. In their study of 56 hypertensive participants, Floras et al. (1987) concluded that variability of laboratory-based reactivity BP was a poor predictor of ABP variability. Results from these studies are difficult to interpret as they often did not control for covariates or were based on correlational data. Knox, Hausforff, & Markovitz (2002) found that the reactivity response to two stressors (cold pressor and star racing) significantly predicted ABP 3 years following laboratory measurement in African American, normotensives participants but not in White participants. These mixed findings may reflect the methodological challenges inherent in the measurement of reactivity BP. Indeed, various authors have argued that reactivity measures may be neither reliable predictors of 24-h ABP nor reliable predictors of diurnal variation in BP (Kamarck & Lovallo, 2003; Manuck, Kasprowicz & Muldoon, 1990; Parati et al., 1991).
To date, studies examining the relationship between recovery BP and ABP are limited. In a sample of 22 normotensives and 30 hypertensives, Guasti et al. (1998) found that DBP and SBP recovery were correlated to ABP, although their predictive power was not examined. Conversely, Seibt et al. (1998) found that laboratory recovery measures did not significantly improve predictive models of ABP despite finding that hypertensives demonstrated impaired recovery compared with normotensives. Recent prospective studies suggest that SBP and DBP recovery provide incremental validity in daily ABP measurements 3 years following baseline measurements, after controlling for baseline BP and reactivity (Moseley & Linden, 2006; Rutledge et al., 2000). In their sample of young, healthy college students, Rutledge et al. (2000) found that while reactivity, recovery, and resting BP were predictive of ABP, only resting and recovery BP remained significant in models which simultaneously assessed all three measures. Moseley & Linden (2006) also found recovery to be a significant predictor of ABP at 3-year follow-up. Whereas both studies provide evidence regarding the value of recovery BP, their generalizability is limited as both samples consisted of normotensives participants and were primarily Caucasian.
The purpose of the current investigation was two-fold: first, to determine the generalizability of laboratory measures of BP in predicting ambulatory daytime and nighttime BP, and second, to further evaluate the unique contribution of BP recovery to the prediction of ABP in a biracial sample of men and women with BP extending from normal through Stage II hypertension. We hypothesized that recovery BP measured in the laboratory would predict daytime and nighttime ABP after accounting for resting BP and BP reactivity.
Methods
Participants
One hundred and eighty-two employed men and women (85 females) were enrolled in the Duke Biobehavioral Investigation of Hypertension Study. Participants were self-selected in response to printed advertisements. Volunteers were excluded if they reported use of exogenous reproductive hormones (e.g., hormone replacement therapy, oral contraceptives); hysterectomy; history of cardiovascular disease or other systemic disease affecting the cardiovascular system; BP >180/100 mm Hg on BP screening examination; chronic use of drugs which alter systemic hemodynamics (e.g., antihypertensives, antidepressants, sympathomimetic agents); and current tobacco or recreational drug use. Eligibility following screening was determined by obtaining seated BP on three separate occasions, one week apart. At each clinic visit, 3 readings were taken by a trained technician such that readings were separated by 2 minute intervals.
All assessment procedures were reviewed and approved by the Duke University Medical Center’s Institutional Review Board. Participants provided verbal and written consent prior to participation.
Procedure
Participants completed laboratory and ABP assessments on separate days, less than one week apart. All laboratory test procedures were conducted in a private, electrically shielded, sound attenuated, temperature-controlled (24°C) room. Participants were seated in a comfortable chair and fitted with an appropriately sized occlusion cuff. BP during laboratory visits was measured via a Suntech 4240 monitor (Suntech Corporation, Raleigh, NC), which is an automated auscultatory BP monitor.
The laboratory protocol involved an initial 20 minute resting period, followed by the alternating presentation of a task and a 10 minute recovery period. During the initial resting period, BP was measured every 5 minutes for the first 15 minutes, and at minutes 18, 19 and 20. BP was measured throughout each task, and at minutes 1, 5 and 10 during the recovery period following the completion of a task. Systolic BP (SBP) and diastolic BP (DBP) were measured. Task order was fully balanced across participants, using a Williams’ Square Design. The tasks are briefly described below (for a detailed description of each task, see Sherwood et al., 2002 and Sherwood et al., 1997).
Anger Recall Interview: Participants had 3 minutes to describe an interpersonal situation which had made them angry during the previous week. A 2-minute preparation preceded this task and participants were encouraged to adhere to an outline describing the situation, their responses, and their satisfaction regarding the outcome.
Reaction Time-Shock Avoidance: Participants were presented with a loud audible tone presented at varying, unpredictable intervals over a 3 minute period. Participants were required to press a key as fast as possible on hearing each tone and were instructed that if the reaction time was considered too slow, a “painful but harmless” electric shock would be delivered immediately by electrodes previously applied to the leg. In fact, shocks were never delivered.
Foot Cold Pressor: Participants placed one foot, up to the ankle, in a bucket containing a mixture of two parts ice to one part water (0°C to 4°C) for 2 minutes.
Mirror Trace: Participants had 3 minutes to outline a star while viewing its reflection in a mirror, as many times as possible, while making a minimum of errors. Deviating from the star activated an aversive buzzer and a counter which recorded the number of errors.
ABP Monitoring
Participants were fitted with an Accutracker II ABP monitor, a programmable auscultatory BP monitor (Suntech, Raleigh, NC), during the morning on a typical workday. Calibration procedures conducted in the laboratory ensured that the Accutracker II was within 5 mm Hg of stethoscopic measurements. The monitor was programmed such that measurements occurred at variable intervals averaging every 15 minutes during the participant’s self-reported waking period, and every 30 minutes during their sleep period. All readings were reviewed and artifactual readings were edited by trained staff members using criteria described in Hinderliter et al. (1991).
Data Reduction and Analyses
The last three minutes of the initial rest period were averaged to obtain resting BP values. BP reactivity was calculated as the difference between mean BP value during a task and the resting BP level. BP recovery levels were defined as the difference between resting BP and the recovery period BP at 1, 5, and 10 minute following completion of a task. To calculate BP recovery, minute 1, 5, and 10 readings were individually subtracted from the resting BP to yield simple change scores. Following this, three aggregate recovery measures were obtained by averaging minute 1, 5, and 10 recovery BPs across tasks. Calculating BP recovery and reactivity scores in this manner has been shown to reduce the effects of measurement error and improve reliability (Kamarck et al., 2000; Rutledge et al., 2000).
The use of simple changes scores when addressing unique contributions of recovery follows established traditions in the recovery literature (Moseley & Linden, 2006; Stewart et al., 2006). The absence of frequent or moment-to-moment recovery readings in our data precluded the utility of curve-fitting methods, which have been proposed as optimal ways of operationalizing recovery (Christenfeld, Glynn, & Gerin, 2000). While residualized change scores are often undertaken in the absence of frequent data points, correlations between residualized changes scores and simple change scores are known to be high and yield similar findings (Swain & Suls, 1996). Therefore, simple change scores were used to operationalize recovery BP in this study.
Pearson product moment correlations were calculated to assess the relationship between daytime and nighttime ABP with aggregate BP reactivity and recovery values. One-way ANOVAs were conducted to ensure the continuation of recovery between minute 1 and minute 10. Hierarchical regression analyses were used to assess the relative contribution of BP recovery towards predicting daytime and nighttime ABP readings. In Step 1, resting BP was entered. In Step 2, reactivity BP was entered. Finally, minute 10 recovery BP was entered in Step 3 to assess whether recovery enhanced the prior models.
In ABP monitoring, the intermittent BP measurement has a greater probability of capturing the hemodynamic events following a stressor (i.e., recovery) than the response produced in the presence of a stressor (i.e., reactivity). Because the minute 10 recovery BP was the most distant point from the cessation of the stressor, this data point was chosen to best estimate the persistent state of arousal likely being captured by the ABP measures. Although this methodology does not include the rapid recovery effects documented elsewhere (Linden et al., 1997), it has been suggested that rapid recovery from an acute stress has less prognostic value than delayed recovery (Schwartz et al., 2003). Using minute 10 BP may therefore capture the pathogenic effects of delayed recovery when compared to minute 1 or minute 5 recovery measures and was chosen a priori for inclusion in our hierarchical analyses.
Statistical significance was defined as effects of p< .05. All analyses were conducted using the SAS® Version 8.2 statistical package (SAS Institute, Cary, NC).
Results
Sample Characteristics
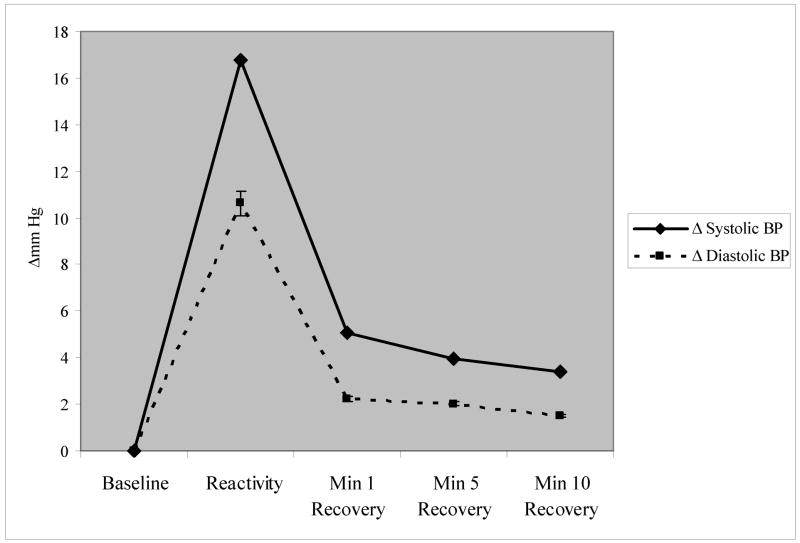
Demographic composition and baseline hemodynamic characteristics are summarized in Table 1. Results of one-way ANOVAs conducted between minute 1, 5, and minute 10 recovery BP were statistically significant (p’s <.0001), suggesting that recovery had continued to take place after the first minute. The graphical representation of stressor-induced BP change from resting BP is seen in Figure 1. The referent, mean resting BP, is transformed to 0 to represent baseline.
Table 1.
Demographic Characteristics
| N | 182 |
| Gender | 97 Males, 85 Females |
| Ethnicity | 92 African Americans, 90 Caucasian Americans |
| Age, yr | 33.48 ± 5.85 |
| Weight, lbs. | 170.48 ± 27.14 |
| BMI, kg/m2 | 26.06 ± 3.44 |
| Resting SBP, mm Hg | 110.87 ± 12.64 |
| Resting DBP, mm Hg | 73.78 ± 8.75 |
| Mean Reactivity SBP, Δmm Hg | 16.79 ± 8.13 |
| Mean Reactivity DBP, Δmm Hg | 10.64 ± 5.61 |
| Mean Recovery SBP min. 1, Δmm Hg | 5.08 ± 6.00 |
| Mean Recovery SBP min. 5, Δmm Hg | 3.95 ± 5.40 |
| Mean Recovery SBP min. 10, Δmm Hg | 3.39 ± 5.38 |
| Mean Recovery DBP min. 1, Δmm Hg | 2.24 ± 4.15 |
| Mean Recovery DBP min. 5, Δmm Hg | 2.03 ± 3.94 |
| Mean Recovery DBP min. 10, Δmm Hg | 1.50 ± 3.83 |
SBP=Systolic BP; DBP=Diastolic BP; BMI=Body Mass Index; min.=minute
Figure 1.
Mean Difference Scores from Baseline across the Laboratory Protocol (Baseline=0)
BP Recovery as a Predictor of ABP
Table 2 summarizes Pearson’s product moment correlations between laboratory-based reactivity and recovery BP and daytime and nighttime ABP. For recovery measures, all correlations were significant at the p<.01 level and ranged from r=.23 to r=.39. Correlations between DBP reactivity and daytime and nighttime DBP were .45 and .21 respectively (p<.01). SBP reactivity was correlated with daytime SBP (r =.22; p <.01) but no association was found between SBP reactivity and nighttime SBP.
Table 2.
Correlations of BP reactivity and BP recovery with ABP
| Daytime SBP | Nighttime SBP | |
|---|---|---|
| SBP Reactivity | .22** | −0.004 ns |
| SBP Recovery at Minute 10 | .34*** | .23** |
|
| ||
| Daytime DBP | Nighttime DBP | |
|
| ||
| DBP Reactivity | .45*+ | .21** |
| DBP Recovery at Minute 10 | .39*+ | .27*** |
p< .01,
p <.001,
p< .0001
Hierarchical regression analyses were employed to evaluate the contribution of resting BP, reactivity BP, and recovery BP in the prediction of daytime and nighttime ABP (see Tables 3 and 4). The total variance accounted for by these models ranged from 49% to 70%. Resting BP was the single largest predictor in each model. Reactivity scores accounted for an additional 0–8% of the variance in these models after controlling for resting BP. The inclusion of minute 10 recovery BP significantly improved the prediction model in all four analyses: daytime SBP (ΔR2=.04; p<.001), nighttime SBP (ΔR2=.05; p<.001), daytime DBP (ΔR2=.01, p<.001), nighttime DBP (ΔR2=.01; p<.001). Of note, the effects of reactivity in predicting daytime SBP became non-significant after the inclusion of recovery in the model.
Table 3.
Hierarchical Regression Analyses Predicting Daytime and Nighttime SBP.
| Predictors | b | p | Model R2 | Increase in R2 | Model F | Model p |
|---|---|---|---|---|---|---|
| Daytime SBP | ||||||
| Step 1: Initial Model | 0.58 | 241.83 | <.001 | |||
| Resting SBP | 0.76 | <.001 | ||||
| Step 2: SBP Reactivity | 0.61 | 0.03 | 136.85 | <.001 | ||
| Resting SBP | 0.76 | <.001 | ||||
| SBP Reactivity | 0.18 | 0.0003 | ||||
| Step 3: SBP Minute 10 Recovery | 0.65 | 0.04 | 109.22 | <.001 | ||
| Resting SBP | 0.82 | <.001 | ||||
| SBP Reactivity | 0.1 | 0.19 | ||||
| SBP Minute 10 recovery | 0.24 | <.001 | ||||
| Nighttime SBP | ||||||
| Step 1: Initial Model | 0.44 | 131.23 | <.001 | |||
| Resting SBP | 0.66 | <.001 | ||||
| Step 2: SBP Reactivity | 0.44 | 0 | 65.46 | <.001 | ||
| Resting SBP | 0.66 | <.001 | ||||
| SBP Reactivity | 0.03 | 0.6021 | ||||
| Step 3: SBP Minute 10 Recovery | 0.49 | 0.05 | 54.17 | <.001 | ||
| Resting SBP | 0.74 | <.001 | ||||
| SBP Reactivity | −0.09 | 0.1199 | ||||
| SBP Minute 10 recovery | 0.28 | <.001 | ||||
Note: For each step, variable added is in italics; b=standardized beta weights
Table 4.
Hierarchical Regression Analyses Predicting Daytime and Nighttime DBP.
| Predictors | b | P | Model R2 | Increase in R2 | Model F | Model p |
|---|---|---|---|---|---|---|
| Daytime DBP | ||||||
| Step 1: Initial Model | 0.61 | 276.58 | <.001 | |||
| Resting DBP | 0.78 | <.001 | ||||
| Step 2: DBP Reactivity | 0.69 | 0.08 | 196.45 | <.001 | ||
| Resting DBP | 0.79 | <.001 | ||||
| DBP Reactivity | 0.28 | <.001 | ||||
| Step 3: DBP Minute 10 Recovery | 0.7 | 0.01 | 136.2 | <.001 | ||
| Resting DBP | 0.81 | <.001 | ||||
| DBP Reactivity | 0.21 | <.001 | ||||
| DBP Minute 10 recovery | 0.12 | 0.02 | ||||
| Nighttime DBP | ||||||
| Step 1: Initial Model | 0.46 | 145.8 | <.001 | |||
| Resting DBP | 0.68 | <.001 | ||||
| Step 2: DBP Reactivity | 0.49 | 0.03 | 80.8 | <.001 | ||
| Resting DBP | 0.69 | <.001 | ||||
| DBP Reactivity | 0.17 | 0.0033 | ||||
| Step 3: DBP Minute 10 Recovery | 0.5 | 0.01 | 57.72 | <.001 | ||
| Resting DBP | 0.72 | <.001 | ||||
| DBP Reactivity | 0.07 | 0.3095 | ||||
| DBP Minute 10 recovery | 0.17 | 0.0129 | ||||
Note: For each step, variable added is in italics; b=standardized beta weights
We also wish to note that we performed additional hierarchical regression analyses, expanding upon the a priori models depicted in Tables 3 and 4. When BPs measured at post-task recovery minute 1 and minute 5 were added to the hierarchical regression models, they did not show significant independent associations with ABP, yielding models that accounted for no additional explanatory variance than had already been accounted for by the inclusion of recovery BP captured at the post-task minute 10 time point.
Discussion
We found that post-stress recovery BP was an independent predictor of real-life blood pressure, measured according to daytime and nighttime ABP, after controlling for resting BP and BP reactivity. While examining the ecological validity of laboratory-based stress responses, the recovery phase may be a more useful analog of real-life stress response patterns than the reactivity phase. Recovery BP may be especially important in predicting aggregate nighttime ABP, now considered a more important prognostic marker than daytime ABP (Palatini et al., 1992; Rizzoni et al., 1992). The absence of a nighttime drop in SBP, or “non-dipping”, is associated with higher cardiovascular morbidity and mortality (Staessen et al., 1999). While the current study did not examine the relationship between recovery BP and nighttime dipping, the association between recovery BP and nighttime ABP provides further impetus to the study of persistent arousal states.
Delayed BP recovery from stress is believed to be a potential marker of chronic sympathetic activation and low parasympathetic activity (Amerena & Julius, 1995; Julius, 1993). Yet, the mechanism resulting in sustained arousal following the cessation of a stressor has not been definitively established. One theory suggests that perseverative cognitions may not only mediate the effects of psychosocial stressors, but may serve as stressors themselves (Brosschot et al., 2006). Support for this theory can be found in recent studies demonstrating that ruminative thinking is associated with slower recovery following anger recall tasks (Gerin et al., 2006; Glynn et al., 2002). This may then be the pathogenic pathway through which both acute and chronic life stressors translate into chronic patterns of physiological arousal.
In our study, the combination of reactivity and recovery accounted for greater variance in all four models predicting ABP, compared with either one individually. Examination of previous studies demonstrated similar patterns, where the consideration of both reactivity and recovery yielded more robust predictive models (Moseley & Linden, 2006; Rutledge et al., 2000; Stewart & France, 2001; Stewart et al., 2006). An implication is that while both constructs capture unique information of hemodynamic changes occurring under stressful situations, neither reactivity nor recovery may be independently sufficient to explain the complex psychophysiological processes occurring in the presence of stress. Indeed, recent advances in statistical analyses have advocated the use of curve-fitting techniques that capture the entire stress response, rather than focusing on either aspect alone (Christenfeld et al., 2000; Llabre et al., 2004). Such approaches were precluded in our study as the use of curve-fitting requires frequent BP assessments during the recovery period, optimally involving beat-by-beat BP assessment. Therefore, a limitation of our study was the utilization of simple change scores to measure recovery, as this methodology does not account for the interdependence between the reactivity and recovery measures. By forcing the recovery BP as the last step in hierarchical regression models, we statistically controlled for the effects of resting BP and reactivity BP. As our eventual goal was to examine the additional variance explained by recovery BP, the collinearity was considered acceptable despite the subsequent loss of power (Moseley & Linden, 2006). Furthermore, the known interdependence between these measures would in reality reduce the probability of finding independent effects for recovery. The additional explained variance of recovery BP in these analyses emphasizes its potency in predicting ABP.
Using the final recovery BP may capture the effects of delayed recovery and improve prediction of both ABP and clinical disease states. However, it may also raise concern that the BP elevations observed reflect anticipatory anxiety of the next task, rather than recovery from the preceding task. The cardiovascular effects of anticipatory anxiety have been documented in anticipation of psychological laboratory tasks such as math problems (Contrada, Wright, & Glass, 1984). However, these results are not consistently reproduced (Gerin, Peiper, & Pickering, 1994). Furthermore, participants in these studies were aware of the nature of the tasks in which they would next participate, which potentially increased the anxiety response. In our study, this issue was addressed in two ways. One, the tasks were presented in a Williams’ Square counter-balanced design. By presenting tasks in this manner we ensured that within each of the 4 randomized groups of participants, each task was presented first, last, preceded each task, and followed each task. Second, participants were unaware which task would follow their recovery period. The aggregate effects of anticipatory anxiety were likely to be diminished due to the unfamiliarity of the tasks and the counterbalanced task presentation.
A potential criticism of our study is our use of the minute 10 recovery BP in our hierarchical regression models as it does not take into account the rapidity through which recovery may occur following a stressor. Because the effects of delayed recovery are considered pathogenic, we were most interested in examining the effects of recovery BP at the farthest point following the cessation of the stressor. In our study, this was captured by the minute 10 BP. Moreover, the 10 minute interval was more likely to correspond with the intermittent ABP readings, thereby enhancing the generalizability of a laboratory-based measure of stress responsivity.
In summary, the addition of recovery measures may potentially enhance our understanding of the stress response. Optimally, the simultaneous consideration of the acute and chronic phases may be more valuable than the consideration of either phase in isolation. Future studies may also focus on understanding the pathophysiological mechanisms which mediate the relationship between the presence of stress and the development of disease states.
Acknowledgments
This study was supported by NIH grant HL49427, and by M01-RR-30, National Center for Research Resources, General Clinical Research Centers Program, NIH, Bethesda, MD, The study’s principal investigator, Dr. Sherwood, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amerena J, Julius S. The role of the autonomic nervous system in hypertension. Hypertension Research-Clinical & Experimental. 1995;18:99–110. doi: 10.1291/hypres.18.99. [DOI] [PubMed] [Google Scholar]
- 2.Borghi C, Costa FV, Boschi S, Mussi A, Ambrosioni E. Predictors of stable hypertension in young borderline subjects: A 5-year follow-up study. Journal of Cardiovascular Pharmacology. 1986;8(Suppl 5):S138–141. doi: 10.1097/00005344-198608005-00030. [DOI] [PubMed] [Google Scholar]
- 3.Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. Journal of Psychosomatic Research. 2006;60:113–124. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- 4.Carroll D, Smith GD, Shipley MJ, Steptoe A, Brunner EJ, Marmot MG. Blood pressure reactions to acute psychological stress and future blood pressure status: A 10-year follow-up of men in the Whitehall II study. Psychosomatic Medicine. 2001;63:737–743. doi: 10.1097/00006842-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Christenfeld N, Glynn LM, Gerin W. On the reliable assessment of cardiovascular recovery: An application of curve-fitting techniques. Psychophysiology. 2000;37:543–550. [PubMed] [Google Scholar]
- 6.Contrada RJ, Wright RA, Glass DC. Task difficulty, type A behavior pattern, and cardiovascular response. Psychophysiology. 1984;21:638–46. doi: 10.1111/j.1469-8986.1984.tb00250.x. [DOI] [PubMed] [Google Scholar]
- 7.Cornish PJ, Blanchard EB, Jaccard J. The relationship between 24-hour ambulatory blood pressures and laboratory measures of cardiovascular reactivity. Biofeedback and Self-regulation. 1994;19:193–209. doi: 10.1007/BF01721067. [DOI] [PubMed] [Google Scholar]
- 8.Devereux RB, Pickering TG. Relationship between the level, pattern and variability of ambulatory blood pressure and target organ damage in hypertension. Journal of Hypertension. 1991;9(Suppl 8):S34–S38. [PubMed] [Google Scholar]
- 9.Falkner B, Kushner H. Race differences in stress-induced reactivity in young adults. Health Psychology. 1989;8(5):613–627. doi: 10.1037//0278-6133.8.5.613. [DOI] [PubMed] [Google Scholar]
- 10.Floras JS, Hassan MO, Jones JV, Sleight P. Pressor responses to laboratory stresses and daytime blood pressure variability. Journal of Hypertension. 1987;5:715–719. doi: 10.1097/00004872-198712000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Frederikson M, Engel BT. Cardiovascular and electrodermal adjustments during a vigilance task in patients with borderline and established hypertension. Journal of Psychosomatic Research. 1985;29(3):235–246. doi: 10.1016/0022-3999(85)90050-9. [DOI] [PubMed] [Google Scholar]
- 12.Gerin W, Davidson KW, Christenfeld NJ, Goyal T, Schwartz JE. The role of angry rumination and distraction in blood pressure recovery from emotional arousal. Psychosomatic Medicine. 2006;68:64–72. doi: 10.1097/01.psy.0000195747.12404.aa. [DOI] [PubMed] [Google Scholar]
- 13.Gerin W, Pickering TJ. Association between delayed recovery of blood pressure after acute mental stress and parental history of hypertension. Journal of Hypertension. 1995;13:603–610. doi: 10.1097/00004872-199506000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Gerin W, Pieper C, Pickering TG. Anticipatory and residual effects of an active coping task on pre- and post-stress baselines. Journal of Psychosomatic Research. 1994;38:139–149. doi: 10.1016/0022-3999(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 15.Gillin JL, Mills PJ, Nelesen RA, Dillon E, Ziegler MG, Dimsdale JE. Race and sex differences in cardiovascular recovery from acute stress. International Journal of Psychophysiology. 1996;23:83–90. doi: 10.1016/0167-8760(96)00041-4. [DOI] [PubMed] [Google Scholar]
- 16.Glynn LM, Christenfeld N, Gerin W. The role of rumination in recovery from reactivity: Cardiovascular consequences of emotional states. Psychosomatic Medicine. 2002;64(5):714–726. doi: 10.1097/01.psy.0000031574.42041.23. [DOI] [PubMed] [Google Scholar]
- 17.Guasti L, Diolisi A, Gaudio G, Grimoldi P, Petrozzino R, Uslenghi S, Bertolini A, Grandi AM, Venco A. Reactivity of blood pressure to mental arithmetic stress test, stress-test recovery time, and ambulatory blood pressure in hypertensive and normotensive subjects. Blood Pressure Monitoring. 1998;3:275–280. [PubMed] [Google Scholar]
- 18.Haynes SN, Gannon LR, Orimoto L, O'Brien WH, Brandt M. Psychophysiological assessment of post-stress recovery. Psychological Assessment. 1991;3:1–10. [Google Scholar]
- 19.Hinderliter AL, Light KC, Willis PW., IV Left ventricular mass index and diastolic filling. Relation to blood pressure and demographic variables in a healthy biracial sample. American Journal of Hypertension. 1991;4:579–585. doi: 10.1093/ajh/4.7.579. [DOI] [PubMed] [Google Scholar]
- 20.Ironson GI, Gellman MD, Spitzer SB, Llabre MM, Pasin RDC, Weidler DJ, Schneiderman N. Predicting home and work blood pressure measurements from resting baselines and laboratory reactivity in Black and White Americans. Psychophysiology. 1989;26:174–184. doi: 10.1111/j.1469-8986.1989.tb03151.x. [DOI] [PubMed] [Google Scholar]
- 21.Jackson RW, Treiber FA, Turner JR, Davis H, Strong WB. Effects of race, sex, and socioeconomic status upon cardiovascular stress responsivity and recovery in youth. International Journal of Psychophysiology. 1999;31:111–119. doi: 10.1016/s0167-8760(98)00044-0. [DOI] [PubMed] [Google Scholar]
- 22.Julius S. Sympathetic hyperactivity and coronary risk in hypertension. Hypertension. 1993;21(6 Pt 2):886–893. doi: 10.1161/01.hyp.21.6.886. [DOI] [PubMed] [Google Scholar]
- 23.Kamarck TW, Debski TT, Manuck SB. Enhancing the laboratory-to-life generalizability of cardiovascular reactivity using multiple occasions of measurement. Psychophysiology. 2000;37:533–542. [PubMed] [Google Scholar]
- 24.Kamarck TW, Lovallo WR. Cardiovascular reactivity to psychological challenge: Conceptual and measurement considerations. Psychosomatic Medicine. 2003;65:9–21. doi: 10.1097/01.psy.0000030390.34416.3e. [DOI] [PubMed] [Google Scholar]
- 25.Knox S, Hausdorff J, Markovitz JH. Reactivity as a predictor of subsequent blood pressure: Racial differences in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Hypertension. 2002;40:914–919. doi: 10.1161/01.hyp.0000041417.94797.57. [DOI] [PubMed] [Google Scholar]
- 26.Manuck SB, Kasprowicz AI, Muldoon MF. Behaviorally-evoked cardiovascular reactivity and hypertension: Conceptual issues and potential associations. Annals of Behavioral Medicine. 1990;12:17–29. [Google Scholar]
- 27.Langewitz W, Ruddel H, Schachinger H, Schmeider R. Standardized testing in the cardiovascular laboratory: Has it any bearing on ambulatory blood pressure values? Journal of Hypertension. 1989;7(Suppl 3):541–548. [PubMed] [Google Scholar]
- 28.Light KC, Obrist PA, Sherwood A, James SA, Strogatz DS. Effects of race and marginally elevated blood pressure on responses to stress. Hypertension. 1987;10:555–563. doi: 10.1161/01.hyp.10.6.555. [DOI] [PubMed] [Google Scholar]
- 29.Linden W, Earle TL, Gerin W, Christenfeld N. Physiological stress reactivity and recovery: Conceptual siblings separated at birth? Journal of Psychosomatic Research. 1997;42:117–135. doi: 10.1016/s0022-3999(96)00240-1. [DOI] [PubMed] [Google Scholar]
- 30.Linden W, Gerin W, Davidson K. Cardiovascular reactivity: Status quo and a research agenda for the new millennium. Psychosomatic Medicine. 2003;65:5–8. doi: 10.1097/01.psy.0000046076.93591.ad. [DOI] [PubMed] [Google Scholar]
- 31.LLabre MM, Spitzer S, Siegel S, Saab PG, Schneiderman N. Applying latent growth curve modeling in the investigation of individual differences in cardiovascular recovery from stress. Psychosomatic Medicine. 2004;66:29–41. doi: 10.1097/01.psy.0000107886.51781.9c. [DOI] [PubMed] [Google Scholar]
- 32.Mancia G, Frattola A, Ulian L, Santucciu C, Parati G. Blood pressures other than the one at the clinic. Blood Pressure Supplement. 1997;2:81–85. [PubMed] [Google Scholar]
- 33.Manuck SB, Kasprowicz AL, Muldoon MF. Behaviorally evoked cardiovascular reactivity and hypertension: Conceptual issues and potential associations. Annals of Behavioral Medicine. 1990;12:17–29. [Google Scholar]
- 34.Ming EW, Adler GK, Kessler RC, Fogg LF, Matthews KA, Herd A, Rose RM. Cardiovascular reactivity to work stress predicts subsequent onset of hypertension: The Air Traffic Controller Health Change Study. Psychosomatic Medicine. 2004;66:459–465. doi: 10.1097/01.psy.0000132872.71870.6d. [DOI] [PubMed] [Google Scholar]
- 35.Morales-Ballejo HM, Eliot RS, Boone JL, Hughes JS. Psychophysiologic stress testing as a predictor of mean daily blood pressure. American Heart Journal. 1988;116:673–681. doi: 10.1016/0002-8703(88)90568-6. [DOI] [PubMed] [Google Scholar]
- 36.Moseley JA, Linden W. Predicting blood pressure and heart rate change with cardiovascular reactivity and recovery: Results from 3-year and 10-year follow-up. Psychosomatic Medicine. 2006;68:833–843. doi: 10.1097/01.psy.0000238453.11324.d5. [DOI] [PubMed] [Google Scholar]
- 37.Obrist PA. Cardiovascular psychophysiology: A perspective. Plenum Press; NY: 1981. [Google Scholar]
- 38.Palatini P, Penzo M, Racioppa A, Zugno E, Guzzardi G, Anaclerio M, Pessina AC. Clinical relevance of nighttime blood pressure and of daytime blood pressure variability. Archives of Internal Medicine. 1992;152:1855–1860. [PubMed] [Google Scholar]
- 39.Perloff D, Sokolow M, Cowan D. The prognostic value of ambulatory blood pressures. JAMA. 1983;249:2792–2798. [PubMed] [Google Scholar]
- 40.Pickering TG, Gerin W. Cardiovascular reactivity in the laboratory and the role of behavioral factors in hypertension: A critical review. Annals of Behavioral Medicine. 1990;12:3–16. [Google Scholar]
- 41.Rizzoni D, Muiesan ML, Montani G, Zulli R, Calebich S, Agabiti-Rosei E. Relationship between initial cardiovascular structure and daytime and nighttime blood pressure monitoring. American Journal of Hypertension. 1992;5:180–186. doi: 10.1093/ajh/5.3.180. [DOI] [PubMed] [Google Scholar]
- 42.Rutledge T, Linden W, Paul D. Cardiovascular recovery from acute laboratory stress: Reliability and concurrent validity. Psychosomatic Medicine. 2000;62:648–654. doi: 10.1097/00006842-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Schuler JLH, O'Brien WH. Cardiovascular recovery from stress and hypertension risk factors: A meta-analytic review. Psychophysiology. 1997;34:649–659. doi: 10.1111/j.1469-8986.1997.tb02141.x. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz AR, Gerin W, Davidson KW, Pickering TG, Brosschot JF, Thayer J, Christenfeld N, Linden W. Toward a causal model of cardiovascular responses to stress and the development of cardiovascular disease. Psychosomatic Medicine. 2003;65:22–35. doi: 10.1097/01.psy.0000046075.79922.61. [DOI] [PubMed] [Google Scholar]
- 45.Seibt R, Boucsein W, Scheuch K. Effects of different stress settings on cardiovascular parameters and their relationship to daily life blood pressure in normotensives, borderline hypertensives and hypertensives. Ergonomics. 1998;41:634–648. doi: 10.1080/001401398186801. [DOI] [PubMed] [Google Scholar]
- 46.Sherwood A, Girdler SS, Bragdon EE, West SG, Brownley KA, Hinderliter AL, Light KC. Ten-year stability of cardiovascular responses to laboratory stressors. Psychophysiology. 1997;34:185–191. doi: 10.1111/j.1469-8986.1997.tb02130.x. [DOI] [PubMed] [Google Scholar]
- 47.Sherwood A, Gullette EC, Hinderliter AL, Georgiades A, Babyak M, Waugh RA, Blumenthal JA. Relationship of clinic, ambulatory, and laboratory stress blood pressure to left ventricular mass in overweight men and women with high blood pressure. Psychosomatic Medicine. 2002;64:247–257. doi: 10.1097/00006842-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 48.Staessen JA, Beilin L, Parati G, Waeber B, White W the Participants of the 1999 Consensus Conference on Ambulatory Blood Pressure Monitoring. Task Force IV: Clinical use of ambulatory blood pressure monitoring. Blood Pressure Monitoring. 1999;4(6):319–331. doi: 10.1097/00126097-199912000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Stewart JC, France CR. Cardiovascular recovery from stress predicts longitudinal changes in blood pressure. Biological Psychology. 2001;58:105–120. doi: 10.1016/s0301-0511(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 50.Stewart JC, Janicki DL, Kamarck TW. Cardiovascular reactivity to and recovery from psychological challenge as predictors of 3-year change in blood pressure. Health Psychology. 2006;25:111–118. doi: 10.1037/0278-6133.25.1.111. [DOI] [PubMed] [Google Scholar]
- 51.Swain A, Suls J. Reproducibility of blood pressure and heart rate reactivity: A meta-analysis. Psychophysiology. 1996;33:162–174. doi: 10.1111/j.1469-8986.1996.tb02120.x. [DOI] [PubMed] [Google Scholar]
- 52.Treiber FA, Kamarck TW, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease States. Psychosomatic Medicine. 2003;65:46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 53.Verdecchia P, Clement D, Fagard R, Palatini P, Parati G. Task Force III: Target-organ damage, morbidity and mortality. Blood Pressure Monitoring. 1999;4:303–317. doi: 10.1097/00126097-199912000-00004. [DOI] [PubMed] [Google Scholar]