Abstract
Cultured human lung epithelial cells (BEAS-2B) were treated in vitro with PM2.5-enriched particles of soil-derived mineral dust from nine sites in the western United States. The particle samples simulate windblown dust and vehicle-generated emissions from unpaved roads. Five of the sites yielded relatively benign dust. Particles from three sites caused IL-6 release when cells were treated for 24 h at doses from 20 to 80 μg/cm2, and particles from one site were highly cytotoxic. The particle components or characteristics that caused the IL-6 release were stable at temperatures below 150°C, but were inactivated by treatment at 300–550°C. The active factors were also associated predominantly with the insoluble fraction, and were partially attenuated by leaching with aqueous and organic solvents. The IL-6 release caused by the particles was much greater than the cytokine response to either lipopolysaccharide (LPS) or to surrogate particles of titanium dioxide mixed with LPS, suggesting that endotoxin was not a major factor in the inflammatory response. The release of IL-8 in response to particle treatment was qualitatively similar to the IL-6 response, but release of TNF-α was not detected at the 24-h time point. The combined results support the hypothesis that some ambient dusts from geological sources can cause cell death and cytokine release in a lung cell line that is widely used as an in vitro model to study mechanisms of environmental respiratory injury.
Keywords: geological dust, physical treatment, cell culture, interleukin-6, IL-6, interleukin-8, IL-8, endotoxin, vanilloid receptor
Ambient particulate matter (PM) is a complex mixture containing geological minerals, biological materials, fossil fuel combustion products, and secondary PM. Particle mass in the ambient air correlates with a wide range of adverse effects, but the importance of geological dust inhalation to human health has not been resolved. Pope et al. (1999) reported that differences in adverse health effects associated with PM10 mass in nearby Utah communities were due to the relative soil dust fraction. Schenker (2000) associated high exposure to agricultural dust with chronic bronchitis, but epidemiology studies in Spokane, WA found no correlation between ambient soil dust and mortality (Schwartz et al., 1999) or hospital visits for asthma (Claiborn et al., 2002).
In vitro toxicology studies, motivated by specific biochemical signaling hypotheses, have attempted to link specific particle sources with cellular responses. Typically, lung cells are exposed to 10–100 μg/cm2 of ambient particles or laboratory surrogates for specific components of air pollution. Cell culture models include immortalized lung cell lines such as BEAS-2B (Frampton et al., 1999; Ghio et al., 1999; Steerenberg et al., 1998) and A549 (Seagrave and Nikula, 2000; Smith et al., 2000), normal human bronchial epithelial cells (Carter et al., 1997), macrophages, and cocultures of macrophages with epithelial cells (Tao and Kobzik, 2002).
Mills et al. (1999) and Driscoll (1999) reviewed cytokine responses in airway epithelial cells. The release of both IL-6 and IL-8 by lung cells is transcriptionally controlled by NF-κB (Fan et al., 2001) as the result of an inflammation-regulating signal cascade involving TNF-α and other cytokines (Nelson and Martin, 2000). Induction of IL-6 has been observed in BEAS-2B cells treated with diesel exhaust particles (Steerenberg et al., 1998), residual oil fly ash (ROFA) (Veronesi et al., 1999), a range of ambient and combustion particles (Veronesi et al., 2002b), negatively charged particles ( Agopyan et al., 2003; Veronesi et al., 2002a), and capsaicin-related compounds (Reilly et al., 2003). Titanium dioxide particles larger than 1 μm are inert and serve as a PM negative control (Steerenberg et al., 1998; van Maanen et al., 1999), but mineral dust from stone quarries induces cytotoxicity and IL-6 release in A549 cells (Hetland et al., 2000) and in rat lung type 2 alveolar cells (Becher et al., 2001).
Identifying the most toxic particle types requires understanding the effects of the low solubility particle core compared to the soluble and adsorbed species that are rapidly released from particles under physiological conditions. Studies that associated effects with the insoluble particle fraction include work with reactive oxygen species (ROS) generation by polymorphonuclear leukocytes treated with a range of mineral dusts and ambient particles (Prahalad et al., 1999) and work with alveolar macrophages treated with concentrated ambient particles (Imrich et al., 2000). Evidence for effects from soluble metals comes from studies of BEAS-2B cells treated with aqueous extracts of Utah Valley filters (Frampton et al., 1999), A549 cells treated with mineral particles (Hetland et al., 2001), rat tracheal epithelial cells treated with residual oil fly ash (Dye et al., 1999), and urban particles instilled into mouse lung (Adamson et al., 1999). Ghio et al. (1999) reported oxidative stress and IL-8 induction by BEAS-2B cells in response to both the soluble and insoluble fractions from filter samples. Physical treatments to modify particle toxicological properties include leaching particles with the metal chelator desferrioxamine (Smith et al., 2000), and applying surface-modifying coatings (Schins, 2002).
Biogenic components, including viable organisms, endotoxin, fungal toxins, and proteins in ambient particles directly affect lung cells (Monn and Koren, 1999). Lipopolysaccharide (LPS) pretreatment is used to model inflammation in animal inhalation experiments (Elder et al., 2000), and LPS induces IL-6 in BEAS-2B and A549 cells (Schulz et al., 2002). Procedures to remove endotoxin from medical devices typically involve repeated washing or heating (Williams, 2001). Heat or chemical sterilization eliminates viable organisms but may change other particle characteristics.
Veronesi et al. (1999) used the receptor antagonists capsazepine (CPZ) and amiloride in BEAS-2B cell culture studies and concluded that the acidic, soluble components of ROFA cause immediate increases in [Ca + 2] influx, followed by IL-6 and IL-8 release through activation of both TRPV1 receptors and acid sensitive ion channels (ASICs). CPZ suppressed IL-6 release in response to St Louis and Ottawa urban particles, wood stove deposits, and coal fly ash (Veronesi et al., 2002b). Experiments with a BEAS-2B-derived cell line that overexpressed TRPV1 showed that overexpression resulted in a 100-fold decrease in the concentration of capsaicinoids needed to cause cell death and IL-6 release (Reilly et al., 2003).
Soil dusts are sometimes assumed to be benign compared to anthropogenic emissions, but preliminary experiments in our laboratory suggested that soil dust induced cytokine release at concentrations comparable to that induced by coal fly ash, a widely studied combustion particle source. The objective of this study was to test the hypothesis that specific soil dusts could induce proinflammatory cytokine signaling in an immortalized lung cell line that is widely used to study the mechanisms by which specific types of atmospheric particulate matter induce proinflammatory signaling in airway tissues. Soil dusts, representative of fugitive dust emissions from unpaved roads, from a range of sites in the western United States were tested for IL-6 release response. A subset of four soils was selected for additional experiments that used particlemodifying treatments to gain insight into the chemical component classes (semivolatile, water soluble, etc.) in the soil that affect the observed cell responses.
MATERIALS AND METHODS
Environmental and surrogate particle sources
Field samples, approximately 10 kg each, were collected as part of a chemical characterization study of dust sources in the western United States (Labban et al., 2004). Table 1 lists the sample identification codes and the geographic setting of the collection sites. For this toxicology study we selected three dusts (DD, WM, and R4) that produced strong, reproducible IL-6 responses in preliminary experiments and one dust (UN) that was exceptionally cytotoxic.
TABLE 1.
Environmental and Surrogate Samples Selected for Detailed Study
| ID Code | Name | Type | Description |
|---|---|---|---|
| DD | Desert dust | Unpaved road | High-calcium, fine, dusty soil from a sparsely vegetated site south of the salt flats in Tooele County, UT. Arid, ≈18 cm rain/year. |
| WM | West mesa | Wind-generated dust area | Sandy soil from a rural grazing land site 10 km upwind of Sunland Park, NM. Arid, ≈ 22 cm rain/year. |
| R4 | Range 40 | Unpaved road | Gravel soil in the foothills of the Doña Ana mountains north of El Paso, TX. Arid, ≈ 25 cm rain/year. |
| UN | Uinta | Wind and recreation activity | Sandy soil from a high elevation site in the Uinta mountains, Duchesne County, UT. Subalpine, periodic flooding. |
| LPS | Positive control | P. aeruginosa LPS in LHC-9 culture media. | |
| TiLPS | Surrogate particle | TiO2 mixed with LPS, dried, weighed, and resuspended in culture media. | |
| Ti + LPS | Surrogate mixture | TiO2 and LPS added separately to cell culture media. |
PM2.5-enriched soil dusts were prepared in the laboratory by screen sifting of the bulk soil samples, followed by mechanical resuspension of the fines by tumbling, and aerodynamic separation of thedust cloud usinga cascade impactor. This process is a laboratory representation of the dust generated by mechanical attrition when tires contact the road surface, or when coarse particle bombardment generates fine dust during high-wind events. Depending on the soil, a 10-kg field sample generated from 50 mg to over a gram of particles highly enriched in the 0.4–3 μm size range. Veranth et al. (2000) describe the equipment, procedures, and microscopy verification of product size distribution.
Surrogate treatments were prepared from 1.0 –2.0 μm APS TiO2 (Alfa Aesar, stock #43047) and Pseudomonas aeruginosa lipopolysaccharide (Sigma, catalog # L9143). Koyama et al. (2000) tested several commercial LPS materials, and P. aeruginosa LPS induced the highest levels of IL-8 in A549 cells. The particles identified as ‘‘TiLPS’’ were prepared by mixing TiO2 and LPS in water, drying the suspension with constant stirring overnight in a vacuum oven at 60°C, and recovering the particles by scraping.
Preparation of cell treatments
Particle samples were weighed, sterilized with 70%ethanol (approximately 50 μl alcohol for 1–10 mg of particles), vacuum dried, and resuspended in cell culture media, typically at 200 μg/ml. The treatment concentrations were prepared by serial dilution. We routinely used a combination of 5 min of sonication in an ultrasonic cleaner and vortexing immediately before each transfer to insure complete dispersion of the particles. Scanning electron microscopy verified that this procedure dispersed clusters but preserved the primary particles.
We physically modified samples of DD, WM, and R4 dusts. The thermally treated particles were prepared by heating in loosely covered borosilicate glass tubes in air in a muffle furnace at 150, 300, or 550°C for 1 h. Leaching treatments involved suspending the particles in 5 ml of liquid and rotating the tubes overnight at 6 rpm. The samples were centrifuged at 750 ×g for 10 min and decanted, and the cycle was repeated three times. The leaching liquids were LHC-9 cell culture media, 1 mM desferrioxamine (Sigma #D9533) in phosphate-buffered saline (PBS), (Biofluids, Camarillo CA), unbuffered demineralized water, and 2:1 (v/v) mixture of chloroform and methanol. The pH values of the particle suspensions were 7.6, 7.0, and 6.0 for the LHC-9, desferrioxamine in PBS, and water, respectively. The supernatant from the first 24 h leaching with LHC-9 was recovered and used to measure the effects of the soluble fraction of the original particles. After leaching, residual solvent was removed from the chloroform-methanol-treated samples by vacuum evaporation. Desferrioxamine was removed by washing the particles with LHC-9.
Some weight change during physical treatment was expected due to evaporation of volatiles, oxidation, and dissolution, so we calculated cell exposures in terms of the original sample mass. The actual amount of particles applied to the cell culture was estimated by light absorbance using a plate reader.
Particle characterization
Endotoxin was measured using the chromogenic Limulus Amebocyte Lysate assay (LAL) kit (QCL-1000, Cambrex Bio-Products, Walkersville, MD). A dilution series was prepared for each particle type to find a concentration within the range of the assay. Values are reported as endotoxin units (EU) per mg of dry particle sample or EU/ml for liquid suspensions of purchased LPS. The Veterinary Pathology Laboratory at Utah State University performed elemental analysis on samples using nitric acid digestion and ion-coupled plasma mass spectrometry.
Cell culture
BEAS-2B human bronchial epithelial cells (Reddel et al., 1989) (American Type Culture Collection, Rockville, MD) were used at passage numbers 60–80. The cells were cultured in Lechner and LaVeck media (LHC-9) containing retinoic acid (33 nM) and epinephrine (2.75 μM). Culture flasks and multiwell plates were coated with LHC-basal media containing BSA (100 μg/ ml), collagen (30 μg/ml), and fibronectin (10 μg/ml) for at least 4 h at 37°C. The cells were maintained in 75 cm2 flasks at 37°C and 6% CO2. Media was replaced every second day, and cells were passaged when <85% confluent by washing with Ca- and Mg-free PBS and dislodging with 0.05% trypsin.
Cell treatment experiments used 48-well polystyrene plates (Costar, Fisher Scientific) containing 0.3 ml of media per well, with cells seeded at an initial density of 20,000/cm2. After one day we applied new media containing the treatments. On the third day we harvested the media for cytokine assays and measured viable cell count. Experiments used triplicate wells for each treatment level and allocated six or nine wells per culture plate as controls. Positive controls were included to monitor changes in the BEAS-2B cell response. All experiments were replicated with at least two independent cell passages.
The role of the TRPV1 receptor was tested by pretreating the cells with the receptor antagonist capsazepine (CPZ) at 1, 2, and 4 μM for 30 min followed by cotreatment with particles and the same concentration of CPZ. The role of reactive oxygen species was tested by adding 2 mM dimethylthiourea to the particle-containing culture media.
Cytotoxicity assay
Cell viability was assessed using the cell counting kit (CCK-8, Dojindo Laboratories, Gaithersberg MD). We incubated the cells for 2h in culture media containing 4% of the reagent, then transferred media containing the reacted dye to a 96-well plate. Viable cell count relative to control was calculated by absorbance at 450 nm minus the absorbance at 630 nm, corrected for the slight absorbance (approximately 0.05–0.07) of cell-free reagent in LHC-9. Preliminary experiments showed that the particles did not interfere with this assay.
ELISA assays
The concentrations of IL-6, IL-8, and TNF-α in the cell culture media were determined using sandwich ELISA assays. For IL-6, we used both a commercial kit (R&D Systems, Minneapolis MN), and plates prepared with anti-human IL-6, biotin-conjugated anti-human IL-6, and avidin-horserad-ish peroxidasefrom eBioscience(SanDiego CA).All IL-6 values werequantified using an R&D Systems recombinant human IL-6 standard. The IL-8 ELISA used the R&D Systems DuoSet IL-8 development kit antibodies and standard. TNF-α was determined using a kit from R&D Systems.
Statistics
Student’s t-test and the Dunnett’s test option in the JMP statistical package (SAS Institute) were used to determine the statistical significance (p < 0.05) of differences between treatment conditions.
RESULTS
The experimental results support the hypothesis that certain soil-derived dusts induce proinflammatory responses in lung epithelial cells. The DD, WM, and R4 sites yielded dusts that reproducibly caused several-fold increase in IL-6 release by BEAS-2B cells, and dust from the UN site was highly cytotoxic. Table 2 shows the elemental analysis for these soil-derived samples. Major elements are given as weight percent, and trace transition metals are given in ppm. Micron-sized TiO2 and Al2O3 and five other soil-derived dusts showed essentially no IL-6 release compared to control in preliminary experiments (data not shown).
TABLE 2.
Elemental Analysis of Soil-Derived PM2.5-Enriched Samples
| DD | WM | R4 | UN | |
|---|---|---|---|---|
| Major elements in % | ||||
| Al | 0.66 | 1.74 | 1.23 | 0.46 |
| Si | 0.83 | 1.14 | 1.34 | 0.16 |
| Mg | 2.06 | 0.79 | 1.09 | 0.13 |
| Ca | 19.3 | 5.76 | 15.8 | 0.53 |
| Fe | 0.82 | 1.38 | 0.85 | 3.43 |
| Organic C | 8.1 | 5.2 | 3.3 | 8.5 |
| Elemental C | 0.26 | 0.18 | 0.08 | 0.89 |
| Trace elements in ppm | ||||
| V | 12 | 23 | 20 | 27 |
| Cr | 3 | 15 | 5 | 6 |
| Mn | 524 | 1019 | 835 | 2369 |
| Co | 5 | 14 | 10 | 24 |
| Ni | 15 | 32 | 23 | 12 |
| Cu | 50 | 665 | 72 | 349 |
| Zn | 89 | 469 | 94 | 49 |
| Pb | 24 | 300 | 32 | 27 |
Note. Metals analysis by nitric acid digestion and ICP-MS. Elements do not add to the total due to, for example, moisture, acid-insoluble minerals, and oxygen. EC and OC data from (Labban et al., 2004).
Figure 1 presents the cytotoxicity, IL-6, and IL-8 response data for the soil dusts used in this study. The top graph shows that UN and WM are the most cytotoxic, while DD is the least cytotoxic. The D D and R4 particles induce an eight-fold increase in IL-6 at the highest particle treatment level. The WM sample shows a peak IL-6 induction at 40 μg/cm2, and the UN particles show similar IL-6 concentration for all treatments above 10 μg/ cm2. Cell death may limit the amount of IL-6 in the media after 24-h treatment at the higher concentrations of WM and UN. The IL-8 release in response to particle treatment was qualitatively similar to the IL-6 response, as shown in the bottom panel of Figure 1. The DD and WM dusts induced IL-8 in a concentration-dependent manner. The IL-8 release from the higher concentrations of the WM and UN dusts appears to be limited by toxicity. The R4 dust was not used in the IL-8 experiments due to the small amount of PM2.5-enriched material extracted from this soil.
FIG. 1.

Geological dusts are cytotoxic to BEAS-2B cells and induce IL-6 and IL-8 release at particle concentrations from 10 to 160 μg/cm2. x¯±SD, n = 6. # designates not statistically different from control; > and <, respectively, designate statistically greater than and statistically less than the response for the same concentration of DD, p < 0.05.
The release of TNF-α was not detected at the 24-h time point when cells were treated with DD, WM, and UN at 80 or 40 μg/cm2. The lowest TNF-α standards showed good reproducibility (4% coefficient of variation), so the limit of detection was nominally <16 pg/ml.
The IL-6 response to the LPS-containing mixtures was primarily due to the LPS, and the response to plausible amounts of LPS was much less than the response to the environmental dust particles. Figure 2 shows that the TiLPS produced less than a two-fold increase in IL-6 over control compared to over sevenfold increase observed with the soil dust positive control. There was no significant difference between treatment with TiO2 and LPS added separately and treatment with LPS alone. The treatments containing commercial LPS were less cytotoxic than DD, and the viable cell count was greater than 70% of control at all treatment levels. The TiLPS and the 2000 EU/ml soluble LPS treatment both induced IL-8 release that was significantly above control but much less than the response to the DD particles.
FIG. 2.

BEAS-2B cells treated with surrogates containing P. aeruginosa LPS showed no cytotoxicity and a minimal increase in IL-6 and IL-8 release compared to the particle positive control. TiLPS, micron-size TiO2 spiked with LPS and dried, doses in μg of dry mixture/cm2 of culture well; Ti + LPS, 80 μg/cm2 of TiO2 and 2000 EU/ml of LPS added separately to the culture media; LPS, 2000 EU/ml; DD80, positive control, 80 μg/cm2 of soil dust. x¯±SD, n = 6. * designates statistically different from control, p < 0.05.
Endotoxin content of the treatments did not correlate with the IL-6 released by the cells. Figure 3 shows the IL-6 response (left axis scale) for the four environmental samples and the three surrogate samples ranked in order of increasing endotoxin content (right axis scale). The endotoxin in the soil dust samples was converted to EU/ml based on a 200 μg/ml particle concentration, which is equivalent to 80 μg/cm2 for the culture conditions. The soil dusts have low measured endotoxin but cause high IL-6 release, and the opposite is true for the surrogates prepared with commercial LPS. The LPS positive control was 2000 EU/ml based on the manufacturer’s nominal activity of 3 million EU/mg, and the LAL assay gave a value of 1500 EU/ml.
FIG. 3.
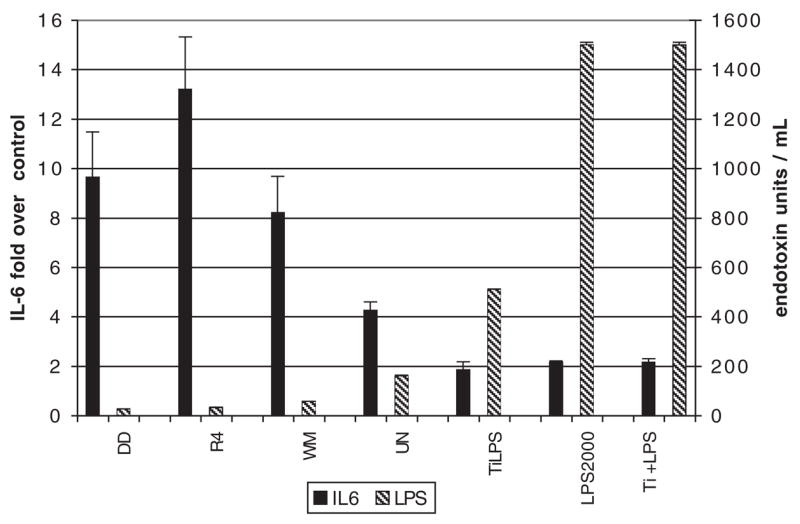
IL-6 fold increase over control (left axis) and endotoxin concentration in the soil dusts and surrogate materials (right axis) have an inverse correlation, demonstrating that the response is not due to endotoxin. IL-6 x¯±SD, n = 6. Endotoxin n = 2.
The particle characteristics or components that cause cell death and IL-6 release are robust, but can be altered by physical treatments. Figure 4 shows the effect of the thermal and leaching treatments of DD, WM, and R4. Heating of the particles had no effect on cytotoxicity, but 3-day aqueous washing did reduce the cytotoxicity of the particles. However, as discussed below, the effect of leaching was likely a combination of mechanical loss of particles during washing and any selective dissolution. Separation of the samples into an LHC-9 leached solid and a supernatant containing the components that dissolve in media showed that cytotoxicity is associated with the solid phase.
FIG. 4.
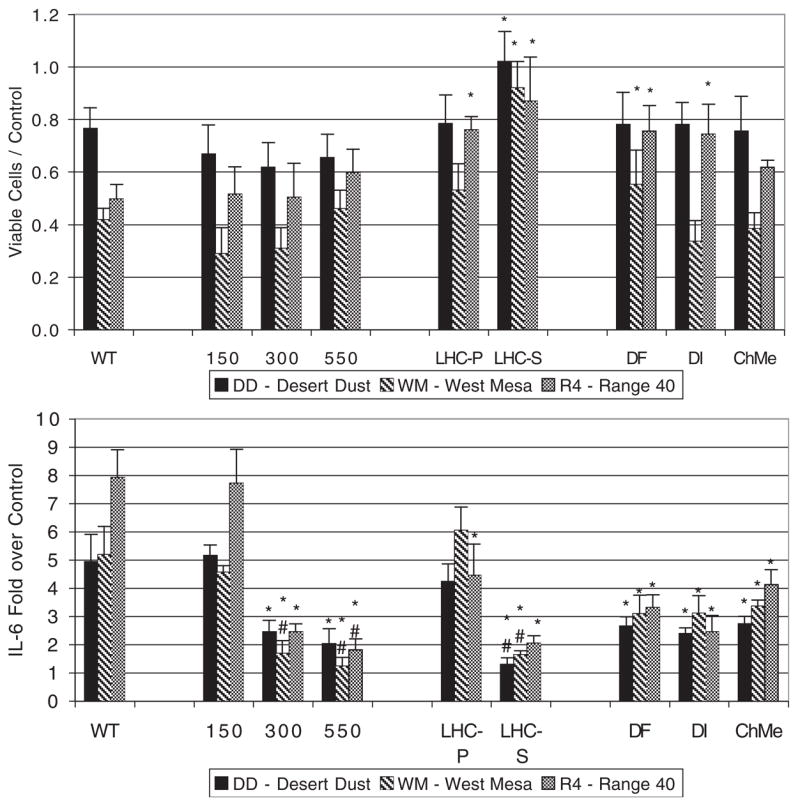
Cytotoxicity and IL-6 response of cells exposed to untreated and physically modified particles of soil dust. All exposures were 200 μg of the original particles/ml of media. Identification: WT, wild-type unmodified particles, 150, 300, 550, oxidizing thermal treatment at indicated temperature for one hour; LHC-9 S, supernatant from initial leaching in cell culture media; LHC-9 P, DF, DI, ChMe, particles recovered after three cycles of leaching in LHC-9, 1 mM desferrioxamine, ion-exchange treated water, and chloroform-methanol, respectively. x¯±SD, n = 6. * designates statistically different from untreated particles, # designates not statistically different from media-only control, p < 0.05.
Physical treatments also affect the particle factors controlling the IL-6 response (Fig. 4). Thermal treatment at 150°C for 1 h had no significant effect on the IL-6 response. Heating to 300 and 550°C attenuated the IL-6 release. Treatment of the DD and WM dust at 550°C reduced the IL-8 response to 5.6- and 1.7-fold over control, respectively, compared to 16- and 10-fold increases for the wild-type particles at the maximum-response treatment concentration. The IL-8 response to 550°C treated WM dust was not significantly different than control. Heating to 550°C is used to prebake quartz filters for trace organic analysis and is sufficient to oxidize most organic materials, but 550°C treatment did not reduce the IL-6 and IL-8 responses to the control level for DD. The 3-day leaching treatments attenuated the IL-6 release, but did not reduce it to control level. The IL-6 release response was predominantly associated with the solid phase for all three soil dusts. The LHC-9 supernatant caused a small, but statistically significant, IL-6 response for R4. All three leaching treatments, the metal chelator desferrioxamine, the slightly acidic water, and the lipid solvent chloroform-methanol, had similar effects, suggesting that the leaching process did not involve selective dissolution of basic organic components, metals, or hydrophobic components.
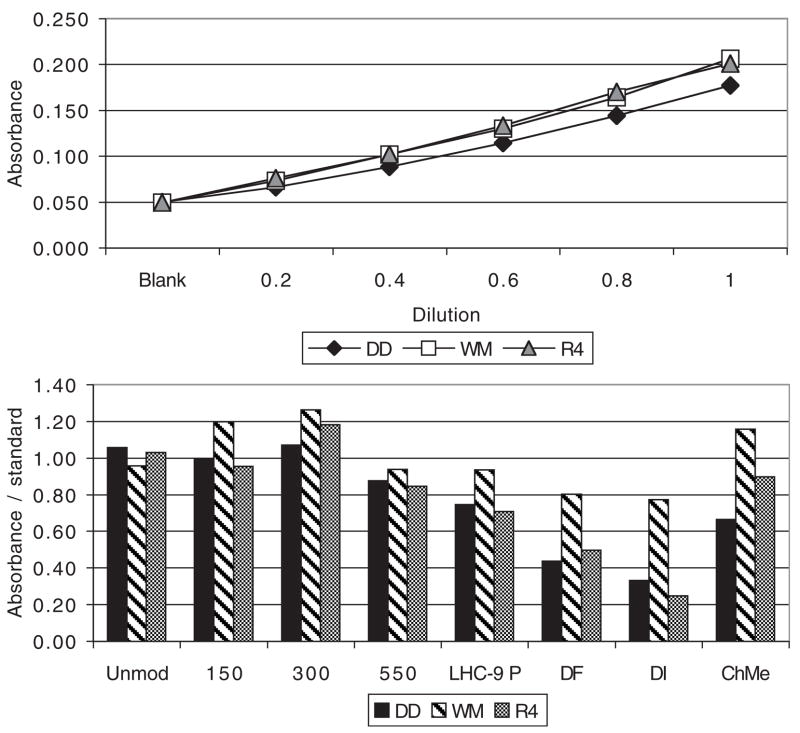
In order to check for material loss from physical treatment of the particles, the absorbance versus mass concentration of treated particles was obtained by a standard curve using a dilution series of each of the untreated suspensions (Fig. 5a). The concentrations of the washed particles appeared to be within ±25% of the unmodified particle treatment with the exception of two leaching treatments for DD and R4. This suggested that the effect of desferrioxamine and water leaching on the DD and R4 soil dusts was due to material loss.
FIG. 5.
Light absorbance indicates that physically modified exposure concentrations were generally within ± 25% of the standard containing 200 μg/ml, but some material loss occurred during leaching. Top (5a): standard curves obtained with a dilution series for each particle type. Bottom (5b): Absorbance of the treated particle suspensions compared to unmodified particles; n = 2.
The experiments to test for TRPV1 receptor involvement in IL-6 release were confounded by the toxicity of the CPZ antagonist, and results were inconclusive (data not shown). Addition of the antioxidant and reactive oxygen species scavenger DMTU to the media also did not have a statistically significant effect on the IL-6 release, in response to the four soil dust types (data not shown).
DISCUSSION
This study of soil-derived PM2.5, particles that are representative of vehicle- and wind-generated dusts, contributes to the growing body of evidence that proinflammatory signaling cascades are activated in lung epithelial cells exposed to specific types of particulate air pollution. Kaolin clay, TiO2, Al2O3, and five other soil dusts used in preliminary experiments were relatively benign when tested with the BEAS-2B cell culture model. However, some simulated road dust samples induced significant response, and this study focused on three soils that induced high levels of IL-6 release and one soil that was highly cytotoxic. This study used physical treatments of these particles as a way to elucidate the chemical components or physical particle characteristics that may be most important for inducing the observed responses. The components or characteristics of the soil-derived particles that cause the IL-6 release (1) were associated predominantly with the solid particles, (2) were only partially attenuated by organic or aqueous washing, and (3) were stable at temperatures below 150°C. Treatment at 550°C reduced the IL-6 response, but not the cytotoxicities of the particles, potentially implying that independent factors are responsible for cell death and IL-6 release.
The endpoints commonly measured with in vitro studies of ambient and mineral particles include the cytokines IL-6 (Becher et al., 2001; Carter et al., 1997; Hetland et al., 2000), IL-8 (Carter et al., 1997; Hetland et al., 2000; Smith et al., 2000) and TNF-α (Carter et al., 1997; Imrich et al., 2000), second messenger signals such as Ca influx (Veronesi et al., 2002a), and oxidative damage (Schins, 2002). The focus of this study was on cell death and IL-6 release. Release of IL-8 in response to the soil dust and LPS treatments was qualitatively similar to the IL-6 responses, suggesting a common or closely related mechanism. The lack of detectable TNF-α is somewhat surprising since this cytokine has been reported to be involved in the regulation of both IL-6 and IL-8 (Nelson and Martin, 2000). The suite of PM2.5-enriched soil dusts in the as-collected and surface-modified condition provide model particles that can be used in future mechanistic in vitro studies to elucidate the pathways by which particles interact with lung cells.
We previously reported necrotic cell death in normal BEAS-2B cells treated with capsaicin at concentrations associated with the maximal IL-6 induction (Reilly et al., 2003). The Vybrant Apoptosis Detection Kit #3 (Molecular Probes), which uses propidium iodide (PI) and annexin-FITC, was used in an attempt to assess the cell death mechanism in response to particle treatments. Control cells, cell-free particles, and particle-treated cells were examined using a fl uorescence activated cell sorter (FACScan, Becton-Dickinson). However, the soil dusts contain particles of fluorescent minerals that are detected in the PI and FITC channels. Also, particles associated with cells increase the side scatter signal. Attempts to eliminate the particle signals by gating appeared to also eliminate dead cells, presumably because the dead cells were the cells associated with surface-bound or internalized particles. These particle effects seriously confounded the cell death assay data, and no conclusions could be drawn.
The physical treatment experiments supported our assumption that handling procedures such as collection of particles from filters by washing, and the subsequent drying and sterilizing of the sample, do not confound experiments with soil and mineral dusts.
New insights have been gained from the physical treatment experiments, but the specific components or characteristics of the soil-derived particles that cause the IL-6 and IL-8 release remain elusive. Many papers have been published reporting in vitro cytokine induction in response to particles. The novel contributions of this paper are that a significant range of responses, from benign to highly inflammatory and cytotoxic, is caused by soil particles that are representative of fugitive dust from unpaved roads, and that physical treatments can be used to modify soil-derived PM2.5 particles as a means of testing toxicology hypotheses related to potential active components or particle characteristics.
Hypotheses for particle-induced toxicity and cytokine release that are in the current literature include metal-dependent ROS generation, vanilloid receptor activation, and LPS-mediated events. We have investigated whether these mechanisms are involved in the responses to soil dust. The studies using soluble LPS, particle-associated LPS, heat-treated particles, and solvent-washed particles all implied that LPS was not the dominant component inducing the response. Treatments with the metal chelator desferrioxamine, the ROS scavenger dimethyl-thiourea, and the TRPV1 antagonist CPZ gave mixed results. The evidence from this study implies that the soil-dust-induced IL-6 release and cytotoxicity result from interactions between solid-phase particle components and the cells. Potential alternative hypotheses include activation of receptors other than TRPV1 by chemicals or sites on the particle surface and processes involving particle uptake by the cells.
It was unfortunate that the TRPV1 nonspecific antagonist, CPZ, was highly cytotoxic under our experimental conditions, because amelioration of IL-6 release has been reported in particle studies by others (Veronesi, 1999). Current studies with more selective TRPV1 antagonists, which are not cytotoxic at low concentrations, may provide more compelling experimental evidence for vanilloid receptor participation in the inflammatory process in response to mineral dust particles.
The physical treatment results also put constraints on the nature of the active components of the particle mixture. For example, most semivolatile and biogenic organic compounds would be removed or inactivated by heating or by washing with solvents. Speculation concerning other components of the particles that could be responsible for release of IL-6 and IL-8 by these immortalized lung cells could focus on organic components (redox-active quinones for example) or redox-active metals. However, these components would have to be very tightly associated with the particles, not affected by dimethyl-thiourea, and stable enough to survive heating to 150°C. We are not aware of any immediately compelling examples of such components.
Endotoxin is known to cause release of IL-6 and IL-8 and is associated with lung inflammation, but this study demonstrated that the response to these soil dusts was not due to particle-associated endotoxin. The evidence includes the limited response to surrogate particles containing LPS (Fig. 2), the lack of correlation between measured endotoxin and IL-6 release (Fig. 3), and the lack of an effect from thermal treatment (Fig. 4) at temperatures higher than those that are used to remove LPS from manufactured medical products.
The concentrations of particles and LPS used in this study are comparable to those used by other investigators. Table 3 indicates typical particle and LPS treatment concentrations used for IL-6 and IL-8 response experiments with BEAS-2B cells grown submerged in media. Particle concentrations of 10 to 80 μg/cm2 convert to 25–200 μg/ml for the cell culture wells used in this study. The LPS concentration of 2000 EU/ml converts to 7 × 105 pg/ml. These concentrations are high compared to plausible lung deposition doses. A calculation (Lighty et al., 2000) based on typical values for particle size distribution, ventilation rate, and deposition fraction shows that a 10 μg/m3 increase in ambient PM 2.5concentration results in an increment of 0.02–0.05 mg of particles deposited in the lung per day (approximately 70 m2). However, particles in vitro are diluted by 3–5 mm of cell culture media compared to a typical lung surfactant liquid layer of 0.05–0.2 μm (Miller et al., 1985). Immortalized cells in submerged culture also have reduced response to particles compared to other, presumably more realistic, systems such as cells grown on an air–liquid interface (Seagrave et al., 2004) or macrophage–epithelial cell coculture (Tao and Kobzik, 2002). Cell culture experiments using high treatment concentrations are most appropriate for mechanistic toxicology experiments where the goal is to induce and modify specific signaling responses in a simplified biological model. Cell culture is also proposed as an expedient method of screening large numbers of environmental samples to select materials for animal toxicology studies.
TABLE 3.
Treatment Concentrations Used in BEAS-2B Cell Culture Cytokine Experiments
| Reference | Treatment materials | Concentration | Signaling endpoints | Results |
|---|---|---|---|---|
| (Veronesi et al., 2002a) | Mt. St. Helens ash | 20–200 mg/ml | IL-6 | Response correlates with surface charge. |
| Oil fly ash | [Ca+2] | |||
| Coal fly ash | ||||
| Urban PM, St. Louis | ||||
| Urban PM, Ottawa | ||||
| Wood stove | ||||
| (Steerenberg et al., 1998) | Diesel particulate | 70–333 mg/ml | IL-6 | Silica produced higher response than diesel. TiO2 was inert. |
| SiO2 | IL-8 | |||
| TiO2 | ||||
| (Drumm et al., 2000) | Soot, FR101 | 10–100 mg/ml | IL-6 | Dose response with soot, enhanced by coculture. |
| Coculture with blood monocytes | IL-8 | |||
| (Carter et al., 1997) | Residual oil fly ash | 5–200 mg/ml | IL-6 | Vanadium, but not iron or nickel compounds mimic ROFA response. |
| IL-8 | ||||
| TNF-α | ||||
| (Schulz et al., 2002) | E.coli LPS | 10−1–107 pg/ml | IL-6 | Dose response for treatments >104 pg/ml in presence of serum. |
| IL-8 |
Geological dusts have often been assumed to be a benign or inert component of ambient air pollution. This study supports the hypothesis that the solid-phase PM2.5 component of certain desert soil dusts is responsible for both proinflammatory and cytotoxic responses in lung epithelial cells exposed to particles in vitro. Thus, studies of the health effects associated with the complex mixtures in ambient air pollution need to include assessment of the fine particles of geological origin.
Acknowledgments
This study was supported by the following grants: NIEHS Research Career Development Award K25 ES011281 (J.M.V.); NIH grant HL069813 (C.A.R. and G.S.Y.); and NIH grant HL13645 (G.S.Y.). Technical assistance from Dr. Jeff Hall for metals analysis, Dr. Wayne Green for flow cytometry, and Erin Kaser for sample generation is acknowledged.
References
- Adamson IY, Prieditis H, Vincent R. Pulmonary toxicity of an atmospheric particulate sample is due to the soluble fraction. Toxicol Appl Pharmacol. 1999;157:43–50. doi: 10.1006/taap.1999.8658. [DOI] [PubMed] [Google Scholar]
- Agopyan N, Li L, Yu S, Simon SA. Negatively charged 2 and 10-μm particles activate vanilloid receptors, increase cAMP and induce cytokine release. Toxicol Appl Pharmacol. 2003;186:63–76. doi: 10.1016/s0041-008x(02)00013-3. [DOI] [PubMed] [Google Scholar]
- Becher R, Hetland RB, Refsnes M, Dahl JE, Dahlman HJ, Schwarze PE. Rat lung inflammatory responses after in vivo and in vitro exposure to various stone particles. Inhal Toxicol. 2001;13:789–805. doi: 10.1080/08958370118221. [DOI] [PubMed] [Google Scholar]
- Carter JD, Ghio AJ, Samet JM, Devlin RB. Cytokine production by human airway epithelial cells after exposure to an air pollution particle is metal-dependent. Toxicol Appl Pharmacol. 1997;146:180–188. doi: 10.1006/taap.1997.8254. [DOI] [PubMed] [Google Scholar]
- Claiborn CS, Larson T, Sheppard L. Testing the metals hypothesis in Spokane, Washington. Environ Health Perspect. 2002;110:547–552. doi: 10.1289/ehp.02110s4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll KE. Cytokines and Regulation of Pulmonary Inflammation. In: Gardner DE, Crapo JD, McClellan RO, editors. Toxicology of the Lung. Taylor and Francis; Philadelphia, PA: 1999. pp. 149–172. [Google Scholar]
- Drumm K, Attia DI, Kannt S, Micke P, Buhl R, Kienast K. Soot-exposed mononuclear cells increase inflammatory cytokine mRNA expression and protein secretion in bronchial epithelial cells. Respiration. 2000;67:291–297. doi: 10.1159/000029513. [DOI] [PubMed] [Google Scholar]
- Dye JA, Alder KB, Richards JH, Dreher KL. Role of soluble metals in oil fly ash-induced airway epithelial injury and cytokine gene expression. Am J Physiol. 1999;3(1):L498–L510. doi: 10.1152/ajplung.1999.277.3.L498. [DOI] [PubMed] [Google Scholar]
- Elder ACP, Gelein R, Finkelstein JN, Cox C, Oberdörster G. Endotoxin priming affects the lung response to ultrafine particles and ozone in young and old rats. Inhal Toxicol. 2000;12(Suppl 1):85–98. [Google Scholar]
- Fan J, Ye RD, Malik AB. Transcriptional mechanisms of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1037–L1050. doi: 10.1152/ajplung.2001.281.5.L1037. [DOI] [PubMed] [Google Scholar]
- Frampton MW, Ghio AJ, Samet JM, Carson JL, Carter JD, Devlin RB. Effects of aqueous extracts of PM10 filters from the Utah Valley on human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 1999;21:L960–L967. doi: 10.1152/ajplung.1999.277.5.L960. [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Stonehurner J, Dailey LA, Carter JD. Metals associated with both the water-soluble and insoluble fractions of an ambient air particle catalyze an oxidative stress. Inhal Toxicol. 1999;11:37–49. doi: 10.1080/089583799197258. [DOI] [PubMed] [Google Scholar]
- Hetland RB, Refsnes M, Myran T, Johansen BV, Uthus Nand Schwartz PE. Mineral and/or metal content as critical determinants of particle-induced release of IL-6 and IL-8 from A549 cells. J Toxicol Environ Med. 2000;60:47–65. doi: 10.1080/009841000156583. [DOI] [PubMed] [Google Scholar]
- Hetland RB, Myhre O, Lag M, Hongve D, Schwarze PE, Refsnes M. Importance of soluble metals and reactive oxygen species for cytokine release induced by mineral particles. Toxicology. 2001;165:133–144. doi: 10.1016/s0300-483x(01)00418-8. [DOI] [PubMed] [Google Scholar]
- Imrich A, Ning Y, Kobzik L. Insoluble components of concentrated air particles mediate alveolar macrophage responses in vitro. Toxicol Appl Pharmacol. 2000;167:140–150. doi: 10.1006/taap.2000.9002. [DOI] [PubMed] [Google Scholar]
- Koyama S, Sato E, Nomura H, Kubo K, Miura M, Yamashita T, Nagai S, Izumi T. The potential of various lipopolysaccharides to release IL-8 and G-CSF. Am J Physiol Lung Mol Physiol. 2000;278:L658–L666. doi: 10.1152/ajplung.2000.278.4.L658. [DOI] [PubMed] [Google Scholar]
- Labban R, Veranth JM, Chow JC, Englebrecht J, Watson J. Size and geographical variation in PM1, PM2.5, and PM10 source profiles from soils in the western United States. Water Air Soil Pollut. 2004 accepted for publication. [Google Scholar]
- Lighty JS, Veranth JM, Sarofim AF. Combustion aerosols: Factors governing their size and composition and implications to human health. J Air Waste Manag Assoc. 2000;50:174–227. doi: 10.1080/10473289.2000.10464197. [DOI] [PubMed] [Google Scholar]
- Miller FJ, Overton JHJ, Jaskot RH, Menzel DB. A model of the regional uptake ofgaseous pollutants in the lung. Toxicol Appl Pharmacol. 1985;79:11–27. doi: 10.1016/0041-008x(85)90364-3. [DOI] [PubMed] [Google Scholar]
- Mills PR, Davies RJ, Devalia JL. Airway epithelial cells, cytokines, and pollutants. Am J Respir Crit Care Med. 1999;160:S38–S43. doi: 10.1164/ajrccm.160.supplement_1.11. [DOI] [PubMed] [Google Scholar]
- Monn C, Koren HS. Bioaerosols in ambient air particulates: A review and research needs. Rev Environ Health. 1999;14:79–89. doi: 10.1515/reveh.1999.14.2.79. [DOI] [PubMed] [Google Scholar]
- Nelson S, Martin TR. Cytokines in Pulmonary Disease: Infection and Inflammation. Vol. 141. Marcel Dekker; New York: 2000. [Google Scholar]
- Pope AC, Hill RW, Villegas GM. Particulate Air Pollution and Daily Mortality on Utah’s Wasatch Front. Environ Health Perspect. 1999;107:567–573. doi: 10.1289/ehp.99107567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prahalad AK, Soukup JM, Inmon J, Willis R, Ghio AJ, Becker S, Gallagher JE. Ambient air particles: Effects on cellular oxidant radical generation in relation to particulate elemental chemistry. Toxicol Appl Pharmacol. 1999;158:81–91. doi: 10.1006/taap.1999.8701. [DOI] [PubMed] [Google Scholar]
- Reddel RR, Yang K, Rhim JS, Brash D, Su RT, Lechner JF, Gerwin BI, Harris CC, Amstad P. Immortalized human bronchial epithelial mesothelial cell lines. USA: Department of Health and Human Services; 1989. [Google Scholar]
- Reilly CA, Taylor JL, Lanza DL, Carr BA, Crouch DJ, Yost GS. Capsaicinoids cause inflammation and epithelial cell death through activation of vanilloid receptors. Toxicological Sciences. 2003;73:170–181. doi: 10.1093/toxsci/kfg044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenker M. Exposures and health effects from inorganic agricultural dusts. Environ Health Perspect. 2000;108:661–664. doi: 10.1289/ehp.00108s4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schins RPF. Surface modification of quartz inhibits toxicity particle uptake and oxidative DNA damage in human lung epithelial cells. Chemical Research in Toxicology. 2002;15:1166–173. doi: 10.1021/tx025558u. [DOI] [PubMed] [Google Scholar]
- Schulz C, Farkas L, Wolf K, Krätzel K, Eissner G, Pfeifer M. Differences in LPS-induced activation of bronchial epithelial cells (BEAS-2B) and type II-like pneumocytes (a-549) Scan J Immunol. 2002;56:294–302. doi: 10.1046/j.1365-3083.2002.01137.x. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Norris G, Larson T, Shepphard L, Claiborne C, Koenig J. Episodes of high coarse particle concentrations are not associated with increased mortality. Environ Health Perspect. 1999;107:339–342. doi: 10.1289/ehp.99107339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagrave J, McDonald JD, Mauderly JL. Air-liquid interface culture: Towards more physiological in vitro toxicology of aerosols. Society of Toxicology; Baltimore, MD: 2004. p. Abstract 1558. [Google Scholar]
- Seagrave JC, Nikula KJ. Multiple modes of responses to air pollution particulate materials in A549 alveolar type II cells. Inhal Toxicol. 2000;12(Suppl 4):247–260. doi: 10.1080/089583700750019594. [DOI] [PubMed] [Google Scholar]
- Smith KR, Veranth JM, Hu AA, Lighty JS, Aust AE. Interleukin-8 levels in human lung epithelial cells are increased in response to coal fly ash and vary with bioavailability of iron, as a function of particle size and source of coal. Chem Res Toxicol. 2000;13:118–125. doi: 10.1021/tx9901736. [DOI] [PubMed] [Google Scholar]
- Steerenberg PA, Zonnenberg JA, Dormans JA, Joon PN, Wouters IM, van Bree L, Scheepers PT, Van Loveren H. Diesel exhaust particles induced release of interleukin 6 and 8 by (primed) human bronchial epithelial cells (BEAS 2B) in vitro. Exp Lung Res . 1998;24:85–100. doi: 10.3109/01902149809046056. [DOI] [PubMed] [Google Scholar]
- Tao F, Kobzik L. Lung macrophage-epithelial cell interactions amplify particle-mediated cytokine release. Am J Respir Cell Mol Biol. 2002;26:499–505. doi: 10.1165/ajrcmb.26.4.4749. [DOI] [PubMed] [Google Scholar]
- van Maanen JM, Borm PJ, Knaapen A, van Herwijnen M, Schilderman PA, Smith KR, Aust AE, Tomatis M, Fubini B. In vitro effects of coal fly ashes: Hydroxyl radical generation, iron release, and DNA damage and toxicity in rat lung epithelial cells. Inhal Toxicol. 1999;11:1123–1141. doi: 10.1080/089583799196628. [DOI] [PubMed] [Google Scholar]
- Veranth JM, Smith KR, Aust AE, Dansie SL, Griffith JB, Hu AA, Huggins ML, Lighty JS. Coal fly ash and mineral dust for toxicology and particle characterization studies: Equipment and methods for PM2.5- and PM1-enriched samples. Aerosol Sci Technol. 2000;32:127–141. [Google Scholar]
- Veronesi B, Oortgiesen M, Carter JD, Devlin RB. Particulate matter initiates inflammatory cytokine release by activation of capsaicin and acid receptors in a human bronchial epithelial cell line. Toxicol Appl Pharmacol. 1999;154:106–115. doi: 10.1006/taap.1998.8567. [DOI] [PubMed] [Google Scholar]
- Veronesi B, de Haar C, Lee L, Oortgiesen M. The surface charge of visible particulate matter predicts biological activation in human bronchial epithelial cells. Toxicol Appl Pharmacol. 2002a;178:144–154. doi: 10.1006/taap.2001.9341. [DOI] [PubMed] [Google Scholar]
- Veronesi B, de Haar C, Roy J, Oortgiesen M. Particulate matter inflammation and receptor sensitivity are target cell specific. Inhal Toxicol. 2002b;14:159–183. doi: 10.1080/089583701753403971. [DOI] [PubMed] [Google Scholar]
- Williams KL. Endotoxins, Pyrogens, LALTesting and Depyrogenation. Marcel Dekker; New York: 2001. [Google Scholar]