Abstract
Purpose
To quantify the retinal thickness and the refractive error of the healthy human eye during hyperglycemia by means of optical coherence tomography (OCT) and Hartmann–Shack aberrometry.
Methods
Hyperglycemia was induced in five healthy subjects who were given a standard oral glucose tolerance test (OGTT) after a subcutaneous injection of somatostatin. Main outcome parameters were the central, pericentral and peripheral thickness of the fovea, measured by means of optical coherence tomography (OCT3). Ocular refractive error was determined with Hartmann-Shack aberrometry. Measurements at baseline and during maximal hyperglycemia were analyzed, and a change was considered clinically significant if the difference between the measurements exceeded the threshold of 50 μm for retinal thickness and 0.2 D for refractive error.
Results
During hyperglycemia (mean blood glucose level at baseline: 4.0 mmol/l; mean maximal blood glucose level: 18.4 mmol/l) no significant changes could be found in the central, pericentral, or peripheral foveal thickness in any of the five subjects. One of the subjects had a hyperopic shift of 0.4 D, but no significant change in refractive error was found in any of the other subjects.
Conclusions
The present study shows that in healthy subjects induced hyperglycemia does not affect retinal thickness, but it can cause a small hyperopic shift of refraction.
Keywords: Hyperglycemia, Diabetes mellitus, Optical coherence tomography, Refractive errors
Patients with diabetes mellitus (DM) often experience subjective symptoms of blurred vision associated to hyperglycemia. The nature and origin of this phenomenon are still unclear. Blurred vision during hyperglycemia could be a result of transient refractive alterations due to changes in the lens [5, 12, 15, 25, 27, 36, 37], but it could also be caused by changes in the retina. Macular edema, or retinal thickening due to abnormal fluid accumulation within the macula, is a common cause of visual loss [1, 14, 22]. The degree of retinal thickening has been found to be significantly correlated with visual acuity [24]. Furthermore, a change in retinal thickness, resulting in a change in axial eye length, could also induce a change in ocular refractive error. For instance, it can be calculated that with a 50 μm increase in retinal thickness, the ocular refractive error becomes 0.15 D more hyperopic [30].
Several studies have demonstrated that retinal thickness is affected by DM [2, 7, 8, 18, 21, 23, 26, 32, 33, 35, 38]. In general, an increase in retinal thickness has been reported in patients with long-term DM and advanced stages of retinopathy [7, 8, 18, 32, 33, 35]. However, in diabetic patients with and without minimal diabetic retinopathy, a decrease in retinal thickness has been observed [2, 23]. In healthy subjects, it has been shown that the average retinal thickness did not change during normo-insulinaemic hyperglycemia [13].
It is unclear whether the thickness of the different retinal areas, such as the foveal area, the pericentral foveal area, and the peripheral foveal area, changes during acute hyperglycemia and suppression of insulin. A change in retinal thickness and/or ocular refractive error could explain the subjective symptoms of blurred vision in patients with DM and hyperglycemia. Therefore, in the present study the effect of hyperglycemia on retinal thickness and ocular refractive error was investigated in healthy subjects during suppression of endogenous insulin. Retinal thickness was measured by means of optical coherence tomography (OCT), which is a non-invasive technique that provides cross-sectional retinal images, and produces an objective measurement of the retinal thickness, independent of the refractive status of the eye [10, 11, 29]. Furthermore, aberrometry was used to measure the ocular refractive error. This technique makes it possible to detect small changes in ocular refraction [19].
Subjects and methods
Five healthy subjects (two males and three females) participated in the study. The mean age of the subjects was 24.8 years (range 21.2–32.6), and their mean body mass index (BMI) was 24.2 kg/m2 (range 21.4–29.7). The subjects were screened during a first visit, which included medical history-taking, a physical examination (measurement of visual acuity, weight, height and blood pressure) and collecting a fasting blood sample. Exclusion criteria were a history of DM (or a fasting plasma glucose >5.5 mmol/l), a BMI of >30 kg/m2, elevated blood pressure (>140 / 85 mmHg), a visual acuity of <0.5 (Snellen) or a history of ocular pathology. The investigators of the ocular parameters (NW and MD) were not informed about the blood glucose levels. Furthermore, the investigators who induced hyperglycemia (EE and SS) were not informed about the results of the ocular measurements. The study protocol was approved by the Medical Ethics Committee of the VU University Medical Center in Amsterdam, and written informed consent was obtained from all subjects after the purpose and nature of the study had been explained to them.
Procedure to induce hyperglycemia
After a 10-hour overnight fast, the subjects were given a subcutaneous injection of a low dose (100 μg) of synthetic somatostatin (Sandostatin, Novartis, Basel, Switzerland) in order to suppress endogenous insulin secretion. Each subject underwent an oral glucose tolerance test (OGTT) (75 g glucose) 30 minutes after the somatostatin injection, and blood glucose levels were measured with a blood glucose analyzer (HemoCue Diagnostics BV, Oisterwijk, the Netherlands). Endogenous insulin levels were measured by means of immunometric assays (Luminescence, Bayer Diagnostics, Mijdrecht, the Netherlands) in the Endocrinology Laboratory at the Department of Clinical Chemistry of the VU University Medical Center. The subjects remained in fasting state during the entire procedure.
Ocular measurements
Retinal thickness was measured with the Stratus OCT (Model 3000, Carl Zeiss Meditec, Dublin, CA, USA), which combines a low-coherence scanning interferometer (wavelength 820 nm) with a video camera to visualize the fundus of the eye. The fast macular thickness OCT scan protocol was used to obtain six cross-sectional macular scans, 6 mm in length, which were positioned at equally spaced angular orientations (30°) centred on the fovea. The cross-sectional images were analyzed with OCT3 mapping software that uses an edge-detection technique to locate the vitreoretinal interface and the anterior surface of the retinal pigment epithelium. Retinal thickness was defined as the distance between these two surfaces. Two OCT scans were made of each subject before, and every 30 minutes during the period of hyperglycemia. In order to quantify the retinal thickness, the foveal map constructed by the software was divided into nine Early Treatment Diabetic Retinopathy Study (ETDRS) areas [6]: the central fovea (central circle with a diameter of 1 mm), and the pericentral area (donut-shaped ring with an inner diameter of 1 mm and an outer diameter of 3 mm) and peripheral area (donut-shaped ring with an inner diameter of 3 mm and an outer diameter of 6 mm), both of which were divided into four quadrants. Retinal thickness was calculated for all separate areas, and for the average pericentral and peripheral regions.
Ocular refractive error was determined with an IRX3 aberrometer (Imagine Eye Optics, Paris, France), which performs wavefront analysis of the eye according to the Hartmann-Shack principle [19]. After pupil dilation and paralysis of accommodation with 1.0% cyclopentolate and 5.0% phenylephrine eye-drops, a series of three aberrometry measurements was made before, and every 30 minutes during the hyperglycemic condition. From these measurements, the equivalent refractive error was calculated as: equivalent refractive error (ERE) = sphere + (cylinder / 2).
The measurements at baseline and during maximal hyperglycemia were analyzed, and any change was considered to be meaningful if the difference between the measurements was greater than the threshold of 50 μm for retinal thickness and 0.2 diopters (D) for ERE. The threshold of 50 μm exceeded the 95% confidence interval for the detection of a change in retinal thickness, which has been reported to be approximately 40 μm [4, 20, 28]. A refractive change of more than 0.2 D also surpasses the precision (defined as 95% confidence interval) of the aberrometer for measuring sphere, cylinder, and consequently ERE [3, 31]. In each subject, the significance of a change was obtained from the precision of the measurement instruments and the difference in the ocular parameters at baseline and during hyperglycemia. In the whole group, the significance of a change could be determined by means of Wilcoxon matched pairs signed rank sum tests. P-values ≤0.05 were considered to be statistically significant.
Results
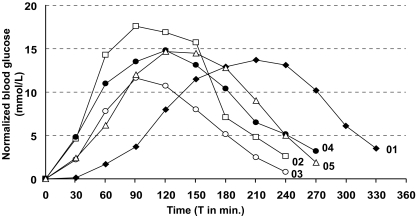
The changes in blood glucose after the administration of somatostatin and glucose are shown in Fig. 1. Mean blood glucose levels rose from 4.0 mmol/l (range 3.4–4.5 mmol/l) to 18.4 mmol/l (range 16.1–22.0 mmol/l) after the OGTT. Endogenous insulin was suppressed by the subcutaneous injection of somatostatin during the glucose load to a mean value of 2.1 pmol/l (range 0.4–4.5 pmol/l), and remained below basal secretion level (<110.0 pmol/l) for 147 minutes (range 75–270 minutes). Subject 01 had a delayed elevation of blood glucose level, compared to the other subjects. This person received a second 75 g oral glucose load after 30 minutes in order to induce a rise in the blood glucose level. In all subjects, the blood glucose and endogenous insulin levels normalized within 6 hours after the OGTT.
Fig. 1.
Graph of normalized blood glucose levels in the five subjects after the administration of somatostatin and glucose. Data are normalized by subtracting the value at baseline from the measured value in each subject. The oral glucose load (75 g) was administered at T 0. Subject 01 received an extra 75 g oral glucose load at T 30
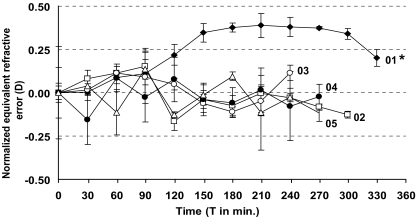
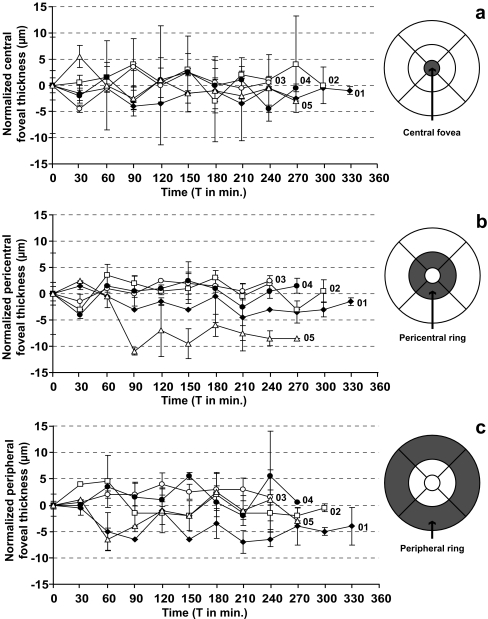
Figure 2 shows the normalized ERE of the five subjects during hyperglycemia. Mean ERE at baseline was 0.6 D (SD 0.6) and 0.7 D (SD 0.6) during maximal hyperglycemia; no significant change was found in the group as a whole. A small, but significant hyperopic shift of 0.4 D (SD 0.2) in ERE was measured in subject 01 during maximal hyperglycemia, compared to the start of the procedure (p < 0.001). No significant change in ERE was found in any of the other subjects. Normalized retinal thickness parameters are shown in Fig. 3a (central foveal area), Fig. 3b (average pericentral foveal area) and Fig. 3c (average peripheral foveal area). Average central foveal thickness, average pericentral foveal thickness, and average peripheral foveal thickness at baseline were 202 μm (SD 8), 277 μm (SD 5), and 243 μm (SD 8). During maximal hyperglycemia, average central foveal thickness, average pericentral foveal thickness, and average peripheral foveal thickness were 203 μm (SD 7), 275 μm (SD 3), and 242 μm (SD 9). No significant differences were found in the group as a whole. Furthermore, none of the subjects had any significant changes in the thickness of the central fovea, the pericentral fovea, or the peripheral fovea during maximal hyperglycemia, compared to baseline. The nine separate ETDRS areas were not affected by hyperglycemia. At baseline and during hyperglycemia, any change in retinal thickness that occurred in the various areas was less than 15 μm, which was within the previously defined threshold of 50 μm.
Fig. 2.
Graph of the normalized equivalent refractive error (ERE) in diopters (D) of the five subjects. Data are presented as mean±SD; three measurements were made of each subject every 30 minutes during the procedure. Data are normalized by subtracting the value at baseline from the measured value in each subject. The oral glucose load was administered at T 0. * Significant difference between ERE at T 0 and T 210 (maximal hyperglycemia), p < 0.001
Fig. 3.
Graphs and maps of normalized retinal thickness parameters in the five subjects during hyperglycemia: (a) central fovea, (b) pericentral fovea, (c) peripheral fovea. Data are normalized by subtracting the value at baseline from the measured value in each subject. Each measured area has been indicated by a dark grey area on the retinal maps. No significant changes in retinal parameters were found in any of the subjects. The oral glucose load was administered at T 0
Discussion
Blurred vision is a symptom that occurs frequently in patients with DM and hyperglycemia. The underlying mechanism is still unclear, and therefore the present study was carried out in an attempt to identify a possible cause of this symptom. The effect of reproducible hyperglycemia on retinal thickness and refractive error was studied in healthy young subjects who did not suffer from the systemic effects of DM.
No changes in the thickness of the central, pericentral or peripheral foveal areas were found in any of the subjects during hyperglycemia. In addition, no significant change was measured in any of the nine different ETDRS areas of the macula. In their study, Jeppesen et al. [13] also found no significant difference in retinal thickness in healthy subjects during normo-insulinaemic hyperglycemia. Before and 180 minutes after the start of a hyperglycemic clamp they measured the average thickness of the retina, and found that retinal thickness was not affected by hyperglycemia. Although in the present study retinal thickness was measured under different circumstances than in the study of Jeppesen et al. (hypo-insulinaemic hyperglycemia instead of normo-insulinaemic hyperglycemia), the results confirm those of Jeppesen et al.
Retinal thickness has been reported to change in patients with long-term DM and retinopathy. A morphological change in the retina may even occur in the early stages of diabetic retinopathy [2, 7, 8, 18, 21, 23, 26, 32, 33, 35, 38]. These changes in retinal thickness are usually due to abnormal fluid accumulation resulting from a breakdown of the blood-retinal barrier [34]. Goebel et al. [8] measured retinal thickness by means of OCT in 136 patients with different stages of diabetic retinopathy and with a mean DM duration of 16 years. Mean foveal thickness was 307 ± 136 μm in the diabetic subjects, compared to 153 ± 15 μm in healthy subjects. It seems that only long-term hyperglycemia and/or long-term fluctuations in blood glucose levels have any significant influence on the blood–retina barrier and retinal thickness. From the findings of the present study, it appears that the blood–retina barrier does not seem to be affected by a single episode of acute hyperglycemia. Nevertheless, the fact that no change in retinal thickness could be determined does not exclude the possibility that there could be early dysfunction of the blood retina barrier. Other means of examination could evidence such a dysfunction of the blood retina barrier following acute hyperglycemia.
A factor that could have biased the results of this study is the administration of a synthetic somatostatin analogue to the subjects. Somatostatin is a peptide hormone that inhibits several hormones, including IGF-1 and insulin. IGF-1 is a growth factor that is produced by the hypoxic retina to mediate angiogenesis, resulting in neovascularisation. Somatostatin analogues not only inhibit neovascularisation in patients with advanced diabetic retinopathy, but also stabilize the blood–retinal barrier in patients with diabetic macular edema [16, 17]. It could have been possible that in the present study an increase in retinal thickness during hyperglycemia was prevented by somatostatin. Nevertheless, the efficacy of synthetic somatostatin in the treatment of advanced diabetic retinopathy was investigated by Grant et al. [9]. With maximally tolerated doses of somatostatin (ranging from 200 to 5000 μg/day), after a period of 15 months one out of 22 eyes required panretinal photocoagulation, compared to nine of 24 eyes that were not treated with somatostatin. From the results of the Grant et al. study, it seems that only frequent, large doses of somatostatin over a long period of time have any significant effect on the progression of diabetic retinopathy. Although the effect of somatostatin on the healthy retina has not been investigated yet, it seems to be unlikely that the results of the present study were biased by the administration of one single, low dose (100 μg) of somatostatin.
In conclusion, the results of this study indicate that in healthy subjects, hyperglycemia does not cause any change in retinal thickness. Furthermore, ocular refraction in general was not affected by hyperglycemia. However, there were interindividual variations, as illustrated by subject 01, who had a hyperopic shift of refraction during hyperglycemia. Therefore, it seems that a refractive change during hyperglycemia cannot be explained by a change in retinal thickness. It could well be that other refractive components, such as the lens, are involved in causing blurred vision and refractive alterations during hyperglycemia.
Acknowledgments
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
The authors had no financial support for this study. There are no conflicts of interest.
References
- 1.Aiello LP, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL 3rd, Klein R (1998) Diabetic retinopathy. Diabetes Care 21(1):143–156 [DOI] [PubMed]
- 2.Biallosterski C, Van Velthoven ME, Michels RP, Schlingemann RO, De Vries JH, Verbraak FD (2007) Decreased OCT-measured pericentral retinal thickness in patients with diabetes mellitus type 1 with minimal diabetic retinopathy. Br J Ophthalmol 91(9):1135–1138 [DOI] [PMC free article] [PubMed]
- 3.Cheng X, Himebaugh NL, Kollbaum PS, Thibos LN, Bradley A (2003) Validation of a clinical Shack-Hartmann aberrometer. Optom Vis Sci 80(8):587–595 [DOI] [PubMed]
- 4.Diabetic Retinopathy Clinical Research Network, Danis RP, Glassman AR, Aiello LP, Antoszyk AN, Beck RW, Browning DJ, Ciardella AP, Kinyoun JL, Murtha TJ, Topping TM, Shami M, Sharuk GS, Wells JA 3rd (2006) Diurnal variation in retinal thickening measurement by optical coherence tomography in center-involved diabetic macular edema. Arch Ophthalmol 124(12):1701–1707 [DOI] [PMC free article] [PubMed]
- 5.Duke-Elder S (1925) Changes in refraction in diabetes mellitus. Br J Ophthalmol 9:167–187 [DOI] [PMC free article] [PubMed]
- 6.Early Treatment Diabetic Retinopathy Study Research Group (1985) Photocoagulation for diabetic macular edema: Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol 103(12):1796–1806 [PubMed]
- 7.Fritsche P, Van der Heijde R, Suttorp-Schulten MS, Polak BC (2002) Retinal thickness analysis: an objective method to assess and quantify the retinal thickness in healthy controls and in diabetics without diabetic retinopathy. Retina 22(6):768–771 [DOI] [PubMed]
- 8.Goebel W, Kretzchmar-Gross T (2002) Retinal thickness in diabetic retinopathy: a study using optical coherence tomography. Retina 22(6):759–767 [DOI] [PubMed]
- 9.Grant MB, Mames RN, Fitzgerald C, Hazariwala KM, Cooper-DeHoff R, Caballero S, Estes KS (2000) The efficacy of octreotide in the therapy of severe nonproliferative and early proliferative diabetic retinopathy. Diabetes Care 23(4):504–509 [DOI] [PubMed]
- 10.Hee MR, Izatt JA, Swanson EA, Huang D, Schuman JS, Lin CP, Puliafito CA, Fujimoto JG (1995) Optical coherence tomography of the human retina. Arch Ophthalmol 113(3):325–332 [DOI] [PubMed]
- 11.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA (1991) Optical coherence tomography. Science 254(5035):1178–1181 [DOI] [PMC free article] [PubMed]
- 12.Huggert A (1954) The appearance of the crystalline lens during different stages of transitory changes of refraction. Acta Ophthalmol (Copenh) 32(4):375–389 [DOI] [PubMed]
- 13.Jeppesen P, Knudsen ST, Poulsen PL, Mogensen CE, Schmitz O, Bek T (2007) Response of retinal arteriole diameter to increased blood pressure during acute hyperglycaemia. Acta Ophthalmol Scand 85(3):280–286 [DOI] [PubMed]
- 14.Klein R, Klein BE, Moss SE (1984) Visual impairment in diabetes. Ophthalmology 91(1):1–9 [PubMed]
- 15.Kluxen G, Scholz A (1987) Evaluation of Scheimpflug photographs in transitory hypermetropia (in German). Klin Monatsbl Augenheilkd 191(2):129–132 [DOI] [PubMed]
- 16.Lang GE (2004) Therapy of diabetic retinopathy with somatostatin analogues (in German). Ophthalmologe 101(12):290–293 [DOI] [PubMed]
- 17.Lang GE (2007) Pharmacological treatment of diabetic retinopathy. Ophthalmologica 221(2):112–117 [DOI] [PubMed]
- 18.Lattanzio R, Brancato R, Pierro L, Bandello F, Iaccher B, Fiore T, Maestranzi G (2002) Macular thickness measured by optical coherence tomography in diabetic patients. Eur J Ophthalmol 12(6):482–487 [DOI] [PubMed]
- 19.Liang J, Grimm B, Goelz S, Bille JF (1994) Objective measurement of wave aberrations of the human eye with the use of a Hartmann-Shack wave-front sensor. J Opt Soc Am A Opt Image Sci Vis 11(7):1949–1957 [DOI] [PubMed]
- 20.Massin P, Vicaut E, Haouchine B, Erginay A, Paques M, Gaudric A (2001) Reproducibility of retinal mapping using optical coherence tomography. Arch Ophthalmol 119(8):1135–1142 [DOI] [PubMed]
- 21.Massin P, Erginay A, Haouchine B, Mehidi AB, Paques M, Gaudric A (2002) Retinal thickness in healthy and diabetic subjects measured using optical coherence tomography mapping software. Eur J Ophthalmol 12(2):102–108 [DOI] [PubMed]
- 22.Moss SE, Klein R, Klein BE (1998) The 14-year incidence of visual loss in a diabetic population. Ophthalmology 105(6):998–1003 [DOI] [PubMed]
- 23.Nilsson M, Von Wendt G, Wanger P, Martin LM (2007) Early detection of Macular changes in Diabetic Patients using Rarebit Fovea Test and Optical Coherence Tomography. Br J Ophthalmol 9: DOI 10.1136/bjo.2007.124461 [DOI] [PMC free article] [PubMed]
- 24.Nussenblatt RB, Kaufman SC, Palestine AG, Davis MD, Ferris FL 3rd (1987) Macular thickening and visual acuity. Measurement in patients with cystoid macular edema. Ophthalmology 94(9):1134–1139 [DOI] [PubMed]
- 25.Okamoto F, Sone H, Nonoyama T, Hommura S (2000) Refractive changes in diabetic patients during intensive glycaemic control. Br J Ophthalmol 84(10):1097–1102 [DOI] [PMC free article] [PubMed]
- 26.Pires I, Bernardes RC, Lobo CL, Soares MA, Cunha-Vaz JG (2002) Retinal thickness in eyes with mild nonproliferative retinopathy in patients with type 2 diabetes mellitus: comparison of measurements obtained by retinal thickness analysis and optical coherence tomography. Arch Ophthalmol 120(10):1301–1306 [DOI] [PubMed]
- 27.Planten JT, Kooijman AC, De Vries B, Woldringh JJ (1978) Pathological-optic approach of cataract and lens. Ophthalmologica 176(6):331–334 [DOI] [PubMed]
- 28.Polito A, Del Borrello M, Isola M, Zemella N, Bandello F (2005) Repeatability and reproducibility of fast macular thickness mapping with stratus optical coherence tomography. Arch Ophthalmol 123(10):1330–1337 [DOI] [PubMed]
- 29.Puliafito CA, Hee MR, Lin CP, Reichel E, Schuman JS, Duker JS, Izatt JA, Swanson EA, Fujimoto JG (1995) Imaging of macular diseases with optical coherence tomography. Ophthalmology 102(2):217–229 [DOI] [PubMed]
- 30.Rabbetts RB (1998) Clinical visual optics. Butterworth-Heinemann, Oxford
- 31.Salmon TO, Van de Pol C (2005) Evaluation of a clinical aberrometer for lower-order accuracy and repeatability, higher-order repeatability, and instrument myopia. Optometry 76(8):461–472 [DOI] [PubMed]
- 32.Sanchez-Tocino H, Alvarez-Vidal A, Maldonado MJ, Moreno-Montanes J, Garcia-Layana A (2002) Retinal thickness study with optical coherence tomography in patients with diabetes. Invest Ophthalmol Vis Sci 43(5):1588–1594 [PubMed]
- 33.Schaudig UH, Glaefke C, Scholz F, Richard G (2000) Optical coherence tomography for retinal thickness measurement in diabetic patients without clinically significant macular oedema. Ophthalmic Surg Lasers 31(3):182–186 [PubMed]
- 34.Smith R, Lee C, Charles H, Farber M, Cuncha-Vaz J (1987) Quantification of diabetic macular edema. Arch Ophthalmol 105(2):218–222 [DOI] [PubMed]
- 35.Sugimoto M, Sasoh M, Ido M, Wakitani Y, Takahashi C, Uji Y (2005) Detection of early diabetic change with optical coherence tomography in type 2 diabetes mellitus patients without retinopathy. Ophthalmologica 219(6):379–385 [DOI] [PubMed]
- 36.Tai MC, Lin SY, Chen JT, Liang CM, Chou PI, Lu DW (2006) Sweet hyperopia: refractive changes in acute hyperglycemia. Eur J Ophthalmol 16(5):663–666 [DOI] [PubMed]
- 37.Turtz CA, Turtz AI (1958) Reversal of lens changes in early diabetes. Am J Ophthalmol 46(2):219 [DOI] [PubMed]
- 38.Yang CS, Cheng CY, Lee FL, Hsu WM, Liu JH (2001) Quantitative assessment of retinal thickness in diabetic patients with and without clinically significant macular oedema using optical coherence tomography. Acta Ophthalmol Scand 79(3):266–270 [DOI] [PubMed]