Abstract
The folate derivatives folic acid (FA) and folinic acid (FNA) decrease the in vivo and in vitro activities of antifolate drugs in Plasmodium falciparum. However, the effects of 5-methyl-tetrahydrofolate (5-Me-THF) and tetrahydrofolate (THF), the two dominant circulating folate forms in humans, have not been explored yet. We have investigated the effects of FA, FNA, 5-Me-THF, and THF on the in vitro activity of the antimalarial antifolates pyrimethamine and chlorcycloguanil and the anticancer antifolates methotrexate (MTX), aminopterin, and trimetrexate (TMX), against P. falciparum. The results indicate that these anticancers are potent against P. falciparum, with IC50 < 50 nM. 5-Me-THF does not significantly decrease the activity of all tested drugs, and none of the tested folate derivatives significantly decrease the activity of these anticancers. Thus, malaria folate metabolism has features different from those in human, and the exploitation of this difference could lead to the discovery of new drugs to treat malaria. For instance, the combination of 5-Me-THF with a low dose of TMX could be used to treat malaria. In addition, the safety of a low dose of MTX in the treatment of arthritis indicates that this drug could be used alone to treat malaria.
Electronic supplementary material
The online version of this article (doi:10.1007/s00436-008-0897-4) contains supplementary material, which is available to authorized users.
Introduction
Antifolates inhibit the synthesis and conversion of folate derivatives (FDs). Rapidly dividing cells such as cancer cells, bacteria, or malaria parasites rely heavily on the availability of folate to grow. Thus, blockade of synthesis of these factors greatly affects cell division. This feature has been exploited for the development of antifolate drugs. FDs is a generic term that consists of ten different forms of folate. The chemical structure of one of the FDs, folic acid (FA), is shown in Fig. 1, and the role of FDs has been discussed previously (Nzila et al. 2005a, b).
Fig. 1.
Chemical structures of folic acid and of the antifolate drugs we analyzed
Among all the FDs, 5-methyl-tetrahydrofolate (5-Me-THF) is the most important one. It is the predominant FD in mammals, representing 80–90% of the total folate content (Belz and Nau 1998; Wagner 1995). The predominance of 5-Me-THF reflects its role as a cofactor in the conversion of homocysteine to methionine, which in turn is the precursor of S-adenosylmethionine, the methylation cofactor involved in almost 100 reactions (Priest and Bunni 1995). Tetrahydrofolate (THF) is the second most represented FD in mammals (10% of the total content); it is the product of the main folate enzyme dihydrofolate reductase (DHFR; Nzila et al. 2005a). The FDs FA and folinic acid (FNA) are used clinically to lower the toxicity of antifolate drugs in the treatment of cancer and rheumatoid arthritis (RA; Jackson 1999).
In Plasmodium falciparum parasites, studies have clearly demonstrated that the addition of FA or FNA decreases the activity of antifolate drugs, both in vitro and in vitro (Kinyanjui et al. 1999; van Hensbroek et al. 1995). Likewise, the lowering of the folate concentration in in vitro culture medium enhances the activity of antifolate antimalarial agents (Wang et al. 1997). By increasing the folate derivative pools, the parasite can bypass the inhibition of folate synthesis, leading to a decrease in antifolate activity. However, the impact of the other FDs on antifolate activity has not been explored in P. falciparum. In the present paper, we explore the impact of 5-Me-THF and THF along with FA and FNA on the standard antifolates pyrimethamine (PM) and chlorcycloguanil (CCG) against P. falciparum and the anticancer antifolates methotrexate (MTX), aminopterin (AMP), and trimetrexate (TMX; Fig. 1). We have included these anticancer drugs because there have been indications that these agents are potent against malaria parasite (Elslager et al. 1983; Fidock et al. 1998; Walter et al. 1991). Our study indicates that these anticancer drugs alone (for MTX) or in combination with 5-Me-THF (for TMX and AMP) could be used to treat malaria. The potential of MTX as an antimalarial has led us to explore the interaction of this antifolate with other antimalarial drugs.
Materials and methods
FA, FNA, THF, 5-Me-THF, PM, dapsone (DDS), MTX, AMP, chloroquine (CQ), mefloquine (MFQ), primaquine (PRQ), quinine (QN), proguanil (PG), and probenecid (PBN) were purchased from Sigma Chemical Co. (Poole, UK). CCG was a gift from AstraZeneca (Cheshire, UK). Amodiaquine (AQ), desethyl-amodiaquine (DEAQ), dihydroartemisinin (DHA), piperaquine (PQ), lumefantrine (LM), pyronaridine (PRN), halofantrine (HLF), and chlorproguanil (CPG) were gifts from Professor Steve Ward, Liverpool School of Tropical Medicine, Liverpool, UK. Trimetrexate was a gift from Professor Andre Rosowsky, Dana-Farber Cancer Institute, Boston, MA, USA.
Antimalarial activity was measured in the presence of varying concentrations of each compound using radioisotopic incorporation (Sixsmith et al. 1984). Results were expressed as the drug concentration required for 50% inhibition of [3H]hypoxanthine incorporation into parasite nucleic acid (IC50), using nonlinear regression analysis of the dose–response curve. These IC50 values were determined in the presence or absence of increasing concentrations of folate derivatives. Two reference P. falciparum laboratory isolates were tested: M24, a fully pyrimethamine-sensitive isolate, and V1/S, a highly pyrimethamine-resistant isolate. M24 carries a wild-type dhfr gene, but the V1/S isolate has four mutations at codons 108, 51, 59, and 164 in its dhfr gene (Nzila et al. 2003). Cultures were carried out in Roswell Park Memorial Institute (RPMI) 1640 (GIBCO BRL, UK) medium supplemented with 10% (v/v) normal human serum, 25 mM bicarbonate, 2 mM glutamine, 25-mM 4-(2-hydroxyethyl)-1-piperazine-ethanesulfonic acid buffer, and 3.6 nM para-aminobenzoic acid. Human blood used to culture the parasites in vitro was obtained from healthy subjects and washed three times with RPMI culture medium nonsupplemented with serum.
Synergy Synergy analyses were carried out using V1/S isolate in RPMI medium containing folic acid at physiological concentration (23 nM). Synergy was assessed by combining MTX with another drug in the following ratios: 1:0, 0.8:0.2, 0.6:0.4, 0.4:0.6, and 0.2:0.8. Synergy was measured in vitro both geometrically by construction of isobolograms (with a minimum of five coordinates) and algebraically by calculating the sum of the minimum fractional inhibitory concentration (FIC; Berenbaum 1978). Synergy is demonstrated when the FIC is <0.5. A FIC value of >4.0 denotes an antagonistic effect and a FIC value between 0.5 and 4.0 indicates either nonsynergistic or nonantagonistic interaction.
Results
Folate effect
We tested the in vitro activity of the anticancer agents MTX, AMP, and TMX and the antimalarials PM and CCG in the presence of increasing concentrations of FA, the commonly used folate derivative, and THF, FNA, and 5-Me-THF, using the multidrug-resistant V1/S and the fully sensitive isolate M24. We used four folate concentrations: 0.023 μM, which is the physiological folate concentration (normal value in the human body), and concentrations representing 10 (0.23 μM), 100 (2.3 μM) and 1,000 (23 μM) times the physiological concentration. The 2.3-μM concentration is the highest concentration of folate that can be achieved in vivo when high doses of folate derivatives are administered (Barredo et al. 1999).
Table S1 shows the results of FA addition on the antimalarial activity of PM and CCG using the multidrug-resistant isolate V1/S. At the physiological FA concentration (0.023 μM), the IC50 values of the antimalarial antifolates PM and CCG and the anticancer antifolates MTX, AMP, and TMX were 875, 12, 31, 59, and 15 nM, respectively. Increasing the concentration of FA to 0.23, 2.3 and 23 μM was associated with a significant decrease in PM and CCG activity, and this decrease in activity was proportional to the concentration of FA added to the medium (Table S1). For instance, PM and CCG were reduced by a factor of >45 in the presence of 23 μM of FA. Surprisingly, however, the activities of MTX, AMP, and TMX were virtually unchanged at 0.23 and 2.3 μM of FA, and only a slight decrease was noticed at a concentration of 23 μM of FA, especially with TMX. Likewise, the same trend was observed against the fully sensitive isolate M24. At the physiological FA concentration, the IC50 values of PM, CCG, MTX, AMP, and TMX were 0.3, 0.22, 36, 48, and 4 nM, respectively (Table S2). PM and CCG activities were decreased by 2.5- to 33-fold when 0.023, 2.3, and 2.3 μM of FA were used, while the activities of AMP, MTX, and TMX remained unchanged up to 23 μM of FA (Table S2).
We have also tested the effect of THF. The activities of PM and CCG in the presence of THF at physiological folate concentrations were 797 and 10 nM, respectively (Table S1). These activities were decreased by between 5- and 100-fold at the three highest folate concentrations tested. On the other hand, MTX, AMP, and TMX activities remained unchanged (24, 68, and 4 nM, respectively) at all THF concentrations (Table S1). The same trend was observed when M24 was used (Table S2). We tested the effect of FNA, another form of folate that the parasite can salvage. Similar to previous observations, FNA does not affect the activity of MTX, AMP, and TMX, even at concentrations 500 times higher than the physiological folate concentration, unlike the effect on the antimalarials PM and CCG. The use of 5-Me-THF yielded the most surprising results. Indeed, this folate derivative, even when used at high concentration, did not change the activity of any of the tested drugs, including the antimalarials PM and CCG (Tables S1 and S2).
Synergy between MTX and sulfa drug
The combination of a sulfa drug, an inhibitor of dihydropteroate synthase (DHPS) and an inhibitor of DHFR, produces a synergistic inhibition of the folate pathway. To explore the mode of action of MTX further, we have tested the synergistic properties of MTX in combination with the DHPS inhibitor dapsone. All FICs were between 1 and 1.82, a clear indication that MTX does not synergize with dapsone, while FICs of the combination CCG–DDS were <0.5 (Table 1). Likewise, we did not observe any synergy between TMX and DDS, and all FICs were in the range 1.2–1.5.
Table 1.
Activity of MTX in the presence of inhibitors of malaria dihydrofolate reductase (CCG and WR99210); inhibitors of dihydropteroate synthase (DDS), triazine-based compounds, synergizers of atovaquone (PG and CPG), quinoline, and aryl amino-alcohol related drugs (CQ, PQ, PRQ, LM, MFQ, HLF, DEAQ, and QN); the sesquiterpene dihydroartemisinin; the antifolate anticancer TMX; the benzonaphthyridine PRN and the naphthoquinone derivative, ATV
| Combination | Drug ratio | Score | ||||
|---|---|---|---|---|---|---|
| 1:1 | 0.8:0.2 | 0.6:0.4 | 0.4:0.6 | 0.2:0.8 | ||
| Sum FIC | ||||||
| CCG–DDS | 0.36 ± 0.18 | 0.46 ± 0.0 | 0.40 ± 0.0 | 0.38 ± 0.0 | 0.48 ± 0.1 | Synergistic |
| MTX–DDS | 1.0 ± 0.1 | 1.17 ± 0.36 | 1.37 ± 0.12 | 1.82 ± 0.72 | 1.78 ± 0.27 | Additive |
| ATV–PG | ND | 0.25 ± 0.0 | 0.22 ± 0.1 | 0.24 ± 0.0 | 0.28 ± 0.0 | Synergistic |
| MTX–PG | 1.16 ± 0.23 | 1.48 ± 0.64 | 1.84 ± 0 | 1.55 ± 0.30 | 1.32 ± 0.41 | Additive |
| MTX–CPG | ND | 0.92 ± 0.45 | 1.49 + 0.0 | 1.57 + 1.0 | .98 + 0.0 | Additive |
| MTX–CCG | 1.1 ± 0.3 | 1.09 ± 0.29 | 1.06 ± 0.16 | 1.18 ± 0.12 | 1.81 ± 0.81 | Additive |
| MTX–WR99210 | ND | 1.10 ± 0.3 | 1.20 ± 0.3 | 1.37 ± 0.4 | 1.40 ± 0.5 | Additive |
| MTX–CQ | ND | 1.40 ± 0.34 | 1.48 ± 0.44 | 1.08 ± 0.18 | 1.17 ± 0.0 | Additive |
| MTX–DHA | ND | 1.47 ± 0.2 | 1.33 ± 0.7 | 1.20 ± 0.30 | 0.84 ± 0.22 | Additive |
| MTX–LM | 1.57 ± 0.18 | 1.07 + 0.0 | 1.77 + 0.25 | 1.89 + 0.19 | 1.82 + 0.0 | Additive |
| MTX–QN | ND | 1.47 ± 0.41 | 1.73 ± 0.24 | 1.56 ± 0.31 | 1.36 ± 0.0 | Additive |
| MTX–TMX | ND | 1.0 ± 0.1 | 1.33 ± 0.0 | 1.35 ± 0.0 | 1.43 ± 0.0 | Additive |
| MTX–PRQ | ND | 1.23 ± 0.2 | 1.34 ± 0.1 | 1.61 ± 0.4 | 1.14 ± 0.1 | Additive |
| MTX–HLF | ND | 2.36 ± 0.0 | 2.32 ± 1.1 | 1.59 ± 0.5 | 1.03 ± 0.2 | Additive |
| MTX–DEAQ | ND | 1.39 ± 0.9 | 1.14 ± 0.3 | 0.87 ± 0.5 | 1.21 ± 0.1 | Additive |
| MTX–MFQ | ND | 1.90 ± 0.7 | 1.67 ± 0.8 | 1.40 ± 0.9 | 1.38 ± 1.2 | Additive |
| MTX–ATV | ND | 1.26 ± 0.2 | 1.63 ± 0.0 | 1.15 ± 0.3 | 1.58 ± 0 | Additive |
| MTX–PRN | ND | 3.52 ± 1.2 | 3.24 ± 1.6 | 3.14 ± 0.4 | 3.36 ± 1.0 | Additive? |
| MTX–PQ | ND | 2.40 ± 0.3 | 3.00 ± 0.3 | 3.33 ± 0.1 | 1.58 ± 0.1 | Additive? |
CCG–DDS and ATV–PG were used as control synergistic combinations. Data are represented as the total fractional inhibitory concentration. Synergy is demonstrated when the FIC is <0.5. A FIC value of >4.0 denotes an antagonistic effect and a FIC value between 0.5 and 4 indicates either nonsynergistic or nonantagonistic interaction
CCG Chlorcycloguanil, DDS dapsone, MTX methotrexate, ATV atovaquone, PG proguanil, CPG chlorproguanil, WR99210 Walter Reed compound 99210, CQ chloroquine, DHA dihydroartemisinin, LM lumefantrine, QN quinine, TMX trimetrexate, PRQ primaquine, HLF halofantrine, DEAQ desethyl-amodiaquine, MFQ mefloquine, PRN pyronaridine
Probenecid effect
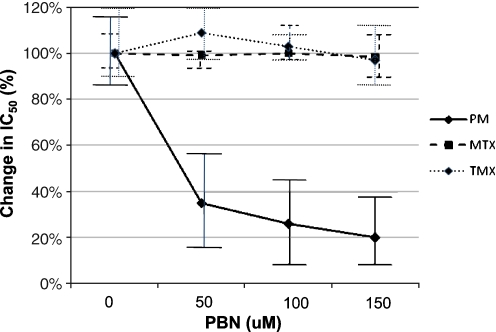
Lastly, we tested the effect of PBN on the activity of anticancers MTX and TMX in P. falciparum. As part of our previous work, we have demonstrated that PBN increases the in vitro activity of antifolates, and this increase is associated with a decrease in folate uptake (Nzila et al. 2003). We assessed the effect of PBN on the activity of MTX and the results are summarized in Fig. 2. PBN alone is a very weak antimalarial with a mean IC50 > 1,500 μM against V1/S parasites. We have tested the effect of noninhibitory concentrations of 50, 100, and 150 μM PBN on the activity of MTX, TMX, and PM. The PM IC50 against V1/S was 1,200 nM, and this IC50 decreased by a factor between 2.5 and 5 as PBN concentration increased from 50 to 150 μM; however, MTX and TMX IC50 remained unchanged (at around 30 and 7 nM, respectively). The data clearly show the absence of a PBN effect on MTX and TMX in P. falciparum.
Fig. 2.
Effect of probenecid (PBN) on the activity of PM, MTX, and TMX. Y axis represents the percentage decrease in IC50 in the presence of PBN. One hundred percent (100%) represents the IC50 in the absence of PBN. In the absence of PBN, the MTX, TMX, and PM IC50 values were 30, 7, and 1,200 nM, respectively
Synergy of MTX with other antimalarials
We also explored the in vitro interaction of MTX with existing antimalarials by assessing its activity in the presence of (1) inhibitors of malaria dihydrofolate reductase (CCG and Walter Reed compound 99210); (2) triazine-based compounds, synergizers of atovaquone (ATV; PG and CPG), quinoline, and aryl amino-alcohol-related drugs (CQ, PQ, LM, MFQ, HLF, DEAQ, QN, and PRQ); (3) the antifolate anticancer TMX; (4) the sesquiterpene DHA; (5) the benzonaphthyridine PRN; and (6) the naphthoquinone derivative ATV. We used two synergistic combinations as controls: CCG and DDS (see previous section) and ATV and PG. Triazine compounds (PG) synergize with inhibitors of electron transport to the cytochrome bc1 complex (ATV; Jones and Ward 2002; Nzila 2006). The data are summarized in Table 1. The combinations of CCG–DDS and ATV–PG were synergistic, as one would predict, with total FIC < 0.5. All of the tested MTX combinations were additive (except two), with the FIC value falling between 0.9 and 1.6, a clear indication that the activity of MTX is neither decreased nor increased in the presence of any of these drugs. However, the total FIC of the combination of MTX with PNR was between 3.14 and 3.52, and two out of the four total FIC values of MTX with PQ (at ratios of 0.4:0.6 and 0.6:04) were 3.00 and 3.15. These values are closer to 4, the cutoff point used to identify antagonistic combinations, an indication that PNR and PQ may antagonize MTX activity.
Discussion
Our data clearly indicate that malaria folate metabolism has features different from those in human. Indeed, the antimalarial activity of the anticancer drugs were not decreased by FA, FNA, and THF. All of these forms of folate increase the concentrations of dihydrofolate and THF, the substrate and the product of DHFR reactions, respectively. Under these conditions, one would expect the activity of the inhibitors of DHFR to decrease. This is what we observed with the standard antimalarial antifolates PM and CCG but not with the anticancers MTX, AMP, and TMX. Yet, evidence indicates that these anticancer drugs, at least MTX, target malaria DHFR. Indeed, kinetics studies on purified plasmodial DHFR have demonstrated that MTX binds efficiently to P. falciparum and Plasmodium vivax DHFR (Tahar et al. 2001; Toyoda et al. 1997), and transfection of malaria parasite with human DHFR has further demonstrated that the antimalarial activity of MTX is primarily borne by the inhibition of DHFR (Fidock et al. 1998). Thus, we would expect that addition of folate derivative would decrease the activity of these anticancer drugs in P. falciparum. In addition, there is evidence that MTX uses folate receptor–transporters to enter into the cell (Wang et al. 2007); thus, the addition of FD would lower MTX transport, leading to a decrease in MTX activity. However, even at high concentrations, FDs do not decrease MTX activity. Clearly, further studies are required to understand the absence of the “folate effect” against these anticancer antifolates.
The most unexpected effect was observed with 5-Me-THF. The use of this folate derivative did not change the activity of any of the drugs we tested, including the antimalarials. 5-Me-THF is utilized to synthesize methionine, and this reaction generates THF, the product of DHFR reaction and this THF would modulate the activity of antifolates as has been clearly demonstrated in mammalian cells (Dudman et al. 1982; Etienne et al. 1993; Hilton et al. 1983; Mini et al. 1984; Reggev and Djerassi 1986). Our data indicate that either 5-Me-THF is not transported into the parasite cell or that the de novo methionine pathways may not efficiently exist in the parasite. It is well established that the parasite obtains its amino acid supply, including methionine, from hemoglobin degradation. Thus, under these conditions, the parasite may not need to synthesize it de novo, though studies have indicated that the methionine de novo pathway may exist in P. falciparum, (Asawamahasakda and Yuthavong 1993; Krungkrai et al. 1989). However, because the parasite can obtain this amino acid from hemoglobin degradation or exogenous medium (Lew et al. 2003), this pathway can be of relative importance, a possibility that has already been proffered (Nzila et al. 2005a).
Because the addition of FA, FNA, and especially 5-Me-THF does not decrease the activity of the anticancer drugs, these folate derivatives could be used as adjuvants to increase the antifolate therapeutic index. For instance, we propose to use 5-Me-THF in combination with TMX to treat malaria. This folate derivative would protect the host against drug toxicity while it will not negate the antimalarial activity of these anticancer drugs. For instance, the same rational has been developed with the combination TMX + FNA in the treatment of Pneumocystis jiroveci infection (an opportunistic infection commonly found with human immunodeficiency virus infection). TMX is a potent drug against P. jiroveci and this microorganism cannot transport folate derivatives; as a result, the combination of TMX + FNA is as potent as TMX alone (Walzer et al. 1992). These observations led scientists to propose the use of TMX + FNA to treat P. jiroveci infection. This combination is safe, and it is now the mainstay of P. jiroveci treatment (Amsden et al. 1992; Fulton et al. 1995). In fact, TMX was discovered as an antimalarial drug (Elslager et al. 1983) but was developed as an anticancer because it is also active against human cells. Because TMX is potent against the malaria parasite (with IC50 < 15 nM), including highly PM-resistant isolates (Table S1), and because TMX + 5-Me-THF is as potent as TMX alone, thus, TMX + 5-Me-THF could be used to treat malaria. It is interesting to note that, based on the pharmacokinetics of TMX, the dose that would be required to treat malaria would be lower than that used in the treatment of cancer or P. jiroveci infection (Marshall and DeLap 1994).
We also demonstrated that MTX is potent against P. falciparum isolates with IC50 < 50 nM; thus, we propose this drug could be used to treat malaria. MTX is used at high doses up to 5–12 g/m2 per week (130–300 mg/kg) for several weeks for the treatment of cancer (Barnhart et al. 2001). This dose can yield concentrations of >1,000 μM MTX, which can have life-threatening toxicity (Barnhart et al. 2001). On the other hand, a low dose of MTX (LD-MTX; 0.1 to 0.35 mg/kg (7.5 to 25 mg per adult)) is used for the treatment of RA on a chronic basis, for up to 5 years. At this dose, MTX is safe and increasingly becoming the mainstay in the treatment of RA in the Western world (Borchers et al. 2004; Cronstein 2005; Ostor 2005; Suzuki et al. 2005; Swierkot and Szechinski 2006). LD-MTX is also becoming the drug of choice for the treatment of arthritis in children and multiple sclerosis, and under this regime, MTX is safe and well tolerated, (Niehues and Lankisch 2006; Gray et al. 2004, 2006; Krishna Sumanth et al. 2007).
All this information has led us to propose the use of LD-MTX in the treatment of malaria. The proof of the concept that LD-MTX can be used to treat malaria in humans has already been provided. Indeed, two small clinical trials have demonstrated that doses as low as 2.5 mg per day for 3–5 days are effective to treat malaria infection in humans (Sheehy and Dempsey 1970; Wildbolz 1973). However, MTX has not come into widespread use because of concerns over toxicity (Ferone 1971; Laing 1972). At the time the aforementioned clinical trials were carried out (which was in the 1970s), no information was available on the safety of LD-MTX. LD-MTX has been widely used for the treatment of arthritis since the 1980s, and its safety has now been proven. Thus, this drug could be revived as an antimalarial. Plans for clinical trials of this drug are under way in our Unit.
We have also provided evidence that MTX does not synergize with the DHPS inhibitor dapsone, yet all DHFR inhibitors described as far synergize with inhibitor of DHPS (Nzila et al. 2005a) and that its activity is not increased by the addition of PBN, a well-known potentiator of antifolate activity (Hooijberg et al. 1999; Nzila et al. 2003). All these observations support further that some features of antifolate anticancers in P. falciparum are different from those found in mammalian cells. To further study the mechanism of action of MTX, we explored the in vitro interaction of MTX with 16 existing antimalarials. We did not find any synergistic combination; MTX has an additive effect with all tested antimalarials, except with PNR and PQ. Thus, MTX could be developed with any of the tested drugs as combination therapy, except with PNR and PQ.
We have demonstrated that P. falciparum has unique features as regards folate metabolism, and some of these features could be exploited to develop antifolate anticancers to treat malaria. For instance, TMX could be combined with 5-Me-THF as an antimalarial, and the use of MTX in the treatment of rheumatoid arthritis indicates that this drug has the potential to become an antimalarial. Plans to test MTX in vivo for the treatment of malaria are under way.
Electronic supplementary material
Below is the link to the electronic supplementary material.
In vitro effect of the addition of FA, THF, and 5-Me-THF on the activity of the antimalarial antifolates PM and CCG and the anticancer antifolates MTX, AMP, and TMX, against the multidrug-resistant isolate V1/S (PPT 122 KB)
In vitro effect of the addition of FA, THF, and 5-Me-THF on the activity of the antimalarial antifolates PM and CCG, and the anticancer antifolates MTX, AMP, and TMX, against the multidrug-resistant isolate M24 (PPT 122 KB)
Acknowledgments
We thank the director of the Kenya Medical Research Institute for permission to publish these data. Part of this work was supported by Pfizer-Royal Society Award, UK (to AN) and the EU Commission under Framework 6 as part of the AntiMal Integrated Project 018834. AN is a European & Developing Countries Clinical Trials Partnership (EDCPT) senior fellow and LM is an EDCPT Ph.D.-funded student. The experiments comply with the current laws of Kenya.
Open Access This article is distributed under the terms of the Creative Commons Attribution NonCommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- 5-Me-THF
5-methyl-tetrahydrofolate
- ATV
atovaquone
- CCG
chlorcycloguanil
- CQ
chloroquine
- CPG
chlorproguanil
- DDS
dapsone
- DHFR
dihydrofolate reductase
- DHPS
dihydropteroate synthase
- DEAQ
desethyl-amodiaquine
- DHA
dihydroartemisinin
- FA
folic acid
- FD
folate derivatives
- FNA
folinic acid
- HLF
halofantrine
- LD-MTX
low dose of methotrexate
- LM
lumefantrine
- QN
quinine
- MFQ
mefloquine
- MTX
methotrexate
- nM
nanomolar
- PG
proguanil
- PQ
piperaquine
- PRB
probenecid
- PRQ
primaquine
- PRN
pyronaridine
- THF
tetrahydrofolate
- TMX
trimetrexate
- uM
micromolar
- WR99210
Walter reed compound 99210
Footnotes
This study was supported by Pfizer-Royal Society Award, UK (to AN), the EU Commission under Framework 6 as part of the AntiMal Integrated Project 018834, and the European & Developing Countries Clinical Trials Partnership (EDCPT).
Electronic supplementary material
The online version of this article (doi:10.1007/s00436-008-0897-4) contains supplementary material, which is available to authorized users.
References
- Amsden GW, Kowalsky SF, Morse GD (1992) Trimetrexate for Pneumocystis carinii pneumonia in patients with AIDS. Ann Pharmacother 26:218–226 [DOI] [PubMed]
- Asawamahasakda W, Yuthavong Y (1993) The methionine synthesis cycle and salvage of methyltetrahydrofolate from host red cells in the malaria parasite (Plasmodium falciparum). Parasitology 107(Pt 1):1–10 [DOI] [PubMed]
- Barnhart K, Coutifaris C, Esposito M (2001) The pharmacology of methotrexate. Expert Opin Pharmacother 2:409–417 [DOI] [PubMed]
- Barredo J, Bunni MA, Kamasamudram R, Priest D (1999) In: Jackman A (ed) Antifolate drugs in cancer therapy. Humana Press, Totawa, pp 323–337
- Belz S, Nau H (1998) Determination of folate patterns in mouse plasma, erythrocytes, and embryos by HPLC coupled with a microbiological assay. Anal Biochem 265:157–166 [DOI] [PubMed]
- Berenbaum MC (1978) A method for testing for synergy with any number of agents. J Infect Dis 137:122–130 [DOI] [PubMed]
- Borchers AT, Keen CL, Cheema GS, Gershwin ME (2004) The use of methotrexate in rheumatoid arthritis. Semin Arthritis Rheum 34:465–483 [DOI] [PubMed]
- Cronstein BN (2005) Low-dose methotrexate: a mainstay in the treatment of rheumatoid arthritis. Pharmacol Rev 57:163–172 [DOI] [PubMed]
- Dudman NP, Slowiaczek P, Tattersall MH (1982) Methotrexate rescue by 5-methyltetrahydrofolate or 5-formyltetrahydrofolate in lymphoblast cell lines. Cancer Res 42:502–507 [PubMed]
- Elslager EF, Johnson JL, Werbel LM (1983) Folate antagonists. 20. Synthesis and antitumor and antimalarial properties of trimetrexate and related 6-[(phenylamino)methyl]-2,4-quinazolinediamines. J Med Chem 26:1753–1760 [DOI] [PubMed]
- Etienne MC, Fischel JL, Formento P, Schneider M, Guillot T, Bardon M, Milano G (1993) Combination of reduced folates with methotrexate or 5-fluorouracil. Comparison between 5-formyltetrahydrofolate (folinic acid) and 5-methyltetrahydrofolate in vitro activities. Biochem Pharmacol 46:1767–1774 [DOI] [PubMed]
- Ferone R (1971) Methotrexate therapy for P. vivax malaria. JAMA 215:117 [DOI] [PubMed]
- Fidock DA, Nomura T, Wellems TE (1998) Cycloguanil and its parent compound proguanil demonstrate distinct activities against Plasmodium falciparum malaria parasites transformed with human dihydrofolate reductase. Mol Pharmacol 54:1140–1147 [DOI] [PubMed]
- Fulton B, Wagstaff AJ, McTavish D (1995) Trimetrexate. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in the treatment of Pneumocystis carinii pneumonia. Drugs 49:563–576 [DOI] [PubMed]
- Gray O, McDonnell GV, Forbes RB (2004) Methotrexate for multiple sclerosis. Cochrane Database Syst Rev CD003208 [DOI] [PMC free article] [PubMed]
- Gray OM, McDonnell GV, Forbes RB (2006) A systematic review of oral methotrexate for multiple sclerosis. Mult Scler 12:507–510 [DOI] [PubMed]
- Hilton MA, Hoffman JL, Sparks MK (1983) Effect of methotrexate with 5-methyltetrahydrofolate rescue and dietary homocysteine on survival of leukemic mice and on concentrations of liver adenosylamino acids. Cancer Res 43:5210–5216 [PubMed]
- Hooijberg JH, Broxterman HJ, Kool M, Assaraf YG, Peters GJ, Noordhuis P, Scheper RJ, Borst P, Pinedo HM, Jansen G (1999) Antifolate resistance mediated by the multidrug resistance proteins MRP1 and MRP2. Cancer Res 59:2532–2535 [PubMed]
- Jackson RC (1999) In: Jackman A (ed) Antifolate drugs in cancer therapy. Human Press, Totawa, pp 1–12
- Jones K, Ward SA (2002) Biguanide–atovaquone synergy against Plasmodium falciparum in vitro. Antimicrob Agents Chemother 46:2700–2703 [DOI] [PMC free article] [PubMed]
- Kinyanjui SM, Mberu EK, Winstanley PA, Jacobus DP, Watkins WM (1999) The antimalarial triazine WR99210 and the prodrug PS-15: folate reversal of in vitro activity against Plasmodium falciparum and a non-antifolate mode of action of the prodrug. Am J Trop Med Hyg 60:943–947 [DOI] [PubMed]
- Krishna Sumanth M, Sharma VK, Khaitan BK, Kapoor A, Tejasvi T (2007) Evaluation of oral methotrexate in the treatment of systemic sclerosis. Int J Dermatol 46:218–223 [DOI] [PubMed]
- Krungkrai J, Webster HK, Yuthavong Y (1989) Characterization of cobalamin-dependent methionine synthase purified from the human malarial parasite, Plasmodium falciparum. Parasitol Res 75:512–517 [DOI] [PubMed]
- Laing AB (1972) Methotrexate in malaria. Trans R Soc Trop Med Hyg 66:518–519 [DOI] [PubMed]
- Lew VL, Tiffert T, Ginsburg H (2003) Excess hemoglobin digestion and the osmotic stability of Plasmodium falciparum-infected red blood cells. Blood 101:4189–4194 [DOI] [PubMed]
- Marshall JL, DeLap RJ (1994) Clinical pharmacokinetics and pharmacology of trimetrexate. Clin Pharmacokinet 26:190–200 [DOI] [PubMed]
- Mini E, Mazzei T, Coronnello M, Criscuoli L, Gualtieri M, Periti P (1984) Modulation of fluoropyrimidine cytotoxicity by methotrexate or 5-methyltetrahydrofolate in human leukemia cells in vitro. Chemioterapia 3:343–349 [PubMed]
- Niehues T, Lankisch P (2006) Recommendations for the use of methotrexate in juvenile idiopathic arthritis. Paediatr Drugs 8:347–356 [DOI] [PubMed]
- Nzila A (2006) The past, present and future of antifolates in the treatment of Plasmodium falciparum infection. J Antimicrob Chemother 57:1043–1054 [DOI] [PubMed]
- Nzila A, Mberu E, Bray P, Kokwaro G, Winstanley P, Marsh K, Ward S (2003) Chemosensitization of Plasmodium falciparum by probenecid in vitro. Antimicrob Agents Chemother 47:2108–2112 [DOI] [PMC free article] [PubMed]
- Nzila A, Ward SA, Marsh K, Sims PF, Hyde JE (2005a) Comparative folate metabolism in humans and malaria parasites (part I): pointers for malaria treatment from cancer chemotherapy. Trends Parasitol 21:292–298 [DOI] [PMC free article] [PubMed]
- Nzila A, Ward SA, Marsh K, Sims PF, Hyde JE (2005b) Comparative folate metabolism in humans and malaria parasites (part II): activities as yet untargeted or specific to Plasmodium. Trends Parasitol 21:334–339 [DOI] [PMC free article] [PubMed]
- Ostor AJ (2005) Beyond methotrexate: biologic therapy in rheumatoid arthritis. Clin Med 5:222–226 [DOI] [PMC free article] [PubMed]
- Priest DG, Bunni MA (1995) In: Bailey LB (ed) Folate in health and disease. Marcel Dekker, New York, pp 379–403
- Reggev A, Djerassi I (1986) Rescue from high-dose methotrexate with 5-methyltetrahydrofolate. Cancer Treat Rep 70:251–253 [PubMed]
- Sheehy TW, Dempsey H (1970) Methotrexate therapy for Plasmodium vivax malaria. JAMA 214:109–114 [DOI] [PubMed]
- Sixsmith DG, Watkins WM, Chulay JD, Spencer HC (1984) In vitro antimalarial activity of tetrahydrofolate dehydrogenase inhibitors. Am J Trop Med Hyg 33:772–776 [DOI] [PubMed]
- Suzuki Y, Wakabayashi T, Jackson K (2005) Methotrexate for therapy of rheumatoid arthritis. Nippon Rinsho 63(Suppl 1):498–502 [PubMed]
- Swierkot J, Szechinski J (2006) Methotrexate in rheumatoid arthritis. Pharmacol Rep 58:473–492 [PubMed]
- Tahar R, de Pecoulas PE, Basco LK, Chiadmi M, Mazabraud A (2001) Kinetic properties of dihydrofolate reductase from wild-type and mutant Plasmodium vivax expressed in Escherichia coli. Mol Biochem Parasitol 113:241–249 [DOI] [PubMed]
- Toyoda T, Brobey RK, Sano G, Horii T, Tomioka N, Itai A (1997) Lead discovery of inhibitors of the dihydrofolate reductase domain of Plasmodium falciparum dihydrofolate reductase-thymidylate synthase. Biochem Biophys Res Commun 235:515–519 [DOI] [PubMed]
- van Hensbroek MB, Morris-Jones S, Meisner S, Jaffar S, Bayo L, Dackour R, Phillips C, Greenwood BM (1995) Iron, but not folic acid, combined with effective antimalarial therapy promotes haematological recovery in African children after acute falciparum malaria. Trans R Soc Trop Med Hyg 89:672–676 [DOI] [PubMed]
- Wagner C (1995) In: Bailey LB (ed) Folate in health and disease. Marcel Dekker, New York
- Walter RD, Bergmann B, Kansy M, Wiese M, Seydel JK (1991) Pyrimethamin-resistant Plasmodium falciparum lack cross-resistance to methotrexate and 2,4-diamino-5-(substituted benzyl) pyrimidines. Parasitol Res 77:346–350 [DOI] [PubMed]
- Walzer PD, Foy J, Steele P, White M (1992) Treatment of experimental pneumocystosis: review of 7 years of experience and development of a new system for classifying antimicrobial drugs. Antimicrob Agents Chemother 36:1943–1950 [DOI] [PMC free article] [PubMed]
- Wang P, Sims PF, Hyde JE (1997) A modified in vitro sulfadoxine susceptibility assay for Plasmodium falciparum suitable for investigating Fansidar resistance. Parasitology 115:223–230 [DOI] [PubMed]
- Wang P, Wang Q, Sims PF, Hyde JE (2007) Characterisation of exogenous folate transport in Plasmodium falciparum. Mol Biochem Parasitol 154:40–51 [DOI] [PMC free article] [PubMed]
- Wildbolz A (1973) Methotrexate in the therapy of malaria. Ther Umsch 30:218–222 [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
In vitro effect of the addition of FA, THF, and 5-Me-THF on the activity of the antimalarial antifolates PM and CCG and the anticancer antifolates MTX, AMP, and TMX, against the multidrug-resistant isolate V1/S (PPT 122 KB)
In vitro effect of the addition of FA, THF, and 5-Me-THF on the activity of the antimalarial antifolates PM and CCG, and the anticancer antifolates MTX, AMP, and TMX, against the multidrug-resistant isolate M24 (PPT 122 KB)