Abstract
Due mainly to the extensive use of antibiotics, the spread of multiresistant bacterial strains is one of the most worrying threats to public health. One strategy that can be used to overcome potential shortcomings might be the inactivation of these microorganisms by 5-aminolevulinic acid (5-ALA) or 5-ALA derivative-mediated photodynamic therapy (PDT). 5-ALA has no photoactive properties, but when it is given exogenously, it acts as a precursor of photosensitive porphyrins predominantly in tissues or organisms that are characterized by a high metabolic turnover, such as tumors, macrophages, and bacteria. However, the weak ability of 5-ALA to cross biological barriers has led to the introduction of more lipophilic derivatives, such as methyl aminolevulinate or hexyl aminolevulinate, which display improved capacities to reach the cytoplasm. Starting from the hypothesis that more lipophilic compounds carrying only a permanent positive charge under physiological conditions may more easily cross the bacterial multilayer barrier, we have tested the efficacies of some 5-ALA n-alkyl esters for the inactivation of bacteria. For this purpose, different bacterial strains were incubated with 5-ALA or its corresponding esters of different lipophilicities. Then, the bacteria were irradiated with light and the numbers of CFU post-PDT were counted and compared to those for the controls, which were kept in the dark. Furthermore, the total amount of accumulated porphyrins was quantified by high-pressure liquid chromatography analysis. In our studies, analysis of the bacterial extracts revealed the presence of all the porphyrins involved in heme biosynthesis, from uroporphyrin to protoporphyin IX. The efficacy of bacterial inactivation was a function of the total amount of porphyrins produced, independently of their nature. The 5-ALA methyl and butyl esters were the most effective compounds with respect to the photodynamic inactivation of bacteria. We observed significant differences in terms of the optimal drug concentration, bactericidal activities, and porphyrin production.
In 1998, the World Health Organization voted on a resolution to classify antimicrobial resistance as one of the major threats against human health (31). During the last few decades, only a few new drugs and even fewer new antibiotic classes have reached the market; the many promises of biotechnology and genetics failed to deliver results. In this context, photodynamic therapy (PDT) may be an interesting alternative. PDT is the result of the use of three autonomously nonactive elements in combination: (i) a nontoxic photoactive molecule called a photosensitizer (PS); (ii) light of the appropriate wavelength to excite the PS; and finally, (iii) oxygen, which is transformed into the highly reactive singlet oxygen species upon energy transfer from the light-activated PS.
The use of PDT for antimicrobial purposes is not recent, as PDT was the predominant step leading to the discovery of this treatment modality at the beginning of the 20th century (16, 29). However, PDT never achieved real success in its native domain, in contrast to other therapeutic fields, where it is now used in routine clinical practice, including the treatment of some forms of cancer and age-related macular degeneration (2, 3).
The renewed interest in antimicrobial PDT originates from two main factors: first, the promising results obtained by PDT in the fields mentioned above and, second, the critical need for new antimicrobial therapies that has arisen from the spread of multiresistant microorganisms.
One of the main observations made from the first attempts to photoinactivate bacteria with conventional PSs was the relative sensitivity of gram-positive strains to photodynamic inactivation (PDI), whereas gram-negative strains were significantly more resistant (8). To overcome the highly impermeable barrier of gram-negative bacteria, pretreatment with EDTA or the use of polycationic polymers has been proposed (15). Furthermore, the use of positively charged porphyrins, phenotiazines, or phthalocyanines enabled the photoinactivation of both gram-positive and -negative strains without any permeabilization pretreatment (9).
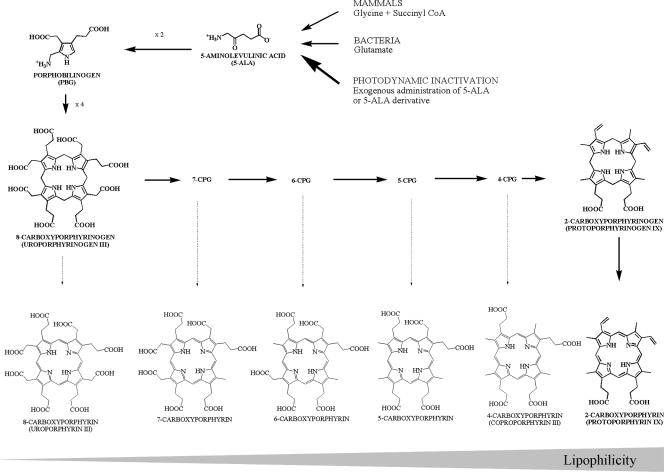
Besides the classical PS, one molecule of particular interest, 5-aminolevulinic acid (5-ALA), is gaining importance in the field of PDI. 5-ALA is not a PS by itself but is an endogenous component in the heme biosynthesis pathway and is ubiquitous in nearly all cells (Fig. 1). When it is provided exogenously, 5-ALA results in the accumulation of photoactive porphyrins (PAPs) within the targeted cells. This accumulation of PAPs is more pronounced in cells with high rates of metabolic activity (4), like cancer cells, inflammatory cells, and bacteria, resulting in high selectivity. However, the low capacity of 5-ALA to cross biological barriers led to the development of more lipophilic derivatives with improved local bioavailability (5). As 5-ALA has been shown to be moderately efficient against gram-negative bacteria (19), these derivatives might also show a clear improvement in their ability to photoinactivate these microorganisms. In the present study, we compared the bacterial PAP formation induced by 5-ALA to that induced by different 5-ALA derivatives in several gram-positive and gram-negative bacterial strains and investigated whether the 5-ALA derivatives may be interesting candidates for use in the PDI of bacteria.
FIG. 1.
Heme biosynthesis cycle. In mammalian cells, 5-ALA is formed from the condensation of glycine and succinyl coenzyme A (CoA), while most bacteria use glutamate as the substrate. Two molecules of 5-ALA condense to form porphobilinogen, catalyzed by 5-ALA dehydratase. Four molecules of porphobilinogen then condense to form a linear tetrapyrrole, which cyclizes to form 8-carboxyporphyrinogen (uroporphyrinogens I and III). Plants and bacteria can use uroporphyrinogen III as an intermediate in the synthesis of various molecules (e.g., chlorophyll and vitamin B12). Mammals exclusively use PPIX, obtained after numerous enzymatic decarboxylations and desaturations, while different CPs (8-CP, 7-CP, 6-CP, 5-CP, 4-CP) can be found in bacteria.
MATERIALS AND METHODS
Chemicals.
5-ALA was obtained from Fluka (Buchs, Switzerland); and its methyl ester (MAL), butyl ester (BAL), pentyl ester (PAL), hexyl ester (HAL), and octyl ester (OAL) were from Organix (Colchester, United Kingdom). The porphyrin standards were purchased from Frontier Scientific (Logan, UT). All solvents and other chemicals were of analytical grade and were used without further purification.
Bacterial strains and growth conditions.
This study was conducted with three gram-negative strains and one gram-positive strain. The gram-negative strains were (i) Escherichia coli K-12, a nonpathogenic laboratory strain; (ii) E. coli Ti05, a uropathogenic strain; and (iii) Pseudomonas aeruginosa. The gram-positive strain was methicillin-resistant Staphylococcus aureus. All strains were provided by the Istituto Cantonale di Microbiologia (Bellinzona, Switzerland) and are described in Table 1.
TABLE 1.
Characteristics of the bacterial strains tested in this study
| Strain | Gram staining result | Identification | Source | Resistance |
|---|---|---|---|---|
| E. coli K-12 | Negative | MG1655 | Laboratory collection | None |
| E. coli Ti05 | Negative | 03-039705 | Blood | Ampicillin, co-trimoxazole, trimethoprim, streptomycin |
| P. aeruginosa | Negative | 04-022545 | Expectorate | None of the P. aeruginosa-specific antibioticsa |
| S. aureus, methicillin resistant | Positive | 04-022798 | Wound puncture | Penicillin, ampicillin, oxacillin, cefazolin, ciprofloxacin, tetracycline, amoxicillin-clavulanic acid (Augmentin), trimethoprim-sulfamethoxazole |
Ceftazidime, cefepime, piperacillin, imipenem-cilastatin sodium (Tienam), aztreonam, gentamicin, tobramycin, amikacin, and ciprofloxacin.
All strains were grown for 48 h on Columbia plates (Columbia agar base with 5% [vol/vol] defibrinated sheep blood; Oxoid, Basel, Switzerland). The colonies were transferred into 100 ml of tryptone soy broth (Oxoid) and were incubated overnight at 37°C. The bacterial suspension was diluted with tryptone soy broth to an optical density at 660 nm of ∼0.10. The bacterial suspensions were incubated at 37°C to an optical density at 600 nm of ∼0.30 and washed twice with phosphate-buffered saline (0.1 N, pH 6.5) before incubation with the substrate.
Photosensitization.
Stock solutions of 5-ALA and the 5-ALA derivatives MAL, BAL, PAL, HAL, and OAL were freshly prepared directly before use by dissolving the different substrates in phosphate-buffered saline (0.1 N, pH 6.5) and were kept in the dark after proper dilution. The bacterial suspension was centrifuged at 2,500 × g for 10 min; the bacterial pellets were then resuspended in the substrate solutions and incubated in the dark for 4 h at 37°C in a shaking incubator (100 rpm). One fraction of the suspension was used for the PDI procedure, while the other was collected for PAP analysis.
PDI.
Samples (2.5 ml) containing approximately 5 × 107 bacteria were introduced into sterile 35-mm-diameter petri dishes and illuminated with white light for 40 min, corresponding to a light dose of 120 J·cm−2. The light source was a 400-W halogen lamp, and the illumination surface was controlled so that the light intensity was homogeneous and constant.
Bacterial cell survival assay.
The numbers of CFU of a bacterial suspension were determined by plating appropriate dilutions (from 10−1 to 10−5) on Columbia agar plates. The survival fraction was calculated as NPDI/N0, where NPDI is the number of CFU per ml after PDI and N0 is the number of CFU per ml in the initial sample. The dark toxicity of the substrates, defined as the intrinsic toxicity of the compounds in the absence of light, was monitored by evaluating the survival fraction of incubated but nonilluminated bacterial samples and was calculated as Ndark/N0, where Ndark is the number of CFU per ml of the nonilluminated samples. The results were expressed as mean values (n = 4) with their standard deviations.
High-pressure liquid chromatography analysis.
Ten milliliters of the bacterial suspensions was centrifuged at 2,500 × g for 10 min. The supernatant was discarded, and the bacteria were resuspended in 1.0 ml of extraction solvent (ethanol, dimethyl sulfoxide, acetic acid, 80:20:1; vol/vol/vol) and stored at −80°C until analysis. For the extraction of porphyrins, the bacterial wall was disrupted by five sonication cycles of 5 s each at 0°C with a sonicator probe (digital sonifier; amplitude, 30%; Branson). After centrifugation (at 13,500 × g for 4 min), the supernatant was collected and injected into the high-pressure liquid chromatography instrument.
Porphyrins were separated by reverse-phase chromatography with a 125/4 Nucleodur C18 gravity 3-μm column (Macherey-Nagel, Oensingen, Switzerland) protected with the corresponding precolumn by using a gradient elution with solvent A (acetate buffer [pH 5.1, 0.5 M] and acetonitrile [90:10; vol/vol]) and solvent B (methanol and acetonitrile [90:10; vol/vol]). Detection was performed with a fluorescence detector (La Chrom L-7480; Merck-Hitachi) with an excitation wavelength of 407 nm and an emission wavelength of 620 nm (Fig. 2).
FIG. 2.
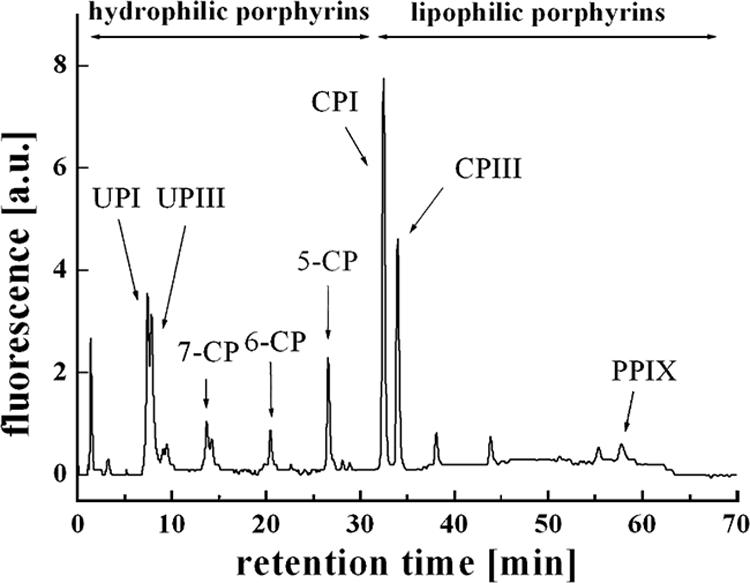
Chromatogram obtained with extracted bacterial porphyrins after gradient elution with solvent A (acetate buffer and acetonitrile, 90:10) and solvent B (methanol and acetonitrile, 90:10) and fluorescence detection (excitation wavelength, 407 nm; emission wavelength, 620 nm). The elution order corresponds to the formation order in heme biosynthesis: 8-CP (UPI and UPIII), 7-CP, 6-CP, 5-CP (coproporphyrin I and III; CPI and CPIII, respectively), and 2-CP (PPIX).
RESULTS
Effect of 5-ALA PDI on E. coli K-12.
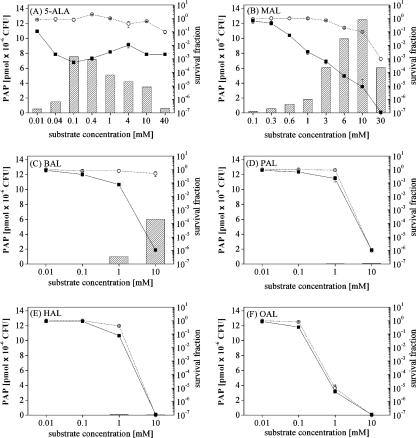
Figure 3A shows the results obtained after incubation of the E. coli K-12 laboratory strain with increasing concentrations of 5-ALA. Without irradiation, bacterial survival was not affected until the 5-ALA concentration reached 40 mM. In contrast, irradiated samples reacted in a drastically different manner, as two phases could be observed. At the lowest 5-ALA concentration, a low efficiency of PDI was observed, and the PDI increased to a maximum at the optimal concentration of 0.1 mM. Beyond this optimal concentration, the efficiency of PDI declined, resulting in greater bacterial viability. In the second phase, with higher 5-ALA concentrations (40 mM) a decrease in bacterial viability was observed. This was presumably related to dark toxicity, a non-light-mediated process.
FIG. 3.
Results for E. coli K-12 incubated for 4 h in the dark with 5-ALA and different n-alkyl esters (MAL, BAL, PAL HAL, and OAL). Bars represent the total bacterial PAP produced. Dotted lines (○) represent the survival fraction after incubation without illumination (dark toxicity), while solid lines (▪) represent the survival fraction after PDI (120 J·cm−2).
To understand this phenomenon, the total formation of the PAPs was analyzed in parallel with the PDI experiments (Fig. 3A). The PAP formation profile seemed to be inversely correlated to bacterial survival after PDI, except at the higher concentrations. Small amounts of PAPs were produced in the presence of low 5-ALA concentrations, increasing to a maximum of 7.5 pmol·10−6 CFU at the optimal concentration of 0.1 mM and then decreasing, despite the presence of higher substrate concentrations. This particular bell-shaped profile is commonly observed in 5-ALA-related photomedicine (27).
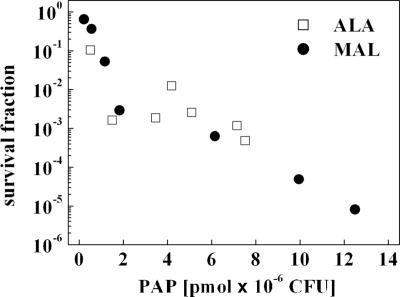
Figure 4 shows a direct correlation between PDI and the total amount of PAP produced in a range within which no or only few dark toxicities were observed, i.e., 0.1 to 10 mM for 5-ALA and MAL.
FIG. 4.
Survival of E. coli K-12 after irradiation (120 J·cm−2) decreases with the increase of the bacterial total PAP formation. □, 5-ALA; •, MAL. The concentration ranges were 0.1 to 10 mM for 5-ALA and MAL.
Types of porphyrins produced by E. coli K-12.
Analysis of the bacterial extracts revealed the presence of all of the porphyrins involved in heme biosynthesis, from uroporphyrin (UP) to protoporphyrin IX (PPIX). These porphyrins were present in various proportions and were dependent upon several factors like the substrate type and the substrate concentration (data not shown). Comparison of the porphyrins induced by 5-ALA- and MAL at their optimal concentrations showed that the majority were lipophilic porphyrins (carboxyporphyrin [CP] and PPIX) when 5-ALA was used, while hydrophilic porphyrins (UP, 7-CP, 6-CP, and 5-CP) were predominant in bacteria incubated with MAL.
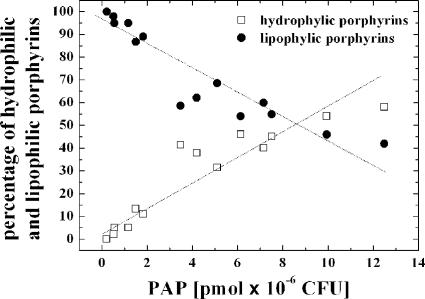
Figure 5 represents the percentage of hydrophilic and lipophilic porphyrins as a function of the total PAP induced. When small amounts of porphyrin were induced, CP and PPIX were the most frequent types of PAPs. In contrast, when larger amounts of PAP were produced, the proportion of hydrophilic porphyrins drastically increased.
FIG. 5.
5-ALA- and MAL-induced porphyrin distribution in E. coli K-12 as a function of total PAP formation. The increased proportion of hydrophilic porphyrins (•) toward lipophilic porphyrins (□) when large amounts of PAP are produced may be explained by the saturation of the decarboxylases involved in the heme biosynthesis cycle.
Effects of 5-ALA derivatives on PDI of E. coli K-12.
A series of 5-ALA n-alkyl esters with increasing chain lengths were tested for their abilities to photoinactivate E. coli K-12 under the same conditions. Major differences in the behaviors of the short- and long-chain derivatives were observed. MAL and BAL induced high levels of PAP formation and efficient photoinactivation at concentrations of 10 mM (Fig. 3B and C). In contrast, PAL, HAL, and OAL did not induce PAP formation or effective photoinactivation, presumably due to the strong dark toxicity observed at concentrations ranging from 1 to 10 mM (Fig. 3D to F). This intrinsic toxicity was also observed with 5-ALA and MAL when the concentrations were increased to 40 and 30 mM, respectively, suggesting the hypothesis that the dark toxicities of the 5-ALA derivatives increase with chain length and, thus, lipophilicity.
Effects of 5-ALA and MAL on different gram-positive and gram-negative strains.
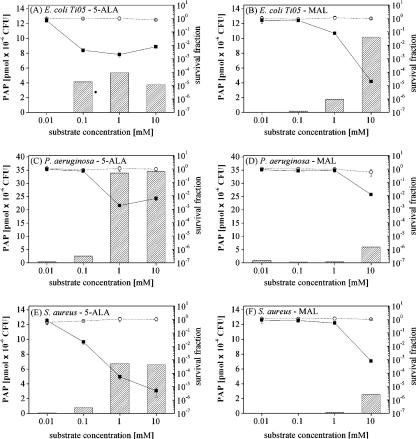
We investigated the efficacy of 5-ALA- and MAL-mediated PDI on two gram-negative strains, pathogenic strain E. coli Ti05 and P. aeruginosa, and on multiresistant S. aureus, a gram-positive strain. The results obtained with E. coli Ti05 were very similar to those obtained with laboratory strain K-12 in terms of PDI and PAP induction after incubation with 5-ALA or MAL. However, compared to the results obtained with E. coli K-12, higher substrate concentrations (Fig. 6A and B) were needed to maintain optimal PAP formation.
FIG. 6.
E. coli Ti05, P. aeruginosa, and methicillin-resistant S. aureus were incubated for 4 h in the dark with 5-ALA and MAL. Bars represent the total bacterial PAPs produced. Dotted lines (○) represent the survival fraction after incubation (dark toxicity), while solid lines (▪) represent the survival fraction after PDT (120 J·cm−2). Note the different scales used for Pseudomonas.
Although P. aeruginosa produced four to six times larger amounts of porphyrins than E. coli K-12 and Ti05 when it was incubated with 5-ALA, a proportional reduction in bacterial survival was not observed. Indeed, an inactivation rate of 99.9%, similar to that achieved for both E. coli strains, was obtained. In addition, at the concentrations tested, MAL did not induce substantial PAP accumulation, although induction with MAL still led to a survival fraction of about 1.2 × 10−2 upon irradiation (Fig. 6C and D). Under all experimental conditions, the majority of the porphyrins that accumulated in P. aeruginosa in response to both substrates was PPIX (data not shown).
The gram-positive organism S. aureus appeared to be significantly more sensitive to 5-ALA-mediated PDI than the gram-negative strains (survival fraction, <10−5), although the levels of PAP produced by S. aureus were comparable to those produced by the gram-negative organisms. MAL also induced sufficient PAP formation to achieve effective photoinactivation of >99.9% of this strain (Fig. 6E and F). The majority of the porphyrins in the different porphyrin extracts from S. aureus were UPs (>80%).
An overview of these results can be seen in Table 2, which compares the optimal concentration, the survival fraction, and PAP formation induced by 5-ALA and MAL for the different strains tested.
TABLE 2.
Comparison of 5-ALA- and MAL-mediated PDI of different bacterial strainsa
| Strain | 5-ALA
|
MAL
|
||||
|---|---|---|---|---|---|---|
| Optimal concn (mM) | Survival fraction | PAP concn (pmol·10−6 CFU) | Optimal concn (mM) | Survival fraction | PAP concn (pmol·10−6 CFU) | |
| E. coli K-12 | 0.1 | 4.8 × 10−4 | 7.5 | 10 | 8.1 × 10−6 | 12.5 |
| E. coli Ti05 | 1.0 | 5.0 × 10−5 | 5.4 | 10 | 2.0 × 10−5 | 10.2 |
| P. aeruginosa | 1.0 | 1.9 × 10−3 | 34.5 | 10 | 1.2 × 10−2 | 6.0 |
| S. aureus | 10 | 5.0 × 10−6 | 6.6 | 10 | 7.8 × 10−4 | 2.5 |
The optimal substrate concentration at which the highest PDI at a light dose of 120 J·cm−2 was achieved, the survival fraction, and the total amount PAP formation induced are reported.
DISCUSSION
The present study demonstrated the possibility of photodynamically inactivating gram-positive and gram-negative bacteria after incubation with 5-ALA acid or MAL. The characteristic profile of 5-ALA-induced porphyrin production as a function of the substrate concentration underlines the strong relationship between these two parameters and the importance of determining the optimal effective concentrations of each substrate. The bell-shaped curve observed is in agreement with the curves obtained in cell culture studies of 5-ALA-and 5-ALA derivative-induced porphyrins (27).
It is known that, in addition to PPIX, porphyrins in general can inhibit different enzymes involved in the heme biosynthetic cycle in order to avoid the accumulation of large amounts of intracellular PAPs (1). However, this negative feedback would result in the appearance of a plateau in terms of total porphyrin production with increasing 5-ALA concentrations and cannot explain the bell-shaped curve. Our results showed that at concentrations above the optimal concentration, 5-ALA itself appears to have an inhibitory effect on porphyrin formation, even though toxic drug concentrations were not reached. Furthermore, the same bacteria incubated with MAL produced significantly larger amounts of PAPs than the parent compound, suggesting that the maximal PAP production capacities were not reached.
The differences in the optimal concentrations observed for 5-ALA and MAL may result from different uptake mechanisms. It is well known that in animal cells, 5-ALA and MAL are transported into the cytosol via different active transport mechanisms (23); however, more lipophilic derivatives enter via passive diffusion through the membranes or endocytosis. In gram-negative bacteria, the double membrane prevents the penetration of exogenous molecules, while porins, which are transmembrane channels, allow only small hydrophilic molecules, usually nutrients, to enter the bacteria (18). Thus, the entrance of 5-ALA into gram-negative bacteria is not problematic (28), as demonstrated by the low concentration necessary for optimal PAP induction. In the case of MAL, the addition of a methyl chain on the carboxylic group increases the log oil-water partition coefficient from −1.52 to −0.94 (27), which is still considered hydrophilic. Hence, this compound may also enter the bacterial cytosol through porin channels. However, the high concentrations (3 to 10 mM) necessary to induce sufficient PAP formation indicate that either the penetration or the availability may vary from that of the parent compound.
The fact that more lipophilic derivatives, such as PAL, HAL, and OAL, do not induce any significant PAP formation might be explained by the trapping of these highly lipophilic compounds in the bacterial membranes or by the fact that the highly amphiphilic properties of these derivatives may induce membrane disruption, causing dark toxicity.
We have seen a strong correlation between the PDI efficiency and the total amount of PAPs formed in E. coli K-12. However, this cannot be extrapolated to other strains due to the different types of porphyrins produced, protection mechanisms (12), and porphyrin localization. All types of porphyrins observed in our study have shown the capacity to absorb light and generate reactive oxygen species (22). The main difference between these endogenous porphyrins is related to the number of carboxylic moieties, which influence solubility, lipophilicity, aggregation, and localization. In the case of E. coli, it was seen that the type of PAP depends on the quantity of porphyrins produced. When small amounts of porphyrins are synthesized, the enzymatic machinery of the bacteria was able to proceed to the decarboxylation of a high proportion of these porphyrins, leading to the accumulation of mainly lipophilic porphyrins. When very large quantities of PAP are produced, the enzymatic process may be overwhelmed, leading to an increased proportion of hydrophilic porphyrins. This tendency was observed only in the E. coli strains (data not shown for the other species). In contrast, S. aureus nearly exclusively produced UPs and was efficiently photoinactivated, while P. aeruginosa produced four to five times more porphyrins, mainly PPIX, but was not more efficiently photoinactivated.
To obtain efficient 5-ALA- or 5-ALA derivative-mediated PDI, the two main conditions necessary are the accumulation of sufficient amounts of PAPs in the targeted bacteria and effective irradiation to activate the PS. In fact, when this type of PS prodrug is used, PDI conducted under suboptimal conditions may lead to contradictory results. For example, Karrer et al. (10) have shown that 5-ALA-mediated PDI induced significant inhibition of the growth of S. aureus but not Staphylococcus epidermidis. In contrast, Nitzan et al. (19) observed that 5-ALA-mediated PDI was effective against both S. aureus and S. epidermidis but not against gram-negative strains, including E. coli and P. aeruginosa. The last two strains were successfully photoinactivated by Szocs et al. (24) and Lee et al. (14), respectively, after incubation with 5-ALA. Therefore, the present study clearly demonstrates not only that the concentrations must be optimized and adapted to the specific bacteria but also that the lipophilicity of the PAP precursor itself must be optimized. While MAL and BAL seemed to be more effective at inactivating E. coli, 5-ALA been demonstrated to have the ability to inactive efficiently S. aureus at high concentrations. In the present study we have mainly focused on PAP precursors that have gained marketing approval for topical administration (5-ALA, MAL, and HAL), two compounds of intermediate lipophilicity (BAL and PAL), and one more lipophilic compound (OAL) that was still water soluble at the concentrations used in our study. However, in future studies more moderately lipophilic compounds, such as the ethyl, propyl, and benzyl esters, should be considered.
In our study, both 5-ALA and MAL have been shown to induce increased amounts of PAPs in all bacteria treated when the optimal conditions were applied. In E. coli, the total inhibitory effect observed after incubation with MAL, although it was observed at higher drug concentrations, was up to 2 orders of magnitude higher than achieved after incubation with 5-ALA. However, this advantage was not observed in the other strains used in the present study. In clinical practice, MAL might have other advantages, especially with respect to its pharmacokinetic and pharmacodynamic properties, which have been established in clinical trials for the treatment of basal cell carcinomas and actinic keratosis (6, 7, 17). As 5-ALA presented a biphasic inactivation curve, intermediate concentrations resulted in the suboptimal inactivation of the targeted bacteria. A more desirable behavior was obtained with MAL, as bacterial survival continuously decreased with increasing MAL concentrations. In this case, PAP formation, and thus, PDI, was inhibited only when toxic drug concentrations (greater than 10 mM) were achieved.
Not only is the ability to induce PAPs important, but 5-ALA derivatives, including MAL, may also have already established advantages with respect to local bioavailability after topical administration for the treatment of other pathologies. The topical administration of MAL has been shown to result in a more selective and deeper distribution of PAPs in patients with thick basal cell carcinomas (20). Therefore, deeper infections can be treated more effectively with this compound. In some cases, MAL was shown to be more selective than 5-ALA for diseased tissue, such as solar keratoses (6), potentially leading to less collateral damage in the surrounding healthy tissue. Furthermore, MAL has been shown to have limited systemic uptake and has been reported to induce less pain during irradiation, which is an important parameter when multiple PDT sessions are needed (11).
The most effective concentrations used in this study may seem relatively high compared to the concentrations of other PS compounds, which are used at micromolar concentrations; indeed, for some other PS compounds, the application time is minutes instead of hours (25, 26). However, the high selectivities of the compounds that we have tested, as demonstrated in other medical domains, may provide the means to potentially eradicate bacterial infections without harming healthy surrounding tissues.
The use of the compounds tested in our studies will be limited to bacterial infections that are accessible to the local administration of both the drug and light, including the skin, the lungs, the oral cavity, the urogenital area, and the gastrointestinal tract. As an example, early clinical trials have demonstrated promising results from 5-ALA-mediated PDI of Helicobacter pylori (30). Current developments in this area focus on the development of systemically applicable derivatives of 5-ALA with prolonged half-lives, which will circumvent the necessity to apply the compounds locally. Furthermore, repeatability may be a major asset of PDI, as cumulative toxicity (21) and induced bacterial resistance upon repeated treatments (13) have not been reported to date.
Therefore, when optimal concentrations were used, 5-ALA and 5-ALA short-chain derivatives (MAL and BAL) induced high levels of PAP accumulation in both the gram-positive and gram-negative bacterial strains that we tested, attaining the first prerequisite for a successful PDI. In contrast, long-chain 5-ALA derivatives (PAL, HAL, and OAL) demonstrated high dark toxicities that increased with lipophilicity, and induction with these compounds showed no significant PAP formation. As MAL efficiently induced PAP formation in bacteria, it might show improved efficacy in vivo, especially due to its advantageous pharmacokinetic and pharmacodynamic properties. Its ability to be used for the treatment of local infections should be further investigated in in vivo experiments.
Footnotes
Published ahead of print on 14 January 2008.
REFERENCES
- 1.Afonso, S. G., R. Enriquez de Salamanca, and A. M. Batlle. 2001. Photodynamic and light independent action of 8 to 2 carboxylic free porphyrins on some haem-enzymes. Int. J. Biochem. Cell Biol. 33:1208-1214. [DOI] [PubMed] [Google Scholar]
- 2.Barnes, R. M., L. Gee, S. Taylor, M. C. Briggs, and S. P. Harding. 2004. Outcomes in verteporfin photodynamic therapy for choroidal neovascularisation— “beyond the TAP study.” Eye 18:809-813. [DOI] [PubMed] [Google Scholar]
- 3.Brown, S. B., E. A. Brown, and I. Walker. 2004. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 5:497-508. [DOI] [PubMed] [Google Scholar]
- 4.Collaud, S., A. Juzeniene, J. Moan, and N. Lange. 2004. On the selectivity of 5-aminolevulinic acid-induced protoporphyrin IX formation. Curr. Med. Chem. Anti-Cancer Agents 4:301-316. [DOI] [PubMed] [Google Scholar]
- 5.Fotinos, N., M. A. Campo, F. Popowycz, R. Gurny, and N. Lange. 2006. 5-Aminolevulinic acid derivatives in photomedicine: characteristics, application and perspectives. Photochem. Photobiol. 82:994-1015. [DOI] [PubMed] [Google Scholar]
- 6.Fritsch, C., B. Homey, W. Stahl, P. Lehmann, T. Ruzicka, and H. Sies. 1998. Preferential relative porphyrin enrichment in solar keratoses upon topical application of delta-aminolevulinic acid methylester. Photochem. Photobiol. 68:218-221. [PubMed] [Google Scholar]
- 7.Fritsch, C., and T. Ruzicka. 2006. Fluorescence diagnosis and photodynamic therapy in dermatology from experimental state to clinic standard methods. J. Environ. Pathol. Toxicol. Oncol. 25:425-439. [DOI] [PubMed] [Google Scholar]
- 8.Hamblin, M. R., and T. Hasan. 2004. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 3:436-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jori, G., C. Fabris, M. Soncin, S. Ferro, O. Coppellotti, D. Dei, L. Fantetti, G. Chiti, and G. Roncucci. 2006. Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications. Lasers Surg. Med. 38:468-481. [DOI] [PubMed] [Google Scholar]
- 10.Karrer, S., R. M. Szeimies, S. Ernst, C. Abels, W. Bäumler, and M. Landthaler. 1999. Photodynamic inactivation of staphylococci with 5-aminolaevulinic acid or photofrin. Lasers Med. Sci. 14:54-61. [DOI] [PubMed] [Google Scholar]
- 11.Kasche, A., S. Luderschmidt, J. Ring, and R. Hein. 2006. Photodynamic therapy induces less pain in patients treated with methyl aminolevulinate compared to aminolevulinic acid. J. Drugs Dermatol. 5:353-356. [PubMed] [Google Scholar]
- 12.Kim, S. Y., E. J. Kim, and J. W. Park. 2002. Control of singlet oxygen-induced oxidative damage in Escherichia coli. J. Biochem. Mol. Biol. 35:353-357. [DOI] [PubMed] [Google Scholar]
- 13.Lauro, F. M., P. Pretto, L. Covolo, G. Jori, and G. Bertoloni. 2002. Photoinactivation of bacterial strains involved in periodontal diseases sensitized by porphycene-polylysine conjugates. Photochem. Photobiol. Sci. 1:468-470. [DOI] [PubMed] [Google Scholar]
- 14.Lee, C. F., C. J. Lee, C. T. Chen, and C. T. Huang. 2004. δ-Aminolaevulinic acid mediated photodynamic antimicrobial chemotherapy on Pseudomonas aeruginosa planktonic and biofilm cultures. J. Photochem. Photobiol. B 75:21-25. [DOI] [PubMed] [Google Scholar]
- 15.Malik, Z., H. Ladan, and Y. Nitzan. 1992. Photodynamic inactivation of gram-negative bacteria: problems and possible solutions. J. Photochem. Photobiol. B 14:262-266. [DOI] [PubMed] [Google Scholar]
- 16.Moan, J., and Q. Peng. 2003. An outline of the hundred-year history of PDT. Anticancer Res. 23:3591-3600. [PubMed] [Google Scholar]
- 17.Moloney, F. J., and P. Collins. 2007. Randomized, double-blind, prospective study to compare topical 5-aminolaevulinic acid methylester with topical 5-aminolaevulinic acid photodynamic therapy for extensive scalp actinic keratosis. Br. J. Dermatol. 157:87-91. [DOI] [PubMed] [Google Scholar]
- 18.Nikaido, H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67:593-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nitzan, Y., M. Salmon-Divon, E. Shporen, and Z. Malik. 2004. ALA induced photodynamic effects on gram positive and negative bacteria. Photochem. Photobiol. Sci. 3:430-435. [DOI] [PubMed] [Google Scholar]
- 20.Peng, Q., A. M. Soler, T. Warloe, J. M. Nesland, and K. E. Giercksky. 2001. Selective distribution of porphyrins in skin thick basal cell carcinoma after topical application of methyl 5-aminolevulinate. J. Photochem. Photobiol. B 62:140-145. [DOI] [PubMed] [Google Scholar]
- 21.Peng, Q., T. Warloe, K. Berg, J. Moan, M. Kongshaug, K. E. Giercksky, and J. M. Nesland. 1997. 5-aminolevulinic acid-based photodynamic therapy—clinical research and future challenges. Cancer 79:2282-2308. [DOI] [PubMed] [Google Scholar]
- 22.Redmond, R. W., and J. N. Gamlin. 1999. A compilation of singlet oxygen yields from biologically relevant molecules. Photochem. Photobiol. 70:391-475. [PubMed] [Google Scholar]
- 23.Rud, E., O. Gederaas, A. Hogset, and K. Berg. 2000. 5-Aminolevulinic acid, but not 5-aminolevulinic acid esters, is transported into adenocarcinoma cells by system BETA transporters. Photochem. Photobiol. 71:640-647. [DOI] [PubMed] [Google Scholar]
- 24.Szocs, K., F. Gabor, G. Csik, and J. Fidy. 1999. δ-Aminolaevulinic acid-induced porphyrin synthesis and photodynamic inactivation of Escherichia coli B. J. Photochem. Photobiol. B 50:8-17. [DOI] [PubMed] [Google Scholar]
- 25.Tegos, G. P., M. Anbe, C. Yang, T. N. Demidova, M. Satti, P. Mroz, S. Janjua, F. Gad, and M. R. Hamblin. 2006. Protease-stable polycationic photosensitizer conjugates between polyethyleneimine and chlorin(e6) for broad-spectrum antimicrobial photoinactivation. Antimicrob. Agents Chemother. 50:1402-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tome, J. P., M. G. Neves, A. C. Tome, J. A. Cavaleiro, M. Soncin, M. Magaraggia, S. Ferro, and G. Jori. 2004. Synthesis and antibacterial activity of new poly-S-lysine-porphyrin conjugates. J. Med. Chem. 47:6649-6652. [DOI] [PubMed] [Google Scholar]
- 27.Uehlinger, P., M. Zellweger, G. Wagnières, L. Juillerat-Jeanneret, H. van den Bergh, and N. Lange. 2000. 5-Aminolevulinic acid and its derivatives: physical chemical properties and protoporphyrin IX formation in cultured cells. J. Photochem. Photobiol. B 54:72-80. [DOI] [PubMed] [Google Scholar]
- 28.Verkamp, E., V. M. Backman, J. M. Bjornsson, D. Soll, and G. Eggertsson. 1993. The periplasmic dipeptide permease system transports 5-aminolevulinic acid in Escherichia coli. J. Bacteriol. 175:1452-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Von Tappeiner, H., and A. Jodlbauer. 1904. Über Wirkung der photodynamischen (fluorieszierenden) Stoffe auf Protozoan und Enzyme. Dtsch. Arch. Klin. Med. 80:427-487. [Google Scholar]
- 30.Wilder-Smith, C. H., P. Wilder-Smith, P. Grosjean, H. van den Bergh, A. Woodtli, P. Monnier, G. Dorta, F. Meister, and G. Wagnières. 2002. Photoeradication of Helicobacter pylori using 5-aminolevulinic acid: preliminary human studies. Lasers Surg. Med. 31:18-22. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization. 1998. Emerging and other communicable diseases: antimicrobial resistance. Report A51/9. World Health Organization, Geneva, Switzerland.