Abstract
Sulfadiazine, pyrimethamine, and atovaquone are widely used for the treatment of severe toxoplasmosis. Their in vitro activities have been almost exclusively demonstrated on laboratory strains belonging to genotype I. We determined the in vitro activities of these drugs against 17 strains of Toxoplasma gondii belonging to various genotypes and examined the correlations among 50% inhibitory concentrations (IC50s), growth kinetics, strain genotypes, and mutations on drug target genes. Growth kinetics were determined in THP-1 cell cultures using real-time PCR. IC50s were determined in MRC-5 cell cultures using a T. gondii-specific enzyme-linked immunosorbent assay performed on cultures. Mutations in dihydrofolate reductase (DHFR), dihydropteroate synthase (DHPS), and cytochrome b genes were determined by sequencing. Pyrimethamine IC50s ranged between 0.07 and 0.39 mg/liter, with no correlation with the strain genotype but a significant correlation with growth kinetics. Several mutations found on the DHFR gene were not linked to lower susceptibility. Atovaquone IC50s were in a narrow range of concentrations (mean, 0.06 ± 0.02 mg/liter); no mutation was found on the cytochrome b gene. IC50s for sulfadiazine ranged between 3 and 18.9 mg/liter for 13 strains and were >50 mg/liter for three strains. High IC50s were not correlated to strain genotypes or growth kinetics. A new mutation of the DHPS gene was demonstrated in one of these strains. In conclusion, we found variability in the susceptibilities of T. gondii strains to pyrimethamine and atovaquone, with no evidence of drug resistance. A higher variability was found for sulfadiazine, with a possible resistance of three strains. No relationship was found between drug susceptibility and strain genotype.
Sulfonamides in combination with pyrimethamine are a mainstay of chemotherapy of toxoplasmosis (34). These drugs have a remarkable synergistic activity against the replicating form of Toxoplasma gondii through the sequential inhibition of parasite dihydropteroate synthase (DHPS) and dihydrofolate reductase (DHFR). These two major enzymes are responsible for the synthesis of the folate compounds that are essential for parasite survival and replication. DHFR is also present in humans so that treatment with DHFR inhibitors may induce a folate deficiency, which is possibly responsible for severe hematological side effects and embryopathies.
In immunocompromised patients, particularly those with AIDS, treatment with sulfonamides and DHFR inhibitors is frequently associated with side effects despite the preventive administration of folinic acid (29). In these patients, possible therapeutic alternative therapies are the combination of clindamycin and pyrimethamine, which proved as efficient as the reference pyrimethamine-suldafiazine combination (12) or the administration of atovaquone alone or combined with pyrimethamine (9, 21). Atovaquone is a broad-spectrum antiparasitic drug that is active against T. gondii through the inhibition of mitochondrial electron transport processes, competing with the biological electron carrier ubiquinone (32, 45). The in vitro and in vivo inhibitory effect of atovaquone on T. gondii is well demonstrated at very low concentrations (4, 43); moreover, atovaquone is potentially active against tissue cysts (5).
Treatment failures have been reported for most drug regimens used for the treatment of toxoplasmic encephalitis, chorioretinitis, and congenital toxoplasmosis (7, 12, 21, 36, 46). Whether these failures are related to host factors (drug intolerance and malabsorption) and/or to the development of drug resistance parasites or a lower susceptibility of the parasite strain is debated. Up to now, very few data document the two latter hypotheses. Drug-resistant mutants have been obtained in vitro by mutagenesis under drug pressure for sulfonamides (38), pyrimethamine (41, 42), and atovaquone (32, 39). However, the demonstration of a mutation responsible for substantial resistance to sulfonamides was demonstrated for only one clinical isolate (6).
Similarly, very little is known on the natural resistance or lower susceptibility of T. gondii strains. Genetic analyses agree to class most of T. gondii strains into three clonal lineages: types I, II, and III (1, 19). Less than 5% of the isolates are strains recombining with a mixture of the two alleles. All the genotypes seem to be able to infect humans. Nevertheless, the type II strains are predominant in the European and North American populations (1), whereas type III and recombinant strain type I/III are predominant in South America. The role of the parasite genotype in the severity of the disease is well established in mice. Type I strains are considered to be highly virulent, while type II strains are nonvirulent and type III strains are intermediate. In humans, the correlate between genotype and clinical presentation is more complex, but the higher virulence of some genotypes is suspected from the higher incidence of severe toxoplasmosis in patients infected with type I or recombinant and atypical strains (1, 17, 26, 27). Up to now, most pharmacological studies have been performed with the RH strain (genotype I), which was chosen for its capacity to grow in culture. To our knowledge, only two studies examined the in vitro susceptibilities of strains belonging to other genotypes. One was performed with one isolate from each of the three major clonal lineages (types I, II, and III) of T. gondii, which were tested for their susceptibilities to pyrimethamine (41). Another study examined the in vitro susceptibilities of seven strains of T. gondii to atovaquone (4).
Taking into account newer knowledge on genetic diversity and genotyping of T. gondii and thanks to the availability of well-characterized T. gondii strains in the French Biological Toxoplasma Resource Centre, we have been able to analyze the susceptibilities of T. gondii strains belonging to various genotypes to sulfadiazine (SDZ), pyrimethamine, and atovaquone. Results of drug susceptibility studies were examined with regard to parasitic genotypes, including mutations on drug target genes, and kinetics of growth in order to document (or refute) the possible need for the adaptation of treatment according to the genotype or the phenotype of the infecting strain.
MATERIALS AND METHODS
Toxoplasma strains.
Strains of T. gondii were provided by the French Biological Toxoplasma Resource Centre (BRC Toxoplasma, France), which has collected and preserved human and animal isolates of T. gondii since 2002. After their isolation from clinical samples, strains were propagated on human acute monocytic leukemia THP-1 cells (48), cultured in RPMI 1640 medium (Sigma, St. Quentin, France) with 2% fetal calf serum, 1% l-glutamine, and antibiotics (100 IU/ml penicillin and 0.1 mg/ml streptomycin), and then kept frozen until use. At inclusion in the BRC Toxoplasma collection, strain genotypes were determined by a multilocus analysis on SAG1, SAG2, and GRA7 genes and microsatellite analysis (2, 19).
The 17 strains selected for this study belonged to the three classical genotypes (types I, II, and III) and recombinant (type I/III) or atypical genotypes. Fifteen were of human origin, and two had been isolated from animals (B1 and ME49). Besides the laboratory strains RH, B1, ENT, and ME49, 10 strains were clinical isolates originating from patients with congenital toxoplasmosis, one was from a patient with primary acquired infection, and two were from immunocompromised patients with toxoplasmic encephalitis (Table 1).
TABLE 1.
Strain characteristics
| Strain | Type | Origin | Sample | Type of infection | Drug exposure before isolationa |
|---|---|---|---|---|---|
| RH | I | Human | Brain and neural tissues | Encephalitis | No |
| B1 | I | Bovine | Aborted fetus | No | |
| ENT | I | Human | Placenta | Congenital toxoplasmosis; no clinical symptoms | No |
| RMS-1995-ABE | II | Human | Placenta | Congenital toxoplasmosis; chorioretinitis | Prenatal treatment, spiramycin for 2 wk, PYR + SDX for 12 wk |
| ME49 | II | Ovine | Placenta | Ovine toxoplasmosis | No |
| TRS-2004-REV | II | Human | Brain | Congenital toxoplasmosis; brain calcifications | Prenatal treatment, spiramycin for 5 wk, PYR + SDZ for 2 wk |
| TOU-1998-TRI | II | Human | Placenta | Congenital toxoplasmosis; chorioretinitis | Prenatal treatment, spiramycin 6 wk, PYR + SDX 14 wk. |
| RMS-2005-HAG | II | Human | Placenta | Congenital toxoplasmosis; chorioretinitis; hydrocephaly, brain calcifications | Prenatal treatment, spiramycin for 27 wk |
| GRE-1995-MAE | II | Human | Brain | Congenital toxoplasmosis; hydrocephaly; disseminated toxoplasmosis | Prenatal treatment, spiramycin for 16 wk |
| PSP-2005-MUP | III | Human | Brain | Encephalitis; HIV positive | No |
| GRE-1998-TRA | III | Human | Placenta | Congenital toxoplasmosis; no clinical symptoms | Prenatal treatment, spiramycin for 2 wk |
| RMS-2003-TOU | III | Human | Placenta | Congenital toxoplasmosis; no clinical symptoms | Prenatal treatment, spiramycin for 19 wk |
| NED | III | Human | Placenta | Congenital toxoplasmosis; no clinical symptoms | No |
| RMS-1994-LEF | I/III | Human | Cerebrospinal fluid | Congenital toxoplasmosis; chorioretinitis | Prenatal treatment, spiramycin for 6 wk, PYR + SDX for 5 wk |
| RMS-2003-DJO | I/III | Human | Brain | Encephalitis; HIV positive | No |
| RMS-2001-MAU | Atypical | Human | Placenta | Congenital toxoplasmosis; chorioretinitis | Prenatal treatment, spiramycin for 6 wk |
| GUY-2003-MEL | Atypical | Human | Blood | Toxoplasmosis acquired in French Guyana | No |
PYR, pyrimethamine; SDX, sulfadoxine.
Before the growth kinetics and drug study experiments, a frozen aliquot was cultured and passaged weekly for 2 weeks on THP-1 cells in RPMI culture medium containing 2% fetal calf serum. Cultures were performed using 25-cm2 tissue culture flasks at 37°C in an atmosphere of 95% air and 5% CO2.
Growth kinetics.
Growth kinetics were determined in THP-1 cell cultures using 24-well plates. The inoculum was prepared from the flask cultures used for maintaining the strain. When the cells appeared to be massively infected and close to bursting, tachyzoites were released by forcing them through a 27-gauge needle and then filtered through a 5-μm filter (sterile Millex filter unit; Millipore, Bedford, MA), washed by centrifugation in RPMI, suspended in RPMI medium containing 2% fetal calf serum, and counted in a hemocytometer. In preliminary experiments, we checked that the growth rates of a given strain inoculated at various parasite/cell ratios did not differ significantly when only the linear portion of the growth curves was considered for calculation. Therefore, the inoculum size and tachyzoite/cell ratio were determined to allow continuous log-linear growth for 7 days in a 24-well culture plate with the persistence of noninfected THP-1 cells at the end of the experiment. The selected ratio was 1 tachyzoite per 50 THP-1 cells for the rapidly growing type I strains, and 1 tachyzoite per 10 cells for all the other strains. For each strain, six replicate wells containing 105 THP-1 cells in a total volume of 1 ml RPMI medium containing 2% fetal calf serum were inoculated with tachyzoites at day 0 and then incubated in moist 5% CO2-95% air at 37°C until the end of the experiment (day 7).
Quantification of tachyzoites was performed by real-time PCR. At day 0, 3 or 4, and 7, 200-μl culture samples (supernatant and cells in suspension) were collected in two wells and then immediately frozen at −20°C. At the end of the experiment, quantification of T. gondii DNA in the samples was performed by quantitative real-time PCR targeting the gene reported under GenBank accession number AF146527, as previously described (8). All sequential samples were tested in the same run. Each run was comprised of a control without DNA and four standard dilutions of T. gondii DNA extracted from tachyzoites of the RH strain (1 to 1,000 tachyzoites per reaction). Crossing-point values obtained by real-time PCR, i.e., the cycle numbers at which a significant signal was observed, were converted into tachyzoite equivalents (EquTachy)/ml by interpolation from the standard parasite dilution curve. We checked that this conversion could also be applied for non-type-I strains since the detection limit of the PCR and the slopes of the standard curves obtained with serial dilutions of tachyzoites of strains RH (type I), RMS-1995-ABE (type II), and NED (type III) were not significantly different (data not shown).
Growth curves were calculated from the three relevant points (days 0, 3, 4, and 7) in a semi-log-linear regression model using GraphPad Prism, version 4.03, for Windows (GraphPad Software, San Diego, CA). In preliminary experiments, we checked that growth curves did not differ significantly from linearity. Using this model, parasite growth is represented by the equation log(EquTachy ml−1) = I + S × days of culture, where I is the intercept, i.e., the measured EquTachy ml−1 at day 0, and S is the slope of the regression curve, i.e., the growth rate of the parasite strain.
Only curves with a regression coefficient (r2) of >0.94 were retained for calculation. Experiments were repeated at least twice for each strain. Strain growth was expressed by the slope (S) ± the standard deviation of the regression curve.
Drug susceptibility study.
In vitro drug susceptibility studies were carried out in MRC-5 fibroblast tissue cultures with quantification of parasites by an enzyme-linked immunosorbent assay (ELISA) as previously described (13, 14), with some minor modifications concerning the inoculum size and the antibody used for ELISA.
Atovaquone (Wellcome Foundation Ltd.), pyrimethamine, and SDZ (Sigma, St. Quentin, France) were dissolved in 50% methanol-50% acetone at a concentration of 1 mg/ml. Serial dilutions of each drug were prepared in RPMI medium such that the addition of 25 μl of each dilution to a culture well produced the required final concentration. Preliminary studies indicated that the final concentrations of methanol and acetone used for the dilution of drugs did not inhibit the growth of T. gondii.
Cultures of MRC-5 fibroblasts (Biomérieux, Lyon, France) were prepared in 96-well tissue culture plates and grown to confluence as previously described (13). At confluence, the mean number of cells per well is comprised of between 3.5 × 104 and 4 × 104 cells and remains stable thereafter (data not shown). Each inoculum was prepared from freshly harvested tachyzoites grown in THP-1 cells as described above. Its size was adapted to each strain in order to obtain continuous growth during the experiment and the observation of nonconfluent parasitic foci in control cultures (without drug) at 72 h. By this method, the levels of infection in control cultures were comparable for all strains at 72 h, and the 50% inhibitory concentrations (IC50s) for rapid- and slow-growing strains could be compared.
From the results of preliminary experiments, the inoculum (25 μl in RPMI) ranged from 0.4 to 1 tachyzoite/10 cells according to the strain.
Three hours after parasite inoculation, drugs were added in four to eight replicate wells for each dilution (25 μl), and the plates were then incubated for 72 h. Plates were examined microscopically for cytopathic effects, fixed with cold methanol for 5 min, and air dried. Toxoplasma growth was evaluated by ELISA performed directly on the infected cultures as previously described, with minor modifications (14, 43). In this study, we used an anti-Toxoplasma immune serum obtained from a New Zealand White rabbit that had been experimentally infected with 200 cysts of strain ME49 (type II) by gavage 3 months earlier. This serum was diluted 1/1,000 in phosphate-buffered saline and incubated for 90 min (50 μl/well). After washing with phosphate-buffered saline, the peroxidase-labeled anti-rabbit immunoglobulin conjugate at a 1/2,000 dilution (50 μl/well; Sigma) was added and incubated for 90 min. After five washings, 3,3′,5,5′-tetramethylbenzidine substrate (Sigma) was added (200 μl/well). After the addition of the stopping solution (1 N H2SO4 at 100 μl/well), spectrophotometric readings of the supernatant were performed at a λ of 405 nm using the negative control wells as blanks. For each well, the results were expressed as optical density values. Experiments were done in duplicate for each strain and for each drug.
The effect of each drug at various concentrations was described by plotting the optical density values as a function of the logarithm of the concentration. A linear regression model was used to summarize the concentration-dose effect relationship and to determine the IC50 as previously described (13).
In order to examine the possible interference of exogenous folates in IC50 determinations, additional experiments were conducted with some strains under folate-free conditions. MRC-5 cell cultures were grown to confluence for 3 days in RPMI medium with the addition of 10% fetal calf serum, and the medium was discarded and replaced with folate-free RPMI medium (Sigma) supplemented with 2% Ultroser (IBF, Villeneuve-la Garenne, France), which is a fetal bovine serum substitute. Tachyzoites and drug solutions were also prepared in folate-free RPMI medium and were added sequentially into the cultures as described above. Plates were incubated for 72 h and then examined by ELISA as described above.
Analysis of drug target genes.
Identification of polymorphic sites of DHPS, DHFR, and cytochrome b genes was carried out by using PCR amplification and direct sequencing. Amplifications were performed in a final volume of 50 μl containing 5 μl of 10× PCR buffer (20 mM Tris-HCl, 50 mM KCl), 2 mM MgCl2, 200 μM each deoxynucleoside triphosphates, 1 μM each forward and reverse primer, 1.25 U of Taq DNA polymerase (Invitrogen Life Technologies, Cergy Pontoise, France), and 20 ng of template DNA. The following cycle program was carried out: an initial denaturation step at 94°C for 3 min and then 40 cycles consisting of 20 s of denaturation at 94°C, 20 s of annealing at primer-dependent temperatures, and 30 s of extension at 72°C. A final extension step was carried out at 72°C for 5 min (PCR thermal cycler; Hybaid, France). PCR-amplified DNA fragments were then sequenced with their corresponding primers by a double-stranded DNA sequencing method by using an automated DNA sequencer (Qiagen, Hilden, Germany). Strain polymorphisms were analyzed by alignment of the nucleotide sequences according to the ClustalW multiple sequence alignment program at the EMBL-European Bioinformatics Institute website (http://www.ebi.ac.uk//clustalw/index.html). Primer pairs of DHPS and DHFR were described previously (6). Primers of cytochrome b (GenBank accession number AF023246) were designed using Primer Pro 3.4 software (http://www.changbioscience.com/primo/primo.html). The three following primers pairs were used to sequence the coding region of cytochrome b: Ato-F1 (5′-GGCACACCTTGTCTTTTATCGGT-3′) and Ato-R1 (5′-GGACATATCCGAGGAAGGCA-3′), Ato-F2 (5′-TTGCATGCTACAACAGCCTC-3′) and Ato-R2 (5′-AAGTGGTGTTACGAACCGGTTG-3′), and Ato-F3 (5′-AATCCTGCAGGTATTGATACCGC-3′) and Ato-R3 (5′-GAACCAATCCGGTAGTAAGG-3′). Annealing temperatures were 52°C for Ato-F2/Ato-R2 and 60°C for Ato-F1/Ato-R1 and Ato-F3/Ato-R3.
Statistical analysis.
Statistical analysis was performed using GraphPad Prism software. Distribution of data was tested by the D'Agostino and Pearson omnibus normality test. For growth rates, comparisons of data from strains or groups of strains were made by one-way analysis of variance (ANOVA) followed by Bonferroni's posttests for growth rates. As the frequency distribution of IC50s failed to pass normality tests for SDZ, data for IC50s were analyzed using the nonparametric Kruskal-Wallis one-way ANOVA to compare medians with Dunn's posttests. Relationships between growth rates and IC50s were analyzed by nonparametric Spearman's rank correlation analysis.
RESULTS
Growth kinetics.
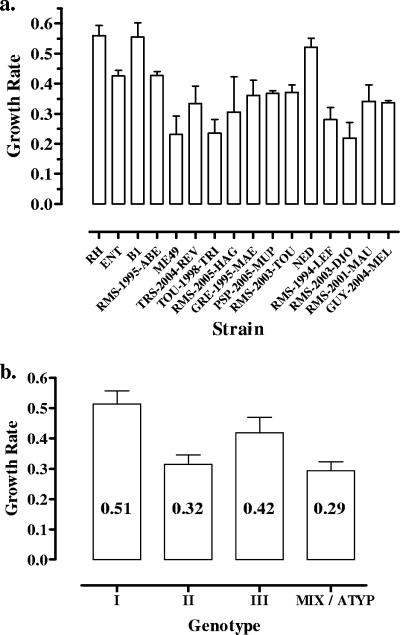
Growth kinetics in THP-1 cells were determined for 16 strains (Fig. 1a), as one strain (GRE-1998-TRA) could not be repeatedly cultured on THP-1 cells. Significant differences in growth rates were observed between strains (P < 0.0001). Mean growth rates ranged from 0.24 for strain RMS-2003-TOU to 0.56 for strain RH, resulting in an increase from 104 to 5 × 105 and 1.7 × 108 EquTachy ml−1 during the 7 days of culture, respectively. This corresponds to a replication time comprised of between 30 h for the slowest-growing strain and 12.9 h for strain RH. Growth rates also varied depending on the strain genotype (P = 0.006) (Fig. 1b). Type I strains showed the highest growth rates, with a mean of 0.51, corresponding a replication time of 14 h, which was found to be significantly different from those of type II and mixed/atypical genotypes (means of 0.29 and 0.32, with replicating times of 22 and 24 h, respectively; P < 0.05).
FIG. 1.
Growth rates (values of regression line slopes) of Toxoplasma in THP-1 cells over a 7-day period of culture. (a) Growth rates of the 16 strains. (b) Mean growth rates of parasites from different genotypes. ATYP, atypical.
Drug susceptibility.
The inhibitory effects of SDZ, pyrimethamine, and atovaquone were determined for 16 strains, as one strain (RMS-2003-TOU) could not be repeatedly cultured on MRC-5 cells. The mean IC50s were calculated from at least two replicate experiments and are presented in Table 2.
TABLE 2.
IC50s for pyrimethamine, SDZ, and atovaquonea
| Strain | Type | IC50 (mg/liter) ± 95% confidence interval
|
Mutation(s) in gene:
|
|||||
|---|---|---|---|---|---|---|---|---|
| Pyrimethamine | SDZ | Atovaquone | DHPS | DHPS at position 407 | DHFR | Cytochrome b | ||
| RH | I | 0.1 ± 0.05 | 6.52 ± 5.09 | 0.05 ± 0.01 | Ex2, E474D | 0 | Ex3, 156 sil Leu | 0 |
| Ex4, R560K | ||||||||
| Ex5, 580 sil Gly; A597E, 627 sil Glu | ||||||||
| ENT | I | 0.34 ± 0.07 | 4.28 ± 5.55 | 0.07 ± 0.04 | Ex2, E474D | 0 | Ex3, 156 sil Leu | 0 |
| Ex4, R560K | ||||||||
| Ex5, 580 sil Gly; A597E 627 sil Glu | ||||||||
| B1 | I | 0.35 ± 0.18 | >50 | 0.10 ± 0.03 | Ex2, E474D | 0 | Ex3, 156 sil Leu | 0 |
| Ex4, R560K | ||||||||
| Ex5, 580 sil Gly; A597E, 627 sil Glu | ||||||||
| RMS-1995-ABE | II | 0.17 ± 0.06 | >50 | 0.11 ± 0.02 | Ex5, A587V | 0 | 0 | 0 |
| ME49 | II | 0.09 ± 0.02 | 6.55 ± 2.76 | 0.05 ± 0.02 | 0 | 0 | 0 | 0 |
| TRS-2004-REV | II | 0.18 ± 0.02 | 6.26 ± 7.41 | 0.07 ± 0.03 | 0 | 0 | 0 | 0 |
| TOU-1998-TRI | II | 0.15 ± 0.11 | 2.95 ± 1.63 | 0.07 ± 0.02 | 0 | 0 | 0 | 0 |
| RMS-2005-HAG | II | 0.11 ± 0.04 | 6.7 ± 1.13 | 0.04 ± 0.03 | 0 | 0 | 0 | 0 |
| GRE-1995-MAE | II | 0.11 ± 0.10 | 14.95 ± 7.14 | 0.03 ± 0 | 0 | 0 | 0 | 0 |
| PSP-2005-MUP | III | 0.14 ± 0 | 18.8 ± 11.60 | 0.06 ± 0.02 | 0 | 0 | 0 | 0 |
| GRE-1998-TRA | III | 0.39 ± 0.04 | 16.85 ± 5.87 | 0.05 ± 0.01 | 0 | 0 | 0 | 0 |
| RMS-2003-TOU | III | ND | ND | ND | 0 | 0 | 0 | 0 |
| NED | III | 0.19 ± 0.09 | 9.25 ± 5.73 | 0.03 ± 0.01 | 0 | 0 | 0 | 0 |
| RMS-1994-LEF | I/III | 0.07 ± 0 | 9.65 ± 5.73 | 0.04 ± 0.01 | Ex2, E474D | 0 | Ex3, 204 sil Ala | 0 |
| Ex4, R560K | ||||||||
| Ex5, 580 sil Gly; A597E, 627 sil Glu | ||||||||
| RMS-2003-DJO | I/III | 0.09 ± 0 | 4.9 ± 4.33 | 0.08 ± 0.02 | 0 | 0 | 0 | 0 |
| RMS-2001-MAU | Atypical | 0.12 ± 0.08 | >50 | 0.06 ± 0.01 | 0 | 0 | 0 | 0 |
| GUY-2003-MEL | Atypical | 0.25 ± 0.02 | 5.35 ± 0.49 | 0.04 ± 0.02 | Ex2, E474D | 0 | Ex2, 145 sil Val | 0 |
| Ex4, R560K | ||||||||
| Ex5, 580 sil Gly; A597E, 627 sil Glu | ||||||||
Mean value and 95% confidence intervals from three separate determinations and corresponding mutations on drug target genes. sil, silent mutation; Ex2, exon 2; ND, not determined.
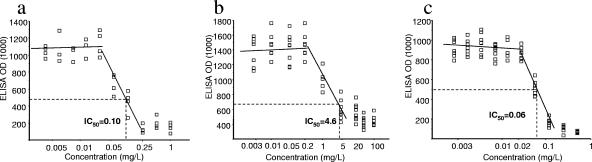
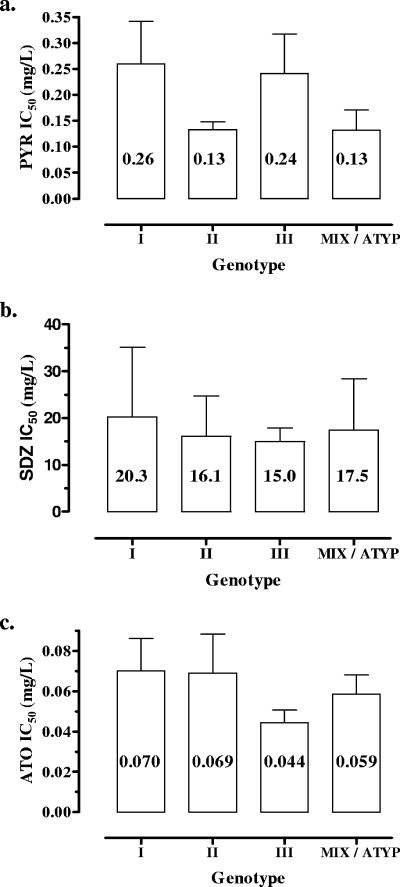
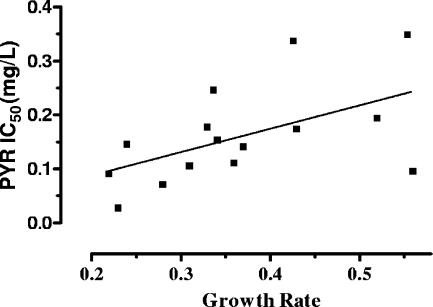
Pyrimethamine was examined at 10 concentrations ranging from between 0.002 and 1 mg/liter. For all strains, the same concentration/inhibition profile was observed, consisting of a lack of inhibition until a threshold concentration was reached. Beyond this concentration, the inhibitory effect sharply increased and usually reached its maximum value at the next concentration tested (Fig. 2a). IC50s ranged from between 0.07 and 0.39 mg/liter (mean, 0.18 ± 0.10), with no significant relationship with strain genotype (P = 0.26) (Fig. 3). However, a significant correlation between IC50s and growth rates was found (Spearman r = 0.55; P = 0.03) (Fig. 4).
FIG. 2.
Representative experiment of in vitro activities of pyrimethamine, SDZ, and atovaquone (strain ME49, type II). Optical density (OD) values for the ELISA with infected monolayers were plotted versus the concentrations of antimicrobial agents (mg/liter). IC50s are estimated from regression analyses. (a) Pyrimethamine. (b) SDZ. (c) Atovaquone.
FIG. 3.
Mean IC50s (mg/liter) according to strain genotypes. (a) Pyrimethamine (PYR) (P = 0.26). (b) SDZ (P = 0.79). (c) Atovaquone (ATO) (P = 0.64). ATYP, atypical.
FIG. 4.
Correlation between IC50s (mg/liter) of pyrimethamine (PYR) and strain growth rate (Spearman r = 0.55; P = 0.03).
Atovaquone was examined at 10 concentrations ranging from between 0.001 and 0.5 mg/liter. As for pyrimethamine, a sharp increase in inhibition was observed over a narrow range of concentrations (Fig. 2c). Calculated IC50s were comprised of between 0.03 and 0.11 mg/liter (mean, 0.06 ± 0.02 mg/liter). No relationship was found between IC50 values and strain genotype (P = 0.74) (Fig. 3) or growth kinetics (P = 0.51).
SDZ was examined at 10 concentrations ranging between 0.0005 and 100 mg/liter. SDZ was inhibitory for 13 strains, with IC50s ranging between 3 and 18.9 mg/liter (mean, 8.8 ± 5.0 mg/liter). In contrast with pyrimethamine and atovaquone, the inhibitory effect of SDZ increased more progressively for increasing concentrations in the cultures (Fig. 2b).
For three strains, B1, RMS-1995-ABE, and RMS-2001-MAU, IC50s were estimated to be >50 mg/liter. A more precise estimation was not possible since concentrations of >100 mg/liter were toxic for the MRC-5 monolayers. These results were confirmed in two additional experiments. High IC50s were not correlated with the strain genotype (one strain each of genotypes I, II, and III) (P = 0.79) (Fig. 3) or growth kinetics (P = 0.44).
Additional experiments were conducted in folate-free RPMI medium supplemented with Ultroser comparatively to RPMI medium supplemented with 10% fetal calf serum in order to explore the possible interference of exogenous folates on drug susceptibility to SDZ or pyrimethamine. The use of folate-free medium did not alter MRC-5 cell growth and had no effect on parasite growth or on IC50s of pyrimethamine for the three strains tested, RH, ENT, and B1. For SDZ, IC50s in folate-free medium were still >50 mg/liter for strains B1 and RMS-1995-ABE and increased for two other strains, RH and ENT, that had low IC50s for SDZ (data not shown). From these results, we assumed that the lower susceptibilities of some strains to SDZ were not related to the presence of folate in the medium.
Analysis of drug target genes.
Gene sequence analysis of DHFR, DHPS, and cytochrome b were obtained for all strains.
The complete sequences of the six exons of the DHPS gene showed several identical mutations in exons 2 (E474D), 4 (R560K), and 5 (A597E, with two silent mutations) of all type I strains as well as one recombinant type I/III strain (RMS-1994-LEF) and one atypical strain isolated in French Guyana (GUY-2003-MEL) (Table 2). In exon 5, a mutation, A587V, was found in strain RMS-1995-ABE, which had a high IC50 for SDZ. No mutation was found at the position 407 in all strains analyzed.
Several mutations were found on the DHFR gene according to the different genotypes of strains. One silent mutation in exon 3 (156L) was constantly found in all type I strains; one other silent mutation was found in exon 3 (204A) for recombinant strain RMS-1994-LEF and in exon 2 (145V) for atypical strain GUY-2003-MEL. None of these mutations was linked to a lower susceptibility to pyrimethamine. No mutation was found on the cytochrome b gene.
DISCUSSION
The variable susceptibilities of T. gondii strains to antiparasitic drugs and the hypothesis that this susceptibility could be linked to the parasite growth rate were suspected a few years after the discovery of the efficacy of sulfonamides and pyrimethamine against T. gondii (10, 22). However, until the 1970s, this variability and the possible mechanisms of resistance to drugs were not thoroughly studied. New insights on the genetic diversity of T. gondii and better knowledge on the genetic basis of resistance of Plasmodium and Pneumocystis to pyrimethamine and atovaquone (23, 24, 35, 42) offered new tracks for the study of resistance of T. gondii to antiparasitic drugs.
Little is known about the possible relationship between genotype and drug susceptibility. In a study comparing the in vitro susceptibilities of one strain each of genotypes I, II, and III, IC50s ranging between 0.22 mg/liter for strain RH (type I) and 0.005 mg/liter for strains P(LK) and VEG (types II and III, respectively) were found, suggesting a 40-fold-higher susceptibility of type II and type III strains to pyrimethamine than the RH type strain (41). A higher variability was found with atovaquone in one study (4). In that study, six strains belonging to various genotypes were tested in vitro for their susceptibilities to atovaquone; IC50s were not determined, but complete growth inhibition was observed with 1.7 mg/liter of atovaquone for strain RH (type I), 0.17 mg/liter for ME49 (type II) and POE (type III), 0.017 mg/liter for CAS (atypical), and 0.017 mg/liter for VEL (type I) and HART (type II/III). This represents a 1- to 100-fold range in susceptibility to atovaquone (4). We found no previously reported study on the possible relationships between genotype and susceptibility to SDZ.
In our study, we did not find such a wide range of IC50s for pyrimethamine since individual IC50 values were comprised of between 0.07 and 0.39 mg/liter, i.e., a one- to sixfold range between strains. These concentrations were in the range of our previously reported results (14) and those obtained using other test conditions with strain RH (3, 18, 28, 31). Higher individual values (≥0.25 mg/liter) were observed with two type I strains, one type III strain, and one atypical strain. However, ANOVA showed no significant relationship between IC50s and strain genotypes but showed a significant negative correlation between susceptibility to pyrimethamine and parasite growth rates. Indeed, a high replication rate is already considered to be a marker of virulence under genetic control (16, 40). Our results suggest that it could also interfere with the susceptibility of T. gondii to drugs that target nucleic acid synthesis, such as pyrimethamine. The range of IC50 values for atovaquone was even lower, with individual values comprised of between 0.03 and 0.11 mg/liter, i.e., a one- to fourfold range between strains. ANOVA showed that genotype and growth rate had no influence on susceptibility to atovaquone.
We found a much higher variability in IC50 values for SDZ. IC50s ranged from between 3 and 18.8 mg/liter for 13 strains and were >50 mg/liter for three strains. The possible interference of exogenous folate incorporation by T. gondii, as suggested previously by Massimine et al. (30), was excluded, since high IC50s were not reduced when the test was repeated under folate-free conditions.
For pyrimethamine and atovaquone, we observed a lower range of IC50s between strains than reported previously. This can be explained by the conditions that we used to compare the drug susceptibilities between strains that do not have the same growth rates. For each strain, we chose to adapt the inoculum size (0.4 to 1 parasite/10 cells) in order to obtain similar levels of infection in control cultures (without drug) for all the strains at the end of the test, i.e., close but nonconfluent parasitic foci in the cultures. In this way, the duration of drug exposure and the end point of the assay were the same for all strains. Furthermore, drug susceptibility tests were performed during the log-linear growth rate period, during which the slope should not be influenced by the inoculum size. This allowed a comparative assessment of drug effects between strains.
Another objective of our study was to identify alterations on drug target genes that could explain variations in drug susceptibility.
Several mutations related to resistance to pyrimethamine had already been identified in the DHFR-TS gene using site-directed mutagenesis and transgenic experiments. Most are mutations that were known to confer drug resistance in Plasmodium falciparum. Among these mutations, the T83N mutation (S108N in P. falciparum) was found to probably confer resistance to pyrimethamine (15). This resistance is even increased when this mutation is associated with a mutation of S36R and F245S (42). Additional mutations not previously found in P. falciparum were also produced in T. gondii (W25R, L98S, and L134H) and were found to generate low levels of resistance (41). Other mutations not related to drug resistance (41, 42) or not affecting the amino acid sequence encoded by the DHFR gene have also been described (6); here, two of these silent mutations were found in two strains (one atypical strain and one recombinant strain).
In the four strains that presented the highest IC50s for pyrimethamine, only silent mutations were found in three strains, i.e., 156-L in the two type I (B1 and ENT) strains and 145-V in one atypical strain, GUY-2003-MEL.
Resistance of T. gondii to sulfonamide can be induced experimentally and has been related to a mutation at position 407 in exon 3 of DHPS (40). This mutation was also evidenced on a clinical isolate (among 32 human strains) obtained from a congenitally infected child; it was suggested that this mutation was “natural” since no treatment had been administered during pregnancy and before the isolation of T. gondii (6). This mutation was not found among the 17 strains studied here. This was also the case in a study performed on 29 strains isolated from patients with congenital toxoplasmosis (37). In our study, various mutations not affecting the amino acid sequence encoded by the DHPS gene were found, mainly in the type I and type I-related strains. One resistant strain presented mutations found in all type I strains, and one had no detectable mutation. A mutation in exon 5, at position 587, converting alanine to valine was demonstrated for one strain that was resistant to SDZ. It is noteworthy that this strain was isolated from the placenta in a case of congenital toxoplasmosis and that prenatal treatment with pyrimethamine and sulfadoxin had been administered for 12 weeks. In this case, the possible selection of a drug-resistant mutant cannot be excluded. Whether the mutation that we found in this strain can be selected under drug pressure and related to resistance to SDZ needs to be investigated experimentally.
Concerning atovaquone, there is genetic evidence that this drugs targets the cytochrome BC1 complex of T. gondii by binding to the Q0 domain of cytochrome b and that mutations within this region are probable mediators of drug resistance (31, 32). M129L and I254L mutations have been identified after selection of N-ethyl-N-nitrosourea-mutagenized T. gondii and related to atovaquone resistance (25, 32). In our study, none of these mutations was detected.
Whether the strains that presented higher IC50s for pyrimethamine, atovaquone, and SDZ can be considered to be resistant is debatable. In most studies, resistance is arbitrarily defined as a marked decrease in in vitro susceptibility. In a genetic study of resistance to pyrimethamine, Reynolds et al. (41, 42) based their definition of resistance on the capacity of strains to grow in the presence of 0.6 μM drug (i.e., 0.15 mg/liter) in a 24-h uracil uptake assay; in that study, sensitive strains exhibited ≥50% inhibition at this concentration, whereas resistant strains were less inhibited (≤35% for moderate resistance and ≤25% for high resistance). Although this narrow range between sensitive and resistant strains can be assumed for mutants derived from the same strain (RH) and under very stringent experimental conditions, this threshold might not be relevant for clinical isolates, which likely present a higher level of natural diversity. Other authors arbitrarily used an eightfold increase in the inhibitory concentration compared to that of sensitive strains (15). For SDZ and atovaquone, no criterion has been defined, but resistant mutants obtained by mutagenesis have IC50s that are 20- to 300-fold higher than that of the wild type (6, 38, 39).
Rather than selecting an arbitrary value, we propose a new approach for a better definition of resistance based on the distribution of observed IC50s. For pyrimethamine and atovaquone, IC50 values of the 16 studied strains follow a log-normal distribution (P = 0.24 and P = 0.11, respectively, by normality test). We can estimate that less than 0.1% of strains will have an IC50 greater than the mean IC50 plus 3.3 standard deviations, i.e., 0.52 mg/liter for pyrimethamine and 0.14 mg/liter for atovaquone. These concentrations (or higher) can be considered to be unusually high and indicative of resistance. For SDZ, IC50s did not fit a log-normal distribution (P = 0.03), as three strains showed outstandingly high values (IC50 of >50 mg/liter), possibly synonymous with resistance. A 1% threshold for abnormally distributed strains can be calculated from the mean value of “sensitive” strains (excluding the three strains that have an IC50 of ≥50 mg/liter) plus 3.3 standard deviations, giving a value of 25.6 mg/liter. Under this condition, none of the strains tested in this study were resistant to pyrimethamine or atovaquone, and the three strains with IC50s for SDZ of >50 mg/liter can actually be considered to be resistant to this drug.
In conclusion, we found some variability in the susceptibilities of T. gondii strains to pyrimethamine and atovaquone but with no clear evidence of drug resistance to these drugs and no relationships with strain genotype or defined mutations in drug target genes. A higher variability was found for SDZ, with possible resistance for three strains. The presence of a new mutation on the DHPS gene of one of these strains could possibly be related to this resistance, but such a link needs to be explored by testing additional strains and confirmed by directed mutagenesis experiments. Our proposal of threshold concentrations for the definition of in vitro resistance could be helpful to facilitate the screening for resistance for a large number of strains. In addition, the relationship between in vitro susceptibility and clinical resistance remains to be examined. Pharmacokinetic studies have revealed marked individual variations in pyrimethamine concentrations in human immunodeficiency virus (HIV)-infected patients (20, 49) and in children treated for congenital toxoplasmosis (11, 33, 44, 47). In HIV-infected patients with toxoplasmic encephalitis, a positive relationship between clinical and radiological responses and median atovaquone plasma concentrations has been shown (46). Due to these individual variations in drug pharmacokinetics, the differentiation between treatment failures due to insufficient plasma or tissue drug concentration and true drug resistance should rely on a better documentation of these failures, with determinations of plasma levels and attempts to isolate strains in patients under treatment.
Acknowledgments
This study was supported by grants from the Region Champagne-Ardennes (France), the BRC Toxoplasma, and the Centre d'Etudes et de Recherches en Infectiologie.
We thank all the BRC members who provided T. gondii strains. We are grateful to Marie-Laure Dardé for helpful discussions and Annie Sulahian for reviewing the manuscript.
Footnotes
Published ahead of print on 22 January 2008.
REFERENCES
- 1.Ajzenberg, D., N. Cogne, L. Paris, M. H. Bessieres, P. Thulliez, D. Filisetti, H. Pelloux, P. Marty, and M. L. Dardé. 2002. Genotype of 86 Toxoplasma gondii isolates associated with human congenital toxoplasmosis, and correlation with clinical findings. J. Infect. Dis. 186:684-689. [DOI] [PubMed] [Google Scholar]
- 2.Ajzenberg, D., A. Dumetre, and M. L. Dardé. 2005. Multiplex PCR for typing strains of Toxoplasma gondii. J. Clin. Microbiol. 43:1940-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allegra, C. J., J. A. Kovacs, J. C. Drake, J. C. Swan, B. A. Chabner, and H. Masur. 1987. Potent in vitro and in vivo anti Toxoplasma activity of the lipid-soluble antifolate trimetrexate. J. Clin. Investig. 79:478-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araujo, F. G., J. Huskinson, and J. S. Remington. 1991. Remarkable in vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against tachyzoites and tissue cysts of Toxoplasma gondii. Antimicrob. Agents Chemother. 35:293-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Araujo, F. G., J. Huskinson-Mark, W. E. Gutteridge, and J. S. Remington. 1992. In vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against the cyst form of Toxoplasma gondii. Antimicrob. Agents Chemother. 36:326-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aspinall, T. V., D. H. Joynson, E. Guy, J. E. Hyde, and P. F. Sims. 2002. The molecular basis of sulfonamide resistance in Toxoplasma gondii and implications for the clinical management of toxoplasmosis. J. Infect. Dis. 185:1637-1643. [DOI] [PubMed] [Google Scholar]
- 7.Baatz, H., A. Mirshahi, J. Puchta, H. Gumbel, and L. O. Hattenbach. 2006. Reactivation of Toxoplasma retinochoroiditis under atovaquone therapy in an immunocompetent patient. Ocul. Immunol. Inflamm. 14:185-187. [DOI] [PubMed] [Google Scholar]
- 8.Cassaing, S., M. H. Bessieres, A. Berry, A. Berrebi, R. Fabre, and J. F. Magnaval. 2006. Comparison between two amplification sets for molecular diagnosis of toxoplasmosis by real-time PCR. J. Clin. Microbiol. 44:720-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chirgwin, K., R. Hafner, C. Leport, J. Remington, J. Andersen, E. M. Bosler, C. Roque, N. Rajicic, V. McAuliffe, P. Morlat, D. T. Jayaweera, J. L. Vildé, B. J. Luft, et al. 2002. Randomized phase II trial of atovaquone with pyrimethamine or sulfadiazine for treatment of toxoplasmic encephalitis in patients with acquired immunodeficiency syndrome. Clin. Infect. Dis. 34:1243-1250. [DOI] [PubMed] [Google Scholar]
- 10.Cook, M. K. 1958. The development of a pyrimethamine-resistant line of Toxoplasma under in vitro conditions. Am. J. Trop. Med. 7:400-402. [DOI] [PubMed] [Google Scholar]
- 11.Corvaisier, S., B. Charpiat, C. Mounier, M. Wallon, G. Leboucher, M. Al Kurdi, J. F. Chaulet, and F. Peyron. 2004. Population pharmacokinetics of pyrimethamine and sulfadoxine in children treated for congenital toxoplasmosis. Antimicrob. Agents Chemother. 48:3794-3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dannemann, B., J. A. McCutchan, D. Israelski, D. Antoniskis, C. Leport, B. Luft, J. Nussbaum, N. Clumeck, P. Morlat, J. Chiu, et al. 1992. Treatment of toxoplasmic encephalitis in patients with AIDS. A randomized trial comparing pyrimethamine plus clindamycin to pyrimethamine plus sulfadiazine. Ann. Intern. Med. 116:33-43. [DOI] [PubMed] [Google Scholar]
- 13.Derouin, F., and C. Chastang. 1988. Enzyme immunoassay to assess effect of antimicrobial agents on Toxoplasma gondii in tissue culture. Antimicrob. Agents Chemother. 32:303-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derouin, F., and C. Chastang. 1989. In vitro effects of folate inhibitors on Toxoplasma gondii. Antimicrob. Agents Chemother. 33:1753-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donald, R. G., and D. S. Roos. 1993. Stable molecular transformation of Toxoplasma gondii: a selectable dihydrofolate reductase-thymidylate synthase marker based on drug-resistance mutations in malaria. Proc. Natl. Acad. Sci. USA 90:11703-11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Hajj, H., M. Lebrun, S. T. Arold, H. Vial, G. Labesse, and J. F. Dubremetz. 2007. ROP18 is a rhoptry kinase controlling the intracellular proliferation of Toxoplasma gondii. PLoS Pathog. 3:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grigg, M. E., J. Ganatra, J. C. Boothroyd, and T. P. Margolis. 2001. Unusual abundance of atypical strains associated with human ocular toxoplasmosis. J. Infect. Dis. 184:633-639. [DOI] [PubMed] [Google Scholar]
- 18.Harris, C., M. P. Salgo, H. B. Tanowitz, and M. Wittner. 1988. In vitro assessment of antimicrobial agents against Toxoplasma gondii. J. Infect. Dis. 157:14-22. [DOI] [PubMed] [Google Scholar]
- 19.Howe, D. K., and L. D. Sibley. 1995. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J. Infect. Dis. 172:1561-1566. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson, J. M., M. Davidian, P. M. Rainey, R. Hafner, R. H. Raasch, and B. J. Luft. 1996. Pyrimethamine pharmacokinetics in human immunodeficiency virus-positive patients seropositive for Toxoplasma gondii. Antimicrob. Agents Chemother. 40:1360-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katlama, C., B. Mouthon, D. Gourdon, D. Lapierre, F. Rousseau, et al. 1996. Atovaquone as long-term suppressive therapy for toxoplasmic encephalitis in patients with AIDS and multiple drug intolerance. AIDS 10:1107-1112. [PubMed] [Google Scholar]
- 22.Kaufman, H. E., J. S. Remington, M. L. Melton, and L. Jacobs. 1959. Relative resistance of slow-growing strains of Toxoplasma gondii to pyrimethamine. AMA Arch. Ophthalmol. 62:611-615. [DOI] [PubMed] [Google Scholar]
- 23.Kazanjian, P., W. Armstrong, P. A. Hossler, W. Burman, J. Richardson, C. H. Lee, L. Crane, J. Katz, and S. R. Meshnick. 2000. Pneumocystis carinii mutations are associated with duration of sulfa or sulfone prophylaxis exposure in AIDS patients. J. Infect. Dis. 182:551-557. [DOI] [PubMed] [Google Scholar]
- 24.Kazanjian, P., W. Armstrong, P. A. Hossler, C. H. Lee, L. Huang, C. B. Beard, J. Carter, L. Crane, J. Duchin, W. Burman, J. Richardson, and S. R. Meshnick. 2001. Pneumocystis carinii cytochrome b mutations are associated with atovaquone exposure in patients with AIDS. J. Infect. Dis. 183:819-822. [DOI] [PubMed] [Google Scholar]
- 25.Kessl, J. J., K. H. Ha, A. K. Merritt, S. R. Meshnich, and B. L. Trumpower. 2006. Molecular basis of Toxoplasma gondii atovaquone resistance modeled in Saccharomyces cerevisiae. Mol. Biochem. Parasitol. 146:255-258. [DOI] [PubMed] [Google Scholar]
- 26.Khan, A., C. Su, M. German, G. A. Storch, D. B. Clifford, and L. D. Sibley. 2005. Genotyping of Toxoplasma gondii strains from immunocompromised patients reveals high prevalence of type I strains. J. Clin. Microbiol. 43:5881-5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan, A., C. Jordan, C. Muccioli, A. L. Vallochi, L. V. Rizzo, R. Belfort, Jr., R. W. Vitor, C. Silveira, and L. D. Sibley. 2006. Genetic divergence of Toxoplasma gondii strains associated with ocular toxoplasmosis, Brazil. Emerg. Infect. Dis. 12:942-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovacs, J. A., C. J. Allegra, J. Beaver, D. Boarman, M. Lewis, J. E. Parrillo, B. Chabner, and H. Masur. 1989. Characterization of de novo folate synthesis in Pneumocystis carinii and Toxoplasma gondii: potential for screening therapeutic agents. J. Infect. Dis. 160:312-320. [DOI] [PubMed] [Google Scholar]
- 29.Luft, B. J., and J. S. Remington. 1992. Toxoplasmic encephalitis in AIDS. Clin. Infect. Dis. 15:211-222. [DOI] [PubMed] [Google Scholar]
- 30.Massimine, K. M., L. T. Doan, C. A. Atreya, T. T. Stedman, K. S. Anderson, K. A. Joiner, and I. Coppens. 2005. Toxoplasma gondii is capable of exogenous folate transport. A likely expansion of the BT1 family of transmembrane proteins. Mol. Biochem. Parasitol. 144:44-54. [DOI] [PubMed] [Google Scholar]
- 31.McFadden, D. C., F. Seeber, and J. C. Boothroyd. 1997. Use of Toxoplasma gondii expressing β-galactosidase for colorimetric assessment of drug activity in vitro. Antimicrob. Agents Chemother. 41:1849-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McFadden, D. C., S. Tomavo, E. A. Berry, and J. C. Boothroyd. 2000. Characterization of cytochrome b from Toxoplasma gondii and Q(o) domain mutations as a mechanism of atovaquone-resistance. Mol. Biochem. Parasitol. 108:1-12. [DOI] [PubMed] [Google Scholar]
- 33.McLeod, R., D. Mack, R. Foss, K. Boyer, S. Withers, S. Levin, J. Hubbell, and the Toxoplasmosis Study Group. 1992. Levels of pyrimethamine in sera and cerebrospinal and ventricular fluids from infants treated for congenital toxoplasmosis. Antimicrob. Agents Chemother. 36:1040-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montoya, J. G., and O. Liesenfeld. 2004. Toxoplasmosis. Lancet 363:1965-1976. [DOI] [PubMed] [Google Scholar]
- 35.Nagesha, H. S., S. Din, G. J. Casey, A. I. Susanti, D. J. Fryauff, J. C. Reeder, and A. F. Cowman. 2001. Mutations in the pfmdr1, dhfr and dhps genes of Plasmodium falciparum are associated with in-vivo drug resistance in West Papua, Indonesia. Trans. R. Soc. Trop. Med. Hyg. 95:43-49. [DOI] [PubMed] [Google Scholar]
- 36.Petersen, E. 2007. Prevention and treatment of congenital toxoplasmosis. Expert Rev. Anti Infect. Ther. 5:285-293. [DOI] [PubMed] [Google Scholar]
- 37.Peyron, F., N. Eudes, F. de Monbrison, M. Wallon, and S. Picot. 2004. Fitness of Toxoplasma gondii is not related to DHFR single-nucleotide polymorphism during congenital toxoplasmosis. Int. J. Parasitol. 34:1169-1175. [DOI] [PubMed] [Google Scholar]
- 38.Pfefferkorn, E. R., S. E. Borotz, and R. F. Nothnagel. 1992. Toxoplasma gondii: characterization of a mutant resistant to sulfonamides. Exp. Parasitol. 74:261-270. [DOI] [PubMed] [Google Scholar]
- 39.Pfefferkorn, E. R., S. E. Borotz, and R. F. Nothnagel. 1993. Mutants of Toxoplasma gondii resistant to atovaquone (566C80) or decoquinate. J. Parasitol. 79:559-564. [PubMed] [Google Scholar]
- 40.Radke, J. R., B. Striepen, M. N. Guerini, M. E. Jerome, D. S. Roos, and M. W. White. 2001. Defining the cell cycle for the tachyzoite stage of Toxoplasma gondii. Mol. Biochem. Parasitol. 115:165-175. [DOI] [PubMed] [Google Scholar]
- 41.Reynolds, M. G., J. Oh, and D. S. Roos. 2001. In vitro generation of novel pyrimethamine resistance mutations in the Toxoplasma gondii dihydrofolate reductase. Antimicrob. Agents Chemother. 45:1271-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reynolds, M. G., and D. S. Roos. 1998. A biochemical and genetic model for parasite resistance to antifolates. Toxoplasma gondii provides insights into pyrimethamine and cycloguanil resistance in Plasmodium falciparum. J. Biol. Chem. 273:3461-3469. [DOI] [PubMed] [Google Scholar]
- 43.Romand, S., M. Pudney, and F. Derouin. 1993. In vitro and in vivo activities of the hydroxynaphthoquinone atovaquone alone or combined with pyrimethamine, sulfadiazine, clarithromycin, or minocycline against Toxoplasma gondii. Antimicrob. Agents Chemother. 37:2371-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt, D. R., B. Hogh, O. Andersen, J. Fuchs, H. Fledelius, and E. Petersen. 2006. The national neonatal screening programme for congenital toxoplasmosis in Denmark: results from the initial four years, 1999-2002. Arch. Dis. Child. 91:661-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomavo, S., and J. C. Boothroyd. 1995. Interconnection between organellar functions, development and drug resistance in the protozoan parasite Toxoplasma gondii. Int. J. Parasitol. 25:1293-1299. [DOI] [PubMed] [Google Scholar]
- 46.Torres, R. A., W. Weinberg, J. Stansell, G. Leoung, J. Kovacs, M. Rogers, J. Scott, et al. 1997. Atovaquone for salvage treatment and suppression of toxoplasmic encephalitis in patients with AIDS. Clin. Infect. Dis. 24:422-429. [DOI] [PubMed] [Google Scholar]
- 47.Trenque, T., N. Simon, I. Villena, C. Chemla, C. Quereux, B. Leroux, R. Jaussaud, G. Remy, D. Dupouy, H. Millart, J. M. Pinon, and S. Urien. 2004. Population pharmacokinetics of pyrimethamine and sulfadoxine in children with congenital toxoplasmosis. Br. J. Clin. Pharmacol. 57:735-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsuchiya, S., M. Yamabe, Y. Yamaguchi, Y. Kobayashi, T. Konno, and K. Tada. 1980. Establishment and characterization of a human acute monocytoid leukemia cell line (THP-1). Int. J. Cancer 26:171-176. [DOI] [PubMed] [Google Scholar]
- 49.Winstanley, P., S. Khoo, S. Szwandt, G. Edwards, E. Wilkins, J. Tija, R. Coker, W. McKane, N. Beeching, S. Watkin, and A. Breckenridge. 1995. Marked variation in pyrimethamine disposition in AIDS patients treated for cerebral toxoplasmosis. J. Antimicrob. Chemother. 36:435-439. [DOI] [PubMed] [Google Scholar]