Abstract
We report on a novel CTX-M extended-spectrum β-lactamase (ESBL), designated CTX-M-42, with enhanced activity toward ceftazidime. CTX-M-42 was identified in a hypermutable Escherichia coli nosocomial isolate (isolate Irk2320) and is a Pro167Thr amino acid substitution variant of CTX-M-3. By molecular typing of ESBL-producing E. coli strains previously isolated in the same hospital ward, we were able to identify a putative progenitor (strain Irk1224) of Irk2320, which had a mutator phenotype and harbored the CTX-M-3 β-lactamase. To reproduce the natural evolution of CTX-M-3, we selected for ceftazidime resistance mutations in blaCTX-M-3 gene in vitro both in clinical isolate Irk1224 and in laboratory-derived hypermutable (mutD5) strain GM2995. These experiments yielded CTX-M-3Pro167Ser and CTX-M-3Asn136Lys mutants which conferred higher levels of resistance to ceftazidime than to cefotaxime. CTX-M-3Asn136Lys had a level of low activity toward ampicillin, which may explain its absence from clinical isolates. We conclude that the selection of CTX-M-42 could have occurred in vivo following treatment with ceftazidime and was likely facilitated by the hypermutable background.
The CTX-M family of extended-spectrum β-lactamases (ESBLs) is rapidly growing and currently includes over 60 enzymes (http://www.lahey.org/studies/other.asp#table 1). These enzymes pose a major clinical problem by conferring resistance to expanded-spectrum cephalosporins (4, 6, 23, 29). Many CTX-M ESBLs confer higher levels of resistance to cefotaxime (CTX) than to ceftazidime (CAZ). Recently, however, several CTX-M variants have been reported to contain point mutations at amino acid position 167 or 240 (Ambler's numbering scheme [1]) in association with increased CAZ-hydrolyzing activity, which leads to a higher level of CAZ resistance (2, 3, 5, 8, 21, 22, 25, 27, 28). Substitutions of these amino acids in CTX-M enzymes have been shown in both clinical isolates (3, 8, 25, 28) and mutants selected in vitro by CAZ exposure (10, 22, 24, 30). In recent studies by Karisik et al. (19) and Novais et al. (24), it has been demonstrated that mutations at position 167 in CTX-M-3, which lead to increased CAZ resistance, are readily selected in vitro when mutator host strains are used. The importance of hypermutability in the evolution of ESBLs and the emergence of new resistance mechanisms among CTX-M producers have also been emphasized previously (13, 14).
In this study, we report a case of the apparent in vivo evolution of CTX-M-3 in a hypermutable clinical strain of Escherichia coli to CTX-M-42, a Pro167Thr variant of CTX-M-3 conferring higher-level resistance to CAZ. We also reproduced the natural evolution of CTX-M-3 toward the acquisition of enhanced CAZ-hydrolyzing activity by in vitro selection experiments with clinical and laboratory-derived mutator strains.
(The results of this study were presented in part at the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy, 2005, Washington, DC, and at the 16th European Congress of Clinical Microbiology and Infectious Diseases, 2006, Nice, France.)
MATERIALS AND METHODS
Clinical isolates.
Clinical E. coli isolates Irk2320 and Irk2322 expressing high-level CAZ resistance were isolated from intensive care unit patients with nosocomial pneumonia and an intra-abdominal infection in May and June 2003, respectively. These isolates were included in the study along with 20 other nosocomial E. coli isolates that were recovered 1 to 32 weeks earlier in same ward and that exhibited an ESBL phenotype with lower-level CAZ resistance than CTX resistance due to CTX-M β-lactamase production. One of the latter group of isolates, isolate Irk1224, which was isolated in March 2003, was found to be related to Irk2320 and Irk2322 and to produce a CTX-M-3 β-lactamase.
Susceptibility testing.
The MICs of ampicillin, amoxicillin-clavulanic acid (2:1), CTX, CAZ, ceftriaxone, cefepime, cefotaxime-clavulanic acid, and CAZ-clavulanic acid (4-μg/ml fixed inhibitor concentration) were determined by the agar dilution method, according to the guidelines of the CLSI (9).
Resistance transfer by conjugation.
Isolates Irk1224, Irk2320, and Irk2322 were mated in broth with E. coli AB1456 Rifr F− (20) to determine the transferability of the CTX-M-coding plasmids. Transconjugants were selected on Mueller-Hinton (MH) agar plates containing rifampin (100 μg/ml) and CTX (1 μg/ml).
IEF of β-lactamases.
Crude β-lactamase extracts were obtained by the sonication method and were analyzed by isoelectric focusing (IEF) with a PhastSystem apparatus and precast polyacrylamide gels containing ampholytes (Amersham Biosciences, Piscataway, NJ), as described earlier (11, 18).
Molecular typing.
The clonality between Irk2320, Irk2322, and the 20 CTX-M-producing isolates with low-level CAZ resistance was assessed by arbitrarily primed PCR (AP-PCR) with primer ERIC1, as described previously (12), and primers AP7 (5′-GTGGATGCGA-3′), OPA4 (5′-AATCGGGCTG-3′), and M13 (5′-GAGGGTGGCGGTTCT-3′). The compositions of the reaction mixtures and the amplification conditions were the same with each of the primers, except that the annealing temperatures were 47°C for primers ERIC1 and M13 and 35°C for primers AP7 and OPA4. To aid with the identification of clonally related isolates, cluster analysis of combined AP-PCR profiles was performed by using GelCompar software (version 4.1; Applied Maths, Sint-Martens-Latem, Belgium) with the Pearson correlation coefficient and the unweighted pair group method with arithmetic averages algorithm.
The relatedness between isolates Irk1224, Irk2320, Irk2322, and Irk1737 (Irk1737 was included as an unrelated isolate) was further assessed by pulsed-field gel electrophoresis (PFGE) of XbaI-cleaved genomic DNA with a GenePath group 6 reagent kit and a CHEF Mapper XA system (Bio-Rad Laboratories, Hercules, CA) according to a standard protocol (17).
Plasmid analysis.
Plasmid DNA was extracted from isolates Irk1224, Irk2320, and Irk2322 and their respective transconjugants with a plasmid midi kit (Qiagen, Hilden, Germany). Undigested plasmids were analyzed by field inversion gel electrophoresis. The plasmids isolated from the transconjugants were digested with PvuII, and the digests were analyzed by electrophoresis in a 1.0% agarose gel.
PCR-based replicon typing of the plasmids was performed with the transconjugants, as described by Carattoli et al. (7).
Determination of mutation rate.
The rates of spontaneous mutations to rifampin resistance were determined as described elsewhere (16). Briefly, two independent colonies of each isolate were inoculated in MH broth tubes and were incubated with rotary movement at 37°C for 24 h. Different dilutions (100 μl) were then seeded onto MH agar plates with and without rifampin (100 μg/μl). The colonies were counted after 48 h of incubation, and mutation frequency values were estimated as the ratio of the number of rifampin-resistant colonies to the total number of CFU.
Amplification, cloning, and sequencing of blaCTX-M genes.
DNA fragments containing the entire blaCTX-M gene and the upstream part of ISEcp1, including the putative promoter region, were amplified by PCR with primers 5′-TGTCTGGTATAATAAGAATATCATC-3′ and 5′-CTATTACAAACCGTCGGTGAC-3′ from the sequences of isolates Irk2320 and Irk1224; ligated into the pCC1 vector (Epicenter Biotechnologies, Madison, WI), which lacks β-lactamase genes; and cloned into E. coli EPI300 (Epicenter Biotechnologies). Plasmids isolated from the respective transformants were used as templates in sequencing reactions with primer M13 and primers specific for an internal fragment of blaCTX-M. The presence of any nucleotide changes relative to the known blaCTX-M-3 gene sequence was confirmed by direct sequencing of PCR products independently amplified from the original strains. Sequencing was performed with an ABI Prism BigDye Terminator (version 3.1) cycle sequencing kit and an ABI Prism 310 genetic analyzer (Applied Biosystems, Stafford, TX).
Mutagenesis and selection.
In vitro CAZ resistance selection experiments were conducted with both clinical E. coli isolate Irk1224 and laboratory-derived hypermutable strain GM2995 (mutD5). The former isolate exhibited a mutator phenotype (26, 27), showing an increased rate of spontaneous rifampin resistance mutations (1 × 10−5 compared to < 5 × 10−8 for the normomutable strains [16]). The latter strain lacks the proofreading activity of DNA polymerase III (as a result of a mutation in the dnaQ gene) and thus confers up to a 105-fold proportional increase in the rates of occurrence of all common point mutations (15). Both isolate Irk1224 carrying blaCTX-M-3 on a natural plasmid and GM2995 into which blaCTX-M-3 was cloned in the pCC1 vector were subcultured in MH broth for 14 to 16 h at 37°C and plated on MH agar containing CAZ at concentrations of two times the MICs: 64 μg/ml for Irk1224 (CTX-M-3) and 2 μg/ml for GM2995 (CTX-M-3). The mutation frequencies were then determined as described elsewhere (16). Thirty-two colonies of each strain grown on selective plates were randomly selected for determination of their susceptibilities to ampicillin, CAZ, and CTX and characterization of the mutations in the blaCTX-M genes. The blaCTX-M genes were amplified from CAZ-resistant mutants, cloned into plasmid pCC1, and sequenced as described above. The resulting plasmids were introduced into E. coli EPI300 by transformation.
Nucleotide sequence accession number.
The nucleotide sequence of the blaCTX-M-42 gene was deposited in GenBank under accession number DQ061159.
RESULTS AND DISCUSSION
During a survey of ESBL producers, we identified an E. coli clinical isolate (isolate Irk2320) which was moderately resistant to cefotaxime (MIC, 8 μg/ml) but highly resistant to CAZ (MIC, 128 μg/ml). Notably, this isolate was obtained from a patient who had been treated with CAZ. Another E. coli isolate (isolate Irk2322) with a phenotype of resistance identical to that of Irk2320 was isolated from another patient in the same ward 10 days later. By PCR amplification with oligonucleotide primers specific for an internal fragment of blaCTX-M, both isolates were shown to harbor a CTX-M β-lactamase. IEF showed the expression of a β-lactamase with an isoelectric point (pI) of about 8.4, consistent with that of CTX-M-type enzymes, and revealed an additional β-lactamase of pI 5.4 in the aforementioned isolates and in Irk1224 (described below). The latter β-lactamase was identified as TEM-1 by PCR amplification of the blaTEM gene and direct sequencing of the PCR products (data not shown).
In mating experiments, the resistance to CAZ was transferred from isolate Irk2320 to the recipient E. coli strain at a frequency of 7 × 10−3. The β-lactam resistance patterns of the transconjugants (AB1456-Irk2320) resembled the β-lactam resistance pattern of the original isolate (Table 1), and PCR and IEF demonstrated that the isolates carried only a CTX-M ESBL. Thus, it appeared that the resistance of Irk2320 to CAZ was likely mediated by a CTX-M enzyme with an enhanced activity for CAZ. Direct sequencing of the internal blaCTX-M gene fragment amplified by PCR from the transconjugants and clinical isolates Irk2320 and Irk2322 revealed a blaCTX-M-3-like gene carrying a single point mutation that led to a Pro167Thr substitution in the deduced amino acid sequence.
TABLE 1.
Susceptibilities of E. coli clinical isolates, transconjugants, and transformants
| Strain | β-Lactamase (mutation)a | MIC (μg/ml)b
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| AMP | AMC | CTX | CTX-CLA | CAZ | CAZ-CLA | CRO | FEP | ||
| Irk1224 | CTX-M-3 (WT) | ≥256 | 32/16 | ≥256 | 2/4 | 32 | 4/4 | ≥256 | 32 |
| Irk2320 | CTX-M-42 (Thr167) | ≥256 | 16/8 | 8 | 0.125/4 | 128 | 2/4 | 8 | 1 |
| Irk2322 | CTX-M-42 (Thr167) | ≥256 | 16/8 | 8 | 0.125/4 | 128 | 2/4 | 8 | 1 |
| AB14156-Irk2320 | CTX-M-42 (Thr167) | ≥256 | 16/8 | 8 | 0.125/4 | 128 | 2/4 | 8 | 1 |
| EPI300 pCC1-1224 | CTX-M-3 (WT) | ≥256 | 8/4 | 8 | 0.06/4 | 1 | 0.25/4 | 4 | 0.5 |
| EPI300 pCC1-2320 | CTX-M-42 (Thr167) | ≥256 | 8/4 | 0.5 | 0.06/4 | 32 | 1/4 | 1 | 0.25 |
| EPI300 pCC1 | None | 2 | 2/1 | 0.06 | 0.06/4 | 0.25 | 0.25/4 | 0.06 | 0.06 |
All mutations are indicated relative to the CTX-M-3 amino acid sequence. The numbering of the amino acid residues is according to Ambler's scheme for class A β-lactamases (1). WT, wild type.
AMP, ampicillin; AMC, amoxicillin-clavulanic acid; CTX, cefotaxime; CTX-CLA, cefotaxime-clavulanic acid; CAZ, ceftazidime; CAZ-CLA, ceftazidime-clavulanic acid; CRO, ceftriaxone; FEP, cefepime.
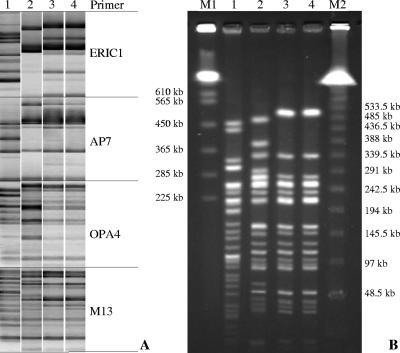
On the basis of these initial findings, we hypothesized that the Pro167Thr mutation could have been acquired by isolate Irk2320 in vivo following treatment of the patient with CAZ. In order to verify this hypothesis and to identify a possible progenitor of Irk2320 lacking the mutation in the blaCTX-M gene, we investigated the genetic relatedness between Irk2320, Irk2322 and 20 CTX-M-producing E. coli isolates with lower-level CAZ resistance that had been isolated in the same intensive care unit up to 8 months before the isolation of Irk2320. Cluster analysis of the combined AP-PCR profiles revealed that one isolate (isolate Irk1224) was clonally related to isolates Irk2320 and Irk2322. As shown in Fig. 1A, the former isolate differed from the last two isolates by only two bands in the ERIC1-PCR profile but shared the same global pattern of bands obtained with the other three primers. In contrast, the AP-PCR profiles of other isolates (the profile of one of which, Irk1737, is shown in Fig. 1 for comparison) were clearly distinct with all four primers used. To confirm the relatedness between Irk1224, Irk2320, and Irk2322, PFGE analysis was performed with DNA from these isolates cleaved with the XbaI endonuclease. The PFGE profile of Irk1224 differed by four bands from the profiles of Irk2320 and Irk2322, which were, in turn, identical to each other. Nevertheless, the profiles of all three isolates were substantially similar, having at least 16 common bands. It is worth mentioning that Irk1224, Irk2320, and Irk2322 demonstrated an elevated frequency of spontaneous rifampin-resistant mutants (∼1 × 10−5) and were therefore suggested to be mutators. Taking into account the hypermutable phenotype of these isolates and the fact that they were obtained from different patients over a 3-month period, we concluded that the observed level of genetic similarity was consistent with the hypothesis of a common ancestry.
FIG. 1.
AP-PCR (A) and PFGE (B) patterns of CTX-M-producing E. coli isolates. Lanes: M1, Saccharomyces cerevisiae marker; M2, bacteriophage lambda ladder PFGE standard; 1, E. coli Irk1737; 2, E. coli Irk1224; 3, E. coli Irk2320; 4, E. coli Irk2322.
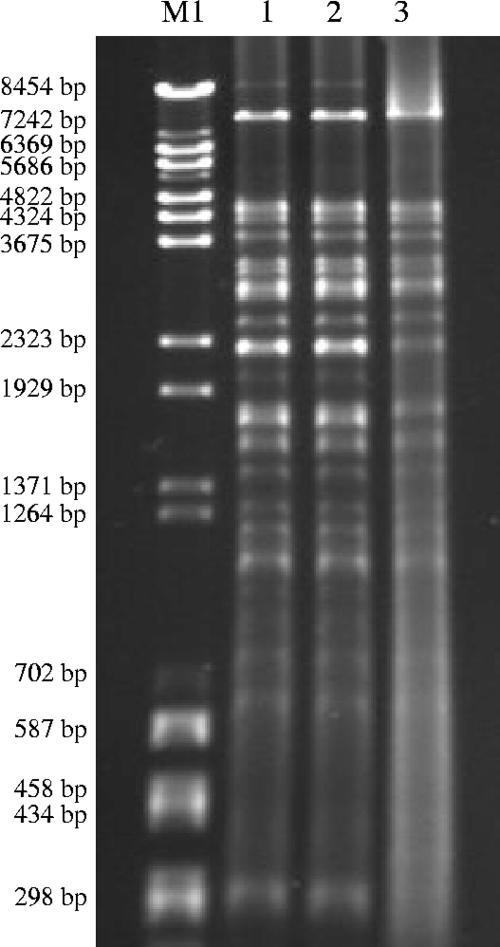
In addition to the similarities of the genomic fingerprints, analysis of the plasmid contents also supported the relatedness of the isolates mentioned above and the common origin of the blaCTX-M genes. Electrophoresis of plasmids isolated from Irk1224, Irk2320, and Irk2322 and their transconjugants revealed a plasmid of approximately 250 kb in all of them. Irk1224 was found to carry an additional plasmid of approximately 150 kb that was lacking in its transconjugant (data not shown). The 250-kb CTX-M-encoding plasmids belonged to the IncL/M group, according to PCR replicon typing, and had identical PvuII restriction profiles (Fig. 2).
FIG. 2.
RFLP patterns of PvuII-digested plasmids isolated from transconjugants. Lanes: M, size standard (a mixture of bacteriophage lambda BstEII and pUC18 HaeIII digests); 1, E. coli AB1456-Irk1224; 2, E. coli AB1456-Irk2320; 3, E. coli AB1456-Irk2322.
A 1,225-bp DNA fragment, which included the complete blaCTX-M open reading frame and part of the upstream ISEcp1 sequence, was amplified from the DNA of both isolate Irk2320 and isolate Irk1224 and was cloned in the pCC1 single-copy vector. The sequence of the cloned 1,225-bp DNA fragment from Irk1224 was identical to the previously published sequence of blaCTX-M-3 and the 5′ adjacent part of ISEcp1 (GenBank accession no. AF550415), while the fragment isolated from Irk2320 contained only the previously identified single nucleotide mutation that leads to the Pro167Thr substitution in the deduced amino acid sequence. No mutations were detected in the promoter region of the Irk2320 blaCTX-M. The respective β-lactamase differing from CTX-M-3 at position 167 was designated CTX-M-42.
The pCC1-Irk2320 and pCC1-Irk1224 plasmids were introduced into E. coli EPI300 by transformation. When they were expressed in the isogenic background of E. coli EPI300, CTX-M-3 and CTX-M-42 conferred the “cefotaximase” and “ceftazidimase” phenotypes, respectively (Table 1), thus clearly supporting the involvement of the Pro167Thr substitution in the modification of the substrate profile of the enzyme.
The Pro167Thr substitution was previously detected in CTX-M-23, which is derived from CTX-M-1 (28). Our study provides an example of the convergent evolution of CTX-M-3 toward the acquisition of CAZ-hydrolyzing activity. The in vivo acquisition of the Pro167Thr mutation in CTX-M-42 is strongly supported by the identification of genetically related clinical isolates that produced CTX-M-3 and CTX-M-42, respectively, and that were consecutively isolated in the same hospital unit. It seems likely, although it has not been proven, that this mutation arose in a hypermutable E. coli strain and was selected by CAZ treatment.
To further address this hypothesis we conducted experiments for the in vitro selection of mutations in blaCTX-M-3 gene supporting elevated CAZ resistance. For the selection of CAZ-resistant mutants, we used both the original clinical isolate, isolate Irk1224, and a laboratory-derived mutator strain, strain GM2995, which had been transformed with pCC1-Irk1224 plasmid DNA. For antibiotic susceptibility testing, the blaCTX-M genes from CAZ-resistant mutants were recloned into pCC1 and expressed in E. coli EPI300.
The total frequencies of mutants with CAZ resistance at least twice the MICs of the original strains were 2 × 10−8 and 2 × 10−6 for isolate Irk1224 and strain GM2995, respectively. Thirty-two mutants obtained from each strain were selected and analyzed.
As shown in Table 2, the majority of mutants (mutant type 1) with elevated CAZ MICs also had very high CTX MICs (≥256 μg/ml). The blaCTX-M genes from these mutants contained no changes in the promoter or coding region relative to the sequence of blaCTX-M-3, as shown by PCR amplification and sequencing, and conferred the same levels of resistance to β-lactams when they were cloned and expressed in E. coli EPI300. Thus, the increased resistance of the first type of mutant to CAZ was not related to the β-lactamase and most likely resulted from alterations in outer membrane permeability. The selection of such mutants was previously reported by Ellington et al. in experiments with mutator strains producing TEM-type β-lactamases (13). Another group of mutants was characterized by CAZ MICs greater than those of CTX. Of this group, one and eight clones derived from Irk1224 and GM2995, respectively, contained a single C→T transition in the blaCTX-M gene that corresponded to the Pro167Ser substitution in the deduced amino acid sequence. This mutation is found in the naturally occurring enzymes of the CTX-M-2 and CTX-M-9 clusters (CTX-M-35 and CTX-M-19) and, as shown by Welsh et al. (30), can be readily selected with CAZ following the in vitro mutagenesis of CTX-M-2. It is interesting to note that our in vitro experiments as well as similar studies by other authors (10, 19, 24, 30) yielded only the Pro→Ser mutation at position 167, whereas a variety of substitutions at this position have been found in the naturally occurring CTX-M ceftazidimases (e.g., Thr in CTX-M-23 and CTX-M-42, Ser in CTX-M-52, and Gln in CTX-M-54). Although the reason for this remains unclear, it may be at least partially explained by the fact that a nucleotide transition required for the Pro167Ser substitution is more likely to occur in almost all types of mutators, including the mutD5 strain used in our experiments and the mutS strains employed in the other studies (19, 24).
TABLE 2.
Susceptibilities of E. coli clinical and laboratory mutator strains and recombinant clones producing the wild-type (CTX-M-3) and mutant CTX-M β-lactamases
| Strains and selected mutants | na | β-Lactamase (mutation)b | MIC (μg/ml)c
|
|||||
|---|---|---|---|---|---|---|---|---|
| Mutator strains
|
E. coli EPI300d
|
|||||||
| AMP | CTX | CAZ | AMP | CTX | CAZ | |||
| Irk1224 | CTX-M-3 (WT) | ≥256 | ≥256 | 32 | ≥256 | 8 | 1 | |
| Irk1224 mutant type 1 | 30 | CTX-M-3 (WT) | ≥256 | ≥256 | ≥256 | ≥256 | 8 | 1 |
| Irk1224 mutant type 2 | 1 | CTX-M-3 (Ser167) | ≥256 | 64 | ≥256 | 128 | 1 | 16 |
| Irk1224 mutant type 3 | 1 | CTX-M-3 (Lys136) | ≥256 | 64 | ≥256 | 32 | 1 | 8 |
| GM2995 pCC1-1224 | CTX-M-3 (WT) | ≥256 | 4 | 1 | ≥256 | 8 | 1 | |
| GM2995 pCC1-1224 mutant type 1 | 24 | CTX-M-3 (WT) | ≥256 | ≥256 | 32 | ≥256 | 8 | 1 |
| GM2995 pCC1-1224 mutant type 2 | 8 | CTX-M-3 (Ser167) | 128 | 1 | 32 | 128 | 1 | 16 |
n, number of mutants of each type selected.
All mutations are indicated relative to the CTX-M-3 amino acid sequence. The numbering of the amino acid residues is according to Ambler's scheme for class A β-lactamases (1), WT, wild type.
AMP, ampicillin; CTX, cefotaxime; CAZ, ceftazidime.
Susceptibilities of E. coli EPI300 strains carrying the blaCTX-M genes from the respective mutants.
A single CAZ-resistant mutant (mutant type 3) of Irk1224 was found to carry a previously unknown substitution, Asn136Lys. Although this mutation increased the MIC of CAZ, it reduced the MIC of CTX and, especially, that of ampicillin, which may explain the absence of this substitution in the CTX-M enzymes from clinical isolates.
None of the selected mutants contained mutations in the promoter region of the blaCTX-M gene or in the codon for Asp240.
In conclusion, we described a new CTX-M variant, CTX-M-42, which was first detected in an E. coli isolate from a patient treated with CAZ. CTX-M-42 confers a ceftazidimase phenotype and is a Pro167Thr variant of CTX-M-3. By examining several clinical isolates from the same hospital unit, we have identified a putative progenitor of the CTX-M-42-carrying isolates that expressed CTX-M-3. We suppose that the mutator phenotype of these isolates was an important factor in the emergence of the novel CTX-M variant with increased ceftazidimase activity. In addition, we were able to reproduce the natural evolution of the CTX-M-3 β-lactamase by selecting for CAZ resistance mutations in blaCTX-M-3 in both clinical and laboratory-derived hypermutable hosts.
Overall, the data presented in this report suggest that because of the high risk of resistance selection, CAZ should not be used for the treatment of infections caused by CTX-M β-lactamase producers, even when in vitro tests indicate susceptibility to this drug.
Acknowledgments
We thank Larisa N. Ikryannikova (Research Institute for Physical-Chemical Medicine, Moscow, Russia) for assistance with plasmid replicon typing.
Footnotes
Published ahead of print on 22 January 2008.
REFERENCES
- 1.Ambler, R. P., A. F. Coulson, J. M. Frere, J. M. Ghuysen, B. Joris, M. Forsman, R. C. Levesque, G. Tiraby, and S. G. Waley. 1991. A standard numbering scheme for the class A beta-lactamases. Biochem. J. 276(Pt 1):269-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bae, I. K., B. H. Lee, H. Y. Hwang, S. H. Jeong, S. G. Hong, C. L. Chang, H. S. Kwak, H. J. Kim, and H. Youn. 2006. A novel ceftazidime-hydrolysing extended-spectrum beta-lactamase, CTX-M-54, with a single amino acid substitution at position 167 in the omega loop. J. Antimicrob. Chemother. 58:315-319. [DOI] [PubMed] [Google Scholar]
- 3.Baraniak, A., J. Fiett, W. Hryniewicz, P. Nordmann, and M. Gniadkowski. 2002. Ceftazidime-hydrolysing CTX-M-15 extended-spectrum beta-lactamase (ESBL) in Poland. J. Antimicrob. Chemother. 50:393-396. [DOI] [PubMed] [Google Scholar]
- 4.Bonnet, R. 2004. Growing group of extended-spectrum beta-lactamases: the CTX-M enzymes. Antimicrob. Agents Chemother. 48:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnet, R., C. Recule, R. Baraduc, C. Chanal, D. Sirot, C. De Champs, and J. Sirot. 2003. Effect of D240G substitution in a novel ESBL CTX-M-27. J. Antimicrob. Chemother. 52:29-35. [DOI] [PubMed] [Google Scholar]
- 6.Canton, R., and T. M. Coque. 2006. The CTX-M beta-lactamase pandemic. Curr. Opin. Microbiol. 9:466-475. [DOI] [PubMed] [Google Scholar]
- 7.Carattoli, A., A. Bertini, L. Villa, V. Falbo, K. L. Hopkins, and E. J. Threlfall. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63:219-228. [DOI] [PubMed] [Google Scholar]
- 8.Cartelle, M., T. M. del Mar, F. Molina, R. Moure, R. Villanueva, and G. Bou. 2004. High-level resistance to ceftazidime conferred by a novel enzyme, CTX-M-32, derived from CTX-M-1 through a single Asp240-Gly substitution. Antimicrob. Agents Chemother. 48:2308-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial susceptibility testing; 16th informational supplement M100-S16. Clinical and Laboratory Standards Institute, Wayne, PA.
- 10.Delmas, J., F. Robin, F. Carvalho, C. Mongaret, and R. Bonnet. 2006. Prediction of the evolution of ceftazidime resistance in extended-spectrum beta-lactamase CTX-M-9. Antimicrob. Agents Chemother. 50:731-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edelstein, M., M. Pimkin, T. Dmitrachenko, V. Semenov, N. Kozlova, D. Gladin, A. Baraniak, and L. Stratchounski. 2004. Multiple outbreaks of nosocomial salmonellosis in Russia and Belarus caused by a single clone of Salmonella enterica serovar Typhimurium producing an extended-spectrum β-lactamase. Antimicrob. Agents Chemother. 48:2808-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edelstein, M., M. Pimkin, I. Palagin, I. Edelstein, and L. Stratchounski. 2003. Prevalence and molecular epidemiology of CTX-M extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in Russian hospitals. Antimicrob. Agents Chemother. 47:3724-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellington, M. J., D. M. Livermore, T. L. Pitt, L. M. Hall, and N. Woodford. 2006. Development of extended-spectrum activity in TEM beta-lactamases in hyper-mutable, mutS Escherichia coli. Clin. Microbiol. Infect. 12:800-803. [DOI] [PubMed] [Google Scholar]
- 14.Ellington, M. J., D. M. Livermore, T. L. Pitt, L. M. Hall, and N. Woodford. 2006. Mutators among CTX-M beta-lactamase-producing Escherichia coli and risk for the emergence of fosfomycin resistance. J. Antimicrob. Chemother. 58:848-852. [DOI] [PubMed] [Google Scholar]
- 15.Fowler, R. G., G. E. Degnen, and E. C. Cox. 1974. Mutational specificity of a conditional Escherichia coli mutator, mutD5. Mol. Gen. Genet. 133:179-191. [DOI] [PubMed] [Google Scholar]
- 16.Galan, J. C., M. Tato, M. R. Baquero, C. Turrientes, F. Baquero, and J. L. Martinez. 2004. Fosfomycin and rifampin disk diffusion tests for detection of Escherichia coli mutator strains. J. Clin. Microbiol. 42:4310-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gautom, R. K. 1997. Rapid pulsed-field gel electrophoresis protocol for typing of Escherichia coli O157:H7 and other gram-negative organisms in 1 day. J. Clin. Microbiol. 35:2977-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huovinen, S. 1988. Rapid isoelectric focusing of plasmid-mediated beta-lactamases with Pharmacia PhastSystem. Antimicrob. Agents Chemother. 32:1730-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karisik, E., M. J. Ellington, R. Pike, D. M. Livermore, and N. Woodford. 2006. Development of high-level ceftazidime resistance via single-base substitutions of blaCTX-M-3 in hyper-mutable Escherichia coli. Clin. Microbiol. Infect. 12:803-806. [DOI] [PubMed] [Google Scholar]
- 20.Kholodii, G., O. Yurieva, S. Mindlin, Z. Gorlenko, V. Rybochkin, and V. Nikiforov. 2000. Tn5044, a novel Tn3 family transposon coding for temperature-sensitive mercury resistance. Res. Microbiol. 151:291-302. [DOI] [PubMed] [Google Scholar]
- 21.Kimura, S., M. Ishiguro, Y. Ishii, J. Alba, and K. Yamaguchi. 2004. Role of a mutation at position 167 of CTX-M-19 in ceftazidime hydrolysis. Antimicrob. Agents Chemother. 48:1454-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimura, S., Y. Ishii, K. Tateda, and K. Yamaguchi. 2007. Predictive analysis of ceftazidime hydrolysis in CTX-M-type beta-lactamase family members with a mutational substitution at position 167. Int. J. Antimicrob. Agents 29:326-331. [DOI] [PubMed] [Google Scholar]
- 23.Livermore, D. M., R. Canton, M. Gniadkowski, P. Nordmann, G. M. Rossolini, G. Arlet, J. Ayala, T. M. Coque, I. Kern-Zdanowicz, F. Luzzaro, L. Poirel, and N. Woodford. 2007. CTX-M: changing the face of ESBLs in Europe. J. Antimicrob. Chemother. 59:165-174. [DOI] [PubMed] [Google Scholar]
- 24.Novais, A., R. Canton, R. Moreira, T. M. Coque, F. Baquero, and J. C. Galan. 2007. Ceftazidime resistance evolution in ESBLs belonging to CTX-M-1 cluster (CTX-M-1, CTX-M-3, CTX-M-10) in a hypermutator background, abstr. 1732_131. Abstr. 17th Eur. Congr. Clin. Microbiol. Infect. Dis.
- 25.Poirel, L., T. Naas, I. Le Thomas, A. Karim, E. Bingen, and P. Nordmann. 2001. CTX-M-type extended-spectrum beta-lactamase that hydrolyzes ceftazidime through a single amino acid substitution in the omega loop. Antimicrob. Agents Chemother. 45:3355-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stepanova, M., and M. Edelstein. 2006. Convergent selection of ceftazidime resistance mutations at position 167 of CTX-M-3 beta-lactamase in hypermutable Escherichia coli strains, abstr. p1238. Abstr. 16th Eur. Congr. Clin. Microbiol. Infect. Dis. [DOI] [PMC free article] [PubMed]
- 27.Stepanova, M., O. Shevchenko, and M. Edelstein. 2005. In vivo evolution and emergence of a new CTX-M beta-lactamase with “ceftazidimase” activity in a hypermutable clinical strain, abstr. C1-60. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 28.Sturenburg, E., A. Kuhn, D. Mack, and R. Laufs. 2004. A novel extended-spectrum beta-lactamase CTX-M-23 with a P167T substitution in the active-site omega loop associated with ceftazidime resistance. J. Antimicrob. Chemother. 54:406-409. [DOI] [PubMed] [Google Scholar]
- 29.Walther-Rasmussen, J., and N. Hoiby. 2004. Cefotaximases (CTX-M-ases), an expanding family of extended-spectrum beta-lactamases. Can. J. Microbiol. 50:137-165. [DOI] [PubMed] [Google Scholar]
- 30.Welsh, K. J., M. Barlow, F. C. Tenover, J. W. Biddle, J. K. Rasheed, L. A. Clark, and J. E. McGowan, Jr. 2005. Experimental prediction of the evolution of ceftazidime resistance in the CTX-M-2 extended-spectrum β-lactamase. Antimicrob. Agents Chemother. 49:1242-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]