Abstract
Innate antifungal defense in Drosophila melanogaster relies on the activation of the Toll molecule and the release of drosomycin, a defensin-like molecule with antifungal properties. Ten human homologues of Toll have been described, with central roles in activation of the innate host defense. In the present study, we report a putative human homologue of the Drosophila-derived drosomycin, designated drosomycin-like defensin (DLD). Synthetic DLD displays a broad spectrum of activity against Aspergillus spp. and other clinically relevant filamentous fungi. These effects are specific for filamentous fungi; no activity has been found against yeasts or gram-positive or gram-negative bacteria. Synthetic DLD also displays immunomodulatory effects on Aspergillus-stimulated cytokine production. In addition, we show the expression of DLD mRNA in several human tissues, particularly in the skin, consistent with its putative role as a defensin against invading microorganisms. This is the first indication of an endogenous human peptide with specific antifungal activity, which is probably central in the defense against infections with molds.
Drosophila melanogaster, like other insects, relies on both cellular and humoral mechanisms to mount its antimicrobial host defense. The mainstay of its humoral defense is the injury-induced secretion of an array of antimicrobial peptides by the fat body, the functional equivalent of the liver (9, 10). To date, seven distinct families of antimicrobial peptides from immune-challenged Drosophila flies have been characterized, each with a specific spectrum of activity. Among these peptides, drosomycin is highly active against filamentous fungi, protecting Drosophila from infections by Aspergillus fumigatus, whereas no activity against gram-positive or gram-negative bacteria has been found (9). The release of drosomycin is under the control of signals mediated by Toll, a transmembrane receptor activated by a cytokine-like protein, Spaetzle (16).
Comparison of the Toll complex with that of mammalian innate defense mechanisms has revealed that the intracellular tail of Toll shows a striking homology with the intracellular domain of interleukin-1 receptor type I (IL-1RI), later designated the Toll/IL-1R (TIR) domain (23). Moreover, the intracellular signaling pathway induced by Drosophila Toll is highly similar to the intracellular pathways activated by IL-1RI (12). Eleven different Toll-like receptors (TLRs) have been identified in mammals and have been demonstrated to be crucial for the recognition of pathogenic microorganisms and the activation of the innate immune response in general (24), including antifungal defense (2, 20, 21). Where Toll activation leads to the direct release of drosomycin, engagement of TLRs induces the release of cytokines and α- and β-defensins (4, 6), but to date no human homologue of drosomycin has been reported.
In the present study, we report a putative human peptide with high homology with drosomycin, and we demonstrate potent antifungal properties for this peptide, designated drosomycin-like defensin (DLD). In addition, we report the presence of mRNA for DLD in various tissues of human origin.
MATERIALS AND METHODS
Sequence homology.
BLAST searches were performed with the protein sequence of Drosophila melanogaster drosomycin (PDB entry code 1MYN) using the BLAST Server and the GenBank database at the National Center for Biotechnology Information. ClustalW multiple alignment was performed using Biology Workbench 3.2 software (http://workbench.sdsc.edu).
Synthetic DLD peptide.
We synthesized three peptides: a 42-residue peptide corresponding to the putative human drosomycin homologue (DLD) as shown in Fig. 1, a 42-residue peptide identical to Drosophila drosomycin, and a random control peptide of similar length and similar (but scrambled) amino acid composition (Bioworld, Dublin, OH). The number of free sulfhydryl groups in the DLD peptide was determined using reversed-phase high-performance liquid chromatography (RP-HPLC) and electrospray ionization mass spectrometry (ESI-MS). Specifically, 1 mg of DLD peptide was resuspended in 2 ml of a 0.2 M NaHCO3 (pH 8.5) buffer and stirred for 4 h. The solution was then split into two equal parts. To one part was added 0.5 mg of iodoacetamide, and the solution was stirred an additional 1 h. Aliquots (10 μl) of each DLD peptide solution were then injected onto a Zorbax 300SB-C18 column (2.1 mm [inner diameter] by 150 mm [length], 300 Å [pore size], 5 μm [particle size]) and eluted with a linear AB gradient of 1% B/min where eluent A is 0.05% aqueous trifluoroacetic acid and eluent B is 0.05% trifluoroacetic acid in acetonitrile. The post-column solvent flow was directly fed into an Agilent SL ESI-MS for peptide mass determination.
FIG. 1.
BLAST result for DLD. The query sequence is the protein sequence of Drosophila melanogaster drosomycin (1MYN). “Subject” is a translated protein sequence from Homo sapiens 2.19 gene mRNA (XM_049333). The statistical results of this match are as follows: score, 37.7; expect, 0.077; identities, 17/40 (42%); positives, 21/40 (52%).
Fungi, yeasts, and bacterial strains.
The filamentous fungi, yeasts, and bacterial strains used in this study either were purchased from ATCC or were generous gifts from various institutions. The filamentous fungi used were Aspergillus fumigatus (ATCC MYA 1163), Aspergillus nidulans (AZN 2867), Aspergillus ustus (clinical isolate), Fusarium solani (AZN 6836), Fusarium oxysporum (clinical isolate), and Rhizopus oryzae (AZN 8892); the yeasts were Candida albicans (ATCC 24433), Candida glabrata (clinical isolate), Candida tropicalis (clinical isolate), Candida krusei (clinical isolate), and Cryptococcus neoformans (CM133); and the bacteria were Staphylococcus aureus (ATCC 43300, ATCC 29213, ATCC 25923), Streptococcus group A (ATCC 43202), Enterococcus faecalis (ATCC 29212), Escherichia coli (ATCC 35218, ATCC 25922), Klebsiella pneumoniae (ATCC 10031), Pseudomonas aeruginosa (ATCC 27853), and Salmonella enterica serovar Enteritidis (clinical isolate).
Antifungal assays and determination of MICs.
Fungal spores (final concentration, 104/ml) were suspended in 1/2 potato dextrose broth (Difco), and the yeast strains were suspended at a starting A600 of 0.001 in the yeast complete medium YPG (1% yeast extract, 1% peptone, 2% glucose). The medium was supplemented with tetracycline (10 μg/ml) and cefotaxime (100 μg/ml) and was dispensed by 80-μl aliquots into the wells of a microplate containing 20 μl of either water or the sample to be analyzed. Growth of fungi and yeast was evaluated after 24 h at 30°C by light microscopy and after 48 h by measuring the culture absorbance at 595 nm using a microplate reader.
When the antifungal assay was performed in the presence of salt, the 1/2 potato dextrose broth medium was prepared in phosphate-buffered saline with 137 mM NaCl.
The procedure used for MIC determination was identical to that for the antifungal assay. The MIC is expressed as the lowest concentration that causes 100% growth inhibition. The fungicidal effects of the synthetic peptides in the MIC assay were verified by reinoculation of the yeasts into potato dextrose broth at the end of the incubation time.
Several dilutions of DLD or drosomycin (50 to 0.05 μM) were tested using the liquid growth inhibition assay as described above in order to determine MICs. The control peptide was used as a negative control, at a concentration of 50 μM. The MIC is expressed as a final (micromolar) concentration.
Antibacterial assay.
During the various purification steps, antibacterial activity was monitored by a liquid growth inhibition assay with the gram-positive and gram-negative microorganisms. Ninety microliters of a suspension of a mid-logarithmic-phase culture of bacteria at a starting A600 of 0.001 in Poor Broth nutrient medium (1% Bacto tryptone and 0.5% [wt/vol] NaCl, pH 7.5) was added to 10 μl of sample for analysis. Microbial growth was assessed by the increase in A600 after a 24-h incubation at 30°C using a microtitration plate reader.
Stimulation of PBMCs.
Peripheral blood mononuclear cells (PBMCs) from five healthy individuals were isolated as described elsewhere (8). Briefly, venous blood was drawn into 10-ml tubes containing 0.2 mg of EDTA (Monoject's-Hertogenbosch, The Netherlands). The PBMC fraction was obtained by density centrifugation of blood using Ficoll-Paque (Pharmacia Biotech AB, Sweden). The PBMCs were washed twice in saline and resuspended in culture medium (RPMI 1640, Dutch modification; ICN Biomedicals, Costa Mesa, CA) supplemented with 1% gentamicin, 1% l-glutamine, and 1% pyruvate. The PBMCs were incubated in 96-well tissue culture plates (Greiner, Alphen, The Netherlands) at a concentration of 5 × 105 cells per well in a total volume of 200 μl, in the presence or absence of a set of stimuli in different experiments. These stimuli included 1 μg/ml DLD, 1 μg/ml control peptide, 1 μg/ml synthetic drosomycin, 1 ng/ml lipopolysaccharide (from E. coli 055:B5; Sigma, St. Louis, MO), or 1 × 106 heat-killed Aspergillus fumigatus conidia, prepared as described elsewhere (22). After 24 h of incubation, the supernatants were collected and stored at −80°C until analysis. In a number of experiments, the incubated PBMCs were used to isolate RNA with RNAzol B (Campro Scientific, Veenendaal, The Netherlands) using standard protocols; the preparations were stored at −20°C until further analysis.
Cytokine measurements.
IL-6 and tumor necrosis factor (TNF) were measured by an enzyme-linked immunosorbent assay according to the manufacturer's protocol (Pelikine, CLB; Amsterdam, The Netherlands).
CHO cells.
Chinese hamster ovary (CHO) fibroblasts stably transfected with human CD14 and TLR2 (3E10-TLR2) or TLR4 (3E10-TLR4) were a kind gift from Robin Ingalls (17). Cell lines express inducible membrane CD25 under the control of a region from the human E-selectin (ELAM-1) promoter containing NF-κB binding sites. Cells were maintained at 37°C under 5% CO2 in Ham's F-12 medium (Gibco, Invitrogen, Breda, The Netherlands) supplemented with 10% fetal calf serum, 0.01% l-glutamine, 50 μg/ml gentamicin, 400 U/ml hygromycin, and 0.5 mg/ml of G418 (for 3E10-TLR2) or 0.05 mg/ml of puromycin (for 3E10-TLR4) as additional selection antibiotics. TLR2 and TLR4 expression was confirmed by flow cytometry (Coulter Epics XL-MCL; Beckman Coulter, Mijdrecht, The Netherlands) using phycoerythrin-labeled anti-TLR2 (clone TL2.1) or anti-TLR4 (clone HTA125) (Immunosource, Halle-Zoersel, Belgium).
For stimulation experiments, 500 μl of cells in culture medium at a density of 1 × 105/ml were plated in 24-well culture plates. After an overnight incubation, cells were incubated with either a control peptide, peptidoglycan (10 μg/ml; Sigma), Pam3Cys (10 μg/ml; EMC Microcollections, Tübingen, Germany), DLD (100 μg/ml), or drosomycin (100 μg/ml). After 20 h of incubation, cells were harvested using trypsin-EDTA (Cambrex, East Rutherford, NJ) and were prepared for flow cytometry (Coulter FACScan). The CD25 expression of the CHO cells was measured using fluorescein isothiocyanate-labeled anti-CD25 (Dako, Glostrup, Denmark).
mRNA expression.
mRNA of human tissues was obtained from autopsy material provided by the Department of Pathology, Radboud University, Nijmegen Medical Center. Reverse transcription-PCR (RT-PCR) of isolated RNA was performed using standard protocols. The resulting cDNA was used in PCR with the following primers for DLD: forward, 5′-GCAACTCTTCACCAGTCCAGAGA-3′; reverse, 5′-GCAGGCCACACTTGTACTTCCT-3′. RT-PCR for Harp (HepA-related protein) was used as a control with the following primers: forward, 5′-CACCATTGAAATCCTGAGTGATGT-3′; reverse, 5′-TGACCAGCCCAAAGGAGAAG-3′.
RESULTS
Sequence homology.
A TBLASTN search identified a DNA sequence with an open reading frame that would upon translation produce a 42-amino-acid peptide, which matches the drosomycin sequence with 42% identity and 52% homology (Fig. 1). The DNA sequence identified (NCBI accession no. AK024601) is a full cDNA clone (FLJ20948) previously catalogued as the human 2.19 gene and subsequently designated the FAM3A gene, located on chromosome Xq28 (7, 29). This putative sequence is not part of the presumed protein that has a methionine as a start codon. We designated this peptide drosomycin-like defensin (DLD). The complete protein containing the 42-residue sequence identified by BLAST with drosomycin remains to be elucidated. The homology between drosomycin and DLD is greater than that with antifungal peptides from plants and other insects, which have previously been characterized as analogues (18, 22) (Fig. 2).
FIG. 2.
Rooted tree made by Clustal W alignment of several plant and insect antifungal peptides and DLD, showing relatedness.
Disulfide bridge formation.
To test whether DLD forms disulfide bridges as do drosomycin and other defensins, we analyzed the refolding of a synthetically prepared DLD peptide under oxidative conditions (0.2 M NaHCO3 [pH 8.5]). MS analysis showed a decrease in molecular size from 4,793.8 ± 0.5 Da to 4,787.3 ± 0.3 Da, consistent with the formation of three intramolecular disulfide bridges. Furthermore, addition of iodoacetamide (reactive against free cysteine side chains) resulted neither in an observable shift in the RP-HPLC elution time nor in an increase in the molecular size of the refolded DLD peptide. This would imply that each DLD molecule is in a full oxidative state.
Antifungal effects of DLD.
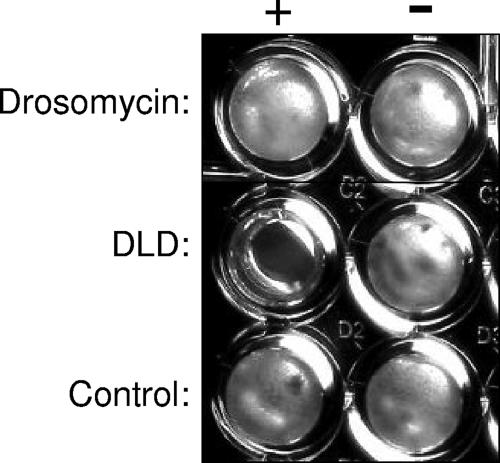
To investigate whether DLD has antifungal properties similar to those of drosomycin, both DLD and drosomycin were synthesized, and their antifungal properties were compared in vitro. As a control, we tested an irrelevant 40-amino-acid scrambled peptide prepared by a similar methodology. As shown in Fig. 3 and Table 1, both DLD and drosomycin, when tested in their oxidized state, which preserves their 3-dimensional structure, exerted potent antifungal activities against filamentous fungi. The inhibition of fungal growth was accompanied by fungicidal effects, as assessed by reinoculation of the yeasts into potato dextrose broth at the end of the incubation time. The results of these assays demonstrated that DLD exerted strong fungicidal effects against the important human pathogen Aspergillus fumigatus but also against Fusarium and Rhizopus spp. DLD was also active against Rhizopus oryzae and Aspergillus nidulans, against which drosomycin was ineffective. In contrast, neither DLD nor drosomycin was active against the yeasts Candida albicans and Cryptococcus neoformans or against gram-negative and gram-positive bacteria (Table 1). In addition, when the linear structure of DLD in the reduced state was tested, no inhibitory effects on the fungal pathogens were observed, demonstrating that the tertiary structure of the peptide is essential for its biological activity.
FIG. 3.
Antifungal effect of DLD on Aspergillus nidulans. This example shows complete growth inhibition in the presence of DLD, whereas the samples with drosomycin or the control peptide are overgrown with the fungus.
TABLE 1.
Antifungal properties of DLDa
| Microorganism | MIC (μM)b of:
|
||
|---|---|---|---|
| DLD | Drosomycin | Control peptide | |
| Filamentous fungi | |||
| A. fumigatus | 6.25 | 6.25 | >50 |
| A. nidulans | 6.25 | >50 | >50 |
| A. ustus | 12.5 | 25 | >50 |
| F. solani | 25 | 25 | >50 |
| F. oxysporum | 6.25 | 6.25 | >50 |
| R. oryzae | 6.25 | >50 | >50 |
| Yeasts | |||
| C. albicans | >50 | >50 | >50 |
| C. glabrata | >50 | >50 | >50 |
| C. tropicalis | >50 | >50 | >50 |
| C. krusei | >50 | >50 | >50 |
| C. neoformans | >50 | >50 | >50 |
| Gram-positive bacteria | |||
| S. aureus | >50 | >50 | >50 |
| Streptococcus group A | >50 | >50 | >50 |
| E. faecalis | >50 | >50 | >50 |
| Gram-negative bacteria | |||
| E. coli | >50 | >50 | >50 |
| K. pneumoniae | >50 | >50 | >50 |
| Salmonella serovar Enteritidis | >50 | >50 | >50 |
| P. aeruginosa | >50 | >50 | >50 |
Several dilutions of DLD and drosomycin (50 to 0.05 μM) were tested using the liquid growth inhibition assay. An irrelevant peptide was used as a negative control at a concentration of 50 μM, at which no inhibitory effects were apparent.
Final concentration.
Immunological effects of DLD.
Several human defensins have been shown to activate the immune system, by interacting with TLRs and inducing dendritic cell maturation (3) or by exerting chemotactic effects (27). We have tested the cytokine-inducing capacity of DLD by incubation of PBMCs with either DLD or drosomycin. Neither DLD nor drosomycin induced cytokine production by human PBMCs. The lack of interaction of DLD with TLR2 and TLR4 was confirmed by the lack of activation of CHO cells transfected with either human TLR2 or TLR4 (data not shown). In addition, DLD did not influence lipopolysaccharide-induced cytokine production by human PBMCs (data not shown). However, DLD inhibited the stimulation of both IL-6 and TNF induced by conidia of Aspergillus fumigatus (1 × 107/ml) in human PBMCs (Fig. 4). This effect was similar to that of the synthetic drosomycin, whereas the control peptide had no effect.
FIG. 4.
Modulation of cytokine production by DLD. PBMCs isolated from five healthy volunteers were stimulated with 1 × 107 heat-killed Aspergillus fumigatus conidia. The effects on TNF and IL-6 release by the following modulators were investigated: control peptide (open bars), DLD (solid bars), and drosomycin (striped bars), each at a concentration of 1 μg/ml. After 24 h of incubation at 37°C, the supernatants were collected and stored at −80°C until analysis. *, P < 0.05 for comparison to the result with the control peptide.
Expression of DLD in human tissues.
The presence of DLD mRNA in various samples of normal human tissue was assessed by RT-PCR. DLD mRNA was detected in samples from several tissues, including skin, liver, muscle, and pancreas (Fig. 5). However, by far the most abundant DLD mRNA was present in the skin. Surprisingly, an inflammatory reaction in the skin, such as that found in psoriasis, significantly reduced the expression of DLD mRNA. Moreover, no DLD mRNA was found in the lung, the target organ of infections with filamentous fungi (Fig. 5). Incubation of isolated PBMCs with conidia or hyphae of Aspergillus fumigatus (1 × 107/ml) did not result in an increase in DLD mRNA expression (data not shown). Harp was used as a control gene, and its expression was similar in all samples (Fig. 5). In addition, a control sample in which RNA was not reverse transcribed displayed no signal, demonstrating that no contaminating DNA was present in the samples (data not shown).
FIG. 5.
mRNA expression of DLD in diverse human tissues. RT-PCRs for DLD were performed with several human tissues, as described in Materials and Methods. The most abundant expression of DLD has been found in the skin, whereas the expression of Harp was similar in all samples. Lanes: 1, lung; 2, blood; 3, liver; 4, pancreas; 5, testis; 6, heart; 7, muscle; 8, normal skin; 9, psoriatic skin.
DISCUSSION
In the present study, we report a human homologue of Drosophila-derived drosomycin, designated DLD, which displays a broad spectrum of activity against filamentous fungi. In addition, we show the expression of DLD mRNA in several human tissues, particularly in the skin, consistent with its putative role as a defensin against invading microorganisms. Our data add to the body of evidence that the Toll/drosomycin-pathway in innate immunity, initially described for Drosophila, is an ancient antifungal defense mechanism that has been conserved during evolution. In this study we present the first evidence that not only are the homologues of Toll—the TLRs—to be found in the mammalian host, but homologues of Drosophila drosomycin may also be involved in the host defense against pathogenic fungi in humans.
Several families of mammalian antimicrobial proteins have been described, such as defensins, cathelicidins, and others (SLPI, SKALP, some cystatins, S100 proteins, dermcidin) (28). These proteins contribute to the host innate defense against bacteria by disrupting the integrity of the bacterial cell membrane, and some also have chemotactic effects on host cells. Defensins are small cationic proteins with six highly conserved cysteine residues that form three pairs of intramolecular disulfide bonds (15, 28), and the structure of DLD looks similar in this respect. These molecules are strikingly homologous to insect and plant defensins, and they represent an ancient protection mechanism against invading pathogens. Drosomycin is a defensin from the insect Drosophila melanogaster, with mainly antifungal properties. The presence of a human homologue of drosomycin, which has retained a high level of homology during evolution, suggests an important role for drosomycin-like defensins in mammalian antifungal responses.
An important question is whether the homology between DLD and drosomycin is present at the significant amino acids required for the defensin function. Landon et al. have proposed that the activity of drosomycin and a number of other antifungal proteins is associated with the presence of a basic/hydrophobic residue pair (Lys38/Trp40 in drosomycin), while the nature of the surrounding residues modulates this activity (14). They hypothesized that the presence of a large number of hydrophobic residues in the vicinity of this residue pair is associated with morphological changes in fungus hyphae (14). An arrangement of hydrophobic and basic residues that resembles drosomycin can be discerned in the predicted protein sequence of DLD as presented here (Fig. 1). While drosomycin contains eight cysteine residues that form four disulfide bridges (19), human DLD contains six cysteine residues. Our data revealed the presence of three intramolecular disulfide bridges per DLD molecule. In addition, it is tempting to speculate whether drosomycin may participate in multimerization, as preliminary data in our laboratory suggest (data not shown) and as is the case for other mammalian defensins (5, 11). These data suggest that DLD and drosomycin share homology not only at the amino acid sequence level but also at the level of 3-dimensional structure. This observation is supported by the virtually identical antifungal activities of the two defensins (Table 1).
An important point is the lack of antibacterial activity of DLD, similar to the lack of effect of drosomycin (9, 13). In contrast, DLD has strong inhibitory effects against a broad range of clinically relevant filamentous fungi, such as A. fumigatus and Rhizopus and Fusarium spp., all of which can cause life-threatening disseminated infections in the immunocompromised host. However, both DLD and drosomycin lack activity against yeasts such as C. albicans or C. neoformans. The reasons for the differences between DLD activities against filamentous fungi and yeasts is unclear at present, although one may speculate that differences at the level of cell wall architecture may be involved, since the modulation of cytokine stimulation by DLD-treated Aspergillus strongly suggests changes at the level of the cell wall.
The discovery of DLD as a human antifungal defensin has important consequences. First, it contributes to better understanding of normal antifungal defense. In this respect, an important argument for the role of DLD as a human defensin is the abundant expression of its mRNA in the skin, known to be rich in defensins, probably because of its barrier function. Second, DLD may have potential value in the therapy of fungal infections. Because the filamentous fungal pathogens are relatively resistant to the current antimycotic drugs (26), alternative treatment strategies are urgently needed, and the use of DLD as an antifungal agent could open new therapeutic avenues.
Because human defensins have been described to have immunomodulatory activities by themselves, we also tested the immunostimulatory capacity of DLD. In contrast to the interaction of β-defensin-2 with TLR4, we did not observe an interaction between either TLR2 and DLD or TLR4 and DLD. Similarly, DLD was not able to stimulate cytokine production by itself, arguing against direct immunostimulatory activities. However, DLD inhibited A. fumigatus-stimulated production of TNF and IL-6, an effect most likely due to modification of the properties of the fungal cell wall by DLD.
Our study is the first to report a homologue of drosomycin in humans. There are still several unanswered questions about DLD. First, this 42-amino-acid peptide is likely to be the core of a larger molecule, but the complete length of the protein is not known. It is tempting to hypothesize that a precursor to DLD exists, since drosomycin is also formed from a precursor protein (18). Second, it is still unknown whether DLD expression is under the control of TLRs. β-Defensin-2 has been reported to be stimulated by TLR2 or TLR4 activation, but its expression is in fact mediated by intermediary release of proinflammatory cytokines (4, 25). We were unable to observe potentiation of DLD mRNA expression by Aspergillus antigens in leukocytes, but experiments using specific TLR agonists in human keratinocytes, known to express both TLRs (1) and DLD mRNA, are warranted. Finally, the role of DLD should be investigated with in vivo experimental models of fungal infections.
In conclusion, a predicted human homologue of Drosophila melanogaster drosomycin, which we designated DLD, shows specific antifungal activities against Aspergillus fumigatus and other filamentous fungi. RT-PCR results indicate that the mRNA for this human drosomycin analogue is expressed primarily in skin, in line with its putative function as a defensin. In contrast, DLD mRNA has not been found in lung tissue, and one may speculate whether this absence could contribute to the lung tropism that characterizes infections with filamentous fungi. In addition, inflammatory reactions in the skin, such as in psoriasis, significantly lowered the expression of DLD. This is the first indication of an endogenous human peptide with specific antifungal activity against molds that is probably involved in the defense against infections with this class of pathogens. Its homology with Drosophila melanogaster drosomycin further expands the cross-species conservation of the Toll/drosomycin/DLD pathway as an ancient mechanism of antifungal defense.
Acknowledgments
M.G.N. was supported by a Vidi grant from The Netherlands Organization for Scientific Research. A.S. was supported by a Veni grant from The Netherlands Organization for Scientific Research.
Footnotes
Published ahead of print on 22 January 2008.
REFERENCES
- 1.Baker, B. S., J.-M. Ovigne, A. V. Powles, S. Corcoran, and L. Fry. 2003. Normal keratinocytes express Toll-like receptors (TLRs) 1, 2 and 5: modulation of TLR expression in chronic plaque psoriasis. Br. J. Dermatol. 148:670-679. [DOI] [PubMed] [Google Scholar]
- 2.Bellocchio, S., C. Montagnoli, S. Bozza, R. Gaziano, G. Rossi, S. S. Mambula, A. Vecchi, A. Mantovani, S. M. Levitz, and L. Romani. 2004. The contribution of Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J. Immunol. 172:3059-3069. [DOI] [PubMed] [Google Scholar]
- 3.Biragyn, A., P. A. Ruffini, C. A. Leifer, E. Klyushnenkova, A. Shakhov, O. Chertov, A. K. Shirakawa, J. M. Farber, J. J. Oppenheim, and L. W. Kwak. 2002. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science 298:1025-1029. [DOI] [PubMed] [Google Scholar]
- 4.Birchler, T., R. Seibl, K. Büchner, S. Loeliger, R. Seger, J. P. Hossle, A. Aguzzi, and R. P. Lauener. 2001. Human Toll-like receptor 2 mediates induction of the antimicrobial peptide human beta-defensin 2 in response to bacterial lipoprotein. Eur. J. Immunol. 31:3131-3138. [DOI] [PubMed] [Google Scholar]
- 5.Campopiano, D. J., D. J. Clarke, N. C. Polfer, P. E. Barran, R. J. Langley, J. R. Govan, A. Maxwell, and J. R. Dorin. 2004. Structure-activity relationships in defensin dimers: a novel link between β-defensin tertiary structure and antimicrobial activity. J. Biol. Chem. 279:48671-48679. [DOI] [PubMed] [Google Scholar]
- 6.Chalifour, A., P. Jeannin, J.-F. Gauchat, A. Blaecke, M. Malissard, T. N′Guyen, N. Thieblemont, and Y. Delneste. 2004. Direct bacterial PAMP recognition by human NK cells involves TLRs and triggers alpha-defensin production. Blood 104:1778-1783. [DOI] [PubMed] [Google Scholar]
- 7.Chen, E. Y., M. Zollo, R. Mazarella, A. Ciccodicola, C. N. Chen, L. Zuo, C. Heiner, F. Burough, M. Repetto, D. Schlessinger, and M. D'Urso. 1996. Long-range sequence analysis in Xq28: thirteen known and six candidate genes in 219.4 kb of high GC DNA between the RCP/GCP and G6PD loci. Hum. Mol. Genet. 5:659-668. [DOI] [PubMed] [Google Scholar]
- 8.Drenth, J. P. H., S. H. M. Van Uum, M. Van Deuren, G. J. Pesman, J. Van der Ven-Jongekrijg, and J. W. M. Van der Meer. 1995. Endurance run increases circulating IL-6 and IL-1ra but downregulates ex vivo TNF-α and IL-1β production. J. Appl. Physiol. 79:1497-1503. [DOI] [PubMed] [Google Scholar]
- 9.Fehlbaum, P., P. Bulet, L. Michaut, M. Lagueux, W. F. Broekaert, C. Hetru, and J. A. Hoffmann. 1994. Insect immunity. Septic injury of Drosophila induces the synthesis of a potent antifungal peptide with sequence homology to plant antifungal peptides. J. Biol. Chem. 269:33159-33163. [PubMed] [Google Scholar]
- 10.Hergannan, J. A., and J. V. Rechhart. 1997. Drosophila immunity. Trends Cell Biol. 7:309-316. [DOI] [PubMed] [Google Scholar]
- 11.Hill, C. P., J. Yee, M. E. Selsted, and D. Eisenberg. 1991. Crystal structure of defensin HNP-3, an amphiphilic dimer: mechanisms of membrane permeabilization. Science 251:1481-1485. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann, J. A. 2003. The immune response of Drosophila. Nature 426:33-38. [DOI] [PubMed] [Google Scholar]
- 13.Lamberty, M., S. Ades, S. Uttenweiler-Joseph, G. Brookhart, D. Bushey, J. A. Hoffmann, and P. Bulet. 1999. Isolation from the lepidopteran Heliothis virescens of a novel insect defensin with potent antifungal activity. J. Biol. Chem. 274:9320-9326. [DOI] [PubMed] [Google Scholar]
- 14.Landon, C., A. Pajon, F. Vovelle, and P. Sodano. 2000. The active site of drosomycin, a small insect antifungal protein, delineated by comparison with the modelled structure of Rs-AFP2, a plant antifungal protein. J. Pept. Res. 56:231-238. [DOI] [PubMed] [Google Scholar]
- 15.Lehrer, R. I., and T. Ganz. 1999. Antimicrobial peptides in mammalian and insect host defence. Curr. Opin. Immunol. 11:23-27. [DOI] [PubMed] [Google Scholar]
- 16.Lemaitre, B., E. Nicolas, L. Michaut, J.-M. Reichhart, and J. A. Hoffmann. 1996. The dorsoventral regulatory gene cassette Spaetzle/Toll/Cactus controls the potent antifungal response in Drosophila adults. Cell 86:973-983. [DOI] [PubMed] [Google Scholar]
- 17.Lien, E., T. K. Means, H. Heine, A. Yoshimura, S. Kusumoto, K. Fukase, M. J. Menton, M. Oikawa, M. Qureshi, B. Monks, R. W. Finberg, R. R. Ingalls, and D. T. Golenbock. 2000. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J. Clin. Investig. 105:497-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ligoxygakis, P., N. Pelte, J. A. Hoffmann, and J. M. Reichhart. 2002. Activation of Drosophila Toll during fungal infection by a blood serine protease. Science 297:114-116. [DOI] [PubMed] [Google Scholar]
- 19.Michaut, L., P. Fehlbaum, M. Moniatte, A. Van Dorsselaer, J. M. Reichhart, and J. A. Hoffmann. 1996. Determination of disulfide array of the first inducible antifungal peptide from insects: drosomycin from Drosophila melanogaster. FEBS Lett. 395:6-10. [DOI] [PubMed] [Google Scholar]
- 20.Netea, M. G., C. de Graaf, A. Vonk, I. Verschueren, J. W. M. Van der Meer, and B. J. Kullberg. 2002. The role of Toll-like receptors in the defense against disseminated candidiasis. J. Infect. Dis. 185:1483-1489. [DOI] [PubMed] [Google Scholar]
- 21.Netea, M. G., N. A. Gow, C. A. Munro, S. Bates, C. Collins, G. Ferwerda, R. P. Hobson, G. Bertram, H. B. Hughes, T. Jansen, L. Jacobs, E. T. Buurman, K. Gijzen, D. L. Williams, R. Torensma, A. McKinnon, D. M. MacCallum, F. C. Odds, J. W. Van der Meer, A. J. Brown, and B. J. Kullberg. 2006. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J. Clin. Investig. 116:1642-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Netea, M. G., A. Warris, J. W. M. Van der Meer, M. J. Fenton, L. Jacobs, T. Verver-Jansen, T. Andressen, P. Verweij, and B. J. Kullberg. 2003. Aspergillus fumigatus evades immune recognition during germination through loss of TLR4-mediated signal transduction. J. Infect. Dis. 188:320-326. [DOI] [PubMed] [Google Scholar]
- 23.Rock, F. L., G. Hardiman, J. C. Timans, J. A. Kastelein, and J. F. Bazan. 1998. A family of human receptors structurally related to Drosophila Toll. Proc. Natl. Acad. Sci. USA 95:588-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335-376. [DOI] [PubMed] [Google Scholar]
- 25.Tsutsumi-Ishii, Y., and I. Nagaoka. 2003. Modulation of human beta-defensin-2 transcription in pulmonary epithelial cells by lipopolysaccharide-stimulated mononuclear phagocytes via proinflammatory cytokine production. J. Immunol. 170:4226-4236. [DOI] [PubMed] [Google Scholar]
- 26.Walsh, T. J., A. Groll, J. Hiemenz, R. Fleming, E. Roilides, and E. Anaissie. 2004. Infections due to emerging and uncommon medically important fungal pathogens. Clin. Microbiol. Infect. 10(Suppl. 1):48-66. [DOI] [PubMed] [Google Scholar]
- 27.Yang, D., O. Chertov, S. N. Bykovskaia, Q. Chen, M. J. Buffo, J. Shogan, M. Anderson, J. M. Schroder, J. M. Wang, O. M. Howard, and J. J. Oppenheim. 1999. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 286:525-528. [DOI] [PubMed] [Google Scholar]
- 28.Yang, D., O. Chertov, and J. J. Oppenheim. 2001. Participation of mammalian defensins and cathelicidins in antimicrobial immunity: receptors and activities of human defensins and cathelicidin (LL-37). J. Leukoc. Biol. 69:691-697. [PubMed] [Google Scholar]
- 29.Zollo, M., R. Mazzarella, S. Bione, D. Toniolo, D. Schlessinger, M. D'Urso, and E. Y. Chen. 1995. Sequence and gene content in 52 kb including and centromeric to the G6PD gene in Xq28. DNA Seq. 6:1-11. [DOI] [PubMed] [Google Scholar]