Abstract
The clinical value of antimonial drugs, the mainstay therapy for leishmaniasis, is now threatened by the emergence of acquired drug resistance, and a comprehensive understanding of the underlying mechanisms is required. Using the model organism Leishmania tarentolae, we have examined the role of trypanothione S-transferase (TST) in trivalent antimony [Sb(III)] resistance. TST has S-transferase activity with substrates such as chlorodinitrobenzene as well as peroxidase activity with alkyl and aryl hydroperoxides but not with hydrogen peroxide. Although S-transferase activity and TST protein levels were unchanged in Sb(III)-sensitive and -resistant lines, rates of metabolism of hydrogen peroxide, t-butyl hydroperoxide, and cumene hydroperoxide were significantly increased. Elevated peroxidase activities were shown to be both trypanothione and tryparedoxin dependent and were associated with the overexpression of classical tryparedoxin peroxidase (TryP) in the cytosol of L. tarentolae. The role of TryP in Sb(III) resistance was verified by overexpression of the recombinant Leishmania major protein in Sb(III)-sensitive promastigotes. An approximate twofold increase in the level of TryP activity in this transgenic cell line was accompanied by a significant decrease in sensitivity to Sb(III) (twofold; P < 0.001). Overexpression of an enzymatically inactive TryP failed to result in Sb(III) resistance. This indicates that TryP-dependent resistance is not due to sequestration of Sb(III) and suggests that enhanced antioxidant defenses may well be a key feature of mechanisms of clinical resistance to antimonial drugs.
The protozoan parasite Leishmania elicits a broad range of human and animal infections ranging from life-threatening kala azar to disfiguring mucocutaneous and cutaneous forms of the disease. The World Health Organization reports over 12 million cases of all forms of leishmaniasis worldwide with 500,000 new infections each year, resulting in an annual death toll of 59,000 (39). Chemotherapy has proven to be the only effective way of controlling infections and is highly dependent upon antimony-containing drugs such as sodium stibogluconate (Pentostam). However, after more than half a century as the mainstay therapy for leishmaniasis, the clinical value of antimonials is now threatened due to the emergence of acquired drug resistance (5). In the past 15 years, failure of antimonials in regions of Bihar, India, where the disease is endemic has become an escalating problem, with primary unresponsiveness reportedly of over 50% (35). Identifying ways in which current antimony therapies can be adapted and improved to circumvent resistance is now a matter of urgency.
The unique thiol metabolism of Leishmania is thought to play a pivotal role in the mechanisms of action of antimonial drugs. In these parasites, the major low-molecular-mass thiol is trypanothione (T[SH]2) [N1,N8-bis(glutathionyl)spermidine], in contrast to most other organisms, which utilize glutathione (γ-l-glutamyl-l-cysteinylglycine) (GSH) (7). Key functions of this essential metabolite include maintenance of thiol redox homeostasis, as well as well as defense against chemical (37) and oxidative stress (7). Antimonial drugs are administered as pentavalent antimony [Sb[V]), a prodrug requiring conversion to the trivalent form [Sb(III)], before becoming biologically active. However, the site of reduction (host macrophage, amastigote, or both) and mechanism of reduction (enzymatic or nonenzymatic) remain unclear (10, 31). Sb(III) interferes directly with thiol metabolism, decreasing thiol-buffering capacity in drug-sensitive Leishmania donovani by inducing rapid efflux of intracellular T[SH]2 and GSH (41). Sb(III) also inhibits T[SH]2 reductase in intact cells, resulting in the accumulation of the disulfide forms of both T[SH]2 (T[S]2) and GSH. These two mechanisms act synergistically against Leishmania parasites, leading to a lethal imbalance in thiol homeostasis.
The ability to generate drug-resistant Leishmania lines in the laboratory, through stepwise exposure to Sb(III), has greatly facilitated the study of antimonial resistance mechanisms (2, 26). Indeed, several key features of in vitro drug resistance have since been identified in resistant clinical isolates (23, 25). A considerable body of evidence places thiol metabolism at the center of both clinical and laboratory-generated resistance mechanisms (2, 23). Elevated levels of T[SH]2 and GSH, resulting from the overexpression of the rate-limiting enzymes involved in the synthesis of GSH (γ-glutamylcysteine synthase [γGCS]) (11) and polyamines (ornithine decarboxylase [ODC]) (13), the two precursor metabolites of T[SH]2, have been observed in several laboratory-induced resistant lines of Leishmania. Interestingly, elevated thiols alone do not result in Sb(III) resistance; however, modulation of T[SH]2 levels, through the use of inhibitors of thiol biosynthesis, reverts resistance (12) indicating that antimonial drug resistance is multifactorial. Overexpression of PgpA, an intracellular metal-thiol transporter from the ATP-binding cassette transporter family, has also been demonstrated to play a key role in resistance (11, 12). Cotransfection of genes encoding γGCS and PgpA confers antimony resistance in a synergistic manner in partially revertant Leishmania parasites (12). Collectively, these studies have led researchers to hypothesize that Sb(III) is detoxified from resistant parasites via the formation of Sb(III)-thiol complexes which are then sequestered in an intracellular vacuolar compartment by PgpA.
In addition to elevated intracellular thiols and elevated PgpA, a third resistance determinant has been identified. In work with the model organism Leishmania tarentolae, Haimeur and colleagues demonstrated that, despite having lost amplification of γGCS, ODC, and PgpA (12), a formerly Sb(III)-resistant revertant cell line retained significant resistance. These researchers hypothesized that an enzyme facilitating the formation of thiol-Sb(III) conjugates for subsequent sequestration by PgpA may well be responsible. Indeed, GSH S-transferase, which catalyzes the conjugation of xenobiotics to GSH in mammalian cells, is elevated in arsenite-resistant Chinese hamster ovary cells (22). Since GSH S-transferase is not detectable in Leishmania spp., it has been suggested that the recently identified T[SH]2 S-transferase (TST) enzyme may facilitate this conjugation in Sb(III)-resistant Leishmania parasites (12). Unusually, TST activity in these cells is associated with the eukaryotic translation elongation factor 1B (38). In the current study we investigate the potential role of TST activity in antimony resistance.
MATERIALS AND METHODS
Cell lines and culture conditions.
The cell lines used in this study were wild-type L. tarentolae (TarII), the Sb(III)-resistant TarII Sb1.1, and its partially resistant revertant TarII Sb1.1rev (12). Promastigotes were propagated in SDM-79 medium supplemented with 10% (vol/vol) fetal calf serum and grown at 24°C with shaking. TarII Sb1.1 cells were grown in the presence of 250 μM potassium antimony tartrate to maintain resistance.
In order to examine the effects of antimony on growth, triplicate cultures containing Sb(III) (as potassium antimony tartrate) were seeded at 5 × 105 promastigotes ml−1. Cell densities were determined microscopically after culture for 72 h, and 50% effective concentrations (EC50s) were determined using the EC50 four-parameter equation provided with GraFit.
Analysis of intracellular thiols.
Mid-log-phase promastigotes (5 × 107) were collected by centrifugation (1,600 × g, 10 min, 4°C) and derivatized with monobromobimane, as described previously (32). Acid-soluble thiols were separated by ion-paired, reverse-phase high-pressure liquid chromatography on a Beckman Ultrasphere C18 column using a Beckman System Gold instrument fitted with a Gilson-121 fluorometer.
Cell lysis.
Frozen L. tarentolae cell pellets were thawed on ice and resuspended in an equal volume of ice-cold lysis buffer (50 mM potassium phosphate [pH 7.0], 1 mM EDTA). The organisms were lysed under pressure (30 kpsi) using a one-shot cell disruptor (Constant Systems). After centrifugation (100,000 × g, 1 h, 4°C), the clarified extracts were dialyzed against the same buffer (4 times, 50 volumes) and assayed immediately for enzyme activity.
Enzyme assays.
T[SH]2 reductase was assayed spectrophotometrically at 340 nm in 40 mM Na+ HEPES (pH 7.5), 1 mM EDTA, 150 μM NADPH, and 100 μM T[S]2 (6). TST was assayed in 100 mM Na+ phosphate (pH 6.5) with 400 μM 1-chloro-2,4-dinitrobenzene and 400 μM T[SH]2 as substrates. The rate of formation of the T[SH]2 S-dinitrobenzene conjugate was followed at 340 nm, using the published extinction coefficient of 9.2 mM cm−1 (36). T[SH]2-dependent peroxidase activities were measured as previously described (15). Briefly, the rates of metabolism of hydrogen peroxide (H2O2), t-butyl hydroperoxide (tBuOOH), and cumene hydroperoxide (CuOOH) were monitored in assays containing 50 mM K+ phosphate (pH 7.0), 0.05 mM T[SH]2, 0.05 mM peroxide, 0.25 mM NADPH, and 0.3 U ml−1 T[SH]2 reductase. Where specified, assays were supplemented with 1 μM recombinant L. major tryparedoxin. Peroxidase activity was measured by the consumption of NADPH at 340 nm. All enzymatic activities were proportional to the amount of protein assayed and heat labile.
Western analysis of whole-cell lysates.
Polyclonal antisera against Leishmania major tryparedoxin, tryparedoxin peroxidase (type I), tryparedoxin peroxidase (type II), and T[SH]2 synthetase and Trypanosoma brucei T[SH]2 reductase were raised in adult male Wistar rats. An initial injection of 100 μg of purified antigen, emulsified in complete Freund's adjuvant, was followed by two identical booster injections of antigen emulsified in Freund's incomplete adjuvant at 2-week intervals.
Mid-log-phase L. tarentolae promastigotes (1 × 107 ml−1) were pelleted by centrifugation (1,600 × g, 10 min, 4°C), washed twice in phosphate-buffered saline (PBS), and resuspended directly in 2× Laemmli buffer containing 200 mM dithiothreitol. Whole-cell extracts (1 × 107 parasites per lane) were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and subsequently transferred onto nitrocellulose. After blocking with 5% skim milk in PBS at room temperature for 1 h, blots were incubated with polyclonal antiserum at room temperature for 1 h, washed in PBS containing 0.1% (vol/vol) Tween 20, and then incubated with a secondary rabbit anti-rat (immunoglobulin G [IgG]) antibody (1/10,000 dilution). Immunoblots were developed using the ECL Plus enhanced chemiluminescence system from Amersham Biosciences. Identical immunoblots were prepared and probed with the following primary antisera: tryparedoxin (1/700 dilution), T[SH]2 synthetase (1/500 dilution), T[SH]2 reductase (1/500 dilution), tryparedoxin peroxidase type I (1/5000 dilution), tryparedoxin peroxidase type II (1/1000 dilution), and TST (1/300 dilution) (38). The relative intensities of protein bands in each Western blot were determined by densitometry using Labworks software (UVP).
Subcellular fractionation.
Subcellular fractions of L. tarentolae were prepared as previously described (27). Each fraction (30 μg) was then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto nitrocellulose. After blocking, the blot was probed with L. major tryparedoxin peroxidase (type I) polyclonal antiserum and detected by chemiluminescence, as described above. Identical immunoblots were probed using the following primary antibodies: anti-Hsp60 monoclonal antibody (1/5,000 dilution; Stressgen); anti-L. major T[SH]2 synthetase antiserum (1/1,000 dilution) (27), and anti-T. brucei BiP antiserum (1/5,000 dilution) (1). Rabbit anti-rat (IgG) antibody (Dako; 1/10,000 dilution) and anti-mouse (IgG-horseradish peroxidase) antibody (Sigma; 1/10,000 dilution) were used as secondary antibodies where appropriate, and immunoblots were once again developed by enhanced chemiluminescence.
Cloning and expression of L. major tryparedoxin peroxidase in L. tarentolae.
The L. major tryparedoxin peroxidase gene (LmjF15.1120) was amplified by PCR from L. major Friedlin genomic DNA using the sense primer 5′-GGATCCATGTCCTGCGGTAACGCCAAGATCAAC-3′ and the antisense primer 5′-GGATCCTTACTGCTTGCTGAAGTATCCCTCGAC-3′, both with additional BamHI sites (underlined). The PCR product was then cloned into the pCR-Blunt II-TOPO vector (Invitrogen) and sequenced. The pCR-Blunt II-TOPO-LmTryP construct was then digested with BamHI and the fragment cloned into the pIR1SAT expression vector, resulting in a pIR1SAT-LmTryP construct. Mid-log-phase L. tarentolae promastigotes (wild type, TarII) were transfected with either pIR1SAT-LmTryP or with the empty vector by electroporation using a high-voltage protocol (28). Following transfection, cells were allowed to grow for 16 to 24 h in SDM-79 medium with 10% fetal calf serum and then plated on semisolid medium containing 1% Noble agar (Difco) and 100 μg ml−1 nourseothricin (Jena Bioscience, Germany). Individual colonies were picked and grown in liquid medium. Clones were maintained in selective medium and then removed from drug selection for one passage prior to experiments.
Cloning and expression of an inactive L. major tryparedoxin peroxidase gene mutant in L. tarentolae.
Site-directed mutagenesis was carried out following the QuikChange protocol (Stratagene) and using the KOD HotStart DNA polymerase (Novagen). Using the pIRSAT-LmTryP construct as a template, an R128D mutant of L. major TryP was generated by PCR with the sense primer 5′ GCCAGGGCGTGCCTACGACGGTCTCTTCATCATCG and antisense primer 5′ CGATGATGAAGAGACCGTCGTAGGCCACGCCCTGGC, with mutated bases underlined. The modified construct, pIR1SAT-LmTryP(R128D), was transfected into mid-log-phase L. tarentolae promastigotes (wild type, TarII) as described above.
RESULTS
Intracellular thiol levels in Sb(III)-sensitive and -resistant L. tarentolae.
A comprehensive understanding of the mechanisms of resistance to antimonials is crucial, not only in the ongoing fight to save these mainstay therapies but also for the future design of more effective anti-Leishmania drugs. With this in mind, the previously characterized L. tarentolae TarII (wild type), TarII Sb1.1 [Sb(III)-resistant] and TarII Sb1.1rev [Sb(III)-resistant revertant] cell lines were used to examine the putative role of TST in Sb(III) resistance (12). Initially, the relative Sb(III) sensitivities of all three cell lines were determined in potassium antimony tartrate. In keeping with previously published data (12), the Sb(III)-resistant cell line was approximately 50-fold less sensitive to potassium antimony tartrate than the parental wild-type cells, while the revertant cell line was 8-fold less sensitive (Table 1).
TABLE 1.
Intracellular thiol levels in Sb(III)-sensitive L. tarentolae TarII (wild type), Sb(III)-resistant TarII Sb1.1, and partially resistant revertant TarII Sb1.1rev
| Cell line | EC50 (μg ml−1) | Thiol content (nmol 108 cells−1)a
|
|||
|---|---|---|---|---|---|
| GSH | GspdSH | T[SH]2 | Total SH | ||
| TarII (wild type) | 7.35 ± 0.14 | 0.75 ± 0.04 | 0.325 ± 0.08 | 1.6 ± 0.03 | 4.28 |
| TarII Sb1.1rev (revertant) | 56.5 ± 1.9c | 0.8 ± 0.05c | 0.37 ± 0.02c | 1.70 ± 0.07c | 4.56c |
| TarII Sb1.1 (resistant) | 377 ± 38.4d | 8.76 ± 0.11d | 1.4 ± 0.01d | 10.05 ± 0.1d | 30.26d |
| TarII Sb1.1 − Sbb | Not applicable | 11.1 ± 0.2d | 1.8 ± 0.09d | 12.1 ± 0.15d | 37.1d |
Thiol levels represent the means ± standard deviations from triplicate determinations.
Cells grown in the absence of Sb(III) for two passages prior to analysis.
Not significantly different from wild-type values (P > 0.05).
Significantly different from wild-type value (P < 0.0001).
Intracellular thiol levels in the parental wild-type, revertant, and resistant cell lines were determined (Table 1). In contrast to the data of Haimeur and Ouellette (14), we could detect no significant difference in the thiol levels of revertant versus wild-type cells. However, Sb(III)-resistant cells maintained significantly higher levels of GSH, GspdSH, and T[SH]2 (P < 0.0001). Indeed, the levels of total acid-soluble low-molecular-mass thiol groups were approximately 7.5-fold higher in TarII Sb1.1 than in revertant and wild-type cells, suggesting that elevated intracellular thiol levels are associated with high levels of Sb(III) resistance but are not responsible for the residual resistance of the Sb1.1rev cell line.
Since Sb(III) is known to induce the rapid efflux of intracellular GSH and T[SH]2 in Leishmania donovani (41), thiols in Sb(III)-resistant promastigotes were reanalyzed following growth for two passages in the absence of Sb(III). Interestingly, these cells maintained even higher levels of intracellular thiols (37.1 nmol 108 cells−1 total SH) than the original resistant cell line (30.3 nmol 108 cells−1 total SH), presumably due to continued thiol synthesis in the absence of thiol-Sb(III) conjugate efflux.
TST activity in clarified L tarentolae lysates.
Clarified extracts of all three L. tarentolae cell lines were prepared and assayed directly for TST activity (Table 2). Using 1-chloro-2,4-dinitrobenzene as a substrate, no difference in the TST activities of extracts of wild-type, revertant, and resistant promastigotes could be detected, suggesting that TST overexpression is not associated with Sb(III) resistance in these parasites. T[SH]2 reductase (TryR) activity was similar in all three extracts, suggesting that an elevated TryR level is not responsible for residual resistance either.
TABLE 2.
Specific activities of T[SH]2 reductase, TST, and T[SH]2-dependent peroxidase in L. tarentolae soluble extracts
| Enzymatic activity | Substrate | Sp act (nmol min−1 mg−1)a
|
||
|---|---|---|---|---|
| Wild type | Revertant strain | Resistant strain | ||
| T[SH]2 reductase | T[S]2 | 112 ± 9 | 120 ± 9b | 114 ± 7.9b |
| TST | CDNB | 1.2 ± 0.5 | 1.6 ± 0.8b | 1.0 ± 0.4b |
| T[SH]2-dependent peroxidase | H2O2 | 3.5 ± 0.4 | 14.4 ± 0.9c | 25.7 ± 1.0c |
| CuOOH | 1.0 ± 0.1 | 3.1 ± 0.1c | 8.9 ± 0.4c | |
| tBuOOH | 1.9 ± 0.2 | 8.8 ± 0.2c | 22.4 ± 0.4c | |
All enzymatic activities were assayed as described in Materials and Methods and were corrected for nonenzymatic background rates. Values are means ± standard deviations.
P > 0.05 compared to wild-type value.
P < 0.0001 compared to wild-type value.
In our previous studies we demonstrated that L. major TST displays T[SH]2-dependent peroxidase activity against a variety of organic hydroperoxides. To further verify that TST is not an Sb(III) resistance determinant in L. tarentolae, the rates of metabolism of H2O2, CuOOH, and tBuOOH were measured in dialyzed extracts. Unexpectedly, the rates of metabolism of H2O2 and tBuOOH were considerably higher in the resistant and revertant lysates than in the wild type (Table 2). Specifically, H2O2 metabolism was enhanced 4-fold and 8.5-fold in Sb1.1rev and Sb1.1 extracts, respectively, in keeping with the 8-fold- and 50-fold-increased levels of Sb(III) resistance found in whole cells. CuOOH was less efficiently reduced. Since L. major TST is known to preferentially metabolize hydrophobic hydroperoxides and is unable to utilize hydrogen peroxide as a substrate (38), it is unlikely that this enzyme is responsible for the peroxidase activities detected in these extracts.
The apparent lack of TST involvement in Sb(III) resistance is in marked contrast to the resistance mechanisms employed by mammalian cells, in which GSH S-transferases accelerate the detoxification of the related metalloid As(III) via conjugation to GSH (18). Although the spontaneous formation of Sb(III)-thiol (GSH and T[SH]2) complexes has been demonstrated (34), some researchers have suggested that an enzyme such as TST may be required to accelerate this reaction in vivo (12). In light of the elevated levels of intracellular thiols and overexpression of the metal-thiol transporter PgpA in resistant Leishmania parasites, the ability to more rapidly form Sb(III)-thiol conjugates for detoxification would be predicted to enhance Sb(III) resistance. However, the absence of accompanying TST overexpression in these cell lines implies that TST is not responsible for the resistance phenotype.
Tryparedoxin-dependent peroxidase activity in clarified extracts.
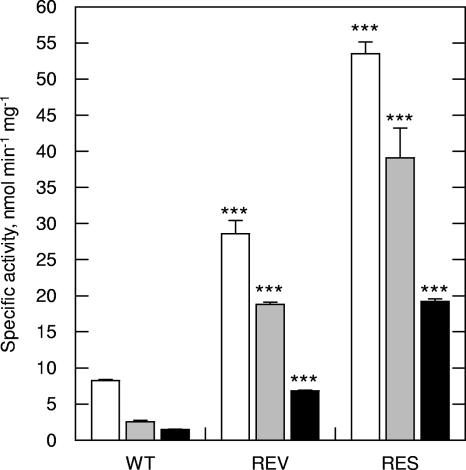
In Leishmania and other trypanosomatids, enzymes of the tryparedoxin peroxidase family are thought to be principally responsible for detoxification of peroxides (9). This family consists of two classes of 2-Cys peroxidases, the decameric type I tryparedoxin peroxidase (TryP) (8, 20) and the monomeric type II tryparedoxin peroxidase (TDPX), a cysteine homologue of the classical GSH peroxidases (16, 29, 30). Both enzymes obtain their reducing equivalents from T[SH]2 via the dithiol protein tryparedoxin (TryX). To establish whether a TryX-dependent peroxidase was involved in Sb(III) resistance, clarified extracts were assayed for peroxidase activity following the addition of exogenous recombinant L. major TryX (Fig. 1). In the presence of TryX, peroxidase activities in all three extracts, and against all substrates, were at least doubled (Table 2). However, metabolism of H2O2, tBuOOH, and CuOOH remained significantly higher in revertant and resistant cell extracts compared to wild type (P < 0.0001). These observations suggest that enhanced peroxide metabolism in Sb(III)-resistant cells is TryX dependent and that TryX is rate limiting under these assay conditions. TryPs from several Leishmania spp. have shown susceptibility to inactivation following exposure to high concentrations of hydrophobic peroxides (3). Therefore, it was interesting to note that preincubation with 1 mM CuOOH inactivated the elevated peroxidase activities in revertant and resistant extracts (data not shown).
FIG. 1.
Tryparedoxin-dependent peroxidase activity in L. tarentolae clarified lysates. Metabolism of hydrogen peroxide (white bars), tBuOOH (gray bars), and CuOOH (black bars) in wild-type (WT), revertant (REV), and resistant (RES) extracts were measured in the presence of 1 μM recombinant L. major tryparedoxin. Data represent the means ± standard deviations from triplicate determinations. ***, P < 0.0001.
Western analysis of whole cell lysates.
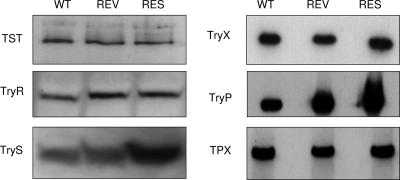
Immunoblots of L. tarentolae promastigote whole-cell lysates were probed with a series of polyclonal antisera against all the enzymes of the tryparedoxin peroxidase pathway (Fig. 2). In keeping with our enzymatic analysis of whole-cell lysates, TST and TryR protein levels were similar in wild-type, revertant, and resistant cell lines. Surprisingly, levels of TryS protein were increased 2.5-fold in the resistant cell line but not in the revertant. Sb(III) up to 1 mM had no inhibitory effect on TryS activity, indicating that TryS, like ODC (spermidine synthesis) and γGCS (GSH synthesis) is a critical, rate-limiting step in T[SH]2 biosynthesis in highly resistant lines. Although there was no apparent association between Sb(III) tolerance and the levels of TryX or the recently characterized TDPX (19, 30), levels of TryP were elevated 2.8-fold and 8.9-fold (as determined by densitometry) in the revertant and resistant lysates, respectively. Overexpression of TryP protein in the revertant and resistant parasites correlates well with the fourfold and eightfold increases in peroxidase activity (Table 2 and Fig. 1).
FIG. 2.
Immunoblot analysis of L. tarentolae whole-cell lysates. Blots of cell extracts from L. tarentolae wild-type (WT), revertant (REV), and Sb(III)-resistant (RES) cell lines (1 × 107 parasites in each lane) were probed with antisera to L. major TST, T. brucei T[SH]2 reductase (TryR), L. major T[SH]2 synthetase (TryS), L. major tryparedoxin (TryX), L. major tryparedoxin peroxidase type I (TryP), and L. major tryparedoxin peroxidase type II (TDPX).
Western analysis of Sb(III)-sensitive and -resistant L. tarentolae subcellular fractions.
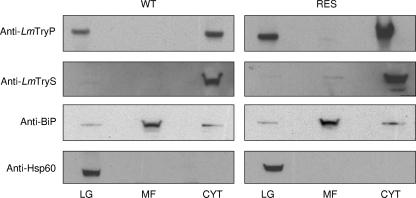
TryP localizes to both the mitochondria and cytosol of Leishmania parasites (4). To determine whether overexpression of TryP in a specific cellular location was associated with Sb(III) resistance, crude subcellular fractions of both wild-type and resistant L. tarentolae were prepared and immunoblots of each fraction were probed with an L. major TryP-specific polyclonal antiserum known to cross-react with both cytosolic and mitochondrial enzymes (Fig. 3). The purity of each fraction was demonstrated using antibodies against marker proteins in each subcellular location. The endoplasmic reticulum-localized BiP protein (immunoglobulin heavy chain-binding protein) was used as a marker for the microsomal fraction, while Hsp60 and TryS were used as marker proteins for the large granular and cytosolic fractions, respectively (27). As expected, TryP was found in both the cytosolic and large granular fractions of both cell lines. The levels of mitochondrial TryP were marginally higher in resistant than in wild-type L. tarentolae (1.8-fold); however, cytosolic TryP levels were considerably higher in the Sb(III)-resistant line (6.5-fold). Specific overexpression of cytosolic TryP was also observed in revertant parasites (data not shown). These data suggest that overexpression of a TryP localized to the cytosol of L. tarentolae is associated with Sb(III) resistance. Interestingly, Southern analysis of genomic DNA isolated from wild-type, revertant, and resistant cells confirmed that increased TryP expression was due to gene amplification (data not shown).
FIG. 3.
Cellular location of elevated tryparedoxin peroxidase. Subcellular fractions of L. tarentolae promastigotes containing the large-granule (LG), microsomal (MF), and cytosolic (C) fractions were prepared as described in Materials and Methods. Western blots of these equally loaded fractions (30 μg of protein in each lane) were probed with antiserum to L. major tryparedoxin peroxidase (type I). In addition, blots were stripped and reprobed with antiserum to marker proteins for each subcellular fraction to demonstrate the purity of each fraction (LG, anti-Hsp60; C, anti-L. major T[SH]2 synthetase; MF, anti BiP).
An association between TryP and heavy metal resistance has previously been established. In recent studies, Lin and colleagues described the concomitant overexpression of cytosolic and mitochondrial TryP in laboratory-generated, arsenite-resistant Leishmania amazonensis (17, 21). Arsenite-resistant Leishmania parasites are often found to be cross-resistant to Sb(III), and the mechanisms of resistance to both metalloids are believed to share many common features (12, 14).
Overexpression of recombinant tryparedoxin peroxidase.
To confirm the role of TryP in Sb(III) resistance, the cytosolic enzyme from L. major was overexpressed in wild-type (TarII), Sb(III)-sensitive L. tarentolae. Western analysis of parasites transfected with L. major TryP revealed an approximately 2.2-fold increase in TryP at the protein level compared with wild-type parasites and those transfected with the pIR1SAT vector alone (Table 3). The overexpression of TryP was further confirmed when metabolism of H2O2 in crude lysates of these pIR1SAT-LmTryP cloned transfectants (8.3 nmol min−1 mg−1) was found to be approximately twofold higher than that in the vector-only control (4.3 nmol min−1 mg−1). Most importantly, the TryP-overexpressing L. tarentolae cell line was found to be significantly more resistant to Sb(III) (EC50, 22.7 ± 0.8 μg ml−1; P < 0.001) than either the wild-type cell line (EC50, 7.3 ± 0.4 μg ml−1) or promastigotes containing the empty vector (EC50, 9.24 ± 0.4 μg ml−1) (Table 3).
TABLE 3.
Comparison of Sb(III) sensitivity and tryparedoxin peroxidase levels in wild-type, revertant, and transgenic L. tarentolae cell lines
| Cell Line | Vector | Relative protein levela | Mean ± SD
|
|
|---|---|---|---|---|
| Peroxidase activity (nmol min−1 mg−1) | EC50 (μg ml−1) | |||
| Wild type | None | 1 | 3.5 ± 0.4 | 7.3 ± 0.4 |
| pIR1SAT | 1 | 4.3 ± 0.2 | 9.3 ± 0.4 | |
| pIR1SAT-LmTryP | 2.2 | 8.3 ± 0.6b | 22.7 ± 0.8b | |
| pIR1SAT-LmTryP (R128D) | 3.3 | 4.4 ± 0.1 | 8.3 ± 0.5 | |
| Revertant | None | 2.8 | 14.4 ± 0.9b | 56.5 ± 1.9b |
As determined by densitometry of Western blots and compared to wild-type level.
P < 0.0001 compared to wild-type value.
Overexpression of an inactive tryparedoxin peroxidase.
In previous studies, Flöhe and colleagues have demonstrated that a highly conserved arginine residue at position 128 of both the Crithidia fasiculata (24) and L. donovani (8) TryPs is essential for peroxidase activity. In both organisms, replacement of R128 with an acidic residue resulted in a correctly folded TryP devoid of activity. To determine whether peroxidase activity itself is central to the Sb(III) resistance conferred by L. major TryP overexpression, a pIRSAT-LmTryP(R128D) construct was generated and transfected into wild-type L. tarentolae. Western analysis of parasites expressing L. major TryP(R128D) revealed an approximately 3.3-fold increase in TryP at the protein level, in keeping with the levels of overexpression seen in the pIR1SAT-L. major TryP transfectants (Table 3). However, in this instance L. major TryP(R128D) overexpression was not accompanied by a concomitant increase in the metabolism of H2O2 (4.45 nmol min−1 mg−1) or by an increase in Sb(III) tolerance (EC50, 8.3 ± 0.5 μg ml−1).
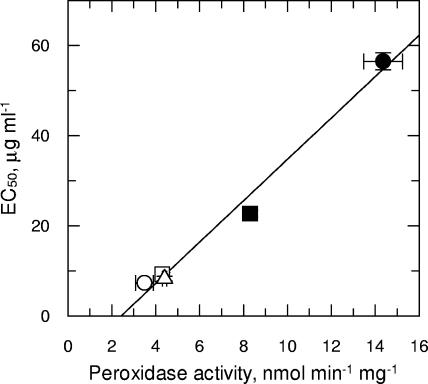
TryP activity (H2O2) in these cloned, transgenic lines and also in the parental wild-type and revertant lines was found to directly correlate with Sb(III) resistance, with a correlation coefficient of 0.99 (Fig. 4). The direct correlation between peroxidase activity and resistance is of particular interest, since it suggests that TryP overexpression may be the principal resistance determinant in the revertant cell line. Not surprisingly, TryP activity did not directly correlate with Sb(III) resistance in the highly resistant Sb1.1 line, since resistance in this cell line is known to be multifactorial (12). These findings confirm that an enhanced ability to metabolize specific peroxide substrates plays a direct and quantifiable role in the mechanisms of Sb(III) resistance in these Leishmania parasites.
FIG. 4.
Analysis of EC50 values for Sb(III) against TryP activity. Peroxidase activities (H2O2) from the cell lysates of wild-type (open circles), REV (closed circles), vector-only control (open squares), TryP-overexpressing (closed squares), and inactive TryP(R128D)-overexpressing (open triangles) cloned cell lines were plotted against the EC50s for Sb(III). A linear regression was fitted to these data with a correlation coefficient of 0.99. Data are the mean of triplicate measurements ± standard deviations.
DISCUSSION
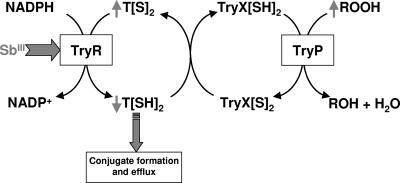
The relevance of elevated intracellular thiol levels, due to overexpression of γGCS and ODC, and an increased efflux of thiol-Sb(III) conjugates, due to the overexpression of PgpA, in high-level resistance is well defined (12, 25). However, the involvement of TryP in resistance has not been reported previously. The data presented here establish for the first time that overexpression of an active TryP directly contributes to the multifactorial Sb(III) resistance mechanisms in Leishmania tarentolae. Our observations with Sb(III)-resistant L. tarentolae are broadly supported by the recent studies of Hsu and colleagues, who reported the overexpression of several components of both the cytosolic and mitochondrial tryparedoxin pathways in laboratory-generated, arsenite-resistant Leishmania amazonensis (17, 21). TryPs form the backbone of parasite antioxidant defenses. In our previous studies we have demonstrated that Sb(III) has pleiotropic effects on Sb T[SH]2 metabolism which severely compromise thiol redox homeostasis within Sb(III)-sensitive parasites, leading to accumulation of reactive oxygen species (ROS) (Fig. 5) (23, 41). Thus, inhibition of TryR, depletion of intracellular T[SH]2, and inhibition of TryP, alone or in combination, could account for the increased levels of ROS.
FIG. 5.
Model for the mode of action of Sb(III) on Leishmania. Sb(III) is known to attack the thiol metabolism of Leishmania parasites in two distinct ways (41). First, Sb(III) inhibits TryR in intact cells, resulting in accumulation of the disulfide forms of T[S]2. Second, Sb(III) decreases thiol buffering capacity by inducing rapid efflux of intracellular T[SH]2. These two mechanisms combine to profoundly compromise the thiol redox potential in drug-sensitive parasites and lead to the accumulation of ROS (23).
Overexpression of TryP could confer resistance to Sb(III) by two mechanisms: by sequestration of Sb(III) to this abundant protein (1 to 4% of the total protein in L. major [19]) or by increased enzyme activity to reduce levels of ROS induced by exposure to Sb(III). The former mechanism can be excluded, since overexpression of a mutant TryP devoid of peroxidase activity but maintaining a full complement of cysteine residues failed to provide protection from Sb(III). Thus, peroxidase activity of TryP is essential to confer resistance in these cell lines. Preliminary studies indicate that Sb(III) binds to TryP sulfydryl groups as well as the other components of the tryparedoxin peroxidase system (TryR, T[SH]2, and TryX), but we have yet to establish whether TryP is a specific target for antimonials (S. Wyllie, J. König, and A. H. Fairlamb, unpublished data). Although TryP has not yet been shown to be essential in Leishmania parasites, RNA interference studies have demonstrated that it is essential for the viability of bloodstream T. brucei (40). Further studies should clarify these possibilities.
The use of Leishmania tarentolae is open to the criticism that it is not a relevant model for clinically observed antimony resistance. However, elevated thiol levels and amplification of γGCS and PgpA have been identified in clinical isolates (25, 33). In preliminary studies with some of these Sb-resistant isolates, we have noted elevated levels of tryparedoxin peroxidase (S. Wyllie, G. Mandal, M. Chatterjee, and A. H. Fairlamb, unpublished data). Collectively, these data suggest that enhanced antioxidant defenses, through overexpression of TryP, may well be a key feature of mechanisms of clinical resistance to antimonial drugs.
Acknowledgments
This work was funded by a grant from the Burroughs Wellcome Fund and Wellcome Trust Infectious Disease Initiative.
We thank Janine König for providing L. major tryparedoxin, Stephen Beverley for providing the vector pIR1SAT, and Marc Ouellette for the L. tarentolae cell lines. Our appreciation also goes to Adel Ibrahim of the University of Dundee Cloning Service for help with site-directed mutagenesis.
Footnotes
Published ahead of print on 4 February 2008.
REFERENCES
- 1.Bangs, J. D., L. Uyetake, M. J. Brickman, A. E. Balber, and J. C. Boothroyd. 1993. Molecular cloning and cellular localization of a BiP homologue in Trypanosoma brucei. Divergent ER retention signals in a lower eukaryote. J. Cell Sci. 105:1101-1113. [DOI] [PubMed] [Google Scholar]
- 2.Borst, P., and M. Ouellette. 1995. New mechanisms of drug resistance in parasitic protozoa. Annu. Rev. Microbiol. 49:427-460. [DOI] [PubMed] [Google Scholar]
- 3.Castro, H., H. Budde, L. Flohé, B. Hofmann, H. Lunsdorf, J. Wissing, and A. M. Tomás. 2002. Specificity and kinetics of a mitochondrial peroxiredoxin of Leishmania infantum. Free Radic. Biol. Med. 33:1563-1574. [DOI] [PubMed] [Google Scholar]
- 4.Castro, H., C. Sousa, M. Santos, A. Cordeiro-Da-Silva, L. Flohé, and A. M. Tomas. 2002. Complementary antioxidant defense by cytoplasmic and mitochondrial peroxiredoxins in Leishmania infantum. Free Radic. Biol. Med. 33:1552-1562. [DOI] [PubMed] [Google Scholar]
- 5.Croft, S. L., S. Sundar, and A. H. Fairlamb. 2006. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 19:111-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham, M. L., and A. H. Fairlamb. 1995. Trypanothione reductase from Leishmania donovani—purification, characterisation and inhibition by trivalent antimonials. Eur. J. Biochem. 230:460-468. [DOI] [PubMed] [Google Scholar]
- 7.Fairlamb, A. H., and A. Cerami. 1992. Metabolism and functions of trypanothione in the Kinetoplastida. Annu. Rev. Microbiol. 46:695-729. [DOI] [PubMed] [Google Scholar]
- 8.Flohé, L., H. Budde, K. Bruns, H. Castro, J. Clos, B. Hofmann, S. Kansal-Kalavar, D. Krumme, U. Menge, K. Plank-Schumacher, H. Sztajer, J. Wissing, C. Wylegalla, and H. J. Hecht. 2002. Tryparedoxin peroxidase of Leishmania donovani: molecular cloning, heterologous expression, specificity, and catalytic mechanism. Arch. Biochem. Biophys. 397:324-335. [DOI] [PubMed] [Google Scholar]
- 9.Flohe, L., H. Budde, and B. Hofmann. 2003. Peroxiredoxins in antioxidant defense and redox regulation. BioFactors 19:3-10. [DOI] [PubMed] [Google Scholar]
- 10.Frezard, F., C. Demicheli, C. S. Ferreira, and M. A. P. Costa. 2001. Glutathione-induced conversion of pentavalent antimony to trivalent antimony in meglumine antimoniate. Antimicrob. Agents Chemother. 45:913-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grondin, K., A. Haimeur, R. Mukhopadhyay, B. P. Rosen, and M. Ouellette. 1997. Co-amplification of the γ-glutamylcysteine synthetase gene gsh1 and of the ABC transporter gene pgpA in arsenite-resistant Leishmania tarentolae. EMBO J. 16:3057-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haimeur, A., C. Brochu, P. A. Genest, B. Papadopoulou, and M. Ouellette. 2000. Amplification of the ABC transporter gene PGPA and increased trypanothione levels in potassium antimonyl tartrate (SbIII) resistant Leishmania tarentolae. Mol. Biochem. Parasitol. 108:131-135. [DOI] [PubMed] [Google Scholar]
- 13.Haimeur, A., C. Guimond, S. Pilote, R. Mukhopadhyay, B. P. Rosen, R. Poulin, and M. Ouellette. 1999. Elevated levels of polyamines and trypanothione resulting from overexpression of the ornithine decarboxylase gene in arsenite-resistant Leishmania. Mol. Microbiol. 34:726-735. [DOI] [PubMed] [Google Scholar]
- 14.Haimeur, A., and M. Ouellette. 1998. Gene amplification in Leishmania tarentolae selected for resistance to sodium stibogluconate. Antimicrob. Agents Chemother. 42:1689-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson, G. B., A. H. Fairlamb, and A. Cerami. 1987. Trypanothione dependent peroxide metabolism in Crithidia fasciculata and Trypanosoma brucei. Mol. Biochem. Parasitol. 24:39-45. [DOI] [PubMed] [Google Scholar]
- 16.Hillebrand, H., A. Schmidt, and R. L. Krauth-Siegel. 2003. A second class of peroxidases linked to the trypanothione metabolism. J. Biol. Chem. 278:6809-6815. [DOI] [PubMed] [Google Scholar]
- 17.Hsu, J. Y., Y. C. Lin, S. C. Chiang, and S. T. Lee. 2008. Divergence of trypanothione-dependent tryparedoxin cascade into cytosolic and mitochondrial pathways in arsenite-resistant variants of Leishmania amazonensis. Mol. Biochem. Parasitol. 157:193-204. [DOI] [PubMed] [Google Scholar]
- 18.Ishikawa, T. 1992. The ATP-dependent glutathione S-conjugate export pump. Trends Biochem. Sci. 17:463-468. [DOI] [PubMed] [Google Scholar]
- 19.Konig, J., and A. H. Fairlamb. 2007. A comparative study of type I and type II tryparedoxin peroxidases in Leishmania major. FEBS J. 274:5643-5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levick, M. P., E. Tetaud, A. H. Fairlamb, and J. M. Blackwell. 1998. Identification and characterisation of a functional peroxidoxin from Leishmania major. Mol. Biochem. Parasitol. 96:125-137. [DOI] [PubMed] [Google Scholar]
- 21.Lin, Y. C., J. Y. Hsu, S. C. Chiang, and S. T. Lee. 2005. Distinct overexpression of cytosolic and mitochondrial tryparedoxin peroxidases results in preferential detoxification of different oxidants in arsenite-resistant Leishmania amazonensis with and without DNA amplification. Mol. Biochem. Parasitol. 142:66-75. [DOI] [PubMed] [Google Scholar]
- 22.Lo, J. F., H. F. Wang, M. F. Tam, and T. C. Lee. 1992. Glutathione S-transferase π in an arsenic-resistant Chinese hamster ovary cell line. Biochem. J. 288:977-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandal, G., S. Wyllie, N. Singh, S. Sundar, A. H. Fairlamb, and M. Chatterjee. 2007. Increased levels of thiols protect antimony unresponsive Leishmania donovani field isolates against reactive oxygen species generated by trivalent antimony. Parasitology 134:1679-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montemartini, M., H. M. Kalisz, H. J. Hecht, P. Steinert, and L. Flohé. 1999. Activation of active-site cysteine residues in the peroxiredoxin-type tryparedoxin peroxidase of Crithidia fasciculata. Eur. J. Biochem. 264:516-524. [DOI] [PubMed] [Google Scholar]
- 25.Mukherjee, A., P. K. Padmanabhan, S. Singh, G. Roy, I. Girard, M. Chatterjee, M. Ouellette, and R. Madhubala. 2007. Role of ABC transporter MRPA, gamma-glutamylcysteine synthetase and ornithine decarboxylase in natural antimony-resistant isolates of Leishmania donovani. J. Antimicrob. Chemother. 59:204-211. [DOI] [PubMed] [Google Scholar]
- 26.Ouellette, M., D. Légaré, and B. Papadopoulou. 2001. Multidrug resistance and ABC transporters in parasitic protozoa. J. Mol. Microbiol. Biotechnol. 3:201-206. [PubMed] [Google Scholar]
- 27.Oza, S. L., M. P. Shaw, S. Wyllie, and A. H. Fairlamb. 2005. Trypanothione biosynthesis in Leishmania major. Mol. Biochem. Parasitol. 139:107-116. [DOI] [PubMed] [Google Scholar]
- 28.Robinson, K. A., and S. M. Beverley. 2003. Improvements in transfection efficiency and tests of RNA interference (RNAi) approaches in the protozoan parasite Leishmania. Mol. Biochem. Parasitol. 128:217-228. [DOI] [PubMed] [Google Scholar]
- 29.Schlecker, T., M. A. Comini, J. Melchers, T. Ruppert, and R. L. Krauth-Siegel. 2007. Catalytic mechanism of the glutathione peroxidase-type tryparedoxin peroxidase of Trypanosoma brucei. Biochem. J. 405:445-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlecker, T., A. Schmidt, N. Dirdjaja, F. Voncken, C. Clayton, and R. L. Krauth-Siegel. 2005. Substrate specificity, localization, and essential role of the glutathione peroxidase-type tryparedoxin peroxidases in Trypanosoma brucei. J. Biol. Chem. 280:14385-14394. [DOI] [PubMed] [Google Scholar]
- 31.Shaked-Mishan, P., N. Ulrich, M. Ephros, and D. Zilberstein. 2001. Novel intracellular Sb-V reducing activity correlates with antimony susceptibility in Leishmania donovani. J. Biol. Chem. 276:3971-3976. [DOI] [PubMed] [Google Scholar]
- 32.Shim, H., and A. H. Fairlamb. 1988. Levels of polyamines, glutathione and glutathione-spermidine conjugates during growth of the insect trypanosomatid Crithidia fasciculata. J. Gen. Microbiol. 134:807-817. [DOI] [PubMed] [Google Scholar]
- 33.Singh, N., R. Almeida, H. Kothari, P. Kumar, G. Mandal, M. Chatterjee, S. Venkatachalam, M. K. Govind, S. K. Mandal, and S. Sundar. 2007. Differential gene expression analysis in antimony-unresponsive Indian kala azar (visceral leishmaniasis) clinical isolates by DNA microarray. Parasitology 134:777-787. [DOI] [PubMed] [Google Scholar]
- 34.Sun, H. Z., S. C. Yan, and K. Y. Ding. 2001. Complexation of antimony(III) and trypanothione: formation of a novel tertiary complex. J. Inorg. Biochem. 86:446. [Google Scholar]
- 35.Sundar, S., D. K. More, M. K. Singh, V. P. Singh, S. Sharma, A. Makharia, P. C. Kumar, and H. W. Murray. 2000. Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic. Clin. Infect. Dis. 31:1104-1107. [DOI] [PubMed] [Google Scholar]
- 36.Vickers, T. J., and A. H. Fairlamb. 2004. Trypanothione S-transferase activity in a trypanosomatid ribosomal elongation factor 1B. J. Biol. Chem. 279:27246-27256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vickers, T. J., N. Greig, and A. H. Fairlamb. 2004. A trypanothione-dependent glyoxalase I with a prokaryotic ancestry in Leishmania major. Proc. Natl. Acad. Sci. USA 101:13186-13191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vickers, T. J., S. H. Wyllie, and A. H. Fairlamb. 2004. Leishmania major elongation factor 1B complex has trypanothione S-transferase and peroxidase activity. J. Biol. Chem. 279:49003-49009. [DOI] [PubMed] [Google Scholar]
- 39.WHO. 1999. Tropical disease research: progress 1997-98. UNDP/World Bank/WHO Special Programme for Reasearch & Training in Tropical Diseases (TDR). WHO, Geneva, Switzerland.
- 40.Wilkinson, S. R., D. Horn, S. R. Prathalingam, and J. M. Kelly. 2003. RNA interference identifies two hydroperoxide metabolizing enzymes that are essential to the bloodstream form of the African trypanosome. J. Biol. Chem. 278:31640-31646. [DOI] [PubMed] [Google Scholar]
- 41.Wyllie, S., M. L. Cunningham, and A. H. Fairlamb. 2004. Dual action of antimonial drugs on thiol redox metabolism in the human pathogen Leishmania donovani. J. Biol. Chem. 279:39925-39932. [DOI] [PubMed] [Google Scholar]