Abstract
The malaria parasite Plasmodium falciparum has at least five putative histone deacetylase (HDAC) enzymes, which have been proposed as new antimalarial drug targets and may play roles in regulating gene transcription, like the better-known and more intensively studied human HDACs (hHDACs). Fourteen new compounds derived from l-cysteine or 2-aminosuberic acid were designed to inhibit P. falciparum HDAC-1 (PfHDAC-1) based on homology modeling with human class I and class II HDAC enzymes. The compounds displayed highly potent antiproliferative activity against drug-resistant (Dd2) or drug sensitive (3D7) strains of P. falciparum in vitro (50% inhibitory concentration of 13 to 334 nM). Unlike known hHDAC inhibitors, some of these new compounds were significantly more toxic to P. falciparum parasites than to mammalian cells. The compounds inhibited P. falciparum growth in erythrocytes at both the early and late stages of the parasite's life cycle and caused altered histone acetylation patterns (hyperacetylation), which is a marker of HDAC inhibition in mammalian cells. These results support PfHDAC enzymes as being promising targets for new antimalarial drugs.
Malaria is one of the most significant infectious diseases of the 21st century, resulting in an estimated 500 million infections and 2 million deaths annually, predominantly in sub-Saharan Africa and Asia (32). There is currently no effective vaccine against malaria, and mosquito control and chemotherapy are the main approaches to the prevention and treatment of this disease. Unfortunately, the principal malarial protozoan parasite in humans, Plasmodium falciparum, has become increasingly resistant to currently used drugs (27, 28). Therefore, there is an urgent need for new antimalarial agents that act on different parasite targets, as they have the potential to combat resistance. The histone deacetylases (HDACs) of P. falciparum (PfHDACs) may be promising targets for new classes of antimalarial drugs (6, 20).
HDAC enzymes play crucial roles in modulating the chromatin structure in eukaryotes through regulating the degree of “packaging/unpackaging” of chromosomal DNA for transcription (3). Histone proteins form the fundamental core unit of chromatin and are reversibly acetylated on the ɛ-amino side chain of several of their lysine residues. Together with other modifications (e.g., methylation), interactions between acetylated/deacetylated histones and DNA are crucial for gene expression (31). The degree of histone acetylation, regulated by the opposing action of histone acetyltransferases and HDACs, is critical for transcriptional control. HDACs also regulate gene expression through the acetylation of other proteins such as transcription factors (13). There are at least 18 HDAC genes in the human genome: human HDAC-1 (hHDAC-1), hHDAC-2, hHDAC-3, and hHDAC-8 belong to class I hHDACs, and hHDAC-4, hHDAC-5, hHDAC-6, hHDAC-7, hHDAC-9, and hHDAC-10 are class II hHDACs, while SIRT1 to SIRT7 are members of the class III HDAC Sir2 (silent information regulator 2) protein family (16). hHDAC-11 has recently been classified as a separate class (reviewed in reference 40).
Two of five putative deacetylase-encoding genes in P. falciparum have now been partially characterized: PfHDAC-1 (20) (PlasmoDB accession number PFI1260c) and PfSir2 (7, 11) (PlasmoDB accession number PF13_0152). PfHDAC-1 has homology with the class I RPD3 family of HDACs from human (54% sequence identity), chicken, frog, and Saccharomyces cerevisiae (20). The PfSir2 homologue (30) has been shown to bind telomeres and cause gene silencing but over larger distances than yeast Sir2 (55 kb, versus 3 kb in yeast) (11, 29). PfSir2, via histone deacetylation, appears to be involved in the silencing of a multigene family encoding P. falciparum variant surface antigens (var genes) (11). Recent annotation of the complete genome of P. falciparum has revealed additional putative HDAC-encoding gene homologues, although these have not been characterized (PlasmoDB accession numbers PF14_0690, PF10_0078, and PF14_0489) (20).
The fungal metabolites apicidin and trichostatin A (TSA) (4, 5) and recent analogues have been shown to inhibit P. falciparum growth and to cause the hyperacetylation of histones in vitro (6). These compounds are, however, readily metabolized in vivo into inactive forms. Other less potent, but more bioavailable, inhibitors of hHDACs also have antimalarial activity against P. falciparum at low micromolar concentrations (e.g., azelaic bishydroxamic acid [50% inhibitory concentration {IC50}, 3.2 to 4.9 μM]) and suberohydroxamic acid [SBHA] [IC50, 0.8 to 1.3 μM]) (1), with SBHA being apparently cytostatic against the acute rodent malaria organism Plasmodium berghei in mice (1). Although all of these compounds nonselectively inhibit the growth of malarial parasites, cancer cells, and mammalian cells in vitro (12, 21), their antiparasitic activities support the idea that PfHDACs may be valuable new antimalarial targets (4, 5).
In this paper, we describe the properties of new compounds that have been identified with the aid of a homology model to have micromolar-to-nanomolar affinity for the active site of PfHDAC-1. They were examined for (i) antiproliferative activity against drug-resistant (Dd2) and drug-sensitive (3D7) strains of P. falciparum compared with toxicity to human cells, (ii) differential effects on parasite growth at specific stages in the parasite intraerythrocytic life cycle, and (iii) the capacity to induce the hyperacetylation of P. falciparum histones. The antimalarial activities were also compared in vitro with known compounds reported to specifically inhibit mammalian HDACs of classes I, I/II, II and III. The new compounds displayed enhanced antiproliferative potencies against P. falciparum (IC50, 3 to 339 nM) and higher selectivities in killing parasites over mammalian cells (up to 100-fold). These impressive in vitro potencies and selectivities suggest a promising new approach to combating malaria and provide powerful new tools for studying the functions of HDACs in P. falciparum.
MATERIALS AND METHODS
Materials.
TSA and sirtinol were purchased from Sigma (St. Louis, MO). The synthesis and characterization of the various hydroxamate derivatives of 2-aminosuberic acid and cysteine have been reported elsewhere in connection with their antitumor activities (12, 21). The HDAC-1 inhibitor MS-275 (26) was obtained commercially from Calbiochem (EMD Chemicals Inc., Darmstadt, Germany), HDAC-6 inhibitor 2664-12 was synthesized and characterized in-house as described previously (34). All compounds were prepared as stocks in 100% dimethyl sulfoxide (DMSO) at 5 to 20 mM and stored at −20°C. TSA was aliquoted and stored at −80°C. Human red blood cells (O positive) and sera were provided by the Brisbane Red Cross Blood Service. Polyclonal rabbit anti-acetyl histone H4 sera were purchased from Upstate (Millipore, Billerica, MA). These antisera recognize tetra-acetylated H4 and cross-react with P. falciparum H2A (25). Anti-rabbit horseradish peroxidase-conjugated antisera were purchased from Zymed (Invitrogen Corp., Carlsbad, CA). Calf thymus histones were purchased from Sigma (St. Louis, MO).
Homology model of PfHDAC-1.
The 499-amino-acid sequence of PfHDAC-1 (PlasmoDB accession number PFI1260c) was submitted to UniProt (http://www.ebi.uniprot.org/index.shtml), and a WU-Blast2 search of the Protein Data Bank (PDB) was performed. Twelve proteins with >30% identity with PfHDAC-1 were identified. Four of these sequences were too short for useful homology modeling, leaving a yeast HDAC-like protein (HDLP) (PDB accession numbers 1C3R, 1C3S, and 1C3P, all with 31% identity) and hHDAC-8 (accession numbers 1T64, 1T67, 1T69, 1VKG, and 1W22, all with 41% identity). These crystal structures were used as templates for the construction of a homology model using the comparative protein structure modeling program Modeller (http://www.salilab.org/modeler/) within InsightII (Accelrys Software Inc.). The PfHDAC-1 amino acid sequence and template crystal sequences were read into InsightII, and the multiple sequence alignment was used to predict structurally conserved regions and loop regions of the target PfHDAC-1 sequence. The aligned sequences were used in Modeller to create a homology model. Parameters were set to cut overhanging amino acids and generate a high level of accuracy for the model, with each loop region being modeled three times and with the final model containing the loop of best fit. Finally, the homology model of PfHDAC-1 was checked using ProCheck (22) and had an overall average G factor of −0.2 (acceptable scores were >−0.5). The structure was used for flexible ligand docking studies with GOLD.
Ligand docking.
InsightII was used to add the catalytic zinc atom to the homology model of PfHDAC-1 and to construct ligands 1 to 14 and TSA. GOLD (CCDC Software Limited), a genetic algorithm for protein-ligand docking, was used to flexibly dock ligands into the active site of PfHDAC-1 using standard parameters. One distance constraint (1.5 to 3.0 Å) was set between the ligand hydroxamic acid hydroxyl oxygen and the zinc atom of PfHDAC-1. GOLD generated 10 docked conformations for each ligand and ranked their relative binding conformations, and Ludi was used to qualitatively assess ligand fit to the active site of PfHDAC-1.
P. falciparum in vitro growth inhibition assays.
P. falciparum Dd2 (39) and 3D7 (38) were cultured in vitro with O-positive human erythrocytes in RPMI 1640 medium (Gibco BRL) supplemented with 10% heat-inactivated human serum, as previously described (36). Growth inhibition of synchronous ring-stage parasitized erythrocytes starting at 0.25% parasitemia and 2.5% hematocrit was assessed using [3H]hypoxanthine incorporation (1). Chloroquine was included in all assays as an internal control. The final concentrations of each compound required to inhibit [3H]hypoxanthine incorporation by 50% (IC50), in comparison to DMSO controls, was determined by linear interpolation of inhibition curves as described previously (18). Each assay was performed in triplicate wells on at least four separate occasions. Data are presented as IC50s (± standard deviations). To calculate the statistical significance (P ≤ 0.05) of IC50 values between strains, a two-tailed Student's t test (two-sample equal variance) was carried out.
Stage-specific activity assays.
The effect of TSA and compounds 9 and 14 on the growth and development of P. falciparum blood-stage parasites was determined by exposing synchronous-ring-, trophozoite-, or schizont-stage P. falciparum Dd2-infected erythrocytes to 200 nM TSA or 50 and 200 nM of compound 9 or 14. Starting parasitemias varied from 0.2 to 1.0% depending on the experiment. In each case, parasitemia was monitored at different intervals over 2 to 4 days via microscopic examination of Giemsa-stained thin smears. Percent parasitemia was determined for at least 1,000 erythrocytes (counted by two independent persons) at each time point and compared to matched DMSO (<0.05%)-treated vehicle controls. Three independent experiments were carried out (representative results are shown). Mean parasitemias at 48 h after beginning treatment for each treatment group were tested using analysis of variance with the experimental replicate included as a covariate. Significance was determined to be a P value of <0.01.
Histone hyperacetylation.
The effect of test compounds on histone acetylation status was determined by incubating 3 × 109 late-trophozoite-stage Dd2-infected erythrocytes with different concentrations of drug, or vehicle (0.01% DMSO), for 3.5 h at 37°C. Cells were lysed in 0.15% saponin (Sigma) and then washed extensively in phosphate-buffered saline (PBS) (pH 7.4) before preparation of histones (as described in instructions provided with a commercially purchased anti-acetyl histone H4 antiserum [Upstate]). Briefly, parasite pellets were resuspended in 5 volumes of lysis buffer (10 mM HEPES [pH 7.9], 1.5 mM NaCl, 10 mM KCl), and a final concentration of 0.5 M HCl was added. Acid extraction was carried out on ice for 1.5 h, with frequent agitation. Acid-insoluble proteins were pelleted, and the acid-soluble protein fraction was precipitated in 1 volume of acetone at −20°C overnight. Acid-soluble proteins were pelleted, air dried, and resuspended in 50 μl 1× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. Histone extracts (approximately 6 × 108 parasites per lane) were resolved by 15% SDS-PAGE and subjected to silver staining to confirm equal loading. To detect changes in acetylation patterns following treatment with compound, SDS-PAGE-separated samples were transferred onto a polyvinylidene difluoride membrane (Roche) at 80 V for 1 h in 25 mM Tris-0.2 M glycine-20% methanol. Membranes were blocked in PBS containing 3% skim milk powder for at least 1 h before adding anti-acetyl H4 histone antibody (1:2,000 dilution in PBS-3% skim milk powder; Upstate) and incubating the solution for 2 h at room temperature. Following three washes in deionized water, membranes were incubated for 1 h in goat anti-rabbit horseradish peroxidase (1:5,000 dilution in PBS-3% skim milk powder; Zymed). Membranes were again washed, and signal was detected with ECL (GE Healthcare, United Kingdom) and autoradiography.
RESULTS
Effect of inhibitors of hHDACs on P. falciparum growth in vitro.
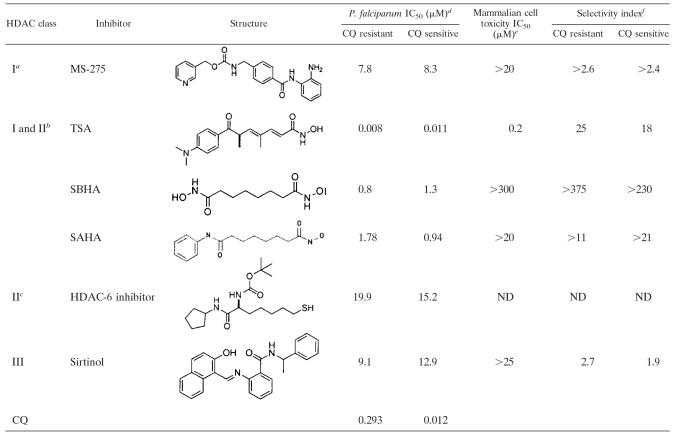
To benchmark this study, we first examined the antiproliferative activities of known inhibitors of class I, class II, and class III mammalian HDACs in vitro against cultured P. falciparum-infected erythrocytes using an isotopic microtest. There are few compounds for which selective action on mammalian HDACs has been reported. The compounds TSA, suberoylanilide hydroxamic acid (SAHA), and SBHA are inhibitors of class I and class II hHDACs and were previously shown to inhibit the growth of drug-resistant (Dd2) and drug-sensitive (3D7) strains of P. falciparum at low micromolar concentrations (1, 4-6) (Table 1), with SBHA being the least potent but displaying a better selectivity index for killing parasites over normal cells than did TSA and SAHA (Table 1). Here, we demonstrate only modest antiproliferative activity (IC50 of 8 to 20 μM) against P. falciparum in vitro for compounds known to be specific inhibitors of hHDAC enzymes of class I (MS-275), class II (2664-12), and class III (sirtinol) (Table 1). MS-275 selectively inhibits hHDAC-1 (26), and compound 2664-12 is a thiolate analogue of an hHDAC-6-selective substrate and is a 40-fold-more-potent inhibitor of hHDAC-6 than hHDAC-1 or hHDAC-4 in enzyme assays (34), while sirtinol is a selective inhibitor of class III mammalian HDACs (15). One of our objectives was to rationally select HDAC inhibitors that might more potently inhibit PfHDACs.
TABLE 1.
Comparative antiproliferative potencies against P. falciparum in vitro for known inhibitors of class I, II, and III hHDAC enzymes
See reference 17.
See reference 34.
P. falciparum line Dd2 is chloroquine (CQ) resistant; P. falciparum line 3D7 is chloroquine sensitive. For SAHA, P. falciparum chloroquine-resistant and -sensitive lines were W2 and D6, respectively (24).
Mammalian cell toxicity was tested against neonatal foreskin fibroblast cells for TSA (26), SBHA (2), and sirtinol (our unpublished results); against Vero cells for SAHA (24); and against WI-38 lung fibroblasts and breast fibroblasts for MS-275 (37).
Selectivity calculated as the difference between P. falciparum and mammalian cell toxicity IC50 values (IC50 of mammalian cells/IC50 of P. falciparum). ND, not determined.
Sequence and structure of the PfHDAC-1 protein.
To develop a homology structural model of PfHDAC-1, we first compared the sequence of PfHDAC-1 with those of mammalian HDACs. hHDAC-1 (61% sequence identity), HDLP (1c3p.pdb) (32% sequence identity), and hHDAC-8 (40% sequence identity) have a well-conserved core deacetylase amino acid sequence in common with PfHDAC-1. Using sequence alignment analysis and crystal structures of HDLP and hHDAC-8 (class I HDACs), we developed a putative structure for PfHDAC-1. ClustalW (35) was used to align the sequences (see Table S1 in the supplemental material). The active-site Zn2+ binding residues in PfHDAC-1 (D174, D262, and H176) were identical in all four proteins. Catalytically important residues identified in HDLP and hHDAC-8 crystal structures were also identical in the four proteins (e.g., H138, H139, D172, D179, and Y301 in PfHDAC-1). The active-site wall from the entrance to the catalytic zinc is lined with identical hydrophobic residues (G147, F148, H176, F203, and Y301 in PfHDAC-1), while PfHDAC-1 Leu269 was conservatively substituted by Met in hHDAC-8.
Sequence alignment of the loop 1 region of PfHDAC-1 (residues 15 to 30), hHDAC-1, and HDLP show good residue conservation, whereas hHDAC-8 has a large insert, P22 to C28, that appeared to produce a longer loop 1, although the crystal structures of HDLP and hHDAC-8 showed the opposite (9, 33). HDLP has a longer loop 1 due to the insertion of HDLP residues H21 and P22 that also appear in homologous regions of PfHDAC-1 and hHDAC-1 but not in hHDAC-8. Therefore, it was expected that PfHDAC-1 loop 1 would be more similar to that of HDLP. Loop 2 of the PfHDAC-1 sequence at N91 to G103 appears to be longer than those of the other proteins. PfHDAC-1 loop 2 has a two-residue insert, at A95 and T96, relative to hHDAC-1 and hHDAC-8. It is also one residue longer than the HDLP sequence, with A95 missing. PfHDAC-1 loop 2 potentially crowds the area around the active-site surface. Loop 5 of PfHDAC-1 at G200 to D211 is less conserved overall, with exceptions being residues F203 and P204, which are either a part of the hydrophobic tunnel or adjacent to it. PfHDAC-1 and hHDAC-1 both have two deletions relative to HDLP and one deletion relative to hHDAC-8. This implies a smaller loop region that is more open at the active site.
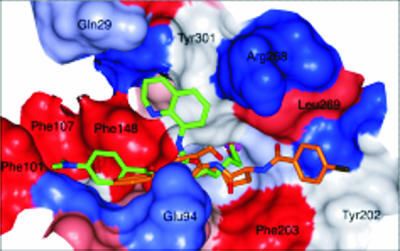
The homology structure has a relatively open binding site and appears to have at least three binding pockets at the surface entrance to the zinc-bound groove (Fig. 1). Using GOLD (19), compounds 1 to 14 (see below) were flexibly docked into the active site of the PfHDAC-1 homology model; the highest-ranking conformation of inhibitors 8 and 13 is shown in Fig. 1. The compounds had predicted binding affinities of 1 μM to 1 nM for PfHDAC-1, suggesting that they would inhibit the enzyme.
FIG. 1.
Homology model of PfHDAC-1 active site (for the hydrophobicity surface, red is hydrophobic, and blue is hydrophilic) with docked inhibitors 8 (orange, carbon atoms) and 13 (green, carbon atoms; blue, nitrogen atoms; red, oxygen atoms), demonstrating three potential binding pockets at the entrance to the active-site tunnel containing the catalytic zinc atom (purple).
Antimalarial efficacy of new HDAC inhibitors.
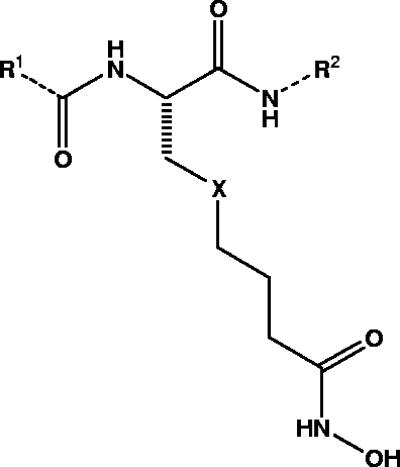
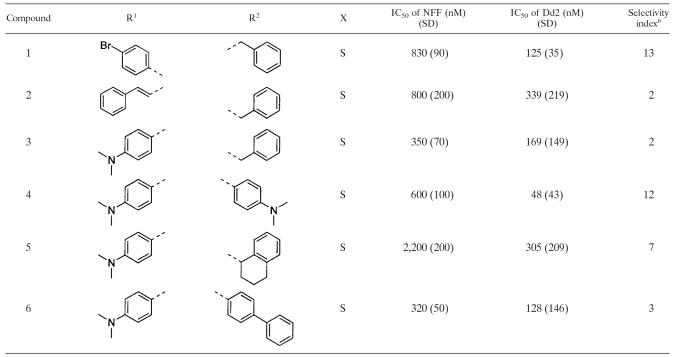
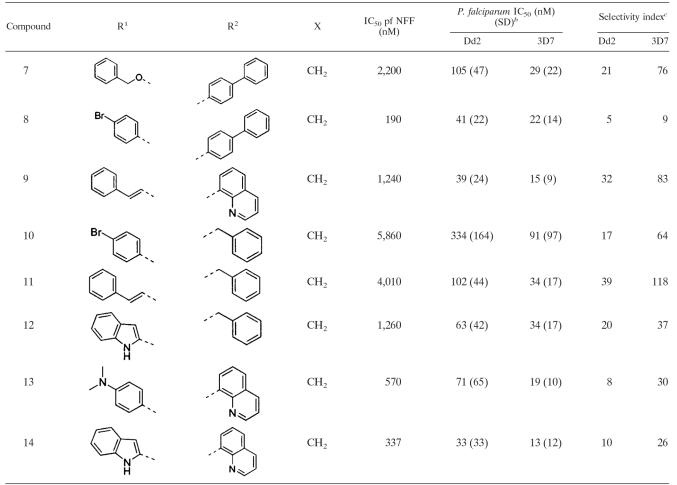
Fourteen nonpeptidic compounds (compounds 1 to 14) derived from cysteine or 2-aminosuberic acid (Fig. 2) were examined for toxicity against a drug-resistant (Dd2) and/or a drug-sensitive (3D7) P. falciparum line in vitro. They were found to exhibit IC50s against strain Dd2 ranging from 33 to 339 nM (Tables 2 and 3). With the exception of compound 10 (Dd2 IC50 of 334 nM), the hydroxamates derived from 2-aminosuberic acid were generally more potent (IC50 of 33 to 105 nM) (Table 3) than those derived from cysteine (IC50 of 48 to 339 nM) (Table 2). This represents up to a ∼100-fold increase in activity compared to that of SBHA (Table 1), which was tested in our previous study (1). These values approach the low nanomolar IC50 of TSA (1, 6). The selectivity of the 2-aminosuberic acid derivatives for P. falciparum parasites over mammalian cells (NNF) (12, 21) was also greater (up to 39-fold) than those derived from cysteine (up to 13-fold) for the Dd2 strain. This prompted us to test the 2-aminosuberic acid derivatives against a second P. falciparum line, 3D7 (Table 3), which is sensitive to chloroquine (Table 1) and other antimalarial drugs. IC50 values for 3D7 ranged from 13 to 91 nM, and with the exception of compounds 7 (P = 0.015) and 11 (P = 0.016), these values were not significantly different from those obtained for Dd2 (P > 0.05).
FIG. 2.
Nonpeptidic compounds derived from 2-aminosuberic acid (X is CH2) and l-cysteine (X is S) (see Tables 2 and 3 for R groups).
TABLE 2.
Comparative toxicities of cysteine derivatives against P. falciparum versus normal cellsa
R1, acid group side chain; R2, amine group side chain; X, atom or group on compound shown in Fig. 1.
Selectivity index was calculated as the difference between P. falciparum and mammalian cells (neonatal foreskin fibroblast cell toxicity IC50 values/IC50 of P. falciparum).
TABLE 3.
Comparative toxicities of 2-aminosuberic acid derivatives against two P. falciparum strains versus a normal cell linea
R1, R2, and X refer to variable groups on the compound shown in Fig. 1.
IC50 values for 3D7 versus Dd2 were significantly different for compounds 7 (P = 0.015) and 11 (P = 0.016) but not for the remaining compounds (P > 0.05).
Selectivity index was calculated as the difference between P. falciparum and mammalian cells (neonatal foreskin fibroblast cell [NFF] toxicity IC50 values/IC50 of P. falciparum).
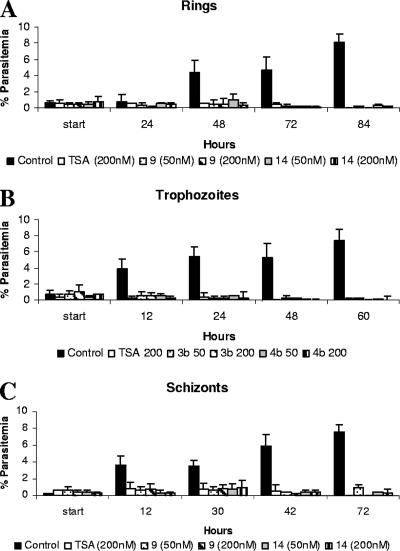
Stage-specific effect of HDAC inhibitors on P. falciparum growth in vitro.
The effect of TSA and compounds 9 and 14 on the growth and development of P. falciparum blood-stage parasites was determined by exposing ring-stage (∼18 h postinvasion), trophozoite-stage (∼26 h postinvasion), or schizont-stage (∼38 h postinvasion) P. falciparum-infected erythrocytes to each compound and monitoring parasitemia over 2 to 4 days. Ring-stage parasites were not able to mature normally into trophozoites and schizonts, even after two complete rounds of asexual reproduction in matched control cultures (Fig. 3A). Similarly, trophozoite-stage parasites were not able to mature into viable schizonts capable of producing invasive merozoites as in untreated, matched control cultures (Fig. 3B). When schizont-stage parasites at ∼38 h postinvasion were treated with TSA or compound 9 or 14, some ring stages were detected after 6 to 12 h but only at relatively low levels compared to those of control cultures. In each case, (ring, trophozoite, and schizont assays), a statistical analysis was carried out on data collected at ∼48 h for three independent experiments, and there was a significant reduction in parasitemia in TSA-, compound 9-, and compound 14-treated groups compared to controls (P < 0.001).
FIG. 3.
Stage-specific effect of TSA and compounds 9 and 14 against P. falciparum-infected erythrocytes. P. falciparum-infected erythrocytes (Dd2) starting at the ring (A), trophozoite (B), or schizont (C) stage were grown in the presence of 200 nM TSA or 50 and 200 nM compound 9 or 14. Parasitemia was determined by microscopic examination of Giemsa-stained thin blood smears over ∼2 to 4 days (representative experiments are shown). Error bars indicate standard deviations between replicate counts on the same slide.
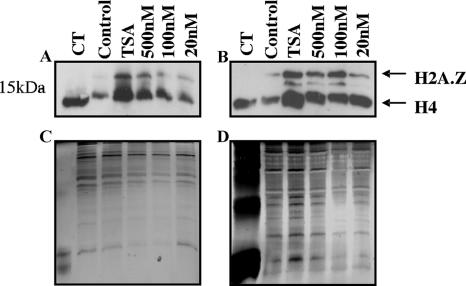
Inhibitors of mammalian HDACs alter histone acetylation in P. falciparum parasites.
Two representative compounds from the 2-aminosuberic acid series, compounds 9 and 14, which display antimalarial potency at low nanomolar concentrations, were tested for their abilities to hyperacetylate histones in P. falciparum parasites (Dd2). Histones prepared from in vitro-cultured, infected erythrocytes treated with each compound (or controls) at various concentrations for 3.5 h were analyzed by Western blotting using a commercial polyclonal anti-tetra-acetyl histone H4 antibody (Upstate). The antibody recognized a single predominant protein of ∼13 kDa in control parasites treated with the vehicle alone (DMSO), which corresponds to H4. The ∼13-kDa band comigrated with control histones from calf thymus (Sigma). These bands represent the natural histone acetylation state of the organism. Exposure of P. falciparum parasites to TSA, compound 9, or compound 14 for 3.5 h resulted in altered parasite histone acetylation profiles compared to those of matched controls, consistent with the inhibition of PfHDAC activity (Fig. 4).
FIG. 4.
Hyperacetylation of P. falciparum histones. P. falciparum-infected erythrocytes (Dd2) were cultured in vitro in the presence of TSA (500 nM) or 500 nM, 100 nM, and 20 nM of compound 9 (A and C) or compound 14 (B and D) for 3.5 h. Matched controls received no drug (control) (0.1% DMSO). Histones were acid extracted in 0.5 M HCl and separated on 15% SDS-polyacrylamide gels. Hyperacetylation was determined by Western blotting using polyclonal anti-tetra-acetyl histone H4 antisera (Upstate) (A and B). Silver staining was carried out as a loading control (C and D). Arrows indicate different histones. CT, calf thymus histones.
DISCUSSION
New, improved treatments for malaria are a high priority to address the global problem of parasite resistance to existing antimalarial drugs (8). HDAC enzymes in malarial parasites may be a promising new target for antimalarial drug development (6). HDAC inhibitors that can arrest growth and induce differentiation and/or apoptotic cell death in various human cancer cell lines also show antimalarial activity in vitro (1, 4-6, 24). These include compounds that are antiproliferative in vitro against P. falciparum at low micromolar-to-nanomolar concentrations but that are rapidly metabolized in vivo (e.g., TSA, apicidin, and trapoxin) (6) and others that are more bioavailable but 10- to 1,000-fold less potent (e.g., azelaic bishydroxamic acid, SAHA, and SBHA) (1). One of these compounds, SAHA, which has modest potency against P. falciparum (IC50 of 0.9 to 1.8 μM) (24) was recently approved by the FDA for the treatment of T-cell lymphomas (14). However, all these inhibitors have relatively poor selectivity for P. falciparum versus normal mammalian cells (1, 4-6, 24) (Table 1).
Toward a better understanding of the role of HDAC inhibitors in P. falciparum, we first examined the antiproliferative properties of known inhibitors of mammalian HDACs against P. falciparum growth in vitro. We found that the specific inhibitors of classes I, II, and III did inhibit P. falciparum growth but were less potent (IC50 of ∼8 to 20 μM) than the more broad-spectrum inhibitors of class I/II hHDAC enzymes (<1 μM) (Table 1). However, none of these known HDAC inhibitors were able to kill the malarial parasite at concentrations much below those that were cytotoxic to normal human cells. This prompted us to examine some structure-activity relationships of alternative HDAC inhibitors with a view to identifying compounds that were more potent and more selective in killing P. falciparum than in killing normal human cells. We generated a homology model of PfHDAC-1 (PlasmoDB accession number PFI1260c) by comparing its protein sequence with those of structurally characterized HDACs. PfHDAC-1 shares >50% amino acid homology with hHDACs, whereas the other putative class I/II PfHDAC homologues (PlasmoDB accession numbers PF14_0690 and PF10_0078) have very limited sequence similarity (20). Furthermore, while PfHDAC-2 is predicted to be expressed in blood stages (www.plasmoDB.org) and may still be a possible target of these compounds, PfHDAC-3 has been shown by mass spectroscopy to be present only in gametocytes (10). The class III HDAC homologue PfSir2 is unlikely to be a target of these compounds, as it can be genetically disrupted in in vitro-cultured P. falciparum parasites (11). Also, we observed no difference in antimalarial IC50 values for compound 9 or 14 for this disruption mutant compared to wild-type parasites (not shown).
By comparing amino acid sequences of PfHDAC-1 and structurally characterized mammalian HDACs, we derived a putative three-dimensional structure for PfHDAC-1 and docked a range of broad-spectrum hHDAC inhibitors into the active site. While quantitative predictions and rankings of ligand affinities in homology structures are notoriously unreliable, we did find that the compounds presented herein had reasonably good molecular fits for the catalytic zinc-bound active site of PfHDAC-1 and were predicted to have micromolar-to-nanomolar affinities for this enzyme. These qualitative predictions were tested in vitro using a proliferative assay for in vitro growth of the malarial parasite P. falciparum in human erythrocytes (Tables 2 and 3), and compounds 7 to 14 in particular were found to have IC50 potencies of 13 to 334 nM against two strains.
The first category of HDAC inhibitor analogues, compounds 1 to 6, were based on a simple cysteine scaffold fused to benzylamine at the C terminus and 4-butanoyl hydroxamate at the S terminus and with different carboxylic acids at the N terminus (Table 2). We have reported antitumor activity at nanomolar concentrations for these inhibitors, which also cause the hyperacetylation of mammalian histones, induce p21 expression, and revert tumor cell morphology to a more normal phenotype (12, 21). The antimalarial IC50s for these compounds ranged from 48 to 339 nM against a multidrug-resistant (Dd2) P. falciparum line (Table 2). Selectivity indices for these compounds ranged from ∼2 to 13, which are similar to those of SAHA (Table 1). The second class of HDAC inhibitors was based on 2-aminosuberic acid, and we have previously found them to be even more selective for tumor cells versus normal cell lines than the compound series derived from l-cysteine (21). We find this class of compounds to be highly potent inhibitors of P. falciparum growth in vitro against multidrug-resistant strain Dd2 (IC50, 33 to 334 nM) and drug-sensitive strain 3D7 (IC50, 13 to 91 nM) of P. falciparum and to display good selectivity for P. falciparum versus a mammalian cell line (Table 3).
In order to determine how these compounds act in the parasite, we examined the effects of two representative compounds from the 2-aminosuberic acid series (compounds 9 and 14) (Table 3) in comparison to those of TSA and untreated controls. In stage-specific growth assays of in vitro-cultured P. falciparum-infected erythrocytes, we found that ring-, trophozoite-, and schizont-stage-infected erythrocytes were all significantly affected by exposure to these compounds. Some new ring forms were observed in schizont cultures following exposure to these compounds after 6 and 12 h but only at low levels. The schizont preparations used were synchronous to a window of about 4 h, and we speculate that the appearance of these ring forms may result from a subpopulation of mature schizonts close to bursting as compound was added and perhaps not affected by these compounds. This will need to be confirmed experimentally in the future. Taken together, these data suggest that these compounds have a broad activity range on asexual P. falciparum forms, although it remains to be seen whether this corresponds to specific activity against PfHDAC and/or a more general cytotoxicity.
We found that compounds 9 and 14 cause the hyperacetylation of P. falciparum histones, using an antibody that recognizes tetra-acetylated H4 (and likely other histone species such as H2A.Z) (23, 25). A similar histone acetylation pattern was observed for controls treated with TSA. Given that hyperacetylation is a marker of HDAC inhibition in mammalian cells and other organisms, including Plasmodium (6, 12, 21), this finding, combined with our in silico modeling studies, suggests that these compounds may act on PfHDAC(s).
Here, we have shown that a series of new compounds derived simply from amino acids (l-cysteine or 2-aminosuberic acid) show potent in vitro activity against P. falciparum-infected erythrocytes. The best antimalarial activity obtained is an order of magnitude greater than that previously reported for synthetic HDAC inhibitors, and unlike most known HDAC inhibitors, some of these compounds are significantly more toxic to P. falciparum parasites than to normal cells. This enhanced selectivity in their killing action makes these inhibitors attractive candidates for further development into antimalarial drugs. Previously, we found that compound 1 had oral bioavailability in rats (12), which augers well for the development of these types of compounds as new antimalarials, with an apparently different mechanism of action from that of established antimalarial drugs. We have also shown that treatment of infected erythrocytes with compounds 9 and 16 results in histone hyperacetylation, which, together with our in silico molecular fitting of these compounds to the active site of PfHDAC-1, supports a mechanism of action involving the inhibition of PfHDAC activity and underscores the potential value of this class of enzymes as possible new pharmaceutical targets in the malaria parasite.
Supplementary Material
Acknowledgments
This work was supported by the Australian Research Council (Federation Fellowship fellowship to D.P.F.), the National Health and Medical Research Council of Australia (development grant to D.P.F.), Griffith University (GURG to K.T.A.), the IMB, and the Queensland Institute of Medical Research. T.N.T. was supported by an ANZ Trustees Medical Research Scholarship.
Blood and sera for P. falciparum culture were provided by the Brisbane Red Cross Blood Service. We thank Michelle Gatton (Queensland Institute of Medical Research) for advice on statistical analysis.
Footnotes
Published ahead of print on 22 January 2008.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Andrews, K. T., A. Walduck, M. J. Kelso, D. P. Fairlie, A. Saul, and P. G. Parsons. 2000. Anti-malarial effect of histone deacetylation inhibitors and mammalian tumour cytodifferentiating agents. Int. J. Parasitol. 30:761-768. [DOI] [PubMed] [Google Scholar]
- 2.Brinkmann, H., A. L. Dahler, C. Popa, M. M. Serewko, P. G. Parsons, B. G. Gabrielli, A. J. Burgess, and N. A. Saunders. 2001. Histone hyperacetylation induced by histone deacetylase inhibitors is not sufficient to cause growth inhibition in human dermal fibroblasts. J. Biol. Chem. 276:22491-22499. [DOI] [PubMed] [Google Scholar]
- 3.Cairns, B. R. 2001. Emerging roles for chromatin remodeling in cancer biology. Trends Cell Biol. 11:S15-21. [DOI] [PubMed] [Google Scholar]
- 4.Colletti, S. L., R. W. Myers, S. J. Darkin-Rattray, A. M. Gurnett, P. M. Dulski, S. Galuska, J. J. Allocco, M. B. Ayer, C. Li, J. Lim, T. M. Crumley, C. Cannova, D. M. Schmatz, M. J. Wyvratt, M. H. Fisher, and P. T. Meinke. 2001. Broad spectrum antiprotozoal agents that inhibit histone deacetylase: structure-activity relationships of apicidin. Part 1. Bioorg. Med. Chem. Lett. 11:107-111. [DOI] [PubMed] [Google Scholar]
- 5.Colletti, S. L., R. W. Myers, S. J. Darkin-Rattray, A. M. Gurnett, P. M. Dulski, S. Galuska, J. J. Allocco, M. B. Ayer, C. Li, J. Lim, T. M. Crumley, C. Cannova, D. M. Schmatz, M. J. Wyvratt, M. H. Fisher, and P. T. Meinke. 2001. Broad spectrum antiprotozoal agents that inhibit histone deacetylase: structure-activity relationships of apicidin. Part 2. Bioorg. Med. Chem. Lett. 11:113-117. [DOI] [PubMed] [Google Scholar]
- 6.Darkin-Rattray, S. J., A. M. Gurnett, R. W. Myers, P. M. Dulski, T. M. Crumley, J. J. Allocco, C. Cannova, P. T. Meinke, S. L. Colletti, M. A. Bednarek, S. B. Singh, M. A. Goetz, A. W. Dombrowski, J. D. Polishook, and D. M. Schmatz. 1996. Apicidin: a novel antiprotozoal agent that inhibits parasite histone deacetylase. Proc. Natl. Acad. Sci. USA 93:13143-13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duraisingh, M. T., T. S. Voss, A. J. Marty, M. F. Duffy, R. T. Good, J. K. Thompson, L. H. Freitas-Junior, A. Scherf, B. S. Crabb, and A. F. Cowman. 2005. Heterochromatin silencing and locus repositioning linked to regulation of virulence genes in Plasmodium falciparum. Cell 121:13-24. [DOI] [PubMed] [Google Scholar]
- 8.Fidock, D. A., P. J. Rosenthal, S. L. Croft, R. Brun, and S. Nwaka. 2004. Antimalarial drug discovery: efficacy models for compound screening. Nat. Rev. Drug Discov. 3:509-520. [DOI] [PubMed] [Google Scholar]
- 9.Finnin, M. S., J. R. Donigian, A. Cohen, V. M. Richon, R. A. Rifkind, P. A. Marks, R. Breslow, and N. P. Pavletich. 1999. Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature 401:188-193. [DOI] [PubMed] [Google Scholar]
- 10.Florens, L., M. P. Washburn, J. D. Raine, R. M. Anthony, M. Grainger, J. D. Haynes, J. K. Moch, N. Muster, J. B. Sacci, D. L. Tabb, A. A. Witney, D. Wolters, Y. Wu, M. J. Gardner, A. A. Holder, R. E. Sinden, J. R. Yates, and D. J. Carucci. 2002. A proteomic view of the Plasmodium falciparum life cycle. Nature 419:520-526. [DOI] [PubMed] [Google Scholar]
- 11.Freitas-Junior, L. H., R. Hernandez-Rivas, S. A. Ralph, D. Montiel-Condado, O. K. Ruvalcaba-Salazar, A. P. Rojas-Meza, L. Mancio-Silva, R. J. Leal-Silvestre, A. M. Gontijo, S. Shorte, and A. Scherf. 2005. Telomeric heterochromatin propagation and histone acetylation control mutually exclusive expression of antigenic variation genes in malaria parasites. Cell 121:25-36. [DOI] [PubMed] [Google Scholar]
- 12.Glenn, M. P., P. Kahnberg, G. M. Boyle, K. A. Hansford, D. Hans, A. C. Martyn, P. G. Parsons, and D. P. Fairlie. 2004. Antiproliferative and phenotype-transforming antitumor agents derived from cysteine. J. Med. Chem. 47:2984-2994. [DOI] [PubMed] [Google Scholar]
- 13.Glozak, M. A., N. Sengupta, X. Zhang, and E. Seto. 2005. Acetylation and deacetylation of non-histone proteins. Gene 363:15-23. [DOI] [PubMed] [Google Scholar]
- 14.Grant, S., C. Easley, and P. Kirkpatrick. 2007. Vorinostat. Nat. Rev. Drug Discov. 6:21-22. [DOI] [PubMed] [Google Scholar]
- 15.Grozinger, C. M., E. D. Chao, H. E. Blackwell, D. Moazed, and S. L. Schreiber. 2001. Identification of a class of small molecule inhibitors of the sirtuin family of NAD-dependent deacetylases by phenotypic screening. J. Biol. Chem. 276:38837-38843. [DOI] [PubMed] [Google Scholar]
- 16.Grozinger, C. M., and S. L. Schreiber. 2002. Deacetylase enzymes: biological functions and the use of small-molecule inhibitors. Chem. Biol. 9:3-16. [DOI] [PubMed] [Google Scholar]
- 17.Hu, E., E. Dul, C. M. Sung, Z. Chen, R. Kirkpatrick, G. F. Zhang, K. Johanson, R. Liu, A. Lago, G. Hofmann, R. Macarron, M. de los Frailes, P. Perez, J. Krawiec, J. Winkler, and M. Jaye. 2003. Identification of novel isoform-selective inhibitors within class I histone deacetylases. J. Pharmacol. Exp. Ther. 307:720-728. [DOI] [PubMed] [Google Scholar]
- 18.Huber, W., and J. C. Koella. 1993. A comparison of three methods of estimating EC50 in studies of drug resistance of malaria parasites. Acta Trop. 55:257-261. [DOI] [PubMed] [Google Scholar]
- 19.Jones, G., P. Willett, R. C. Glen, A. R. Leach, and R. Taylor. 1997. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 267:727-748. [DOI] [PubMed] [Google Scholar]
- 20.Joshi, M. B., D. T. Lin, P. H. Chiang, N. D. Goldman, H. Fujioka, M. Aikawa, and C. Syin. 1999. Molecular cloning and nuclear localization of a histone deacetylase homologue in Plasmodium falciparum. Mol. Biochem. Parasitol. 99:11-19. [DOI] [PubMed] [Google Scholar]
- 21.Kahnberg, P., A. J. Lucke, M. P. Glenn, G. M. Boyle, J. D. Tyndall, P. G. Parsons, and D. P. Fairlie. 2006. Design, synthesis, potency, and cytoselectivity of anticancer agents derived by parallel synthesis from alpha-aminosuberic acid. J. Med. Chem. 49:7611-7622. [DOI] [PubMed] [Google Scholar]
- 22.Laskowski, R. A., M. W. MacArthur, D. S. Moss, and J. M. Thornton. 1993. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26:283-291. [Google Scholar]
- 23.Lin, R., J. W. Leone, R. G. Cook, and C. D. Allis. 1989. Antibodies specific to acetylated histones document the existence of deposition- and transcription-related histone acetylation in Tetrahymena. J. Cell Biol. 108:1577-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mai, A., I. Cerbara, S. Valente, S. Massa, L. A. Walker, and B. L. Tekwani. 2004. Antimalarial and antileishmanial activities of aroyl-pyrrolyl-hydroxyamides, a new class of histone deacetylase inhibitors. Antimicrob. Agents Chemother. 48:1435-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miao, J., Q. Fan, L. Cui, J. Li, J. Li, and L. Cui. 2006. The malaria parasite Plasmodium falciparum histones: organization, expression, and acetylation. Gene 369:53-65. [DOI] [PubMed] [Google Scholar]
- 26.Moradei, O., C. R. Maroun, I. Paquin, and A. Vaisburg. 2005. Histone deacetylase inhibitors: latest developments, trends and prospects. Curr. Med. Chem. Anticancer Agents 5:529-560. [DOI] [PubMed] [Google Scholar]
- 27.Musset, L., O. Bouchaud, S. Matheron, L. Massias, and J. Le Bras. 2006. Clinical atovaquone-proguanil resistance of Plasmodium falciparum associated with cytochrome b codon 268 mutations. Microbes Infect. 8:2599-2604. [DOI] [PubMed] [Google Scholar]
- 28.Ndiaye, D., J. P. Daily, O. Sarr, O. Ndir, O. Gaye, S. Mboup, and D. F. Wirth. 2005. Mutations in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase genes in Senegal. Trop. Med. Int. Health 10:1176-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Renauld, H., O. M. Aparicio, P. D. Zierath, B. L. Billington, S. K. Chhablani, and D. E. Gottschling. 1993. Silent domains are assembled continuously from the telomere and are defined by promoter distance and strength, and by SIR3 dosage. Genes Dev. 7:1133-1145. [DOI] [PubMed] [Google Scholar]
- 30.Scherf, A., L. M. Figueiredo, and L. H. Freitas-Junior. 2001. Plasmodium telomeres: a pathogen's perspective. Curr. Opin. Microbiol. 4:409-414. [DOI] [PubMed] [Google Scholar]
- 31.Schlake, T., D. Klehr-Wirth, M. Yoshida, T. Beppu, and J. Bode. 1994. Gene expression within a chromatin domain: the role of core histone hyperacetylation. Biochemistry 33:4197-4206. [DOI] [PubMed] [Google Scholar]
- 32.Snow, R. W., C. A. Guerra, A. M. Noor, H. Y. Myint, and S. I. Hay. 2005. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434:214-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Somoza, J. R., R. J. Skene, B. R. Katz, C. Mol, J. D. Ho, A. J. Jennings, C. Luong, A. Arvai, J. J. Buggy, E. Chi, J. Tang, B. Sang, E. Verner, R. Wynands, E. M. Leahy, D. R. Dougan, G. Snell, M. Navre, M. W. Knuth, R. V. Swanson, D. E. McRee, and L. W. Tari. 2004. Structural snapshots of human HDAC8 provide insights into the class I histone deacetylases. Structure 12:1325-1334. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki, T., A. Kouketsu, Y. Itoh, S. Hisakawa, S. Maeda, M. Yoshida, H. Nakagawa, and N. Miyata. 2006. Highly potent and selective histone deacetylase 6 inhibitors designed based on a small-molecular substrate. J. Med. Chem. 49:4809-4812. [DOI] [PubMed] [Google Scholar]
- 35.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 37.Ungerstedt, J. S., Y. Sowa, W. S. Xu, Y. Shao, M. Dokmanovic, G. Perez, L. Ngo, A. Holmgren, X. Jiang, and P. A. Marks. 2005. Role of thioredoxin in the response of normal and transformed cells to histone deacetylase inhibitors. Proc. Natl. Acad. Sci. USA 102:673-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walliker, D., I. A. Quakyi, T. E. Wellems, T. F. McCutchan, A. Szarfman, W. T. London, L. M. Corcoran, T. R. Burkot, and R. Carter. 1987. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science 236:1661-1666. [DOI] [PubMed] [Google Scholar]
- 39.Wellems, T., A. Oduola, B. Fenton, R. Desjardins, L. Panton, and V. do Rosario. 1988. Chromosome size and variation occurs in cloned Plasmodium falciparum on in vitro cultivation. Rev. Bras. Genet. 11:813-825. [Google Scholar]
- 40.Yang, X. J., and E. Seto. 2007. HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention. Oncogene 26:5310-5318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.