Abstract
Severe adenovirus infections in transplant recipients undergoing immunosuppressive therapy are of increasing concern. Controversy exists on the contribution of antiviral therapy and the host immune response to recovery from these infections. Here, we established a systemic mouse adenovirus type 1 (MAV-1) infection in cyclophosphamide (CyP)-treated BALB/c mice. CyP was administered at 100 mg per kg of body weight every other day for 2, 3, or 4 weeks, thereby inducing general but reversible leukopenia, with a major suppression of the B-cell numbers and functionality that was more pronounced than that seen with T cells. The outcome of MAV-1 infection was dependent on the duration of CyP therapy, as the mice with the most severe immunosuppression were the most vulnerable to MAV-1-induced hemorrhagic enteritis and mortality. The protective effect of concomitant antiviral therapy with cidofovir depended on the level of immunosuppression. The combination of cidofovir treatment with the withdrawal of immunosuppression was the most successful regimen for increasing survival rates. Survival was clearly correlated with the clearance of virus and increased titers of MAV-1-specific antibodies in sera. In addition, the passive transfer of MAV-1-specific immunoglobulin G into MAV-1-infected SCID BALB/c mice caused a marked delay in mortality, the extent of the delay being dependent on the titer of MAV-1-specific antibodies. Based on the critical role of the humoral immune response in the early defense against disseminated adenovirus infection, the concomitant use of adenovirus-specific immunoglobulins and antiviral therapy should be considered for transplant patients at risk for severe adenovirus infections.
Adenoviruses are common opportunistic pathogens that are rarely associated with severe clinical symptoms in healthy individuals. In contrast, in patients with compromised immunity, adenovirus infections often result in disseminated and potentially life-threatening disease. Among this group are AIDS patients, individuals with hereditary immunodeficiencies, and recipients of bone marrow, solid-organ, or hematopoietic stem cell transplants, the latter accounting for the largest number of severe adenovirus infections (22). Pediatric patients undergoing bone marrow or stem cell transplantations are at three times higher risk for adenovirus infection than their adult counterparts, which may, in part, be explained by the higher incidence of primary infections than of reactivated infections (18). Besides a young age, other reported risk factors for adenovirus infection and disseminated disease include the receipt of a transplant from an unrelated donor, the occurrence of graft-versus-host disease, T-cell depletion of the graft, and the type and extent of immunosuppressive drug treatment (12).
At present, there is no formally approved antiviral therapy for adenovirus infections, nor are there any data from prospective randomized, controlled trials of potentially useful antiadenovirus therapeutics (26). Only two antiviral drugs, i.e., ribavirin and cidofovir, have been used in a number of case studies and a few cohort studies. Treatment with ribavirin has yielded conflicting results and seems to be ineffective in patients who are at high risk for disseminated adenovirus disease (13, 23, 4). Both failures and successes have been described for cidofovir, a potent inhibitor of the replication of several DNA viruses in vitro. Success rates with cidofovir appeared to be highest when antiviral treatment was initiated rapidly after the diagnosis of the infection (4, 15, 17, 24). Unfortunately, the interpretation of the efficacy of antiviral drugs in the treatment of adenovirus infections in the transplantation setting has been hampered by the lack of concomitant data concerning the patient's immunocompetence. Indeed, in several reports, a strong correlation between a positive outcome of adenovirus disease and immunological recovery has been put forward (7, 39, 16), thereby raising the question of whether the immune response and/or antiviral therapy is critical for viral suppression. The reported efficacy of donor leukocyte infusions, along with the fact that the withdrawal of immunosuppression has a beneficial effect on the course of adenovirus infections, points to a potential role for T cells in the immune response to human adenoviruses (6, 19, 7). These findings have provided support for the rationale of adoptive cellular immunotherapy, a strategy that has already been successfully pursued for cytomegalovirus and Epstein-Barr virus infections in the immunocompromised host (30). On the other hand, there is some evidence on the importance of humoral immunity in the protection against adenovirus infection (39, 10, 35). However, as the investigation of the nature of specific immune responses during human adenovirus infections has only recently begun, the relative contributions of virus-specific T cells and virus-neutralizing antibodies in the clearance of adenoviruses are still unclear.
Since adenoviruses are species specific, in vivo models for the study of disseminated adenovirus infections require the use of a nonhuman adenovirus, such as mouse adenovirus type 1 (MAV-1). We previously demonstrated that continued antiviral treatment with cidofovir causes a marked delay in MAV-1-induced disease but cannot prevent a fatal outcome in severe combined immunodeficient (SCID) mice (27). In other studies, mice with genetic deficiencies in specific immunological functions were used to investigate the roles of distinct leukocyte subsets in MAV-1 infection (31, 32). Here, we used cyclophosphamide (CyP) to create a general but reversible immunosuppressive status in MAV-1-infected BALB/c mice, thereby mimicking the clinical situation of immunocompromised patients suffering from opportunistic adenovirus infections. The efficacy of antiviral therapy in this setting of recovering immunity and the effect of the passive transfer of humoral immunity were evaluated.
MATERIALS AND METHODS
Cells and viruses.
The C3H/3T3 mouse embryonic fibroblast cell line was cultured as described previously (27). For the virus neutralization assays, the concentration of heat-inactivated newborn calf serum was reduced to 2%.
MAV-1 strain FL was obtained from the American Type Culture Collection. Virus stocks were prepared in C3H/3T3 cells as described before (27). The titer of the virus stocks, determined by a standard plaque assay, was ∼8 × 105 PFU per ml. For in vivo experiments, MAV-1 particles were purified chromatographically by the Vivapure AdenoPACK purification kit (Sartorius, Vilvoorde, Belgium) (25). The titer of this virus stock was ∼104 PFU per ml. C3H/3T3 cells and virus stocks were free of Mycoplasma contamination, as determined by PCR analysis.
Chemical compounds and antibodies.
The origins of the compounds were as follows: cidofovir [(S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine; Vistide] was obtained from Gilead Sciences (Foster City, CA), and CyP (Endoxan) was obtained from Baxter S.A. (Lessen, Belgium). The hamster monoclonal antibody, directed against the mouse CD3 complex, was prepared from the culture supernatant of 145-2C11 hybridoma cells (28) and tested for the absence of endotoxin. Lipopolysaccharide (LPS) derived from Escherichia coli was purchased from Sigma-Aldrich (Bornem, Belgium). The following antibodies were purchased from BD Biosciences Pharmingen (Erembodegem, Belgium): anti-CD16 and anti-CD32, fluorescein isothiocyanate (FITC)-conjugated anti-CD8, FITC-conjugated anti-CD11b, phycoerythrin (PE)-conjugated anti-CD4, and PE-conjugated anti-Gr-1. PE-conjugated anti-B220 was from eBioscience (Halle, Belgium).
Mice and virus inoculation.
C.B-17/Icr scid/scid (Fox Chase SCID) mice were bred at the Rega Institute under specific-pathogen-free conditions. Female SCID mice were used when they were 5 to 7 weeks old, weighing 16 to 20 g. Female BALB/c mice, 5 weeks old and weighing 15 to 19 g, were obtained from Elevage Janvier (Le Genest Saint Isle, France). All animal experiments were conducted in accordance with the guidelines of the Ethical Committee on Vertebrate Animal Experiments of Katholieke Universiteit Leuven, Leuven, Belgium. For MAV-1 inoculation, mice were given light ether anesthesia and infected intranasally with ∼300 PFU of MAV-1 in a volume of 20 μl of phosphate-buffered saline (PBS).
Induction of immunosuppression.
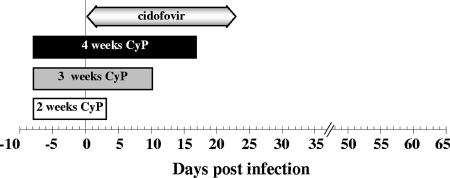
MAV-1-infected BALB/c mice were subjected to immunosuppressive therapy with intraperitoneal (i.p.) injections of CyP at a dose of 100 mg per kg of body weight. CyP treatment was started 8 days before virus inoculation and continued on alternate days for 2 weeks (days −8 to 3) for the CyP-2 group, 3 weeks (days −8 to 10) for the CyP-3 group, or 4 weeks (days −8 to 17) for the CyP-4 group. To assess drug-associated toxicity, uninfected animals were administered CyP according to the same scheme as that described for the virus-infected mice. In addition, the body weights of all mice were recorded daily. In each experiment, two control conditions (untreated and MAV-1 infected and untreated and uninfected) were included.
Antiviral therapy.
CyP-treated BALB/c mice were given antiviral therapy with cidofovir starting 30 min prior to infection. The compound was administered subcutaneously at a dose of 100 mg per kg. Treatment with cidofovir was continued daily for 6 consecutive days postinfection (d.p.i.) and then on alternate days until day 23 postinfection (p.i.). To monitor toxicity associated with the cidofovir-CyP combination therapy, uninfected mice receiving this drug regimen were included and examined daily for clinical signs and body weight changes. Infected, untreated and uninfected, untreated mice were included in each experiment.
Passive transfer of mouse serum immunoglobulin.
A serum immunoglobulin G (IgG) fraction rich in anti-MAV-1 antibodies was obtained by the following immunization protocol. BALB/c mice were immunized by a single intranasal inoculation with MAV-1. At two time points p.i. (15 and 43 d.p.i.), sera were collected by heart puncture. Nonimmune sera were taken from uninfected BALB/c mice. Sera obtained at the same time points were pooled, and the total serum IgG fraction was isolated by affinity purification using the MAbTrap kit (Amersham Biosciences, Diegem, Belgium). The MAV-1-neutralizing IgG titer was determined in the virus neutralization assay described below.
For passive transfer experiments, 1 day before and 9 days after MAV-1 infection, SCID mice were given i.p. injections of ∼100 μg of nonimmune IgG or IgG obtained from MAV-1-infected BALB/c mice (five mice per treatment condition). Infected, untreated and uninfected, untreated mice were included in each experiment.
Virus neutralization assay.
C3H/3T3 cells were seeded into 96-well plates at 17,000 cells per well, and the plates were incubated until confluence was reached. Equal amounts of MAV-1, at ∼4 × 103 PFU per ml, were mixed with serial dilutions of serum samples (diluted at 1:20 to 1:5,120) inactivated by heating (30 min at 56°C) or serum IgG fractions (diluted at 1:2 to 1:781,250), and the mixtures were incubated for 1 h at 37°C. Following incubation, 50-μl aliquots of the mixtures were added to the C3H/3T3 cell monolayers. After 2 h at 37°C, unbound virus was aspirated and replaced by medium (200 μl per well). After 7 days of incubation at 37°C, microscopy was performed to score the virus-induced cytopathic effect. All samples were analyzed in duplicate. Neutralizing antibody titers were expressed as the reciprocal of the highest dilution exerting complete inhibition of the cytopathic effect.
Lymphocyte proliferation assays.
Splenocytes and lymphocytes from pooled spleens or lymph nodes from two mice were prepared as follows. Spleens and lymph nodes (axillary, inguinal, and mesenteric) were harvested, gently cut into small pieces, and passed through cell strainers (Becton Dickinson Labware, Franklin Lakes, NJ). Red blood cells were lysed by two consecutive incubations (5 and 3 min at 37°C) with 0.83% NH4Cl in 0.01 M Tris-HCl, pH 7.2. After washing, the remaining leukocytes were resuspended in cold PBS and cultured in U-bottomed 96-well plates (2 × 105 per well). Cells were incubated in medium, 3 μg of anti-CD3 (αCD3) per ml, or 20 μg of LPS per ml for 48 h at 37°C in 7% CO2. Cultures were pulsed for the last 8 h with 1 μCi of [methyl-3H]thymidine and harvested. The stimulation indices were calculated by dividing the counts per minute from the stimulated cell populations by the counts per minute from unstimulated cell populations, and the final results were expressed as the percentages relative to the control values obtained for normal mice.
Flow cytometry.
Splenocytes and lymphocytes (106) from pooled spleens or lymph nodes obtained as described above were incubated for 15 min with the Fc receptor-blocking antibodies anti-CD16 and anti-CD32. After being washed with PBS containing 2% fetal calf serum, cells were stained for 30 min with FITC-conjugated antibody (anti-CD8-FITC or anti-CD11b-FITC), washed twice, and incubated for 30 min with PE-conjugated antibody (anti-CD4-PE, anti-Gr-1-PE, or anti-B220-PE). Cells were washed, fixed with 0.37% formaldehyde in PBS, and analyzed on a FACScan flow cytometer with Cell Quest software (BD Biosciences).
Quantification of viral DNA.
For the analysis of MAV-1 titers in tissues of infected mice, cardiac perfusion with PBS and dissection were performed and organs were recovered, homogenized, and subjected to DNA extraction according to the tissue protocol of the QIAamp DNA mini kit (Qiagen, Hilden, Germany). MAV-1 DNA was quantified by real-time PCR analysis using an ABI Prism 7000 apparatus (Applied Biosystems, Foster City, CA) and a previously published protocol (27). To correct for experimental variations, the primer-probe set of the 18S genomic endogenous control kit (Eurogentec, Seraing, Belgium) was included in the same PCR mixture as the target primer-probe set. In each individual experiment, standards for the amplification of the MAV-1 hexon gene and the 18S rRNA gene were included and used to convert the respective threshold cycle values for the DNA extracts into the numbers of MAV-1 DNA copies per nanograms of chromosomal DNA. All samples were run in duplicate.
Histopathology and immunohistochemistry.
At predetermined time points, mice were sacrificed and subjected to extensive transcardial perfusion with PBS. From the following organs, parts were fixated in 4% formaldehyde for 24 h and then embedded in paraffin: spleen, kidney, adrenal glands, liver, small intestine, lung, heart, and brain. Tissue sections, 4 μm thick, were stained with hematoxylin and eosin for routine histologic examination and immunohistochemically with a rabbit polyclonal antibody to the MAV-1 E3 class 1 protein by using a standard avidin-biotin immunoperoxidase staining procedure, as previously described (27).
Statistical analysis.
For the statistical analysis of viral DNA titers, P values were calculated with a two-tailed Student t test. For survival analysis, a log rank test, based on a Kaplan-Meier plot, was used. Differences were considered significant at P values of <0.05.
RESULTS
CyP treatment renders BALB/c mice susceptible to MAV-1 infection.
Eight days prior to infection with MAV-1, CyP treatment was started and continued for 2, 3, or 4 weeks (Fig. 1). When CyP treatment was maintained for 3 or 4 weeks, 100% mortality was observed, with mean days of death (± standard deviations) of 18.3 ± 1.1 or 16.7 ± 2.1 d.p.i., respectively. The overall mortality rate decreased to 43% when the duration of CyP therapy was reduced to 2 weeks (mean day of death, 15.0 ± 1.0 d.p.i.). No deaths occurred among nonimmunosuppressed infected BALB/c mice or among uninfected BALB/c mice treated with CyP for 2, 3, or 4 weeks. MAV-1-induced disease manifested by the abrupt onset of lethargy, ruffled fur, anorexia, a hunched posture, an unsteady, mechanical gait, and hyperpnoea. Gross pathological examination revealed no evidence of disease until the onset of clinical illness and showed that all moribund mice succumbed to focal hemorrhagic enteritis.
FIG. 1.
Schedule of treatment of MAV-1-infected BALB/c mice with CyP and/or cidofovir. CyP (100 mg/kg, injected i.p.) was administered on alternate days. Cidofovir (100 mg/kg, injected subcutaneously) was given for 6 consecutive days and thereafter continued on alternate days.
Suppression of leukocyte populations in MAV-1-infected mice treated with CyP.
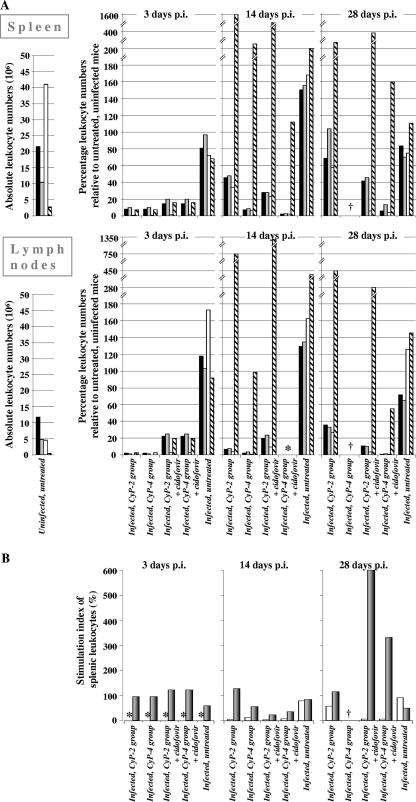
Leukocyte subsets in spleens and lymph nodes from mice receiving CyP were evaluated during the course of MAV-1 infection (Fig. 2A). Three d.p.i. (i.e., 11 days after the commencement of CyP treatment), general leukopenia was observed and was more pronounced in lymph nodes than in spleens. The B cells were more affected than the CD4+, CD8+, and neutrophil cells, the size of the B-cell population being only 0.1 to 1% of that in uninfected, untreated mice. In the CyP-2 group, subpopulations of B and T cells were recovering after the cessation of CyP therapy. Most strikingly, at 14 d.p.i., neutrophil numbers were dramatically increased to 750 and 1,600% of the values for uninfected, untreated spleens and lymph nodes, respectively. This rebound of neutrophils occurred as a consequence of the cessation of CyP therapy and was independent of MAV-1 infection, since similar results for uninfected mice receiving the same immunosuppressive treatment were obtained (data not shown). In the CyP-4 group, the B- and T-cell suppression was maintained throughout the course of infection, although at day 14 p.i., a slight B-cell recovery compared to the marked B-cell suppression at day 3 p.i. was observed. Remarkably, although mice in this group were undergoing sustained immunosuppressive therapy, neutrophil counts approached the normal value at day 14 p.i. By itself, MAV-1 infection appeared to have some temporary effect: at 14 d.p.i., lymph nodes of untreated MAV-1-infected mice contained B-cell and neutrophil numbers amounting to 163 and 433% of the normal values, respectively.
FIG. 2.
Kinetics of leukocyte populations in spleen and lymph nodes and splenic T- and B-cell functionality in MAV-1-infected BALB/c mice undergoing immunosuppressive treatment with CyP. MAV-1-infected mice were subjected to CyP treatment for 2 or 4 weeks with or without antiviral therapy with cidofovir, as depicted in Fig. 1. (A) Flow cytometric examination of CD4+ T cells (black bars), CD8+ T cells (gray bars), B220+ B cells (white bars), and CD11b+ Gr-1+ neutrophils (striped bars) in spleens and lymph nodes. (Left panels) Mean number of leukocyte subtypes in spleens or lymph nodes from uninfected, untreated mice (n = 6). (Right panels) On days 3, 14, and 28 p.i., infected mice (n = 2 per treatment condition) were sacrificed and leukocytes from spleens or lymph nodes were pooled for subsequent analysis. These data are expressed as percentages relative to the corresponding values for uninfected, untreated mice. Note that on day 3 p.i., values for CyP-2 and CyP-4 groups were equal, since conditions for these groups were identical and their leukocytes were pooled. (B) With the same spleens, leukocyte functionality was analyzed in a lymphocyte proliferation assay using αCD3, LPS, or no mitogen. The stimulation index was calculated by dividing the response to mitogen (αCD3, gray bars; LPS, white bars) by the response of unstimulated cell populations and is expressed as the percentage of the value obtained for uninfected, untreated mice. †, no surviving mice; *, not determined.
In addition, we measured the proliferative response of splenic leukocytes toward the T-cell stimulus αCD3 and the B-cell mitogen LPS. CyP treatment severely affected cell proliferation, and the effect was visible even in the absence of mitogen (up to 88% reduction in [methyl-3H]thymidine incorporation compared to the value for untreated, uninfected mice) (data not shown), indicating a general defect of the lymphocytes’ proliferation capacity in CyP-treated mice. Regarding T-cell functionality, the CyP-2 group showed normal T-cell stimulation indices (with αCD3) at all time points (Fig. 2B). When CyP therapy was prolonged (i.e., for the CyP-4 group), this index decreased to 56% of the normal value at 14 d.p.i. A more dramatic functional impairment was seen for the B-cell response to LPS, since at 14 d.p.i., the B-cell stimulation indices in the CyP-2 and CyP-4 groups were only 4 and 12%, respectively, of the value obtained in control mice. By day 28 p.i. (i.e., 25 days after the cessation of CyP therapy), the B-cell proliferation capacity in mice receiving CyP for 2 weeks had started to recover. MAV-1 infection in untreated, asymptomatic mice had marginal (if any) effects on B- and T-cell functionality.
Analysis of pathological, viral, and immunological parameters of MAV-1-induced disease in CyP-treated mice.
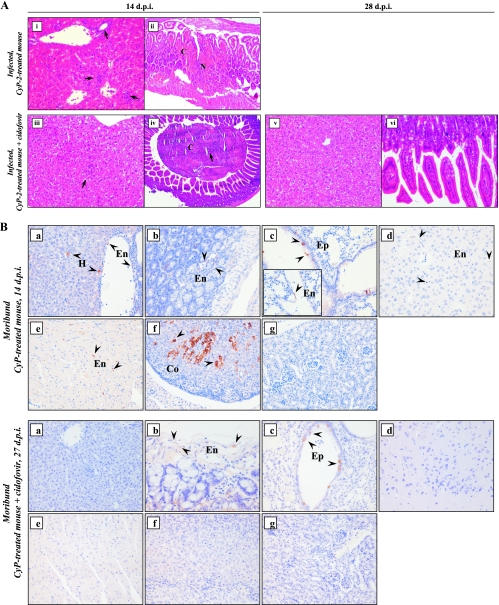
At several time points p.i., mice from each group were sacrificed for detailed histopathologic evaluation. At the time of severe illness, the infected mice showed acute and focal enteritis characterized by hemorrhagic necrosis, which was associated with a neutrophilic infiltrate (Fig. 3A, panel ii). Mice in the CyP-2 group also exhibited prominent portal and parenchymal inflammation in their livers (Fig. 3A, panel i). This was not the case for the CyP-3 and CyP-4 groups, in which occasionally a few inflammatory foci were observed in the livers (data not shown). No pathology in any other tissue was observed (data not shown). Except for kidneys, all organs examined (livers, lungs, brains, hearts, and adrenal and intestinal tissues) stained positive for the MAV-1 E3 antigen, which was consistently detected in the endothelial cells (Fig. 3B, upper panels a to g). In addition, airway epithelia of lungs and parenchymal cells of livers and adrenal glands also stained positive for the viral antigen. In untreated, MAV-1-infected mice, no pathology was observed and the MAV-1 early antigen was not detected in any of the organs examined (data not shown).
FIG. 3.
(A) Hematoxylin and eosin staining of liver (left panels [panels i, iii, and v]) and intestinal (right panels [panels ii, iv, and vi]) tissue sections taken at 14 and 28 d.p.i. from mice receiving CyP treatment for 2 weeks with or without cidofovir, showing representative MAV-1-induced pathology. The arrows indicate neutrophilic infiltrates. Original magnification, ×200. C, congestion; N, necrosis. (B) Immunohistochemical staining for MAV-1 E3 protein in tissues from moribund MAV-1-infected CyP-3 mice at 14 or 27 d.p.i. receiving, respectively, CyP treatment (upper panels) and CyP-plus-cidofovir therapy (lower panels): (a) liver; (b) small intestine; (c) lung; (d) brain; (e) heart; (f) adrenal; and (g) kidney tissue. Infected cells show dark brown cytoplasmic staining for MAV-1 E3 and are indicated by an arrowhead. Original magnification, ×200 (original magnification of the inset in the upper panel c, ×400). Co, cortical cell; En, endothelial cell; Ep, epithelial cell; H, hepatocyte.
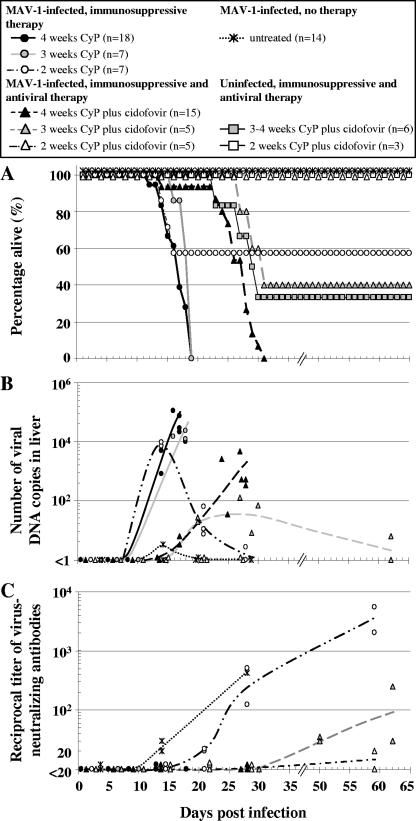
As a marker for viral replication in CyP-treated MAV-1-infected mice, real-time PCR analysis was performed to monitor the viral DNA loads in livers in the period preceding and coinciding with the onset of fatal disease (Fig. 4A and B). Livers were chosen because MAV-1 titers in the livers were representative of viral titers in other organs during MAV-1 dissemination (data not shown). Also, MAV-1 distribution in liver tissue is homogeneous, in contrast to the focal areas of MAV-1 replication in small-intestinal tissue (leading to lethal enteritis), making it difficult to collect a representative specimen from the intestine. Until 9 d.p.i., MAV-1 DNA levels in livers from CyP-treated mice were very low (close to the detection limit). Seven to nine days later, mice from the CyP-3 and CyP-4 groups were moribund and their viral DNA contents reached the maximums (range, 104 to 105 viral genome copies per ng of chromosomal DNA), which were comparable for the two dosing schedules (P = 0.24; two-sided Student's t test). Similar virus titers in moribund mice from the CyP-2 group were seen (P > 0.1 for moribund CyP-2 group mice versus moribund CyP-3 or CyP-4 group mice; two-sided t test). However, in the fraction of mice in this group that survived MAV-1 infection, viral titers then declined to almost undetectable values at 28 d.p.i. In MAV-1-infected mice that were not immunosuppressed, little virus replication occurred during the course of the infection: viral DNA titers passed the detection limit only temporarily at 14 d.p.i.
FIG. 4.
Evolution of overall survival, virus replication, and virus-neutralizing antibody titers in MAV-1-infected, immunocompromised BALB/c mice receiving either antiviral or no therapy. BALB/c mice infected with MAV-1 were treated with CyP and/or cidofovir according to various schemes (see Fig. 1 and Materials and Methods for dosing schedules). (A) Survival curve (the total number of mice for each treatment condition is given in the legend box). (B) Viral DNA loads in livers were measured by means of real-time PCR. Data are presented as the numbers of viral DNA copies per nanogram of chromosomal DNA. (C) Titers of MAV-1-neutralizing antibodies in sera were determined in a virus neutralization assay using 1:20 to 1:5,120 serum dilutions. Symbols in panels B and C represent the data obtained from individual mice, with the curves representing trend lines.
In parallel, the humoral immune response was investigated by measuring the levels of MAV-1-specific antibodies in sera (Fig. 4C). All CyP-treated mice (whether treated for 2, 3, or 4 weeks) that succumbed to MAV-1 disease failed to raise an antibody response. The fraction of mice that had been treated for 2 weeks and that survived MAV-1 infection had developed MAV-1-specific antibodies by 21 d.p.i., the titer rising to 1:5,120 at 62 d.p.i.
Effect of antiviral therapy on MAV-1 infection in CyP-treated mice.
The MAV-1/CyP model was used to evaluate the effect of antiviral therapy with cidofovir, based on its similar activities (50% effective concentration ≈ 2 μM) against MAV-1 and human adenovirus in cell culture (27). In analogy with our previous data from the MAV-1/SCID model, we administered cidofovir at its maximum tolerated dose (100 mg/kg) (27). In MAV-1-infected mice receiving CyP for 3 or 4 weeks, cidofovir caused a significant delay in mortality (Fig. 4A), with P values of <0.005 (log rank test) for the analysis of the survival of the cidofovir-treated groups versus those receiving no antiviral therapy. In the cidofovir-treated CyP-4 group, 100% mortality from MAV-1 infection was recorded, whereas cidofovir reduced the death toll to 60% among the cidofovir-treated CyP-3 group. In the CyP-2 group receiving cidofovir, no clinical symptoms from the infection were visible, resulting in a 100% survival rate. Histopathologic examination revealed that cidofovir delayed MAV-1-induced pathology. At the time CyP-treated mice were dying (14 d.p.i.) (Fig. 3A, upper panels), few (if any) signs of pathology or inflammation in the livers or intestines of mice receiving cidofovir were observed (Fig. 3A, panels iii and iv; the necrotic and purulent debris in the lumen of the intestine suggests transient enteritis). However, 10 days later, in cidofovir-treated mice succumbing to MAV-1 disease, the gross pathology and inflammation in the intestinal tissue and livers were similar to those in the moribund mice receiving no antiviral therapy (data not shown). Cidofovir-treated mice that survived the infection no longer showed pathology (28 d.p.i.) (Fig. 3A, panels v and vi). The detection of the MAV-1 early antigen was confined mainly to lung, liver, and intestinal tissue, and the antigen levels were markedly lower than those in the mice receiving no antiviral therapy (Fig. 3B).
These clinical observations were confirmed by real-time PCR analysis of viral DNA levels (Fig. 4B). Cidofovir clearly delayed viral replication in the CyP-3 and CyP-4 groups. However, even upon continuous antiviral therapy, viral DNA titers finally increased, coinciding with disease evolution. Surprisingly, at the time of death, the viral DNA levels in the mice in these groups were significantly (∼40- to ∼400-fold) lower than those in the corresponding groups receiving no antiviral therapy (P < 0.02; two-sided t test). In the mice of the CyP-3 group that survived because of cidofovir therapy, the virus titers in the livers declined to become almost undetectable at 62 d.p.i. Furthermore, in the cidofovir-treated mice from the CyP-2 group, MAV-1 replication was completely suppressed (Fig. 4B). A more detailed determination of MAV-1 titers in several tissues revealed that, on days 21 and 28 p.i., viral DNA was not detected in any of the organs, except at low levels in the lungs, which probably reflects the presence of viral DNA residues after intranasal inoculation (data not shown).
No MAV-1-neutralizing antibodies were raised in CyP-3 and CyP-4 mice receiving antiviral therapy and succumbing to MAV-1 disease (Fig. 4C). However, survivors in the cidofovir-treated CyP-3 group developed protective antibodies within 50 d.p.i., the titer rising to 1:240 by 62 d.p.i. In cidofovir-treated mice that were immunosuppressed for 2 weeks, antibody titers were practically undetectable, in contrast to the high titers in mice receiving the same CyP treatment but no antiviral therapy.
In mice receiving either CyP (for 2, 3, or 4 weeks) or cidofovir, we observed no serious signs of toxicity. The two-drug combination gave minor toxicity in the CyP-2-plus-cidofovir group, as evidenced by some body weight loss and pronounced leukopenia (Fig. 2A, 28 d.p.i.), yet the T-cell stimulation index was markedly higher after the withdrawal of cidofovir therapy (Fig. 2B, 28 d.p.i.). Possibly, lymphocytes undergo cell cycle inhibition and hence synchronization as a result of the cytostatic side effect of cidofovir (29). Upon drug withdrawal, this effect is reversed, which leads to enhanced lymphocyte proliferation. The toxic effects of the CyP-cidofovir combination therapy were more prominent in the CyP-3 and CyP-4 groups than in the CyP-2 group, resulting in some mortality (Fig. 4A). The mortality curve of this toxicity control coincided with that of MAV-1-infected mice receiving the same two-drug combination. This result means that the mortality in the infected mice was at least partially due to drug toxicity, although this conclusion is contradicted by the relatively high virus titers and the histopathologic evidence of enteritis.
The passive transfer of anti-MAV-1 IgG significantly prolongs the survival of MAV-1-infected SCID mice.
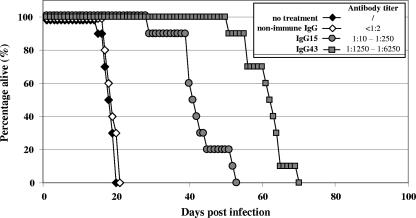
The protective role of humoral immunity during an MAV-1 infection was evaluated by the passive transfer of MAV-1-specific antibodies into MAV-1-infected SCID mice. For this study, we preferred to use SCID mice (rather than CyP-treated mice) because of their pure B- and T-cell deficiencies and because the patterns of pathology induced by MAV-1 in the two models proved to be identical. MAV-1-specific IgG markedly delayed MAV-1-induced morbidity and mortality (Fig. 5), the extent of the delay being dependent on the titer of MAV-1 antibodies in the antiserum (P < 0.0001 for the anti-MAV-1 IgG-treated groups versus the group without treatment; log rank test). IgG from uninfected BALB/c mice had no protective effect. However, all treated mice ultimately succumbed within 70 d.p.i. Histochemical analysis showed that the pathology and virus distribution in moribund IgG-treated SCID mice infected with MAV-1 were comparable to those described previously for untreated infected SCID mice (27), although the enteritis was less pronounced in mice receiving MAV-1-specific IgG therapy. Also, real-time PCR quantification of MAV-1 DNA in liver tissue revealed that virus titers in moribund anti-MAV-1 IgG-treated mice reached levels nearly similar to those in untreated MAV-1-infected SCID mice (data not shown).
FIG. 5.
Suppression of MAV-1 infection in SCID mice receiving virus-specific IgG. Immunocompetent BALB/c mice were challenged with MAV-1, and at 15 and 43 d.p.i., the IgG fractions (designated IgG15 and IgG43, respectively) were isolated from pooled immune sera. Nonimmune IgG was obtained from noninfected BALB/c mice. MAV-1-neutralizing IgG titers were determined by a virus neutralization assay (see inset). Nonimmune IgG, IgG15 (mean titer, 1:130), or IgG43 (mean titer, 1:3,750) was transferred into SCID mice by i.p. injection (in a 200-μl volume) 1 day before and 9 days after infection with MAV-1. Data for each treatment condition were compiled from the results of two independent experiments, each including five mice per group.
DISCUSSION
The MAV-1/CyP model presented here was established to unravel the controversy on the impact of antiviral therapy and/or host immunity on recovery from severe adenovirus infections. As MAV-1 causes lethal infection only in adult BALB/c mice that are immunodeficient (14, 34), CyP was used to induce general but reversible immunosuppression. CyP treatment is one of the most common conditioning regimens prior to bone marrow or hematopoietic stem cell transplantation in leukemia patients. In this regard, CyP has been applied previously for the development of mouse models for severe herpes- and poxvirus infections in the context of immunosuppression (37, 36). This study is the first to demonstrate the course of an MAV-1 infection in CyP-treated mice. Following an intensive CyP regimen for 2, 3, or 4 weeks, MAV-1-infected BALB/c mice succumbed to hemorrhagic gastro-enteritis, with a disease course, virus titers, and patterns of virus dissemination in organs similar to those previously described for MAV-1-infected SCID mice (8, 27). The outcome was dependent on the duration of immunosuppressive therapy, since about half of the mice receiving the shortest CyP treatment were able to clear the infection, in contrast to the 100% mortality rate obtained with the other two regimens. Antiviral treatment with cidofovir proved to be effective, as evidenced by protection against virus lethality or a delay in MAV-1-induced disease, although its effect depended on the level of immunosuppression. In the mice receiving the most profound immunosuppressive therapy, i.e., 4 weeks of CyP, cidofovir treatment was not able to eradicate the virus, since these mice ultimately succumbed to infection despite continued antiviral therapy. Survival was clearly correlated with the clearance of virus and increased titers of MAV-1-specific antibodies in sera after the withdrawal of CyP immunosuppression, supporting the essential role of an active humoral response in controlling an MAV-1 infection. Together, these results demonstrate that the combination of a recovering immune system together with antiviral therapy significantly improves the course of the MAV-1 infection. Early antiviral intervention is necessary to control MAV-1 replication prior to the eradication of the virus by the recovering immune response. These results provide an explanation for our previous findings that sustained cidofovir treatment is not sufficient to protect SCID mice from fatal disseminated MAV-1 disease, due to the lack of an adequate adaptive immune response in the absence of functional B and T cells in these mice (27).
The host immunity was monitored by a time-dependent analysis of leukocyte numbers and lymphocyte functionality in lymphoid tissues from MAV-1-infected mice receiving the minimum or maximum CyP dosing. All leukocyte subsets were affected by CyP therapy, but the strongest suppression was noted for B lymphocytes, since both the absolute numbers and proliferative capacity of B cells were heavily reduced, even after the withdrawal of immunosuppressive therapy. In contrast, CD4+- and CD8+-T-cell populations were also markedly depleted during immunosuppressive treatment, yet their functionality was impaired only when CyP treatment was given beyond 2 weeks. The finding that B cells were much more vulnerable to the cytotoxic effect of CyP than T lymphocytes is in agreement with results in earlier reports (2, 5). The cessation of CyP therapy was associated with a rapid colonization of the spleen by neutrophils, consistent with data from Angulo et al. (2). The observed correlation between the severe B-cell impairment and the occurrence of fatal MAV-1 infections in CyP-treated mice, together with the detection of MAV-1-neutralizing antibodies only in the mice that survived MAV-1 infection, further stresses the importance of an efficient B-cell component in the immune response against MAV-1 infection. This importance was confirmed by our experiments in which the transfer of MAV-1-specific IgG antibodies caused a considerable delay in mortality in infected SCID mice, the delay being significantly more pronounced than that previously seen with antiviral therapy (27). The protective effect of the MAV-1-specific IgG was not durable, since all treated SCID mice eventually succumbed to infection. It is possible that, in the presence of antibodies, MAV-1 established a state of persistence which continued until the titers of passively transferred antibodies were low enough to allow the resumption of viral replication. MAV-1 persistence in brains, spleens, kidneys, and lymph nodes of infected Swiss outbred mice has been documented before (38). Since the half-life of mouse IgG is reported to be about 5 days (40), a ∼30-fold reduction in the IgG titer is achieved after ∼25 days, which is consistent with our observation that the mice receiving high- versus low-titer anti-MAV-1 IgG showed a delay of ∼20 days in their mortality. Our data concur with those in a previous report on MAV-1 infection in mice lacking B cells or having reductions in immunoglobulin in sera that can be counteracted by the transfer of MAV-1-specific antisera. In this study, survival after acute MAV-1 infection was shown to be T-cell independent but relied on the pivotal role of B-cell function in preventing disseminated infection (32). This conclusion was also supported by the results of our preliminary experiments with cyclosporine A, an immunosuppressive drug that impairs T-cell function by blocking interleukin-2 synthesis. Cyclosporine A treatment did not enhance MAV-1 replication, nor did it induce MAV-1 disease.
Our findings may have important consequences for the clinical situation. Although current strategies for the immunotherapy of adenovirus infections in immunocompromised patients are focusing on the recovery of T-cell immunity, our data point to an important role for virus-specific (neutralizing) antibodies in the protection or control of disseminated adenovirus infections. This idea is supported by case reports of severe adenovirus disease in patients with a rare disorder comprising profound antibody deficiency but an intact T-cell arm of immunity (35, 20). Furthermore, of considerable interest is the association between the increased incidence of adenovirus infections in transplant recipients and the use of the monoclonal anti-CD52 antibody alemtuzumab in the conditioning regimen (3, 7, 33), an observation that has led investigators to assign T cells a vital role in the immune response to human adenoviruses. However, alemtuzumab, of which the main use is for the treatment of chronic B-cell lymphocytic leukemia, heavily depletes T cells as well as B lymphocytes (1), indicating that transplant patients receiving alemtuzumab also suffer the severe depression of B-cell function. Further evidence comes from a case report on fatal adenovirus hepatitis after therapy with rituximab (a B-cell-specific monoclonal antibody) (21). Special consideration should therefore be given to the development of adenovirus-specific immunoglobulin as a more convenient approach to reconstitute the patient's immune status than costly, time-consuming, and labor-intensive adoptive cellular immunotherapy strategies for transplant recipients at risk for adenovirus infections. In this context, intravenous immunoglobulin therapy is sometimes included in treatment schedules, yet with various results (9, 11). Nonetheless, although pooled intravenous immunoglobulin batches are relatively low in adenovirus-specific antibody titers, it is possible that the selection of donor material containing high titers of neutralizing antibodies specific for the infectious adenovirus serotype may favorably alter the course of the infection (10).
In conclusion, our data on MAV-1 infection in mice suffering severe yet transient immunosuppression point to an important role for humoral immunity in protection against disseminated adenovirus infection and emphasize the potential of the prophylactic use of virus-specific immunoglobulin. Depending on the host's immune status, cidofovir therapy was shown to effectively suppress viral replication in our MAV-1 model. In the clinical transplantation setting in which immunosuppression cannot be easily tapered, the preemptive use of immunoglobulin preparations containing adenovirus-specific antibodies, concurrent with antiviral therapy, should be considered.
Acknowledgments
We thank Willy Zeegers, Wim van Dam, Omer Rutgeerts, Lieze Wolput, and Katleen Van Meerbeek for their excellent technical assistance.
These investigations were supported by grants from the Flemish Fonds voor Wetenschappelijk Onderzoek (no. G.0267.04) and the Centers of Excellence of the Katholieke Universiteit Leuven (EF/05/15). L. Lenaerts is a research assistant of the Fonds voor Wetenschappelijk Onderzoek.
Footnotes
Published ahead of print on 11 February 2008.
REFERENCES
- 1.Alinari, L., R. Lapalombella, L. Andritsos, R. A. Baiocchi, T. S. Lin, and J. C. Byrd. 2007. Alemtuzumab (Campath-1H) in the treatment of chronic lymphocytic leukemia. Oncogene 26:3644-3653. [DOI] [PubMed] [Google Scholar]
- 2.Angulo, I., F. G. las Heras, J. F. Garcia-Bustos, D. Gargallo, M. A. Munoz-Fernandez, and M. Fresno. 2000. Nitric oxide-producing CD11b+ Ly-6G(Gr-1)+ CD31(ER-MP12)+ cells in the spleen of cyclophosphamide-treated mice: implications for T-cell responses in immunosuppressed mice. Blood 95:212-220. [PubMed] [Google Scholar]
- 3.Avivi, I., S. Chakrabarti, D. W. Milligan, H. Waldmann, G. Hale, H. Osman, K. N. Ward, C. D. Fegan, K. Yong, A. H. Goldstone, D. C. Linch, and S. Mackinnon. 2004. Incidence and outcome of adenovirus disease in transplant recipients after reduced-intensity conditioning with alemtuzumab. Biol. Blood Marrow Transplant. 10:186-194. [DOI] [PubMed] [Google Scholar]
- 4.Bordigoni, P., A. S. Carret, V. Venard, F. Witz, and A. Le Faou. 2001. Treatment of adenovirus infections in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin. Infect. Dis. 32:1290-1297. [DOI] [PubMed] [Google Scholar]
- 5.Cao, L., A. Martin, N. Polakos, and J. A. Moynihan. 2004. Stress causes a further decrease in immunity to herpes simplex virus-1 in immunocompromised hosts. J. Neuroimmunol. 156:21-30. [DOI] [PubMed] [Google Scholar]
- 6.Chakrabarti, S., K. E. Collingham, C. D. Fegan, D. Pillay, and D. W. Milligan. 2000. Adenovirus infections following haematopoietic cell transplantation: is there a role for adoptive immunotherapy? Bone Marrow Transplant. 26:305-307. [DOI] [PubMed] [Google Scholar]
- 7.Chakrabarti, S., V. Mautner, H. Osman, K. E. Collingham, C. D. Fegan, P. E. Klapper, P. A. Moss, and D. W. Milligan. 2002. Adenovirus infections following allogeneic stem cell transplantation: incidence and outcome in relation to graft manipulation, immunosuppression, and immune recovery. Blood 100:1619-1627. [DOI] [PubMed] [Google Scholar]
- 8.Charles, P. C., J. D. Guida, C. F. Brosnan, and M. S. Horwitz. 1998. Mouse adenovirus type-1 replication is restricted to vascular endothelium in the CNS of susceptible strains of mice. Virology 245:216-228. [DOI] [PubMed] [Google Scholar]
- 9.Crooks, B. N., C. E. Taylor, A. J. Turner, H. K. Osman, M. Abinun, T. J. Flood, and A. J. Cant. 2000. Respiratory viral infections in primary immune deficiencies: significance and relevance to clinical outcome in a single BMT unit. Bone Marrow Transplant. 26:1097-1102. [DOI] [PubMed] [Google Scholar]
- 10.Dagan, R., R. H. Schwartz, R. A. Insel, and M. A. Menegus. 1984. Severe diffuse adenovirus 7a pneumonia in a child with combined immunodeficiency: possible therapeutic effect of human immune serum globulin containing specific neutralizing antibody. Pediatr. Infect. Dis. 3:246-251. [DOI] [PubMed] [Google Scholar]
- 11.Emovon, O. E., A. Lin, D. N. Howell, F. Afzal, M. Baillie, J. Rogers, P. K. Baliga, K. Chavin, V. Nickeleit, P. R. Rajagapalan, and S. Self. 2003. Refractory adenovirus infection after simultaneous kidney-pancreas transplantation: successful treatment with intravenous ribavirin and pooled human intravenous immunoglobulin. Nephrol. Dial. Transplant. 18:2436-2438. [DOI] [PubMed] [Google Scholar]
- 12.Feuchtinger, T., P. Lang, and R. Handgretinger. 2007. Adenovirus infection after allogeneic stem cell transplantation. Leuk. Lymphoma 48:244-255. [DOI] [PubMed] [Google Scholar]
- 13.Gavin, P. J., and B. Z. Katz. 2002. Intravenous ribavirin treatment for severe adenovirus disease in immunocompromised children. Pediatrics 110:e9. [DOI] [PubMed] [Google Scholar]
- 14.Guida, J. D., G. Fejer, L. A. Pirofski, C. F. Brosnan, and M. S. Horwitz. 1995. Mouse adenovirus type 1 causes a fatal hemorrhagic encephalomyelitis in adult C57BL/6 but not BALB/c mice. J. Virol. 69:7674-7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartline, C. B., K. M. Gustin, W. B. Wan, S. L. Ciesla, J. R. Beadle, K. Y. Hostetler, and E. R. Kern. 2005. Ether lipid-ester prodrugs of acyclic nucleoside phosphonates: activity against adenovirus replication in vitro. J. Infect. Dis. 191:396-399. [DOI] [PubMed] [Google Scholar]
- 16.Heemskerk, B., A. C. Lankester, T. van Vreeswijk, M. F. Beersma, E. C. Claas, L. A. Veltrop-Duits, A. C. Kroes, J. M. Vossen, M. W. Schilham, and M. J. van Tol. 2005. Immune reconstitution and clearance of human adenovirus viremia in pediatric stem-cell recipients. J. Infect. Dis. 191:520-530. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman, J. A., A. J. Shah, L. A. Ross, and N. Kapoor. 2001. Adenoviral infections and a prospective trial of cidofovir in pediatric hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 7:388-394. [DOI] [PubMed] [Google Scholar]
- 18.Howard, D. S., G. L. Phillips II, D. E. Reece, R. K. Munn, J. Henslee-Downey, M. Pittard, M. Barker, and C. Pomeroy. 1999. Adenovirus infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 29:1494-1501. [DOI] [PubMed] [Google Scholar]
- 19.Hromas, R., K. Cornetta, E. Srour, C. Blanke, and E. R. Broun. 1994. Donor leukocyte infusion as therapy of life-threatening adenoviral infections after T-cell-depleted bone marrow transplantation. Blood 84:1689-1690. [PubMed] [Google Scholar]
- 20.Iyengar, S. R., B. G. Blackburn, and D. B. Lewis. 2007. Severe hypogammaglobulinemia and absent B cells in an adult patient: a case report. J. Allergy Clin. Immunol. 119:S256-. [Google Scholar]
- 21.Iyer, A., R. Mathur, B. V. Deepak, and J. Sinard. 2006. Fatal adenoviral hepatitis after rituximab therapy. Arch. Pathol. Lab. Med. 130:1557-1560. [DOI] [PubMed] [Google Scholar]
- 22.Kojaoghlanian, T., P. Flomenberg, and M. S. Horwitz. 2003. The impact of adenovirus infection on the immunocompromised host. Rev. Med. Virol. 13:155-171. [DOI] [PubMed] [Google Scholar]
- 23.Lankester, A. C., B. Heemskerk, E. C. Claas, M. W. Schilham, M. F. Beersma, R. G. Bredius, M. J. van Tol, and A. C. Kroes. 2004. Effect of ribavirin on the plasma viral DNA load in patients with disseminating adenovirus infection. Clin. Infect. Dis. 38:1521-1525. [DOI] [PubMed] [Google Scholar]
- 24.Legrand, F., D. Berrebi, N. Houhou, F. Freymuth, A. Faye, M. Duval, J. F. Mougenot, M. Peuchmaur, and E. Vilmer. 2001. Early diagnosis of adenovirus infection and treatment with cidofovir after bone marrow transplantation in children. Bone Marrow Transplant. 27:621-626. [DOI] [PubMed] [Google Scholar]
- 25.Lenaerts, L., D. Daelemans, N. Geukens, E. De Clercq, and L. Naesens. 2006. Mouse adenovirus type 1 attachment is not mediated by the coxsackie-adenovirus receptor. FEBS Lett. 580:3937-3942. [DOI] [PubMed] [Google Scholar]
- 26.Lenaerts, L., and L. Naesens. 2006. Antiviral therapy for adenovirus infections. Antivir. Res. 71:172-180. [DOI] [PubMed] [Google Scholar]
- 27.Lenaerts, L., E. Verbeken, E. De Clercq, and L. Naesens. 2005. Mouse adenovirus type 1 infection in SCID mice: an experimental model for antiviral therapy of systemic adenovirus infections. Antimicrob. Agents Chemother. 49:4689-4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leo, O., M. Foo, D. H. Sachs, L. E. Samelson, and J. A. Bluestone. 1987. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc. Natl. Acad. Sci. USA 84:1374-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liekens, S., J. Neyts, E. De Clercq, E. Verbeken, D. Ribatti, and M. Presta. 2001. Inhibition of fibroblast growth factor-2-induced vascular tumor formation by the acyclic nucleoside phosphonate cidofovir. Cancer Res. 61:5057-5064. [PubMed] [Google Scholar]
- 30.Lucas, K. G., Q. Sun, R. L. Burton, A. Tilden, W. P. Vaughan, M. Carabasi, D. Salzman, and A. Ship. 2000. A phase I-II trial to examine the toxicity of CMV- and EBV-specific cytotoxic T lymphocytes when used for prophylaxis against EBV and CMV disease in recipients of CD34-selected/T cell-depleted stem cell transplants. Hum. Gene Ther. 11:1453-1463. [DOI] [PubMed] [Google Scholar]
- 31.Moore, M. L., C. C. Brown, and K. R. Spindler. 2003. T cells cause acute immunopathology and are required for long-term survival in mouse adenovirus type 1-induced encephalomyelitis. J. Virol. 77:10060-10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore, M. L., E. L. McKissic, C. C. Brown, J. E. Wilkinson, and K. R. Spindler. 2004. Fatal disseminated mouse adenovirus type 1 infection in mice lacking B cells or Bruton's tyrosine kinase. J. Virol. 78:5584-5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myers, G. D., R. A. Krance, H. Weiss, I. Kuehnle, G. Demmler, H. E. Heslop, and C. M. Bollard. 2005. Adenovirus infection rates in pediatric recipients of alternate donor allogeneic bone marrow transplants receiving either antithymocyte globulin (ATG) or alemtuzumab (Campath). Bone Marrow Transplant. 36:1001-1008. [DOI] [PubMed] [Google Scholar]
- 34.Pirofski, L., M. S. Horwitz, M. D. Scharff, and S. M. Factor. 1991. Murine adenovirus infection of SCID mice induces hepatic lesions that resemble human Reye syndrome. Proc. Natl. Acad. Sci. USA 88:4358-4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegal, F. P., S. H. Dikman, R. B. Arayata, and E. J. Bottone. 1981. Fatal disseminated adenovirus 11 pneumonia in an agammaglobulinemic patient. Am. J. Med. 71:1062-1067. [DOI] [PubMed] [Google Scholar]
- 36.Smee, D. F., K. W. Bailey, M. H. Wong, M. K. Wandersee, and R. W. Sidwell. 2004. Topical cidofovir is more effective than is parenteral therapy for treatment of progressive vaccinia in immunocompromised mice. J. Infect. Dis. 190:1132-1139. [DOI] [PubMed] [Google Scholar]
- 37.Smee, D. F., R. A. Burger, J. Coombs, J. H. Huffman, and R. W. Sidwell. 1991. Progressive murine cytomegalovirus disease after termination of ganciclovir therapy in mice immunosuppressed by cyclophosphamide treatment. J. Infect. Dis. 164:958-961. [DOI] [PubMed] [Google Scholar]
- 38.Smith, K., C. C. Brown, and K. R. Spindler. 1998. The role of mouse adenovirus type 1 early region 1A in acute and persistent infections in mice. J. Virol. 72:5699-5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Tol, M. J., A. C. Kroes, J. Schinkel, W. Dinkelaar, E. C. Claas, C. M. Jol-van der Zijde, and J. M. Vossen. 2005. Adenovirus infection in paediatric stem cell transplant recipients: increased risk in young children with a delayed immune recovery. Bone Marrow Transplant. 36:39-50. [DOI] [PubMed] [Google Scholar]
- 40.Vieira, P., and K. Rajewsky. 1988. The half-lives of serum immunoglobulins in adult mice. Eur. J. Immunol. 18:313-316. [DOI] [PubMed] [Google Scholar]