Abstract
We previously found that caspofungin synergized with amphotericin B lipid complex in treating murine mucormycosis. We now report a similarly enhanced activity of liposomal amphotericin combined with micafungin or anidulafungin in mice with disseminated mucormycosis. The efficacy of combination echinocandin-polyene therapy for mucormycosis is a class effect.
It is well established that echinocandins have minimal activity against the agents of mucormycosis when tested in vitro by standard techniques (1, 2). However, it is now known that Rhizopus oryzae expresses the target enzyme for echinocandins (4), and in a murine model of disseminated mucormycosis, we previously reported that caspofungin did have limited activity against R. oryzae (4). Furthermore, we found that a combination of caspofungin and amphotericin B lipid complex was synergistic in the treatment of disseminated mucormycosis in diabetic ketoacidotic (DKA) mice (9). While either therapy alone mediated no survival benefit, the combination significantly improved survival (50% survival for the combination versus 0% for placebo, caspofungin alone, or amphotericin B lipid complex alone). To further investigate the potential role of combination therapy in the treatment of mucormycosis, we sought to determine whether the synergy between caspofungin and amphotericin B lipid complex reflected a class effect by testing combinations of two other echinocandins with a different lipid polyene.
BALB/c male mice were rendered diabetic with a single intraperitoneal injection of 210 mg of streptozotocin/kg of body weight in 0.2 ml citrate buffer 10 days prior to fungal challenge, as we have previously described (3-6, 9). Glycosuria and ketonuria were confirmed in all mice 7 to 10 days after streptozotocin injection. DKA mice were infected via the tail vein with Rhizopus oryzae 99-892, a clinical isolate known to be virulent in our model (6, 9). Pilot studies of micafungin or anidulafungin monotherapy were performed to determine the doses to be used in subsequent combination therapy studies. While no monotherapy doses of micafungin or anidulafungin significantly improved survival compared to the placebo dose, micafungin at 1 or 3 mg/kg/day and anidulafungin at 1 or 10 mg/kg/day trended toward benefit (data not shown).
To determine the efficacy of combination therapy, DKA mice were infected with R. oryzae and treated with liposomal amphotericin B (LAmB) (5 mg/kg/day [initially dissolved in water and final concentration made in 5% dextrose water]), micafungin (1 or 3 mg/kg/day in phosphate-buffered saline), anidulafungin (1 or 10 mg/kg/day [initially dissolved in 20% ethanol and final concentration made in 5% dextrose water]), combinations thereof, or placebo (5% dextrose water or 5% dextrose water containing the appropriate amount of ethanol). The LAmB dose was chosen based on previously established low efficacy as monotherapy (6), thereby enabling statistical detection of the potentially enhanced efficacy of combination therapy. Treatments were administered intravenously for 4 days starting 24 h after infection.
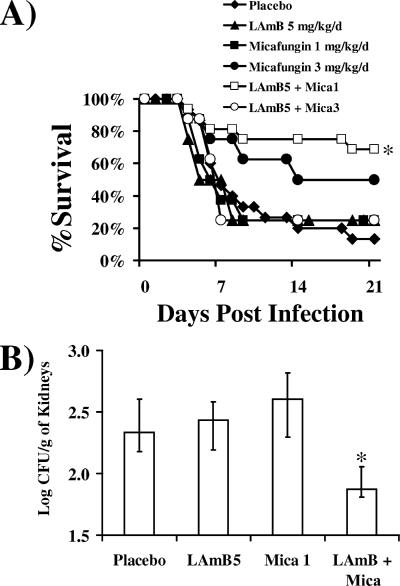
The combination of LAmB and micafungin at 1 mg/kg/day synergistically improved survival compared to either monotherapy arm (Fig. 1A). Combination therapy with micafungin at 3 mg/kg/day was not similarly synergistic, consistent with our previous report of synergy with caspofungin at 1 mg/kg/day but not at 5 mg/kg/day (9). Monotherapy with micafungin at 3 mg/kg/day resulted in more surviving animals than monotherapy with micafungin at 1 mg/kg/day, but the difference in surviving animals or in time to death was not significant (P ≥ 0.1 for both comparisons).
FIG. 1.
Combination therapy of LAmB and micafungin improves survival and reduces kidney fungal burden of DKA mice with mucormycosis. (A) Survival of DKA mice (n = 16 for placebo and combination arms and n = 8 for monotherapy arms) infected with R. oryzae (2.2 × 104 spores). *, P < 0.03 compared to placebo, LAmB, or micafungin by the log rank test. (B) Kidney fungal burden of DKA mice (n = 7 for all arms except for combination arm, which had 8) infected with R. oryzae (2.0 × 104 spores) and treated at 24 h postinfection for three consecutive days. Data are displayed as medians ± interquartile ranges. The y axis reflects the lower limit of detection of the assay. *, P < 0.03 compared to the placebo or monotherapy arms by the Mann-Whitney U test. d, day; Mica, micafungin.
To determine whether combination therapy also reduced kidney fungal burden, as we have previously described for caspofungin (9), DKA mice were again infected with R. oryzae and treated as described above. Mice were sacrificed after 96 h, and kidneys (primary target organ) were gently homogenized, as we have previously described (6), and quantitatively cultured. Combination therapy with micafungin at 1 mg/kg/day significantly reduced tissue fungal burden compared to all other therapies (Fig. 1B).
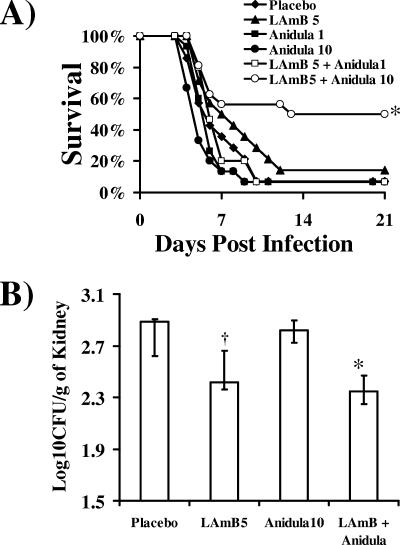
Similarly, the combination of LAmB and anidulafungin synergistically improved survival compared to either monotherapy arm, but the benefit from anidulafungin was seen only at the 10-mg/kg/day dose (Fig. 2A). No benefit of combination therapy was seen at the 1-mg/kg/day dose of anidulafungin. Combination therapy with anidulafungin at 10 mg/kg/day significantly reduced tissue fungal burden compared to therapy with placebo or anidulafungin alone, and LAmB also reduced fungal burden compared to the placebo (Fig. 2B).
FIG. 2.
Combination therapy of LAmB and anidulafungin improves survival and reduces kidney fungal burden of DKA mice with mucormycosis. (A) Survival of DKA mice (n = 16 for two separate experiments with similar results) infected with R. oryzae (2.0 × 104 spores). *, P < 0.05 compared to all other arms by the log rank test. (B) Kidney fungal burden of DKA mice (n = 9) infected with R. oryzae (4.0 × 104 spores) and treated at 24 h postinfection for three consecutive days. Data are displayed as medians ± interquartile ranges. The y axis reflects the lower limit of detection of the assay. *, P < 0.003 compared to placebo or anidulafungin (Anidula) by the Mann-Whitney U test; †, P < 0.05 compared to placebo or anidulafungin by the Mann-Whitney U test.
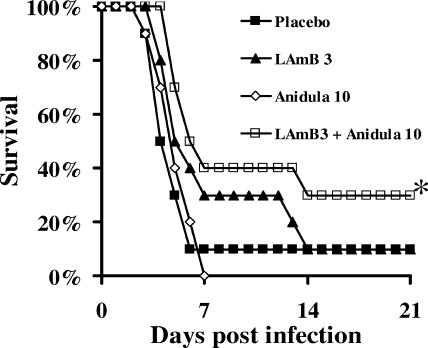
To determine the efficacy of combination therapy in an alternate model, mice were rendered neutropenic by the administration of a single intraperitoneal dose of cyclophosphamide (200 mg/kg). Two days after the treatment (on the day neutropenia began), mice were infected via the tail vein with R. oryzae and treated with placebo, anidulafungin (10 mg/kg/day), LAmB (3 mg/kg/day [based on preliminary data demonstrating limited survival benefit for infected mice treated with this dose]), or a combination for 6 days starting 24 h after infection, reflecting the duration of neutropenia in this model (10). Only mice treated with combination therapy demonstrated survival benefit compared to those treated with the placebo (Fig. 3).
FIG. 3.
Combination therapy of LAmB and anidulafungin improves survival of neutropenic mice (n = 10) infected with R. oryzae (2.1 × 104 spores). *, P = 0.04 compared to placebo by the log rank test. Anidula, anidulafungin.
These data extend our prior findings of the efficacy of the combination of caspofungin and amphotericin B lipid complex therapy for mucormycosis in the same DKA mouse model. The enhanced efficacy of combination therapy of echinocandins and lipid polyenes for murine mucormycosis appears to be a class effect, although optimal doses for this enhanced efficacy differ between caspofungin-micafungin (1 mg/kg/day) and anidulafungin (10 mg/kg/day). It is unclear why a paradoxical loss of efficacy against mucormycosis was seen with higher doses of caspofungin (4, 9) and micafungin but not anidulafungin.
It is not known why echinocandins synergize with polyenes against mucormycosis infections. However, while the effect of combination therapy on tissue fungal burden has varied depending on the polyene and echinocandin used and on the fungal inoculum and technique used to measure fungal burden (quantitative PCR [9] versus colony counts, as used in this study), survival was improved in all experiments. These data suggest that enhanced clearance of fungus is not the predominant mechanism by which combination therapy improves efficacy against mucormycosis. The effect of combination echinocandin-polyene therapy on R. oryzae virulence and on host response (7, 8) to the fungus is under current investigation.
Given the poor outcomes of mucormycosis with current treatments, clinical investigation of the potential for combination echinocandin-polyene therapy to improve survival is warranted.
Acknowledgments
This work was supported by Public Health Service grants R01 AI063503 and R21 AI064716 and research and educational grants from Astellas Pharmaceuticals and Pfizer Inc. to A.S.I. B.S. is supported by Public Health Service grant K08 AI060641, American Heart Beginning grant-in-aid 0665154Y, and the Liu Young Investigator in Biomedical Research. A.S. Ibrahim has received research support from Astellas, Gilead, Enzon, Merck, and Pfizer and has participated in educational programs funded by Astellas. J. E. Edwards, Jr. serves on the scientific advisory boards of Pfizer, Merck, and Gilead, has participated in educational programs regarding fungal infections funded by Pfizer, Merck, and Astellas, and has received research laboratory support from Pfizer, Merck, and Gilead. B. Spellberg has received consulting fees from Pfizer, research support from Astellas, Gilead, Enzon, Merck, and Pfizer, and is on the speakers' bureau for Merck, Pfizer, and Astellas.
The research described in the paper was conducted at the research facilities of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center.
Footnotes
Published ahead of print on 22 January 2008.
REFERENCES
- 1.Del Poeta, M., W. A. Schell, and J. R. Perfect. 1997. In vitro antifungal activity of pneumocandin L-743,872 against a variety of clinically important molds. Antimicrob. Agents Chemother. 41:1835-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Espinel-Ingroff, A. 1998. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J. Clin. Microbiol. 36:2950-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ibrahim, A. S., V. Avanessian, B. Spellberg, and J. E. Edwards, Jr. 2003. Liposomal amphotericin B, and not amphotericin B deoxycholate, improves survival of diabetic mice infected with Rhizopus oryzae. Antimicrob. Agents Chemother. 47:3343-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibrahim, A. S., J. C. Bowman, V. Avanessian, K. Brown, B. Spellberg, J. E. Edwards, Jr., and C. M. Douglas. 2005. Caspofungin inhibits Rhizopus oryzae 1,3-β-d-glucan synthase, lowers burden in brain measured by quantitative PCR, and improves survival at a low but not a high dose during murine disseminated zygomycosis. Antimicrob. Agents Chemother. 49:721-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ibrahim, A. S., J. E. Edwards, Jr., Y. Fu, and B. Spellberg. 2006. Deferiprone iron chelation as a novel therapy for experimental mucormycosis. J. Antimicrob. Chemother. 58:1070-1073. [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim, A. S., T. Gebermariam, Y. Fu, L. Lin, M. I. Husseiny, S. W. French, J. Schwartz, C. D. Skory, J. E. Edwards, Jr., and B. J. Spellberg. 2007. The iron chelator deferasirox protects mice from mucormycosis through iron starvation. J. Clin. Investig. 117:2649-2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinoshita, K., H. Iwasaki, H. Uzui, and T. Ueda. 2006. Candin family antifungal agent micafungin (FK463) modulates the inflammatory cytokine production stimulated by lipopolysaccharide in THP-1 cells. Transl. Res. 148:207-213. [DOI] [PubMed] [Google Scholar]
- 8.Lewis, R. E., G. Chamilos, R. A. Prince, and D. P. Kontoyiannis. 2007. Pretreatment with empty liposomes attenuates the immunopathology of invasive pulmonary aspergillosis in corticosteroid-immunosuppressed mice. Antimicrob. Agents Chemother. 51:1078-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spellberg, B., Y. Fu, J. E. Edwards, Jr., and A. S. Ibrahim. 2005. Combination therapy with amphotericin B lipid complex and caspofungin acetate of disseminated zygomycosis in diabetic ketoacidotic mice. Antimicrob. Agents Chemother. 49:830-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spellberg, B. J., M. Collins, V. Avanesian, M. Gomez, J. E. Edwards, Jr., C. Cogle, D. Applebaum, Y. Fu, and A. S. Ibrahim. 2007. Optimization of a myeloid cell transfusion strategy for infected neutropenic hosts. J. Leukoc. Biol. 81:632-641. [DOI] [PubMed] [Google Scholar]