Abstract
The in vitro and in vivo antifungal activities of T-2307, a novel arylamidine, were evaluated and compared with those of fluconazole, voriconazole, micafungin, and amphotericin B. T-2307 exhibited broad-spectrum activity against clinically significant pathogens, including Candida species (MIC range, 0.00025 to 0.0078 μg/ml), Cryptococcus neoformans (MIC range, 0.0039 to 0.0625 μg/ml), and Aspergillus species (MIC range, 0.0156 to 4 μg/ml). Furthermore, T-2307 exhibited potent activity against fluconazole-resistant and fluconazole-susceptible-dose-dependent Candida albicans strains as well as against azole-susceptible strains. T-2307 exhibited fungicidal activity against some Candida and Aspergillus species and against Cryptococcus neoformans. In mouse models of disseminated candidiasis, cryptococcosis, and aspergillosis, the 50% effective doses of T-2307 were 0.00755, 0.117, and 0.391 mg·kg−1·dose−1, respectively. This agent was considerably more active than micafungin and amphotericin B against candidiasis and than amphotericin B against cryptococcosis, and its activity was comparable to the activities of micafungin and amphotericin B against aspergillosis. The results of preclinical in vitro and in vivo evaluations performed thus far indicate that T-2307 could represent a potent injectable agent for the treatment of candidiasis, cryptococcosis, and aspergillosis.
Invasive mycoses are serious life-threatening infections for immunocompromised patients. Fungal infections are primarily treated with azole derivatives, candin derivatives, or amphotericin B. Azole antifungal agents, such as fluconazole, itraconazole, and voriconazole, are now widely used for the treatment of fungal infections due to their broad-spectrum activities and improved safety profiles. However, there is a great concern because of the increased prevalence of Candida glabrata isolates exhibiting reduced susceptibility to triazole antifungals and of Candida krusei, which has developed an intrinsic resistance to fluconazole and itraconazole due to the empirical use of triazole-based drugs, especially in the case of patients at risk of systemic invasion (1, 9, 19, 27, 28, 29). Candin derivatives, such as micafungin, caspofungin, and anidulafungin (2), exhibit broad-spectrum potent activities against Candida and Aspergillus species and are fungicidal against Candida species. However, they are not active against Cryptococcus neoformans (3). Amphotericin B exhibits broad-spectrum and fungicidal activity; however, due to the significant side effects that it causes, its clinical utility is limited to controlled intravenous administration (24). Therefore, a critical need arises for a new class of antifungal agents that exhibit broad-spectrum activities and novel mechanisms of action.
In order to meet the needs mentioned above, we have focused on arylamidine derivatives that have antifungal potentials. We screened specific compounds from our chemical library and optimized them from the viewpoint of their in vitro and in vivo antifungal activities and in vivo toxicological profiles.
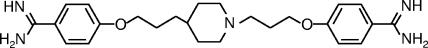
Finally, we selected T-2307, i.e., 4-{3-[1-(3-{4-[amino(imino)methyl]phenoxy}propyl)piperidin-4-yl]propoxy}benzamidine (Fig. 1), as a drug candidate.
FIG. 1.
Chemical structure of T-2307.
This paper deals with the in vitro and in vivo antifungal activities of T-2307 compared with those of other reference antifungal agents.
MATERIALS AND METHODS
In vitro study. (i) Antifungal agents.
T-2307 was synthesized by Toyama Chemical Co. Ltd. (Tokyo, Japan). Fluconazole solution for injection (2 mg/ml) was provided by Toyama Chemical Co. Ltd. Voriconazole, micafungin, and amphotericin B were commercially obtained from Pfizer Japan Inc. (Tokyo, Japan), Astellas Pharma Inc. (Tokyo, Japan), and Bristol-Myers KK (Tokyo, Japan), respectively.
(ii) Organisms.
In total, 216 strains of yeasts and filamentous fungi were used for the in vitro study. Fluconazole-resistant Candida albicans was obtained from the American Type Culture Collection (ATCC).
Clinical isolates of Candida species were obtained from hospitals in Japan. The Teikyo University Institute of Medical Mycology (TIMM) and Institute for Food Microbiology (IFM) strains and clinical isolates of Aspergillus fumigatus and Cryptococcus neoformans strains were kindly provided by Hideyo Yamaguchi (TIMM) and Katsuhiko Kamei (Chiba University Research Center for Pathogenic Fungi and Microbial Toxicoses), respectively.
(iii) Antifungal susceptibility studies.
Broth microdilution testing was performed in accordance with Clinical and Laboratory Standards Institute (CLSI) (formerly the National Committee for Clinical Laboratory Standards) documents M27-A2 (16) and M38-A (17) with RPMI 1640 medium (Sigma-Aldrich Co., St. Louis, MO) buffered to pH 7.0 with 0.165 M 3-(N-morpholino)propanesulfonic acid (MOPS) buffer (Dojindo Laboratories, Kumamoto, Japan) for all organisms except Malassezia furfur. The medium used to test the susceptibility of Malassezia furfur was prepared as described above and was supplemented with glucose (Wako Pure Chemical Industries Ltd., Osaka, Japan), bile salts (Oxoid Limited, Hampshire, United Kingdom), glycerol (Wako Pure Chemical Industries Ltd.), glycerol monostearate (Wako Chemical KK, Osaka, Japan), and Tween 20 (Wako Pure Chemical Industries Ltd.) (26). Stock inoculum suspensions of all yeasts other than Malassezia furfur were obtained from 24-h-old cultures (and from 48-h-old cultures in the case of C. neoformans) grown on potato dextrose agar (PDA; Eiken Chemical Co. Ltd., Tokyo, Japan) at 35°C. A stock inoculum suspension of Malassezia furfur was obtained from 48-h-old cultures grown on Leeming-Notman agar at 35°C by using 0.5% Tween 20 in sterile saline (11). Stock inoculum suspensions of the filamentous fungi were prepared from cultures grown on PDA at 30°C. The final inoculum concentrations of the yeasts and the filamentous fungi ranged from 0.65 × 103 to 2.5 × 103 CFU/ml and 0.87 × 104 to 3.8 × 104 CFU/ml, respectively. The microplates were incubated at 30°C for 24 h and 48 h in the case of Mucor racemosus and Fusarium solani, respectively. For the other strains, the microplates were incubated at 35°C for 24 h (Rhizopus oryzae and Absidia corymbifera), 48 h (Candida species, Malassezia furfur, Saccharomyces cerevisiae, Trichosporon asahii, and Aspergillus species), and 72 h (Cryptococcus neoformans, Pseudallescheria boydii, and Trichophyton rubrum).
In the case of the yeasts, the MICs of T-2307, fluconazole, and voriconazole were recorded as the lowest concentration at which a prominent decrease (approximately 50%) in turbidity relative to the turbidity of the growth control was observed, while the MIC of amphotericin B was read as the lowest concentration at which complete inhibition was observed. In the case of the filamentous fungi, the MICs of T-2307 and fluconazole were recorded as the lowest concentrations at which a prominent decrease in turbidity was observed, while those of voriconazole and amphotericin B were recorded as the lowest concentrations at which complete inhibition was observed. The MIC of micafungin for both yeasts and filamentous fungi was recorded as the lowest concentration at which a prominent decrease in turbidity was observed (8, 20). Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258 were included as quality control strains in each test, as recommended by the CLSI (16, 17).
(iv) MFC.
Following the MIC measurements, the cultures were spread on PDA plates by using spreaders. Leeming-Notman agar plates were used instead of PDA plates in the case of Malassezia furfur. The agar plates were incubated for up to 21 days at 23°C (R. oryzae), 30°C (F. solani), and 35°C (the other species) and were observed at appropriate time points. The minimal fungicidal concentration (MFC) was defined as the lowest concentration at which 99% of the inoculated organisms were killed (8).
In vivo study. (i) Animals.
We used 4-week-old specific-pathogen-free ICR strain male mice (Japan SLC Inc., Shizuoka, Japan) for the in vivo study. The mice were provided food and water ad libitum. All the animal experimental procedures were conducted at Toyama Chemical Co. Ltd. in accordance with the guidelines for the care and use of laboratory animals.
(ii) Systemic infection.
Disseminated candidiasis, cryptococcosis, and aspergillosis were induced by the intravenous inoculation of 0.2 ml of the cell suspension of each test strain via the lateral tail vein. Candida albicans TIMM 1623 and Cryptococcus neoformans ATCC 90112, which were obtained from overnight cultures grown on Sabouraud dextrose agar (Eiken Chemical Co. Ltd.) plates at 35°C and 32°C, respectively, were suspended in sterile saline. The conidia of Aspergillus fumigatus IFM 46895 were prepared by culturing the organisms on PDA for 7 days at 30°C. The conidia were collected in sterile saline containing 0.05% Tween 80 and were stored at −85°C.
Systemic infections with Candida albicans, Cryptococcus neoformans, and Aspergillus fumigatus were induced in neutropenic mice. Transient immunosuppression was induced by intraperitoneal treatment with 200 mg/kg of body weight cyclophosphamide (Shionogi & Co. Ltd., Osaka, Japan) 3 days before the infection and with 100 mg/kg cyclophosphamide 1 day after the infection in the case of Candida albicans and Aspergillus fumigatus and 4 days before and 1 day after the infection in the case of Cryptococcus neoformans. These immunosuppressive conditions were in accordance with those of the method described by Ikeda et al. (7), with slight modifications. The challenge doses of Candida albicans TIMM 1623, Cryptococcus neoformans ATCC 90112, and Aspergillus fumigatus IFM 46895 administered were 3.0 × 104, 9.1 × 104, and 8.4 × 104 CFU/mouse, respectively. For all the experiments, each group comprised 8 to 10 mice, and sterile saline was administered to the control mice. T-2307 and the reference agents were subcutaneously administered once a day for 7 days, beginning at 2 h after the infection. In this study, we adopted the subcutaneous route of administration because repeated administration into the tail vein of mice is difficult. Furthermore, we have confirmed that there is only a marginal difference in the efficacy of subcutaneous and intravenous administration.
The mortality of the mice was recorded daily, and the 50% effective doses (ED50s) were calculated by the probit method, based on the survival rates on day 15 in the case of mice infected with Candida albicans and Aspergillus fumigatus or on day 22 in the case of those infected with Cryptococcus neoformans. The survival curves between the drug-treated and control groups were compared by using log rank tests. Computations for the statistical analysis were carried out by using the statistical analysis system (SAS, version 8.2).
RESULTS
Antifungal spectrum.
Table 1 shows the MICs and MFCs of T-2307 and the reference agents against the various yeasts and filamentous fungi. T-2307 exhibited broad-spectrum and potent activity against a variety of fungal species.
TABLE 1.
MICs and MFCs of T-2307CLH and the reference agents against various fungia
| Strain | MIC/MFC (μg/ml)
|
||||
|---|---|---|---|---|---|
| T-2307 | Fluconazole | Voriconazole | Micafungin | Amphotericin B | |
| Candida albicans ATCC 10261 | 0.001/>64 | 0.125/>64 | 0.0039/>64 | 0.0313/0.25 | 1/2 |
| Candida albicans ATCC 24433 | 0.001/>64 | 0.125/>64 | 0.0078/>64 | 0.0313/0.25 | 1/1 |
| Candida albicans ATCC 90028 | 0.001/>64 | 0.125/>64 | 0.0039/>64 | 0.0313/0.125 | 1/1 |
| Candida albicans TIMM 1623 | 0.001/>64 | 0.125/>64 | 0.0039/>64 | 0.0625/4 | 1/>1 |
| Candida dubliniensis ATCC MYA-577 | 0.001/>64 | 0.125/>64 | 0.0078/>64 | 0.125/8 | 1/2 |
| Candida glabrata ATCC 90030 | 0.0039/>64 | 8/>64 | 0.25/>64 | 0.0313/0.0313 | 1/1 |
| Candida guilliermondii IFO 10279 | 0.002/0.0078 | 4/>64 | 0.0625/0.5 | 0.5/8 | 0.5/1 |
| Candida krusei IFO 0584 | 0.001/0.002 | 32/64 | 0.125/2 | 0.125/0.25 | 2/2 |
| Candida parapsilosis NBRC 10219 | 0.001/>64 | 1/>64 | 0.0313/>64 | 2/>32 | 1/1 |
| Candida tropicalis IFO 1400 | 0.0005/4 | 2/>64 | 0.0625/>64 | 0.0625/>64 | 1/1 |
| Cryptococcus neoformans ATCC 90112 | 0.0078/0.0313 | 1/>64 | 0.0313/>64 | >64/>64 | 1/1 |
| Cryptococcus neoformans ATCC 90113 | 0.0078/0.0625 | 4/>64 | 0.0625/>64 | >64/>64 | 2/2 |
| Cryptococcus neoformans TIMM 0354b | 0.0039/0.0156 | 2/>64 | 0.0313/>64 | >64/>64 | 1/1 |
| Malassezia furfur NBRC 0656c | 0.0313/2 | 4/>64 | 0.0625/>64 | >64/>64 | 0.5/64 |
| Saccharomyces cerevisiae ATCC 24657 | >64/>64 | 8/>64 | 0.125/>64 | 0.5/1 | 1/2 |
| Trichosporon asahii JCM 2466 | 64/>64 | 1/>64 | 0.0625/>64 | >64/>64 | 2/4 |
| Aspergillus flavus NBRC 6343 | 0.5/8 | >64/>64 | 0.5/1 | 0.0625/>64 | 2/2 |
| Aspergillus fumigatus ATCC 16424 | 0.125/>64 | >64/>64 | 0.25/32 | 0.0313/>64 | 1/2 |
| Aspergillus fumigatus IFM 46895 | 0.0625/>64 | >64/>64 | 0.5/4 | 0.0313/>64 | 2/2 |
| Aspergillus fumigatus IFM 49895 | 0.5/>64 | >64/>64 | 2/8 | 0.0313/>64 | 2/2 |
| Aspergillus fumigatus TIMM 0063 | 1/>64 | >64/>64 | 1/1 | 0.0156/>64 | 2/2 |
| Aspergillus nidulans NBRC 33017 | 0.0156/0.0313 | 64/>64 | 0.125/0.25 | 0.0313/>64 | 2/2 |
| Aspergillus niger NBRC 33023 | 0.0625/0.0625 | >64/>64 | 0.5/16 | 0.0313/>64 | 2/2 |
| Aspergillus terreus NBRC 33026 | 0.125/4 | >64/>64 | 0.5/32 | 0.0313/>64 | 2/>64 |
| Fusarium solani IFO 31093d | 0.125/>64 | >64/>64 | 8/8 | >64/>64 | 2/2 |
| Pseudallescheria boydii NBRC 32229b | 4/>64 | 16/>64 | 1/>64 | 0.5/>64 | >64/>64 |
| Rhizopus oryzae NBRC 31005e | >64/>64 | >64/>64 | 8/32 | >64/>64 | 1/1 |
| Absidia corymbifera NBRC4009e | 0.5/>64 | >64/>64 | 32/>64 | 32/>64 | 2/>64 |
| Mucor racemosus NBRC5403d,e | 2/>64 | >64/>64 | >64/>64 | 32/>64 | 1/2 |
| Trichophyton rubrum NBRC 5467b | 2/>64 | 0.25/>64 | 0.0313/>64 | 0.0313/>64 | 1/1 |
Unless indicated otherwise, RPMI 1640-MOPS medium was used, the inoculum sizes were 0.65 × 103 to 2.5 × 103 CFU/ml for the yeasts and 0.87 × 104 to 3.8 × 104 CFU/ml for the filamentous fungi, and incubation was at 35°C for approximately 48 h.
Incubation was for 72 h.
Modified RPMI 1640-MOPS medium was used.
Incubation was at 30°C.
Incubation was for 24 h.
The MICs of T-2307 against most Candida species, Cryptococcus neoformans, Malassezia furfur, and F. solani ranged from 0.0005 to 0.125 μg/ml and were between 2- and 32,000-fold lower than those of fluconazole, voriconazole, micafungin, and amphotericin B. The MFCs of T-2307 against Candida guilliermondii, Candida krusei, and Cryptococcus neoformans were 0.0313 μg/ml or less. The difference between the MIC and MFC of T-2307 against these strains was small, indicating that the action of T-2307 is fungicidal. The MFCs of T-2307 ranged from 2 to >64 μg/ml against Candida albicans, Candida dubliniensis, Candida glabrata, Candida parapsilosis, S. cerevisiae, and Trichosporon asahii. The MFCs of T-2307 for these species were considerably higher than its MICs, indicating that the action of T-2307 is fungistatic.
The MICs of T-2307 against Aspergillus species ranged from 0.0156 to 1 μg/ml and were comparable to those of voriconazole and micafungin. The MFCs of T-2307 against Aspergillus nidulans and Aspergillus niger ranged from 0.0313 to 0.0625 μg/ml, indicating that its action is fungicidal. The MFCs of T-2307 against other Aspergillus species, F. solani, Pseudallescheria boydii, R. oryzae, Absidia corymbifera, Mucor racemosus, and Trichophyton rubrum ranged from 4 to >64 μg/ml and were considerably higher than the corresponding MICs, indicating that that action of T-2307 is fungistatic.
Table 2 shows the MICs of T-2307 and the reference agents against fluconazole-resistant and fluconazole-susceptible-dose-dependent Candida albicans strains. The MICs of T-2307 ranged from 0.0005 to 0.001 μg/ml. T-2307 exhibited no cross-resistance against fluconazole-resistant and fluconazole-susceptible-dose-dependent Candida albicans strains.
TABLE 2.
MICs of T-2307 and the reference agents against fluconazole-resistant and fluconazole-susceptible-dose-dependent Candida albicans strainsa
| Strain | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|
| T-2307 | Fluconazole | Voriconazole | Micafungin | Amphotericin B | |
| ATCC 64124 | 0.001 | >64 | 16 | 0.0625 | 2 |
| ATCC 64550 | 0.0005 | 16 | 0.25 | 0.0625 | 1 |
| ATCC 96901 | 0.001 | >64 | 0.5 | 0.125 | 1 |
| ATCC MYA-573 | 0.001 | >64 | 0.25 | 0.0625 | 2 |
| ATCC MYA-574 | 0.001 | 64 | 0.25 | 0.0625 | 1 |
| ATCC MYA-575 | 0.001 | 16 | 0.0625 | 0.125 | 2 |
| ATCC MYA-1003 | 0.001 | 64 | 0.5 | 0.125 | 2 |
| ATCC MYA-2732 | 0.001 | 16 | 0.25 | 0.125 | 2 |
RPMI 1640-MOPS medium was used, the inoculum size was 1.1 × 103 to 1.5 × 103 CFU/ml, and incubation was at 35°C for approximately 47 h.
Table 3 lists the MIC ranges, MIC50s, and MIC90s of T-2307 against the clinically isolated yeasts and against Aspergillus fumigatus. T-2307 also exhibited potent activity against clinical isolates of Candida species, Cryptococcus neoformans, and Aspergillus fumigatus.
TABLE 3.
MICs of T-2307 and the reference agents against clinical isolates of Candida species, Cryptococcus neoformans, and Aspergillus fumigatus
| Species (no. of isolates) | Antifungal agent | MIC (μg/liter)
|
||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| Candida albicans (40) | T-2307 | 0.00025-0.0039 | 0.0005 | 0.002 |
| Fluconazole | 0.125-0.5 | 0.125 | 0.25 | |
| Voriconazole | 0.0039-0.0156 | 0.0039 | 0.0078 | |
| Micafungin | 0.0313-0.125 | 0.0625 | 0.125 | |
| Amphotericin B | 1-2 | 2 | 2 | |
| Candida glabrata (25) | T-2307 | 0.0039-0.0078 | 0.0039 | 0.0078 |
| Fluconazole | 2-64 | 8 | 16 | |
| Voriconazole | 0.0625-1 | 0.125 | 0.5 | |
| Micafungin | 0.0313-2 | 0.0625 | 0.0625 | |
| Amphotericin B | 1-2 | 1 | 2 | |
| Candida guilliermondii (17) | T-2307 | 0.001-0.0039 | 0.002 | 0.0039 |
| Fluconazole | 2-8 | 4 | 8 | |
| Voriconazole | 0.0313-0.125 | 0.0625 | 0.125 | |
| Micafungin | 0.5-1 | 1 | 1 | |
| Amphotericin B | 0.5-1 | 1 | 1 | |
| Candida krusei (16) | T-2307 | 0.0005-0.002 | 0.001 | 0.002 |
| Fluconazole | 32-64 | 64 | 64 | |
| Voriconazole | 0.25-1 | 0.5 | 0.5 | |
| Micafungin | 0.25-0.5 | 0.5 | 0.5 | |
| Amphotericin B | 2 | 2 | 2 | |
| Candida parapsilosis (20) | T-2307 | 0.00025-0.002 | 0.0005 | 0.0005 |
| Fluconazole | 0.25-8 | 0.5 | 1 | |
| Voriconazole | 0.0078-0.125 | 0.0156 | 0.0313 | |
| Micafungin | 0.5-4 | 2 | 4 | |
| Amphotericin B | 1-2 | 2 | 2 | |
| Candida tropicalis (20) | T-2307 | 0.00025-0.0005 | 0.0005 | 0.0005 |
| Fluconazole | 0.125-8 | 0.5 | 1 | |
| Voriconazole | 0.0156-0.5 | 0.0625 | 0.0625 | |
| Micafungin | 0.0625-0.25 | 0.0625 | 0.125 | |
| Amphotericin B | 1-2 | 1 | 2 | |
| Cryptococcus neoformansb (20) | T-2307 | 0.0078-0.0625 | 0.0156 | 0.0313 |
| Fluconazole | 0.25-4 | 2 | 4 | |
| Voriconazole | 0.0078-0.0625 | 0.0313 | 0.0625 | |
| Micafungin | >64 | >64 | >64 | |
| Amphotericin B | 1 | 1 | 1 | |
| Aspergillus fumigatus (20) | T-2307 | 0.125-4 | 1 | 2 |
| Fluconazole | 64->64 | >64 | >64 | |
| Voriconazole | 0.25-1 | 1 | 1 | |
| Micafungin | 0.0313 | 0.0313 | 0.0313 | |
| Amphotericin B | 1-2 | 2 | 2 | |
Unless indicated otherwise, RPMI 1640-MOPS medium was used, the inoculum sizes were 0.57 × 103 to 2.5 × 103 CFU/ml for the yeasts and 0.4 × 104 to 3.3 × 104 CFU/ml for the filamentous fungi, and incubation was at 35°C for approximately 48 h.
Incubation was for 72 h.
In vivo efficacy in mice.
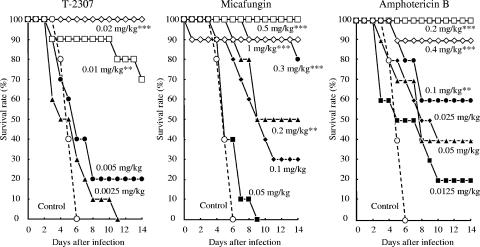
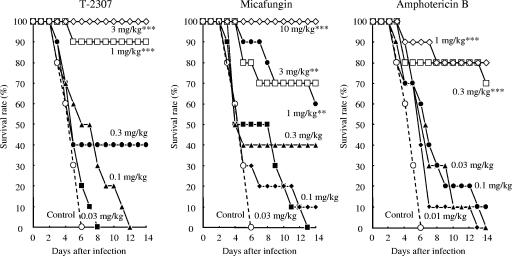
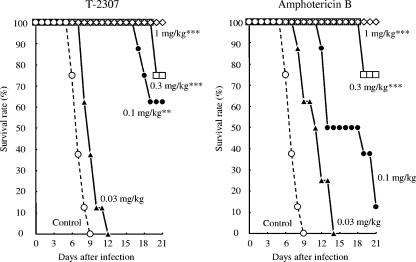
The protective effects of T-2307 on experimental systemic infections caused by Candida albicans, Cryptococcus neoformans, and Aspergillus fumigatus were examined (Fig. 2 to 4 and Table 4). We performed repeated preliminary experiments to define the inoculum size of each strain or to obtain reproducible results. The results reported here represent the final data obtained in these experiments.
FIG. 2.
Therapeutic effects of T-2307, micafungin, and amphotericin B on murine systemic candidiasis caused by Candida albicans TIMM 1623. Mice were intravenously injected with 0.2 ml of a fungal suspension (3.0 × 104 CFU/mouse). T-2307 and the reference agents were subcutaneously administered at 2 h and 1 to 6 days after the infection. Statistical analysis was performed by using the Kaplan-Meier log rank test against the results for the control group (**, P < 0.01; ***, P < 0.001).
FIG. 4.
Therapeutic effects of T-2307, micafungin, and amphotericin B in murine systemic aspergillosis caused by Aspergillus fumigatus IFM 46895. Mice were intravenously injected with 0.2 ml of a fungal suspension (8.4 × 104 CFU/mouse). The agents were subcutaneously administered at 2 h and 1 to 6 days after the infection. Statistical analysis was performed by using the Kaplan-Meier log rank test against the results for the control group (**, P < 0.01; ***, P < 0.001).
TABLE 4.
Therapeutic effects of T-2307 and the other antifungal agents against murine systemic infections caused by Candida albicans TIMM 1623, Cryptococcus neoformans ATCC 90112, and Aspergillus fumigatus IFM46895a
| Strain | Agents | ED50 (mg·kg−1· dose−1) | 95% confidence limit |
|---|---|---|---|
| Candida albicans TIMM | T-2307 | 0.00755 | 0.00562-0.0102 |
| 1623b | Micafungin | 0.182 | 0.122-0.255 |
| Amphotericin B | 0.0466 | 0.0238-0.0798 | |
| Cryptococcus neoformans | T-2307 | 0.117 | 0.0608-0.212 |
| ATCC 90112c | Amphotericin B | 0.199 | 0.115-0.354 |
| Aspergillus fumigatus | T-2307 | 0.391 | 0.241-0.630 |
| IFM 46895d | Micafungin | 0.699 | 0.352-1.42 |
| Amphotericin B | 0.289 | 0.162-0.539 |
Treatment was by subcutaneous administration at 2 h and 1 to 6 days after infection.
Inoculum size, 3.0 × 104 CFU/mouse.
Inoculum size, 9.1 × 104 CFU/mouse.
Inoculum size, 8.4 × 104 CFU/mouse.
The MICs of T-2307 and the reference agents against Candida albicans TIMM 1623, Cryptococcus neoformans ATCC 90112, and Aspergillus fumigatus IFM 46895 are shown in Table 1.
In the systemic infection caused by Candida albicans (Fig. 2), all the control mice died by day 6. Mortality was significantly delayed in mice that were administered T-2307 at a dose of 0.01 mg·kg−1·dose−1 compared with that in the control mice. This dose was considerably lower than the doses of micafungin (0.2 mg·kg−1·dose−1) and amphotericin B (0.1 mg·kg−1·dose−1) administered. The calculated ED50s of T-2307, micafungin, and amphotericin B were 0.00755, 0.182, and 0.0466 mg·kg−1·dose−1, respectively. T-2307 exhibited a superior protective effect over those of micafungin and amphotericin B against systemic candidiasis in mice (Table 4).
In the systemic infection caused by Cryptococcus neoformans (Fig. 3), all the control mice died by day 9. However, mortality was significantly delayed in mice administered T-2307 at a dose of 0.1 mg·kg−1·dose−1 compared with that in the control mice. This dose was lower than the dose of amphotericin B (0.3 mg·kg−1·dose−1) administered. The calculated ED50s of T-2307 and amphotericin B were 0.117 and 0.199 mg·kg−1·dose−1, respectively. T-2307 exhibited a superior protective effect over that of amphotericin B against systemic cryptococcosis in mice (Table 4). Under the same treatment regimen, the ED50 of fluconazole was >20 mg·kg−1·dose−1 (data not shown).
FIG. 3.
Therapeutic effects of T-2307 and amphotericin B on murine systemic cryptococcosis caused by Cryptococcus neoformans ATCC 90112. Mice were intravenously injected with 0.2 ml of a fungal suspension (9.1 × 104 CFU/mouse). The agents were subcutaneously administered at 2 h and 1 to 6 days after the infection. Statistical analysis was performed by using the Kaplan-Meier log-rank test against the results for the control group (**, P < 0.01; ***, P < 0.001).
In the systemic infection caused by Aspergillus fumigatus (Fig. 4), all the control mice died by day 6. However, mortality was significantly delayed in mice that were administered T-2307 at a dose of 1 mg·kg−1·dose−1 compared with that in the control mice. The calculated ED50s of T-2307, micafungin, and amphotericin B were 0.391, 0.699, and 0.289 mg·kg−1·dose−1, respectively (Table 4). T-2307 was slightly less active than amphotericin B but exhibited a superior therapeutic effect compared with that of micafungin against systemic aspergillosis in mice.
DISCUSSION
In the present study, we investigated the in vitro and in vivo antifungal activities of T-2307, a novel arylamidine, using fluconazole, voriconazole, micafungin, and amphotericin B as reference antifungal agents.
T-2307 exhibited broad-spectrum and potent activity against clinically significant pathogenic fungi. An important characteristic of T-2307 was its potent activity against Candida species, including azole-resistant Candida albicans, Cryptococcus neoformans, Malassezia furfur, and F. solani. The in vitro activity of T-2307 was far superior to the activities of fluconazole, voriconazole, micafungin, and amphotericin B. Moreover, T-2307 was active against Aspergillus species, and its in vitro activity against these species was comparable to the activities of micafungin and voriconazole.
In mouse models of disseminated infection, the activity of T-2307 was considerably greater than the activities of micafungin and amphotericin B against candidiasis and the activity of amphotericin B against cryptococcosis, and it was comparable to the activities of micafungin and amphotericin B against aspergillosis.
A previous study that compared the therapeutic effects of fluconazole and micafungin in a model of Candida albicans infection has reported the ED50 of fluconazole to range from 2.15 to 4.51 mg·kg−1·dose−1 (7). Although the ED50 of fluconazole cannot be directly compared with that of T-2307, it can definitely be speculated that T-2307 has a superior effect compared with that of fluconazole because the previously reported ED50 of micafungin (0.14 to 0.21 mg·kg−1·dose−1) is almost consistent with our results (0.182 mg·kg−1·dose−1) (7).
In a previous study, T-2307 exhibited good in vivo efficacy in models of intracerebral cryptococcal infection and disseminated Candida glabrata infection (17a, 29a). In addition, T-2307 exhibited good in vivo efficacy in a model of disseminated Aspergillus infection caused by other strains, with a higher MIC (6a).
We consider that this typical potent antifungal activity of T-2307, which showed no cross-resistance against drug-resistant or intrinsic drug-resistant strains, arises due to its mechanism of action.
At present, the detailed mechanism of action of T-2307 remains unknown. T-2307 belongs to a class of aromatic diamidines, similar to pentamidine, which is used to treat pneumocystosis, leishmaniasis, and trypanosomiasis. Recently, aromatic diamidine derivatives similar to pafuramidine (DB289) (23, 30), which is a prodrug of furamidine (DB75), have been demonstrated to have good efficacy against African trypanosomiasis, Pneumocystis jirovecii pneumonia, and malaria.
Although several mechanisms for the action of pentamidine against different microorganisms have been suggested (6, 4, 21, 22), its detailed mechanism of action is not understood to date. Pentamidine has been shown to be selectively bound in the minor groove of AT-rich DNA duplexes (5, 10, 18); however, its antimicrobial activities are not proven to be due to DNA binding (25). Pentamidine has also been demonstrated to inhibit the self-splicing of various group I introns in vitro, including the nuclear group I introns present in the rRNA genes of Pneumocystis carinii (13, 14), the Ca.LSU nuclear group I intron in the 26S rRNA gene of Candida albicans (15), and the mitochondrial introns of S. cerevisiae (31).
Recently, Lanteri et al. (12) reported the mechanisms of action of DB75. They observed that S. cerevisiae cells grown in a medium containing glycerol, which is the nonfermentative carbon source, were more sensitive to the growth-inhibitory effects of DB75 or pentamidine than cells grown in a medium containing dextrose, suggesting that DB75 and pentamidine inhibit the mitochondrial functions of cells.
Similarly, in a study of T-2307, S. cerevisiae cells cultured in a medium containing glycerol were more susceptible to the growth-inhibitory effects of T-2307 than those cultured in a medium containing dextrose (22a). Therefore, we now consider that one of the mechanisms of action of T-2307 may be similar to that of DB75 or pentamidine. In future, further studies will be required to elucidate the detailed mechanisms of action of T-2307.
In conclusion, T-2307 is a novel antifungal agent that exhibits potent antifungal and growth-inhibitory activities against pathogenic yeasts and filamentous fungi. These characteristics suggest that T-2307 could be useful for the treatment of fungal infections.
Footnotes
Published ahead of print on 28 January 2008.
REFERENCES
- 1.Abi-Said, D., E. Anaissie, O. Uzun, I. Raad, H. Pinzcowski, and S. Vartivarian. 1997. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin. Infect. Dis. 24:1122-1128. [DOI] [PubMed] [Google Scholar]
- 2.Cappelletty, D., and K. Eiselstein-McKitrick. 2007. The echinocandins. Pharmacotherapy 27:369-388. [DOI] [PubMed] [Google Scholar]
- 3.Denning, D. W. 2003. Echinocandin antifungal drugs. Lancet 362:1142-1151. [DOI] [PubMed] [Google Scholar]
- 4.Dykstra, C. C., and R. R. Tidwell. 1991. Inhibition of topoisomerases from Pneumocystis carinii by aromatic dicationic molecules. J. Protozool. 38:78S-81S. [PubMed] [Google Scholar]
- 5.Edwards, K. J., T. C. Jenkins, and S. Neidle. 1992. Crystal structure of a pentamidine-oligonucleotide complex: implications for DNA-binding properties. Biochemistry 31:7104-7109. [DOI] [PubMed] [Google Scholar]
- 6.Grady, R. W., S. H. Blobstein, S. R. Meshnick, P. C. Ulrich, A. Cerami, J. Amirmoazzami, and E. M. Hodnett. 1984. The in vitro trypanocidal activity of N-substituted p-benzoquinone imines: assessment of biochemical structure-activity relationships using the Hansch approach. J. Cell. Biochem. 25:15-29. [DOI] [PubMed] [Google Scholar]
- 6a.Hashimoto, K., E. Yamada, H. Nishikawa, N. Oogake, Y. Ito, N. Nomura, K. Hayashi, J. Mitsuyama, and N. Terashima. 2005. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-486.
- 7.Ikeda. F., Y. Wakai, S. S. Matsumoto, K. Maki, E. Watabe, S. Tawara, T. Goto, Y. Watanabe, F. Matsumoto, and S. Kuwahara. 2000. Efficacy of FK463, a new lipopeptide antifungal agent, in mouse models of disseminated candidiasis and aspergillosis. Antimicrob. Agents Chemother. 44:614-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikeda. F., K. Otomo, T. Nakai, Y. Morishita, K. Maki, S. Tawara, S. Mutoh, F. Matsumoto, and S. Kuwahara. 2002. In vitro activity of a new lipopeptide antifungal agent, micafungin, against a variety of clinically important fungi. Jpn. J. Chemother. 50:8-19. [Google Scholar]
- 9.Iwen, P. C., D. M. Kelly, E. C. Reed, and S. H. Hinrichs. 1995. Invasive infection due to Candida krusei in immunocompromised patients not treated with fluconazole. Clin. Infect. Dis. 20:342-347. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins, T. C., and A. N. Lane. 1997. AT selectivity and DNA minor groove binding: modelling, NMR and structural studies of the interactions of propamidine and pentamidine with d(CGCGAATTCGCG)2. Biochim. Biophys. Acta 1350:189-204. [DOI] [PubMed] [Google Scholar]
- 11.Kaneko, T., K. Makimura, M. Onozaki, K. Ueda, Y. Yamada, Y. Nishiyama, and H. Yamaguchi. 2005. Vital growth factors of Malassezia species on modified CHROMagar Candida. Med. Mycol. 43:699-704. [DOI] [PubMed] [Google Scholar]
- 12.Lanteri, C. A., B. L. Trumpower, R. R. Tidwell, and S. R. Meshnick. 2004. DB75, a novel trypanocidal agent, disrupts mitochondrial function in Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 48:3968-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu, Y., and M. J. Leibowitz. 1993. Variation and in vitro splicing of group I introns in rRNA genes of Pneumocystis carinii. Nucleic Acids Res. 21:2415-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu, Y., R. R. Tidwell, and M. J. Leibowitz. 1994. Inhibition of in vitro splicing of a group I intron of Pneumocystis carinii. J. Eukaryot. Microbiol. 41:31-38. [DOI] [PubMed] [Google Scholar]
- 15.Miletti, K. E., and M. J. Leibowitz. 2000. Pentamidine inhibition of group I intron splicing in Candida albicans correlates with growth inhibition. Antimicrob. Agents Chemother. 44:958-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution testing of yeasts. Approved standard, 2nd ed. M27-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 17.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard. M38-A. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 17a.Nishikawa, H., K. Hashimoto, N. Oogake, E. Yamada, Y. Ito, N. Nomura, K. Hayashi, J. Mitsuyama, and N. Terashima. 2005. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F1-487.
- 18.Nunn, C. M., T. C. Jenkins, and S. Neidle. 1993. Crystal structure of d(CGCGAATTCGCG) complexed with propamidine, a short-chain homologue of the drug pentamidine. Biochemistry 32:13838-13843. [DOI] [PubMed] [Google Scholar]
- 19.Pfaller, M. A., D. J. Diekema, R. N. Jones, H. S. Sader, A. C. Fluit, R. J. Hollis, and S. A. Messer. 2001. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY antimicrobial surveillance program. J. Clin. Microbiol. 36:1886-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfaller, M. A., S. A. Messer, L. Boyken, C. Rice, S. Tendolkar, R. J. Hollis, and D. J. Diekema. 2004. Further standardization of broth microdilution methodology for in vitro susceptibility testing of caspofungin against Candida species by use of an international collection of more than 3,000 clinical isolates. J. Clin. Microbiol. 42:3117-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shapiro, T. A., and P. T. Englund. 1990. Selective cleavage of kinetoplast DNA minicircles promoted by antitrypanosomal drugs. Proc. Natl. Acad. Sci. USA 87:950-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shapiro, T. A. 1993. Inhibition of topoisomerases in African trypanosomes. Acta Trop. 54:251-260. [DOI] [PubMed] [Google Scholar]
- 22a.Shibata, T., T. Takahashi, E. Yamada, H. Nishikawa, H. Hayakawa, N. Nomura, and J. Mitsuyama. 2007. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., abstr F1-943.
- 23.Sturk, L. M., J. L. Brock, C. R. Bagnell, J. E. Hall, and R. R. Tidwell. 2004. Distribution and quantitation of the anti-trypanosomal diamidine 2,5-bis(4-amidinophenyl)furan (DB75) and its N-methoxy prodrug DB289 in murine brain tissue. Acta Trop. 91:131-143. [DOI] [PubMed] [Google Scholar]
- 24.Thomas, A. H. 1986. Suggested mechanisms for the antimycotic activity of the polyene antibiotics and the N-substituted imidazoles. J. Antimicrob. Chemother. 17:269-279. [DOI] [PubMed] [Google Scholar]
- 25.Tidwell, R. R., S. K. Jones, J. D. Geratz, K. A. Ohemeng, M. Cory, and J. E. Hall. 1990. Analogues of 1,5-bis(4-amidiniumphenoxy)pentane (pentamidine) in the treatment of experimental Pneumocystis carinii pneumonia. J. Med. Chem. 33:1252-1257. [DOI] [PubMed] [Google Scholar]
- 26.Velegraki, A., E. C. Alexopoulos, S. Kritikou, and G. Gaitanis. 2004. Use of fatty acid RPMI 1640 media for testing susceptibilities of eight Malassezia species to the new triazole posaconazole and to six established antifungal agents by a modified NCCLS M27-A2 microdilution method and Etest. J. Clin. Microbiol. 42:3589-3593. (Erratum, 43:1014, 2005.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viscoli, C., C. Girmenia, A. Marinus, L. Collette, P. Martino, B. Vandercam, C. Doyen, B. Lebeau, D. Spence, V. Krcmary, B. De Pauw, and F. Meunier. 1999. Candidemia in cancer patients: a prospective, multicenter surveillance study by the Invasive Fungal Infection Group (IFIG) of the European Organization for Research and Treatment of Cancer (EORTC). Clin. Infect. Dis. 28:1071-1979. [DOI] [PubMed] [Google Scholar]
- 28.Wingard, J. R., W. G. Merz, M. G. Rinaldi, T. R. Johnson, J. E. Karp, and R. Saral. 1991. Increase in Candida krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N. Engl. J. Med. 325:1274-1277. [DOI] [PubMed] [Google Scholar]
- 29.Wingard, J. R., W. G. Merz, M. G. Rinaldi, C. B. Miller, J. E. Karp, and R. Saral. 1993. Association of Torulopsis glabrata infections with fluconazole prophylaxis in neutropenic bone marrow transplant patients. Antimicrob. Agents Chemother. 37:1847-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29a.Yamada, E., H. Nishikawa, T. Shibata, N. Fujino, K. Hashimoto, N. Nomura, and J. Mitsuyama. 2006. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F1-1369.
- 30.Yeates, C. 2003. DB-289 Immtech International. IDrugs 6:1086-1093. [PubMed] [Google Scholar]
- 31.Zhang, Y., A. Bell, P. S. Perlman, and M. J. Leibowitz. 2000. Pentamidine inhibits mitochondrial intron splicing and translation in Saccharomyces cerevisiae. RNA 6:937-951. [DOI] [PMC free article] [PubMed] [Google Scholar]