Abstract
Polyomavirus BK is an important pathogen in transplant recipients with no effective therapy. This study demonstrates that alkoxyalkyl esters of (S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine and fatty acid derivatives of 9-[2-(phosphonomethyoxy)ethyl]adenine (P393 and P405) are potent and selective inhibitors of BK virus replication in vitro, with a 50% effective concentration in the micromolar-to-nanomolar range.
Polyomaviruses are widely latent DNA viruses, of which the most important species is BK virus (BKV). BKV is reactivated in 20 to 60% of renal transplant recipients, and nephropathy develops in up to 10% of them. BKV is also associated with hemorrhagic cystitis in up to 60% of bone marrow transplant patients (19, 26). No effective antiviral therapies are available. Although some medical centers have empirically used leflunomide and cidofovir, no proven clinical benefit has resulted (10, 23, 25).
We investigated the antiviral activities of several nucleoside analogs by using the BKV Gardner strain (ATCC VR837) grown in log-phase WI-38 cells (ATCC CCL-75) (7) in a 7-day quantitative PCR assay of viral replication. Toxicity was evaluated by the conventional neutral red assay and by quantifying the housekeeping gene for aspartoacylase. The technical details of these methods have been published previously (6, 19, 20). Selected chemical structures are depicted in Fig. 1 and 2, and the results of testing are summarized in Table 1.
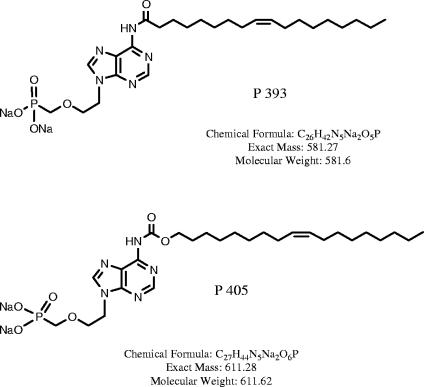
FIG. 1.
Chemical structures of PMEA derivatives P393 and P405.
FIG. 2.
Methylenecyclopropane analogs of nucleoside analogs tested for anti-BKV activity.
TABLE 1.
Anti-BKV activities of selected nucleoside analogsa
| Compound | CC50 (μM)b | EC50 (μM) | SIc |
|---|---|---|---|
| Acyclic nucleoside phosphonates | |||
| PMEA | 124.7 ± 7.8 | 95.8 ± 8.2 | 1.3 |
| PMEA dipivoxil | 3.1 ± 0.7 | 0.7 ± 0.3 | 5.7 |
| PMEA derivative P393 | 165.3 ± 1.6 | 1.0 ± 0.3 | 58.9 |
| PMEA derivative P405 | 528.6 | 2.3 ± 0.03 | 232.8 |
| HDP-(S)-HPMPA | 0.8 ± 0.4 | 0.02 ± 0.006 | 58.5 |
| ODE-(S)-HPMPA | 0.5 ± 0.2 | 0.03 ± 0.01 | 11.7 |
| Cyclosal derivatives | |||
| 5-H-cycloSal-acyclovir monophosphate | >100 | >100 | 1.0 |
| 3-Methyl-cycloSal-3′-OH-bromovinyl deoxyuridine monophosphate | 98.3 | 41.9 | 2.3 |
| Cyclopropane derivatives | |||
| Cyclopropavir | >100 | >100 | 1.0 |
| Synadenol | 259.6 ± 5.5 | 44.2 ± 0.9 | 5.9 |
| Synguanol | >100 | >100 | 1.0 |
| ZSM-I-32-F | 312 ± 2 | 40.8 ± 4.4 | 11.9 |
| Nucleoside reverse transcriptase inhibitors | |||
| Didanosine | >100 | >100 | 1.0 |
| Famciclovir | >0.78 | >0.78 | 1.0 |
| Stavudine | >100 | >100 | 1.0 |
| Lamivudine | >100 | >100 | 1.0 |
| Tenofovir disoproxil | >50 | >50 | 1.0 |
| Azidothymidine | >100 | >100 | 1.0 |
See the text for compound abbreviations. PMEA derivatives P393 and P405 were contributed by M. Bradley, HPMPA derivatives were contributed by K. Y. Hostetler, cycloSal derivatives were contributed by A. Sauerbrei, and cyclopropane derivatives were contributed by J. Zemlicka. All other compounds were purchased from Sigma Chemicals, St. Louis, MO, or from the pharmacy at the University of Pittsburgh Medical Center.
The EC50 and SI data presented were calculated by the neutral red assay. All results expressed as means ± standard deviations are based on at least three experiments, except for PMEA (tested twice).
SI = CC50/EC50.
Acyclic nucleoside phosphonates were tested because this class of compounds encompasses several clinically useful antiviral agents. In our system, 9-[2-(phosphonomethyoxy)-ethyl]adenine (PMEA) showed no significant activity (selectivity index [SI] = 1.3), confirming and extending prior work done with mouse polyomavirus (1). The prodrug form PMEA dipivoxil was approximately 2 logs more potent but showed only a marginal increase in selectivity (SI = 5.74). However, fatty acid derivatives of PMEA, namely, P393 and P405 (Fig. 1), showed striking activity. P393 exhibited a 50% cytotoxic concentration (CC50), a 50% effective concentration (EC50), and an SI of 165.3 ± 11.6 μM, 1.0 ± 0.31 μM, and 58.9, respectively. P405 had a comparable EC50 (2.3 ± 0.03 μM), but the CC50 was >100 μM and >200 μM in the first two experiments. By repeating the experiment in a concentration range of 100 to 1,000 μM, a more precise value of 528.6 μM was obtained and this generated an SI of 232.8. The mechanism by which fatty acid side chains enhance the efficacy of the parent compound was not determined. The possibility of increased transport into infected cells was considered, but one might have expected this to have resulted in lowering of the CC50, and this was not observed. Notably, while PMEA dipivoxil also increased cell permeability compared to the parent compound, as reflected by a lower EC50, it did not have the same selectivity as the PMEA derivatives.
(S)-9-(3-Hydroxy-2-phosphonylmethoxypropyl)-adenine [(S)-HPMPA] is the adenine analog of the broad-spectrum compound cidofovir. (S)-HPMPA is a broad-spectrum antiviral agent with demonstrated activity against orthopoxviruses, cytomegalovirus, human herpesvirus 6, adenovirus, and hepatitis B virus (2, 4, 9, 18, 21, 24). We have previously reported that the hexadecyloxypropyl (HDP) ester of cidofovir is very active against BKV in vitro (19). Our present work shows that the HDP ester of (S)-HPMPA is the most active compound tested to date, with a CC50, an EC50, and an SI of 0.8 ± 0.4 μM, 0.02 ± 0.006 μM, and 58.5. HDP-(S)-HPMPA is roughly nine times more active in vitro against BKV than HDP-cidofovir is (19). The parent compound (S)-HPMPA was recently reported not to be active against multiple strains of mouse and monkey polyomaviruses (11). In the latter study, HPMP derivatives containing a 5-azacytosine moiety were shown to have the best activity; the highest SI (58.3) was found for the compound hexadecyloxyethyl-cHPMP-5-azaC, with other related compounds showing SIs of <30.0.
There has been recent interest in profiling the antiviral activities of methylenecyclopropane analogs of nucleosides (Fig. 2) (27). The rationale is to introduce methylene groups, reduce the number of rotatable bonds, and increase the entropy factor, thereby altering the biologic properties of the compounds (28). Modification of acyclovir and ganciclovir on the basis of this principle has been used to generate a new class of antiherpesvirus compounds. However, in our assay, cyclopropavir, synguanol, synadenol, and ZSM-I-32-F showed no significant antiviral activity.
Nucleoside analogs that inhibit the human immunodeficiency virus enzyme reverse transcriptase were also tested (5). Several of these compounds are active against hepatitis B virus, a DNA virus with a life cycle that includes an RNA intermediate that is reverse transcribed back to the DNA genome (8, 12). We evaluated the anti-BKV activities of these compounds, which are already approved by the Food and Drug Administration. Didanosine, lamivudine, and stavudine were all found to be inactive at a screening concentration of 100 μM. Famciclovir and tenofovir disoproxil also showed no effect within the concentration limits imposed by the solubility of these compounds. Zidovudine (azidothymidine) was inactive.
The nucleoside analogs acyclovir and brivudine have previously been tested against several polyomavirus strains and found to have very low SIs (1). However, the synthesis of cycloSaligenyl (cycloSal)-nucleoside monophosphates results in compounds that have greatly increased activities against orthopoxviruses, herpesviruses, and human immunodeficiency virus (3, 13-16, 22). Hence, we tested cycloSal-nucleoside monophosphates of acyclovir and brivudine (5-H-cycloSal-acyclovir monophosphate and 3-methyl-cycloSal-3′-OH-bromovinyl deoxyuridine monophosphate) in our sys-tem but found no significant antiviral activity (Table 1). The failure of the cycloSal strategy for BKV is likely related to the fact that the small 5-kb genome encodes neither a thymidine kinase nor a DNA polymerase. The presumed increased intracellular uptake of cycloSal compounds with subsequent release of the nucleoside by chemical hydrolysis, therefore, did not translate into reduced BKV replication.
In conclusion, we tested several compounds for anti-BKV activity in vitro. The most active compound was HDP-(S)-HPMPA which had an EC50 of 0.02 μM and an SI of 58. The PMEA derivatives P393 and P403 were less active, with EC50s of 1.0 to 2.3 μM and SIs of 158 and 232, respectively. The other compounds lacked either efficacy or selectivity against BKV in vitro.
ADDENDUM IN PROOF
Additional testing following acceptance of this manuscript showed that 2′,3′-didehydro-3′-deoxythymidine has significant anti-BKV effect, with an SI, CC50, and EC50 of 79.2, 404.3 ± 49.3 μM, and 5.1 ± 1.9 μM, respectively. It is of interest that a related compound, 3′-fluoro-2′-deoxythymidine, is a potent inhibitor of adenovirus (17). Likewise, 3′-azido-thymidine is a well-known compound in clinical use for human immunodeficiency virus infection.
Acknowledgments
This work was supported by NIH grants AI51227 (P.R.), AI63360 (P.R.), AI66499 (M.B.), and CA32779 (J.Z.) and NIAID contract N01-AI-30044 (P.R.).
Footnotes
Published ahead of print on 19 February 2008.
REFERENCES
- 1.Andrei, G., R. Snoeck, M. Vandeputte, and E. De Clercq. 1997. Activities of various compounds against murine and primate polyomaviruses. Antimicrob. Agents Chemother. 41:587-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker, R., M. Bray, and J. W. Huggins. 2003. Potential antiviral therapeutics for smallpox, monkeypox and other orthopoxvirus infections. Antivir. Res. 57:13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balzarini, J., F. Haller-Meier, E. De Clercq, and C. Meier. 2001. Antiviral activity of cycloSaligenyl prodrugs of acyclovir, carbovir and abacavir. Antivir. Chem. Chemother. 12:301-306. [DOI] [PubMed] [Google Scholar]
- 4.Beadle, J. R., K. Y. Hostetler, R. M. Buller, J. Schriewer, K. A. Aldern, M. N. Prichard, K. Keith, and E. R. Kern. 2007. Synthesis and antiviral evaluation of iso-methyl-alkoxyalkyl (S)-HPMPA esters. Antivir. Res. 74:A48. [Google Scholar]
- 5.Daugas, E., J. P. Rougier, and G. Hill. 2005. HAART-related nephropathies in HIV-infected patients. Kidney Int. 67:393-403. [DOI] [PubMed] [Google Scholar]
- 6.Farasati, N. A., R. Shapiro, A. Vats, and P. Randhawa. 2005. Effect of leflunomide and cidofovir on replication of BK-virus in an in vitro culture system. Transplantation 79:116-118. [DOI] [PubMed] [Google Scholar]
- 7.Fishman, J. A. 2002. BK virus nephropathy: polyomavirus adding insult to injury. N. Engl. J. Med. 347:527-530. [DOI] [PubMed] [Google Scholar]
- 8.Fung, J., C. Lai, J. Yuen, D. Wong, Y. Tanaka, M. Mizokami, and M. Yuen. 2007. Adefovir dipivoxil monotherapy and combination therapy with lamivudine for the treatment of chronic hepatitis B in an Asian population. Antivir. Ther. 12:41-46. [PubMed] [Google Scholar]
- 9.Hartline, C., K. Gustin, W. Wan, S. Ciesla, J. Beadle, K. Hostetler, and E. Kern. 2005. Ether lipid-ester prodrugs of acyclic nucleoside phosphonates: activity against adenovirus replication in vitro. J. Infect. Dis. 191:396-399. [DOI] [PubMed] [Google Scholar]
- 10.Josephson, M. A., R. D. Poduval, P. V. Kadambi, B. Javaid, R. C. Harland, P. F. Foster, J. R. Thistlethwaite, S. M. Meehan, and J. W. Williams. 2003. BK nephropathy: can leflunomide control it? J. Am. Soc. Nephrol. 14:43A. [Google Scholar]
- 11.Lebeau, I., G. Andrei, M. Krecmerova, E. De Clercq, A. Holy, and R. Snoeck. 2007. Inhibitory activities of three classes of acyclic nucleoside phosphonates against murine polyomavirus and primate simian virus 40 strains. Antimicrob. Agents Chemother. 51:2268-2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews, G. V., D. A. Cooper, and G. J. Dore. 2007. Improvements in parameters of end-stage liver disease in patients with HIV/HBV-related cirrhosis treated with tenofovir. Antivir. Ther. 12:119-122. [PubMed] [Google Scholar]
- 13.Meier, C., L. Habel, F. Haller-Meier, A. Lomp, M. Herderich, R. Klocking, A. Meerbach, and P. Wutzler. 1998. Chemistry and anti-herpes simplex virus type 1 evaluation of cycloSal-nucleotides of acyclic nucleoside analogues. Antivir. Chem. Chemother. 9:389-402. [DOI] [PubMed] [Google Scholar]
- 14.Meier, C., T. Knispel, E. De Clercq, and J. Balzarini. 1999. cycloSal-pronucleotides of 2′,3′-dideoxyadenosine and 2′,3′-dideoxy-2′,3′-didehydroadenosine: synthesis and antiviral evaluation of a highly efficient nucleotide delivery system. J. Med. Chem. 42:1604-1614. [DOI] [PubMed] [Google Scholar]
- 15.Meier, C., A. Lomp, A. Meerbach, and P. Wutzler. 2002. CycloSal-BVDUMP pronucleotides: how to convert an antiviral-inactive nucleoside analogue into a bioactive compound against EBV. J. Med. Chem. 45:5157-5172. [DOI] [PubMed] [Google Scholar]
- 16.Meier, C., A. Lomp, A. Meerbach, and P. Wutzler. 2001. Synthesis, hydrolysis and anti-EBV activity of a series of 3′-modified cycloSal-BVDUMP pronucleotides. Nucleosides Nucleotides Nucleic Acids 20:307-314. [DOI] [PubMed] [Google Scholar]
- 17.Mentel, R., M. Kinder, U. Wegner, M. von Janta-Lipinski, and E. Matthes. 1997. Inhibitory activity of 3′-fluoro-2′ deoxythymidine and related nucleoside analogues against adenoviruses in vitro. Antivir. Res. 34:113-119. [DOI] [PubMed] [Google Scholar]
- 18.Naesens, L., L. Lenaerts, G. Andrei, R. Snoeck, D. Van Beers, A. Holy, J. Balzarini, and E. De Clercq. 2005. Antiadenovirus activities of several classes of nucleoside and nucleotide analogues. Antimicrob. Agents Chemother. 49:1010-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Randhawa, P., N. A. Farasati, R. Shapiro, and K. Y. Hostetler. 2006. Ether lipid ester derivatives of cidofovir inhibit polyomavirus BK replication in vitro. Antimicrob. Agents Chemother. 50:1564-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Randhawa, P. S., A. Vats, D. Zygmunt, P. A. Swalsky, V. Scantlebury, R. Shapiro, and S. Finkelstein. 2002. Quantitation of viral DNA in renal allograft tissue from patients with BK virus nephropathy. Transplantation 74:485-488. [DOI] [PubMed] [Google Scholar]
- 21.Reymen, D., L. Naesens, J. Balzarini, A. Holy, H. Dvorakova, and E. DeClercq. 1995. Antiviral activity of selected acyclic nucleoside analogues against human herpesvirus 6. Antivir. Res. 28:343-357. [DOI] [PubMed] [Google Scholar]
- 22.Sauerbrei, A., C. Meier, A. Meerbach, and P. Wutzler. 2006. Inhibitory efficacy of cycloSal-nucleoside monophosphates of aciclovir and brivudin on DNA synthesis of orthopoxviruses. Antivir. Chem. Chemother. 17:25-31. [DOI] [PubMed] [Google Scholar]
- 23.Scantlebury, V., R. Shapiro, P. S. Randhawa, K. Weck, and A. Vats. 2002. Cidofovir: a method of treatment for BK virus associated transplant nephropathy. Graft 5(Suppl.):S82-S86. [Google Scholar]
- 24.Smee, D. F., B. B. Barnett, R. W. Sidwell, E. J. Reist, and A. Holy. 1995. Antiviral activities of nucleosides and nucleotides against wild-type and drug-resistant strains of murine cytomegalovirus. Antivir. Res. 26:1-9. [DOI] [PubMed] [Google Scholar]
- 25.Vats, A., R. Shapiro, P. Singh Randhawa, V. Scantlebury, A. Tuzuner, M. Saxena, M. L. Moritz, T. J. Beattie, T. Gonwa, M. D. Green, and D. Ellis. 2003. Quantitative viral load monitoring and cidofovir therapy for the management of BK virus-associated nephropathy in children and adults. Transplantation 75:105-112. [DOI] [PubMed] [Google Scholar]
- 26.Wadei, H. M., A. D. Rule, M. Lewin, A. S. Mahale, H. A. Khamash, T. R. Schwab, J. M. Gloor, S. C. Textor, M. E. Fidler, D. J. Lager, T. S. Larson, M. D. Stegall, F. G. Cosio, and M. D. Griffin. 2006. Kidney transplant function and histological clearance of virus following diagnosis of polyomavirus-associated nephropathy (PVAN). Am. J. Transplant. 6:1025-1032. [DOI] [PubMed] [Google Scholar]
- 27.Zemlicka, J., and X. Chen. 2004. Methylenecyclopropane analogs of nucleosides as antiviral agents, p. 267-307. In R. F. Schinazi and D. C. Liotta (ed.), Frontiers in nucleosides and nucleic acids. IHL Press, Tucker, GA.
- 28.Zhou, S. M., J. M. Breitenbach, K. Z. Borysko, J. C. Drach, E. R. Kern, E. Gullen, Y. C. Cheng, and J. Zemlicka. 2004. Synthesis and antiviral activity of (Z)- and (E)-2,2-[bis(hydroxymethyl)cyclopropylidene]methylpurines and -pyrimidines: second-generation methylenecyclopropane analogues of nucleosides. J. Med. Chem. 47:566-575. [DOI] [PubMed] [Google Scholar]