Abstract
The production of interleukin-8 induced by the activation of protease-activated receptor 2 and its synergism with interleukin-1β were modulated by 14-membered-ring macrolides, namely, roxithromycin, erythromycin, and clarithromycin, in cultured normal human epidermal keratinocytes. Those macrolides may attenuate the protease-activated receptor 2-interleukin-8 axis and thereby modulate proinflammatory responses in the skin.
Besides their antibiotic actions, 14-membered-ring macrolides have immunomodulatory activities (24) that are beneficial for patients with inflammatory skin disorders (10, 11, 23) or with chronic inflammatory airway diseases (12, 15), possibly due to their inhibitory actions on neutrophils and macrophages (15) and/or on inflammatory cytokines from regional epithelial cells (4, 21). Recent findings have suggested that protease-activated receptor 2 (PAR2) is involved in various aspects of skin inflammation, such as allergic and toxic contact dermatitis (18), via the up-regulation of intercellular cell adhesion molecule 1 expression (2) and interleukin-8 (IL-8) in keratinocytes (6); therefore, PAR2 might play a pivotal role in modulating the inflammatory processes of the skin. Here we show that 14-membered-ring macrolides suppress the keratinocyte PAR2-IL-8 axis, which might be a therapeutic target for the control of cutaneous inflammation.
Normal human epidermal keratinocytes (NHEK) plated at a density of 1.0 × 104 cells per cm2 were incubated for 72 h, after which they were transfected with a PAR2-specific small interfering RNA (siRNA) (Qiagen, Inc., Hilden, Germany). After 24 h of incubation, the cells were harvested for analysis by real-time quantitative reverse transcription-PCR. To assess IL-8 production by NHEK, after 72 h of incubation, the medium was changed to KGM2 medium without additives. Cells were preincubated for 24 h with or without each macrolide, and then a PAR2 agonist peptide (SLIGKV-NH2) or a control reverse peptide (VKGILS-NH2) and/or 100 ng/ml IL-1β or IL-18 was added. Forty-eight hours later, IL-8 levels in the medium were assayed by enzyme-linked immunosorbent assay.
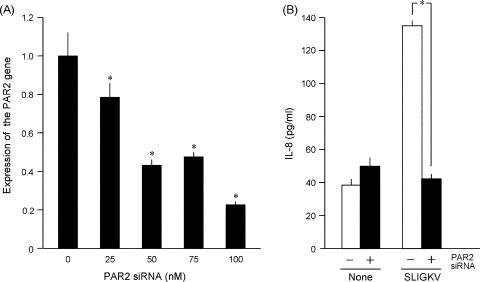
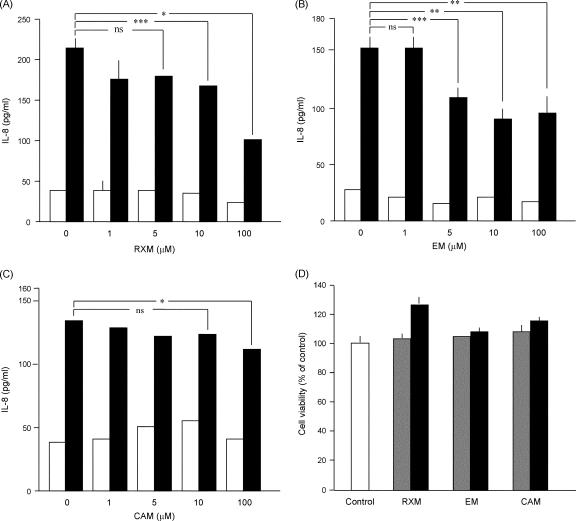
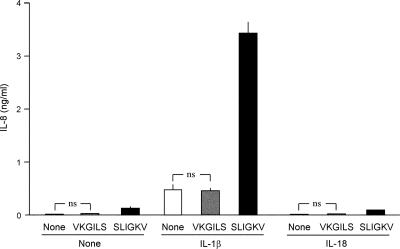
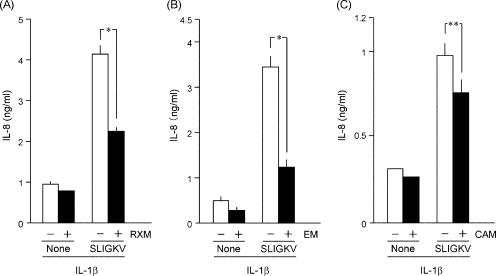
In NHEK transfected with the PAR2-specific siRNA, PAR2 transcript levels were decreased in parallel with the transfected amounts of siRNA (Fig. 1A). At 100 nΜ siRNA, PAR2 transcripts were as little as about 25% of the control, and the SLIGKV-NH2 induction of IL-8 was decreased to the control level (Fig. 1B). This indicated that the increase of IL-8 elicited by SLIGKV-NH2 depends on the expression of the PAR2 gene in NHEK. When NHEK were treated with 14-membered-ring macrolides, the SLIGKV-NH2-induced production of IL-8 was decreased by roxithromycin (RMX) at 10 and 100 μM or by erythromycin (EM) at 5, 10, and 100 μM (Fig. 2A and B). However, clarithromycin (CAM) showed weaker effects than RXM or EM at concentrations up to 100 μM (Fig. 2C). In contrast, 16-membered-ring macrolides (spiramycin, oleandomycin, and josamycin) did not suppress the SLIGKV-NH2-induced production of IL-8 (data not shown). When NHEK were treated with 10 or 100 μM RXM, EM, or CAM, those drugs did not decrease the cell viability as assessed with a colorimetric assay kit (Fig. 2D) or the levels of PAR2 transcripts (data not shown). Therefore, it is unlikely that the effects of 14-membered-ring macrolides on the SLIGKV-NH2-induced production of IL-8 are due simply to their cytotoxic effects or to the down-regulation of the PAR2 gene. As shown in Fig. 3, the treatment of NHEK with both IL-1β and SLIGKV-NH2 synergistically increased IL-8 levels. In contrast, IL-18, a cytokine in the IL-1 family, did not show such activities. The control peptide VKGILS-NH2 did not significantly increase the production of IL-8, regardless of the presence of IL-1β. In NHEK treated with both IL-1β and SLIGKV-NH2, 10 μM RXM or EM reduced the synergistic increase of IL-8 production (Fig. 4A and B). CAM also significantly decreased the synergistic effect but was less effective than RXM or EM (Fig. 4C).
FIG. 1.
(A) Effect of a PAR2-specific siRNA on the expression of the PAR2 gene in NHEK. The indicated concentration of the PAR2-specific siRNA was transfected into NHEK. The expression of the PAR2 gene was inhibited by the PAR2-specific siRNA in a dose-responsive manner. (B) Effect of the PAR2-specific siRNA on the production of IL-8 induced by the agonist peptide SLIGKV-NH2 in NHEK. The SLIGKV-NH2-induced production of IL-8 was completely suppressed by 100 nM PAR2-specific siRNA. An analysis of variance followed by Scheffe's test was performed for the statistical analysis of data. *, P < 0.001 (versus the control).
FIG. 2.
Effect of the 14-membered-ring macrolide RXM (A), EM (B), or CAM (C) on the SLIGKV-NH2-induced production of IL-8 in NHEK. Empty bars, control; filled bars, SLIGKV-NH2-treated cells. An analysis of variance followed by Scheffe's test was performed for the statistical analysis of data. *, P < 0.001; **, P < 0.01; ***, P < 0.05; ns, not significant. (D) Effect of RXM, EM, or CAM on the viability of NHEK. NHEK were incubated with no macrolide (empty bars) or 10 μM (gray bars) or 100 μM (filled bars) RXM, EM, or CAM for 72 h, and then cell viability was examined. None of those 14-membered-ring macrolides reduced the viability of NHEK.
FIG. 3.
Effect of IL-1β or IL-18 on the SLIGKV-NH2-induced production of IL-8 in NHEK. A synergistic increase in IL-8 production was induced by the combination of SLIGKV-NH2 with IL-1β but not with IL-18. No significant changes were observed in the presence of the control peptide VKGILS-NH2. Empty bars, control; filled bars, SLIGKV-NH2-treated cells; gray bars, VKGILS-NH2-treated cells. Student's two-tailed t test was performed for the statistical analysis of data. ns, not significant.
FIG. 4.
Effect of macrolides on the synergistic production of IL-8 induced by both IL-1β and SLIGKV-NH2 in NHEK. The synergistic induction of IL-8 was suppressed by 10 μM RXM (A), EM (B), or CAM (C). Empty bars, control; filled bars, SLIGKV-NH2-treated cells. Student's two-tailed t test was performed for the statistical analysis of data. *, P < 0.005; **, P < 0.05.
For several cases of inflammatory skin disorders, eruptions and/or symptoms are improved by treatment with macrolides. Especially in psoriasis vulgaris, the activation of PAR2 may contribute to the pathological condition of the disease, in which IL-8 recruits neutrophils into the epidermis to form microabscesses (16). The activation mechanism of PAR2 in the psoriatic epidermis is unknown, but it is possible that serine proteinases released from keratinocytes or from infiltrating inflammatory cells could activate PAR2 in the epidermis (7). It is interesting that itchy sensations are well controlled by RXM, but not by CAM, for patients with psoriasis vulgaris (personal communication from Kunihiko Tamaki, Tokyo University Graduate School of Medicine). The clinical findings of Tamaki et al. correlate with our in vitro results that CAM is not as effective as RXM or EM for inducing the production of IL-8 in NHEK, although the reason for that is unknown. The biological effects of those macrolides seem to not necessarily be coordinated as has been shown for other types of cells (14, 17).
In a case with chronic prurigo nodularis, the combination of RXM with tranilast was effective (5). That case implies that the action of RXM is not so strong that it completely reduces the disease activity. When therapeutic doses of those macrolides are taken, their peak concentrations in human skin range from 0.5 to 30 μM (1, 3, 22). The mild anti-inflammatory effect of macrolides on those skin diseases seems to be reflected in our results; the suppressive effect of RXM or EM on IL-8 remains 25 to 30% at pharmacological concentrations ranging up to 10 μM. The effect of those macrolides on the PAR2 system in other types of cells has not been tested, but we speculate that RXM and EM might show similar effects on the PAR2 system, at least in some epithelial cells, because the release of IL-8 from tracheal or nasal epithelial cells is decreased by the administration of those macrolides (8, 19).
As shown in this study and in a previous report (7), the combination of IL-1β with a PAR2 agonist, SLIGKV-NH2, shows a synergistic induction of IL-8 in NHEK. Possibly, this synergistic effect boosts inflammation in the epidermis in vivo. RXM, EM, and CAM significantly decrease the synergistic induction of IL-8. It has not been examined whether signals elicited by the activation of PAR2 cross talk with those originating from IL-1β in keratinocytes, but at least some signals might activate nuclear factor κB (2, 13), which is a possible target of RXM as well as p38 and AP-1 in keratinocytes (9, 20). Hence, it is likely that signals from PAR2 might be mediated by those signaling molecules and transcription factors.
Acknowledgments
RXM was kindly provided by Eisai Pharmaceutical Co., Ltd., and CAM was from Taisho Toyama Pharmaceutical Co., Ltd. (Tokyo, Japan). We thank Shen-Chun Shen and members of the Joint-Use Research Facilities of the Hyogo College of Medicine for their technical assistance.
This work was partially supported by a Ministry of Education, Science, Sports, and Culture Grant-in-Aid for Scientific Research and for Young Scientists; by a High-Tech Research Center grant; by a Grant-in-Aid from the Japan Foundation for Applied Enzymology; and by a Grant-in-Aid for Researchers, Hyogo College of Medicine.
Footnotes
Published ahead of print on 22 January 2008.
REFERENCES
- 1.Arata, J. 1992. Newer macrolide antibiotics. Jpn. J. Clin. Dermatol. 46:145-150. [Google Scholar]
- 2.Buddenkotte, J., C. Stroh, I. H. Engels, C. Moormann, V. M. Shpacovitch, S. Seeliger, N. Vergnolle, D. Vestweber, T. A. Luger, K. Schulze-Osthoff, and M. Steinhoff. 2005. Agonists of proteinase-activated receptor-2 stimulate upregulation of intercellular cell adhesion molecule-1 in primary human keratinocytes via activation of NF-kappa B. J. Investig. Dermatol. 124:38-45. [DOI] [PubMed] [Google Scholar]
- 3.Campa, M., I. Zolfino, S. Senesi, N. Bernardini, R. Danesi, M. Ducci, M. Oleggini, R. Di Stefano, F. Mosca, and A. Lazzarini. 1990. The penetration of roxithromycin into human skin. J. Antimicrob. Chemother. 26:87-90. [DOI] [PubMed] [Google Scholar]
- 4.Fujita, K., T. Shimizu, Y. Majima, and Y. Sakakura. 2000. Effects of macrolides on interleukin-8 secretion from human nasal epithelial cells. Eur. Arch. Otorhinolaryngol. 257:199-204. [DOI] [PubMed] [Google Scholar]
- 5.Horiuchi, Y., S. Bae, and I. Katayama. 2006. Uncontrollable prurigo nodularis effectively treated by roxithromycin and tranilast. J. Drugs Dermatol. 5:363-365. [PubMed] [Google Scholar]
- 6.Hou, L., S. Kapas, A. T. Cruchley, M. G. Macey, P. Harriott, C. Chinni, S. R. Stone, and G. L. Howells. 1998. Immunolocalization of protease-activated receptor-2 in skin: receptor activation stimulates interleukin-8 secretion by keratinocytes in vitro. Immunology 94:356-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwakiri, K., M. Ghazizadeh, E. Jin, M. Fujiwara, T. Takemura, S. Takezaki, S. Kawana, S. Yasuoka, and O. Kawanami. 2004. Human airway trypsin-like protease induces PAR-2-mediated IL-8 release in psoriasis vulgaris. J. Investig. Dermatol. 122:937-944. [DOI] [PubMed] [Google Scholar]
- 8.Kawasaki, S., H. Takizawa, T. Ohtoshi, N. Takeuchi, T. Kohyama, H. Nakamura, T. Kasama, K. Kobayashi, K. Nakahara, Y. Morita, and K. Yamamoto. 1998. Roxithromycin inhibits cytokine production by and neutrophil attachment to human bronchial epithelial cells in vitro. Antimicrob. Agents Chemother. 42:1499-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komine, M., T. Kakinuma, S. Kagami, Y. Hanakawa, K. Hashimoto, and K. Tamaki. 2005. Mechanism of thymus- and activation-regulated chemokine (TARC)/CCL17 production and its modulation by roxithromycin. J. Investig. Dermatol. 125:491-498. [DOI] [PubMed] [Google Scholar]
- 10.Komine, M., and K. Tamaki. 2000. An open trial of oral macrolide treatment for psoriasis vulgaris. J. Dermatol. 27:508-512. [DOI] [PubMed] [Google Scholar]
- 11.Komiya, N., and K. Tamaoki. 2001. Therapeutic effects of macrolides on patients with psoriasis vulgaris, pigmentary prurigo, or eosinophilic pustule folliculitis. Jpn. J. Antibiot. 54(Suppl. A):100-102. (In Japanese.) [PubMed] [Google Scholar]
- 12.Kudoh, S., A. Azuma, M. Yamamoto, T. Izumi, and M. Ando. 1998. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am. J. Respir. Crit. Care Med. 157:1829-1832. [DOI] [PubMed] [Google Scholar]
- 13.Macfarlane, S. R., C. M. Sloss, P. Cameron, T. Kanke, R. C. McKenzie, and R. Plevin. 2005. The role of intracellular Ca2+ in the regulation of proteinase-activated receptor-2 mediated nuclear factor kappa B signalling in keratinocytes. Br. J. Pharmacol. 145:535-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitsuyama, T., K. Hidaka, T. Furuno, and N. Hara. 1997. Neutrophil-induced endothelial cell damage: inhibition by a 14-membered-ring macrolide through the action of nitric oxide. Int. Arch. Allergy Immunol. 114:111-115. [DOI] [PubMed] [Google Scholar]
- 15.Oishi, K., F. Sonoda, S. Kobayashi, A. Iwagaki, T. Nagatake, K. Matsushima, and K. Matsumoto. 1994. Role of interleukin-8 (IL-8) and an inhibitory effect of erythromycin on IL-8 release in the airways of patients with chronic airway diseases. Infect. Immun. 62:4145-4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozawa, M., and S. Aiba. 2004. Immunopathogenesis of psoriasis. Curr. Drug Targets Inflamm. Allergy 3:137-144. [DOI] [PubMed] [Google Scholar]
- 17.Sato, Y., K. Kaneko, and M. Inoue. 2007. Macrolide antibiotics promote the LPS-induced upregulation of prostaglandin E receptor EP2 and thus attenuate macrolide suppression of IL-6 production. Prostaglandins Leukot. Essent. Fatty Acids 76:181-188. [DOI] [PubMed] [Google Scholar]
- 18.Seeliger, S., C. K. Derian, N. Vergnolle, N. W. Bunnett, R. Nawroth, M. Schmelz, P. Y. von der Weid, J. Buddenkotte, C. Sunderkotter, D. Metze, P. Andrade-Gordon, E. Harms, D. Vestweber, T. A. Luger, and M. Steinhoff. 2003. Proinflammatory role of proteinase-activated receptor-2 in humans and mice during cutaneous inflammation in vivo. FASEB J. 17:1871-1885. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki, H., A. Shimomura, K. Ikeda, M. Furukawa, T. Oshima, and T. Takasaka. 1997. Inhibitory effect of macrolides on interleukin-8 secretion from cultured human nasal epithelial cells. Laryngoscope 107:1661-1666. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi, H., Y. Hashimoto, A. Ishida-Yamamoto, and H. Iizuka. 2005. Roxithromycin suppresses involucrin expression by modulation of activator protein-1 and nuclear factor-kappaB activities of keratinocytes. J. Dermatol. Sci. 39:175-182. [DOI] [PubMed] [Google Scholar]
- 21.Takizawa, H., M. Desaki, T. Ohtoshi, S. Kawasaki, T. Kohyama, M. Sato, M. Tanaka, T. Kasama, K. Kobayashi, J. Nakajima, and K. Ito. 1997. Erythromycin modulates IL-8 expression in normal and inflamed human bronchial epithelial cells. Am. J. Respir. Crit. Care Med. 156:266-271. [DOI] [PubMed] [Google Scholar]
- 22.Vaillant, L., G. Lorette, J. Loulergue, and A. Audurier. 1987. Erythromycin ethyl succinate: diffusion through interstitial dermal fluid. Eur. J. Clin. Pharmacol. 33:529-530. [DOI] [PubMed] [Google Scholar]
- 23.Yazawa, N., H. Ihn, K. Yamane, T. Etoh, and K. Tamaki. 2001. The successful treatment of prurigo pigmentosa with macrolide antibiotics. Dermatology 202:67-69. [DOI] [PubMed] [Google Scholar]
- 24.Zalewska-Kaszubska, J., and D. Gorska. 2001. Anti-inflammatory capabilities of macrolides. Pharmacol. Res. 44:451-454. [DOI] [PubMed] [Google Scholar]