Abstract
The antibacterial effects (ABE) of tomopenem (formerly RO4908463/CS-023) against seven Staphylococcus aureus strains (methicillin-resistant S. aureus [MRSA] strain tomopenem MICs, 0.5 to 16 mg/liter; methicillin-sensitive S. aureus [MSSA] strain tomopenem MIC, 0.06 mg/liter) were studied in an in vitro pharmacokinetic model. Initially, two human doses were simulated, 750 mg every 8 hours (8hly) and 1,500 mg 8hly intravenously, using S. aureus at a standard inoculum of 106 CFU/ml. There was a rapid clearance of bacteria from the model by 12 h after drug exposure with most strains. Clearance was not related to the tomopenem MIC. The ABE of these two tomopenem dose regimens were also tested at a high inoculum, 108 CFU/ml; in all simulations, there was a >4-log drop in viable count at 24 h. Strains were not cleared from the model at 108 CFU/ml, in contrast to what was seen for the standard inoculum. When the ABE of tomopenem at 750 mg 8hly was compared to those of vancomycin, tomopenem was seen to have a superior effect, as measured by the area under the bacterial kill curve at 24 h (AUBKC24) and 48 h (P < 0.05). Dose ranging studies were performed to provide time-above-MIC (T>MIC) drug exposures of 0 to 100% (8 to 10 doses per strain) with five MRSA/MSSA strains. The T>MIC for a 24-h bacteriostatic effect was 8% ± 5% (range, 1.3% to 15.4%); the T>MIC for a 4-log drop in viable count was 32% ± 18% (range, 12.8% to 36.2%). The T>MIC for a 90% maximum response using AUBKC24 as ABE was 24.9% ± 15.7%. Inoculum had little impact on T>MIC exposures for ABE. There was emergence of resistance to tomopenem in the dose ranging studies, with increased growth of subpopulations on plates containing tomopenem at 2× and 4× the MIC compared to what was seen for preexposure population analysis at T>MICs of <20%. The pharmacodynamics of tomopenem against S. aureus is similar to those of other members of the carbapenem class, with the exception that MRSA is included. These data indicate that tomopenem will have clinically useful activity against MRSA at T>MICs achievable in humans.
Tomopenem (formerly RO4908463/CS-023) is an injectable 2-substituted 1-β-methyl carbapenem. It has a guanidine-pyrrolidine side chain and binds with high affinity to penicillin binding protein 1 (PBP1), PBP2, and PBP4 from Staphylococcus aureus. The MIC50 and MIC90 against methicillin-sensitive S. aureus (MSSA) are 0.12 and 0.12 to 0.25 mg/liter (17, 19, 32). For methicillin-resistant S. aureus (MRSA), the MIC50 and MIC90 are 2 and 4 to 16 mg/liter (17, 19, 32). This contrasts to imipenem and meropenem, which have MRSA MIC90 values of 32 and 16 to 32 mg/liter. Tomopenem retains in vitro potency against extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella spp. as well as Pseudomonas aeruginosa (8, 17, 19, 33).
The pharmacokinetics studies with male healthy volunteers indicate relatively typical carbapenem pharmacokinetics except for a prolonged serum half-life of about 2 h, which is related to a lack of renal tubular secretion (29). Tomopenem is stable to digestion by human renal DHP-1 (18) and produces peak serum concentrations of about 44 mg/liter after a 700-mg dose and 100 mg/liter after a 1,400-mg dose in humans (29). Around 70% of the dose is excreted unchanged in the urine, but a pharmacologically inactive open ring metabolite (R131624) has been described (30). Protein binding in humans is <10% (29) and the volume of distribution is 15 to 17 liters. Autoradioluminograms of animals injected with 14C-tomopenem indicated wide tissue distribution except for the cerebrospinal fluid and testes (30).
The aim of this study is to describe the antibacterial effects (ABE) of tomopenem against a range of MRSA strains at high and low inocula, establish the time-above-MIC (T>MIC)-ABE relationship, and compare the effects of tomopenem to vancomycin. The impact of the maximum concentration of drug in serum/MIC and area under the concentration-time curve/MIC on ABE was not studied.
A dilutional single-compartment in vitro pharmacokinetic model was used.
MATERIALS AND METHODS
In vitro pharmacokinetic model.
A New Brunswick (Hatfield, Hertfordshire, England) Bioflo 1000 in vitro pharmacokinetic model was used to simulate free drug serum concentrations associated with dosings of tomopenem and vancomycin. The apparatus, which has been described before, consists of a single central chamber connected to a reservoir containing broth. The central chamber is connected to a collecting vessel for overflow (23). The contents of the central chamber were diluted with broth by using a peristaltic pump (Ismatec, Bennett & Co, Weston-super-Mare, England) at a flow rate of 132 ml/h for tomopenem and 47.9 ml/h for vancomycin. The temperature was maintained at 37°C and the broth in the central chamber was agitated by a magnetic stirrer.
Media.
Ten-percent Mueller-Hinton broth (MHB) was used in experiments with both tomopenem and vancomycin. Previous experiments had shown that 10% broth was able to sustain the growth of S. aureus and produced consistent and reproducible results in the model systems. Nutrient agar plates (Oxoid, Basingstoke, England) were used to recover S. aureus from the in vitro model. Five microliters β-lactamase/ml was used to neutralize tomopenem. The β-lactamase neutralized tomopenem up to a concentration of 100 mg/liter. Tomopenem was added to nutrient agar plates in the studies on the emergence of resistance.
Strains.
Six MRSA strains of S. aureus were provided by M. Jones, Eurofins Medinet Inc., Herndon, VA. The tomopenem MICs ranged from 0.5 to 16 mg/liter for MRSA strains. An MSSA strain MIC of 0.06 mg/liter was used for comparison.
Antibiotics.
Tomopenem was supplied by Hoffman-La Roche Inc., Nutley, NJ. Vancomycin was supplied by Alpharm Ltd., Basingstoke, England. Stock solutions were prepared according to British Society of Antimicrobial Chemotherapy guidelines (7) and stored at −70°C.
MICs.
MICs were determined by a standard broth dilution method according to CLSI guidelines (9). MICs were performed in 10% MHB and at nondoubling dilutions to more accurately determine MICs.
Pharmacokinetics.
A number of tomopenem free drug simulations were performed. The pharmacokinetic parameters simulated were associated with the following doses of intravenous tomopenem: 750 mg every 8 hours (8hly) and 1,500 mg 8hly with maximum concentrations of free drug in serum of 45 mg/liter and 90 mg/liter and a serum half-life of 2 h. Six doses were given over 48 h. In addition, between 7 and 13 doses were simulated per strain in a dose ranging design to achieve a T>MIC range of 0 to 100% for each strain in experiments to determine the T>MIC-ABE relationship.
Free drug concentrations associated with 1 g intravenous vancomycin given 12hly were an initial peak of 15 mg/liter and a trough at 12 h of 3.8 mg/liter, with a half-life of 6 h (22). Drug concentrations for tomopenem were determined using a bioassay method (4). For tomopenem, the indicator organism was E. coli NCTC 10418, the medium was Penassay seed agar with wells, the standard curve range was 2 to 128 mg/liter, and the limit of detection was 0.5 mg/liter. The coefficient of variation was 12.2%. Vancomycin was assayed by polarization fluoroimmunoassay (Abbott, Berkshire, England). The coefficient of variation was 5.9%.
ABE.
Experiments were performed at inoculum densities of 106 CFU/ml and 108 CFU/ml with tomopenem. For the 106 inoculum, 720 μl of a 109-CFU/ml bacterial suspension from a 24-h plate culture was added to the sample chamber 45 min before dosing. For the 108 inoculum, 1 ml of 0.5 McFarland standard prepared from a 24-h plate culture was added to the central chamber and the model run overnight. Samples were taken throughout the 48-h period for determination of viable counts. Bacteria were quantified by using a spiral plater (Don Whitley Spiral Systems, West Yorkshire, United Kingdom). The minimum level of detection was 102 CFU/ml. Additional aliquots were also stored at −70°C for measurement of the antibiotic concentration using bioassay (4) and polarization fluoroimmunoassay.
Emergence of resistance.
Resistance to tomopenem was assessed as before using population analysis profiles (22) at time zero (preexposure) and at 24 h and 48 h (postexposure). Samples were plated onto agar containing no antibiotic and antibiotic at 1×, 2×, 4×, and 8× the MIC to quantify any resistant subpopulation. The limit of detection of growth was 2 log10.
All pharmacokinetic simulations of human doses to determine ABE and the emergence of resistance were performed at least in triplicate.
Pharmacodynamics and measurement of ABE.
The ABE of the antibiotics were calculated by determining the log change in viable counts between time zero and 12 h (Δ12), 24 h (Δ24), 36 h (Δ36), and 48 h (Δ48). The times for the inoculum to fall to 99% and 99.9% of its value at time zero were recorded as T99 and T99.9, respectively. The area under the bacterial kill curve (AUBKC; log CFU/ml·h) was calculated using the log-linear trapezoidal rule for the periods 0 to 24 h (AUBKC0-24) and 0 to 48 h (AUBKC0-48). The relationship between T>MIC and ABE was delineated using sigmoid Emax models (GraphPad Prism; GraphPad Software Incorporated, San Diego, CA).
RESULTS
MICs and initial tomopenem population analysis profiles.
The tomopenem MICs by CLSI methods and in 10% MHB are shown on Table 1. The MICs in 10% MHB were significantly lower than the MICs by conventional CLSI methods. When T>MIC was calculated for tomopenem in the in vitro model, the MICs in 10% MHB were used. The vancomycin MICs for MRSA strains 33922, 33827, and 33815 were 1.5, 0.5, and 0.25 mg/liter, respectively. The population analysis profiles for each MRSA strain with tomopenem are also shown on Table 1. These indicated some variability in the population profiles before the strains are exposed to tomopenem. Strains with similar MICs but differing population analysis profiles were selected for study in the in vitro pharmacokinetic model.
TABLE 1.
Tomopenem MICs for the S. aureus strains according to CLSI and 10% MH broth methods and population analysis profile
| Strain type and no. | CLSI MIC (mg/liter) | 10% MH broth MIC (mg/liter) | Preexposure population analysis profile growth (log CFU/ml) at:
|
||
|---|---|---|---|---|---|
| 2× MIC | 4× MIC | 6× MIC | |||
| MSSA 5956 | 0.06 | 0.04 | |||
| MRSA 33921 | 0.5 | 0.06 | 4.3 | <2 | <2 |
| MRSA 33922 | 0.5 | 0.25 | 8.3 | 6.0 | 4.1 |
| MRSA 33827 | 2 | 0.75 | 4.3 | <2 | <2 |
| MRSA 33829 | 2 | 0.5 | 5.8 | 5.4 | 2.7 |
| MRSA 33820 | 16 | 6 | 2.3 | <2 | <2 |
| MRSA 33815 | 16 | 6 | <2 | <2 | <2 |
Pharmacokinetic curves and pharmacodynamic parameters.
There was good agreement between target and achieved tomopenem and vancomycin concentrations. In all situations, the measured antibiotic concentration 95% confidence interval (CI) limits encompassed the target concentration (data not shown).
The T>MICs for the 750-mg 8hly and 1,500-mg 8hly simulations were 100% for all strains except MRSA 33820 and 33815. The T>MICs for both strains with the 750-mg 8hly simulations were 73% and with the 1,500-mg 8hly simulation were 98%.
ABE: tomopenem human dose simulations of 750 mg 8hly and 1,500 mg 8hly.
The first series of simulations was performed with tomopenem at an initial inoculum of 106 CFU/ml against the MSSA strain and all six MRSA strains. Irrespective of tomopenem MIC, rapid clearance occurred in the model, with counts being undetectable by 12 h with all strains except MRSA 33921 (Table 2).
TABLE 2.
Antibacterial effect of tomopenem at standard (106 CFU/ml) inoculum
| Strain/CLSI MIC (mg/liter) | Dose (mg)a | Log change in viable count (log CFU/ml) at:
|
Time (h) to kill:
|
AUBKC (log CFU/ml·h)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 12 h | 24 h | 36 h | 48 h | Max | 99% | 99.9% | 0-24 | 0-48 | ||
| 5956/0.06 | 750 | −4.1 ± 0.5 | −4.3 ± 0.4 | −4.5 | −4.5 | −4.5 | 4 | 6 | 27.6 ± 4.5 | 27.6 ± 4.5 |
| 1,500 | −4.4 ± 0.1 | −4.4 ± 0.1 | −4.4 ± 0.1 | −4.4 ± 0.1 | −4.4 ± 0.1 | 5 | 8 | 22.7 ± 2.2 | 22.7 ± 2.2 | |
| 33921/0.5 | 750 | −3.6 ± 1.0 | −4.0 ± 0.9 | −3.9 ± 1.2 | −3.8 ± 1.3 | −4.4 ± 0.1 | 8 | 12 | 30.9 ± 7.2 | 41.0 ± 33.6 |
| 1,500 | −3.0 ± 0.1 | −4.2 ± 0.1 | −4.2 ± 0.1 | −3.7 ± 0.4 | −4.2 ± 0.1 | 8 | 12 | 32.9 ± 2.1 | 38.1 ± 5.9 | |
| 33922/0.5 | 750 | −4.4 ± 0.4 | −4.1 ± 0.6 | −4.0 ± 0.9 | −3.9 ± 1.1 | −4.4 ± 0.1 | 4 | 5 | 20.2 ± 1.2 | 27.7 ± 15.7 |
| 1,500 | −4.2 ± 0.1 | −4.2 ± 0.1 | −4.2 ± 0.1 | −4.2 ± 0.1 | −4.2 ± 0.1 | 4 | 5 | 16.9 ± 1.0 | 16.9 ± 1.0 | |
| 33827/2 | 750 | −4.4 ± 0.1 | −3.6 ± 1.6 | −3.7 ± 1.7 | −3.3 ± 2.2 | −4.4 ± 0.1 | 4 | 7 | 27.4 ± 7.3 | 44.9 ± 42.3 |
| 1,500 | −4.3 ± 0.1 | −4.0 ± 0.6 | −4.0 ± 0.6 | −3.6 ± 0.7 | −4.3 ± 0.1 | 6 | 12 | 28.1 ± 2.0 | 35.5 ± 10.3 | |
| 33829/2 | 750 | −4.5 ± 0.1 | −4.5 ± 0.1 | −4.5 ± 0.1 | −4.5 ± 0.1 | −4.5 ± 0.1 | 5 | 12 | 25.0 ± 3.2 | 25.0 ± 3.2 |
| 1,500 | −4.5 ± 0.1 | −4.2 ± 0.4 | −4.0 ± 0.8 | −3.8 ± 1.2 | −4.5 ± 0.1 | 7 | 12 | 30.0 ± 1.4 | 37.4 ± 11.5 | |
| 33820/16 | 750 | −3.9 ± 0.3 | −4.2 ± 0.2 | −4.2 ± 0.2 | −4.2 ± 0.2 | −4.2 ± 0.2 | 7 | 12 | 28.6 ± 2.8 | 28.6 ± 2.8 |
| 1,500 | −4.2 ± 0.1 | −4.2 ± 0.1 | −3.8 ± 0.6 | −4.2 ± 0.1 | −4.2 ± 0.1 | 6 | 12 | 23.4 ± 0.9 | 23.4 ± 0.9 | |
| 33815/16 | 750 | −4.2 ± 0.1 | −4.2 ± 0.1 | −4.2 ± 0.1 | −4.2 ± 0.1 | −4.2 ± 0.1 | 6 | 12 | 22.7 ± 2.8 | 22.7 ± 2.8 |
| 1,500 | −4.2 ± 0.1 | −4.2 ± 0.1 | −4.2 ± 0.1 | −4.2 ± 0.1 | −4.2 ± 0.1 | 3 | 5 | 12.5 ± 0.4 | 12.5 ± 0.4 | |
Doses were given 8hly.
Analysis of variance of the AUBKC0-24 and AUBKC0-48 for the 750-mg 8hly simulations indicated no significant differences between the strains (P > 0.05). A similar analysis of the 1,500-mg 8hly simulation indicated that strain MRSA 33815 had a smaller AUBKC0-24 and AUBKC0-48 than the other strains (P < 0.001). This indicates that tomopenem had a greater activity against this strain than the others tested. There was no difference between the ABE of the 750-mg 8hly simulations and the 1,500-mg 8hly simulations.
The ABE of tomopenem was also tested against three strains (33922, 33827, and 33815) at a high inoculum (108 CFU/ml). At the higher inoculum, S. aureus was not cleared from the model, even over 48 h later (Table 3). However, in all cases, a >4 log10 drop in viable count was observed by 24 h with minimal growth back up to 48 h. As might be expected, the times to clear 99% or 99.9% of the initial inoculum were longer with the high inoculum, as were the AUBKC0-24 and AUBKC0-48, compared to what was seen for equivalent experiments at 106 CFU/ml (Table 3).
TABLE 3.
Antibacterial effect of tomopenem at high (108 CFU/ml) inoculum
| Strain/MIC (mg/liter) | Dose (mg)a | Log change in viable count (log CFU/ml) at:
|
Time (h) to kill:
|
AUBKC (log CFU/ml·h)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 12 h | 24 h | 36 h | 48 h | Max | 99% | 99.9% | 0-24 | 0-48 | ||
| 33922/0.5 | 750 | −4.5 ± 0.4 | −5.0 ± 0.3 | −5.5 ± 0.5 | −5.8 ± 0.2 | −6.0 ± 0.2 | 12 | 12 | 71.5 ± 3.1 | 81.5 ± 10.8 |
| 1,500 | −1.5 ± 0.1 | −5.0 ± 0.3 | −5.2 ± 0.5 | −4.1 ± 0.1 | −5.5 ± 0.4 | 16 | 24 | 100.1 ± 1.7 | 124.7 ± 8.8 | |
| 33827/2 | 750 | −3.8 ± 0.2 | −5.2 ± 0.2 | −5.0 ± 0.3 | −5.9 ± 0.4 | −6.2 ± 0.1 | 12 | 12 | 77.4 ± 4.3 | 87.9 ± 9.2 |
| 1,500 | −3.4 ± 0.1 | −5.8 ± 0.6 | −6.0 ± 0.2 | −5.9 ± 0.1 | −6.1 ± 0.1 | 12 | 12 | 84.4 ± 3.4 | 93.5 ± 1.8 | |
| 33815/16 | 750 | 1.7 ± 0.2 | −4.3 ± 0.6 | −5.5 ± 1.3 | −5.0 ± 1.5 | −5.5 ± 1.2 | 16 | 24 | 100.0 ± 4.8 | 119.0 ± 28.9 |
| 1,500 | −1.5 ± 0.2 | −4.1 ± 0.1 | −3.9 ± 0.2 | −3.9 ± 0.1 | −4.2 ± 0.1 | 16 | 24 | 103.5 ± 5.5 | 152.6 ± 7.1 | |
Doses were given 8hly.
ABE: vancomycin.
Vancomycin did not result in clearance by 24 h from the model challenged at a 106-CFU/ml inoculum; there was a 1.5- to 3-log10 drop in viable counts with the three MRSA strains tested (Table 4). Only with one strain, MRSA 33827, did clearance occur at 48 h. When the ABE of vancomycin and tomopenem were compared in terms of AUBKC0-24 and AUBKC0-48, then tomopenem had an ABE superior to that of vancomycin (P < 0.05).
TABLE 4.
Antibacterial effect of vancomycin at a standard (106 CFU/ml) inoculum
| Strain/vancomycin MIC (mg/liter) | Log change (log CFU/ml) at:
|
Time (h) to kill:
|
AUBKC (log CFU/ml.h)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| 12 h | 24 h | 36 h | 48 h | Max | 99% | 99.9% | 0-24 | 0-48 | |
| 33922/3 | −2.2 ± 0.1 | −2.6 ± 0.4 | −3.0 ± 0.5 | −2.9 ± 0.6 | −3.1 ± 0.5 | 12 | >48 | 54.8 ± 1.5 | 80.1 ± 13.3 |
| 33827/3 | −1.8 ± 0.1 | −3.2 ± 0.2 | −4.0 ± 0.1 | −4.2 ± 0.1 | −4.2 ± 0.1 | 24 | 24 | 56.7 ± 1.4 | 61.9 ± 3.5 |
| 33815/3 | −3.3 ± 0.4 | −2.0 ± 1.0 | −1.7 ± 1.3 | −1.6 ± 1.7 | −3.5 ± 0.5 | 12 | 12 | 46.2 ± 5.5 | 97.4 ± 39.1 |
Dose ranging.
A range of doses (n = 8 to 11) was used to provide a T>MIC range of 0 to 100% for each strain at an inoculum of 106 CFU/ml. ABE was measured by Δ24, Δ48, AUBKC0-24, and AUBKC0-48 for each strain and related to T>MIC in a sigmoid Emax model. Using Δ24 as the main ABE measure, the T>MIC for static effect was 8% ± 5% (95% CI, 2.0% to 13%), increasing to 32% ± 18% (95% CI, 9% to 55%) for a 4-log drop in count (Table 5; Fig. 1).
TABLE 5.
Relationship between T>MIC (% of dosing interval) and Δ24 for tomopenem against MSSA (one strain) and MRSA (five strains) - inoculum 106 CFU/ml
| T>MIC (%) for: | Strain
|
Mean ± SD (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| MSSA 5965 | MRSA 33922 | MRSA 33829 | MRSA 33827 | MRSA 33820 | MRSA 33815 | ||
| Static effect | 6.0 | 1.3 | 12.1 | 15.4 | 5.9 | 5.3 | 8 ± 5 (2-13) |
| 1-log drop in count | 7.4 | 3.4 | 16.8 | 24.4 | 9.9 | 8.6 | 12 ± 8 (4-20) |
| 2-log drop in count | 8.7 | 5.4 | 20.8 | 31.2 | 14.5 | 12.5 | 16 ± 9 (6-25) |
| 3-log drop in count | 10.1 | 9.4 | 21.5 | 43.0 | 20.4 | 17.1 | 21 ± 13 (8-35) |
| 4-log drop in count | 12.8 | 21.5 | - | 61.0 | 36.2 | 29.6 | 32 ± 18 (9-55) |
| R2 model | 0.999 | 0.989 | 0.968 | 0.997 | 0.998 | 0.989 | 0.936 |
FIG. 1.
T>MIC relationship to Δ24 for S. aureus at an initial inoculum of 106 CFU/ml using MSSA strains 5965 and MRSA strains 33922, 33829, 33827, 33820, and 33815. d24, Δ24.
T>MIC could also be related to Δ48 and AUBKC0-24 or AUBKC0-48 at an R of >0.9 for each strain. The T>MIC to produce a 90% maximum response using AUBKC0-24 as the ABE was 23% ± 13% (95% CI, 9% to 36%). The T>MIC to produce a 4-log drop in count at 48 h was 27% ± 28% (95% CI, 43% to 98.4%) and a 90% maximum response using AUBKC0-48 as ABE was 25% ± 16% (95% CI, 8% to 41%).
A range of doses (n = 7 to 13) was also modeled at a higher (108-CFU/ml) inoculum for MSSA strain 5965 and for three MRSA strains, 22922, 33817, and 33815. A static-effect T>MIC cannot be usefully calculated in these experiments, as 108 CFU/ml is the maximum supportable inoculum in the model, beyond which no further growth occurs. The T>MIC to produce a 1-log10 drop in viable count was 6.4% ± 8.7% of the dosing interval by use of Δ24 as the ABE measure (Table 6) . The T>MIC to produce a 4-log drop at 24 h was 30.1% ± 33.1% of the dosing interval.
TABLE 6.
Relationship between T>MIC (% of dosing interval) and Δ24 for tomopenem against MSSA (1 strain) and MRSA (3 strains) - inoculum 108 CFU/ml
| T>MIC% for: | Strain
|
Mean ± SD (95% CI) | |||
|---|---|---|---|---|---|
| MSSA 5965 | MRSA 33922 | MRSA 33827 | MRSA 33815 | ||
| Static effect | |||||
| 1-log drop in count | 3.4 | 2.0 | 19.5 | 1 | 6.4 ± 8.7 (−7.4 to 20.4) |
| 2-log drop in count | 7.4 | 4.7 | 39.6 | 2 | 13.4 ± 17.6 (−14.6 to 41.4) |
| 3-log drop in count | 12.8 | 8.7 | 59.3 | 3.3 | 20.9 ± 25.7 (−19.3 to 61.9) |
| 4-log drop in count | 20.1 | 15.0 | 79.1 | 6.5 | 30.1 ± 33.1 (−22.4 to 82.9) |
| R2 model | 0.98 | 0.89 | 0.96 | 0.96 | |
Emergence of resistance to tomopenem.
With the 750-mg and 1,500-mg 8hly simulations at 106 CFU/ml, there was no emergence of resistance as there was clearance from the models. At the 108-CFU/ml initial inoculum, no S. aureus organisms were recovered from antibiotic plates containing 4× the MIC of tomopenem (MIC-×4 plates) or above.
In the dose ranging studies, emergence of resistance was noted in some experiments. At an initial inoculum of 106 CFU/ml, no strains produced subpopulations able to grow on MIC-×2 plates prior to dosing with tomopenem.
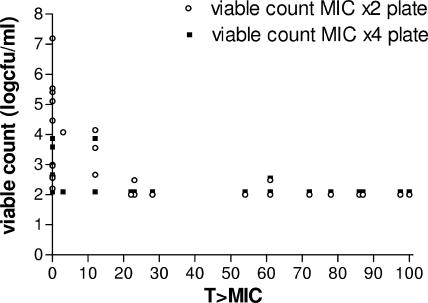
After 24 h of exposure, all strains except MRSA 33815 produced growth on MIC-×2 plates (Fig. 2). Only MRSA 33922 and MRSA 33927 produced subpopulations able to grow on MIC-×4 plates (Fig. 2). A score of 2 logs for Fig. 2 indicates no growth on the MIC-×2 or MIC-×4 plates. Bacterial counts of >2 logs were noted primarily with T>MICs of <25%. The lower the T>MIC, the higher the bacterial count (Fig. 2). The pattern of growth on tomopenem after 48 h exposure was similar to that at 24 h except for MSSA 5956, MRSA 33922, and MRSA 33927, which produced subpopulations capable of growth on MIC-×4 plates.
FIG. 2.
T>MIC relationship to growth on MIC-×2 and MIC-×4 plates after 24 h with a 106 inoculum (data from all strains were pooled).
At the higher inoculum (108 CFU/ml), growth was noted on MIC-×2 plates prior to drug dosing with strains MSSA 5956 and MRSA 33827. Growth on MIC-×4 plates was noted at 24 h with MSSA 5956 and MRSA 33827 and was also noted for MIC-×8 plates at 48 h.
There was a clear relationship between T>MIC and growth on MIC-×2 or MIC-×4 plates at 24 h and 48 h, with T>MIC values of ≤20 to 30% being much more likely to produce resistant subpopulations.
DISCUSSION
The pharmacodynamics of β-lactams and in particular carbapenems are well understood (10). Carbapenems show concentration-dependent killing at concentrations of up to 8 to 16 times the pathogen MIC (13) but not at high concentrations (15) and also show persistent antibiotic effects in animal and in vitro experiments (5, 16, 26, 32). The postantibiotic effect is related to both the concentration of carbapenem employed and the duration of pathogen exposure (27). There is a lack of inoculum effect on antibacterial activity compared to other β-lactams (28). The dominant pharmacodynamic index is T>MIC for both gram-positive and gram-negative bacteria (6, 15, 16, 20, 34), as shown in in vitro and in vivo pharmacodynamic models.
The pharmacodynamics of carbapenems against S. aureus follows this pattern, showing concentration-dependent killing at low concentrations (14) but not high, with a longer postantibiotic effect than against E. coli (16). T>MIC drives ABE (1).
The initial part of this study was to describe the ABE of tomopenem against a range of MRSA strains with MICs from 0.5 to 16 mg/liter. Two dosing regimens were simulated, 750 mg or 1,500 mg 8hly intravenously. Both regimens produced rapid clearance of all MRSA strains and the single MSSA strain from the model at a standard inoculum. It was not possible to relate clearance to initial strain MIC. Previous work simulating human serum carbapenem concentrations against MSSA has shown significant ABE in in vitro models, with 3- to 4-log drops in viable counts up to 6 h and 4- to 5-log drops at 12 to 24 h (6, 11, 14, 24, 25). Our data are in keeping with this, with the exception that the strains we used were mainly MRSA.
Increasing the initial bacterial inoculum from 106 CFU/ml to 108 CFU/ml resulted in around a 5-log reduction in count by 24 h with approximately 3 to 4 log units of bacteria being detected to 48 h. This has also been observed when meropenem was tested against MSSA at an initial inoculum of 108 CFU/ml (6).
There is clinical evidence to suggest that β-lactam antibiotics such as isoxazyl penicillins (penicillinase-resistant penicillins) may offer advantages over vancomycin in the therapy of human MSSA infection (21, 31). Recently, this has been related to the high in vitro and in vivo activity of cloxacillin compared to that of vancomycin (12). Our comparison of tomopenem with vancomycin by use of three strains of MRSA clearly indicated that tomopenem had an ABE superior to that of vancomycin in terms of clearance of pathogens from the model.
The second part of the study employed a dose ranging design to determine the T>MIC for a range of ABE. These data indicated that for both 106 and 108 CFU/ml inocula, the T>MIC for a 1- to 2-log drop in viable count at 24 h was on average 10 to 20% of the dosing interval, with the maximum response occurring at about 30% of the dosing interval. Of importance, however, is the strain-to-strain variation in T>MIC which results in significant spread from mean T>MICs. These values are somewhat lower than some T>MICs reported for animal models with S. aureus and doripenem, where the static effect occurred in the range from 27 to 35% and a 2-log kill was between 31 and 41%. In contrast, the anti MRSA carbapenem PZ601 had a T>MIC of 8% ± 5% for a static effect against S. aureus, including MRSA, and required a T>MIC of 19.5% ± 3.47% for a 2-log kill (2). These values are very close to those reported here. In addition, animal studies of the anti-MRSA cephalosporin PPI 0903/TAK 599 indicated static-effect T>MIC in the range from 15 to 36% and a 2-log kill between 23 and 51%; the maximum effect occurred at a free drug concentration of >40% (3). Our data are similar to these results, with a maximal effect at a free drug T>MIC of >30% of the dosing interval being slightly lower with the carbapenem than with cephalosporin, as might be expected. Inoculum did not have an impact on the T>MIC-ABE relationship.
Studies on emergence of resistance to carbapenems in S. aureus in in vitro pharmacokinetic models are rare. Our data indicate that at T>MICs of >30%, the risk of resistance is very low. This contrasts to situations where the T>MIC is <10%.
In conclusion, these preclinical pharmacokinetic model data are supportive of the use of tomopenem to treat MRSA in humans. Tomopenem shows good ABE against MRSA strains, and this effect is not impaired significantly by high inoculum. Comparative studies with vancomycin indicated superiority, while the T>MIC for a maximum effect was around 30% and the T>MIC for a static effect was less than 10%.
A T>MIC target of 30% can be used to set clinical breakpoints for tomopenem against MRSA; at this level of exposure, there is little risk of the emergence of resistance.
Acknowledgments
This study was funded by Hoffman La Roche Inc., Nutley, NJ.
In particular, we would like to thank Brian Dannemann for his support of this project.
Footnotes
Published ahead of print on 28 January 2008.
REFERENCES
- 1.Andes, D. R., S. Kiem, and W. A. Craig. 2003. In vivo pharmacodynamic activity of a new carbapenem, doripenem, against multiple bacteria in a murine thigh infection model, A-308, p. 10. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 2.Andes, D. R., and W. A. Craig. 2006. Pharmacodynamics of a new cephalosporin PPI 0903 (TAK 599) activity against methicillin-resistant Staphylococcus aureus in murine thigh and lung infection models: identification of an in vivo pharmacokinetic-pharmacodynamic target. Antimicrob. Agents Chemother. 50:1376-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andes, D. R., and W. A. Craig. 2006. In vivo pharmacodynamic activity of a new carbapenem, PZ601, against multiple bacteria in murine thigh and lung infection models. F1-232, p. 199. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, San Francisco, CA.
- 4.Andrews, J. 1999. Microbiological assays, p. 35-45. In D. S. Reeves, R. Wise, J. M. Andrews, and L. O. White (ed.), Clinical antimicrobial assays. Oxford University Press, Oxford, United Kingdom.
- 5.Bowker, K. E., H. A. Holt, D. S. Reeves, and A. P. MacGowan. 1996. Bactericidal activity, post antibiotic effect and modified effective regrowth time of meropenem at high concentrations. J. Antimicrob. Chemother. 38:1055-1060. [DOI] [PubMed] [Google Scholar]
- 6.Bowker, K. E., H. A. Holt, R. J. Lewis, D. S. Reeves, and A. P. MacGowan. 1998. Comparative pharmacodynamics of meropenem using an in vitro model to simulate once, twice and three times daily dosing in humans. J. Antimicrob. Chemother. 42:461-467. [DOI] [PubMed] [Google Scholar]
- 7.British Society for Antimicrobial Chemotherapy, Working Party. 2001. Antimicrobial susceptibility testing. J. Antimicrob. Chemother. 46(Suppl. S1):5-16. [DOI] [PubMed] [Google Scholar]
- 8.Brown, N. P., C. M. Pillar, D. C. Draghi, M. E. Jones, B. Dannemann, and D. F. Sahm. 2007. Baseline profile of RO4908463 (CS-23) against recent isolates of target gram-negative pathogens exhibiting β-lactam resistant phenotypes from Europe 2003-2006, abstr. P-16663. Abstr. 17th Eur. Congr. Clin. Microbiol. Infect. Dis./25th Int. Congr. Chemother., Munich, Germany.
- 9.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing. CLSI document M100-S16. Clinical and Laboratory Standards Institute, Wayne, PA.
- 10.Craig, W. A. 2002. Pharmacodynamics of antimicrobials: general concepts and applications, p. 1-22. In C. H. Nightingale, T. Murakawa, and P. G. Ambrose (ed.). Antimicrobial pharmacodynamics in theory and clinical practice. Marcel Dekker Inc., Basel, Switzerland.
- 11.Della Bruna, C., D. Jabes, R. Rossi, G. Younes, and P. Castellani. 1989. FCE 22101 and FCE 22891: in vitro antibacterial activity at concentrations simulating human plasma levels following intravenous, intramuscular and oral administration. J. Antimicrob. Chemother. 23(Suppl. C):119-128. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes Guerrero, M. L., and M. de Gorgolas. 2006. Comparative activity of cloxacillin and vancomycin against methicillin-susceptible Staphylococcus aureus experimental endocarditis. J. Antimicrob. Chemother. 58:1066-1069. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari, A., G. Grassi, F. A. Grassi, P. D. Piccioni, and G. Gialdroni Grassi. 1989. Bactericidal activity of meropenem and interactions with other antibiotics. J. Antimicrob. Chemother. 24(Suppl. A):239-250. [DOI] [PubMed] [Google Scholar]
- 14.Firsov, A. A., and H. Mattie. 1997. Relationships between antimicrobial effect and area under the concentration time curve as a basis for comparison of modes of antibiotic administration: meropenem bolus injections versus continuous infusions. Antimicrob. Agents Chemother. 41:352-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fluckiger, U., C. Segessenmann, and A. U. Gerber. 1991. Integration of pharmacokinetics and pharmacodynamics of imipenem in a human-adapted mouse model. Antimicrob. Agents Chemother. 35:1905-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuentes, F., M. M. Martin, J. Izquierdo, M. L. Gomez-Lius, and J. Prieto. 1995. In vivo and in vitro study of several pharmacodynamic effects of meropenem. Scand. J. Infect. Dis. 27:469-474. [DOI] [PubMed] [Google Scholar]
- 17.Jones, M. E., M. A. Cohen, N. P. Brown, B. Dannemann, and D. F. Sahm. 2006. RO4908463 demonstrates a low potential for in vitro selection of resistance in S. aureus, E. coli, and P. aeruginosa, abstr. C1-35, p. 65. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, San Francisco, CA.
- 18.Kawamoto, I., Y. Skinoji, O. Kanno, K. Kojima, K. Ishikawa, E. Matsuyama, Y. Ashida, T. Shibayama, T. Fukuoka, and S. Ohya. 2003. Synthesis and structure-activity relationships of novel parenteral carbapenems, CS-023 (R-115685) and related compounds containing an amidine moiety. J. Antibiot. (Tokyo) 56:565-579. [DOI] [PubMed] [Google Scholar]
- 19.Koga, T., T. Abe, H. Inove, T. Takenouchi, A. Kitayama, T. Yoshida, et al. 2005. In vitro and in vivo antibacterial activities of CS-023 (RO4908463), a novel parenteral carbapenem. Antimicrob. Agents Chemother. 49:3239-3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leggett, J. E., B. Fantin, S. Ebert, K. Totsuka, B. Vogelman, W. Calame, H. Mattie, and W. A. Craig. 1989. Comparative antibiotic dose effect relations at several dosing intervals in murine pneumonitis and thigh-infection models. J. Infect. Dis. 159:281-292. [DOI] [PubMed] [Google Scholar]
- 21.Levine, D. P., B. S. Fromm, and B. R. Reddy. 1991. Slow response to vancomycin or vancomycin plus rifampicin in methicillin-resistant Staphylococcus aureus endocarditis. Ann. Intern. Med. 115:674-680. [DOI] [PubMed] [Google Scholar]
- 22.MacGowan, A. P., D. S. Reeves, and R. Wise. 1999. Clinical interpretation of antimicrobial assays. In D. S. Reeves, R. Wise, J. M. Andrews, and L. O. White (ed.), Clinical antibiotic assays. Oxford University Press, Oxford, United Kingdom.
- 23.MacGowan, A. P., C. A. Rogers, H. A. Holt, and K. E. Bowker. 2003. Activities of moxifloxacin against, and emergence of resistance in, Streptococcus pneumoniae and Pseudomonas aeruginosa in an in vitro pharmacokinetic model. Antimicrob. Agents Chemother. 47:1088-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maggiolo, F., A. Tarus, S. Frontespezi, F. Bottari, M. C. Legnani, and F. Suter. 1993. Bactericidal activity of two different dosage regimens of imipenem in an in vitro dynamic model. J. Antimicrob. Chemother. 27:295-300. [DOI] [PubMed] [Google Scholar]
- 25.Mattie, H., L.-C. Zhang, E. van Strigen, B. R. Sekin, and A. E. A. Douwes-Idema. 1997. Pharmacokinetic and pharmacodynamic models of the anti staphylococcal effects of meropenem and cloxacillin in vitro and in experimental infection. Antimicrob. Agents Chemother. 41:2083-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGrath, B. J., C. R. Marchbanks, D. Gilbert, and M. N. Dudley. 1993. In vitro postantibiotic effect following repeated exposure to imipenem, temafloxacin, and tobramycin. Antimicrob. Agents Chemother. 37:1723-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munckhof, W. J., D. Olden, and J. D. Turnidge. 1997. The postantibiotic effect of imipenem relationship with drug concentration, duration of exposure, and MIC. Antimicrob. Agents Chemother. 41:1735-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Periti, P., and P. Nicoletti. 1999. Classification of betalactam antibiotics according to their pharmacodynamics. J. Chemother. 11:323-330. [DOI] [PubMed] [Google Scholar]
- 29.Shibayama, T., Y. Matsushita, T. Hirota, T. Ikeda, and S. Kuwahara. 2006. Pharmacokinetics of CS-023 (RO4908463), a novel parenteral carbapenem, in healthy male Caucasian volunteers. Antimicrob. Agents Chemother. 50:4186-4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shibayama, T., Y. Matsushita, K. Kawal, T. Hirota, T. Ikeda, and S. Kuwahara. 2007. Pharmacokinetics and disposition of CS-023 (RO4908463), a novel parenteral carbapenem, in animals. Antimicrob. Agents Chemother. 50:257-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Small, P. M., and H. F. Chambers. 1990. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob. Agents Chemother. 34:1227-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svensson, E., H. Hanberger, M. Nilsson, and L. E. Nilsson. 1998. Pharmacodynamic effects of amikacin, ciprofloxacin and imipenem on growing and non-growing Escherichia coli and Pseudomonas aeruginosa. Clin. Microbiol. Infect. 5:140-148. [DOI] [PubMed] [Google Scholar]
- 33.Thomson, K. S., and E. S. Moland. 2004. CS-023 (R-115685), a novel carbapenem with enhanced in vitro activity against oxacillin-resistant Staphylococci and Pseudomonas aeruginosa. J. Antimicrob. Chemother. 54:557-562. [DOI] [PubMed] [Google Scholar]
- 34.Walker, R., D. Andes, R. Comlin, S. Ebert, and W. Craig. 1994. Pharmacodynamic activities of meropenem in an animal model, abstr. A91, p. 147. Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Orlando, FL.