Abstract
Eighty adults in areas of Kenya where malaria is holoendemic received presumptive treatment with atovaquone-proguanil and were followed closely. The time to the first Plasmodium falciparum parasitemia was 32 days. This prolonged prophylaxis period has implications for study design when used in malaria intervention trials and cautiously suggests clinical investigation of potential preexposure prophylaxis of malaria.
Malaria intervention trials in study populations subject to continuous mosquito transmission often measure the time to parasitemia as the primary efficacy end point (1, 2, 8). In order to maximize comparability, presumptive antimalarial treatment is given to control and intervention groups to eliminate subpatent parasitemia. This may involve the use of drugs such as atovaquone and proguanil that exert causal prophylactic activity by eliminating the development of hepatic-stage parasites. The selection of presumptive drug treatment is based upon considerations of drug registration status, parasite susceptibility, practicality of administration under direct observation, half-life, and tolerability. Careful timing is required to avoid interference. Drugs must be given early enough to maximize the elimination of parasites and to avoid an antiparasitic effect during efficacy determination. Conversely, drugs must be given late enough such that there is no interval during which new parasitemias might arise prior to the start of the period of efficacy determination.
A fixed combination of atovaquone and proguanil (Malarone; GlaxoSmithKline, United Kingdom) is licensed in many countries for the prevention and treatment of acute, uncomplicated Plasmodium falciparum malaria. Three daily doses are almost 100% effective for the treatment of falciparum malaria, including highly drug-resistant strains from Southeast Asia (4). However, this dosage regimen has not been studied or received regulatory approval for presumptive treatment of malaria, especially for travelers. We report the use of atovaquone-proguanil as presumptive treatment in the context of a malaria vaccine trial in malaria-immune adults.
The protocol was approved by the Kenya Medical Research Institute Ethical Review Committee and the U.S. Army Medical Research and Materiel Command's Human Subjects Research Review Board, Fort Detrick, MD. The ClinicalTrials.gov identifier is NCT00197054. Subjects were recruited from Kombewa Division of the Kisumu West District within 1-mile-radius catchment areas around each of 13 field stations. The field stations were staffed 24 h and had radio communications and vehicles throughout the study to facilitate referral medical care. Malaria is holoendemic with peak malaria transmission periods from April to August and from October to December. Area residents receive 100 to 300 infectious mosquito bites/year (3). Malaria is mostly due to P. falciparum. After giving written informed consent, 255 healthy adults, 18 to 35 years of age, were enrolled and randomized to receive either one of two malaria vaccines or a rabies vaccine as the comparator on a 0-, 4-, or 8-week schedule. At week 7, each subject received four tablets (as a single dose), each containing 250 mg of atovaquone and 100 mg of proguanil, for three days, administered by study personnel to ensure compliance. Only the subjects in the rabies comparator group are reported here. Subjects were considered to be at risk from the day of conclusion of atovaquone-proguanil treatment until one of the following conditions occurred: development of parasitemia, loss to follow-up, emigration from the study area, withdrawal, or end of follow-up period. Passive detection of infection began immediately. Active detection of infection by scheduled weekly malaria blood films began at week 2 postcompletion of atovaquone-proguanil. The time at risk took into account absences from the study area. Subjects were followed for the first instance of P. falciparum parasitemia detected microscopically by malaria blood film. Each slide was read independently by two microscopists, each of whom examined 100 oil immersion fields before declaring a slide to be negative. Discrepancies in slide reading were adjudicated by a senior, third microscopist.
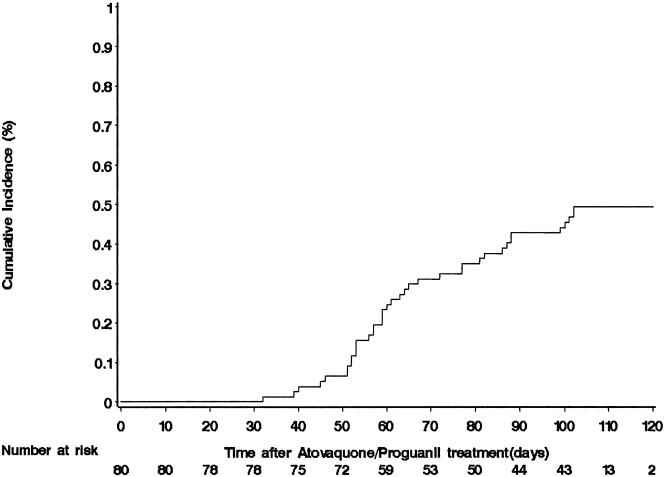
Eighty malaria-immune subjects (11 female, 69 male) completed 3 days of atovaquone-proguanil in September 2005 and were followed through January of 2006. P. falciparum parasitemia-free survival is depicted in Fig. 1. The first instance of P. falciparum parasitemia occurred 32 days after the completion of atovaquone-proguanil. The intervals for 95%, 90%, and 80% parasitemia-free survival were 45, 51, and 57 days. At the conclusion of the 17-week follow-up period, 38 of 80 (49.4%) subjects had had a first instance of P. falciparum parasitemia.
FIG. 1.
Kaplan-Meier curve showing the time delay until P. falciparum parasitemia following treatment with atovaquone-proguanil, 1,000 mg and 400 mg, respectively, daily for 3 days in 80 malaria-immune adults in areas of western Kenya where malaria is holoendemic.
An additional ad hoc analysis examined whether treatment for malaria during the 7 weeks from the receipt of the first immunization to the time of the first dose of atovaquone-proguanil affected malaria attack rates. All malaria cases in this interval received standard oral artemether-lumefantrine treatment. There was a nonsignificant trend by log-rank analysis (P = 0.155) toward earlier new infection in those who had received artemether-lumefantrine, suggesting no confounding of results by persistent artemether-lumefantrine effects.
Our findings are consistent with a study of 65 adults in western Kenya which reported a 29-day time to initial infection after an identical 3-day treatment regimen of daily atovaquone-proguanil (10). This long antimalarial effect may be partially explained by persistent serum schizonticidal activity attributable to serum atovaquone, as was shown in in vitro assays in two serologic studies (5, 6). Future use of atovaquone-proguanil for the radical/suppressive cure of malaria in the setting of interventional trials should allow at least 28 days before initiating surveillance for efficacy.
These data may also be viewed as preexposure chemoprophylaxis using a 3-day daily treatment regimen of atovaquone-proguanil (11), an approach that might benefit short-term malaria-naïve travelers to areas where malaria is endemic. We caution that the prophylactic efficacy described here with malaria-immune subjects probably reflects both drug effect and some anti-blood-stage immunity and might be greater than would be found in malaria-naïve travelers. The use of an antimalarial drug combination in intervals that exceed the therapeutic persistence of one of the two drugs would promote the emergence and selection of parasites resistant to the drug with the longer half-life. This is a specific risk for such use of atovaquone-proguanil, since the more persistent drug atovaquone is well described to engender resistance when employed as a single agent for malaria treatment (7) or when P. falciparum is cultured in the presence of low concentrations of atovaquone (9).
Careful clinical challenge and field studies would be required to define a period of time for short-term malaria-naïve travelers to areas where malaria is endemic, likely to be in the range of 2 to 4 weeks, during which adequate protection could be expected following a single preexposure treatment course of atovaquone-proguanil. Strict one-time use and prompt return to an area where malaria is nonendemic might limit or prevent the emergence or the propagation of atovaquone-proguanil-resistant parasites.
Acknowledgments
We thank Colonel Alan Magill and G. Dennis Shanks and Dennis E. Kyle for their critical review and commentary. However, the opinions expressed herein are solely those of the authors.
This study was funded by the U.S. Army Medical Materiel Development Activity, Fort Detrick, MD.
Marc Lievens and W. Ripley Ballou are employees of and own stock in GlaxoSmithKline, the manufacturer of Malarone, the combination drug described in this report.
The views expressed herein are private and do not reflect official positions of the U.S. Army or U.S. Department of Defense or GlaxoSmithKline. The opinions expressed are personal and are not to be construed as use guidelines or to contradict or replace current labeling indications and guidelines for the use of atovaquone-proguanil.
Footnotes
Published ahead of print on 11 February 2008.
REFERENCES
- 1.Alonso, P. L., J. Sacarlal, J. J. Aponte, A. Leach, E. Macete, J. Milman, I. Mandomando, B. Spiessens, C. Guinovart, M. Espasa, Q. Bassat, P. Aide, O. Ofori-Anyinam, M. M. Navia, S. Corachan, M. Ceuppens, M. C. Dubois, M. A. Demoite, F. Dubovsky, C. Menedez, N. Tornieporth, W. R. Ballou, and J. Cohen. 2004. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet 364:1411-1420. [DOI] [PubMed] [Google Scholar]
- 2.Bejon, P., J. Mwacharo, O. Kai, T. Mwangi, P. Milligan, S. Todryk, S. Keating, T. Lang, B. Lowe, C. Gikonyo, C. Molyneux, G. Fegan, S. C. Gilbert, N. Peshu, K. Marsh, and A. V. Hill. 2006. A phase 2b randomised trial of the candidate malaria vaccines FP9 ME-TRAP and MVA ME-TRAP among children in Kenya. PLoS Clin. Trials 1:e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloland, P. B., T. K. Ruebush, J. B. McCormick, J. Ayisi, D. A. Boriga, A. J. Oloo, R. Beach, W. Hawley, A. Lal, B. Nahlen, V. Udhayakumar, and C. C. Campbell. 1999. Longitudinal cohort study of the epidemiology of malaria infections in an area of intense malaria transmission. I. Description of study site, general methodology, and study population. Am. J. Trop. Med. Hyg. 60:635-640. [DOI] [PubMed] [Google Scholar]
- 4.Boggild, A. K., M. E. Parise, L. S. Lewis, and K. C. Kain. 2007. Atovaquone-proguanil: report from the CDC expert meeting on malaria chemoprophylaxis (II). Am. J. Trop. Med. Hyg. 76:208-223. [PubMed] [Google Scholar]
- 5.Butcher, G. A., and R. E. Sinden. 2003. Persistence of atovaquone in human sera following treatment: inhibition of Plasmodium falciparum development in vivo and in vitro. Am. J. Trop. Med. Hyg. 68:111-114. [PubMed] [Google Scholar]
- 6.Edstein, M. D., B. M. Kotecka, K. L. Anderson, D. J. Pombo, D. E. Kyle, K. H. Rieckmann, and M. F. Good. 2005. Lengthy antimalarial activity of atovaquone in human plasma following atovaquone-proguanil administration. Antimicrob. Agents Chemother. 49:4421-4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Looareesuwan, S., C. Viravan, H. K. Webster, D. E. Kyle, D. B. Hutchinson, and C. J. Canfield. 1996. Clinical studies of atovaquone, alone or in combination with other antimalarial drugs, for treatment of acute uncomplicated malaria in Thailand. Am. J. Trop. Med. Hyg. 54:62-66. [DOI] [PubMed] [Google Scholar]
- 8.Moorthy, V. S., E. B. Imoukhuede, P. Milligan, K. Bojang, S. Keating, P. Kaye, M. Pinder, S. C. Gilbert, G. Walraven, B. M. Greenwood, and A. S. Hill. 2004. A randomised, double-blind, controlled vaccine efficacy trial of DNA/MVA ME-TRAP against malaria infection in Gambian adults. PLoS Med. 1:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rathod, P. K., T. McErlean, and P. C. Lee. 1997. Variations in frequencies of drug resistance in Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 94:9389-9393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shanks, G. D., B. O. Ragama, and A. J. Oloo. 1999. Time to reappearance of malaria parasites following various drug treatment regimens in a holoendemic area of western Kenya. Trans. R. Soc. Trop. Med. Hyg. 93:304-305. [DOI] [PubMed] [Google Scholar]
- 11.Shanks, G. D., A. J. Magill, D. O. Freedman, J. S. Keystone, D. J. Bradley, and R. Steffen. 2007. Drug-free holidays: pre-travel versus during travel malaria chemoprophylaxis. Am. J. Trop. Med. Hyg. 77:1-2. [PubMed] [Google Scholar]