Abstract
Summary: Infection of humans with the nematode worm parasite Anisakis simplex was first described in the 1960s in association with the consumption of raw or undercooked fish. During the 1990s it was realized that even the ingestion of dead worms in food fish can cause severe hypersensitivity reactions, that these may be more prevalent than infection itself, and that this outcome could be associated with food preparations previously considered safe. Not only may allergic symptoms arise from infection by the parasites (“gastroallergic anisakiasis”), but true anaphylactic reactions can also occur following exposure to allergens from dead worms by food-borne, airborne, or skin contact routes. This review discusses A. simplex pathogenesis in humans, covering immune hypersensitivity reactions both in the context of a living infection and in terms of exposure to its allergens by other routes. Over the last 20 years, several studies have concentrated on A. simplex antigen characterization and innate as well as adaptive immune response to this parasite. Molecular characterization of Anisakis allergens and isolation of their encoding cDNAs is now an active field of research that should provide improved diagnostic tools in addition to tools with which to enhance our understanding of pathogenesis and controversial aspects of A. simplex allergy. We also discuss the potential relevance of parasite products such as allergens, proteinases, and proteinase inhibitors and the activation of basophils, eosinophils, and mast cells in the induction of A. simplex-related immune hypersensitivity states induced by exposure to the parasite, dead or alive.
INTRODUCTION
More than 1 billion people worldwide are infected with one or more species of gastrointestinal nematode worm parasite (282), which cause a wide range of conditions from the mild to the lethal. Related parasites of domestic animals impose a significant economic burden, reducing productivity and requiring elaborate and expensive control methods (56, 199). Humans can also be accidental hosts for nematode parasites that cannot progress their life cycles in humans but nevertheless can cause debilitating diseases directly or by initiating immune hypersensitivity states. In the latter category, the nature of the deleterious immune response is determined by the cytokine bias that typifies the normal protective response reactions against worm parasites. The subject of this review is a case in point, realized first in the 1960s as an unusual infection and then increasingly recognized as a cause of severe hypersensitivity reactions in the 1990s.
The consumption of raw or undercooked fish may lead to infection with several helminths, but the nematode worm species most commonly involved in human infections is Anisakis simplex, and Pseudoterranova decipiens is less frequent but still common (131, 281, 285) (Fig. 1 illustrates a heavy infection of fish muscle). Infection with the closely related Anisakis physeteris and Contracaecum spp. has been reported in only a very few cases (21, 57, 124). The first case of human infection by a member of the family Anisakidae was reported more than 50 years ago in The Netherlands by Van Thiel. This author described the presence of a marine nematode in the center of an eosinophilic intestinal phlegmon from a patient suffering from acute abdominal pain as a “very unusual finding.” Later, the nematode was identified as Anisakis spp., a common parasite of marine fish and mammals, and the human parasitosis was named anisakiasis (273). Since then, the majority of anisakiasis cases have been described by Japanese authors (124), reflecting the frequent consumption of raw fish in that country. Cases have now been reported from the five continents of Asia (Korea), Europe (The Netherlands, France, the United Kingdom, Spain, Germany, Italy, and others), Africa (Egypt), and the Americas (the United States, including Alaska and Hawaii; Canada; and South American countries) as well as from New Zealand (see references 40, 49, 57, 77, 121, 154, 169, 194, 219, 233, and 278 for reviews).
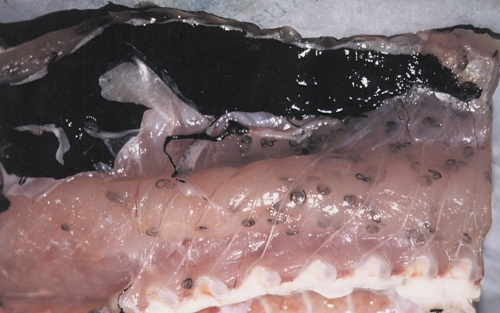
FIG. 1.
High-level parasitism by Anisakis simplex L3 in the flesh of a hake (Merluccius merluccius). Larvae persist in an arrested development stage (hypobiosis) prior to ingestion by the final host. Embedded larvae are dark in color due to a protective cuticle and different from the pinkish-white color typical of free and motile L3 (see Fig. 4).
Since the 1960s, the term anisakiasis has been used to designate not only the human disease caused by the third-stage larvae (L3) of Anisakis spp. but also the human disease caused by L3 of other members of the Anisakidae family. In 1988, a group of experts on the standardized nomenclature of animal parasite diseases recommended the use of three different terms: (i) anisakidosis for disease caused by any member of the family Anisakidae, (ii) anisakiasis for disease caused by members of the genus Anisakis, and (iii) pseudoterranovosis for disease caused by members of the genus Pseudoterranova (137). This terminology will be used in this review.
Human anisakid infections frequently cause gastrointestinal symptoms, which may be associated with mild to severe immunological, usually allergic-type, reactions. In addition, some patients show more-generalized hypersensitivity reactions, without any associated digestive disorders. We therefore consider that anisakid larvae may be responsible for four clinical forms of illness in humans: gastric, intestinal, and ectopic anisakidosis and allergic forms. A. simplex extracts are now included in the standard sets of allergens for the investigation of food allergies, anaphylaxis, and even drug allergies (189). Recently, moreover, Daschner et al. suggested its possible involvement in chronic urticaria (75). Rheumatic symptoms may appear in the course of anaphylaxis but are extremely rare (68). Alarmingly, some episodes of allergy have been described in association with exposure to even very small doses of A. simplex antigens and without the involvement of living parasites: two episodes of anaphylaxis have been reported in direct association with skin prick testing using an A. simplex extract (25, 50). It has also been reported that A. simplex allergy behaves as other severe food allergies in that it can be provoked by skin contact or inhalation of minute quantities of the allergen (258), causing occupational hazards such as conjunctivitis (12) and asthma (17, 220, 241), protein contact dermatitis, and conjunctivitis (51, 61). More recently, a cross-sectional epidemiologic analysis of South African fish-processing workers revealed a high prevalence (8%) of sensitization to A. simplex (higher than sensitization to fish) associated with dermatitis and nonspecific bronchial hyperreactivity (201). Furthermore, allergic reactions attributable to A. simplex sensitivity can arise even from the consumption of chicken meat, presumably from birds fed with fish meal contaminated with parasite material (16).
EPIDEMIOLOGY
Anisakidosis
Infective-stage larvae found within the flesh of sea fish or cephalopods can be ingested alive by humans, causing anisakidosis as a zoonotic disease (29, 281; http://www.nhm.ac.uk/research-curation/projects/host-parasites) (Fig. 2 shows the life cycle of the parasite). A. simplex infects humans accidentally when raw or undercooked fish contaminated with larvae is consumed. These larvae are in their third developmental stage (L3) and are developmentally arrested until ingested by sea mammals such as seals and dolphins, whereupon they progress through two more developmental stages until adulthood is achieved. Once in the human gastrointestinal tract, L3 of A. simplex, and particularly those of P. decipiens, may progress to L4, but only in exceptional cases may the immature adult stage be reached (29; reviewed by Kliks [155]).
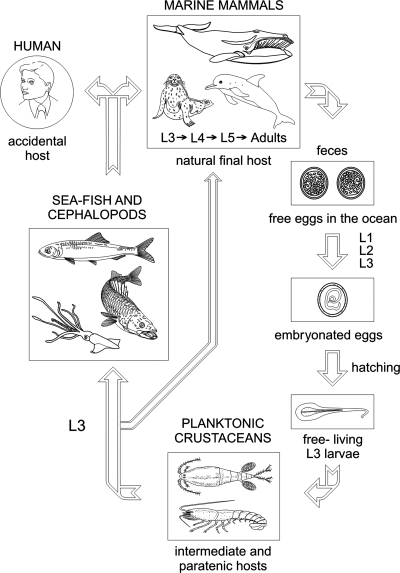
FIG. 2.
Life cycle of Anisakis simplex including accidental human hosts. Adult parasites live in the stomach of marine mammals and, following copulation, fertilized but unembryonated eggs are expelled with the feces. The eggs develop and then hatch, releasing free-living Anisakis simplex L3. These L3 are ingested by euphausiid oceanic krill and copepods (intermediate hosts). Sea fish and cephalopods (paratenic hosts) ingest planktonic crustaceans or other fish and cephalopods infected with L3, contributing to the dissemination of the parasite. The infective L3 (embedded in the viscera and muscle or free in the body cavity) are transferred to the final hosts (marine mammals) by ingestion of sea fish and cephalopods (in the case of dolphins, porpoises, seals, sea lions, and walruses) or via oceanic krill (in the case of whales). In the final host, two molts occur (from L3 to adult) before sexual maturity to produce eggs, and a further life cycle is initiated. If L3-infected raw fish or cephalopods are eaten by humans, larvae present in the flesh produce a zoonotic infection, and humans act then as accidental hosts, since L3 usually do not develop any further and the cycle cannot be completed.
The transmission of these food-borne pathogens is particularly associated with traditions of consumption of raw or undercooked fish. A number of fish dishes are considered to be high risk for the contraction of anisakidosis, including Japanese sushi and sashimi, Filipino bagoong, Dutch salted or smoked herring, Scandinavian gravlax, Hawaiian lomi-lomi and palu, South American ceviche, and Spanish boquerones en vinagre (pickled anchovies) (see references 29, 80, 205, 233, and 276 for reviews). The preparation of these dishes may involve methods (salting, curing, marinating, pickling, and smoking at 40°C, etc.) which are generally sterilizing for other food-borne pathogens but not for anisakids (see references 11, 29, 136, 217, 233, and 251 for reviews).
Therefore, the use of (i) temperatures of ≥60°C for at least 1 min when cooking fish conventionally or smoking fish, (ii) heating to ≥74°C for at least 15 seconds when microwave cooking (3), and (iii) freezing at temperatures lower than −20°C for at least 24 h (European and Japanese species) of fish to be eaten raw (including marinated fish, fish smoked at low temperatures, or fish prepared by other techniques which do not guarantee L3 death) has been recommended to kill the parasites and prevent live infections. In some studies carried out with A. simplex and Pseudoterranova spp. consumed in the United States, the parasites have been found to survive −20°C for short periods (37, 80) and therefore prolonged freezing (−20°C or below) for at least 1 week or blast freezing (−35°C or below) for at least 15 h is recommended by the FDA. These preventive measures have been adopted by the fish industry as Hazard Analysis and Critical Control Points (HACCP) systems (11, 59, 267) and as EEC (81, 86), Canadian (280), and FDA (97) legislation.
Usually within a few hours after the ingestion of a living worm, A. simplex causes an acute and transient infection that may lead to abdominal pain, nausea, vomiting, and/or diarrhea. Some patients develop syndromes simultaneously exhibiting clinical manifestations of allergy and infection after eating living parasites; this was first described by Kasuya and coworkers (138, 139) and represents a borderline disease between parasitic infection and allergy termed “gastroallergic anisakiasis” (71).
Epidemiological studies in Japan have found that anisakiasis is more frequent in coastal populations and among 20- to 50-year-old males (20, 251). The main fish species transmitting anisakidosis are the spotted chub mackerel (Scomber japonicus) and the Japanese flying squid (Todarodes pacificus) (196, 197, 205, 256).
There is a study reported by Cheng (1971) that was unable to find human anisakiasis in Taiwan (reviewed by Smith and Wootten [251]), and we are aware of only a single case published since then. It is not clear why there is a difference between Chinese and Japanese populations in the prevalence of the condition, given that fish consumption is common in both. Cheng suggested that anisakidosis cases are not common in China because Chinese eat fish at the end of meals, when the stomach is full, unlike Japanese. Other authors investigating the killing activities of vegetable condiments used in both traditional Chinese “raw fish cooking” and medicine have found that perilla leaves (Perilla frutescens) and ginger rhizomes (Zingiber officinale) are lethal for L3 (106).
In the United States, the majority of reported anisakidosis cases have been due to ingestion of Pacific salmon (Oncorhynchus spp.) (77, 78, 79, 205), and one study has shown that up to 10% of the salmon consumed in the sushi bars of the Seattle region contained larvae of Anisakis spp. (2). In Western Europe, herring (Clupea arengus) is the main species involved (195, 274, 275), although cases with other species that were insufficiently cooked (microwaved, grilled, or shallow fried) have also been reported. In Spain, most cases have been related to the consumption of pickled anchovies (Engraulis encrasicholus) (10, 69).
Development of better diagnostic tools and greater awareness has resulted in an increase in the frequency of reports in many parts of the world, including the United States (77, 79, 205) since the 1980s and Canada (63) and Europe (40, 69, 208, 219, 249) since the 1990s. Sakanari and McKerrow (233) pointed out reasons for the increased number of cases in the United States in the 1980s that are still applicable worldwide today, summarized as follows: (i) the well-known worldwide distribution of anisakid nematodes (reviewed in references 251 and 281), which have been reported from all major oceans and seas; (ii) the increase in marine mammal populations (hosts of the adult parasites) as a result of conservation measures; (iii) human migratory movements and globalization and the consequent increase in consumer interest in exotic dishes; (iv) the use of faster cooking tools (microwave cookers, etc.) and an increasing trend not to overcook food; and (v) the acknowledged benefits of the Mediterranean diet (characterized in part by high fish consumption) to prevent heart diseases (29, 233).
Pseudoterranovosis (caused by the so-called “codworm”) is unusual in south Japan and Europe but it is more frequent in the United States and Canada and has also been reported in Korea and Chile (29, 131, 183, 205, 233, 252). The main transmitters of pseudoterranovosis appear to be Pacific cod (Gadus macrocephala) and the Pacific halibut (Hippoglossus stenolepis) in Japan and the red snapper (Sebastes spp.) in the United States (203).
Allergic Symptoms
Anisakis-associated hypersensitivity cases have been particularly noted in northern Spain. In the allergic cases from this region, the consumption of cooked hake (Merluccius merluccius) predominates, closely followed by cooked or raw anchovies (29, 95) (Table 1). It is becoming apparent that A. simplex is the most important hidden food allergen in the adult population suffering acute urticaria (82) and anaphylaxis (28, 29) of the Basque Country in northern Spain, and this recognition has now spread to other regions in Spain (13). It is also the etiologic factor most commonly associated with urticaria for any specific food allergy in the adult population and comprises as much as 10% of the anaphylaxis previously diagnosed as idiopathic (28) (Fig. 3). Although food allergy is most frequent in atopic patients and children, A. simplex also induces allergic reactions in nonatopic middle-aged adults. Soon after the first description of allergic cases in the Basque Country, reports appeared from the central region of Spain but were there associated with gastrointestinal anisakidosis and with raw anchovies in vinegar (69). Moreover, a significant number of fishmongers and fishermen in Italy and African fish-processing workers, as well as 13% of healthy blood donors, are sensitized to this parasite (82, 201, 221). It should be borne in mind, however, that specific immunoglobulin E (IgE) detection by ImmunoCAP assay can overestimate the number of sensitized subjects (165, 167), so the above estimates may be exaggerated. However, there is clearly cause for concern, and a need for further analysis using more-refined methods and serodiagnostic tools.
TABLE 1.
Food fish and cephalopods reported to have been eaten by allergic patients suffering from anaphylaxis in Spain
| Host species | No. of outbreaks from indicated citya
|
||||||
|---|---|---|---|---|---|---|---|
| Total no. of patients
|
C
|
NC
|
Ca (A) | ||||
| A | B | A | B | A | B | ||
| Hake (Merluccius merluccius) | 23 | 2 | 22 | 2 | 2 | ||
| Anchovy (Engraulis encrasicholus) | 16 | 8 | 5 | 11 | 8 | ||
| Codfish (Gadus morhua) | 7 | 7 | |||||
| Tuna (Sarda sarda) and striped tunny (Thunnus thynnus) | 6 | 6 | 4 | ||||
| Sardine (Sardina pilchardus) | 3 | 1 | 3 | 1 | 1 | ||
| Squid (Illex coindetii and Toradopsis eblanae) | 3 | ||||||
| Horse mackerel (Trachurus picturatus) | 3 | 1 | |||||
| Megrim (Lepidorhombus boscii) | 2 | ||||||
C, cooked fish eaten; NC, fish eaten was not cooked; Ca, canned fish eaten; A, Vitoria-Gasteiz (Basque Country, Spain) (27); B, Burgos (Castilla-León Comunity, Spain) (99). Note that the total number of patients is not necessarily the sum of C plus NC plus Ca because some cases presented more than one episode of allergy with different fish preparations.
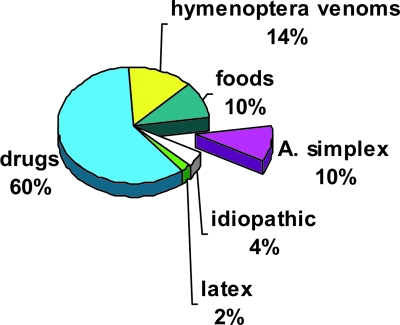
FIG. 3.
Attributions of anaphylaxis before and after screening for anti-Anisakis simplex responses. Adult patients studied in the Allergy Department of the Santiago Apóstol Hospital (Vitoria-Gasteiz, Basque Country, northern Spain). Cases of anaphylaxis (n = 625) were reviewed during six consecutive years (1994 to 1999). Allergic diagnoses of those cases were as follows: drug (n = 389), hymenoptera venom (n = 88), food (n = 67), parasites (n = 62), idiopathic (n = 32), and latex (n = 12). Note that the incidence of A. simplex allergy is similar to that of all food allergies combined (10%) and idiopathic causes are reduced from 14% (before A. simplex screening) to 4% when this parasite is considered. (Based on data from reference 27.)
PATHOGENICITY
Infections with parasitic helminths are characterized by the development of both allergic-type and immunomodulatory responses (52, 93, 109, 129, 152, 153, 209). Although fulminant allergic reactions to infections are rare, they can be important in the accidental release of parasite antigens into the general circulation during, for instance, surgery to remove hydatid cysts. Protective responses to intestinal nematodes are associated with the production of Th2 cytokines and the resulting mastocytosis, IgE response, and eosinophilia, all of which are typical of allergic reactions and immune responses to tissue-parasitic helminths (269). Which elements of these responses are associated with parasite expulsion remain to be determined, but mast cells, for example, appear to be involved (89, 181).
In addition to promoting Th2 responses, helminth infections have been shown to modulate immune responses to nonparasite or bystander heterologous antigens, and they can cause either potentiation or suppression of such responses (7, 52, 90, 109, 115, 171, 185, 191, 246, 286). These infections have been shown to be involved in breaking T-cell tolerance, with serious implications for the host (36, 88, 227). The pathologies associated include autoimmune diseases, nontolerance of oral antigens, increasing susceptibility to secondary infections, and decreasing vaccine efficacy (1, 227, 286). The ability to modulate immune responsiveness is not dependent on the presence of infections with a live parasite, because antigens derived from the parasite have similar properties, albeit seldom with the potency of a live infection (7, 34, 45, 62, 113, 171, 209).
Most helminth infections induce chronic rather than acute disease, even in cases of very high levels of parasites, and parasitic helminths have developed mechanisms to overcome and evade host immune responses to secure their survival. Human anisakidosis is peculiar because this parasite is not adapted to live in humans and infection is transitory (Table 2). Furthermore, more than 90% of the cases described are caused by a single larva (71, 120, 134). Differences may therefore be expected between A. simplex pathogenesis and that caused by other helminths in humans. An example of this is filariasis, in which there is a high and persistent burden of parasites, and it has been argued that the parasites and the hosts have coevolved in order to optimize their mutual survival (110, 185, 246, 260). Overt hypersensitivity reactions are rare unless provoked by natural or drug-induced death of parasites residing in tissues.
TABLE 2.
Summary chronological physiopathology of Anisakis simplex infection in humans
| Time after ingestion | Infection event(s) | Factors released or immune response | Tissue events |
|---|---|---|---|
| <1 h | Mucous adhesion | Proteolytic enzymes | Hemorrhagic lesions; worm burrowing and tunnel formation |
| 4 h to 6 days | Mucosa and submucosa penetration | Chemotactic factors | Eosinophilic phlegmon; erosive lesions |
| 7-14 days | Granuloma formation | Hypersensitivity response induction | Ulcerous lesions |
| >14 days | Larval death | Persistent inflammation or granuloma | Loss of parasite or chronic ulceration around remains |
Over the last few years, studies have indicated that, as with other helminth infections, the pathological changes occurring within the gastrointestinal tract during infection with A. simplex are the combined result of the direct action of the larva during tissue invasion and of the complex interaction between the host immune system and the substances released by, or contained within, the parasite. In Fig. 4 we provide a schematic illustrating the pathogenesis involving innate and adaptive immune responses to the parasite.
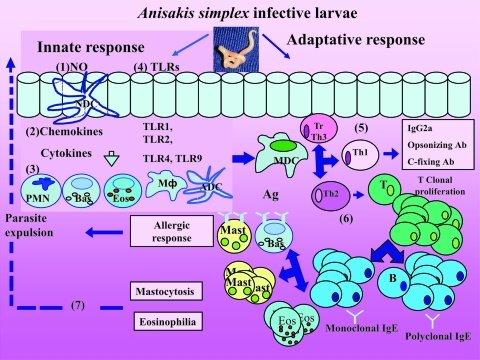
FIG. 4.
Schematic representation of Anisakis simplex pathogenicity in the alimentary tract. The human patient can be exposed to A. simplex antigens from several sources: ES antigens from living larvae and somatic and cuticular antigens from dead and disintegrating larvae present in food. Epithelial cells might secrete cytotoxic molecules such as NO (1) and also chemokines and cytokines (2), which attract polymorphonuclear leukocytes (PMN), tissue macrophages (MΦ), naïve dendritic cells (NDC), basophils (Bas), and eosinophils (Eos) (3). Innate responses may also involve TLRs (4) from epithelial cells and activated dendritic cells (ADC). In the adaptive response, antigen presentation by mature dendritic cells (MDC) stimulates a double response of Th1 (5) and Th2 (6). Other T cells can be recruited as T-regulatory cells and Th3. Th1 cytokines (IFN-γ, tumor necrosis factor beta, IL-2, and IL-3) (5) induce IgG2a, opsonizing and complement-fixing antibodies, macrophage activation, antibody-dependent cell-mediated cytotoxicity, and delayed-type hypersensitivity. Th2 cytokines (IL-4, IL-5, IL-6, IL-9, IL-10, and IL-13) promote IgG1 and IgA production (6), and by T-lymphocyte (T) stimulation, antigen-specific IgE and total/polyclonal IgE are produced. Mastocytosis and eosinophilia are induced by a Th2 response and chemoattractive cytokines and may be responsible for parasite expulsion (7). Basophils are crucial for the initiation of a Th2 response. Eosinophilia may be due to the release of numerous chemotactic factors by epithelial cells, T lymphocytes, mast cells, basophils, and factors derived directly from the parasites.
Invasive Mechanisms
In order to invade the gastrointestinal mucosa, the L3 of A. simplex probably use mechanical disruption of tissue combined with the release of potent proteolytic enzymes that are capable of degrading the extracellular matrix (142, 178, 182, 232, 234). These proteases are probably produced by the dorsal esophageal gland and the excretory cell of the A. simplex larva to be secreted through the excretory pore and the oral opening, respectively (46, 179, 231). Proteolytic entities of 54.3 kDa (179), 23.4 and 46 kDa (149), and 25 to 26 kDa (234, 235) have been isolated from excretory-secretory (ES) products released by A. simplex L3 in vitro. The latter is probably the same as the 23.4-kDa protein described by Kennedy and colleagues (149) and is similar to mammalian trypsin in its enzymatic activity (193). In 1994, a 40-kDa enzyme that degrades chondroitin sulfate-A and hyaluronic acid (114) and a 30-kDa serine protease similar to that present in the bacterium Dichelobacter nodosus (193) were characterized. The invasive capacity of the larvae, together with the presence of anticoagulant substances in the ES products, explains the existence of the multiple, well-defined, erosive, and/or hemorrhagic lesions usually detected near the main lesion within the gastric mucosa of patients suffering from anisakidosis (198, 213, 217).
The metabolic products released by the larva are also important from the immunological point of view. Humoral and cellular responses observed for acute lesions are mainly localized to the oral region of the parasite, where products are released by the larva and form insoluble immune complexes with antibody (38, 122). It has been demonstrated that certain ES as well as surface and somatic components of the parasites not only induce immune responses but also have other effects, such as the direct, IgE-independent degranulation of mast cells in sensitized mice, observed by Kobayashi in 1972 (reviewed by Smith and Wootten [251]). Other reported effects include immunosuppression associated with thermolabile components of 66 and 95 kDa in size (223, 224), mutagenic properties by components of 10 kDa (55, 217), anticoagulant activity (213, 217), and chemotaxis of eosinophils associated with thermolabile factors from the parasites (84, 259) that do not damage the surface of larvae but are partly responsible for the tissue damage induced in the host.
Immunopathologic Mechanisms
The involvement of immunologic mechanisms in the pathogenesis of acute anisakiasis was first proposed in 1964 by Kuipers with the “double-hit” hypothesis, proposed to explain why more-severe pathological changes occurred after reinfection in rabbits (160; reviewed by Smith and Wootten [251]). Subsequent experimental studies in orally infected guinea pigs and rabbits confirmed that sensitization (quantified as antibody response) and lesions were of greater severity following reinfection. These lesions were of a nature consistent with type I, type III, and type IV hypersensitivity reactions that may be involved in the immunopathology of anisakiasis (19, 123, 206).
Eosinophilia
One of the main features of the local inflammatory lesions produced by A. simplex larvae is the presence of a conspicuous eosinophilic infiltration in the tissues surrounding the parasite. These cells adhere to the nematode's epicuticle in the presence of antibodies (particularly in the oral region where the ES products are localized) releasing cytotoxic factors which, though apparently not capable of damaging the nematode's cuticle (76), are probably responsible for a great deal of tissue damage observed surrounding the parasite in both acute and chronic infections. Eosinophil concentration in damaged areas may be due not only to the release of numerous chemotactic factors by T lymphocytes, mast cells, and basophils but also to the secretion of chemotactic substances for these cells by the parasite. Surprisingly, although systemic eosinophilia is frequently associated with helminthic diseases, for anisakidosis it is described in fewer than 30% of cases (122, 176, 203).
Eosinophils appear to be incapable of destroying A. simplex larvae directly in vitro (76), but eosinophil infiltration of the tissue surrounding the parasite is one of the most distinctive features of the local inflammatory lesions observed for anisakiasis. The presence of these cells may reflect the late stage of a type I immune hypersensitivity response following the release of eosinophilic chemotactic factors during the acute stage of the response. The possibility that parasite-derived substances may specifically attract eosinophils is consistent with the observation made by Iwasaki and Torisu (126) that A. simplex extracts applied to the ilea of nonsensitized rabbits induce lesions characterized by local eosinophilia but a scant presence of mast cells and polymorphonuclear leukocytes. In summary, the effect of the eosinophil response lies mainly at the local (digestive tract) level and not systemically, in association with infection with live parasites or exposure to ingested antigens of A. simplex.
Innate Immune Responses
Epithelial cells.
Epithelial cells are often the first responders to immune and inflammatory stimuli in the recruitment and activation of inflammatory cells in response to enteric parasites (246). An increase in the rate of epithelial turnover in the large intestine, for example, is thought to displace worms from their optimal niche, leading to their expulsion, and the rate of epithelial cell movement is under interleukin-13 (IL-13) control (58). The response to an oral acquisition of a parasite includes the activation of neutrophils, tissue macrophages, monocytes, dendritic cells, basophils, and eosinophils, as has been recently reviewed for Toxoplasma gondii (44).
NO.
Nitric oxide (NO) is recognized as a potent microbicidal agent, and its role in host defense against intra- and extracellular parasites has been demonstrated against larvae of nematodes such as A. simplex. Inducible NO synthesis (iNOS) requires stimulation by bacterial lipopolysaccharide and/or cytokines which upregulate the transcription of iNOS enzymes, leading to the conversion of l-arginine to l-citrulline and NO. Counteractive evasive mechanisms by parasitic worms are thought to include scavenging of l-arginine via arginase-1, making l-arginine unavailable for iNOS enzyme, thus reducing the final production of parasiticidal NO (66, 128, 162).
Autonomic control.
Ingestion of both living and dead A. simplex L3 can rapidly induce (within 4 h) cholinergic hyperactivity and adrenergic blockade in the rat intestine and, as a consequence, digestive symptoms ensue (237). Viable larvae will first elicit reactions in the duodenum at the site of tissue penetration and exposure to ES materials. If the parasites become ruptured or disintegrate, then there is the likelihood of the whole small bowel being exposed to internal components of the worms. This may be of significance because 70% of symptoms in A. simplex anaphylactic reactions involve the digestive tract (27, 29).
Basophils.
Basophils can be activated to release cytokines through specific and/or nonspecific mechanisms. For example, various lectins have been demonstrated to activate basophils by cross-linking of specific carbohydrate side chains or direct cross-linking of their high-affinity IgE receptor (FcɛRI). A role for basophils in parasitic infections has been indicated from animal models, especially during the expulsion period, and for humans it has also been suggested for filarial infections and other parasitoses (93). Apart from their role in innate immunity, basophils are an important cellular source for early IL-4 production, which is strongly influential in the initiation of a Th2 response. Curiously, Cuellar et al. have shown by enzyme-linked immunosorbent assay (ELISA) that IL-4-like molecules are present in L3 ES products and crude extracts from A. simplex (67). Among dyspeptic patients with Helicobacter pylori infection, for instance, those seropositive to A. simplex presented with increased eosinophil and basophil counts as determined histologically (264).
TLRs.
Toll-like receptors (TLRs) form a major family of phylogenetically conserved transmembrane receptors involved in nonself recognition. At the moment, more than 10 TLRs have been described for humans and more than 12 for mice (135). It is well established that TLRs are the main triggers of the innate immune system for bacterial and viral infections. They are expressed in several epithelial cells (skin, respiratory, intestinal, and genitourinary tract cells) associated with routes of entry of pathogens into the host organism and can contribute to protection against infection by signaling to activate effector mechanisms. TLRs have also been identified in immune system cells such as macrophages and dendritic cells. The involvement of TLR signaling pathways in parasitic infections is emerging such as with Entamoeba histolytica (173) and other protozoa (Leishmania spp., Trypanosoma cruzi, Trypanosoma brucei, Plasmodium spp., and Toxoplasma gondii) (102). As far as the helminth parasites are concerned, although there are no studies yet with A. simplex, the role of TLRs in infection by Schistosoma blood flukes and filarial nematodes is beginning to be investigated. Curiously, several species of filarial nematode possess endosymbiotic Wolbachia bacteria, the products of which can interact with the innate immune system through TLR2 and TLR4 (30, 42). Similarly, schistosomes have been shown to induce immune responses through TLR2, TLR3, and TLR4 (109). This new focus of research has important implications for nematode infections, particularly since nematodes appear to contain glycolipids (reviewed by Kennedy [142] and Lorenzo et al. [167]) that are of types confined to them and since detection systems that recognize nematodes generically, such as TLRs, would be selectively advantageous (109). Although the TLR family is an important class of sensors for innate recognition of parasites, other non-TLR pattern recognition systems may also be involved (64).
Adaptative Immune Response
Cellular response.
The granulomatous lesions observed for the chronic gastrointestinal and ectopic forms of anisakiasis are typical of type IV hypersensitivity reactions. Kikuchi et al. (151) demonstrated the role of cell-mediated immunity (type IV) in anisakidosis experimentally in rabbits and guinea pigs. Mice infected with the nematode Nippostrongylus brasiliensis and treated with gamma interferon (IFN-γ) exhibited downregulation of Th2-typical responses (blood and pulmonary eosinophilia, intestinal mastocytosis, and intestinal expulsion of worms), while the nematode's fecundity (egg production) increased (268), consistent with the Th2 response being protective against this nematode in mice. Similarly, in a human immunopathogenesis study of anisakidosis, a Th2 response was found, as indicated by local detection of mRNA for IL-4, IL-5, and eotaxin but without detectable transcripts for IFN-γ, IL-2, or IL-8 (84). In all cases, an inflammatory infiltrate composed of eosinophils and lymphocytes was found in the mucosa and submucosa and the larva was identifiable in some cases. They also found eosinophilic mediators potentially involved in parasite killing, such as iNOS, major basic protein, eosinophil cationic protein, and eosinophil protein X, in 13 intestinal biopsy samples and lymphocyte cell cultures and sera. Most of the patients were positive for A. simplex-specific IgE. Thus, A. simplex should be considered in the differential diagnosis of eosinophilic gastroenteritis and acute abdomen (204). No granulomatous lesions were observed in a study of an immunosuppressed patient suffering from both AIDS and intestinal anisakidosis, arguing in favor of the involvement of cellular immunity in these lesions (94). Another example of immunomodulation effected by nematodes is the finding that Onchocerca volvulus (the causative agent of river blindness) downregulates cellular responsiveness to mycobacterial infections (Mycobacterium tuberculosis and M. leprae), which is consistent with a doubled incidence of lepromatous leprosy in regions of onchocerciasis endemicity (253).
Antibody kinetics.
Anti-A. simplex antibodies in infected patients reflect a broad immunologic stimulation including both Th1 and Th2 responses (74). Infection causes a strong immune response, and a typical progression from IgM antibody to other isotypes (IgG, IgA, and IgE, etc.) has been observed during the first month of initial infection, as analyzed from serial blood sampling and immunoblotting (5, 29, 70, 84) in which the highest antigen-specific IgE, IgG, and IgA levels are seen to occur after 1 month of infection; specific IgM shows the highest levels within 24 h and a progressive decay over the following 6 months (74).
In humans, low-level worm infestations tend to be tolerated with a degree of eosinophilia, but this level of exposure does not usually induce strong T- or B-cell responses. In mice, low antigen doses tend to favor type 1 responses and susceptibility to the infestation, whereas high antigen doses favor a type 2 response and resistance (90). Generally, the responses to nematode ES antigens occur earlier and tend to be stronger than those induced by somatic antigens (39, 148, 172).
Several research groups have investigated the kinetics of specific antibody production in mice and rabbits (118, 119, 133, 149), but these results cannot be directly extrapolated to allergic responses in humans. In 2006, Cho et al. described the specific antibody response in rats that were infected orally with L3 on two occasions at intervals of 9 weeks. The results suggested that the allergic response was elicited by the reinfection (peaking after 1 week) and was accompanied by an elevated IgM level (53). Iglesias and colleagues (117) found some antigens captured by monoclonal antibodies (MAbs) that contain epitopes recognized by human IgE antibodies. The best results were obtained with two MAbs that recognized a doublet with molecular mass of 130 kDa, similar to the findings of Yagihashi et al. in 1990 (284).
Several experimental rodent models have been used to investigate the immune mechanisms controlling allergic responses to A. simplex, the survival of which is transient, as in human. A murine model of anaphylaxis in mice sensitized by intravenous or oral challenge confirmed a mixed Th1/Th2 pattern of cytokines in both cases and anaphylaxis only in those mice intravenously challenged (31). It has been demonstrated experimentally that rats infected by a single parasite develop higher IgE levels than do rats infected with a high number. Differences have been described between infection and antigen exposure to A. simplex in the sense that reinfection induces a strong Th2 response, whereas antigens alone do so to a lesser degree or not at all (53). In contrast, it has been shown recently that both live parasites and crude extracts of L3 cause allergic reaction in sensitized mice challenged orally with A. simplex proteins, resulting in a striking allergic reaction within as little as 1 hour, accompanied by scratching, irritability, diarrhea, and puffiness around the eyes (237). This is clearly relevant to the debate about whether antigens from dead parasites can cause allergy (28, 201).
Antibodies and proteases.
Antibodies produced during the infection can inhibit the biochemical functioning of proteases secreted by a number of other nematodes, such as Ascaris suum, Dictyocaulus viviparus, and Trichinella spiralis (42, 144, 149, 168, 182). The antibody response to proteases secreted by A. simplex might be responsible for the eventual death of tissue-invasive larvae through prevention of nutrient acquisition by the parasite and/or restriction of its mobility so that other immune effectors, such as cellular attack, can act. It has been observed, however, that antibodies bound to the surface of living larvae are rapidly shed (M. W. Kennedy, unpublished data) in a fashion similar to that seen with larvae of the human-infective larvae of the dog ascaridid Toxocara canis, for instance (143, 250).
Protease and protease inhibitor involvement in allergic responses.
Apart from their potential role in tissue invasion and immune evasion, proteases may have additional importance in that such enzymes are among the major allergens (defined as being the targets of IgE antibody in more than 50% of allergic patients) of the house dust mites, Dermatophagoides spp. Moreover, recent work on the cleavage of the CD23 cell surface receptor (which is involved in IgE regulation) by Dermatophagoides protease allergens has led to the idea that enzymes of the appropriate specificity could directly bias immune responses toward Th2 dominance (242, 244, 257). In 2000 (142, 163), it was pointed out that proteases could be allergens or otherwise immunologically manipulative as a general phenomenon, but given the large quantities in which A. simplex appears to secrete proteases when it enters the mammalian host, their role in allergic reactions ought to be investigated. When serum CD23 levels were measured at intervals of 24 h, 1 month, and 6 months in gastroallergic anisakiasis patients, levels were unchanged. However, higher values of soluble circulating CD23 were observed for patients with higher total IgE levels (73).
It is now well established that helminth proteases and their inhibitors are prominent among well-characterized allergens (reviewed in references 157, 200, and 245). Protease inhibitors that are also allergens for humans include Der p 9 from dust mite (Dermatophagoides pteronyssinus), Api m 7 from honeybee (Apis mellifera), and Fel d 3 from cat (Felis domesticus) along with food allergens like Sola t 3 and Sola t 4 from potato (Solanum tuberosum), Act d 1 from kiwifruit (Actidinia deliciosa), and Cuc m 1 from muskmelon (Cucumis melo) (http://www.allergen.org). Four major protease inhibitor classes have been identified (Table 3) according to the catalytic type of protease inhibited, namely, (i) serine (serpins and smapins), (ii) cysteine (stefins, cystatins, and kininogens), (iii) aspartic (aspins), and (iv) metalloproteases (reviewed by Knox [156]). Very recently, Ani s 4 and Ani s 6 (Table 4) have been found to be the first protease inhibitors of cystatin and serine classes, respectively, of the class Nematoda that cause allergy in humans (158, 229). Until now, only two protease inhibitors (belonging to the aspin and cystatin classes) had been identified in nematodes as major allergens for sheep (245) and rodents (111), respectively. Table 3 shows how A. simplex-derived protease inhibitors may function in counteracting the host's antiparasite response and that some of these inhibitors are also allergens for humans (156). It would also be interesting to know whether the antibodies produced to A. simplex infection are able to inhibit the biochemical activity of the secreted proteases, as has been shown with other nematode parasites, and thereby limit their possible immunomodulatory effects (43, 157, 262).
TABLE 3.
Presumptive functions of Anisakis simplex-derived proteinase inhibitors
| Function | Point of intervention | Proteinase type inhibited | Inhibitor name | Reference(s) |
|---|---|---|---|---|
| Modulation of antigen presentation by MHC | Antigen processing and peptide loading | Cysteine | Cystatins | 229 |
| Downregulation of T-cell responses | IL-10 production, reduced expression of HLA-DR and CD86 | Cysteine | ||
| Upregulation of NO production | From IFN-γ-stimulated lymphocytes | Cysteine | ||
| Allergenic | Stimulate IgE responses; IgE production | Cysteine | Kininogen | |
| Regulation of digestive proteinases | Inactivation of digestive proteases | Cysteine | ||
| Anti-inflammatory | Induction of TNFa and increased IL-10; reduction of IL-12 | Cysteine | Stefins | |
| Reproduction | Not known | Serine | Serpins | 168 |
| Modulation of proteinases of granulocyte origin | Neutrophil elastase, mast cell protease, and cathepsin G | Serine | 193, 200, 226 | |
| Protection from digestion by host | Trypsin, chymotrypsin, pepsin | Serine | 225, 247 | |
| Anticoagulant | Factor Xa, factor VIIa | Serine | Smapins | 158 |
TNF, tumor necrosis factor.
TABLE 4.
Allergens of Anisakis simplex
| Name | Molecular mass (kDa) | Localization | Allergen class | Reference(s) |
|---|---|---|---|---|
| Ani s 1a | 21-24 | ES | Homology with a Kunitz-type serine protease inhibitor | 18, 47, 104, 187, 230, 247 |
| Ani s 2 | 97 | Somatic | Paramyosin | 108, 212 |
| Ani s 3 | 41 | Somatic | Tropomyosin | 22, 108, 231 |
| Ani s 4 | 9 | ES | Cysteine protease inhibitor | 229 |
| Ani s 5 | 15 | ES | SXP/RAL protein | 158 |
| Ani s 6 | ES | Serine protease inhibitor | 158 | |
| Ani s 7 | 139-154 | ES | Glycoprotein | 164, 228 |
| Ani s 8 | 15 | ES | SXP/RAL protein | 159 |
Ani s 1 is a major allergen that should not be confused with another 21-kDa minor allergen, the encoding cDNA of which was cloned, this protein was also named Ani s 1 at the same time by Arrieta et al. (18) and is a protein of a different structure (belonging to the nematode troponin family).
A. simplex allergy in humans. (i) General aspects.
The potential for type I hypersensitivity responses in acute anisakiasis is indicated by the fact that serum anti-A. simplex IgE levels increase rapidly during the first few days (70, 72, 77, 85, 284) and remain high for months or years (27). Hypersensitivity is usually diagnosed by skin prick tests and in vitro confirmation (specific IgE, histamine release, basophil activation test [BAT]). In the case of A. simplex, the skin prick test was first used in 1995 (25), and since then its use has been widespread among allergy clinicians as a valuable screening test in cases of urticaria and anaphylaxis (29, 50, 69, 82). Other Japanese authors have reported preliminary studies using intradermal and scratch tests to investigate allergic manifestations associated with anisakidosis (125, 139), but these two techniques are no longer recommended as primary tests for the diagnosis of IgE-mediated allergic diseases or for research purposes by the European and American Academies of Allergy.
(ii) Basophils and IgE response.
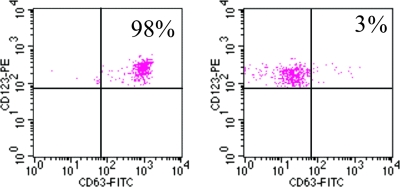
Recently, detection of allergen-induced basophil activation (BAT) by flow cytometry has been proposed as a useful diagnostic technique for allergy. This test uses whole-blood samples and live basophils, detecting activation-associated membrane markers (CD63) of antigen binding to the IgE high-affinity receptor (FcɛRI-bound IgE) (105). A significant activation of basophils was found for patients allergic to A. simplex versus controls (M. T. Audicana and N. Longo, unpublished data). (Fig. 5). Similar findings have been published by others, and the test discriminated between allergics and controls with a 95 to 100% sensitivity and a 100% specificity (105).
FIG. 5.
Human BAT in allergic and normal control subjects. Activation of basophils was detected by flow cytometry using a fluorophore-tagged (phycoerythrin [PE]) MAb against the CD63 cell activation marker. The expression of CD63 (horizontal axis) is plotted against levels of anti-IgE fluorescein isothiocyanate (FITC) (vertical axis) in response to A. simplex crude extract. The left-hand plot shows results from the first patient described 11 years before (25), although the test was carried out 11 years later (2006), and the right-hand panel corresponds to a female subject exhibiting no allergy to A. simplex used as a control. The results are expressed as the percentage of CD63+ basophils. Note that activated basophils from the allergic patient appear in the upper right window encompassing 98% of marked cells. In contrast, the control (right-hand plot) shows that a mere 3% of basophils were activated upon exposure to parasite antigen/allergens. (M. T. Audicana and N. Longo, unpublished data.)
Figure 5 (Audicana and Longo, unpublished) illustrates a cellular phenotype analysis from BAT carried out in 2006 of an anaphylaxis case diagnosed in 1995 (25). IgE was monitored yearly for this patient, and IgE decreased progressively but remained positive for 11 years. During the last test (in 2006), blood samples were also taken for the first time to carry out BAT, and basophil responsiveness was also found to have persisted for 11 years.
(iii) Immunopathologic findings and clinical manifestations.
In the allergic form of anisakidosis, the most relevant immunopathologic finding is the increase in specific and total IgE levels in patients ’ sera (4, 95, 100). Allergic reactions to A. simplex occur within the first few hours after ingestion of infected fish (typically within the first hour). Clinical manifestations are severe in 20 to 60% of cases and may affect several organs (Table 5). This IgE production, a hallmark of helminth infections, is typical of Th2-dominated immune responses (7, 202) and is dependent on IL-4 (161). Anaphylaxis is not triggered by all cytokines; IL-4 exacerbates anaphylaxis by increasing sensitivity to vasoactive mediators in mice infected with T. spiralis and IFN-γ inhibit several IL-4-dependent responses, including IgE production and B-cell class II major histocompatibility complex (MHC) expression (254).
TABLE 5.
Symptoms in allergic patients suffering from anaphylaxis in two Spanish cities
| Symptomsa | Quantity of patients experiencing symptoms in indicated cityb
|
|||
|---|---|---|---|---|
| No.
|
%
|
|||
| A | B | A | B | |
| Generalized anaphylaxis: U/AE + bronchospasm + H/S | 12 | 2 | 19 | 16 |
| Bronchospasm + other implicated organ | 25 | 4 | 40 | 33 |
| H/S + other implicated organ | 20 | 10 | 32 | 83 |
| Digestive symptoms + other implicated organ | 45 | 9 | 72 | 75 |
| U/AE + Rheumatic symptoms | 2 | 0 | 3 | 0 |
Immunoblotting studies have shown that human IgE and IgG antibody responses to A. simplex antigens are highly heterogeneous and vary dramatically between individuals, both quantitatively and qualitatively (100, 116, 147). Diversity in the specificity of the antibody repertoire has also been observed for humans infected with parasites related to A. simplex, and modeling the phenomenon in experimental animals strongly indicates that it is due to genetic differences between individuals, specifically in the class II region of the MHC (145-147, 150, 263). In other nematode infections (such as filariasis), IgG4 responses in small children positively correlate with intensity of infection, while in older children and adults, IgE antibodies appear as the parasite burdens decrease (265).
Anisakis Allergens
Allergenic sources.
Other factors that may control the intensity and nature of the immune response to A. simplex would include the nature and source of the immunogen to which the patient had been exposed. In human anisakidosis, the patient can be hypothetically exposed to A. simplex antigens from three sources: (i) all ES, somatic, and cuticular antigens as a result of tissue penetration and subsequent degeneration of the larvae, leading to exposure to the complete profile of the parasite's antigens; (ii) ES antigens only, in cases where there is a expulsion of the parasite intact, possibly after penetration of gut tissue has occurred; and (iii) cuticular and somatic antigens from dead larvae contained in food, in which case ES antigens would be present only in minimal quantities—this last point has also been suggested by Jackson (127).
Allergen isolation and characterization.
In the 1990s, attempts to improve diagnostic techniques for anisakiasis led to the isolation of a MAb (An2) by Japanese authors that recognized an ES product of 40 to 42 kDa (284). cDNA encoding the target antigen was then cloned by Sugane et al. (255), and Spanish authors developed five further MAbs, among which was UA3, whose target antigen is recognized by antibody in most patients with an antigen capture ELISA (166, 167). Interestingly, UA3 was found to be useful for both anisakidosis and allergy diagnosis, the latter using an O-deglycosylated antigen (165). The predominant epitopes therefore seem not to be carbohydrate in nature, and this is consistent with the observation that crude extracts of A. simplex retained their allergenic activity following treatment with sodium periodate, and the peptide nature of allergen epitopes also applied to defined allergens of a related parasite (54, 186). In the 2000s, there has been an increasing interest in the molecular isolation and characterization of A. simplex L3 allergens, by use of both high-resolution protein purification methods and isolation of allergen-encoding cDNA clones.
To date, eight A. simplex allergens have been described at the molecular level (Ani s 1 to Ani s 8); six of these are ES derived, two are somatic in origin, and none are associated with the cuticle of the worm (Table 4). Previous reports indicated that antibodies from fully sensitized patients recognize several different allergens in a crude extract by immunoblotting and immunoelectrophoresis (15, 100). This recognition of multiple allergens from a given biological source is now a common finding, such as with grass pollens (272).
Ani s 1 is now known to occur in different isoforms (247) and has been found to be highly resistant to heat (boiling for 30- and 10-min intervals) by both Spanish and Japanese research groups, respectively (29, 47, 48, 247, 264). It is not yet known to which family of proteins it belongs because, although it showed certain similarities in amino acid sequence (30 to 40% identity) to Kunitz-type serine protease inhibitors, it lacks appropriate inhibitory activity (247). Ani s 1 (described by Moneo et al. and Shimakura et al.) is a major allergen (47, 187, 230, 247) that should not be confused with another 21-kDa minor allergen (recognized by only 20% of allergic patients), the encoding cDNA of which was cloned, and this protein was also named Ani s 1 at the same time (18) and is a protein of different structure (belonging to the nematode troponin family).
Ani s 2 and Ani s 3 are the two somatic allergens described so far (22, 23, 108, 212). They are paramyosin and tropomyosin proteins, respectively, and are similar to the paramyosins and tropomyosins of other species (reviewed by Weiler [279]). Ani s 2, for instance, is similar to paramyosin allergens from dust mites (Blo t 11 from the mite Blomia tropicalis) (222). The similarity in sequence of these highly conserved proteins is probably the cause of cross-reactivity in IgE binding to A. simplex proteins seen for German cockroaches, chironomids, and dust mites (96, 132, 279). The muscle protein tropomyosin is also involved in cross-reactivity between shrimp (Pen a 1), cockroach (Per a 7), dust mites (Der p 10 and Der f 10), and snail (reviewed by Ferreira et al. [96]). There is another heat-stable tropomyosin allergen (Cha f I) similar to Ani s 3, suggesting that since they share structure they may share thermostability too. Another interesting finding is the presence of the T-cell-binding motif of HLA DRBI*0404 in these allergens and the thermostability of Ani s 3 (108). Recombinant Ani s 3 is already commercially available (http://www.allergome.org) for use in microarray diagnosis.
Ani s 4 (9 kDa) and Ani s 6 are ES allergens and are inhibitors of cysteine and serine protease, respectively, and they were the first nematode protease inhibitors found to be allergens to humans (158, 229). Ani s 4 belongs to the cystatin family of cysteine protease inhibitors, both by its sequence similarity to other cystatins and by its experimentally demonstrable protease activity (against papain) (229). It is located both in the excretory gland and below the cuticle, and it is heat stable (boiling for 30 min) and resistant to pepsin digestion (48). Ani s 6 is not a major allergen, and it is similar in sequence to serine protease inhibitors of other animals and inhibits α-chymotrypsin, but not trypsin, in a dose-dependent manner (158).
Ani s 5 (15 kDa) and Ani s 8 (15 kDa) are heat-stable ES allergens that show antigenic cross-reactivity and share amino acid sequence similarity with several members of the nematode SXP/RAL-2 protein family (158, 159). Ani s 5 is not a major allergen of A. simplex (158). Ani s 7 is a glycoprotein present in ES and is the target of the UA3 MAb, and it seems to be recognized by 100% of allergic patients (228).
Allergen thermostability.
It was noted in the original work that led to the wide recognition of A. simplex allergy that cooking and freezing cannot be relied upon to destroy allergenicity and protect against hypersensitivity reactions to parasite material contaminating ingested fish (24, 27-29, 100). Recently, thermostable allergens have been detected in A. simplex extracts (reviewed in the previous section), and this recalls the controversy about the safety of human consumption of these parasites in cooked fish (48, 188). In an experimental situation, it has been found that crude Ascaris materials remain allergenically active even after autoclaving, which would raise the temperature of a sample to well beyond that routinely encountered in cooking (54, 144). This resilience of allergenic potential could therefore be due to the existence of epitopes comprising amino acid sequence rather than those reliant upon protein conformation. More-detailed work on purified ABA-1 (an allergen of Ascaris for which there is a homologue in A. simplex), however, illustrates another important principle in that this protein requires unusually high temperatures (89°C) before it denatures, and it can renature upon cooling and recover its allergenicity (54, 144, 283).
Antibody repertoire and route of sensitization.
Symptomatic patients suffering from gastroallergic anisakiasis tolerate the ingestion of dead larvae, an observation that led to the suggestion that these patients are probably sensitized to ES antigens of A. simplex (9). Armentia et al. (16) have detected IgE against low-molecular-weight A. simplex allergens in fishmongers with asthma after handling fish (two of them also presented with contact urticaria when the fish was contaminated with A. simplex) and in eight patients previously diagnosed as having A. simplex sensitization after eating chicken meat. Food allergy found in adults could represent a persistent reaction starting early in childhood or be primarily initiated in adulthood. Food allergy in adulthood seems to be commonly associated with sensitization to other allergens, particularly those inhaled. Several terms have been used to define this situation, such as “pollen-associated food sensitivity,” “bird-egg syndrome,” and “pork-cat syndrome” (65). The diversity of A. simplex antigens involved in the potential routes of sensitization (inhalation, mucosal and/or cutaneous contact, and ingestion, etc.) may be relevant in the development of different clinical responses (rhinitis, conjunctivitis, acute and chronic urticaria, contact dermatitis, asthma, anaphylaxis, gastroallergic anisakiasis), which probably also depend on the route of sensitization.
Sensitization without Allergic Symptoms
Serodiagnostic tests available for Anisakis reactivity include procedures based on latex agglutination, Ouchterlony tests, immunoelectrophoresis, indirect immunofluorescence, hemagglutination, complement fixation, radioallergosorbent test and ImmunoCAP systems, immunoblotting, and ELISA, including antigen capture ELISA (4, 140, 215, 217). All of these methods use unfractionated or partially purified antigens and therefore show poor specificity because of cross-reactivity with antigens from many other parasites or allergens (167).
It is not uncommon to find positive IgE values against A. simplex for subjects who do not react allergically to the parasite. In these cases, specific IgE determination against the parasite not only cannot be considered to be a reliable indicator of allergy but also can be a confusing factor, since such antibody has been detected in 25% of otherwise healthy controls (27, 83). This is not surprising, since cross-reactivity has also been described for inhalant allergens from a wide variety of sources, and 43% of subjects with positive IgE to pneumoallergens do not present respiratory symptoms (248). Discrimination between symptomatic and asymptomatic patients presenting with allergen-specific IgE is included among scientific programs of the Global Allergy and Asthma European Network (GA2LEN) (41). A possible reason for IgE detection of antibodies in a healthy population is a subclinical or undiagnosed gastric (without allergy) anisakiasis, and this could explain the high prevalence of specific IgE in both the Spanish and the Japanese populations (4, 69). Other possible explanations for the existence of IgE against A. simplex without clinical manifestations may include (i) cross-reactivity with other nematodes (26, 167); (ii) the presence of a “panallergen,” such as tropomyosin, which occurs in crustaceans, insects, and mites (23, 96, 108)—some authors suggest that IgE can be stimulated by invertebrate tropomyosins in general (22); (iii) cross-reactivity due to carbohydrates or phosphorylcholine (35, 96, 167, 271); and (iv) cross-reactivity with glycans present in glycoproteins of other nematodes or the presence of biotinyl enzymes that can stimulate the production of IgE in some patients (166).
Not only IgE antibodies are detectable in healthy individuals; also detected are high levels of IgG1 antibodies reactive to biotinyl enzymes from nematodes that are adventitiously detected by antigen capture ELISA using streptavidin to capture biotinylated antigens. Biotinyl enzymes are widespread in helminths, as they are in other animals, bacteria, and plants. Since sera from an A. simplex-free population also present these antibodies, A. simplex biotinyl enzymes on their own do not seem to be the cause of sensitization. False positives may also be due to immunodominant carbohydrates that may be present in parasite glycoproteins (167), as was demonstrated using a deglycosylated antigenic fraction (named UA3R) which improved allergy diagnosis specificity (165).
The antigenic cross-reaction problems that can confound currently available in vitro tests (based on crude parasite extracts) for A. simplex allergy may be significantly improved with the use of a cocktail of recombinant proteins as target antigens. These have the advantage over crude extracts or fractions thereof in that they can be purified to homogeneity, provided in quantity, and standardized, providing batch uniformity and a more accurate diagnosis (41, 141).
Immunomodulation and Immune Deviation
A significant number of nematode-derived molecules have immunomodulatory properties, but these have mostly been described in association with long-lasting worm infections. In A. simplex infection, exposure to such factors is likely to be transitory because of the time limitation of the infection in humans, although their influence may persist long after the loss of the parasite. Unfortunately, literature on this topic is scant, but American authors have found that minute quantities (1 μg/24 h) of larval ES protein is enough to inhibit lymphocyte blastogenesis, suggesting that a single larva during 24 h is able to produce an immunomodulatory effect (223).
Complement.
Complement, either alone or in combination with antibodies, might also damage worms. Interestingly, therefore, A. simplex larval products have an anticomplementary activity, presumably as a means of evading host defenses, mainly in this case by attacking the classical pathway acting at the level of C3 (101). Similarly, crude extracts and ES products of A. simplex dose dependently inhibited NO production in bacterial lipopolysaccharide-treated macrophages (66). The same authors demonstrated the presence of IL-4-like factors in the larval antigens of A. simplex and suggested that the parasite thereby can modulate the Th1-Th2 response for its own benefit (67).
Rheumatologic manifestations.
Immunomodulatory factors have been described for other species of nematode and may prove pertinent to A. simplex pathogenicity, since proteins, glycoproteins, glycans, and even RNA have been reported to be influential (109, 211, 225, 226, 270). Several aspects of A. simplex pathogenesis, such as rheumatologic manifestations, carcinogenic effects, and autoimmunity, may be associated with immunomodulatory effects and still remain to be explained, and the link between A. simplex and rheumatologic symptoms (arthralgia/arthritis) remains unclear (14, 68, 91). Such symptoms could be caused by immune complexes and autoantibodies, as has been described for other parasitoses such as trypanosomiasis, malaria, Chagas’ disease, and schistosomiasis (210).
Protective role in inflammatory bowel diseases.
The ability of enteric parasites to downregulate type 1-mediated inflammation and upregulate type 2-mediated allergy continues to be of interest. There is controversy about whether, by triggering a Th2 response, worm infections may have a protective role against inflammatory bowel diseases, which are usually characterized as Th1-dominated conditions. There is experimental evidence to support the idea that materials from nematode parasites may have potential therapeutic benefits (reviewed by Hunter and McKay [115] and Zaccone et al. [286]). Although similar immune response modulation has not been studied for A. simplex, other helminth infections improved inflammatory bowel disease indices in human and in murine models and diminished the allergy-induced changes in pulmonary function and even abrogated anaphylaxis elicited by peanut allergens (246).
Cancer.
The role of A. simplex in cancer is not clear, since both carcinogenic and cytostatic activities have been described as associated with infection or the parasite's secretory products (184, 216). Nevertheless, only a few cases of tumors apparently associated with A. simplex parasitism have been described, despite the thousands of cases of infection reported from Japan.
Th2-associated eosinophilia and IL-4 can decrease tumor growth and initiate antitumor activity, despite the angiogenesis induced by eosinophils (reviewed by Ellyard et al. [88]). Also, as was pointed out in the 1980s, the ES products of A. simplex and Pseudoterranova spp. can exhibit cytostatic activities in vitro (223).
On the other hand, Th2 dominance has been regarded as favoring tumor growth by promoting angiogenesis and inhibiting cell-mediated immunity and tumor cell killing. A. simplex and Fasciola hepatica (liver fluke) have been associated with intravascular lymphomatosis in Japanese patients (reviewed by Aljurf et al. [6]). Studies on carcinogenic mechanisms involving F. hepatica-associated cholangiocarcinoma have suggested that NO, which is produced in some antihelminth responses, is both cytotoxic and genotoxic: it is known, for instance, that nitrates, nitrites, and N-nitroso compounds present in preserved foods are likely carcinogens in liver and gastric cancer (reviewed by Watanapa and Watanapa [277] and Matsuzaka et al. [177]).
Although both gastric cancer and anisakidosis have their highest prevalences in Japan and were previously linked together (216), recent reviews on gastric cancer do not discuss the potential role of A. simplex in causing cancer. Instead, cancer has now been associated with a vigorous Th1 immune response to Helicobacter pylori among other cofactors (177). A Th2-polarizing helminthic infection could, however, downregulate Th1-promoted responses to unrelated antigens such as H. pylori and thereby decrease the risk of gastric cancer later in life (171).
GENETIC VARIATION IN THE PARASITES AND THE ACCIDENTAL HOST
Genetic Diversity in Anisakidae
The virulence of a pathogen can be influenced by its reproductive rate, its avoidance of host defenses (anatomic seclusion, antigenic disguise, and antigenic variation, etc.), and the production of enzymes, antigens, and toxins (243, 261). These strategies are presumably aimed primarily at the natural host species for A. simplex rather than at accidental hosts such as humans (36). Population genetics studies of the pathogen species are therefore important to an understanding of the effect of infection in humans, particularly considering the global oceanic distribution of A. simplex and related parasites and the range of fish, invertebrates, and marine mammal species that act as hosts. Genetic studies have revealed considerable genetic diversity between Anisakidae congeners, with differences in morphology, host species, geographical distribution and reproductive isolation (180). A similar pattern has been observed for Phocanema decipiens nematode species complexes (P. decipiens A, B, and C) (207). Recently, studies around the Portuguese coast reported two reproductively isolated sibling species of the A. simplex complex from Pleuronectiformes (flatfish) by use of DNA restriction fragment length polymorphisms (175). There have been no studies investigating the possibility of diversity within the species, subspecies, or strains of the above-mentioned parasites in the diseases they elicit, which will be further complicated by the high degree of polymorphism of immune genes in humans. However, some authors have suggested that P. decipiens larvae, especially those found in the United States, are less invasive and less pathogenic than are A. simplex larvae (79, 174, 205).
Human Susceptibility Factors
Virulence may also be determined by the susceptibility of the accidental host. Due to the high incidence of infection of fish by anisakids (281), it is striking that only a few individuals appear to be susceptible to both anisakiasis and overt A. simplex allergy. Furthermore, despite the worldwide A. simplex parasitism of fish (281), the first cases of A. simplex allergy were detected in Japan (138, 139) and then in the Basque Country (northern Spain) (25, 28). If we can assume that this is due not merely to higher levels of infection in these regions or to underdiagnosis or a lack of unawareness, there could be some form of genetic predisposition that requires investigation (29, 32, 33, 87). The European population is, except for a few outliers such as the Basques or Sardinians, relatively homogeneous genetically. The Basques are an unusual outlier population, both linguistically and for their genetic distinctiveness, and have been studied using a variety of markers including HLA (60). The HLA haplotypes from populations in northern Spain are significantly more interrelated than those occurring in other subpopulations in Spain (238). The Japanese are also known to be a relatively distinct group, distant from European and African populations (60, 238).
Anisakis simplex allergy: a nonatopic disease.
Atopic allergic diseases are familiar and have a genetic basis, but multiple markers for atopy and allergic diseases have been described (HLA-DR locus, FcRIɛIgE receptor, IL-4 family cytokines, and chromosome 5) (141). However, most patients diagnosed as allergic to A. simplex cannot be classified simply as merely being atopy-susceptible patients but are instead adults of middle age without a previous history of atopic dermatitis, asthma, or rhinitis (28, 98, 266). However, individual resistance to infection varies and may be controlled by a number of immune response genes. A good example of this is that the possession of certain HLA alleles that are widespread in native West Africans, but not in Caucasians, appears to correlate with protection against severe malaria (112). In terms of immune responses to defined antigens of several species of nematode, there is a clear association between with MHC genetics in experimental animals (142). Further investigation of the particular susceptibilities in other populations could be extremely useful in understanding the A. simplex-induced syndromes globally.
Anisakis simplex allergy and HLA.
Recent works have shown a genetic predisposition to A. simplex allergy (HLA class II alleles) studying 46 Caucasian (from Cantabria, northern Spain) patients allergic to A. simplex. The DRB1*1502-DQB1*0601 haplotype was significantly associated with allergy to A. simplex (239). These authors, based on published works by others, discuss the frequency of this haplotype in different populations, which is common in the Asian population and is relatively frequent among Scottish Highlanders, Spanish Gypsies, Romanians, and Italians but absent or very rare in the populations of Norway, Germany, Denmark, Hungary, France, and Spain. The so-called “Thai” allele (DRB1*1502) is frequent in Japan, Korea, Thailand, and Vietnam and in Canadians of Indian ancestry and relatively uncommon in Basques and Cantabrians (from the north of Spain). The “Welsh” allele (DQB1*0601) is frequent among the Welsh, the Wailibri people (Australia), and Asians (214, 239). Furthermore, HLA DRB1*1502, DQB1*0601, and DRBI*0404 are significantly overrepresented (and hence probable genetic risk factors) in subjects allergic to A. simplex (239). The newly characterized allergens Ani s 2 and Ani s 3 (Table 4), when screened for MHC binding motifs, were found to have predicted motifs for HLA DRBI*0404 (Ani s 2, three sites; Ani s 3, one site), which is consistent with the high prevalence of this allele in subjects allergic to A. simplex (108).
As the awareness of anisakiasis and A. simplex as a hidden food allergen is increasing (87), reports from France (214), Italy (98), Portugal (92), and Spain (190, 192) are showing that allergy in Europe is not confined to the Basque Country but is also in Japan, where more cases have also been diagnosed (152). However, Italian and Portuguese cases are similar to those of the Basque Country because they involve true allergy with cooked fish, and reports from other parts of Spain (Madrid and its surrounding provinces) appear to describe gastroallergic cases caused by raw or undercooked fish (71, 99). In other countries, where Pseudoterranova is more frequent (such as the United States), perhaps this parasite's allergens should be tested for when testing for fish allergy. As far as the occupational aspect is concerned, there are reports from the Basque Country (27), other regions of Spain (12, 17, 220), Italy (221), and South Africa (201).
PERSPECTIVE
Exposure to anisakid parasites and their antigens/allergens, either as a living infection or by consumption of dead parasites in food fish, remains a widespread problem with many clinical manifestations that are increasingly being recognized. This review aimed at describing the known routes of sensitization and the range and diversity of clinical symptoms, but the following topics remain to be addressed.
Allergic Sensitization
Is previous infection with A. simplex required for subsequent allergic reactions in humans? Or is exposure to certain A. simplex allergens sufficient? Might people differ in the route of sensitization by which they are susceptible?
Should we fully recognize A. simplex as a source of potent “hidden food allergens” able to cause severe allergies triggered by ingestion, by inhalation, or by skin contact?
Are digestive tract and cutaneous allergic manifestations merely two sides of the same coin but elicited by different allergens or initial routes of sensitization? Alternatively, are they due to different susceptibilities due to other factors (such as genetics of humans and/or parasites) or behavior (fish consumption rates and cooking habits, etc.)?
Parasite Factors
Has A. simplex enough time during infection to release immunomodulatory factors that are influential in both the long and the short terms?
What is the therapeutic potential of A. simplex-derived materials, since other nematodes appear to secrete medically and pharmacologically interesting products? New research work is finding that helminths secrete interesting molecules which may have beneficial effects for cardiovascular disease (170), bowel disease, arthritis (191), and even allergic diseases (246).
Dietary Guidelines for Allergic Patients
What would be the ideal diet for A. simplex-allergic patients? What should it be in order to prevent subsequent sensitization at later ages? Some contradictory dietary guidelines for allergic patients have been proposed. Some authors, for instance, propose the training of subjects to avoid accidentally consuming the parasite by teaching the patients to recognize the worms and not to consume small fish (like anchovies) or hypaxial tissue (ventral muscles next to the abdominal cavity) in large fish (27, 29). These recommendations were based on A. simplex fish parasitology studies (11, 251), and for very severe cases (anaphylaxis), a strict diet with complete avoidance of fish is recommended (27, 29, 107). In contrast, other authors consider that with specific reference to gastrointestinal allergy, the parasite may be consumed if it is dead, based on patient follow-up and oral challenge test with dead L3 (9, 240).
What characteristics of A. simplex allergens are associated with the triggering of allergic episodes or the initiation of the allergic state (thermostability, resistance to pepsin, pathogen-associated molecular patterns, presence of HLA binding motifs, and protein structural features, etc.)?
Could food preparation and storage practices realistically be altered to avoid allergic sensitization and recall?
Occupational Allergy
Anisakis simplex is now associated with occupational seafood allergy (130), and it is important to appreciate that more than 38 million people work in fish production activities (fishery and aquaculture) (201), aside from related sectors (fishmongers, cooks, animal feed production workers) (16, 17). Should skin and mucous membrane barrier protection methods therefore be considered as protective measures for these workers?
Fish Hygienic Quality and Food Safety
Aquaculture could have advantages over extractive fishing in supplying fish guaranteed to be free from A. simplex and related parasites. However, allergen traceability in feed is crucial, especially when fish meal is used to feed fish or domestic animals, and bearing in mind the striking findings of Armentia et al. (16), who found that highly sensitized A. simplex-allergic patients detected worm allergens in chicken meat.
Is it possible to supply food fish and feeds free from A. simplex parasites or allergens and certify them as such? Could commercially viable tests be developed to screen fish products (for human and animal consumption) for allergen content, as is currently done by allergen tracing to prevent peanut allergy?
Desensitization
Is specific immunological desensitization feasible? Standard immunotherapy attempts have been made with several food allergens (peanuts and milk, etc.) with little success. However, new strategies are being developed using wild-type or mutated recombinant protein allergens, overlapping peptides of allergens, or the addition of oligodeoxynucleotide immunostimulatory sequences to proteins (236). In the particular case of A. simplex, this task has not yet begun, but it could be difficult given that the parasite may be antigenically more complex than classical food allergens, a target of innate antinematode immune mechanisms, or cross-reactive with other nematode parasites, particularly those encountered as infections in childhood.
With time, natural desensitization seems to occur in patients allergic to A. simplex that stop eating fish. But can resensitization occur, and what would be a minimal safe withdrawal period?
Hopefully, some of these questions can be addressed using information and materials derived from an analysis of the parasite's antigens and allergens provided through cDNA analysis, the production of recombinant allergen proteins for immunological surveys, and characterization of the natural allergens themselves. Possible direct practical applications of recombinant allergens include diagnostic techniques, the development of allergen vaccines (immunotherapy), and perhaps the development of useful kits for the fish industry or even therapeutic targets to develop new drugs. It is, however, now abundantly clear that close collaboration between clinicians, food technologists, molecular biologists, protein chemists, and even geneticists and sociologists could be crucial to appreciating fully the impact of, and controlling, A. simplex and other marine nematode-related disorders.
Acknowledgments
We are greatly indebted to Lina Audicana for translation of many parts of this article.
M. T. Audicana acknowledges the financial support of Fundación BIO (from the Basque Government).
REFERENCES
- 1.Abbas, A. K., K. M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383:787-793. [DOI] [PubMed] [Google Scholar]
- 2.Adams, A. M., L. L. Leja, K. Jinneman, J. Beeh, G. A. Yuen, and M. M. Wekell. 1994. Anisakis parasites, Staphylococcus aureus and Bacillus cereus in sushi and sashimi from Seattle area restaurants. J. Food Prot. 57:311-317. [DOI] [PubMed] [Google Scholar]
- 3.Adams, A. M., K. S. Miller, M. M. Wekell, and F. M. Dong. 1999. Survival of Anisakis simplex in microwave-processed arrowtooth flounder (Atheresthes stomias). J. Food Prot. 62:403-409. [DOI] [PubMed] [Google Scholar]
- 4.Akao, N., T. Ohyama, and K. Kondo. 1990. Immunoblot analysis of serum IgG, IgA and IgE responses against larval excretory-secretory antigens of Anisakis simplex in patients with gastric anisakiasis. J. Helminthol. 64:310-318. [DOI] [PubMed] [Google Scholar]
- 5.Akao, N., and H. Yoshimura. 1989. Latex agglutination test for immunodiagnosis of gastric anisakiasis, p. 97-102. In H. Ishikura and M. Namiki (ed.), Gastric anisakiasis in Japan. Epidemiology, diagnosis, treatment. Springer-Verlag, Tokyo, Japan.
- 6.Aljurf, M. D., T. W. Owaidah, A. Ezzat, E. Ibrahim, and A. Tbakhi. 2003. Antigen-and/or immune driven lymphoproliferative disorders. Ann. Oncol. 14:1595-1606. [DOI] [PubMed] [Google Scholar]
- 7.Allen, J. E., and R. M. Maizels. 1996. Immunology of human helminth infection. Int. Arch. Allergy Immunol. 109:3-10. [DOI] [PubMed] [Google Scholar]
- 8.Reference deleted.
- 9.Alonso, A., A. Moreno-Ancillo, A. Daschner, and M. C. López-Serrano. 1999. Dietary assesment in five cases of allergic reactions due to gastroallergic anisakiasis. Allergy 54:517-520. [DOI] [PubMed] [Google Scholar]
- 10.Alonso-Gomez, A., A. Moreno-Ancillo, M. C. López-Serrano, J. M. Suarez de Parga, A. Daschner, M. T. Caballero, P. Barranco, and R. Cabañas. 2004. Anisakis simplex only provokes allergic symptoms when the worm parasitises the gastrointestinal tract. Parasitol. Res. 93:378-384. [DOI] [PubMed] [Google Scholar]
- 11.Angot, V., and P. Brasseur. 1995. Les larves d'Anisakidés et leur incidence sur las qualité des poissons et produits de poisson. Rev. Med. Vet. 146:791-804. [Google Scholar]
- 12.Añibarro, B., and F. J. Seoane. 1998. Occupational conjunctivitis caused by sensitization to Anisakis simplex. J. Allergy Clin. Immunol. 102:331-332. [DOI] [PubMed] [Google Scholar]
- 13.Añibarro, B., F. J. Seoane, and M. V. Múgica. 2007. Involvement of hidden allergens on food allergic reactions. J. Investig. Allergol. Clin. Immunol. 17:168-172. [PubMed] [Google Scholar]
- 14.Arenal Vera, J. J., J. L. Marcos Rodriguez, M. H. Borrego Pintado, W. Bowakin Dib, J. Castro Lorenzo, and J. I. Blanco Alvarez. 1991. Anisakiasis como causa de apendicitis aguda y cuadro reumatológico: primer caso en la literatura médica. Rev. Esp. Enferm. Dig. 79:355-358. [PubMed] [Google Scholar]
- 15.Arlian L. G., M. S. Morgan, S. Quirce, F. Marañon, and E. Fernandez-Caldas. 2003. Characterization of allergens of Anisakis simplex. Allergy 58:1299-1303. [DOI] [PubMed] [Google Scholar]
- 16.Armentia, A., A. Callejo, J. M. Vega, and C. Martínez. 2006. Anisakis allergy after eateing chicken meat. J. Investig. Allergol. Clin. Immunol. 16:258-263. [PubMed] [Google Scholar]
- 17.Armentia, A., M. Lombardero, A. Callejo, J. M. Martín Santos, F. J. Martin Gil, J. Vega, M. L. Arranz, and C. Martínez. 1998. Occupational asthma by Anisakis simplex. J. Allergy Clin. Immunol. 102:831-834. [DOI] [PubMed] [Google Scholar]
- 18.Arrieta, I., M. del Barrio, L. Vidarte, V. del Pozo, C. Pastor, J. Gonzalez-Cabrero, B. Cardaba, M. Rojo, A. Minués, I. Cortesano, S. Gallardo, E. Aceituno, P. Palomino, and C. Lahoz. 2000. Molecular cloning and characterization of an IgE-reactive protein from Anisakis simplex: Ani s 1. Mol. Biochem. Parasitol. 107:263-268. [DOI] [PubMed] [Google Scholar]
- 19.Asaishi, K., C. Nishino, T. Ebata, M. Totsuka, H. Hayasaka, and T. Suzuki. 1980. Studies on the etiologic mechanism of anisakiasis. I. Immunological reactions of digestive tract induced by Anisakis larva. Gastroenterol. Jpn. 15:120-127. [PubMed] [Google Scholar]
- 20.Asaishi, K., C. Nishino, and H. Hayasaka. 1989. Geographical distribution and epidemiology, p. 31-36. In H. Ishikura and M. Namiki (ed.), Gastric anisakiasis in Japan. Epidemiology, diagnosis, treatment. Springer-Verlag, Tokyo, Japan.
- 21.Asato, R., M. Wakuda, and T. Sueyoshi. 1991. A case of human infection with Anisakis physeteris larvae in Okinawa, Japan. Jpn. J. Parasitol. 40:181-183. [Google Scholar]
- 22.Asturias, J. A., E. Eraso, and A. Martinez. 2000. Cloning and high level expression in Escherichia coli of an Anisakis simplex tropomyosin isoform. Mol. Biochem. Parasitol. 108:263-267. [DOI] [PubMed] [Google Scholar]
- 23.Asturias, J. A., E. Eraso, I. Moneo, and A. Martinez. 2000. Is tropomyosin an allergen in Anisakis? Allergy 55:898-899. [DOI] [PubMed] [Google Scholar]
- 24.Audicana, L., M. T. Audicana, L. Fernández de Corres, and M. W. Kennedy. 1997. Cooking and freezing may not protect against allergic reactions to ingested Anisakis simplex antigens in humans. Vet. Rec. 140:235. [DOI] [PubMed] [Google Scholar]
- 25.Audicana, M., L. Fernández de Corres, D. Muñoz, E. Fernández, J. A. Navarro, and M. D. Del Pozo. 1995. Recurrent anaphylaxis due to Anisakis simplex parasitizing sea-fish. J. Allergy Clin. Immunol. 96:558-560. [DOI] [PubMed] [Google Scholar]
- 26.Audicana, M., M. García, M. D. Del Pozo, I. Moneo, J. Díez, D. Muñoz, E. Fernández, M. Echenagusia, L. Fernández de Corres, and I. J. Ansotegui. 2000. Clinical manifestations of allergy to Anisakis simplex. Allergy 55(Suppl.):28-33.11291778 [Google Scholar]
- 27.Audicana, M. T. 2002. Anisakis simplex y alergia alimentaria. Ph.D. thesis. Basque Country University, Lejona, Spain.
- 28.Audicana, M. T., I. J. Ansotegui, L. Fernández de Corres, and M. W. Kennedy. 2002. Anisakis simplex: dangerous dead and alive? Trends Parasitol. 18:20-25. [DOI] [PubMed] [Google Scholar]
- 29.Audicana, M. T., M. D. Del Pozo, R. Iglesias, and F. M. Ubeira. 2003. Anisakis simplex and Pseudoterranova decipiens, p. 613-636. In R. Learmonth and M. D. Milliotis (ed.), International handbook of foodborne pathogens, 1st ed. Marcel Dekker, New York, NY.
- 30.Babu, S., C. P. Blauvelt, V. Kumaraswami, and T. B. Nutman. 2005. Cutting edge: diminished expression and function of TLR in lymphatic filariasis: a novel mechanism of immune dysregulation. J. Immunol. 175:1170-1176. [DOI] [PubMed] [Google Scholar]
- 31.Baeza, M. L., L. Conejero, Y. Higaki, E. Martín, C. Pérez, S. Infante, M. Rubio, and J. M. Zubeldia. 2005. Anisakis simplex allergy: a murine model of anaphylaxis induced by parasitic proteins displays a mixed Th1/Th2 pattern. Clin. Exp. Immunol. 142:433-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bahna, S. L. 2001. Unusual presentations of food allergy. Ann. Allergy Asthma Immunol. 86:414-420. [DOI] [PubMed] [Google Scholar]
- 33.Bahna, S. L. 2004. You can have fish allergy and eat it too! J. Allergy Clin. Immunol. 114:125-126. [DOI] [PubMed] [Google Scholar]
- 34.Balic, A., Y. Harcus, M. J. Holland, and R. M. Maizels. 2004. Selective maturation of dendritic cells by Nippostrongylus brasiliensis-secreted proteins drives Th2 immune responses. Eur. J. Immunol. 34:3047-3059. [DOI] [PubMed] [Google Scholar]
- 35.Batanero, E., M. Villalba, R. I. Monsalve, and R. Rodríguez. 1996. Cross-reactivity between the major allergen from olive pollen and unrelated glycoproteins: evidence of an epitope in the glycan moiety of the allergen. J. Allergy Clin. Immunol. 97:1264-1271. [DOI] [PubMed] [Google Scholar]
- 36.Belkaid, Y., R. B. Blank, and I. Suffia. 2006. Natural regulatory T cells and parasites: a common quest for host homeostasis. Immunol. Rev. 212:287-300. [DOI] [PubMed] [Google Scholar]
- 37.Bier, J. W. 1976. Experimental anisakiasis: cultivation and temperature tolerance determinations. J. Milk Food Technol. 39:132-137. [Google Scholar]
- 38.Bier, J. W., and R. B. Raybourne. 1988. Anisakis simplex (nematoda ascaridoidea): formation of immunogenic attachment caps in pigs. Proc. Helminthol. Soc. Wash. 55:91-94. [Google Scholar]
- 39.Blaxter, M. L., A. P. Page, W. Rudin, and R. M. Maizels. 1992. Nematode surface coats: actively evading immunity. Parasitol. Today 8:243-247. [DOI] [PubMed] [Google Scholar]
- 40.Bourée, P., A. Paugam, and J. C. Petithory. 1995. Anisakidosis: report of 25 cases and review of the literature. Comp. Immunol. Microbiol. Infect. Dis. 18:75-84. [DOI] [PubMed] [Google Scholar]
- 41.Bousquet, J., J. M. Anto, C. Bachert, P. J. Bousquet, P. Colombo, R. Crameri, M. Daeon, W. Fokkens, B. Leynaert, C. Lahoz, M. Maurer, P. Passalacqua, R. Valenta, M. Van Hage, and R. Van Ree. 2006. Factors responsible for differences between asymptomatic subjects and patients presenting an IgE sensitisation to allergens. A GA2LEN project. Allergy 61:671-680. [DOI] [PubMed] [Google Scholar]
- 42.Brattig, N. W., C. Bazzocchi, C. J. Kischning, N. Reiling, D. W. Buttner, F. Ceciliani, F. Geisinger, H. Hochrein, M. Ernst, H. Wagner, C. Bandi, and A. Hoerauf. 2004. The major surface protein of Wolbachia endosymbionts in filarial nematodes elicits immune responses through TLR2 and TLR4. J. Immunol. 1:437-445. [DOI] [PubMed] [Google Scholar]
- 43.Britton, C., C. P. Knox, G. J. Canto, G. M. Urquhart, and M. W. Kennedy. 1992. The secreted and somatic proteinases of the bovine lungworm Dictyocaulus viviparus and their inhibition by antibody from infected and vaccinated animals. Parasitology 105:325-333. [DOI] [PubMed] [Google Scholar]
- 44.Buzoni-Gatel, D., J. Schulthess, L. C. Menard, and L. H. Kasper. 2006. Mucosal defences against orally acquired protozoan parsites, emphasis on Toxoplasma gondii infections. Cell. Microbiol. 8:535-544. [DOI] [PubMed] [Google Scholar]
- 45.Buzoni-Gatel, D., and C. Werts. 2006. Toxoplasma gondii and subversion of the immune system. Trends Parasitol. 22:448-452. [DOI] [PubMed] [Google Scholar]
- 46.Buzzell, G. R., and R. I. Sommerville. 1985. The structure of the oesophagus in the third-stage infective larva of Anisakis sp. (Nematoda: Anisakidae). Trans. Am. Microsc. Soc. 104:86-94. [Google Scholar]
- 47.Caballero, M. L., and I. Moneo. 2002. Specific IgE determination to Ani s 1, a major allergen from Anisakis simplex, is a useful tool for diagnosis. Ann. Allergy Asthma Immunol. 89:74-77. [DOI] [PubMed] [Google Scholar]
- 48.Caballero, M. L., and I. Moneo. 2004. Several allergens from Anisakis simplex are highly resistant to heat and pepsin treatments. Parasitol. Res. 93:248-251. [DOI] [PubMed] [Google Scholar]
- 49.Cabrera, R., M. Del Pilar, and T. Altamirano. 2004. Anisakidosis a marine parasitic zoonosis: unknown or emerging in Perú? Rev. Gastroenterol. Peru 24:335-342. [PubMed] [Google Scholar]
- 50.Carretero, P., J. Blanco, F. García, M. Marcos, L. Alonso, M. Garcés, R. Perez, S. Juste, and M. C. Gutierrez. 1997. Protein contact dermatitis caused by Anisakis simplex. Contact Dermatitis 37:247. [DOI] [PubMed] [Google Scholar]
- 51.Carretero, P., C. Rivas, P. Todo, B. Gómez, C. Núñez, E. Alday, and I. Moneo. 1998. Anaphylaxis after a prick test to Anisakis simplex. Rev. Esp. Alergol. Inmunol. Clin. 13:226-228. [Google Scholar]
- 52.Carvalho, E. M., L. S. Bastos, and M. I. Araujo. 2006. Worms and allergy. Parasite Immunol. 28:525-534. [DOI] [PubMed] [Google Scholar]
- 53.Cho, T. H., H. Y. Park, S. Cho, J. Sohn, Y. W. Yoon, J. E. Cho, and S. W. Cho. 2006. The time-course of biological and immunochemical allergy states induced by Anisakis simplex larvae in rats. Clin. Exp. Immunol. 143:203-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Christie, J. F., B. Dunbar, and M. W. Kennedy. 1993. The ABA-1 allergen of the nematode Ascaris suum: epitope stability, mass spectrometry, and N-terminal sequence comparation with its homologue in Toxocara canis. Clin. Exp. Immunol. 92:125-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chung, J. G., H. M. Kuo, T. H. Lin, C. C. Ho, J. H. Lee, J. M. Lai, G. N. Levy, and W. W. Weber. 1996. Evidence for arylamine N-acetyltransferase in the nematode Anisakis simplex. Cancer Lett. 106:1-8. [DOI] [PubMed] [Google Scholar]
- 56.Claerebout, E., and J. Vercruysse. 2000. The immune response and the evaluation of acquired immunity against gastrointestinal nematodes in cattle: a review. Parasitology 120(Suppl.):25-42. [DOI] [PubMed] [Google Scholar]
- 57.Clavel, A., B. Delgado, C. Sánchez Acedo, E. Carbonell, J. Castillo, and J. Ramírez. 1993. A live Anisakis physeteris larva found in the abdominal cavity of a woman in Zaragoza, Spain. Jpn. J. Parasitol. 42:445-448. [Google Scholar]
- 58.Cliffe, L. J., N. E. Humphreys, T. E. Lane, C. S. Potten, C. Booth, and R. K. Grencis. 2005. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science 308:1463-1465. [DOI] [PubMed] [Google Scholar]
- 59.Codex alimentarius commission. 2004. Proposed draft standard for ready-to-eat smoked fish. http://www.codexalimentarius.net.
- 60.Comas, D., E. Mateu, F. Calafell, A. Perez-Lezaun, E. Bosch, R. Matinez-Arias, and J. Bertranpetit. 1998. HLA class I and class II typing and the origin of Basques. Tissue Antigens 51:30-40. [DOI] [PubMed] [Google Scholar]
- 61.Conde-Salazar, L. M., A. González, and D. Guimaraens. 2002. Type I and type IV sensitization to Anisakis simplex in 2 patients with hand eczema. Contact Dermatitis 46:361. [DOI] [PubMed] [Google Scholar]
- 62.Cooper, P. J., M. Chico, C. Sandoval, I. Espinel, A. Guevara, M. M. Levine, G. E. Griffin, and T. B. Nutman. 2001. Human infection with Ascaris lumbricoides is associated with suppression of the interleukin-2 response to recombinant cholera toxin B subunit following vaccination with the live oral cholera vaccine CVD 103-HgR. Infect. Immun. 69:1574-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Couture, C., L. Measures, J. Gagnon, and C. Desbiens. 2003. Human intestinal anisakidosis due to consumption of raw salmon. Am. Surg. Pathol. 27:1167-1172. [DOI] [PubMed] [Google Scholar]
- 64.Creagh, E. M., and L. A. O'Neill. 2006. TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 27:352-357. [DOI] [PubMed] [Google Scholar]
- 65.Crespo, J. F., and J. Rodriguez. 2003. Food allergy in adulthood. Allergy 58:98-113. [DOI] [PubMed] [Google Scholar]
- 66.Cuellar, C., M. J. Perteguer, and B. De Las Heras. 1998. Effects of Anisakis simplex on nitric oxide production in J774 macrophages. Scand. J. Infect. Dis. 30:603-606. [DOI] [PubMed] [Google Scholar]
- 67.Cuellar, C., M. J. Perteguer, and M. Rodero. 2001. Presence of IL-4-like molecules in larval excretory-secretory products and crude extracts from Anisakis simplex. Scand. J. Immunol. 53:483-488. [DOI] [PubMed] [Google Scholar]
- 68.Cuende, E., M. T. Audicana, M. Garcia, M. Anda, L. Fernandez Corres, C. Jimenez, and J. C. Vesga. 1998. Rheumatic manifestations in the course of anaphylaxis caused by Anisakis simplex. Clin. Exp. Rheumatol. 16:303-304. [PubMed] [Google Scholar]
- 69.Daschner, A., A. Alonso-Gomez, P. Barranco, J. M. Suarez de Parga, and M. C. López-Serrano. 1998. Gastric anisakiasis: an underestimated cause of acute urticaria and angio-oedema. Br. J. Dermatol. 139:822-828. [DOI] [PubMed] [Google Scholar]
- 70.Daschner, A., A. Alonso-Gomez, T. Caballero, J. M. Suarez de Parga, and M. C. López-Serrano. 1999. Usefulness of early serial measurement of specific and total IgE in the diagnostic of gastro-allergic anisakiasis. Clin. Exp. Allergy 29:1260-1264. [DOI] [PubMed] [Google Scholar]
- 71.Daschner, A., A. Alonso-Gómez, R. Cabañas, M. Suarez-de-Parga, and M. C. López Serrano. 2000. Gastroallergic anisakiasis: bordeline between food allergy and parasitic disease—clinical and allergologic evaluation of 20 patients with confirmed acute parasitism by Anisakis simplex. J. Allergy Clin. Immunol. 105:176-181. [DOI] [PubMed] [Google Scholar]
- 72.Daschner, A., A. Alonso-Gomez, and M. C. López-Serrano. 2000. What does Anisakis simplex parasitism in gastro-allergic anisakiasis teach us about interpretating specific and total IgE values? Allergol. Immunopathol. 28:67-70. [PubMed] [Google Scholar]
- 73.Daschner, A., C. Cuéllar, A. Alonso-Gomez, C. Y. Pascual, and M. Martín-Esteban. 2001. Serum CD23 is not altered in gastroallergic anisakiasis, but correlates with the production of specific IgE and the amount of polyclonal stimulation. Allergy 56:1003-1007. [DOI] [PubMed] [Google Scholar]
- 74.Daschner, A., C. Cuéllar, S. Sánchez-Pastor, C. Y. Pascual, and M. Martín-Esteban. 2002. Gastro-allergic anisakiasis as a consequence of simultaneous primary and secondary immune response. Parasite Immunol. 24:243-251. [DOI] [PubMed] [Google Scholar]
- 75.Daschner, A., F. Vega de la Osada, and C. Pascual. 2005. Allergy and parasites reevaluated: wide-scale induction of chronic urticaria by the ubiquitous fish-nematode Anisakis simplex in an endemic region. Allergol. Immunopathol. 33:31-37. [DOI] [PubMed] [Google Scholar]
- 76.Deardorff, T. L., R. E. Jones, and S. G. Kayes. 1991. Adherence of eosinophils to the epicuticle of infective juveniles of Anisakis simplex (Nematoda: Anisakidae). J. Helminthol. Soc. Wash. 58:131-137. [Google Scholar]
- 77.Deardorff, T. L., S. G. Kayes, and T. Fukumura. 1991. Human anisakiasis transmitted by marine food products. Hawaii Med. J. 50:9-16. [PubMed] [Google Scholar]
- 78.Deardorff, T. L., and M. L. Kent. 1989. Prevalence of larval Anisakis simplex in pen-reared and wild-caught salmon (Salmonidae) from Puget Sound, Washington. J. Wildl. Dis. 25:416-419. [DOI] [PubMed] [Google Scholar]
- 79.Deardorff, T. L., and R. M. Overstreet. 1990. Seafood-transmitted zoonoses in the United States: the fishes, the dishes, and the worms, p. 211-265. In D. R. Ward and C. Hackney (ed.), Microbiology of marine food products. Van Nostrand Reinhold, New York, NY.
- 80.Deardorff, T. L., R. B. Raybourne, and R. S. Desowivtz. 1984. Behavior and viavility of third-stage larvae of Terranova sp. (type HA) and Anisakis simplex (type I) under coolant conditions. J. Food Prot. 47:49-52. [DOI] [PubMed] [Google Scholar]
- 81.Decision 93/140/EEC. 1993. Decision of the Commission of 19 January 1993 laying down the detailed rules relating to the visual inspection for the purpose of detecting parasites in fishery products. Off. J. L 056(09.03.1993):42. [Google Scholar]
- 82.Del Pozo, M. D., M. T. Audicana, J. Diez, D. Muñoz, I. J. Ansotegui, E. Fernández, M. García, M. Etxenagusia, I. Moneo, and L. Fernández de Corres. 1997. Anisakis simplex, a relevant etiologic factor in acute urticaria. Allergy 52:576-579. [DOI] [PubMed] [Google Scholar]
- 83.Del Pozo, M. D., I. Moneo, L. Fernández de Corres, M. Audicana, D. Muñoz, E. Fernández, J. A. Navarro, and M. García. 1996. Laboratory determinations in Anisakis simplex allergy. J. Allergy Clin. Immunol. 97:977-984. [DOI] [PubMed] [Google Scholar]
- 84.Del Pozo, V., I. Arrieta, T. Tunon, I. Cortegano, B. Gomez, B. Cardaba, S. Gallardo, M. Rojo, G. Renedo, P. Palomino, A. I. Tabar, and C. Lahoz. 1999. Immunopathogenesis of human gastrointestinal infection by Anisakis simplex. J. Allergy Clin. Immunol. 104:637-643. [DOI] [PubMed] [Google Scholar]
- 85.Desowitz, R. S., R. B. Raybourne, H. Ishikura, and M. M. Kliks. 1985. The radioallergosorbent test (RAST) for the serological diagnosis of human anisakiasis. Trans. R. Soc. Trop. Med. Hyg. 79:256-259. [DOI] [PubMed] [Google Scholar]
- 86.Directive 91/493/EEC. 1991. Directive of Council of 22 July 1991 laying down the health conditions for the production and the placing on the market of fishery products. Off. J. L 268(24.09.1991):15-34. [Google Scholar]
- 87.E.C. Scientific Committee on Veterinary Measures Relating to Public Health. 1998. Allergic reactions to ingested Anisakis simplex antigens and evaluation of the possible risk to human health. http://ec.europa.eu/food/fs/sc/scv/out05_en.html.
- 88.Ellyard, J. I., L. Simson, and C. R. Parish. 2007. Th2-mediated antitumour immunity: friend or foe? Tissue Antigens 70:1-11. [DOI] [PubMed] [Google Scholar]
- 89.Else, K. J., F. D. Finckenkelman, C. R. Malizewski, and R. K. Grencis. 1994. Cytokine-mediated regulation of chronic intestinal helminth infection. J. Exp. Med. 179:347-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Elston, D. M. 2006. The hygiene hypothesis and atopy: bring back the parasites? J. Am. Acad. Dermatol. 54:172-179. [DOI] [PubMed] [Google Scholar]
- 91.Fabresse, F. X., H. Essioux, M. Meiran, P. Larroque, and H. Celton. 1984. Polyarthrite de l′anisakiase. Premier cas. Presse Med. 13:1004. [PubMed] [Google Scholar]
- 92.Falcao, H., N. Lunet, E. Neves, and H. Barros. 2002. Do only life larvae cause Anisakis simplex sensitization? Allergy 57:44. [PubMed] [Google Scholar]
- 93.Falcone, F. H., D. Zillikens, and B. F. Gibbs. 2006. The 21st century renaissance of the basophil? Current insights into its role in allergic responses and innate immunity. Exp. Dermatol. 15:855-864. [DOI] [PubMed] [Google Scholar]
- 94.Feldmeier, H., G. Poggensee, and U. Poggensee. 1993. The epidemiology, natural history, and diagnosis of human anisakiasis. Eur. Microbiol. 2:30-38. [Google Scholar]
- 95.Fernández de Corres, L., M. Audicana, M. D. Del Pozo, D. Muñoz, L. Fernández, J. A. Navarro, M. García, and J. Diez. 1996. Anisakis simplex induces not only anisakiasis: report on 28 cases of allergy caused by this nematode. J. Investig. Allergol. Clin. Immunol. 6:315-319. [PubMed] [Google Scholar]
- 96.Ferreira, F., T. Hawranek, P. Gruber, N. Wopfner, and A. Mari. 2004. Allergic cross-reactivity: from gene to the clinic. Allergy 59:243-267. [DOI] [PubMed] [Google Scholar]
- 97.Food and Drug Administration. 2007. Anisakis simplex and related worms. http://www.cfsan.fda.gov/∼mow/chap25.html.
- 98.Foti, C., E. Nettis, N. Cassano, I. Di Mundo, and G. A. Vena. 2002. Acute allergic reactions to Anisakis simplex after ingestion of anchovies. Acta Derm. Venereol. 82:121-123. [DOI] [PubMed] [Google Scholar]
- 99.Garcia, F., J. G. Blanco, M. Garcés, S. Juste, M. Fuentes, and D. Herrero. 2001. Freezing protects against Allergy to Anisakis simplex. J. Investig. Allergol. Clin. Immunol. 11:49-52. [PubMed] [Google Scholar]
- 100.Garcia, M., I. Moneo, M. T. Audicana, M. D. Del Pozo, D. Muñoz, E. Fernández, J. Diez, M. Etxenagusia, I. Ansotegui, and L. Fernández de Corres. 1997. The use of IgE immunoblotting as a diagnostic tool in Anisakis simplex allergy. J. Allergy Clin. Immunol. 99:497-501. [DOI] [PubMed] [Google Scholar]
- 101.Garcia-Hernandez, P., M. Rodero, and C. Cuellar. 2007. Anisakis simplex: the activity of larval products on the complement system. Exp. Parasitol. 115:1-8. [DOI] [PubMed] [Google Scholar]
- 102.Gazzinelli, R. T., and E. Y. Denkers. 2006. Protozoan encounters with Toll-like receptor signalling pathways: implications for host parasitism. Nat. Rev. Immunol. 6:895-906. [DOI] [PubMed] [Google Scholar]
- 103.Reference Deleted.
- 104.Gómez-Aguado, F., A. Picazo, M. L. Caballero, I. Moneo, J. A. Asturias, M. T. Corcuera, I. Casado, and M. J. Alonso. 2003. Ultrastructural localization of Ani s 1, a major allergen from the fish parasite Anisakis simplex. Parasitol. Res. 89:379-380. [DOI] [PubMed] [Google Scholar]
- 105.Gónzalez-Muñoz, M., R. Luque, F. Nauwelaers, and I. Moneo. 2005. Detection of Anisakis simplex-induced basophil activation by flow cytometry. Cytometry Part B 68B:31-36. [DOI] [PubMed] [Google Scholar]
- 106.Goto, C., S. Kasuya, K. Koga, H. Ohtomo, and N. Cagei. 1990. Lethal efficacy of extract from Zingiber officinale (traditional Chinese medicine) or [6]-shogaol and [6]-gingerol in Anisakis larvae in vitro. Parasitol. Res. 76:653-656. [DOI] [PubMed] [Google Scholar]
- 107.Gracia-Bara, M. T., V. Matheu, J. M. Zubeldia, M. Rubio, E. Ordoqui, M. P. López-Sáez, Z. Sierra, P. Tornero, and M. L. Baeza. 2001. Anisakis simplex-sensitized patients: should fish be excluded from their diet? Ann. Allergy Asthma Immunol. 86:679-685. [DOI] [PubMed] [Google Scholar]
- 108.Guarneri, F., C. Guarneri, and S. Benvenga. 2007. Cross-reactivity of Anisakis simplex: possible role of Ani s 2 and Anis s 3. Int. J. Dermatol. 46:146-150. [DOI] [PubMed] [Google Scholar]
- 109.Harnett, W., and M. M. Harnett. 2006. Molecular basis of worm-induced immunomodulation. Parasite Immunol. 28:535-543. [DOI] [PubMed] [Google Scholar]
- 110.Harnett, W., and M. M. Harnett. 2006. Filarial nematode secreted product ES-62 is an anti-inflammatory agent: therapeutic potential of small molecule derivatives and ES-62 peptide mimetics. Clin. Exp. Pharmacol. Physiol. 28:535-543. [DOI] [PubMed] [Google Scholar]
- 111.Hartmann, S., A. Sollwedel, A. Hoffmann, B. Sonnenburg, and R. Lucius. 2003. Characterization of IgE responses in a rodent model of filariasis and the allergenic potential of filarial antigens using an in vitro assay. Parasite Immunol. 25:9-16. [DOI] [PubMed] [Google Scholar]
- 112.Hill, A. V. S., J. Elvin, A. C. Willis, M. Aidoo, C. B. M. Allsopp, F. M. Gotch, X. M. Gao, M. Takiguchi, B. M. Greenwood, A. R. Townsend, A. J. McMichael, and H. W. C. Hilton. 1992. Molecular analysis of the association of HLA-B53 and resistance to severe malaria. Nature 360:434-439. [DOI] [PubMed] [Google Scholar]
- 113.Holland, M. J., Y. M. Harcus, A. Balic, and R. M. Maizels. 2005. Th2 induction by Nippostrongylus secreted antigens in mice deficient in B cells, eosinophils or MHC class I-related receptors. Immunol. Lett. 96:93-101. [DOI] [PubMed] [Google Scholar]
- 114.Hotez, P., M. Cappello, J. Hawdon, C. Beckers, and J. Sakanari. 1994. Hyaluronidases of the gastrointestinal invasive nematodes Ancylostoma caninum and Anisakis simplex: possible functions in the pathogenesis of human zoonoses. J. Infect. Dis. 170:918-926. [DOI] [PubMed] [Google Scholar]
- 115.Hunter, M. M., and D. M. McKay. 2004. Helminths as therapeutic agents for inflammatory bowel disease. Aliment. Pharmacol. Ther. 19:167-177. [DOI] [PubMed] [Google Scholar]
- 116.Hwang, Y. K., J. S. Kim, J. B. Lee, T. J. Song, K. W. Joo, J. S. Lee, and S. W. Cho. 2003. Human anisakiasis: diversity in antibody response profiles to the changing antigens in larval excretions/secretions. Parasite Immunol. 25:1-7. [DOI] [PubMed] [Google Scholar]
- 117.Iglesias, R., J. Leiro, M. T. Santamarina, M. L. Sanmartin, and F. M. Ubeira. 1997. Monoclonal antibodies against diagnostic Anisakis simplex antigens. Parasitol. Res. 83:755-761. [DOI] [PubMed] [Google Scholar]
- 118.Iglesias, R., J. Leiro, F. M. Ubeira, M. T. Santamarina, and M. L. Sanmartin. 1993. Anisakis simplex: antigen recognition and antibody production in experimentally infected mice. Parasite Immunol. 15:243-250. [DOI] [PubMed] [Google Scholar]
- 119.Iglesias, R., J. Leiro, F. M. Ubeira, M. T. Santamarina, and M. L. Sanmartin. 1995. Anisakis simplex: stage-specific antigens recognized by mice. J. Helmithol. 69:319-324. [DOI] [PubMed] [Google Scholar]
- 120.Ikeda, K., R. Kumashiro, and T. Kifune. 1989. Nine cases of acute gastric anisakiasis. Gastrointest. Endosc. 35:305-308. [DOI] [PubMed] [Google Scholar]
- 121.Im, K., H. Shin, B. Kim, and S. Moon. 1995. Gastric anisakiasis cases in Cheju-do, Korea. Korean J. Parasitol. 33:179-186. [DOI] [PubMed] [Google Scholar]
- 122.Ishikura, H. 1990. Clinical features of intestinal anisakiasis, p. 89-100. In H. Ishikura and K. Kikuchi (ed.), Intestinal anisakiasis in Japan. Infected fish, sero-immunological diagnosis, and prevention. Springer-Verlag, Tokyo, Japan.
- 123.Ishikura, H. 1990. Epidemiological aspects of intestinal anisakiasis and its pathogenesis, p. 3-21. In H. Ishikura and K. Kikuchi (ed.), Intestinal anisakiasis in Japan. Infected fish, sero-immunological diagnosis, and prevention. Springer-Verlag, Tokyo, Japan.
- 124.Ishikura, H., K. Kikuchi, K. Nagasawa, T. Ooiwa, H. Takamiya, N. Sato, and K. Sugane. 1993. Anisakidae and anisakidosis, p. 43-102. In T. Sun (ed.), Progress in clinical parasitology, vol. III. Springer-Verlag, New York, NY. [DOI] [PubMed] [Google Scholar]
- 125.Ishikura, H., Y. Kikuchi, O. Tokoyawa, H. Hayasaka, and K. Kikuchi. 1990. Skin (intradermal) testing using several kinds of Anisakis larva antigens, p. 159-165. In H. Ishikura and K. Kikuchi (ed.), Intestinal anisakiasis in Japan. Infected fish, sero-immunological diagnosis, and prevention. Springer-Verlag, Tokyo, Japan.
- 126.Iwasaki, K., and M. Torisu. 1982. Anisakis and eosinophil. II. Eosinophilic phlegmon experimentally induced in normal rabbits by parasite-derived eosinophil chemotactic factor (ECF1-P). Clin. Immunol. Immunopathol. 23:593-605. [DOI] [PubMed] [Google Scholar]
- 127.Jackson, G. J. 1990. Public health and research perpectives on the microbial contamination of foods. J. Anim. Sci. 68:884-891. [DOI] [PubMed] [Google Scholar]
- 128.James, S. L. 1995. Role of nitric oxide in parasitic infections. Microbiol. Rev. 59:533-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jarrett, E. E., and D. M. Haig. 1976. Time course studies on rat IgE production in N. brasiliensis infection. Clin. Exp. Immunol. 24:346-351. [PMC free article] [PubMed] [Google Scholar]
- 130.Jeebhay, M. F., T. G. Robins, S. B. Lehrer, and A. I. Lopata. 2001. Occupational seafood allergy: a review. Occup. Environ. Med. 58:553-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jensen, T. 1997. Experimental infection/transmission of sculpins (Myoxocephalus scorpius) and cod (Gadus morhua) by sealworm (Pseudoterranova decipiens) larvae. Parasitol. Res. 83:380-382. [DOI] [PubMed] [Google Scholar]
- 132.Johansson, E., M. Aponno, M. Lundber, and M. van Hage-Hamsten. 2001. Allergenic cross-reactivity between the nematode Anisakis simplex and the dust mites Acarus siro, Lepidoglyphus destructor, Tyrophagus putrescentiae, and Dermatophagoides pteronyssinus. Allergy 56:660-666. [DOI] [PubMed] [Google Scholar]
- 133.Jones, R. E., T. Deardorff, and S. G. Kayes. 1990. Anisakis simplex: histopatological changes in experimentally infected CBA/J mice. Exp. Parasitol. 70:305-313. [DOI] [PubMed] [Google Scholar]
- 134.Kagei, A., and I. Isogaki. 1992. A case of acute abdominal syndrome caused by the presence of a large number of Anisakis simplex larvae. Int J. Parasitol. 22:251-253. [DOI] [PubMed] [Google Scholar]
- 135.Kaisho, T., and S. Akira. 2006. Toll-like receptor function and signaling. J. Allergy Clin. Immunol. 117:979-987. [DOI] [PubMed] [Google Scholar]
- 136.Karl, H., A. Roepstorff, H. H. Huss, and B. Bloemsma. 1995. Survival of Anisakis larvae in marinated herring fillets. Int. J. Food Sci. Technol. 29:661-670. [Google Scholar]
- 137.Kassai, T., M. Cordero del Campillo, J. Euzeby, S. Gaafar, T. Hiepe, and C. A. Himonas. 1988. Standardized nomenclature of animal parasite diseases (SNOAPAD). Vet. Parasitol. 29:299-326. [DOI] [PubMed] [Google Scholar]
- 138.Kasuya, S., H. Amano, and S. Izumi. 1989. Gastric anisakiasis with anaphylactoid reactions. ACI News 1:13-14. [Google Scholar]
- 139.Kasuya, S., H. Amano, and S. Izumi. 1990. Mackerel-induced urticaria and Anisakis. Lancet 335:665. [DOI] [PubMed] [Google Scholar]
- 140.Kasuya, S., and K. Koga. 1992. Significance of detection of specific IgE in Anisakis-related diseases. Arerugi 41:106-110. [PubMed] [Google Scholar]
- 141.Kay, A. B. 2000. Overview of allergy and allergic diseases: with a view to the future. Br. Med. Bull. 56:853-864. [DOI] [PubMed] [Google Scholar]
- 142.Kennedy, M. W. 2000. Immune response to Anisakis simplex and other ascarid nematodes. Allergy 55(Suppl. 59):7-13.11291780 [Google Scholar]
- 143.Kennedy, M. W. 2006. The larval surface, p. 32-41. In C. V. Holland and H. V. Smith (ed.) Toxocara: the enigmatic parasite, 1st ed. CABI Publishing, Wallingford, United Kingdom.
- 144.Kennedy, M. W., A. Brass, A. B. McCruden. N. C. Price, S. M. Kelly, and A. Cooper. 1995. The ABA-1 allergen of the parasitic nematode Ascaris suum: fatty acid and retinoid binding function and structural characterization. Biochemistry 34:6700-6710. [DOI] [PubMed] [Google Scholar]
- 145.Kennedy, M. W., E. M. Fraser, and J. F. Christie. 1991. MHC class-II (I-A) region control of the IgE antibody repertoire to the Aba-1 allergen of the nematode Ascaris. Immunology 72:577-579. [PMC free article] [PubMed] [Google Scholar]
- 146.Kennedy, M. W., A. M. S. Gordon, L. A. Tomlinson, and F. Qureshi. 1987. Genetic (major histocompatibility complex?) control of the antibody repertoire to the secreted antigens of Ascaris. Parasite Immunol. 9:269-273. [DOI] [PubMed] [Google Scholar]
- 147.Kennedy, M. W., A. E. McIntosh, A. J. Blair, and D. McLaughlin. 1990. MHC (RT1) restriction of the antibody repertoire to infection with the nematode Nippostrongylus brasiliensis in the rat. Immunology 71:317-322. [PMC free article] [PubMed] [Google Scholar]
- 148.Kennedy, M. W., F. Qureshi, E. M. Fraser, M. Haswell-Elkins, D. B. Elkins, and H. V. Smith. 1989. Antigenic relationship between the surface-exposed, secreted, and somatic materials of the nematode parasites Ascaris lumbricoides, Ascaris suum and Toxocara canis. Clin. Exp. Immunol. 75:493-500. [PMC free article] [PubMed] [Google Scholar]
- 149.Kennedy, M. W., J. Tierney, P. Ye, F. A. McMonagle, A. McIntosh, D. McLaughlin, and J. W. Smith. 1988. The secreted and somatic antigens of the third stage larva of Anisakis simplex, and antigenic relationship with Ascaris suum, Ascaris lumbricoides, and Toxocara canis. Mol. Biochem. Parasitol. 31:35-46. [DOI] [PubMed] [Google Scholar]
- 150.Kennedy, M. W., D. L. Wassom, A. E. McIntosh, and J. C. Thomas. 1991. H-2 (I-A) control of the antibody repertoire to secreted antigens of Trichinella-Spiralis in infection and its relevance to resistance and susceptibility. Immunology 73:36-43. [PMC free article] [PubMed] [Google Scholar]
- 151.Kikuchi, Y., H. Saeki, and H. Ishikura. 1990. Detection of cellular immunity by migration inhibition test on rabbits and guinea pigs immunized with Anisakis larval antigens, p. 191-198. In H. Ishikura and K. Kikuchi (ed.), Intestinal anisakiasis in Japan. Infected fish, sero-immunological diagnosis, and prevention. Springer-Verlag, Tokyo, Japan.
- 152.Kimura, S., Y. Takagi, and K. Gomi. 1999. IgE response to Anisakis compared to seafood. Allergy 54:1225-1226. [DOI] [PubMed] [Google Scholar]
- 153.King, C. L., L. Kumaraswami, R. W. Poindexter, S. Kumari, K. Jayaraman, D. W. Allin, E. A. Ottesen, and T. B. Nutman. 1992. Immunologic tolerance in lymphatic filariasis. Diminished parasite-specific T and B lymphocyte precursor frequency in the microfilaremic state. J. Clin. Investig. 89:1403-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kliks, M. M. 1983. Anisakiasis in the western United States: four new case reports from California. Am. J. Trop. Med. Hyg. 32:526-532. [DOI] [PubMed] [Google Scholar]
- 155.Kliks, M. M. 1986. Human anisakiasis: an update. J. Am. Med. Assoc. 255:2605. [DOI] [PubMed] [Google Scholar]
- 156.Knox, D. P. 2007. Proteinase inhibitors and helminth parasite infection. Parasite Immunol. 29:57-71. [DOI] [PubMed] [Google Scholar]
- 157.Knox, D. P., and M. W. Kennedy. 1988. Proteinases released by the parasitic larval stages of Ascaris-suum, and their inhibition by antibody. Mol. Biochem. Parasitol. 28:207-216. [DOI] [PubMed] [Google Scholar]
- 158.Kobayashi, Y., S. Ishizaki, K. Shimakura, Y. Nagashima, and K. Shiomi. 2007. Molecular cloning and expression of two new allergens from Anisakis simplex. Parasitol. Res. 100:1233-1241. [DOI] [PubMed] [Google Scholar]
- 159.Kobayashi, Y., K. Shimakura, S. Ishizaki, Y. Nagashima, and K. Shiomi. 2007. Purification and cDNA cloning of a new heat-stable allergen from Anisakis simplex. Mol. Biochem. Parasitol. 155:138-145. [DOI] [PubMed] [Google Scholar]
- 160.Kuipers, F. C. 1964. Eosinophilic phlegmonous inflammation of the alimentary canal caused by a parasite from the herring. Pathol. Microbiol. 27:925-930. [DOI] [PubMed] [Google Scholar]
- 161.Lawrence, C. E., J. C. M. Paterson, L. M. Higgins, T. T. MacDonald, M. W. Kennedy, and P. Garside. 1998. IL-4 regulated enteropathy in an intestinal nematode infection. Eur. J. Immunol. 28:2627-2684. [DOI] [PubMed] [Google Scholar]
- 162.Liew, F. Y., X. Q. Wei, and L. Proudfoot. 1997. Cytokines and nitric oxide as effector molecules against parasitic infections. Philos. Trans. R. Soc. Lond. B 352:1311-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lobos, E. 1997. The basis of IgE responses to specific antigenic determinants in helminthiasis. Chem. Immunol. 66:1-25. [DOI] [PubMed] [Google Scholar]
- 164.Lorenzo, S., R. Iglesias, M. T. Audicana, R. García-Villaescusa, F. Pardo, M. L. Sanmartín, and F. M. Ubeira. 1999. Human immunoglobulin isotype profiles produced in response to antigens recognized by monoclonal antibodies specific to Anisakis simplex. Clin. Exp. Allergy 29:1095-1101. [DOI] [PubMed] [Google Scholar]
- 165.Lorenzo, S., R. Iglesias, J. Leiro, F. M. Ubeira, I. Ansotegui, M. García, and L. Fernández de Corres. 2000. Usefulness of currently available methods for the diagnosis of Anisakis simplex allergy. Allergy 55:627-633. [DOI] [PubMed] [Google Scholar]
- 166.Lorenzo, S., R. Iglesias, E. Paniagua, J. Leiro, and F. M. Ubeira. 1999. Analysis of the antigenicity in mice of biotinyl-enzymes from Anisakis simplex and other nematodes. Parasitol. Res. 85:441-445. [DOI] [PubMed] [Google Scholar]
- 167.Lorenzo, S., F. Romaris, R. Iglesias, M. T. Audicana, J. M. Alonso, J. Leiro, and F. M. Ubeira. 2000. O-glycans as a source of cross-reactivity in determinations of human serum antibodies to Anisakis simplex antigens. Clin. Exp. Allergy 30:551-559. [DOI] [PubMed] [Google Scholar]
- 168.Lu, C. C., T. Nguyen, S. Morris, D. Hill, and J. A. Sakanari. 1998. Anisakis simplex: mutational bursts in the reactive site centers of serine protease inhibitors from an ascarid nematode. Exp. Parasitol. 89:257-261. [DOI] [PubMed] [Google Scholar]
- 169.Lucas, S. B., J. P. Cruse, and A. A. M. Lewis. 1985. Anisakiasis in the United Kingdom. Lancet ii:843-844. [DOI] [PubMed] [Google Scholar]
- 170.Magen, E., G. Brokow, Z. Bentwich, J. Mishal, and S. Scharf. 2005. Can worms defend our hearts? Chronic helminthic infections may attenuate the development of cardiovascular diseases. Med. Hypotheses 64:904-909. [DOI] [PubMed] [Google Scholar]
- 171.Maizels, R. M. 2005. Infections and allergy—helminths, hygiene and host immune regulation. Curr. Opin. Immunol. 17:656-661. [DOI] [PubMed] [Google Scholar]
- 172.Maizels, R. M., D. de Savigny, and B. M. Ogilvie. 1984. Characterisation of surface and excretory-secretory antigens of Toxocara canis infective larvae. Parasite Immunol. 6:23-37. [DOI] [PubMed] [Google Scholar]
- 173.Maldonado-Bernal, C., C. J. Kirschning, Y. Rosenstein, L. M. Rocha, N. Rios-Sarabia, M. Espinosa-Cantellano, I. Becker, I. Estrada, R. M. Salazar-Gonzalez, C. Lopez-Macías, H. Wagner, J. Sanchez, and A. Isibasi. 2005. The innate immune response to Entamoeba histolytica lipopeptide phosphophoglycan is mediated by toll-like receptors 2 and 4. Parasite Immunol. 27:127-137. [DOI] [PubMed] [Google Scholar]
- 174.Margolis, M. 1977. Public health aspects of “codworm” infection: a review. J. Fish Res. Board Can. 34:887-898. [Google Scholar]
- 175.Marques, J. F., H. M. Cabral, M. Busi, and S. D'Amelio. 2006. Molecular identification of Anisakis species from Pleuronectiformes off the Portuguese coast. J. Helminthol. 80:47-51. [DOI] [PubMed] [Google Scholar]
- 176.Matsui, T., M. Iida, M. Fujishima, and T. Yao. 1990. Radiographic features of intestinal anisakiasis p. 101-108. In H. Ishikura and K. Kikuchi (ed.), Intestinal anisakiasis in Japan. Infected fish, sero-immunological diagnosis, and prevention. Springer-Verlag, Tokyo, Japan.
- 177.Matsuzaka, M., S. Fukuda, I. Takahashi, S. Shimaya, T. Omaya, M. Yaegaki, T. Shimoyama, J. Sakamoto, S. Nakaji, and T. Umeda. 2007. The decreasing burden of gastric cancer in Japan. Tohoku J. Exp. Med. 212:207-219. [DOI] [PubMed] [Google Scholar]
- 178.Matthews, B. E. 1982. Behaviour and enzyme release by Anisakis sp. larvae (Nematoda: Ascaridida). J. Helminthol. 56:177-183. [DOI] [PubMed] [Google Scholar]
- 179.Matthews, B. E. 1984. The source, release and specificity of proteolytic enzyme activity produced by Anisakis simplex larvae (Nematoda: Ascaridida) in vitro. J. Helminthol. 58:175-185. [Google Scholar]
- 180.Mattiucci, S., G. Nascetti, M. Dailey, S. C. Web, N. B. Barros, R. Cianchi, and L. Bullini. 2005. Evidence for a new species of Anisakis Dujardin, 1845: morphological description and genetic relationships between, congeners (nematoda: Anisakidae). Syst. Parasitol. 3:157-171. [DOI] [PubMed] [Google Scholar]
- 181.McDermott, J. R., R. E. Bartram, P. A. Knight, H. R. P. Miller, D. R. Garrod, and R. K. Grencis. 2003. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc. Natl. Acad. Sci. USA 100:7761-7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.McKerrow, J. H. 1989. Parasite proteases. Exp. Parasitol. 68:111-115. [DOI] [PubMed] [Google Scholar]
- 183.Mercado, R., P. Torres, and J. Maira. 1997. Human case of gastric infection by a fouth larval stage of Pseudoterranova decipiens (Nematoda, Anisakidae). Rev. Saude Publica 31:178-181. [DOI] [PubMed] [Google Scholar]
- 184.Mineta, S., K. Shimanuki, A. Sugiura, Y. Tsuchiya, M. Kaneko, Y. Sugiyama, K. Akimaru, and T. Tajiri. 2006. Chronic anisakiasis of the ascending colon associated with carcinoma. J. Nippon Med. Sch. 73:169-174. [DOI] [PubMed] [Google Scholar]
- 185.Mitchell, G. F. 1991. Co-evolution of parasites and adaptative immune responses. Immunol. Today 12:A2-A5. [DOI] [PubMed] [Google Scholar]
- 186.Moneo, I., M. T. Audicana, M. T. Alday, G. Curiel, M. D. Del Pozo, and M. García. 1997. Periodate treatment of Anisakis simplex allergens. Allergy 52:565-569. [DOI] [PubMed] [Google Scholar]
- 187.Moneo, I., M. L. Caballero, F. Gomez, E. Ortega, and M. J. Alonso. 2000. Isolation and characterization of a major allergen from the fish parasite Anisakis simplex. J. Allergy Clin. Immunol. 106:177-182. [DOI] [PubMed] [Google Scholar]
- 188.Moneo, I., M. L. Caballero, M. Gonzalez-Muñoz, A. I. Rodríguez-Mahillo, R. Rodríguez-Perez, and A. Silva. 2005. Isolation of a heat-resistant allergen from the fish parasite Anisakis simplex. Parasitol. Res. 96:285-289. [DOI] [PubMed] [Google Scholar]
- 189.Moneret-Vautrin, D. A., M. Morisset, J. Flabbee, E. Beaudouin, and G. Kanny. 2005. Epidemiology of life-threatening and lethal anaphylaxis: a review. Allergy 60:443-451. [DOI] [PubMed] [Google Scholar]
- 190.Montoro, A., M. J. Perteguer, T. Chivato, R. Laguna, and C. Cuellar. 1997. Recidivous acute urticaria caused by Anisakis simplex. Allergy 52:985-991. [DOI] [PubMed] [Google Scholar]
- 191.Moreels, T. G., and P. A. Pelckmans. 2005. Gastrointestinal parasites. Potential therapy for refactory inflammatory bowel diseases. Inflamm. Bowel Dis. 11:178-184. [DOI] [PubMed] [Google Scholar]
- 192.Moreno-Ancillo, A., M. T. Caballero, R. Cabañas, J. Contreras, J. A. Martín-Barroso, P. Barranco, and M. C. López-Serrano. 1997. Allergic reactions to Anisakis simplex parasitizing seafood. Ann. Allergy Asthma Immunol. 79:246-250. [DOI] [PubMed] [Google Scholar]
- 193.Morris, S. R., and J. A. Sakanari. 1994. Characterization of the serine protease and serine protease inhibitor from the tissue-penetrating nematode Anisakis simplex. J. Biol. Chem. 269:27650-27656. [PubMed] [Google Scholar]
- 194.Moschella, C. M., S. Mattiucci, P. Mingazzini, G. De Angelis, M. Assenza, F. Lombardo, S. Monaco, L. Paggi, and C. Modini. 2004. Intestinal anisakiasis in Italy: case report. J. Helminthol. 78:271-273. [DOI] [PubMed] [Google Scholar]
- 195.Mudry, J., P. Lefebvre, E. Dei-Cas, A. Vernes, J. Poirriez, M. Débat, R. Marti, P. Binot, and A. Cortot. 1986. Anisakiase humaine: 5 cas dans le nord de la France. Gastroenterol. Clin. Biol. 10:83-87. [PubMed] [Google Scholar]
- 196.Muraoka, A., I. Suehiro, M. Fujii, K. Nagata, H. Kusunoki, Y. Kumon, D. Shirasaka, T. Hosooka, and K. Murakami. 1996. Acute gastric anisakiasis: 28 cases during the last 10 years. Dig. Dis. Sci. 41:2362-2365. [DOI] [PubMed] [Google Scholar]
- 197.Nagasawa, K. 1990. Anisakis larvae in intermediate and paratenic hosts in Japan, p. 23-29. In H. Ishikura and K. Kikuchi (ed.), Intestinal anisakiasis in Japan. Infected fish, sero-immunological diagnosis, and prevention. Springer-Verlag, Tokyo, Japan.
- 198.Namiki, N., and Y. Yazaki. 1989. Endoscopic findings of gastric anisakiasis with acute symptoms, p. 47-51. In H. Ishikura and M. Namiki (ed.), Gastric anisakiasis in Japan. Epidemiology, diagnosis, treatment. Springer-Verlag, Tokyo, Japan.
- 199.Newton, S. E., and E. N. Meeusen. 2003. Progress and new technologies for developing vaccines against gastrointestinal nematode parasites of sheep. Parasite Immunol. (Oxford) 25:283-296. [DOI] [PubMed] [Google Scholar]
- 200.Nguyen, T., M. A. Qasim, S. Morris, C. C. Lu, D. Hill, M. Laskowski, Jr., and J. A. Sakanari. 1999. Expression and characterization of elastase inhibitors from the ascarid nematodes Anisakis simplex and Ascaris suum. Mol. Biochem. Parasitol. 102:79-89. [DOI] [PubMed] [Google Scholar]
- 201.Nieuwenhuizen, N., A. L. Lopata, M. F. Jeebhay, D. R. Herbert, T. G. Robins, and F. Brombacher. 2006. Exposure to the fish parasite Anisakis causes allergic airway hyperreactivity and dermatitis. J. Allergy Clin. Immunol. 117:1098-1105. [DOI] [PubMed] [Google Scholar]
- 202.Ogilvie, B. M., M. E. Selkirk, and R. M. Maizels. 1990. The molecular revolution and nematode parasitology: yesterday, today and tomorrow. J. Parasitol. 76:607-618. [PubMed] [Google Scholar]
- 203.Ohtaki, H., and R. Ohtaki. 1989. Clinical manifestation of gastric anisakiasis, p. 37-46. In H. Ishikura and M. Namiki (ed.), Gastric anisakiasis in Japan. Epidemiology, diagnosis, treatment. Springer-Verlag, Tokyo, Japan.
- 204.Ortega-Deballon, P., A. Carabias-Hernandez, A. Martin-Blazquez, P. Garaulet, L. Benoit, B. Kretz, M. Limones-Esteban, and J. P. Favre. 2005. Anisakiasis: an infestation to be known by surgeons. Ann. Chir. 130:407-410. [DOI] [PubMed] [Google Scholar]
- 205.Oshima, M. T. 1987. Anisakiasis—is the sushi bar guilty? Parasitol. Today 3:44-48. [DOI] [PubMed] [Google Scholar]
- 206.Oshima, T. 1972. Anisakis and anisakiasis in Japan and adjacent area, p. 301-393. In K. Morishita, Y. Komiya, and H. Matsubayashi (ed.), Progress of medical parasitology in Japan, vol. IV. Meguro Parasitological Museum, Tokyo, Japan. [Google Scholar]
- 207.Paggi, L., G. Nascetti, R. Cianchi, P. Orecchia, S. Mattiucci, S. D'Amelio, B. Berland, B. Brattey, J. W. Smith, and L. Bullini. 1991. Genetic evidence for three species within Pseudoterranova decipiens (Nematoda, Ascaridida, Ascaridoidea) in the North Atlantic and Norwegian and Barents Sea. Int. J. Parasitol. 21:195-212. [DOI] [PubMed] [Google Scholar]
- 208.Pampiglione, S., F. Rivasi, M. Criscuolo, A. De Benedittis, A. Gentile, S. Russo, M. Testini, and M. Villan. 2002. Human anisakiasis in Italy: a report of eleven new cases. Pathol. Res. Pract. 198:429-434. [DOI] [PubMed] [Google Scholar]
- 209.Paterson, J. C. M., P. Garside, M. W. Kennedy, and C. E. Lawrence. 2002. Modulation of a heterologous immune response by the products of Ascaris suum. Infect. Immun. 70:6058-6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Pavon-Martinez, N., F. Masso-Rojas, R. López-Alcántara, and M. V. Monteón. 2007. Altered pattern of connectivity in Chagas disease. Ann. N. Y. Acad. Sci. 1107:271-279. [DOI] [PubMed] [Google Scholar]
- 211.Peisong, G., A. Yamasaki, X. Q. Mao, T. Enomoto, Z. Feng, and F. Gloria-Bottini. 2004. An asthma-associated genetic variant of STAT6 predicts low burden of Ascaris worm infestation. Genes Immun. 5:58-62. [DOI] [PubMed] [Google Scholar]
- 212.Perez-Perez, J., E. Fernandez-Caldas, F. Marañon, J. Sastre, M. L. Bernal, J. Rodríguez, and C. A. Bedate. 2000. Molecular cloning of paramyosin, a new allergen of Anisakis simplex. Int. Arch. Allergy Immunol. 123:120-129. [DOI] [PubMed] [Google Scholar]
- 213.Perteguer, M. J., R. Raposo, and C. Cuellar. 1996. In vitro study on the effect of larval excretory/secretory products and crude extracts from Anisakis simplex on blood coagulation. Int. J. Parasitol. 26:105-108. [DOI] [PubMed] [Google Scholar]
- 214.Petithory, J. C. 2007. New data on anisakiasis. Bull. Acad. Natl. Med. 191:53-65. [PubMed] [Google Scholar]
- 215.Petithory, J. C., J. Lapiere, M. Rousseau, and M. T. Clique. 1986. Diagnostic sérologique de l'anisakiase (granulome éosinophile digestif) par précipitation en milieu gélifié (ouchterlony, électrosynérèse, immunoélectrophorèse). Med. Mal. Infect. 53:157-162. [Google Scholar]
- 216.Petithory, J. C., B. Paugam, P. Buyet-Rousset, and A. Paugam. 1990. Anisakis simplex, a co-factor of gastric cancer? Lancet 336:1002. [DOI] [PubMed] [Google Scholar]
- 217.Petithory, J. C., M. Rousseau, and F. Siodlak. 1991. Données séroepidémiologiques sur l'anisakiase: conséquences prophylactiques pour les produits de la pêche. Bull. Acad. Natl. Med. 175:273-279. [PubMed] [Google Scholar]
- 218.Phillips, C., R. Coward, D. I. Pritchard, and C. R. Hewitt. 2003. Basophis express a type 2 cytokine profile on exposure to proteases from helminths and house dust mites. J. Leukoc. Biol. 73:165-171. [DOI] [PubMed] [Google Scholar]
- 219.Plath, F., A. Holle, D. Zendeh, F. W. Moller, M. Barten, E. C. Reisinger, and S. Liebe. 2001. Anisakiasis of the stomach—a case report from Germany. Z. Gastroenterol. 39:177-180. [DOI] [PubMed] [Google Scholar]
- 220.Pulido-Marrero, Z., E. Gonzalez, T. Alfaya, B. de la Hoz, and M. Cuevas. 2000. Unusual sensitization to Anisakis simplex. Allergy 55:586-587. [DOI] [PubMed] [Google Scholar]
- 221.Purello-D'Dambrosio, F., E. Pastorello, S. Gangemi, G. Lombardo, L. Ricciardi, O. Fogliani, and R. A. Merendino. 2000. Incidence of sensitivity to Anisakis simplex in a risk population of fishermen/fishmongers. Ann. Allergy Asthma Immunol. 84:439-444. [DOI] [PubMed] [Google Scholar]
- 222.Ramos, J. D. A., A. S. M. Teo, B. W. Lee, N. Cheong, and K. Y. Chua. 2004. DNA immunization for the production of monoclonal antibodies to Blo t 11, a paramyosin homolog from Blomia tropicalis. Allergy 59:539-547. [DOI] [PubMed] [Google Scholar]
- 223.Raybourne, R., T. L. Deardorff, and J. W. Bier. 1986. Anisakis simplex: larvas excretory secretory protein production and cytostatic action in mammalian cell cultures. Exp. Parasitol. 62:92-97. [DOI] [PubMed] [Google Scholar]
- 224.Raybourne, R., R. S. Desowitz, M. M. Kliks, and T. L. Deardorff. 1983. Anisakis simplex and Terranova sp.: inhibition by larval excretory-secretory products of mitogen-induced rodent lymphoblast proliferation. Exp. Parasitol. 55:289-298. [DOI] [PubMed] [Google Scholar]
- 225.Rhoads, M. L., R. H. Fetterer, and D. E. Hill. 2000. Trichuris suis: a secretory serine protease inhibitor. Exp. Parasitol. 94:1-7. [DOI] [PubMed] [Google Scholar]
- 226.Rhoads, M. L., R. H. Fetterer, E. Hill, and J. F. Urban. 2000. Trichuris suis: a secretory chymotrypsin/elastase inhibitor with potential as an immunomodulator. Exp. Parasitol. 95:36-44. [DOI] [PubMed] [Google Scholar]
- 227.Rocken, M., J. F. Urban, and F. Shevach. 1992. Infection breaks T-cell tolerance. Nature 359:79-82. [DOI] [PubMed] [Google Scholar]
- 228.Rodriguez, E., A. M. Anadón, E. García-Bodas, F. Romarís, R. Iglesias, T. Gárate, and F. M. Ubeira. 2008. Novel sequences and epitopes of diagnostic value derived from the Anisakis simplex Ani s 7 major allergen. Allergy 63:219-225. [DOI] [PubMed] [Google Scholar]
- 229.Rodriguez-Mahillo, A. I., M. Gonzalez-Muñoz, F. Gomez-Aguado, R. Rodriguez-Perez, M. T. Corcuera, M. L. Caballero, and I. Moneo. 2007. Cloning and characterisation of the Anisakis simplex allergen Ani s 4 as a cysteine-protease inhibitor. Int. J. Parasitol. 37:907-917. [DOI] [PubMed] [Google Scholar]
- 230.Rodriguez-Perez, R., M. L. Caballero, M. Gonzalez-Muñoz, A. Rodriguez-Mahillo, and I. Moneo. 2007. Cloning and expression of a biologically active Anisakis simplex allergen Ani s 1 in the yeast Pichia pastoris. Mol. Biochem. Parasitol. 154:115-118. [DOI] [PubMed] [Google Scholar]
- 231.Ruitenberg, E. J., and H. J. Loendersloot. 1971. Histochemical properties of the excretory organ of Anisakis sp. larva. J. Parasitol. 57:149-150. [PubMed] [Google Scholar]
- 232.Sajid, M., and J. H. McKerrow. 2002. Cysteine proteases of parasitic organisms. Mol. Biochem. Parasitol. 120:1-21. [DOI] [PubMed] [Google Scholar]
- 233.Sakanari, J. A., and J. H. McKerrow. 1989. Anisakiasis. Clin. Microbiol. Rev. 2:278-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sakanari, J. A., and J. H. McKerrow. 1990. Identification of the secreted neutral proteases from Anisakis simplex. J. Parasitol. 76:625-630. [PubMed] [Google Scholar]
- 235.Sakanari, J. A., C. E. Staunton, A. E. Eakin, C. S. Craik, and J. H. McKerrow. 1989. Serine proteases from nematode and protozoan parasites: isolation of sequence homologs using generic molecular probes. Proc. Natl. Acad. Sci. USA 86:4863-4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Sampson, H. A. 2000. Food anaphylaxis. Br. Med. Bull. 56:925-935. [DOI] [PubMed] [Google Scholar]
- 237.Sanchez-Monsalvez, I., C. De Armas-Serrá, W. Bernadina, and F. Rodriguez-Caabeiro. 2003. Altered autonomic control in rat intestine due to both infection with Anisakis simplex and incubation with the parasite's crude extract. Dig. Dis. Sci. 48:2342-2352. [DOI] [PubMed] [Google Scholar]
- 238.Sanchez-Velasco, P., E. Gomez-Casado, J. Martinez-Laso, J. Moscoso, J. Zamora, E. Lowy, C. Silvera, A. Cemborain, F. Leyva-Cobián, and A. Arnaiz-Villena. 2003. HLA alleles in isolated populations from north Spain: origin of the Basques and the ancient Iberians. Tissue Antigens 61:384-392. [DOI] [PubMed] [Google Scholar]
- 239.Sanchez-Velasco, P., L. Mendizábal, E. M. Antón, G. Ocejo-Vinyals, J. Jerez, and F. Leyva-Cobián. 2000. Association of hypersensitivity to the nematode Anisakis simplex with HLA class II DRB1*1502-DQB1*0601 haplotype. Hum. Immunol. 61:314-319. [DOI] [PubMed] [Google Scholar]
- 240.Sastre, J., M. Lluch-Bernal, S. Quirce, I. Arrieta, C. Lahoz, A. Del Amo, E. Fernández-Caldas, and F. Marañón. 2000. A double blind, placebo-controlled oral challenge study with lyophilized larvae and antigen of the fish parasite, Anisakis simplex. Allergy 55:560-564. [DOI] [PubMed] [Google Scholar]
- 241.Scala, E., M. Giani, L. Pirrotta, E. C. Guerra, S. Cadoni, C. R. Girardelli, O. De Pita, and P. Puddu. 2001. Occupational generalised urticaria and allergic airborne asthma due to Anisakis simplex. Eur. J. Dermatol. 11:249-250. [PubMed] [Google Scholar]
- 242.Schulz, O., B. J. Sutton, R. L. Beavil, J. G. Shi, H. F. Sewell, H. J. Gould, P. Laing, and F. Shakib. 1997. Cleavage of the low-affinity receptor for human IgE (CD23) by a mite cysteine protease: nature of the cleaved fragment in relation to the structure and function of CD23. Eur. J. Immunol. 27:584-588. [DOI] [PubMed] [Google Scholar]
- 243.Scott, P. A., and A. Sher. 1993. Immunoparasitology, p. 1179-1210. In W. E. Paul (ed.), Fundamental immunology, 3rd ed. Raven Press, New York, NY.
- 244.Shakib, F., O. Schulz, and H. Sewell. 1998. A mite subversive: cleavage of CD23 and CD25 by Der p I enhances allergenicity. Immunol. Today 19:313-316. [DOI] [PubMed] [Google Scholar]
- 245.Shaw, R. J., M. M. McNeil, D. R. Maass, W. R. Hein, T. K. Barber, M. Wheeler, C. A. Morris, and C. B. Shoemaker. 2003. Identification and characterization of an aspartyl protease inhibitor homologue as major allergen of Trichostronguylus colubriformis. Int. J. Parasitol. 33:1233-1243. [DOI] [PubMed] [Google Scholar]
- 246.Shea-Donohue, T., and J. F. Urban. 2004. Gastrointestinal parasite and host interactions. Curr. Opin. Gastroenterol. 20:3-9. [DOI] [PubMed] [Google Scholar]
- 247.Shimakura, K., H. Miura, K. Ikeda, S. Ishizaki, Y. Nagashima, T. Shirai, S. Kasuya, and K. Siomi. 2004. Purification and molecular cloning of a major allergen from Anisakis simplex. Mol. Biochem. Parasitol. 135:69-75. [DOI] [PubMed] [Google Scholar]
- 248.Sicherer, S. H. 2001. Clinical implications of cross-reactive food allergens. J. Allergy Clin. Immunol. 108:881-890. [DOI] [PubMed] [Google Scholar]
- 249.Skirnisson, K. 2006. Pseudoterranova decipiens (Nematoda, Anisakidae) larvae reported from humans in Iceland after consumption of insufficiently cooked fish. Laeknabladid 92:21-25. [PubMed] [Google Scholar]
- 250.Smith, H. V., R. Quinn, R. Kusel, and R. W. Girdwood. 1981. The effect of temperature and antimetabolites on antibody-binding to the outer surface of 2nd stage Toxocara-Canis larvae. Mol. Biochem. Parasitol. 4:183-193. [DOI] [PubMed] [Google Scholar]
- 251.Smith, J. W., and R. Wootten. 1978. Anisakis and anisakiasis. Adv. Parasitol. 16:93-163. [DOI] [PubMed] [Google Scholar]
- 252.Sohn, W. M., and S. Y. Seol. 1994. A human case of gastric anisakiasis by Pseudoterranova decipiens. Korean J. Parasitol. 32:53-56. [DOI] [PubMed] [Google Scholar]
- 253.Stewart, G. R., M. Boussinesq, T. Coulson, L. Elson, T. Nutman, and J. E. Bradley. 1999. Onchocerciasis modulates the immune response to mycobacterial antigens. Clin. Exp. Immunol. 117:517-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Strait, R. T., S. C. Morris, K. Smiley, J. F. Urban, Jr., and F. D. Finkelman. 2003. IL-4 exacerbates anaphylaxis. J. Immunol. 170:3835-3842. [DOI] [PubMed] [Google Scholar]
- 255.Sugane, K., S. Sun, and T. Matsuura. 1992. Molecular cloning of the cDNA encoding a 42 kDa antigenic polypeptide of Anisakis simplex larvae. J. Helminthol. 66:25-32. [DOI] [PubMed] [Google Scholar]
- 256.Sugimachi, K., K. Inokuchi, T. Ooiwa, T. Fugino, and Y. Ishii. 1985. Acute gastric anisakiasis. Analysis of 178 cases. JAMA 253:1012-1013. [PubMed] [Google Scholar]
- 257.Takai, T., T. Kato, M. Ota, H. Yasueda, T. Kuhara, K. Okumura, and H. Ogawa. 2005. Recombinant Der p 1 and Der f 1 with in vitro enzymatic activity to cleave human CD23, CD25 and alpha(1)-antitrypsin, and in vivo IgE-eliciting activity in mice. Int. Arch. Allergy Immunol. 137:194-200. [DOI] [PubMed] [Google Scholar]
- 258.Tan, B. M., M. R. Sher, R. A. Good, and S. L. Bahna. 2001. Severe food allergies by skin contact. Ann. Allergy Asthma Immunol. 86:583-586. [DOI] [PubMed] [Google Scholar]
- 259.Tanaka, J., and M. Torisu. 1978. Anisakis and eosinophil. I. Detection of a soluble factor selectively chemotactic for eosinophils in the extract from Anisakis larvae. J. Immunol. 120:745-749. [PubMed] [Google Scholar]
- 260.Taylor, M. D., L. LeGoff, A. Harris, E. Malone, J. E. Allen, and R. M. Maizels. 2005. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J. Immunol. 174:4924-4933. [DOI] [PubMed] [Google Scholar]
- 261.Thompson, R. C. A., and A. J. Lymbery. 1994. Genetic variation in helminths and its epidemiological significance, p. 146-159. In N. Chowdhury and I. Tade (ed.), Helminthology. Springer-Verlag, New York, NY.
- 262.Todorova, V. K., D. P. Knox, and M. W. Kennedy. 1995. Proteinases in the excretory-secretory products (Es) of adult Trichinella-Spiralis. Parasitology 111:201-208. [DOI] [PubMed] [Google Scholar]
- 263.Tomlinson, L. A., J. F. Christie, E. M. Fraser, D. McLaughlin, A. E. McIntosh, and M. W. Kennedy. 1989. MHC restriction of the antibody repertoire to secretory antigens, and a major allergen, of the nematode parasite Ascaris. J. Immunol. 143:2349-2356. [PubMed] [Google Scholar]
- 264.Toro, C., M. L. Caballero, M. Baquero, J. Garcia-Samaniego, I. Casado, P. Martinez, T. Alarcon, and I. Moneo. 2006. Seropositivity to a major allergen of Anisakis simplex, Ani s 1, in dyspeptic patients with Helicobacter pylori infection: histological and laboratory findings and clinical significance. Clin. Microbiol. Infect. 12:453-458. [DOI] [PubMed] [Google Scholar]
- 265.Turner, J. D., H. Faulkner, J. Kamgno, M. W. Kennedy, J. Behnke, M. Boussinesq, and J. E. Brandley. 2005. Allergen-specific IgE and IgG4 are markers of resistance and susceptibility in a human intestinal nematode infection. Microbes Infect. 7:990-996. [DOI] [PubMed] [Google Scholar]
- 266.Umebayashi, Y. 2001. Analysis of factors influencing on Anisakis specific IgE antibodies. Arerugi 50:1090-1095. [PubMed] [Google Scholar]
- 267.University of California Seafood Network Information Center. 2007. Generic HACCP. http://seafood.ucdavis.edu/haccp/Plans.htm.
- 268.Urban, F. J., Jr., K. B. Madden, A. W. Cheever, P. P. Trotta, I. M. Katona, and F. D. Finkelman. 1993. IFN inhibits inflammatory responses and protective immunity in mice infected with the nematode parasite Nippostrongylus brasiliensis. J. Immunol. 143:7086-7094. [PubMed] [Google Scholar]
- 269.Urban, F. J., K. B. Madden, A. Svetic, A. Cheever, P. P. Trotta, W. C. Gause, I. M. Katona, and F. D. Finkelman. 1992. The importance of Th2 cytokines in protective immunity to nematodes. Immunol. Rev. 127:205-220. [DOI] [PubMed] [Google Scholar]
- 270.Van der Kleij, D., and M. Yazdanbakhsh. 2003. Control of inflammatory diseases by pathogens: lipids and the immune system. Eur. J. Immunol. 33:2953-2963. [DOI] [PubMed] [Google Scholar]
- 271.Van der Veen, M. J., R. van Ree, R. C. Aalberse, J. Akkerdaas, S. J. Koppelman, and H. M. Jansen. 1997. Allergens, IgE, mediators, inflammatory mechanisms. Poor biologic activity of cross-reactive IgE directed to carbohydrate determinants of glycoproteins. J. Allergy Clin. Immunol. 100:327-334. [DOI] [PubMed] [Google Scholar]
- 272.Van Neerven, R. J., J. Arnved, and H. Ipsen. 2000. Phleum pratense-specific T cells of allergic rhinitis patients display a broader recognition pattern than Phleum pratense-specific serum immunoglobulin E. Clin. Exp. Allergy 30:242-254. [DOI] [PubMed] [Google Scholar]
- 273.Van Thiel, P. H. 1962. Anisakiasis. Parasitology 52:16-17. [Google Scholar]
- 274.Van Thiel, P. H. 1976. The present state of anisakiasis and its causative worms. Trop. Geogr. Med. 28:75-85. [PubMed] [Google Scholar]
- 275.Verhamme, M. A. M., and C. H. R. Ramboer. 1988. Anisakiasis caused by herring in vinegar: a little known medical problem. Gut 29:843-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Verma, R., J. Gaspaul, and M. Khalili. 2005. Ceviche and abdominal pain: a rare case of Anisakis simplex in the United States. Am. J. Gastroenterol. 100:191. [Google Scholar]
- 277.Watanapa, P., and W. B. Watanapa. 2002. Liver fluke-associated cholangiocarcinoma. Br. J. Surg. 89:962-970. [DOI] [PubMed] [Google Scholar]
- 278.Watt, I. A., N. R. Mclean, R. W. A. Girdwood, L. H. Kissen, and A. H. B. Fyfe. 1979. Eosinophilic gastroenteritis associated with a larval anisakine nematode. Lancet ii:893-894. [DOI] [PubMed] [Google Scholar]
- 279.Weiler, C. R. 2007. Anisakis simplex and cross-reacting antigens. Int. J. Dermatol. 46:224-225. [DOI] [PubMed] [Google Scholar]
- 280.Weir, E. 2005. Sushi, nemotodes and allergies. CMAJ 173:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Williams, H., and A. Jones. 1994. Fish worms and man, p. 371-443. In H. Williams and A. Jones (ed.), Parasitic worms of fish, 1st ed. Taylor and Francis, London, United Kingdom.
- 282.World Health Organization. 2008. Soil-transmitted helminths. World Health Organization, Geneva, Switzerland. http://www.who.int/intestinal_worms/en.
- 283.Xia, Y., H. J. Spence, J. Moore, N. Heaney, L. McDermott, A. Cooper, D. G. Watson, B. Mei, R. Komuniecki, and M. W. Kennedy. 2000. The ABA-1 allergen of Ascaris lumbricoides: sequence polymorphism, stage-and tissue-specific expression, lipid binding function, and protein biophysical properties. Parasitology 120:211-224. [DOI] [PubMed] [Google Scholar]
- 284.Yagihashi, A., N. Sato, S. Takahashi, H. Ishikura, and K. Kikuchi. 1990. A serodiagnostic assay by microenzyme-linked immunosorbent assay for human anisakiasis using a monoclonal antibody specific for Anisakis larvae antigen. J. Infect. Dis. 161:995-998. [DOI] [PubMed] [Google Scholar]
- 285.Yu, J. R., M. Seo, Y. W. Kim, M. H. Oh, and W. M. Sohn. 2001. A human case of gastric infection by Pseudoterranova decipiens larva. Korean J. Parasitol. 39:193-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Zaccone, P., Z. Fehervari, J. M. Phillips, D. W. Dunee, and A. Cooke. 2006. Parasitic worms and inflammatory diseases. Parasite Immunol. 28:515-523. [DOI] [PMC free article] [PubMed] [Google Scholar]