Abstract
Summary: Of the four species of the genus Echinococcus (Cestoda) distinguished by biological and morphological characteristics, two species, E. vogeli and E. oligarthrus, occur widely in the Neotropics. Approximately 200 cases of polycystic echinococcosis (PE) have been recorded from 12 countries in South America. Following early proliferation of E. vogeli in the human host, typically in the liver, the metacestode usually spreads in the peritoneal and pleural cavities, and numerous organs may be invaded. The clinical characteristics of PE in 81 patients with sufficient information are reviewed. Type I disease consists of polycysts in the liver and abdominal cavity (37% of the patients had this characteristic); type II is similar to type I but also includes hepatic insufficiency (26%); type III consists of cysts in liver and chest (14%); type IV consists of cysts only in the mesenteries (16%); and type V consists of cysts calcified in liver and lung (4%). The percentage of patients with polycysts in the liver was 81%, and the percentage of patients with polycysts in the chest was 14%. PE is most ready diagnosed by geographic origin of the patient and by means of ultrasound or computerized tomography scanning showing cysts and calcifications. The highest mortality was for patients with type II disease, due to hepatic failure and its complications. There were five patients who died due to surgical accidents, whereas 35 cases had uncomplicated surgery. Twenty-three patients died of PE, making the total mortality 29% (23 of 78 cases). None of the 13 patients treated only with albendazole, the most efficacious treatment, was completely cured. PE represents a severe medical problem in South America. A reevaluation of the characteristics of the metacestode of E. oligarthrus indicated that it is unicystic. Only three human cases are known (two with infection in the orbit and one with infection in the heart). The metacestode of E. oligarthrus, in contrast with that of E. vogeli, consists of a spherical, fluid-filled vesicle that enlarges concentrically and is not known to undergo exogenous proliferation.
INTRODUCTION
The most pathogenic of cestodes in the human host are those of the family Taeniidae Ludwig 1886 (suborder Taeniata Skriabin et Schulz 1937), when the organism at the asexually reproducing stage, the metacestode, develops in the liver and/or lungs and elsewhere. In addition to the metacestode (cysticercus) of Taenia solium L. 1758, the metacestodes of the four species of the genus Echinococcus Rudolphi 1801 are the most important cause of severe to fatal disease in humans. Investigations in the Neotropics during recent decades have shown that Echinococcus vogeli, the causative agent of polycystic echinococcosis, the main subject of the present discussion, may be recognized as the most pathogenic species of Echinococcus.
The genus Echinococcus contains four species readily distinguished on the basis of morphological characters in both the strobilar and metacestode stages. They also differ markedly in biological characteristics. The strobilar stage of each occurs in the small intestine of a carnivore; the metacestodes develop in organs of an herbivorous intermediate host that is the typical prey of the respective final hosts. Each of the four species can be distinguished morphologically and biologically produce a distinctive form of echinococcosis in humans with the degree of pathogenicity depending on the process involved in asexual reproduction in the metacestode. The species of Echinococcus are E. granulosus (Batsch 1786), which causes cystic echinococcosis; E. oligarthrus (Diesing 1863), which causes unicystic echinococcosis; E. multilocularis Leuckart 1863, which causes alveolar echinococcosis; and E. vogeli Rausch et Bernstein 1972, which causes polycystic echinococcosis.
Taxonomy of Echinococcus spp.
Two species, E. granulosus and E. multilocularis, occur in natural hosts in the northern hemisphere. The former now has a cosmopolitan distribution in livestock-raising countries, having been introduced with animals from Europe beginning in the early 16th century (55). The taxonomy of cestodes of the genus Echinococcus has been complicated by typological descriptions (1) of numerous taxa, the majority now recognized as E. granulosus.
Several genotypes, ostensibly having metacestodes adapted to the various species of domestic ungulates, have been distinguished by molecular-genetic methods (84), but otherwise they are morphologically and biologically like E. granulosus. Such genotypes appear to have arisen after the domestication of ungulates, beginning about 8,000 years before the present (BP) (35). Echinococcus granulosus and its natural hosts (wolf [Canis lupus L.] and deer [family Cervidae]) appear to represent an ancient assemblage in the holarctic zones of tundra and taiga. By contrast, E. multilocularis shows little genetic variation (68), but it also, like E. granulosus, exhibits morphological variation in the strobila, and host-induced variation in the metacestode occurs (62). The recently described E. shiquicus Xiao et al. 2005 resembles E. multilocularis morphologically; the metacestode was found in pikas (family Ochotonidae) and differs morphologically from that developing in the typical intermediate hosts of E. multilocularis, rodents of the family Arvicolidae. Such rodents are sympatric with pikas, from which the metacestode of E. shiquicus was studied. Of the four species of Echinococcus recognized here, the metacestode of E. multilocularis exhibits the least degree of host specificity, having been recorded in natural infections in small, herbivorous mammals of at least eight families (56). A major obstacle to a better understanding of the taxonomic status of genotypes of cestodes in the genus Echinococcus has been the lack of investigations involving experimental infections of ungulates and rodents that serve as intermediate hosts. Thus far, no genotype has been shown to be reproductively isolated, and the status of the various nominal taxa therefore remains uncertain.
Neotropical Species of Echinococcus in the Final Host
The two neotropical species and their respective hosts appear also to represent assemblages of ancient origin, as indicated by the typical occurrence of their metacestodes in hystricognath rodents. Those rodents were the dominant small terrestrial herbivores in South America by the Miocene epoch, from ca. 22 million to 5 million years BP (25). The family Taeniidae is represented in South America by a few species of the genus Taenia, in addition to the indigenous E. vogeli and E. oligarthrus. During the Pliocene epoch, formation of the Panamanian isthmus enabled numerous faunal exchanges between North America and South America; various species of Taenia, but none of Echinococcus, dispersed into South America with their hosts by way of the isthmus of Panama. The cougar, Puma concolor (L.), for example, of North American origin and now having an extensive geographic range in South America (90), harbors at least one nearctic species of Taenia and has become a common final host of the neotropical E. oligarthrus. It is evident that E. oligarthrus is capable of development in any species of felid in the neotropical region and probably in the Nearctic as well.
Of the four species of Echinococcus considered in this review, only E. oligarthrus has wild cats (Felidae) as a final host (60). The strobilar stages of the three others occur in carnivores of the family Canidae, each in a characteristic species of final host under natural conditions, but the domestic dog, Canis lupus forma familiaris, can readily replace their natural final hosts. The domestic dog is the most significant source of infection of humans by the etiologic agents of cystic, alveolar, and polycystic forms of echinococcosis.
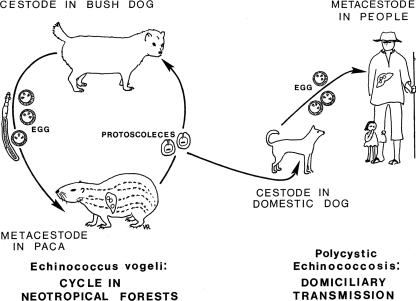
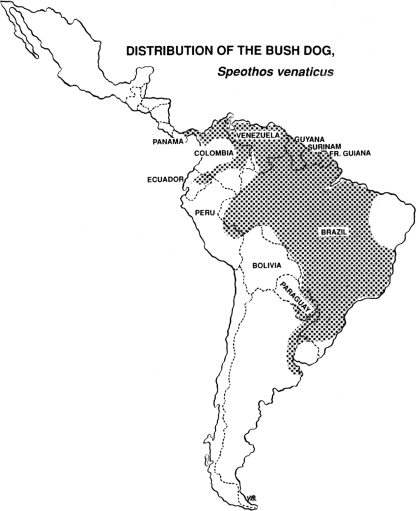
The natural cycle of E. vogeli was first reported by Cabrera et al. (8), who noted that the bush dog, Speothos venaticus (Lund), the natural final host of that cestode, hunted its preferred prey, the paca, Cuniculus paca (L.), in packs, pursuing it on land and in water (Fig. 1, 2, and 3). Although bush dogs and pacas are of similar sizes, single bush dogs may sometimes be capable of overcoming the rodents, as shown by Deutsch (21). A fox-like canid, Cerdocyon thous (L.), which occurs widely in South America, was infected experimentally by D'Alessandro (unpublished data). That canid, an omnivore that feeds also on small mammals (67), does not hunt in packs and therefore appears to be unable to capture mammals as large as a paca. In Argentina, fox-like canids of the genus Dusicyon are often infected by E. granulosus (5, 74). Those animals feed mainly on smaller mammals but evidently become infected by that cestode from scavenging on carcasses of sheep (67).
FIG. 1.
Life cycle of Echinococcus vogeli in neotropical forests and the course of domiciliary transmission to humans.
FIG. 2.
The bush dog, final host of Echinococcus vogeli. (Reprinted with permission of the Los Angeles Zoo.)
FIG. 3.
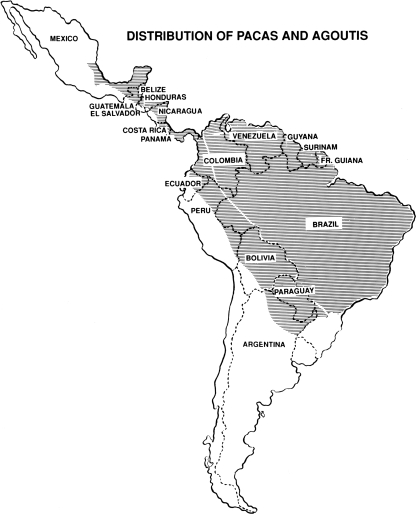
The paca, intermediate host of Echinococcus vogeli. (Courtesy of Ignacio Borero, University del Valle, Cali, Colombia.)
At the present, the bush dog is the only known natural final host of E. vogeli. Few bush dogs have been examined for the presence of intestinal helminths, but the high prevalence of the metacestode of E. vogeli in pacas is indicative of a much higher rate in the final hosts. Such a differential between the respective final and intermediate hosts is typical also for E. granulosus and E. multilocularis (57, 61).
Characteristics of the Strobilar Stages of E. vogeli and E. oligarthrus
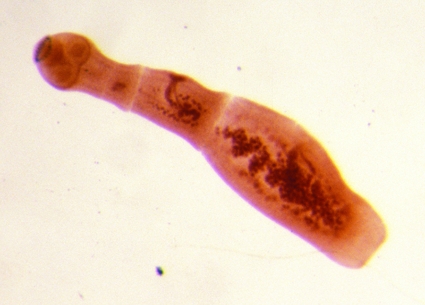
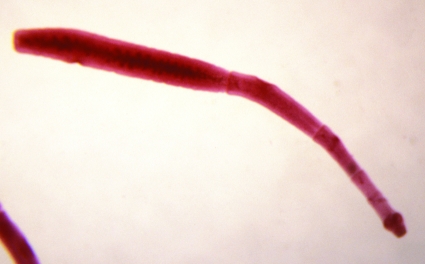
The strobilae of the two neotropical species of Echinococcus are easily identifiable morphologically. That of E. oligarthrus is evidently the smallest of the four species of the genus, with a length of ca. 2 to 3 mm and three proglottids. The rostellar hooks are similar to those of E. vogeli in length but differ in shape (58, 65). The form of the rostellar hooks is diagnostic. In experimentally infected house cats, eggs appeared in the feces 86 to 87 days postinfection (78). By contrast, E. vogeli has the longest strobila of any member of the genus Echinococcus, reaching a length of at least 12 mm in experimentally infected dogs. In fully developed specimens, six proglottids are present (Fig. 4 and Fig. 5). The gravid proglottid is long and cylindrical, making up more than half of the strobilar length. Eggs begin to appear in the uterus at about 90 days postinfection in domestic dogs.
FIG. 4.
Strobilar stage of Echinococcus oligarthrus (length, 2 mm) from a naturally infected jaguarundi from Colombia.
FIG. 5.
Strobilar stage of Echinococcus vogeli (length, 12 mm) from an experimentally infected domestic dog.
The Metacestode of E. vogeli in the Natural Intermediate Host and in Humans
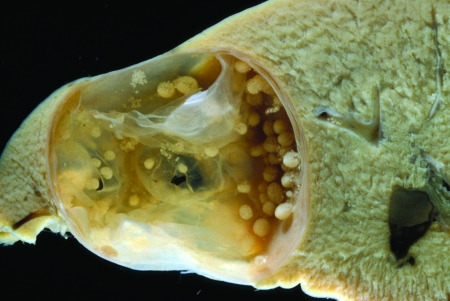
The paca, Cuniculus paca, is the natural intermediate host of E. vogeli. The metacestode occurred almost always in the liver and consisted of few to numerous spherical to subspherical vesicles, isolated or frequently contiguous. In two animals, metacestodes occurred additionally in the hepatic ligament and in the mesentery near the cecum. The greatest numbers were in the left and median lobes, the largest of the four lobes making up the liver of the paca. They were situated at all levels within the hepatic parenchyma but mostly were near the surface, partially covered by Glisson's capsule (64), there protruding slightly above the hepatic surface (Fig. 6). The metacestodes were white and somewhat translucent, contrasting markedly with the dark color of the liver. Within the parenchyma, they were surrounded by connective tissue 70 to 250 μm in thickness, the outer surface of which formed a boundary distinct from the unaffected hepatic tissue. When partially exposed under Glisson's capsule, the covering layer over the metacestode was only 20 to 30 μm in thickness.
FIG. 6.
Metacestode of Echinococcus vogeli in the liver of a paca kept as a pet in a household in Carimagua, Colombia.
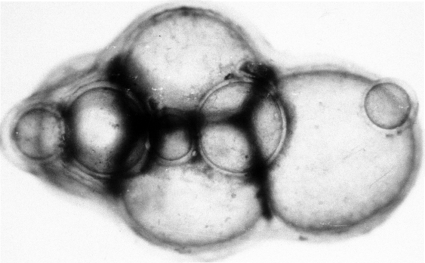
When transected in situ, the metacestodes were seen to be enclosed within a laminated membrane variable in thickness and often exhibiting folding. Intrusions of laminated membrane usually produce a few trabeculae, forming chambers lined by germinal tissue from which the brood capsules arose (Fig. 7). Brood capsules were large and relatively few, more or less spherical to pyriform, with the smaller end of each attached to the germinal tissue. They range from 424 μm to l.56 mm in length by 389 μm to 1.45 mm in maximal diameter. Brood capsules each contained 10 to 480 (average, 81) protoscoleces (64). When fully developed, protoscoleces were 158 to 203 μm in length by 108 to 145 μm in diameter (average, 175 by 133 μm).
FIG. 7.
Transected metacestode of Echinococcus vogeli, showing brood capsules and other tissues, in the liver of a naturally infected paca, El Porvenir, Colombia.
As seen in thick sections of liver, transected metacestodes of E. vogeli, when occurring singly, showed no indication of exogenous proliferation. When two or more were contiguous, their separate boundaries usually could be discerned, and each appeared to have arisen from a single egg. The findings indicated that proliferation in the liver does not occur in the natural intermediate host. However, in one paca, vesicles involving mesentery and cecum formed a mass that could have arisen by means of proliferation (Fig. 8).
FIG. 8.
Vesicles of Echinococcus vogeli in the mesentery near the caecum in a naturally infected paca, Carimagua, Colombia.
A difference in the developmental process and its consequences between the natural intermediate hosts and humans is not unique to E. vogeli, but that relationship is like that pertaining to the metacestode of E. multilocularis. When voles ingest a few eggs of E. multilocularis, development of the metacestode takes place only in the liver and infective protoscoleces are produced within 2 to 3 months; when infections are severe, more hepatic lobes are affected, and exogenous proliferation of germinal tissue may result in formation of masses of metacestode in the peritoneal cavity but other organs usually are not invaded.
The pattern of development of E. multilocularis is also different in the human host. Therein, development takes place in the liver, where the membranes of the metacestode continue to proliferate for the life of the host but protoscoleces are rarely produced. Also in the human host, the membranes of the metacestode proliferate only around the periphery of the lesion; the internal bulk of the lesion is replaced by connective tissue in which areas of necrosis usually develop. Marked hepatomegaly is characteristic, and diffuse calcification is typical in cases with advanced disease. The metacestode of E. multilocularis may extend into contiguous organs (e.g., kidney); metastasis to distant sites (e.g., lung or brain) sometimes may occur.
The metacestodes of E. granulosus and, evidently, of E. oligarthrus are alike in that their developments in the natural intermediate hosts and their developments in humans do not differ. In their respective intermediate hosts, the metacestodes of both species enlarge concentrically, and many protoscoleces are produced.
Histogenesis and Pathogenesis of the Metacestode of E. vogeli in Polycystic Echinococcosis
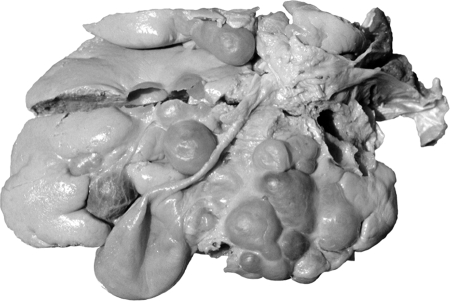
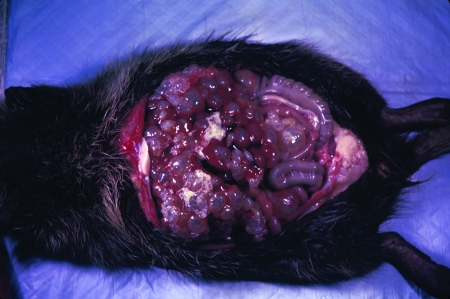
After ingestion of eggs of E. vogeli, the oncospheres released in the intestine are transported to the liver, where the oncosphere forms a primary lacuna and development of the metacestode begins. In hosts other than the natural intermediate host, an initial vesicle, from which successive generations of vesicles arise, is produced (Fig. 9). That process accounts for the invasion of host tissues (59). In the human host, proliferation of vesicles leads initially to extensive involvement of the liver (Fig. 10). After an undefined interval, the proliferating vesicles penetrate Glisson's capsule and invasion of organs in the peritoneal and pleural cavities takes place (12).
FIG. 9.
Proliferating vesicles of Echinococcus vogeli from the peritoneal cavity of an experimentally inoculated Mongolian gerbil. (Reprinted from reference 59 with permission of the publisher.)
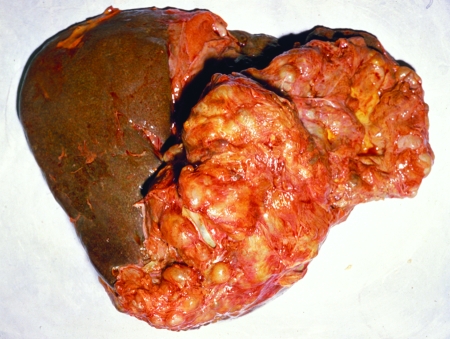
FIG. 10.
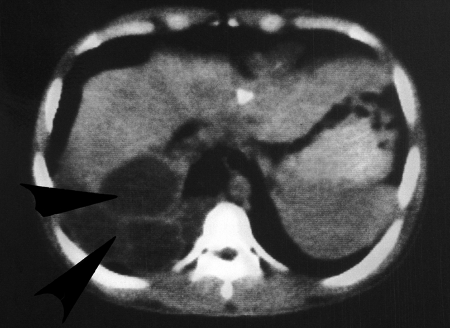
Polycystic echinococcosis in the human liver (fatal case, from a resident of Panama). (Courtesy of Octavio Sousa, Centro de Investigación y Diagnóstico de Enfermedades Parasitarias, Facultad de Medicina, República de Panamá.)
Earlier stages of the disease in humans have not been defined, because patients usually do not seek medical attention until the infection has become symptomatic, by which time the disease is far advanced. An outbreak of polycystic echinococcosis in large primates at the Los Angeles Zoo was a source of useful information, since, according to our observations with experimentally infected nonhuman primates and from descriptions in the literature, infections produced in such animals by E. granulosus, E. multilocularis, and E. vogeli do not appear to differ discernibly from those in the human host. At the zoo, primates of several species were confined as young juveniles in an enclosure adjacent to one in which a bush dog (captured in Colombia) was kept (36). Within 10 years, seven orangutans died from polycystic echinococcosis or were noted to have severe infections if other causes of death supervened. In addition, nine other large primates of various species died from advanced echinococcosis, and two surviving gorillas showed signs of severe disease. Two gorillas having “very pendulous abdomens” were diagnosed by means of ultrasonic examination, and exploratory surgery revealed massive infections (36). Both were euthanized, and necropsy revealed severe involvement of the liver and disseminated disease affecting organs of the peritoneal and pleural cavities (36). Those findings suggest that advanced disease in humans may develop within a period of about a decade (12).
The Metacestode of E. oligarthrus in the Natural Intermediate Host
As reported by D'Alessandro et al. (18), natural infections by the metacestode are known to occur in the following mammals in Central America and South America: opossums [Didelphis marsupialis (L.)]; agoutis {Dasyprocta punctata Gray, D. fuliginosa Wagler, D. aguti [= D. leporina (L.)], and Dasyprocta sp. indet.}; spiny rats {Proechimys semispinosus (Tomes), P. guyannensis [= P. decumanus (Thomas)], and Proechimys sp. indet.}; and pacas. The metacestode described as E. cruzi Brumpt et Joyeux 1924 (7), from agoutis in Brazil, has been shown to be that of E. oligarthrus (63). The metacestode has been reported to occur in rabbits (Sylvilagus floridanus (Allen)) in Venezuela (41). S. floridanus has expanded its range into South America relatively recently, via the Panamanian isthmus.
Using eggs of E. oligarthrus from a cougar in Panama, Sousa and Thatcher (78) experimentally infected laboratory-reared rodents, namely, spiny rat Proechimys semispinosus (Tomes), climbing rat Tylomys panamensis (Gray), and brown agouti D. punctata. Then, using eggs from an experimentally infected house cat, they infected cotton rats (Sigmodon hispidus Say et Ord) and Mongolian gerbils. Probably small felids are not capable of preying on agoutis, so small rodents of various species must serve also as natural intermediate hosts of E. oligarthrus in South America.
In natural intermediate hosts, as well as in rodents infected experimentally, the metacestodes of E. oligarthrus are subspherical to irregular in form. Each is enclosed by a thin laminated membrane lined by germinal tissue containing abundant calcareous corpuscles (few in the metacestodes of E. vogeli). Unicystic metacestodes in subcutaneous muscles in agoutis measured 0.5 to 3 cm in diameter (78). In all infected rodents, the metacestodes developed normally, producing brood capsules and protoscoleces. According to Sousa and Thatcher (78), internal septae were often present, but as we also have observed, proliferation did not occur. In contrast to that of E. vogeli, in which brood capsules are few and scattered (Fig. 7), the germinal tissue in the metacestode of E. oligarthrus usually is covered by brood capsules that, according to our observations of sections, often form contiguous layers that may fill most of the lumen. The protoscoleces are somewhat larger than those of E. vogeli, having a diameter of 218 to 230 μm in spiny rats (78). The rostellar hooks of the unicystic metacestode are consistently shorter than those of E. vogeli, and in that stage, the two species are distinguished as well by strongly defined differences in the shape of the hooks (65). Rodriguez et al. (70) have given a detailed description of the metacestode of E. oligarthrus from the spiny rat Proechimys guairae.
Histogenesis and Pathogenesis of the Metacestode of E. oligarthrus in Unicystic Echinococcosis
Like those of E. granulosus, the oncospheres of E. oligarthrus may pass through the liver and be transported by the systemic circulation to various loci in natural, as well as atypical, intermediate hosts. At the present, in humans, only three cases of unicystic echinococcosis have been recognized; thus, the potential range of loci in the human body is not known. In humans, the metacestode of E. oligarthrus has been described as a more or less spherical structure that enlarges concentrically, and pathogenesis appears to result from pressure effects caused by its expansion. Two of the three known cases involved retro-ocular location of single vesicles, with production of significant exophthalmos (4, 39). In the third case, the autopsy of a patient who died of tetanus at 70 years of age revealed two metacestodes, each about 1.5 cm in diameter, in the left wall of the ventricle (16). In those patients, development of the metacestode was evidently normal, and the rostellar hooks of protoscoleces provided the means of identification of the organism as E. oligarthrus.
MEDICAL ASPECTS OF NEOTROPICAL ECHINOCOCCOSES
Polycystic and unicystic echinococcoses are helminthic infections that occur exclusively in rural areas of the Neotropics (60). As indicated earlier in this article, E. granulosus, the cause of cystic echinococcosis, occurs widely in the southern cone of South America, particularly in sheep and other ungulates in areas in which livestock is raised in Uruguay, Argentina, Chile, the Andean regions of Peru and Bolivia, and the state of Rio Grande do Sul in Brazil. On the other hand, autochthonous human cases of cystic echinococcosis are rare in other South American countries, although dogs and domestic ungulates may sometimes be infected. The reason for this situation is not clear, but perhaps epidemiological conditions do not favor the infection in humans, or less likely, the genotype of the cestode in those regions does not readily infect humans. In those areas, metacestodes of E. granulosus usually are found to occur in immigrants from other countries, especially from Europe. Human cases of infection by the two neotropical species of Echinococcus are seen mostly where E. granulosus is not endemic. E. multilocularis, the etiologic agent of alveolar echinococcosis, is limited in occurrence to the northern hemisphere, and it is widely present in Eurasia and in northern North America. An atypical form of the metacestode of E. granulosus in the liver of ungulates, characterized by small vesicles without protoscoleces in a dense matrix of connective tissue, often has been confused with the metacestode of E. multilocularis, but its identity has been clearly established (47, 89, 93). Vogel proposed that the atypical forms of E. granulosus in the liver of ungulates be designated “multicystic” (89).
Similar multicystic metacestodes have rarely been reported in humans but have been the basis of considerable confusion in South America, where their occurrence has been taken to lend support to the “unicist” concept that one species of Echinococcus (E. granulosus) causes both of the aforementioned forms of echinococcosis, compared with the “dualist” view (now recognized as valid) that two species of Echinococcus are involved (33, 88, 94). In addition to the possible occurrence of the multicystic form of E. granulosus in humans in South America, such cases, considered in retrospect, might have been caused by E. vogeli or, less likely, by E. oligarthrus. Some alveolar lesions have been described in Argentina and reported to have occurred in humans in Chile and Uruguay. Because the anomalous multicystic metacestode of E. granulosus does not produce protoscoleces, rostellar hooks necessary for identification have not been available. As a consequence, we do not know if the neotropical species of Echinococcus occur in those countries (19, 22, 85, 86, 87, 88).
In Costa Rica, a human case of echinococcosis in the liver was studied and tentatively diagnosed as being due to E. oligarthrus, probably because that species had been found in a Costa Rican cougar (6). The patient was an immigrant who had spent 27 of his 53 years in Spain. Later on, the study of the hooks demonstrated that he was infected by E. granulosus, but the metacestode morphologically somewhat resembled a multicystic echinococcus. One of us (A.D.) studied the hooks of the protoscoleces and was in agreement with R. Arroyo and R. Brenes, who kindly sent samples of the specimens and had recognized that the infection was due to E. granulosus (R. Arroyo, personal communication [7 April 1993]).
D'Alessandro et al. (17) reported cases of polycystic echinococcosis in persons from four countries (Colombia, Ecuador, Panama, and Venezuela), and these cases involved tumor-like masses in the liver. The patients had resections or biopsies of the lesions. The presurgical diagnoses were cancer of the gall bladder with metastases, tumor, abscess or hepatic cirrhosis, gastric tumor, and chondrosarcoma of a rib. The clinical and surgical diagnoses were found by pathologists to be erroneous. Those and later reports on other autochthonous cases described alveolar, multicystic, or multilocular echinococcosis, due to E. granulosus or E. multilocularis and, only more recently, due to E. oligarthrus. However, experimental infection of cats and dogs, the only way to obtain the cestodes at the strobilar stage at the time, was not attempted. D'Alessandro et al. (14, 17, 18) and Rausch et al. (65) were able to establish the infections in such mammals, using metacestodes from humans and other animals from Colombia. Findings from experimental animals established that the metacestodes developing in humans were polycystic and were that of E. vogeli and thus represented a new parasitic infection for humans. In addition, they established the criteria for not only the differential diagnosis of infection with the two neotropical species, E. vogeli and E. oligarthrus, but also criteria separating the causative agents from the other two recognized species, E. granulosus and E. multilocularis.
Fortunately, in recent years, investigators have become increasingly aware of and interested in the two zoonoses caused by the neotropical cestodes and have improved their capabilities for recognition and diagnosis of such infections. Due to its frequency, polycystic echinococcosis is no longer a medical curiosity, and it should be considered in the differential diagnosis of polycystic masses in humans. On the other hand, human infections with the unicystic metacestode of E. oligarthrus have been rarely diagnosed.
Clinical Characteristics of Polycystic and Unicystic Echinococcoses
The clinical characteristics of polycystic echinococcosis in patients depend on the location of the metacestode as well as the extent of invasion of tissues, particularly the liver (Table 1).
TABLE 1.
Frequency of clinical characteristics of polycystic and unicystic neotropical echinococcoses according to species
| Clinical type of echinococcosis | Clinical presentation | No. of cases
|
||||
|---|---|---|---|---|---|---|
| E. vogeli | E. cf. vogelia | E. oligarthrus | E. granulosus | Total (%)c | ||
| Polycystic | ||||||
| I | Cysts in the liver and abdominal cavity | 19 | 11 | 0 | 1 | 30 (37) |
| II | Cysts in the liver and abdominal cavity plus hepatic insufficiency | 9 | 12 | 0 | 0 | 21 (26) |
| III | Cysts in the liver and lung/chest | 4 | 7 | 0 | 0 | 11 (14) |
| IV | Cysts only in the mesentery of the intestine or of the stomach | 9 | 4 | 0 | 0 | 13 (16) |
| V | Calcified cysts in the liver and lungb | 1 | 2 | 0 | 0 | 3 (4) |
| Unicystic | ||||||
| VI | Cysts only in the orbit | 0 | 0 | 2 | 0 | 2 (2) |
| VII | Cysts only in the heart | 0 | 0 | 1 | 0 | 1 (1) |
| Total | 42 | 36 | 3 | 1 | 81 (100) | |
E. cf. vogeli causes infections with polycystic lesions but with no hooks found.
The total number of these patients with liver cysts was 66/81 (81%), and the number with lung cysts was 11/81 (14%).
Excludes the E. granulosus case.
D'Alessandro et al. (17) published observations on all reported or known cases of polycystic echinococcosis. In addition, D'Alessandro invited senior parasitologists of Central American and South American countries to report, during various meetings of the Federación Latinoamericana de Parasitologos, the status of echinococcosis in their countries, in particular concerning polycystic echinococcosis in humans and other animals. Their contributions have been reported (12). The present report includes all data, published and unpublished, obtained from reliable sources since that time.
The total number of known cases of neotropical echinococcosis, as of March 2007, is 172. Included in that number are 18 new cases observed by Ulysses Meneghelli (personal communication [October 2006]). In addition, two serological surveys in Brazil demonstrated that 41 of 1,064 and 19 of 40 persons (total, 60 of 1,104 [5%]) showed antibodies for Echinococcus in the counterelectrophoresis test. Whether all those 60 persons had asymptomatic echinococcosis is not known, because one single test may overlook or involve a cross-reaction with other conditions or parasitic infections. Nonetheless, it seems logical to consider that some of those persons from areas in which the infection is endemic may have had asymptomatic infections by E. vogeli. Therefore, the number of infected persons in the neotropical countries may be 232 and, in Brazil alone, 160 (52; U. Meneghelli, personal communication [2006]). Forty Brazilian cases of polycystic echinococcosis occurring in the states of Para and Amapa, in the western Amazonian region, were summarized by Soares et al. (76). Included in that group were 14 cases reported by Orlando Fonseca and Aurelio Costa in 1995 (12).
As of March 2007, the clinical information from 81 patients is available among the 172 known cases (Table 1). By far, the liver was the organ most frequently affected. Indeed, in 81% of the cases, metacestodes were found in the liver alone or with vesicles situated in the abdomen, in the liver and the lungs/pleural cavities, or only as calcified vesicles in the liver. Organs involved in the abdomen included the diaphragm, spleen, pancreas, omentum, mesenteries, rectal-vesical sac, ovaries, uterus, abdominal wall, psoas muscle, and vertebra. In the chest, the lungs were infiltrated in 11 (14%) cases; also involved were ribs, intercostals and subscapular muscle, pleura, pericardium, auricle, vena cava, and other large mediastinal vessels. The remaining 13 polycystic lesions were located only in the mesentery. Of unicystic lesions, due to metacestodes of E. oligarthrus, one was located in the heart and two were in the orbit. Those single-site infections were reported as being not concomitant or secondary to any other lesions located elsewhere in the body.
Clinical Characteristics of Polycystic Echinococcosis (E. vogeli) (Types I to V)
Examination of the patients' case histories offered the opportunity to group them in types so that frequency of clinical features, severity of the illness, complications, types of treatments used, and mortality could be better assessed.
Type I.
The most common type of presentation, type I, included lesions in the liver (Fig. 11) and in the abdomen, seen in 30 of 81 (37%) cases (Table 1). The patients presented with palpable, hard, and rounded masses, painful or not painful, usually within or connected with the liver (the hepatic masses and size of abdomen progressively enlarging), abdominal pain, gastrointestinal problems, marked weight loss, and fever. The illness was first treated with analgesics or other symptomatic drugs. For seven of the patients in this group, a diagnostic laparotomy/biopsy (Table 2) was the only invasive procedure carried out; these patients usually were considered to have intractable disease (carcinoma) and left alone. With recognition of the nature of this tropical illness, surgical intervention was begun. Surgery was well tolerated, and none of 17 cases died during the surgery or as a direct consequence. For at least 4 of those 17 cases, a partial hepatectomy was carried out; for others, the entire metacestode was resected or a partial cystectomy was performed. The entire metacestode could not always be removed. At times, some spilling of its fluid content into the abdominal cavity occurred, with the risk of secondary proliferation causing recurrent disease. Polycystic echinococcosis in most cases takes a chronic course, sometimes over many years. However, surgery that failed to resect the entire metacestode was usually beneficial to the patient, who might then live for a long time with minimal disturbances.
FIG. 11.
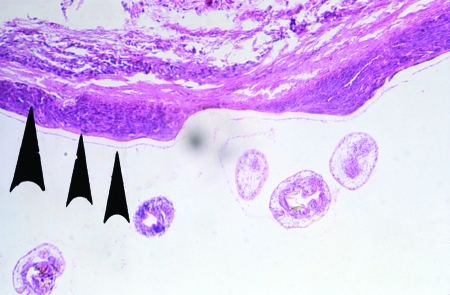
Portion of the wall of a polycystic lesion, showing germinal and laminated membranes (rightmost arrows) and accumulated dead eosinophils (larger arrow) between the metacestode and the fibrotic pericyst (hematoxylin-eosin stain).
TABLE 2.
Management and treatment of 78 cases of polycystic echinococcosis and 3 cases of unicystic echinococcosis with sufficient information
| Clinical disease typea | No. of patients with indicated treatment or outcome
|
No. of patients who died after study completion | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Lapa/biopsyb | Surgery
|
Albendazole alone | Died of PEc | Autopsy finding | Calcified cysts | Total (% of all patients) | |||
| Survived | Died | ||||||||
| I | 7 | 17 | 0 | 5 | 1 | 30 (37) | 1 | ||
| II | 0 | 8 | 4 | 4 | 4 | 1 | 21 (26) | 5 | |
| III | 0 | 3 | 1 | 3 | 3 | 1 | 11 (14) | 3 | |
| IV | 3 | 7 | 0 | 1 | 1 | 1 | 13 (16) | ||
| V | 3 | 3 (4) | |||||||
| VI | 2 (2) | ||||||||
| VII | 1 (1) | ||||||||
| Total | 10d | 35 | 5 | 13 | 9 | 3 | 3 | 81 (100) | 9 |
Note that types VI and VII represent unicystic echinococcosis.
Lapa, laparotomy.
PE, polycystic echinococcosis.
One Costa Rican case was excluded from the total because rostellar hooks were not found.
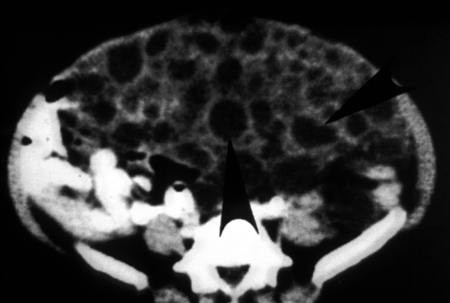
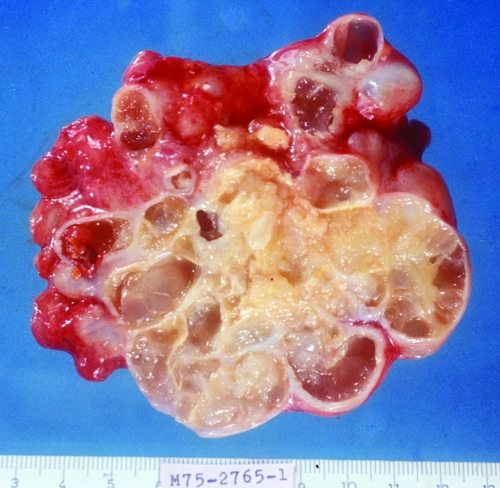
A typical case of type I disease is as follows (12): a 58-year-old female who was born in a rural area and relocated to the cities of Buenaventura and Cali, Colombia, had a 15-year history of an abdominal mass as of presentation in 1981. Within one year, she lost 8 to 10 kg in body weight and the mass had become larger, compressing the thorax, and was movable by hand. The enlarged abdomen gave the impression of advanced pregnancy. The mass was hard and painless; laboratory results were normal. Imaging showed round calcifications. Laparoscopy revealed polycystic masses in the abdominal cavity, liver, omentum, and abdominal wall. Simple X rays, computerized tomography (CT) scan, and ultrasonic examination were performed (Fig. 12, 13, 14, 15, and 16). We recommended palliative surgery because the abdominal cysts were interfering with breathing and eating. The surgeon excised only the vesicles from the sites mentioned. The liver, stomach, and spleen formed a unified mass that was not disturbed. With the removal of 2 kg of cysts, the patient had a short, uneventful recovery after surgery, with relief of intra-abdominal pressure. A year later, she again became symptomatic and for 28 days was treated with albendazole, which was well tolerated. She improved clinically for several months and did not return for a follow-up. Subsequently, it was learned that the patient became cachectic and developed ascites and collateral abdominal circulation compatible with portal hypertension; she died at home in 1999. Serological tests (enzyme-linked immunosorbent assay [ELISA] and immunoblot [IB]) were always positive for echinococcosis.
FIG. 12.
Section of biopsy specimen from a human liver (from the 58-year-old female case from reference 12), containing part of a polycystic lesion, showing a granulomatous reaction with palisaded histiocytes (periodic acid-Schiff stain).
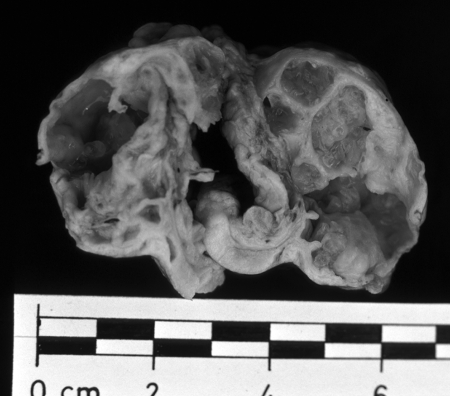
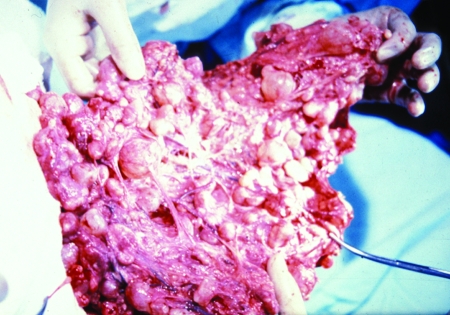
FIG. 13.
Macroscopic appearance of polycystic vesicles removed from the omentum of a patient (58-year-old female case from reference 12) by palliative surgery. (Reprinted from reference 12 with permission from Elsevier.)
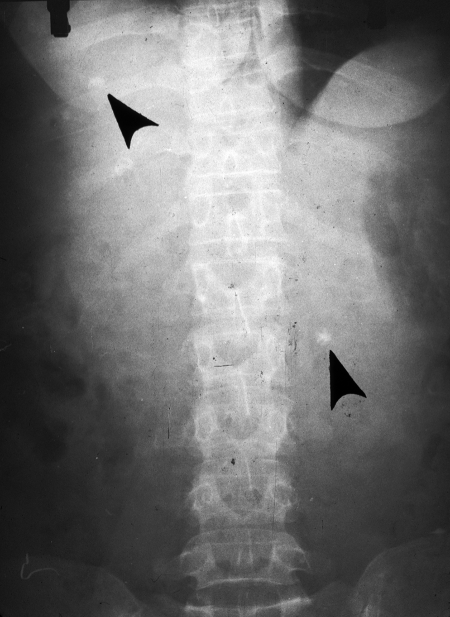
FIG. 14.
Abdominal posteroanterior X ray, showing three small, round calcifications (arrows) in a polycystic lesion involving the liver and omentum (from the 58-year-old female case from reference 12). (Reprinted from reference 12 with permission from Elsevier.)
FIG. 15.
Ultrasonic image of a liver, showing polycystic lesions (from the 58-year-old female case from reference 12). The anechoic vesicles were round and of different sizes, with regular walls. (Reprinted from reference 12 with permission of the publisher.)
FIG. 16.
CT scan, demonstrating vesicles of Echinococcus vogeli in the abdomen (from the 58-year-old female case from reference 12). The vesicles are hypodense and round to oval. The intestine has been displaced against the posterior wall. (Reprinted from reference 12 with permission from Elsevier.)
Ghiotti Siqueira et al. (29) evidently cured a patient from whom all metacestodes in the liver, omentum, and peritoneum were removed by means of partial hepatectomy and omentectomy, after which albendazole was administered for a period of 6 months. At 11 months following surgery, examination by means of CT revealed no evidence of recurrence. Cures were also reported by Chigot et al. (10), who followed a similar course of treatment, including CT scanning, and the patient remained well for at least 3 years. In another series, 1 patient died, but 16 improved after surgery (76).
Chemotherapy sometimes has been used in the treatment of polycystic echinococcosis. In one series of patients, five were treated with albendazole alone, with apparently favorable results (76). The courses of treatment were reportedly for different lengths of time and with different degrees of success. It was not known if the patients followed the regimen of treatment, and usually they did not continue to take the drug or only attended a clinic and returned home. In most cases it was not possible to determine the ultimate fate of those individuals.
Type II.
The second type of clinical presentation, type II, is similar to type I but includes signs that the vesicles are located close to and compressing the portal blood supply and/or the biliary system of the liver. That compression evoked jaundice, hepatosplenomegaly, collateral circulation, and hematemesis due to the rupture of esophageal varices. Laboratory tests revealed abnormalities due to the hepatic injuries not found in type I patients. Twenty-one of the 78 (26%) cases showed that complication. Four died during surgery or because of postsurgical complications (33, 79; unpublished data). Four additional patients died of the disease, and another was found at autopsy to have a pulmonary abscess. Four improved after chemotherapy with albendazole alone. After the management of that group ended, it was reported that five eventually died of serious complications of polycystic echinococcosis, i.e., hepatic failure due to biliary cirrhosis and hematemesis, etc. (12, 22, 26, 34, 38, 50, 77).
Type III.
In the clinical presentation of type III, lesions were present in both liver and pleural cavity of 11 (14%) patients. They complained of pain in the chest and epigastrium, cough, hemoptysis and signs of bacterial infection of the lungs, and jaundice and other signs of hepatic insufficiency and cardiomegaly, as well as congestive heart failure and acute pulmonary edema, which were indicators that the heart was badly damaged by the invading metacestode of E. vogeli. Three patients died of later complications (76). The attempt to clear bile ducts of parasites was unsuccessful, but surgery was followed by treatment with albendazole after which the general condition of the patients improved, although jaundice persisted (patient number 5) (44). In this case, as well as in others, improvement was observed after chemotherapy for up to 30 months, but the patients did not return for follow-up, and their fates remain uncertain.
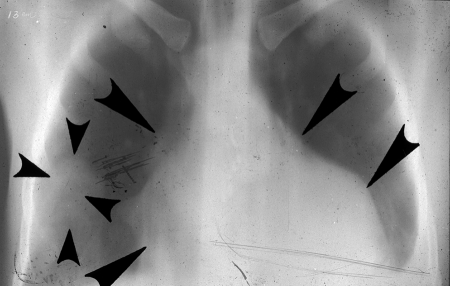
A typical case of type III was that of a 22-year-old male born in a tropical rural area of Colombia, who presented in 1975 with a 4-year history of purulent expectoration with fever and chills (12). He had been treated with antibiotics before he moved to an urban dwelling. An X ray revealed several masses in the chest and also displacement of the ureters. Tentative diagnoses included lymphoma, retroperitoneal sarcoma, seminoma, and pulmonary carcinoma. At laparotomy, small polycystic masses removed from each hepatic lobe led to a diagnosis of polycystic echinococcosis. During two thoracotomies, left and right, cysts were found in the pericardium, superior vena cava and right auricle, inferior pulmonary lobe, central right pulmonary lobe, pleura, and diaphragm. Surgery was uneventful, and all vesicles were removed (Fig. 17, 18, and 19). Six years later, in 1981, lesions had returned, but the patient was asymptomatic. In 1986, due to a protrusion in the right flank, albendazole treatment was prescribed; it was well tolerated and evidently effective, because the mass disappeared and the patient gained weight. CT scanning showed vesicles in the abdomen and in the retroperitoneal region. The patient continued working and had a normal life, but we unfortunately have no photos or images from that period. His serology was always positive (ELISA and IB). The patient tolerated the infection quite well and accepted treatment with albendazole only after he became icteric or had some other disorder. He was killed in a street accident. The autopsy was conducted by a medical examiner. It was not possible to obtain information about the lesions caused by E. vogeli.
FIG. 17.
Polycystic lesion of the pericardium, the frontal section (from the 22-year-old male case from reference 12). (Reprinted from reference 17 with permission of the publisher.)
FIG. 18.
Posteroanterior X ray of the thorax, indicating right pulmonary lobe and left and right pericardial tumors (arrows). From the first X-ray study of case no. 1 (from the 22-year-old male case from reference 12), subsequently proved to be polycystic echinococcosis. (Reprinted from reference 17 with permission of the publisher.)
FIG. 19.
CT scan, made 5 years after surgery (from the 22-year-old male case from reference 12), showing hypodense, round polycystic vesicles, mainly posterior in the right hepatic lobe and right lung. Triangular calcifications are evident in the liver. (Reprinted from reference 12 with permission from Elsevier.)
A relevant case history of one patient who died in a surgical accident also is presented here. This is a remarkable case history of a patient whose disease presentation was classified as type III; one of us (A.D.) had the opportunity to meet this patient. At the end of 1983, while in Bogota, A.D. was invited to discuss with a group of colleagues the case of a Colombian woman of about 35 years of age with polycystic echinococcosis. The diagnosis had been made by means of a liver biopsy and a detailed study of thoracic images, in particular of the large vessels and the heart. The discussion was to determine whether surgery might be indicated or beneficial to the patient. A.D.'s opinion was that surgery would be too risky, particularly to a patient who at the time was asymptomatic. After the meeting, A.D. was able to talk with the patient, who flatly refused to undergo surgery, because she had been well since the hepatic biopsy and was asymptomatic. Twelve years later, in 1996, it was learned that she was working at a local hospital and was still asymptomatic. The case was presented again at a medical meeting, and a new set of thoracic images revealed ever-enlarging lesions in the heart and in the large vessels in the mediastinum. It was strongly recommended that the patient should have surgery and that a description of the unique case should be published. A year later, it was learned that the patient had developed congestive heart failure and had gone to a different hospital, where an auricular resection had been successfully performed. The operation was well tolerated and the patient had become asymptomatic. She was convinced, however, that a complete cure would depend on the excision of the hepatic lesion. In 1998, during surgery to remove the hepatic lesion, the patient died of a surgical accident. A complete report of the case would no doubt be informative.
Type IV.
In the type IV patients, lesions were limited to the mesentery of the small or large intestine (and in one case, in that of the stomach) of 13 (16%) of the 78 patients. The liver and other organs were reported to be free of metacestodes. The lesions and pain were centrally located in the abdomen, and at surgery, the vesicles in the mesenteries could be excised, sometimes along with portions of the intestine surrounding the cystic mass. Surgery was well tolerated by seven patients, but for three, only a biopsy specimen could be obtained because the metacestode involved large vessels, making surgery too risky. In some of the cases involving operations, portions of the polycystic mass remained attached to the posterior wall of the abdomen, too close to vessels to attempt removal. All were treated with albendazole. One was considered cured after surgery and a second after two years of albendazole treatment alone (44).
Nine cases of polycystic echinococcosis recently were reported in Suriname. In four of the nine patients, the metacestodes involved the intestinal mesentery (4, 48, 49). In 1999, the late Beltus Oostburg (University of Suriname in Paramaribo) organized a field trip to visit each of the areas of residence of the patients mentioned above to determine the outcome of their illness. Fortunately, one of the patients had attended the clinic organized for the purpose. Her case history is presented here.
A 25-year-old Amerindian woman, case 2 of the Suriname series (49), had three small cysts in the intestinal mesenteries that were removed surgically at the Disconessen Hospital in Paramaribo in 1985. The diagnosis was echinococcosis. She voluntarily attended the clinic, had been well after surgery, and had taken mebendazole (3 g/day) for three months. At the time of her attendance at the clinic she was well, her serology (by ELISA and IB) was negative, and an ultrasonic examination also was negative. She had been well for 14 years, and it was concluded that she had been cured (48, 49). This case also was exceptional because she could be located and traced. The fates of many of the humans suffering from polycystic echinococcosis considered in this review are unknown, because many returned to their homes from the modern hospitals where they had undergone treatment.
Another case history of a type IV patient is given here (12). A 78-year-old man evaluated in Colombia complained of a painful mass in the left hemiabdomen. He was diagnosed as possibly having an echinococcal cyst of the spleen (based on a calcification observed in the roentgenogram) or a leiomyoma of the small intestine. At surgery, the mass was found to be attached to the posterior wall of the abdomen, surrounded by a loop of the small intestine. The mass, along with 90 cm of the intestine, was resected, but portions of cysts remained attached to the base of the mesenteries; also, fluid from the mass was spilled into the abdominal cavity. The etiologic agent was identified as E. vogeli. The patient was well for seven months and then died of a myocardial infarction.
Type V.
Calcified cysts of the liver and mesentery are categorized as type V. Three cases of dead and calcified cysts, two resembling infections by E. vogeli and the third caused by E. vogeli, have been reported. Meneghelli et al. (45) reported two Brazilian cases of hepatic calcification that resembled lesions in other patients having polycystic echinococcosis. R. L. Rausch thought that the clinical, epidemiological, and radiological data provided were compatible with the suggested diagnosis. One of the two patients had presented with abdominal distention that had been attributed to calculus cholecystitis, but at surgery, the gall bladder was normal. The thoracic X ray revealed small, calcified nodules in the lungs and a large conglomeration of cysts particularly at the margin of the liver. The second patient was asymptomatic but had similar hepatic calcifications. He had lived in rural areas, was familiar with pacas, and always had kept dogs. His case was considered to be similar to that of the first type V patient described.
The third case was reported by Moraes et al. (46). A calcified mass situated in the mesentery was discovered by X ray of the lumbar region for evaluation of possible prolapse of an intervertebral disk. The cystic structure was calcified, but some rostellar hooks were found in the mass after its surgical removal, permitting identification of E. vogeli.
Those three cases, involving dead metacestodes of E. vogeli, illustrate that some patients, when infected, exhibit an unusual tissue response. Total calcification of the metacestodes and their presence only in the mesenteries, may be indicative of a defensive host response to infection by E. vogeli. Such a relationship between the cestode and the host has been explored already in human infections caused by E. multilocularis (30, 32).
Clinical Characteristics of Unicystic Echinococcosis (E. oligarthrus) (Types VI and VII)
The unicystic lesions of E. oligarthrus in humans have been so far reported in only two loci: in the orbit of the eye and in the myocardium. We designate them type VI and type VII, respectively, using the same terminology as that used for cases of E. vogeli.
Type VI.
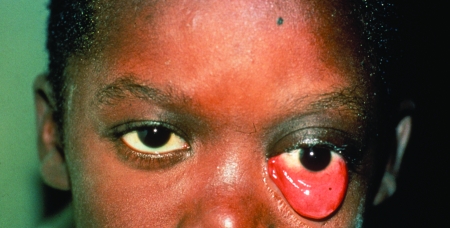
Unicysts in the orbit (two cases due to E. oligarthrus) are categorized as type VI. One case involved a 61-year-old Venezuelan woman who had lived in a rural semiarid area of hot climate, bushes, and thorny plants. Villagers and their animals drank water from small, artificial ponds. Raising goats was the main economic activity in the area. The patient presented with proptosis of the left eye, ptosis of the eyelid, and headache. The CT scan revealed a single orbital, retro-ocular vesicle of 2 cm in diameter. The immunoelectrophoresis Arc 5 test for echinococcosis was positive. The vesicle was evacuated and then removed. The rostellar hooks of the protoscoleces obtained were of E. oligarthrus. Whether the eyesight of that patient was preserved was not stated (39). The second case of E. oligarthrus infection in the orbit was seen in a 6-year-old child from Suriname. The exophthalmos was irreducible, the chemosis was extensive, and only light reception vison functioned (Fig. 20 and 21). Ultrasound (US) and CT showed a large, liquid-filled vesicle 27 by 32 mm, surrounded by a 3-mm-thick fibrotic capsule at the apex of the orbit. The indirect hemagglutination assay (IHA) for echinococcosis was negative. Surgery was carried out in Paris, France, using a fronto-temporal incision. After the fibrous capsule was opened, 10 ml of clear liquid was aspirated from the vesicle and hypertonic saline solution was injected and later replaced by isotonic fluid. Hooks of E. oligarthrus were recognized. Due to the risk to the eye that might accompany the complete removal of the cyst, the surgeon opted to prescribe postsurgical chemotherapy with albendazole. The severe proptosis and chemosis were corrected, but a slight blepharoptosis remained, as well as blindness of the left eye. One year later, back in Suriname, the patient was in good health (4).
FIG. 20.
Palpebral ptosis and chemosis in a child with a retro-ocular unicyst of Echinococcus oligarthrus. (Reprinted from reference 4 with permission of the publisher.)
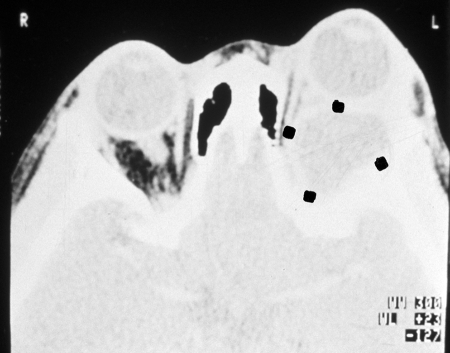
FIG. 21.
CT scan of the young patient shown in Fig. 20, indicating the liquid-filled, retro-ocular unicyst (four dots). (Reprinted from reference 4 with permission of the publisher.)
Type VII.
Unicysts in the heart due to E. oligarthrus are categorized as type VII. Discussed herein is a previously published case (16) of echinococcosis in the heart of a Brazilian person who died of tetanus. The 70-year-old male agricultural worker from Minas Gerais was taken to the hospital with tetanus. He presented in poor health and marked malnutrition and died soon after admission. The autopsy revealed an enlarged pericardium with 50 ml of clear, yellowish liquid in the pericardial space. The heart was increased in volume and weight. In the ventricular wall, two masses, each 1.5 cm in diameter, were seen. When sectioned, those were found to be unicysts that could be easily separated from the myocardium. The vesicles' content was gelatinous and clear and contained brood capsules of E. oligarthrus. The patient also had chagasic myocarditis, but no other Echinococcus vesicles were found at necropsy. As previously observed in long-standing cysts of the two neotropical species, these unicysts showed signs of degeneration next to areas in which the metacestode appeared normal in condition.
Consequences of Medical Treatment
Table 2 indicates that type I lesions were the most frequent (37%) as well as the most frequently operated with no accidents occurring (57%). A single earlier patient died after management was completed, case 4 (12). Type II lesions occurred two-thirds as frequently as those of type I, but mortality was rather high (four surgical accidents, four deaths from polycystic echinococcosis, and five postmanagement deaths). Type III and IV lesions occurred in 11 (14%) and 13 (16%) of the patients. Ten were treated surgically, and only one died (of a surgical accident). Soares et al. (76) reported necropsy findings from three individuals with type III disease who died of severe cardiac, pulmonary, and hepatic failure.
Thirteen patients (16%) were treated only with albendazole. Results are given in Table 3, which summarizes the outcomes of treatment of 78 patients having polycystic echinococcosis and for whom sufficient information was available. Thirty-five cases (45%) involved surgical attempts to remove large, invasive polycystic lesions or attempts to clear biliary obstructions. The patients tolerated surgery well and had no complications; only five (6%) died (due to surgical accidents) (17 [case 13], 33, 76, 80, and the present report).
TABLE 3.
Outcome of 78 human cases of polycystic echinococcosis
| Outcome or parameter | No. (%) of cases |
|---|---|
| Uncomplicated surgery | 35 (45) |
| Death due to surgical accident | 5 (6) |
| Death due to polycystic echinococcosis during the study and after the study ended | 18 (23) |
| Total mortality | 23 (29) |
| Treatment with albendazole alone | 13 (16) |
| Success (Meneghelli et al., 1992 [44]) | 6a |
| No action on cysts (Almeida et al., 1997 [2]) | 1/1 |
| Improved symptoms (Pastora et al., 2003 [52]) | 2/2 |
| Improvement/US negative at 1 yr (Pieres et al. [53]) | 1/1 |
| Improvement (Soares et al., 2004 [76]) | 5/5b |
| Total cured | 5 (6) |
| Total nondead cases | 59 (76) |
Two cases achieved partial success (one later died, one was albendazole resistant), and four achieved full success (patients did not complete follow-up).
Patients did not complete follow-up.
D'Alessandro (12) included by error, among persons who had died from surgical complications of polycystic echinococcosis, those who had died from E. vogeli infections; the confusion has been recognized and corrected.
Of the 13 patients treated only with albendazole, results for the first six were reported as partially or wholly successful. Meneghelli et al. (44) had followed them for 10 to 30 months, the elapsed time now being about 15 years. To obtain additional information, we contacted Ulysses Meneghelli (personal correspondence [27 October 2006]). Unfortunately, he had lost contact with all but two patients. One had died of hemorrhage due to the rupture of esophageal varices, and the second had active polycystic echinococcosis that did not respond to chemotherapy with albendazole.
The observations from the other investigations listed in Table 3 indicated results of albendazole treatment similar to those of Meneghelli et al.: diminished size of the metacestodes and clinical improvement in the patient. It seems therefore that the drug is not parasitocidal but rather parasitostatic. Nonetheless, it may be used to ameliorate the overall condition of patients, both pre- and postsurgery. The experience gained during the last 10 years indicates that surgery is a very important part of treatment for patients who can tolerate the procedure (depending on age, general health, and willingness to undergo the operation, etc.). In the group of patients evaluated in this review, surgery was carried out in almost half of the 78 cases surveyed; only five patients died of surgical accident (as mentioned above). The only five patients that seem to have been cured were treated surgically with the additional chemotherapy with albendazole. CT scan and US, performed after treatment was terminated, were negative (10, 29, 42, 44, 49, 53).
Albendazole has been administered at a dosage of 10 mg/kg of body weight/day, divided into two or three doses daily. The usual schedule has been three months of treatment with a 2-week interval between months, but higher dosages have been used, as well as longer treatment with no interruptions. Side effects discerned have included increased aminotransferase, leucopenia, proteinuria, alopecia, gastrointestinal symptoms, and allergic reactions. Such side effects were transitory and disappeared if the drug was discontinued and treatment could then be restarted with no ill effects. Early during treatment, appetite improved, temperature was reduced, and general well-being improved. After three weeks of treatment, reduction in the number and size of cysts may be evident. Abdominal pain or respiratory signs may disappear in days or weeks. The use of mebendazole has been limited, because it is less soluble (26, 54, 66).
To summarize Table 2 and Table 3, 23 (29%) of 78 patients died. Mortality by type of polycystic echinococcosis was as follows: one patient, four patients, three patients, and one patient had types I, II, III, and IV, respectively. Nine patients died after the study was completed; five of them were had type II, the category with highest mortality, probably due to hepatic failure and its complications. The only patients most probably cured were treated surgically in combination with chemotherapy (albendazole). Apparently, however, albendazole alone was parasitostatic against the metacestode of E. vogeli. As a consequence of past experience, we learned that patients are best handled by surgical intervention followed by a few weeks of therapy with albendazole. When the invasion by the metacestode involves essential organs or especially vulnerable parts of organs, the judgment of the surgeon must determine whether to leave portions of the metacestode and continue therapy with albendazole or, in the future, perhaps to administer other drugs that reduce the invasiveness or are parasitocidal for the metacestode. Liver transplant has also been considered as a possibility (44).
We consider that the metacestode of E. oligarthrus is unicystic, single or multiple (when multiple, each cyst is separate and independent), and that it does not proliferate in the human host but that its structure in humans is evidently identical with that in the natural intermediate host (see above). Microscopic and macroscopic findings from the three known cases of unicystic echinococcosis were unlike those from cases of infection by E. vogeli. Anatomically, the unicystic metacestode exhibits a quite different arrangement of brood capsules, and of course, the size and form of rostellar hooks of protoscoleces are diagnostic. Only a few metacestodes of E. oligarthrus, from a cardiac infection in one individual, have been studied. One cannot certainly predict that lesions of long standing may not exhibit some greater degree of invasiveness, or other E. vogeli-like characteristics in the human host, but interspecific similarities of such magnitude would not be expected to occur among species of Echinococcus.
E. vogeli (and evidently E. oligarthrus) is less organ-specific than is the metacestode of E. multilocularis, which usually remains localized in the liver. E. multilocularis undergoes intrahepatic proliferation, typically not producing protoscoleces in the human host; extension into the peritoneal cavity does not occur, but contiguous organs (kidney) may be invaded, and occasionally metastasis to lung or brain may occur. E. vogeli appears to be the most pathogenic of the four species of the genus Echinococcus.
DIAGNOSIS OF POLYCYSTIC ECHINOCOCCOSIS (E. VOGELI)
Before this illness was recognized as being due to a parasitic infection, the intra-abdominal masses were erroneously considered to represent a variety of disorders, including hepatic tumor, abscess, cirrhosis or cholecystitis, gall bladder cancer, mesenteric tumor, and costal chondrosarcoma (17). With modern diagnostic methods, the recognition of polycystic echinococcosis has been greatly facilitated. Moreover, with an increasing number of published reports concerning this disease, medical personnel in tropical areas, as in Colombia and more recently in Brazil, have been keenly interested in undertaking epidemiological surveys (51, 69).
Patients' Histories
Geographical origin of the patients is very important. They typically are born, or have lived for prolonged periods, in rural tropical areas of continental South America, particularly in regions with past or present abundance of wildlife. Familiarity with pacas, hunting pacas, knowledge of possible presence of “water vesicles” in their livers, and whether domestic dogs were fed viscera of pacas are demographic data that are very useful to the clinician in reaching a correct diagnosis. Prolonged contact with domestic dogs is equally important. (It was mentioned earlier that neotropical echinococcosis occurs outside the geographical area of transmission of E. granulosus to humans.)
Sex and Age Distribution
The number of cases that has been published and those findings from reliable sources make a total of 172. Those for which information is sufficient number 81. Of those cases, 45% were males and 55% were females. The median age was 43 years. That figure is the same as that obtained in our previous review of cases 10 years ago. Ages of patients ranged from 6 to 77 years. The youngest age group, 6 to 22 years, made up 16% of the total. The youngest patient from whom data were recorded was 6 years old; a 12-year-old child died of portal hypertension 1.7 years after the onset of symptoms (26). Of the 81 patients for whom adequate information is available, 6, 12, 16, 19, 17, 4, and 5 patients were in the first, second, third, fourth, fifth, sixth, and seventh decades of life, respectively.
Most of the patients infected with E. vogeli were born and had lived all their lives in rural tropical areas of Central America and South America where wildlife was abundant, and it was impossible to determine the duration of their infection. If the infected individual had moved permanently to a city dwelling, it was possible to determine the approximate age of the Echinococcus infection, which, for three of our patients, were 12, 25, or 60 years. Symptoms had appeared at 2, 10, and 36 years of age, respectively, after the patients left the rural area. The median age at diagnosis of alveolar echinococcosis (E. multilocularis) was 53 years (91).
Laboratory Findings
General observations, including our own, indicate that laboratory findings, outside of the obvious significance of morphological features of the cestodes, are not useful in the diagnosis of neotropical echinococcoses. However, it is important to assess possible injury to the liver when infiltration of the biliary system may be diagnosed. Increases of alkaline phosphatase, bilirubin, liver transaminases, and gamma globulin and diminished albumen and hemoglobin are common findings in cases of polycystic echinococcosis (45). Eosinophilia was reported in about 21% of cases, ranging from 9 to 28%, but it is not considered to have specific diagnostic value.
Physical Examination of the Abdomen
In most instances, the patients affected with polycystic echinococcosis consult for abdominal pain and have recognized the presence of a mass in the abdomen close to the liver. Hepatomegaly was usually present because that organ was certainly involved in 80% of cases of polycystic echinococcosis. When the metacestode is located in the mesentery, it usually can be palpated as a mass separate from the liver.
Radiological Imaging
With the help of the newer radiological techniques, US and CT, the tridimensional form of the metacestode becomes visible, making possible the detection of its polycystic character, which was not demonstrable by palpation and simple X ray only. As a consequence, the differential diagnoses became less complicated and diagnosis required consideration of fewer illnesses. (Definitive diagnosis, however, is accomplished by study of the metacestode itself, obtained by means of biopsy, surgery, or autopsy.) A simple X ray may show the parasitic mass and any calcifications in the parasite suggestive of echinococcosis. Calcifications are annular, 2 to 3 cm in diameter, with a radiodense halo and a clear center. They are located within the polycystic lesions in the liver or elsewhere in the abdomen if there are extensions into other organs (Fig. 14 and 18). Initially, we used simple tomography that could show the lobulated character of lesions.
US of the polycystic lesions shows multiple rounded, unilocular, anechoid formations with regular walls. That picture by itself does not provide the diagnosis of echinococcosis but is useful in assessing serological findings. For those reasons, US has become important in field studies of human populations at the time of confirming serological tests. In surveys of populations, US is considered to be more accurate than serology (27, 75). Portable equipment is available at affordable prices, and therefore, US is also being used in developing countries instead of the much more expensive apparatus for CT.
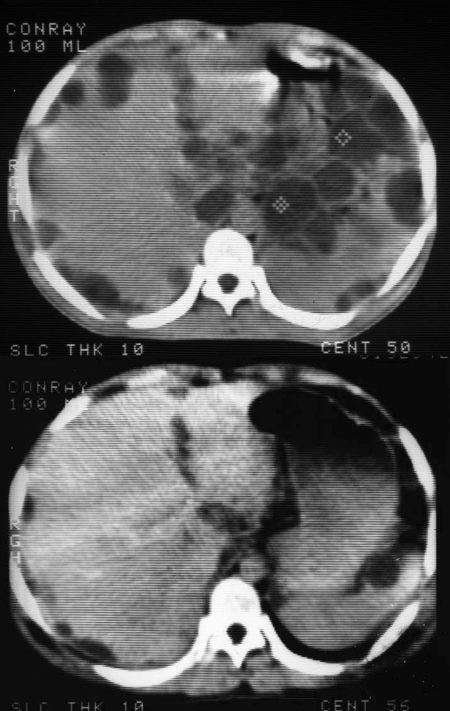
CT scans show multiple, hypodense, cystic structure, round or ovoid, of various sizes, often coalescent in liver, spleen, pancreas, omentum, pelvis, and lung, etc. Calcifications of variable appearance, usually solid and small but at times large and bizarre, can be detected and help to make an accurate diagnosis. Meneghelli et al. (44, 45) published excellent US and CT photos of Brazilian patients with polycystic echinococcosis. Also included in their publications are images showing changes in size and number of cysts following albendazole treatment (Fig. 22). CT scanning is expensive but is the best procedure, so far, for differential diagnosis of polycystic lesions (cystic echinococcosis, polycystic liver and kidney disease, primary or metastatic malignancies, and hepatic amebic abscess [15], pancreatic epithelioma, ovarian cyst and, in the lungs, dermatoid cysts with inclusions, neurinoma, aortic aneurism, primary bronchial carcinoma, metastatic sarcoma, seminoma, and lesions in ovary and uterus, etc.) and for assessment of albendazole treatment. We are not aware of reports on the use of magnetic resonance imaging in diagnosing polycystic echinococcosis.
FIG. 22.
CT scan of a patient with polycystic echinococcosis (from Brazil), treated with albendazole alone. (Top) Pretreatment, rounded, hypoechoid formations in the peritoneum and hepatic parenchyma. (Bottom) Reduction in number and size of vesicles 70 days after the beginning of treatment. (Courtesy of Ulysses G. Meneghelli, Departamento de Clinica Médica, Facultad de Medicina de Ribeirâo Preto, Brazil.)
Serological Tests
Wilson et al. (92) published a report concerned with molecular and immunological diagnosis of parasitic infections, based on their experience at the Centers for Disease Control and Prevention. The authors discussed serological diagnosis as it applies to cystic and alveolar echinococcoses, but the methods also apply to diagnosis of neotropical echinococcoses:
False-positive reactions may occur in persons with other helminthic infections, cancer and chronic immune disorders. Negative test results do not rule out echinococcosis because some cyst-carriers do not have detectable antibodies. Whether the patient has detectable antibodies is dependent on the physical location, structural integrity, and vitality of the larval cysts. Cysts in the liver are more likely to elicit an antibody response than those in the lung, and, regardless of location, tests for antibody are least sensitive for patients with hyaline cysts (p. 561-562 in reference 92).
A patient with senescent, calcified, or dead cysts is generally found to be seronegative. At the present, the best available serological diagnosis is obtained by using a combination of tests. ELISA or IHA is used to screen all specimens; a positive reaction is confirmed by an immunoblot assay or any gel diffusion assay that demonstrates the presence of the echinococcal Arc 5. Although those confirmatory assays give false-positive reactions with sera from 5 to 25% of persons with neurocysticercosis, the clinical and epidemiological presentation of neurocysticercosis patients should rarely be confused with that of echinococcosis. Following successful radical surgery, antibody levels decline and sometimes disappear; antibodies rise again if secondary hydatid cysts develop. Tests for Arc 5 or immunoglobulin E antibodies appear to reflect an antibody decline during the first 24 months postsurgery, whereas the IHA and other tests remain positive for at least 4 years. Chemotherapy has not been followed by consistent declines in antibody levels. Consequently, the usefulness of serology to monitor the course of disease is limited; imaging techniques provide a more accurate assessment of the patient's condition.
The antigen used for serodiagnosis of infection with E. granulosus and E. multilocularis, as well as that with E. vogeli and in one case that with E. oligarthrus, has been the fluid from metacestodes of E. granulosus. In addition, purified antigens of all echinococcal species but E. oligarthrus have been developed and used. From crude tissues, Gottstein et al. (31) derived the E. vogeli Ev2 antigen that differentiates between E. vogeli and E. granulosus but not between E. vogeli and E. multilocularis. The latter two species, however, are not sympatric; therefore, epidemiological data are important for distinguishing them.
Unfortunately, the test using the Ev2 antigen could not be evaluated in a blind serological survey carried out in a large indigenous human population in a South American country. As originally agreed upon, the results of the serological tests were mailed to our colleagues, but the parasitological information concerning the humans tested was never received by us.
Antigen of E. oligarthrus is not available, nor is serum from patients with that infection.
Only the case (orbital) reported by Lopera et al. (39) was positive for Arc 5 by immunoelectrophoresis, indicating cross-reactivity between antigens of E. oligarthrus and E. granulosus, the latter having been used in the test. It was stated 10 years ago that separation of metacestodes of the two neotropical species was not possible serologically, and that situation has not changed (12).
As stated by Wilson et al. (92), use of two serological tests, one very sensitive (ELISA) and another very specific (IB), is convenient. Among the 81 patients with neotropical echinococcosis that we evaluated, 40 were studied serologically; for 12 of them, the two tests gave identical results. For a particular patient undergoing surgery in France, the IHA and the Arc 5 tests were positive, as were the ELISA and IB tests.
Among other patients tested, the results were as follows. IHA was positive for 14 of 19 persons. Of the five negative cases, from one, a metacestode of E. vogeli had been removed 1.5 years earlier, while two had active E. vogeli infections and two were negative, having completely calcified hepatic cysts (42). Counter electrophoresis was positive in 6 of 7 cases, immunoelectophoresis Arc 5 was positive in 7 of 9, ELISA was positive in 4 of 5 (the case with a negative result was also negative by IB test), and 18 of the 19 patients with positive results described above also had positive IB tests. A single serological test for the diagnosis of echinococcosis, either positive or negative, may be misleading: a negative test should not be taken to exclude infection, and the opposite may be true.
Parasitological Diagnosis
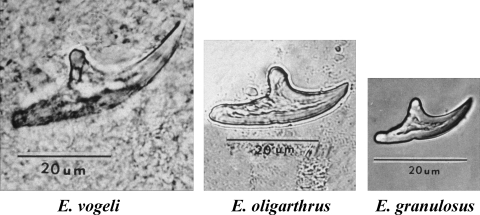
Metacestodes of the three species of Echinococcus occurring in Central America and South America are distinguishable on the basis of form and size of rostellar hooks from protoscoleces (Fig. 23). If rostellar hooks are not observed, definitive identification usually is not possible.
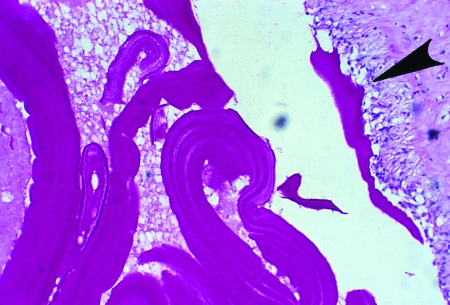
FIG. 23.
Large rostellar hooks from protoscoleces of Echinococcus vogeli, E. oligarthrus, and E. granulosus (left to right, all at the same magnification).
EPIDEMIOLOGY
Geographical Distribution of Neotropical Echinococcoses
The number and distribution of cases of polycystic and unicystic echinococcosis are presented in Tables 1 and 4. Table 1 indicates that of the 81 patients we evaluated, three were determined to be infected by E. oligarthrus and 42 were infected by E. vogeli, and for 36 patients, rostellar hooks were not found and identification of the cestode could not be made. In those 36, the diagnosis was stated to be polycystic echinococcosis. Our judgment now, after more than 20 years of evaluations of lesions produced in polycystic echinococcosis, is that the etiological agent was E. vogeli. The structures of the metacestodes, the pathogenic processes, and the epidemiological information provided by the patients (metacestodes with and without hooks, the patients' knowledge of pacas and their infections, the awareness of feeding liver of pacas, with any cysts present, to domestic dogs, and the presence of domestic dogs around previous or current dwellings of patients) were similar whether or not the species of cestode could be identified. In addition, the lesions observed in the patients with E. vogeli infections in which rostellar hooks were present were similar to those in which hooks could not be found. In our judgment, when rostellar hooks are not found in polycystic lesions, it is diagnostically acceptable to designate the etiologic agent Echinococcus cf. vogeli.
TABLE 4.
Number of cases of echinococcosis in the Neotropics by country and species of Echinococcusa
| Country | No. of cases with indicated infection
|
||||
|---|---|---|---|---|---|
| E. vogeli | E. cf. vogeli | E. oligarthrus | E. granulosus | Total | |
| Nicaragua | 1 | 1 | |||
| Costa Rica | 1 | 1 | 2 | ||
| Panama | 2 | 2 | |||
| Colombia | 15 | 14 | 29 | ||
| Ecuador | 6 | 5 | 11 | ||
| Venezuela | 2 | 1 | 1 | 4 | |
| Peru | 1 | 1 | |||
| Brazil | 21 | 77 | 1 | 99 | |
| Suriname | 7 | 1 | 1 | 9 | |
| Uruguay | 2 | 2 | |||
| Argentinab | 11 | 11 | |||
| Chile | 1 | 1 | |||
| Total | 54 | 114 | 3 | 1 | 172 |
As of March 2007. (Adapted and updated from reference 12 with permission from Elsevier.) E. cf. vogeli, E. vogeli causing infections with polycystic lesions but with no hooks found.
Synanthropic infections by E. granulosus not included.
We have listed in Table 4 a total of 172 cases in 12 Central American and South American countries, from Nicaragua and Costa Rica in the north to Argentina in the south (86, 87, 88), as well as Uruguay (19, 22) and Chile (85). Fifty-four cases were due to E. vogeli, 114 cases were due to Echinococcus cf. vogeli, and 3 cases were due to E. oligarthrus. E. vogeli and E. oligarthrus occur only in the Neotropics, with the exception of one recorded instance of E. oligarthrus in Mexico. Two human cases of submaxillary echinococcosis in India were erroneously reported to have been caused by E. oligarthrus but instead were caused by E. granulosus (13, 37, 71). The number of reported cases of E. vogeli infection has increased within the last 10 years, going from 72 persons in 11 countries to 172 in 12 countries. Peru is the newest country of record. The country with most cases was formerly Colombia but now is Brazil, with 58% of all reported cases. Polycystic echinococcosis has received much attention and emphasis from the medical profession in Brazil, which accounts for the relatively large number of reported cases in that country. We consider that the total number of cases in Central America and South America indicates but a small proportion of actual infections; the number of diagnosed cases may represent only the tip of the iceberg.
Natural Hosts of E. vogeli and E. oligarthrus
The two species of Echinococcus indigenous to the Neotropics, E. vogeli and E. oligarthrus, are sympatric over extensive areas of the New World where their requisite hosts are present. In order to study the epidemiology and transmission of those two neotropical species of Echinococcus, we carried out a survey involving more than 4,000 wild mammals from different regions of Colombia (18), collected by trapping or shooting. In addition, also included in the survey were data from necropsy records from studies carried out by members of the Tulane University-del Valle University International Center for Medical Research in Cali, from collections of the del Valle University-Rockefeller Foundation Virus Program, and from other sources. The investigators, hunters, and others performing the necropsies were aware of the interest in detecting cystic or tumor-like structures in organs, muscles, and subcutaneous tissues.
Animals were collected (from 1962 to 1979) in the following regions of Colombia: oriental plains, eastern piedmont, Pacific coast, and the Andean Cordilleras. Echinococcus-infected animals were found in the oriental plains and piedmont. Figure 24 shows the plains and the gallery forest along rivers and streams of the Orinoco River basin. There, the tropical forest has abundant wildlife, although during the daytime, the forest appears to be deserted because most mammals are not active then. The investigations in Colombia demonstrated a large enzootic area of transmission in the oriental plains of that country.
FIG. 24.
Aerial view of gallery forest in the oriental plains of Colombia. (Reprinted from reference 17 with permission of the publisher.)
Of 4,198 mammals belonging to 8 orders, 67 genera, and 78 species, 156 were carnivores (potential final host). Strobilae of Echinococcus spp. were sought in 121 carnivores and found in 5 of them (1 of 15 domestic dogs, 1 cougar, 1 of 11 ocelots, and 2 of 9 jaguarundis). The dog, which belonged to a hunter, died, probably of an infection of Trypanosoma evansi, and had fully developed strobilae of E. vogeli and a few underdeveloped specimens of E. oligarthrus in the intestine. Additional strobilae of both species were obtained experimentally in appropriate hosts. Carnivores of families other than Felidae and Canidae included in our survey are not discussed here because none was found to be infected. Bush dogs were present in the enzootic area of neotropical echinococcosis, but because they are very wary, none could be obtained for examination. On a few occasions, they could be seen by us out on the plains during daytime. In Suriname, three of four sport hunters never had seen a single Speothus bush dog, despite the frequency of their hunting; the fourth hunter had seen a bush dog one time only. The local people do not hunt bush dogs, since the skins have no market value.
Natural infections by the strobila of E. vogeli have been found only in bush dogs (Speothus venaticus) captured for zoological gardens; no others have been examined for helminths. The detailed work of Drüwa (24) and others has shown that the geographic range of the bush dog encompasses Panama southward, not including Chile, most of Argentina, and Uruguay (Fig. 25). The pattern of transmission of E. vogeli is represented in Fig. 1. Eggs of that cestode expelled by bush dogs in the natural environment would seem to be of little public health significance. However, pacas (intermediate host) are much hunted by the local peoples for food; so far as we have learned, viscera of such pacas are typically fed to domestic dogs, who then serve as the major source of infection in humans (Fig. 1). Evidently, the domestic dog is also the only significant source of infection of humans by E. granulosus and E. multilocularis.
FIG. 25.
Map of Central America and South America, indicating the geographic range of the bush dog, as compiled from various sources.
The human cases of neotropical echinococcosis (i.e., with no hooks present in the metacestode and thus, the species is not determined) in Nicaragua and Costa Rica in Central America and from southern Argentina, Uruguay, and Chile are outside the range of the bush dog. It is possible, therefore, that the metacestode involved could have been E. oligarthrus rather than E. vogeli. The supportive information for this suggestion is, first, the presence of three species of wild cats infected with E. oligarthrus in those countries and, secondly, that E. oligarthrus has been reported from other regions from humans (three only) in whom the metacestodes were unicystic and extrahepatic, rather than polycystic and hepatic, as those of E. vogeli. If E. vogeli is demonstrated in countries where bush dogs are absent, other canids, such as the coyote in Central America or other species in South America, may be responsible for transmission. Only under unusual circumstances may infection be acquired by the ingestion of eggs of that cestode expelled with the feces of a bush dog. The accidental microepidemic in numerous primates in a zoo, described above, is a tragic example. In another example of accidental transmission, nutrias became infected by E. vogeli in a second American zoo where South American carnivores were housed. Infections might also occur in humans in close contact with wild canids or felids kept as pets in people's dwellings (11, 65, 81).
Among the 2,809 rodents surveyed in Colombia, metacestodes of Echinococcus were found in only the pacas and the spiny rats (Proechimys spp.; 6 of 1,168) (18), but agoutis, Dasyprocta spp., are known to be a prominent host of the metacestode of E. oligarthrus. The distribution of pacas and agoutis extends from southern Mexico to Ecuador and, east of the Andes, south to Bolivia, Paraguay, northeastern Argentina, and Santa Catalina, Brazil. They also occur in the Lesser Antilles (Fig. 26).
FIG. 26.
Map of Central America and South America, showing the geographic ranges of the paca and agoutis. (Reprinted from reference 12 with permission from Elsevier.)
Of 325 pacas, 96 (29.5%) were infected. In 61, the species was E. vogeli; in 12, the species was Echinococcus cf. vogeli; and in 3 (0.9%), the species was E. oligarthrus. The identity of metacestodes, all without rostellar hooks, in the remaining 20 pacas could not be determined. The paca (Fig. 3) is a large (weighing up to 13.5 kg) hystricognath rodent of nocturnal habits. When alarmed, it takes readily to water, near which it is usually found. It is hunted (not trapped) for food by local people, who appreciate its meat. Pacas are herbivorous, and their diet consists mainly of fruits of arborescent plants, with some herbaceous vegetation seasonally (25). In the state of Chiapas, in southern Mexico, Gallina (28) determined that pacas consumed the fruits of trees of 18 species, representing 18 families. Periods of fruit production by trees of the various species overlap, making such food resources generally available. Because bush dogs hunt in packs on land (and in water if pacas enter watercourses to escape them), it is considered that their fecal deposits are extensively dispersed in habitat where the rodents forage. Under such conditions, incidental ingestion of eggs must account for the comparatively high prevalence of the metacestodes of E. vogeli in the intermediate host.
Age classes of pacas were estimated: adults were animals of more than 7.5 kg, subadults were animals between 5.5 kg and 7.5 kg, and juveniles were animals of less than 5.5 kg. The frequency of infection in the adult class (28/95; 29.5%) was found to be significantly greater than that of infection in the subadult class (8/50; 16%), and the subadult infection frequency was significantly greater than the juvenile class infection frequency (0/26).
In a field study in Brazil, Pastore et al. (51) asked the local people if they had seen cysts in animals other than pacas. A large proportion of them indicated that domestic pigs frequently showed large cysts in the abdomen. Those authors stated that there were no data indicating that pigs participate in the cycle of E. vogeli. We had the opportunity to study one such cyst, and Gustavo Morales, who reviewed the histopathology, reported that possibly it was a peritoneal reaction to a foreign body. The wall was fibrous and had a pseudoepithelium with no parasitic elements. However, another cestode, Taenia hydatigena, occurs in dogs, and its metacestode may be found in the abdomen of pigs and other ungulates in which the cysticercus is attached to, or in, abdominal organs. The identity of that cestode can be determined from the rostellar hooks of the cysticercus. Pigs also are susceptible to infection by E. granulosus, but the tropical forest is not an environment favoring domestic swine; T. hydatigena possibly occurs in wild pigs (indigenous species) in South America.
In mammals of the four species from which metacestodes of Echinococcus have been recorded in Argentina and Chile, only in the case of the European hare was it well established that the cestode was E. granulosus (73). The taxonomic statuses of metacestodes recorded from rodents of the genera Microcavia, Octodon, and Myocastor are undetermined because at the time of earlier publications, neotropical species of Echinococcus were not well distinguished (3, 20) and E. vogeli had not yet been described. We know that the nutria (Myocastor sp.) is susceptible to experimental infection with E. vogeli (Fig. 27) (59).
FIG. 27.
Metacestodes of Echinococcus vogeli in the liver of an adult nutria (body weight, 5.1 kg), examined 50 days after receiving gravid proglottids from a domestic dog. The dog 129 days previously had received metacestodes from a naturally infected paca captured at Carimagua, Colombia.
The strobilar stage of E. oligarthrus has been reported from 6 of the 10 species of felids occurring in South America and Central America. It was first collected from a cougar examined by J. Natterer in Brazil during the 1830s but incorrectly identified as Taenia crassicollis Rudolphi 1810 (= Taenia taeniaeformis Batsch 1786, a common cestode in the house cat) by Diesing (23). Lühe (40) restudied Diesing's cestodes from felids and correctly recognized that morphologically, they corresponded to Echinococcus spp. E. oligarthrus has been reported from the cougar at various South American and Central American localities, following the earliest record in that host in Brazil (40), namely, Panama (78, 82), Costa Rica (6), and Colombia (18). It has been reported also from the ocelot (Leopardus pardalis (L.)) in Colombia (18); jaguarundi (Puma yagouaroundi (Geoffroy Saint-Hillaire)) in Colombia and Panama and from the London Zoo, geographic origin uncertain (9); jaguar (Panthera onca (L.)) in Panama (83); and pampas cat (Leopardus colocolo (Molina)) in Argentina (designated Echinococcus pampeanus Szidat 1967) (79). A survey by Schantz and Colli added Geoffroy's cat, Leopardus geoffroyi (d'Orbigny et Gervais), in southern Argentina (7 of 47 infected) (73, 74). In felids examined in Colombia, numbers of the cestode ranged from hundreds to thousands in individual animals. E. oligarthrus has been recorded also from a bobcat, Lynx rufus (Schreber), in North America, in northern Mexico, about 100 km south of Brownsville, Texas (72). The bobcat, an endangered species in Mexico, has an extensive geographic range, occurring from the latitude of southern British Columbia south as far as Oaxaca, Mexico (90). The record from North America suggests that if the cestode spreads northward, house cats might become involved in the cycle.
Because only three cases of unicystic echinococcosis have been reported, nothing is known about the epidemiology of the infection of humans. Perhaps it can be assumed that humans may have very rare contact with eggs of E. oligarthrus, through some activity within the natural habitat of the wild felids. According to our observations, the keeping of house cats by residents of the regions where such disease is endemic would be unusual.
Agoutis, Dasyprocta spp., which are medium-sized terrestrial rodents of essentially diurnal habits, are known to be a prominent intermediate host of E. oligarthrus. Eleven species, widely occurring in the Neotropics, have been described (Fig. 27). Agoutis are also hunted for meat. In those rodents, occurrence of metacestodes of E. oligarthrus appears to be well established, and they are considered to be the usual intermediate host of E. oligarthrus, as for example in Panama (78, 82) and elsewhere (63), although none of the 130 agoutis examined in Colombia was infected. However, hunters in Colombia provided a liver from an agouti infected with E. oligarthrus and information about another with cysts in the heart, muscle, and liver. The most frequent locations of the metacestode of that species are in muscle and subcutaneous tissues, where the cysts could be more easily overlooked. Pacas are an occasional intermediate host of E. oligarthrus, according to our data (18). Metacestodes were found in 6 (0.5%) of 1,168 spiny rats.
In Argentina, beyond the geographic range of pacas, agoutis, and spiny rats, other species of rodents must serve as the intermediate host of E. oligarthrus where small felids become infected (73). An opossum, Didelphis marsupialis, has been reported as an intermediate host of E. oligarthrus (81). In our survey, none of the 527 opossums examined was infected, and therefore, the opossum does not appear to be important in transmission of the cestode to the final host.
CONCLUSIONS
In the natural transmission cycle of E. vogeli, the bush dog and the paca are known hosts. That cycle is not responsible for human infections, but a synanthropic association is required. Dogs that have received viscera from pacas become infected by the cestodes, which produce eggs that are expelled in gravid segments and may be ingested by people in close contact with their dogs.
Cestodes in dogs (and cats) could be eliminated as a risk to humans through treatment with an anthelminthic (praziquantel) at the appropriate intervals. The cost of such treatment probably would be prohibitive for most people in the Neotropics. It is known, however, that arecoline hydrobromide is being used in several areas of transmission.
Polycystic echinococcosis and unicystic echinococcosis are natural-focal diseases, the two species of Echinococcus and their respective hosts having existed independently as components of neotropical biocoenoses for many millennia in South America and Central America. Following the establishment of people in the Neotropics, the lack of a mechanism for exposure to eggs of E. vogeli would appear to have precluded infection of human inhabitants. When subsequent immigrants were accompanied by domestic dogs, an event that occurred probably less than 10,000 years BP (25), an interaction between humans and the cestode became at least potentially established. Hunting pacas for food was probably an early practice and was perhaps accomplished with the involvement of dogs. In contrast to E. vogeli, E. oligarthrus is of little public health significance because, so far as we can judge, no mechanism exists for the intrusion of humans into its natural cycle. That situation possibly could change if domestic cats become involved along with the natural final hosts.
The infection of primates and nutrias by eggs of E. vogeli from bush dogs in North American zoos, or other carnivores housed there, suggests that human visitors might also be at risk. As well, canids and felids imported from areas where the disease is endemic and kept as pets might be sources of infection. Cases of polycystic echinococcosis no doubt often escape diagnosis, and significant morbidity and mortality are the consequence. Enthusiastic interest in polycystic echinococcosis by the medical profession, as in Brazil (43), indicates significant progress toward needed action.
Programs to educate populations at risk might eventually be beneficial, but means of establishing such programs seem generally to be unavailable. The risk of acquiring echinococcosis (sensu lato) is seen to be greatly diminished if dogs are not kept (57), but the ancient association between people and their dogs is disrupted only under exceptional demands for changes in lifestyle.
Acknowledgments
We express our thanks to Ulysses Meneghelli, who kindly provided information on the outcome of patients he had treated earlier with albendazole from unpublished results of a human serological survey for echinococcosis in Brazil as well as permission to use some of the imaging from his patients. We also thank Mark Wiser, Maria Frontini, and Kris Swalm, Tulane University, School of Public Health and Tropical Medicine, Department of Tropical Medicine, Epidemiology and Computing Services, respectively, for their help with the preparation of the manuscript due to their expertise with the computer. Special thanks to Virginia Rausch, Burke Museum of Natural History, University of Washington, for her interest and help in this work and for preparing the drawing of the life cycle. We are grateful as well to Mark Derby and David Wolbrecht, University of Washington, who kindly provided technical assistance.
REFERENCES
- 1.Abuladze, K. I. 1964. Teniaty—lentochnye gel'minty zhivotnykh i cheloveka i vyzyvaemye imi zabolevaniia. Osnovy tsestodologii, vol. 4. Nauka, Moskva, U.S.S.R.
- 2.Almeida, S. C. X., R. L. Martins, A. P. Morales, C. A. Viegas, and M. Grilo. 1997. Hidatidosis pulmonar policística mimetizando lesaôs metastáticas: relato de caso. J. Pneumonol. 23:261-263. [Google Scholar]
- 3.Alvarez, V. 1961. Investigaciones sobre echinococcosis silvestre en Chile. Biologica 31:89-94. [PubMed] [Google Scholar]
- 4.Basset, D., C. Girou, I. P. Nozais, F. D'Hermies, C. Hoang, R. Gordon, and A. D'Alessandro. 1998. Neotropical echinococcosis in Suriname: Echinococcus oligarthrus in the orbit and Echinococcus vogeli in the abdomen. Am. J. Trop. Med. Hyg. 59:787-790. [DOI] [PubMed] [Google Scholar]
- 5.Blood, B. D., and J. L. Lelijveld. 1969. Studies on sylvatic echinococcosis in southern South America. Z. Tropenmed. Parasitol. 20:475-482. [PubMed] [Google Scholar]
- 6.Brenes Madrigal, R. R., G. Munoz Montoya, G. E. M. Ocampo, and G. R. Herrera. 1973. Presencia en Costa Rica de Echinococcus oligarthrus Diesing 1863, colectado en el intestino delgado de Felis concolor costaricensis. Rev. Biol. Trop. 21:139-141. [PubMed] [Google Scholar]
- 7.Brumpt, E., and C. Joyeux. 1924. Description d'un nouvel Échinocoque: Echinococcus cruzi n. sp. Ann. Parasitol. Hum. Comp. 2:226-231. [Google Scholar]
- 8.Cabrera, A., J. Yepes, and C. C. Wiedner. 1960. Mamiferos sud americanos. Historia natural, vol. 1, 2nd ed. Ediar S. A. Editores, Buenos Aires, Argentina.
- 9.Cameron, T. W. M. 1926. Observations on the genus Echinococcus Rudolphi 1801. J. Helminthol. 4:13-22. [Google Scholar]
- 10.Chigot, J. J., F. Menegaux, R. Hammoud, J. P. Nozias, and C. Hoang. 1995. A propos d'un cas d'hydatidose à Echinococcus vogeli ayant bénéficié d'un traitement chirurgical complet. Sem. Hosp. Paris 71:429-431. [Google Scholar]
- 11.Cross, J., and R. M. Thomas. 1966. Hydatid disease in the nutria. J. Parasitol. 52:1215-1216. [PubMed] [Google Scholar]
- 12.D'Alessandro, A. 1997. Polycystic echinococcosis in tropical America: Echinococcus vogeli and E. oligarthrus. Acta Trop. 67:43-65. [DOI] [PubMed] [Google Scholar]
- 13.D'Alessandro, A., and R. L. Rausch. 2004. Erroneous report of Echinococcus oligarthrus as a cause of echinococcosis in India. J. Parasitol. 90:202-203. [DOI] [PubMed] [Google Scholar]
- 14.D'Alessandro, A., H. Henao, and C. Cuello. 1978. Una caso colombiano autóctono de hidatidosis poliquistica múltiple de hidago, paricardio, pulmones, pleura y corazon. Acta Med. Valle 9:23-35. [Google Scholar]
- 15.D'Alessandro, A., J. Lega, and M. A. Vera. 1966. Cystic calcifications of the liver in Colombia. Echinococcosis or calcified abscesses? Am. J. Trop. Med. Hyg. 15:908-913. [DOI] [PubMed] [Google Scholar]
- 16.D'Alessandro, A., L. E. Ramirez, E. Chapadeiro, E. R. Lopes, and P. M. De Mesquita. 1995. Second recorded case of human infection by Echinococcus oligarthrus. Am. J. Trop. Med. Hyg. 52:29-33. [DOI] [PubMed] [Google Scholar]
- 17.D'Alessandro, A., R. L. Rausch, C. Cuello, and N. Aristibal. 1979. Echinococcus vogeli in man, with a review of polycystic hydatid disease in Colombia and neighboring countries. Am. J. Trop. Med. Hyg. 28:303-317. [DOI] [PubMed] [Google Scholar]
- 18.D'Alessandro, A., R. L. Rausch, G. A. Morales, S. Collet, and D. Angel. 1981. Echinococcus infections in Colombian animals. Am. J. Trop. Med. Hyg. 30:1263-1276. [DOI] [PubMed] [Google Scholar]
- 19.Dardel, G. 1955. A propósito de la equinococosis alveolaris en el Uruguay. Arch. Urug. Med. Cir. Esp. 46:25-32. [PubMed] [Google Scholar]
- 20.De La Barrera, J. M. 1947. Echinococcus granulosus in Microcavia australis. Arch. Int. Hidat. 7:175-176. [Google Scholar]
- 21.Deutsch, L. A. 1983. An encounter between bush dog (Speothos venaticus) and paca (Agouti paca). J. Mammal. 64:532-533. [Google Scholar]
- 22.Dévé, F., R. Piaggio-Blanco, and F. Garcia-Capurro. 1936. Échinococcose hépatique maligne micropolykystique infiltrate. Forme intermédiara entre l'échinococcose hydatic et l'échinococcose alveolaire. Arch. Urug. Med. Cir. Est. 8:3-28. [Google Scholar]
- 23.Diesing, C. M. 1850. Systema helminthum. Historiae naturalis classica, vol. XI. [Reprint, H. R. Engelmann (J. Cramer), Weinheim, Germany, 1960.] Vindobonae, Braumüller, Germany.
- 24.Drüwa, P. 1982. Perro de grulleiro, der Südamerikanische Waldhund—ein Rätsel für die Hundeforschung. Zeitsch. Kölner Zoo. 25:71-81. [Google Scholar]
- 25.Eisenberg, J. F. 1989. Mammals of the Neotropics. The Northern Neotropics, vol. 1. University of Chicago Press, Chicago, IL.
- 26.Ferreira, M. S., S. A. Nishioka, A. Rocha, and A. D'Alessandro. 1995. Echinococcus vogeli polycystic hydatid disease: report of two Brazilian casesoutside the Amazon region. Trans. Roy. Soc. Trop. Med. Hyg. 89:286-287. [DOI] [PubMed] [Google Scholar]
- 27.Frider, B., J. Moguilensky, J. C. Salvitti, M. Odriozol, and E. Larrieu. 1999. Epidemiological surveillance of human hydatidosis by means of ultrasonography: Its contribution to the evaluation of control programs. Acta Trop. 79:219-223. [DOI] [PubMed] [Google Scholar]
- 28.Gallina, S. 1981. Contribución al conocimiento de los hábitos alimenticios del tapezcuintle (Aguti paca Lin.) en Lancajá-Chansayab, Chiapas, p. 58-67. In P. R. Castillo (ed.), Estudios ecológicos en el trópico Mexicano, publication 6. Instituto de Ecología A. C., Mexico, D.F., Mexico
- 29.Ghiotti Siqueira, N., F. Barbosa de Almeida, S. R. Silva Chalub, J. R. Machadao-Silva, and R. Rodrigues-Silva. 2007. Successful outcome of hepatic polycystic echinococcosis managed with surgery and chemotherapy. Trans. R. Soc. Trop. Med. Hyg. 101:624-626. [DOI] [PubMed] [Google Scholar]
- 30.Gottstein, B. 1992. Molecular and immunological diagnosis of echinococcosis. Clin. Microbiol. Rev. 5:248-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gottstein, B., A. D'Alessandro, and R. L. Rausch. 1995. Immunodiagnosis of polycystic hydatid disease/polycystic echinococcosis due to Echinococcus vogeli. Am. J. Trop. Med. Hyg. 53:558-563. [DOI] [PubMed] [Google Scholar]
- 32.Gottstein, B., B. Mesarina, I. Tannrer, R. D. Ammann, J. F. Wilson, J. Eckert, and A. Lanier. 1991. Specific cellular and humoral immune responses in patients with different long-term courses of alveolar echinococcosis infection with Echinococcus multilocularis. Am. J. Trop. Med. Hyg. 45:734-742. [DOI] [PubMed] [Google Scholar]
- 33.Grases, P., and N. Salazar. 1970. Hidatidosis alveolar. G. E. N. 25:71-89. [Google Scholar]
- 34.Guderian, R., K. R. Kerrigan, M. E. Chico, A. Guevara, and G. Hidalgo. 1990. Hidatidosis humana en el Oriente Ecuatóriano: informe de los primeros casos. Rev. Med. Vozandes 4:57-60. [Google Scholar]
- 35.Herre, W., and M. Röhrs. 1973. Haustiere—zoologisch gesehen. Gustav Fischer Verlag, Stuttgart, Germany.
- 36.Howard, E. B., and A. P. Gendron. 1980. Echinococcus vogeli infection in higher primates at the Los Angeles Zoo, p. 379-382. In R. J. Montali and G. Migaki (ed.), The comparative pathology of zoo animals. Symposia of the National Zoological Park. Smithsonian Institution Press, Washington, DC.
- 37.Kini, U., S. Chariff, and V. Nirmala. 1997. Aspiration cytology of Echinococcus oligarthrus. A case report. Acta Cytol. 41:544-548. [DOI] [PubMed] [Google Scholar]
- 38.Lazo, R., C. Moran, E. Plaza, and A. Mite. 1985. Hidatidosis humana por Echinococcus vogeli en el Ecuador, p. 237-244. In Memorias del VII Congreso Latinoamericano de Parasitología. Trabajos Graficos Juan Montalvo Ciao Ldta., Guayaquil, Ecuador.
- 39.Lopera, R. D., R. D. Melendez, I. Fernandez, J. Sirit, and M. P. Perera. 1989. Orbital hydatid cyst of Echinococcus oligarthrus in a human in Venezuela. J. Parasitol. 75:467-470. [PubMed] [Google Scholar]
- 40.Lühe, M. 1910. Cystotänien südamerikanischer Feliden. Zool. Jahrb. Suppl. 12:687-710. [Google Scholar]
- 41.Meléndez, R. D., M. S. Yépez, and A. Coronado. 1984. Echinococcus oligarthus cysts of rabbits in Venezuela. J. Parasitol. 70:1004-1005. [PubMed] [Google Scholar]
- 42.Meneghelli, U. G. 1985. Multiple hepatic calcifications resulting from polycystic hydatid disease. Rev. Goiania Med. 31:53-60. [Google Scholar]
- 43.Meneghelli, U. G. 2002. Hidatidose policística: a comprovação de sua ocorrência e a possível dimensão epidemiológica da doença no Brasil. A gastroenterología no Brasil II. Lemos Editorial, Sao Paulo, Brazil.
- 44.Meneghelli, U. G., A. L. C. Martinelli, A. D. Bellucci, M. G. Villanova, M. A. S. Llorach Velludo, and J. E. Magro. 1992. Polycystic hydatid disease (Echinococcus vogeli). Treatment with albendazole. Ann. Trop. Med. Parasitol. 86:151-156. [DOI] [PubMed] [Google Scholar]
- 45.Meneghelli, U. G., A. L. C. Martinelli, M. A. S. Llorach Velludo, A. D. Bellucci, J. E. Magro, and M. L. P. Barbo. 1992. Polycystic hydatid disease (Echinococcus vogeli). Clinical, laboratory and morphological findings in nine Brazilian patients. J. Hepatol. 14:203-210. [DOI] [PubMed] [Google Scholar]
- 46.Moraes, M. A. P., M. N. M. Sobreira, P. Medeiros Filho, A. Cavalca Tavares, and M. I. Gomes. 2003. Polycystic hydatidosis: casual finding of calcified hydatid cyst simulating mesenteric neoplasm. Rev. Soc. Bras. Med. Trop. 36:519-521. [DOI] [PubMed] [Google Scholar]
- 47.Ohbayashi, M., R. L. Rausch, and F. H. Fay. 1971. On the ecology and distribution of Echinococcus species (Cestoda: Taeniidae), and characteristics of their development in the intermediate host. II. Comparative studies on the development of E. multilocularis Leuckart, 1863, in the intermediate host. Jpn. J. Vet. Res. 19(Suppl. 3):1-53. [PubMed] [Google Scholar]
- 48.Oostburg, B. F. J., M. A. Vrede, and A. E. Bergen. 2000. The occurrence of polycystic echinococcosis in Suriname. Ann. Trop. Med. Parasit. 94:247-252. [DOI] [PubMed] [Google Scholar]
- 49.Oostburg, B. F. J., M. Eersel, M. A. Vrede, B. Gottstein, R. L. Rausch, and A. D'Alessandro. 2004. Preliminary field observation on neotropical echinococcosis in Suriname. Rev. Pat. Trop. (Goiania) 35:125-134. [Google Scholar]
- 50.Passos, D. M. M., M. Pinho Alves, O. Creuv, G. Fritas, J. P. Matushita, and E. Marchiori. 1982. Equinococcose alveolar hepatica (descrição de um caso). Radiol. Bras. 15:129-132. [Google Scholar]
- 51.Pastore, R., L. H. Vitali, V. O. Macedo, and A. Prata. 2003. A serological survey of the infection by Echinococcus sp. in the municipality of Sena Madureira, Acre. Rev. Soc. Bras. Med. Trop. 36:473-477. [DOI] [PubMed] [Google Scholar]
- 52.Pastore, R., L. H. Vitali, J. Weirich, A. C. Tojal, V. O. Macedo, and A. Prata. 2003. Hidatidosis poliquistica: relato de dois casos procedentes de Sena Madureira, Acre, na Amazônia brasileira. Rev. Soc. Bras. Med. Trop. 36:97-101. [DOI] [PubMed] [Google Scholar]
- 53.Pieres, J. A. A., G. B. Pereira, R. Pastore, and L. Lauria-Pires. 2001. Doença hepática policística. J. Bras. Patol. 37:90-98. [Google Scholar]
- 54.Pinilla, L. G., K. Zamora, and G. Gonzalez. 1982. Hidatidosis poliquística. Tribuna Med. 11:47-50. [Google Scholar]
- 55.Rausch, R. L. 1967. On the ecology and distribution of Echinococcus spp. (Cestoda: Taeniidae), and characteristics of their development in the intermediate host. Ann. Parasitol. Hum. Comp. 42:19-63. [DOI] [PubMed] [Google Scholar]
- 56.Rausch, R. L. 1986. Life cycle patterns and geographic distribution of Echinococcus species, p. 44-80. In R. C. A. Thompson (ed.), The biology of Echinococcus and hydatid disease. George Allen and Unwin, London, United Kingdom.
- 57.Rausch, R. L. 2003. Cystic echinococcus in the Arctic and sub-Arctic. Parasitology 127(Suppl.):S73-S85. [DOI] [PubMed] [Google Scholar]
- 58.Rausch, R. L., and J. J. Bernstein. 1972. Echinococcus vogeli sp. n. (Cestoda: Taeniidae) from the bush dog, Speothos venaticus (Lund). Z. Tropenmed. Parasitol. 23:25-34. [PubMed] [Google Scholar]
- 59.Rausch, R. L., and A. D'Alessandro. 1999. Histogenesis in the metacestode of Echinococcus vogeli and mechanism of pathogenesis in polycystic hydatid disease. J. Parasitol. 85:410-418. [PubMed] [Google Scholar]
- 60.Rausch, R. L., and A. D'Alessandro. 2002. The epidemiology of echinococcosis caused by Echinococcus oligarthrus and E. vogeli in the Neotropics, p. 107-113. In P. Craig and Z. Pawlowski (ed.), Proceedings of the NATO Workshop on Cestode Zoonoses: echinococcosis and cysticercosis—an emergent and global problem. IOS Press, Amsterdam, The Netherlands.
- 61.Rausch, R. L., and F. H. Fay. 2002. Epidemiology of alveolar echinococcosis, with reference to St. Lawrence Island, Bering Sea, p. 309-325. In P. Craig and Z. Pawlowski (ed.), Proceedings of the NATO Workshop on Cestode Zoonoses: echinococcosis and cysticercosis—an emergent and global problem. IOS Press, Amsterdam, The Netherlands.
- 62.Rausch, R. L., and S. R. Richards. 1971. Observations on parasite-host relationships of Echinococcus multilocularis Leuckart, 1863, in North Dakota. Can. J. Zool. 49:1317-1330. [DOI] [PubMed] [Google Scholar]
- 63.Rausch, R. L., A. D'Alessandro, and M. Ohbayashi. 1984. The taxonomic status of Echinococcus cruzi Brumpt and Joyeux, 1924 (Cestoda: Taeniidae) from an agouti (Rodentia: Dasyproctidae) in Brazil. J. Parasitol. 70:295-302. [PubMed] [Google Scholar]
- 64.Rausch, R. L., A. D'Alessandro, and V. R. Rausch. 1981. Characteristics of the larval Echinococcus vogeli Rausch and Bernstein, 1972 in the natural intermediate host, the paca, Cuniculus paca L. (Rodentia: Dasyproctidae). Am. J. Trop. Med. Hyg. 30:1043-1052. [DOI] [PubMed] [Google Scholar]
- 65.Rausch, R. L., V. R. Rausch, and A. D'Alessandro. 1978. Discrimination of the larval stages of Echinococcus oligarthrus (Diesing, 1863) and E. vogeli Rausch and Bernstein, 1972 (Cestoda: Taeniidae). Am. J. Trop. Med. Hyg. 27:1195-1202. [DOI] [PubMed] [Google Scholar]
- 66.Rausch, R. L., J. F. Wilson, B. J. McMahon, and M. A. O'Gorman. 1986. Consequences of continuous mebendazole therapy in alveolar hydatid disease—with a summary of a 10-year clinical trial. Ann. Trop. Med. Parasitol. 80:403-419. [DOI] [PubMed] [Google Scholar]
- 67.Redford, K. H., and J. F. Eisenberg. 1992. Mammals of the Neotropics. The Southern Cone, vol. 2. University of Chicago Press, Chicago, IL.
- 68.Rinder, H., R. L. Rausch, K. Takahashi, H. Kopp, A. Thomschke, and T. Löscher. 1997. Limited range of genetic variation in Echinococcus multilocularis. J. Parasitol. 83:1045-1050. [PubMed] [Google Scholar]
- 69.Rodrigues-Silva, R., J. R. V. Peixoto, R. M. F. Oliveira, R. Maghalaes Pinto, and D. Corrêa-Gomez. 2002. An autochtonous case of Echinococcus vogeli Rausch & Bernstein, 1972. Polycystic echinococcosis in the State of Rôndonia, Brazil. Mem. Inst. Oswaldo Cruz 97:123-126. [DOI] [PubMed] [Google Scholar]
- 70.Rodriguez, G., M. Tamayo, and J. Boshell. 2000. Estructura del quiste hidatico producio por Echinococcus oligarthrus en el hospedero intermediario Proechimys cf. guairae (rata espinosa) en Casanare, Colombia. Biomedica 20:238-247. [Google Scholar]
- 71.Sahni, J. K., M. Jain, Y. Bajaj, V. Kumar, and A. Jain. 2000. Submandibular hydatid cyst caused by Echinococcus oligarthrus. J. Laringol. Otol. 114:473-476. [DOI] [PubMed] [Google Scholar]
- 72.Salinas-López, N., F. Jiménez-Guzmán, and A. Cruz-Reyes. 1996. Presence of Echinococcus oligarthrus (Diesing, 1863) Lÿhe, 1910 in Lynx rufus texensis Allen, 1895. from San Fernando, Tamaulipas State, in north-east Mexico. Int. J. Parasitol. 26:793-796. [DOI] [PubMed] [Google Scholar]
- 73.Schantz, P. M., and C. Colli. 1973. Echinococcus oligarthrus (Diesing, 1863) from Geoffroy's cat (Felis geoffroyi D'Orbigny and Gervais) in temperate South America. J. Parasitol. 59:1138-1140. [PubMed] [Google Scholar]
- 74.Schantz, P. M., A. Cruz-Reyes, C. Colli, and R. D. Lord. 1975. Sylvatic echinococcosis in Argentina. I. On the morphology and biology of strobilar Echinococcus granulosus (Batsch, 1786) from domestic and sylvatic animal hosts. Tropenmed. Parasitol. 26:334-344. [PubMed] [Google Scholar]
- 75.Shambesh, M. A., P. S. Craig, C. N. L. Macpherson, M. T. Rogan, A. B. Gusbi, and F. E. Echtuish. 1999. An extensive ultrasound and serological study to investigate the prevalence of human cystic echinococcosis in Northern Libya. Am. J. Trop. Med. Hyg. 60:462-468. [DOI] [PubMed] [Google Scholar]
- 76.Soares, M. V. C. P., C. A. Moreira-Silva, M. Moreira Alves, H. M. Nunes, I. Abraçado do Amaral, L. J. M. Pereira Móia, S. R. S. Silva Conde, F. Barbosa Almeida, R. Rodrigues-Silva, and J. A. Barletta Crescente. 2004. Equinococose policística na Amazônia oriental brasileira: atualização da casuística. Rev. Soc. Bras. Med. Trop. 37(Suppl.):75-83. [DOI] [PubMed] [Google Scholar]
- 77.Sousa, O. E., and J. Lombardo Ayala. 1965. Informe de un caso de hidatidosis en sujeto nativo panameño. Primer caso autoctono. Arch. Med. Panama 14:79-86. [Google Scholar]
- 78.Sousa, O. E., and V. E. Thatcher. 1969. Observations on the life-cycle of Echinococcus oligarthrus (Diesing, 1863) in the Republic of Panama. Ann. Trop. Med. Parasitol. 63:165-175. [DOI] [PubMed] [Google Scholar]
- 79.Szidat, L. 1967. Echinococcus pampeanus. Una nueva especie de la Argentina, parásita de Felis colocolo pajeros Desmarest, 1916 (Cestoda). Neotrop. 13:90-96. [Google Scholar]
- 80.Szotlender, N., F. Acevedo, E. Cargonell, and E. Sogbe. 1969. Hidatidosis hepática. Presentación de un caso. Arch. Hosp. Vargas (Caracas) 25:71-89. [Google Scholar]
- 81.Thatcher, V. E. 1972. Neotropical echinococcosis in Colombia. Ann. Trop. Med. Parasitol. 66:99-105. [DOI] [PubMed] [Google Scholar]
- 82.Thatcher, V. E., and O. E. Sousa. 1966. Echinococcus oligarthrus Diesing, 1863, in Panama and a comparison with a recent human hydatid. Ann. Trop. Med. Parasitol. 60:405-416. [DOI] [PubMed] [Google Scholar]
- 83.Thatcher, V. E., and O. E. Sousa. 1967. Echinococcus oligarthrus (Diesing, 1863) from a Panamanian jaguar (Felis onca L.). J. Parasitol. 53:1040. [PubMed] [Google Scholar]
- 84.Thompson, R. C. A., and D. P. McManus. 2003. Towards a taxonomic revision of the genus Echinococcus. Trends Parasitol. 18:452-457. [DOI] [PubMed] [Google Scholar]
- 85.Villaroel, L., J. Seitz, and O. Castillo. 1983. Hidatidosis pulmonar por Echinococcus multilocularis. Rev. Chilena Cir. 36:436-437. [Google Scholar]
- 86.Viñas, M. 1903. Quistes hidaticos multiloculares o alveolares. La Semana Méd. (Argentina) 10:1234-1236. [Google Scholar]
- 87.Viñas, M. 1904. Echinococcus alveolar. La Semana Méd. (Argentina) 11:931-932. [Google Scholar]
- 88.Viñas, M. 1905. Parasitologia del Echinococcus alveolar. An. Depart. Nac. Hig. (Buenos Aires) 12:71-82. [Google Scholar]
- 89.Vogel, H. 1957. Über den Echinococcus multilocularis Süddeutschlands. I. Das Bandwurmstadium von Stämmen menschlicher und tierischer Herkunft. Z. Tropenmed. Parasit. 8:404-456. [PubMed] [Google Scholar]
- 90.Wilson, D. E., and D.-A. M. Reeder. 2005. Mammal species of the world. Johns Hopkins University Press, Baltimore, MD.
- 91.Wilson, J. F., R. L. Rausch, and F. R. Wilson. 1995. Alveolar hydatid disease. Review of the surgical experience in 42 cases of active disease among Alaskan eskimos. Ann. Surg. 221:315-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wilson, M., P. M. Schantz, and T. Nutman. 2006. Molecular and immunological approaches to the diagnosis of parasitic infections, p. 557-568. In B. Detrick, R. G. Hamilton, and J. D. Folds (ed.), Manual of molecular and clinical laboratory immunology, 7th ed. American Society for Microbiology, Washington, DC.
- 93.Xiao, N., J. Qui, M. Nakao, K. Makaya, H. Yamashaki, Y. Sako, P. M. Mamuti, P. M. Schantz, P. Craig, and A. Ito. 2003. Identification of Echinococcus species from a yak in the Qinghai-Tibet Plateau region of China. Am. J. Trop. Med. Hyg. 69:445-446. [PubMed] [Google Scholar]
- 94.Zérega, F. 1965. Hidatidosis alveolar. Rev. Ecuador Hig. Med. Trop. 22:115-117. [PubMed] [Google Scholar]