Abstract
Vibrio cholerae non-O1/non-O139 strains have caused several cases of ear, wound, and blood infections, including one lethal case of septicemia in Austria, during recent years. All of these cases had a history of local recreational activities in the large eastern Austrian lake Neusiedler See. Thus, a monitoring program was started to investigate the prevalence of V. cholerae strains in the lake over several years. Genetic analyses of isolated strains revealed the presence of a variety of pathogenic genes, but in no case did we detect the cholera toxin gene or the toxin-coregulated pilus gene, both of which are prerequisites for the pathogen to be able to cause cholera. In addition, experiments were performed to elucidate the preferred ecological niche of this pathogen. As size filtration experiments indicated and laboratory microcosms showed, endemic V. cholerae could rapidly grow in a free-living state in natural lake water at growth rates similar to those of the bulk natural bacterial population. Temperature and the quality of dissolved organic carbon had a highly significant influence on V. cholerae growth. Specific growth rates, growth yield, and enzyme activity decreased markedly with increasing concentrations of high-molecular-weight substances, indicating that the humic substances originating from the extensive reed belt in the lake can inhibit V. cholerae growth.
Vibrio cholerae is both a human pathogen and a natural inhabitant of aquatic environments (10, 13). More than 200 serogroups have been identified to date, but only serogroups O1 and O139 are associated with epidemic cholera (48). Non-O1/non-O139 strains have so far not been found to be involved in epidemic cholera but can cause other diseases in humans. Since the seminal work of Colwell et al. (10), several investigations have traced the potential ecological niches where V. cholerae thrives and survives in aquatic environments, but still, the ecology of this human pathogen is poorly understood (13, 60). V. cholerae has been shown to live mainly in association with crustacean zooplankton (18, 23) and has been detected with algae (16, 27, 28) in a variety of aquatic environments where it is involved in surface biofilm formation (55) and where it degrades the polymeric substances chitin (40) and mucilage (49). In addition, V. cholerae has been isolated from freshwater and marine macrophytes (26) as well as from benthic animals like prawns, oysters (52), crabs (3), and chironomid egg masses (5) and has also been shown to be able to replicate intracellularly in free-living amoebae (1). In contrast to the fact that V. cholerae can grow in the particle-associated state, a few reports have demonstrated that V. cholerae can also grow in water as a free-living organism in the planktonic phase (39, 53, 60). Thus, the existence of at least two main growth strategies of environmental V. cholerae (particle associated versus free living) can be assumed, which has important consequences for mechanisms controlling population size and survival. Food web interactions, such as grazing by protozoa and/or larger zooplankton, viral attack, interactions with the nutrient supply, or the influence of changes in the chemophysical environment, will have dramatically different effects, depending on V. cholerae's growth strategy in a specific ecosystem.
In most developed countries, including Austria, this pathogen is responsible for cholera-like but less severe watery diarrhea and blood, wound, ear, and respiratory tract infections (8, 38), usually without epidemic character. In the past 5 years, 13 cases of V. cholerae non-O1/non-O139 infections were documented in Austria, of which 8 had a local history (22). Five cases could be explicitly associated with recreational activities at the study area, the lake Neusiedler See, with four cases of otitis and one of lethal septicemia. This lake offers ideal conditions for V. cholerae, with moderate salinity between 1 and 3.5‰ and a high pH between 7.8 and 9.1. The main threat, however, is certainly the causation of cholera, which is restricted mainly to developing countries due to poor standards for hygiene and sanitation. However, also in Europe, several countries (Italy, Ukraine, and Russia) were plagued by cholera in the 1990s (32). Cholera can be considered a reemerging disease, as the incidence of this infection in humans has increased within the past 2 decades and threatens to increase in the near future (41). Due to intense travel activity worldwide, the possibilities that O1/O139 strains are transported to European countries and that they may arrive in aquatic environments as well cannot be excluded. In eastern Austria, the lake Neusiedler See is intensively used for recreation activities and is, in addition, a hot spot for migratory-bird-associated microbial import in Europe (30). This region has been heavily afflicted with avian botulism since the 1980s (61) and is identified as a high-risk zone for avian influenza by the Austrian Ministry of Health. Temperature increase due to global warming additionally enhances the probability of the establishment of increased populations of pathogenic Vibrio strains in these aquatic ecosystems. As the basis for a future risk assessment, it is thus of prime importance to understand the ecology of endemic non-O1/non-O139 V. cholerae strains.
The aim of the study was to demonstrate the permanent endemic existence of V. cholerae in the lake over several seasons. Size fractionation experiments were performed to identify the preferred habitat (attached versus free living) of this bacterium in the lake. Based on these findings, two hypotheses were tested in laboratory batch culture experiments. First, V. cholerae is able to grow actively in the water and independently of surface attachment. Second, it may preferably grow on high-molecular-weight (HMW) substrates, as it is known to possess enzymes for the degradation of complex polymeric substances (40, 49) and because the bacterioplankton in the lake have been shown to depend primarily on HMW dissolved organic carbon (DOC) derived from the abundant reed Phragmites australis (45).
MATERIALS AND METHODS
Description of the study area.
The lake Neusiedler See (47°42′N, 16°46′E) (Fig. 1) is the largest shallow alkaline brown-water lake in central Europe (115 m above sea level; surface area, 321 km2; maximum depth, 1.8 m; mean depth, 1.1 m; pH 8.5 to 9.1). About 55% of the lake is covered with reeds (Phragmites australis), and within this vegetation, extended brown-water areas are found. The water level of the lake is controlled mainly by precipitation (500 to 700 mm year−1) and evaporation. Frequent resuspension of the sediment caused by winds and currents results in a high concentration of suspended solids in the water column (secchi depth ≈ 0.2 m). Due to the shallow water column, water temperature changes rapidly in response to weather events (20). A more detailed description of the limnology of the lake is given by Löffler (33).
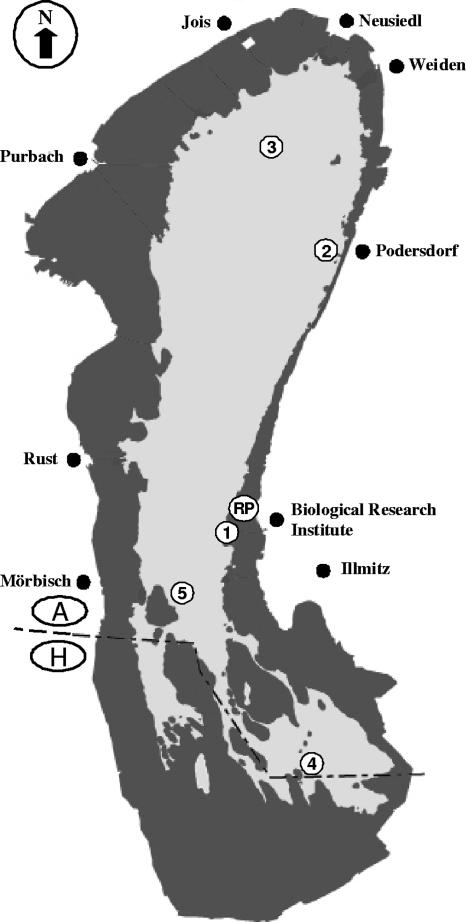
FIG. 1.
View of Neusiedler See, shared by Austria (A) and Hungary (H), with the five routine sampling points (1 to 5) and the extra sampling point (Ruster Poschen [RP]) for the laboratory batch culture experiments indicated. The darkly shaded area indicates the Phragmites australis reed belt; the lightly shaded area indicates the open-water area.
Seasonal cycle (“routine sampling”). (i) Sampling.
During the period from 2001 to 2004, the lake was investigated for the presence of culturable V. cholerae at weekly to biweekly intervals from April to October and at longer intervals (4 to 8 weeks) from November to March. Samples were taken by boat from five representative stations (1 to 5) along a longitudinal transect through the lake in sterilized 500-ml glass bottles with a sampling rod from about 30 cm below the water surface. Three stations were located in the center of the lake, one was located near the eastern shore, and one was located within the reed belt (Fig. 1). Temperature, oxygen level, pH, and conductivity were recorded simultaneously with freshly calibrated portable meters (WTW, Weilheim, Germany) at a water depth of 30 cm. Samples for the determination of DOC, chlorophyll a (CHLA), total suspended solids (TSS), and total phosphorus (TP) levels were taken in cleaned (with 1 N HCl and rinsed three times with water from the sampling site) 5-liter polycarbonate flasks. Zooplankton samples were collected with vertical net hauls (mesh size, 250 μm). This resulted in integrated samples of the whole water column; depending on the water depth at the respective station (between 75 and 180 cm), 50 to 120 liters of lake water was filtered. In the period of ice cover, sampling with vertical net hauls was impossible, and therefore, a 5-liter Schindler sampler was used. Samples were taken at three discrete depths (below ice cover, above lake bottom, and in the middle of the water column) and lumped into one sample. The zooplankton was collected from the net and fixed in a defined volume (100 to 150 ml) of 4% formaldehyde in 200-ml glass bottles. All samples were transferred to the laboratory (Biological Research Institute, Burgenland, Austria) in an isolated box in the dark within 3 h.
(ii) Basic environmental parameters.
For the determination of the TSS level, 100 ml of sample water was filtered through precombusted (465°C, 4 h) glass fiber filters (GF/F; Whatman, England) and dried to a constant weight (25 to 718 mg liter−1). The TP level was determined photometrically after the dissolution of the unfiltered sample with potassium-peroxydisulfate, using the molybdenum blue method according to Strickland and Parsons (51). For the determination of the DOC level, subsamples were filtered through precombusted Whatman GF/F filters and the DOC level was determined using a Shimadzu TOC 5000 carbon analyzer (Shimadzu Corporation, Tokyo, Japan) after sparging the sample with CO2-free air. Standards were prepared with potassium hydrogen phthalate (Kanto Chemical Co., Inc.); a platinum catalyst on quartz was used (44). For the determination of the level of chlorophyll, a defined volume of the sample (250 to 750 ml) was filtered through Whatman GF/F filters, extracted with 90% acetone (4°C overnight), and measured spectrophotometrically (Hitachi U-2000) (42).
(iii) Crustacean zooplankton.
Zooplankton samples were counted in petri dishes under a dissecting and inverted microscope. Dry weight estimations were derived from length-weight relationships for the various species (19). Data from all sampling stations were averaged.
(iv) Vibrio cholerae cultivation and biochemical identification.
The 500-ml samples from the five stations were added to 500 ml of a double-concentration alkaline peptone water enrichment broth (APW), consisting of a 2% final concentration of Bacto peptone buffered with 0.06% Na2HPO4 and adjusted to pH 8.9 ± 0.1. After incubation for 24 h at room temperature, a loop of surface water was streaked onto thiosulfate citrate bile sucrose agar (Merck, Darmstadt, Germany) and incubated for an additional 24-h period at 36°C ± 2°C. Single colonies typical for V. cholerae (2 to 3 mm in diameter, yellow, and flat) were transferred to Difco nutrient agar (containing peptone and beef extract) with and without 3% NaCl. Only strains growing on both agars were considered for further identification and tested for their ability to produce oxidase (Bactident; Merck) and aminopeptidase (Bactident; Merck). Aminopeptidase- and oxidase-positive strains were further identified with the API 20E system (bioMerieux, Nürtingen, Germany) according to the manufacturer's instructions and serologically tested with commercially available V. cholerae O1 and O139 antiserum (Becton Dickinson GmbH, Heidelberg, Germany).
Size fractionation experiments.
In 2003, size fractionation experiments of the lake water were performed to elucidate the preferred habitat of V. cholerae in the lake. From June to September, additional samples beyond the routine sampling number were taken at biweekly intervals in sterile 500-ml glass bottles at stations 1 and 5 (Fig. 1). A total of 250 ml of water was cascade filtered through a 250-μm nylon net and 10-μm, 1.2-μm, and 0.2-μm polycarbonate filters (Millipore, Vienna, Austria) using a 100-mm-diameter filtration device. The 0.2-μm-filter fraction was also investigated to find out whether small Vibrio cells can pass through a 0.2-μm filter. All materials were autoclaved (filters), heat sterilized, or precombusted (glassware) before use. The filters were transferred with sterile forceps to 250 ml of APW, and the filtrate from the 0.2-μm filtration was added to 250 ml of double-concentration APW. V. cholerae strains were isolated and biochemically identified as described above. In addition, strains were frozen at −20°C in 1 ml sterile deionized water in sterile 1.5-ml Eppendorf tubes for molecular biological analysis via PCR and at −80°C in 1 ml of 20% glycerol in 1.5-ml cryovials for long-term storage.
Molecular characterization of isolates.
All isolated and biochemically identified strains were further checked for the presence of genes typical of V. cholerae via PCR. The targeted DNA sequences were hlyA (the ElTor and the classical variant of the gene coding for hemolysin), toxR (a gene coding for a protein which coregulates toxin expression), ompU (a gene coding for an outer membrane protein acting as a putative adherence factor of V. cholerae), ctxA (a gene coding for cholera toxin), tcpI (a gene encoding a colonization factor known as toxin-coregulated pilus), and a designated 16S-23S intergenic spacer region (ISR). The presence of this 16S-23S ISR was shown to be highly specific for V. cholerae and absent from other closely related species, such as Vibrio mimicus (9). For DNA extraction, bacterial cells from the frozen samples were lysed by heating them for 15 min at 100°C to release their nucleic acids and immediately centrifuged for 15 min at 4°C. The lysate supernatant fluid was transferred to a microcentrifuge tube, and 2 μl was used as a template for the PCRs immediately after extraction. As positive controls, a non-O1/non-O139 strain (CIP 106970), an environmental V. cholerae non-O1/non-O139 isolate from France (Jean Lesne and Sandrine Baron, Centre National de la Santé, Rennes), and an O1 strain (NCCB 36033) were used. Vibrio alginolyticus, Vibrio parahaemolyticus, and Vibrio vulnificus strains from Sweden (Alexander Eiler and Stefan Bertilsson, Uppsala University) (15), a V. mimicus strain (CIP 106921), and Escherichia coli were used as negative controls. All oligonucleotide primers were synthesized by MWG Biotech (Ebersberg, Germany). The sequence positions, amplicon sizes, and references are listed in Table 1. The following reagents were added to each sample PCR mixture to yield a total reaction volume of 40 μl: 4 μl amplification buffer (100 mM Tris HCl, 500 mM KCl, 1% Triton X-100 [pH 9]; Promega, Mannheim, Germany), 1 μl of each deoxynucleoside triphosphate (final concentration, 200 μM; Promega), 0.8 μl of each forward and reverse primer (final concentration, 1 μM), 4.8 μl of MgCl2 (final concentration, 1.5 mM), 0.4 μl of Taq DNA polymerase at 5 U μl−1, and 23.2 μl of sterile MilliQ water. The solution was mixed and placed in a Primus 25 thermocycler (MWG Biotech). PCR amplification conditions followed the specifications given by Rivera et al. (46), with minor modifications: denaturation at 94°C for 2 min, annealing at 57°C for 1 min (except for tcpI [3 min]), and extension at 72°C for 1 min, with a final extension step at 72°C for 10 min at the end of 30 cycles, followed by maintenance at 4°C. The PCR products were separated by 1% agarose gel electrophoresis in 1× TAE buffer (0.04 M Tris-acetate, 0.001 M EDTA [pH 8]), stained in 1 μg ml−1 ethidium bromide solution, and visualized under UV light.
TABLE 1.
Gene targets, primers, amplicon sizes, and references used in this study
| Gene | Primer | Sequence (5′-3′) | Amplicon size(s) | Reference |
|---|---|---|---|---|
| ISR | VC-F | TTA AGC STT TTC RCT CAG AAT G | 295 | 9 |
| VCM-R | AGT CAC TTA ACC ATA CAA CCC G | |||
| hlyA (ElTor) | 744F | GAG CCG GCA TTC ATC TGA AT | 481 | 46 |
| 1184R | CTC AGC GGG CTA ATA CGG TTT A | |||
| hlyA (class, ElTor) | 489F | GGC AAA CAG CGA AAC AAA TAC C | 727, 738 (class, ElTor) | 46 |
| 1184R | CTC AGC GGG CTA ATA CGG TTT A | |||
| toxR | 101F | CCT TCG ATC CCC TAA GCA ATA C | 779 | 46 |
| 837R | AGG GTT AGC AAC GAT GCG TAA G | |||
| ompU | 80F | ACG CTG ACG GAA TCA ACC AAA G | 869 | 46 |
| 906R | GCG GAA GTT TGG CTT GAA GTA G | |||
| ctxA | 94F | CGG GCA GAT TCT AGA CCT CCT G | 564 | 46 |
| 614R | CGA TGA TCT TGG AGC ATT CCC AC | |||
| tcpI | 132F | TAG CCT TAG TTC TCA GCA GGC A | 862 | 46 |
| 951R | GGC AAT AGT GTC CAG CTC GTT A |
Laboratory batch culture experiments.
To test whether V. cholerae is able to grow actively in the water without surface attachment and whether it preferably grows on HMW substrates rather than low-molecular-weight (LMW) substances, laboratory batch culture experiments were performed at different temperatures and DOC concentrations and with different DOC compositions. Such laboratory microcosm experiments appeared appropriate because the complexity of the question makes it nearly impractical to accomplish experiments in the field and because microcosms are perfectly suited for testing hypotheses derived from field observations under controlled conditions (14), provided that such studies are of appropriate scale and duration (6).
(i) DOC fractionation.
Water for these experiments was always collected from an additional station (Ruster Poschen [RP]) (Fig. 1), which is located within the center of a reedless area in the reed belt, because of its high concentrations of humic substances. For the fractionation of the DOC into humic and nonhumic components, the protocol of Reitner et al. (45) was followed. Briefly, 1 to 2 liters of the water collected was filtered through precombusted (450°C, 4 h) Whatman GF/F filters, followed by filtration through autoclaved 0.2-μm-pore-size polycarbonate filters (Millipore) and mounted in a combusted-glass filter holder. The pH of the filtrate was measured, and 10 ml of the sample was withdrawn, acidified to pH 2 with 50 μl of 6 N HCl, and stored in combusted-glass scintillation vials with Teflon-lined caps at −20°C for a subsequent DOC concentration analysis (see above). The filtrate was fractionated into a humic fraction and a nonhumic fraction of the DOC using macroporous Amberlite XAD-8 resin (2, 37). The sample water was adjusted to pH 2 ± 0.05 with 6 N HCl, poured through a column filled with Amberlite XAD-8 resin, and subsequently eluted with 0.1 N NaOH. This fraction was designated the humic fraction (45). Both the DOC fraction not retained by the XAD-8 resin (considered the nonhumic fraction) and the fraction eluted from the XAD-8 resin were adjusted to pH 10 ± 0.05 and poured through a cationic-exchange column filled with Amberlite IR-118H. Water flow through the columns was adjusted to a rate of ∼40 ml min−1. Subsequently, the humic and the nonhumic fractions of the DOC were adjusted to the original pH ± 0.05 with 6 N HCl and 2 N NaOH and combined with sterile double-distilled water to reach the original volume of the sample. Samples for the DOC analysis were taken from the humic and the nonhumic fractions as well as from the double-distilled water, acidified, and stored frozen until analysis. The absorbance characteristics of the DOC were measured against those of the double-distilled water at 250 and 365 nm using a Beckmann DU 640I photometer and a 5-cm quartz cuvette. The ratio of the absorption at 250 nm to that at 365 nm was calculated to determine possible shifts in the molecular size spectrum of the DOC during the experiments (45). A higher ratio indicates a higher percentage of LMW substances than HMW substances.
(ii) Preparation of growth cultures and sampling design.
Growth cultures with different ratios of humic to nonhumic substances were prepared in sterile 1-liter Schott flasks. For this, the humic fraction was mixed with the nonhumic fraction at ratios of 2:1, 1:1, and 0.25:1 (the natural ratio of these two fractions in the original water sample) to a final volume of 800 ml. A ratio of 0.5:1 was tested additionally in two of the six experiments. Two control flasks with a ratio of 1:1 were observed in parallel without the addition of V. cholerae. A volume of 800 ml was chosen, as the use of smaller volumes increases the probability of a so-called bottle effect (31), leading to overestimations of bacterial growth. For inoculation, two different V. cholerae isolates (VC030529α2 for experiments 1 to 5 and VC030710γ1 for experiment 6) from the frozen stock were thawed and cultured overnight in liquid Luria-Bertani (LB) medium at 37°C. Preliminary experiments with six selected strains of our strain collection had shown that all of these strains grew at similar velocities and with similar growth yields (data not shown), and thus, the two selected strains can be regarded as representative of the V. cholerae strains in Neusiedler See. Strain numbers and genotype information are provided in Table SA in the supplemental material. An aliquot (100 μl) was transferred to 50 ml LB broth and grown on a rotary shaker at the temperature chosen for the respective experiment until an optical density at 620 nm of 0.6 to 0.7 was reached. Aliquots were centrifuged (8,000 × g, 10 min) and washed three times with autoclaved lake water filtered with a sterile filter. The washed pellet was resuspended in 1 ml of the respective water mixture, and an aliquot (5 to 10 μl) was inoculated into the flasks to yield a final concentration of approximately 1.5 × 104 to 4 × 104 cells ml−1. The cultures were incubated in the dark and subsampled at intervals ranging from 2 to 12 h for a period of 48 to 96 h, depending on the chosen temperature (15, 20, 25, 30, and 37°C). Subsamples for the determination of bacterial numbers (10 ml) were taken with sterile, muffled glass pipettes at each sampling point and fixed with 2% (final concentration) formaldehyde. Subsamples for the determination of enzyme activity (50 ml) were taken twice for each culture during each experiment, shortly after the beginning and at the end of the log phase of bacterial growth. Subsamples for the DOC analysis (10 ml) were taken at the beginning and at the end of the experiment, as well as at the end of the log phase.
(iii) V. cholerae abundance, specific growth rates, and yield.
Ten milliliters (beginning of the experiment) to 0.5 ml (later samples) of the fixed samples were filtered through a black polycarbonate 0.2-μm-pore-size filter (Millipore, Vienna, Austria) and stained with DAPI (4′,6′-diamidino-2-phenylindole; Sigma-Aldrich, Vienna, Austria) according to the method of Porter and Feig (43). Stained filters were examined using UV excitation (340 to 380 nm) under a Nikon Eclipse 8000 microscope, and at least 20 microscopic fields were counted for the estimation of bacterial numbers. From the increase in cell numbers, the specific growth rate (μ) was calculated using the formula μ = (ln BN1 − ln BN0) × (T1 − T0)−1, where BN0 and BN1 are the bacterial numbers at the beginning (time zero [T0]) and at the end (T1) of the exponential growth phase, respectively. The doubling time (t) was calculated accordingly, using the formula t = ln 2/μ. The yield (absolute increase in bacterial numbers) was calculated by subtracting BN0 from BN1.
(iv) Enzyme activities.
The activities of chitinase and beta-glucosidase were measured in four of the six experiments as surrogates for the degradation of HMW substances (21). Artificial substrates (methyl-umbelliferyl-N-acetyl-β-d-glucosamine and methyl-umbelliferyl-β-d-glucose; Sigma-Aldrich, Vienna, Austria) were used as substrate analogues. The increase in fluorescence (366-nm excitation, 464-nm emission) after enzymatic cleavage was monitored in 30-min intervals over 2 h with a spectrofluorometer (model F-2000; Hitachi). The increase in fluorescence was linear during this period. The enzymatic reactions of both enzymes followed Michaelis-Menten kinetics and were tested two times prior to the start of the experiments at concentrations ranging from 5 to 200 μM. A 50 μM concentration was further chosen for all measurements, as this represented approximately the Km value for both enzymes.
Statistical analysis.
Statistical analysis was performed with SPSS 14.0 for Windows. For correlation analysis, the Spearman rank test was applied. For multiple stepwise regression analysis, data were tested for normal distribution. Results were accepted as significant at a probability of ≤0.05.
RESULTS
Seasonal occurrence of V. cholerae and relationship to environmental variables.
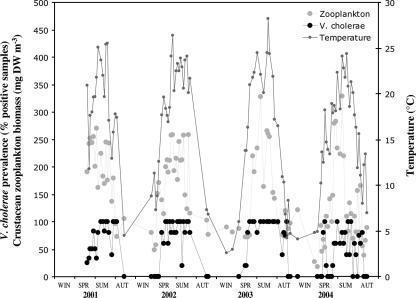
The occurrence of V. cholerae followed similar seasonal patterns during all 4 years (Fig. 2). From the end of April, V. cholerae could be detected by cultivation. Between one (20%) and five (100%) sampling stations were positive for V. cholerae. From June to September, the bacterium could be cultivated from all sampling stations on nearly all sampling occasions, but from December to March, no detection via cultivation was possible. Because each investigated sample yielded either a positive or a negative result for V. cholerae, these data have to be regarded as semiquantitative, as only frequencies of positive samples are available at present. However, a highly significant correlation of the percentage of V. cholerae-positive samples with temperature was found (rho = 0.65; P < 0.001; n = 102) as well as with the zooplankton biomass (rho = 0.43; P < 0.001; n = 100) and conductivity (rho = 0.33; P < 0.01; n = 102) but not with DOC, CHLA, TP, or TSS levels. The zooplankton biomass itself (rho = 0.61; P < 0.001; n = 100) and conductivity (rho = 0.39; P < 0.001; n = 102) were also highly significantly correlated with temperature. Table 2 summarizes the seasonal averages of these variables in the lake. Maximum water temperature during the summer was 28.3°C. This value represents an average of measurements performed between 8 a.m. and noon, but higher values up to 32°C can sometimes be observed locally under calm conditions in the uppermost surface layer (5 to 10 cm). Average conductivity ranged from 1,600 μS cm−1 in winter to 2,850 μS cm−1 in autumn, corresponding to salinity values between 1.3 and 3.2 g liter−1. DOC concentrations did not change markedly over the year due to the facts that CHLA values were also rather constant and that the wide reed belt supplies the lake with organic carbon throughout the year. Also, TP concentrations showed no clear seasonal trend, despite the fact that the mean values were higher in winter and spring.
FIG. 2.
Seasonal pattern of V. cholerae prevalence, crustacean zooplankton biomass, and water temperature in Neusiedler See during the period from 2001 to 2004. V. cholerae prevalence data indicate the percentages of samples that tested positive for V. cholerae that were taken from five sampling points. DW, dry weight; WIN, winter; SPR, spring; SUM, summer; AUT, autumn.
TABLE 2.
Important ecological variables in Neusiedler See during different seasons from 2001 to 2004a
| Season | Temp (°C) | Conductivity at 25°C (mS cm−1) | DOC (mg liter−1) | CHLA (μg liter−1) | TP (μg liter−1) |
|---|---|---|---|---|---|
| Spring (April to June) | 17.8 (8.8-26.4) | 2,255 (1,880-2,580) | 15.5 (12.2-19.4) | 10.0 (1.6-31.1) | 119 (48-262) |
| Summer (July to August) | 21.7 (12.9-28.3) | 2,455 (2,067-2,825) | 15.0 (12.6-17.2) | 10.1 (1.4-21.1) | 81 (38-147) |
| Autumn (September to November) | 12.6 (4.2-21.7) | 2,515 (2,140-2,850) | 15.3 (12.3-18.9) | 8.6 (0.9-19.1) | 73 (32-176) |
| Winter (December to March) | 5.9 (2.6-11.3) | 1,825 (1,600-2,310) | 13.0 (12.5-16.1) | 11.3 (3.2-24.9) | 93 (21-324) |
Variables are expressed as averages, with ranges in parentheses.
Size fractionations.
V. cholerae could be detected via cultivation in all size fractions of the lake water from Neusiedler See. The bacterium was always present in the period from May to September in the total sample and in the fractions with particle sizes of 0.2 μm to 1.2 μm and <0.2 μm. In the fractions with particle sizes of >250 μm (crustacean zooplankton) and 10 μm to 250 μm (small zooplankton and phytoplankton), the bacterium was found in 13 out of 14 cases, and in the fractions with particle sizes of 1.2 μm to 10 μm, it was found in 12 out of 14 cases.
Strain identification.
All strains isolated from the five sampling stations during the period from 2001 to 2004 (>250 isolates) and all strains from the size fractionation experiments (86 isolates), which were identified as V. cholerae by the API 20E system, tested negative for the O1 and O139 antigens and were designated non-O1/non-O139 V. cholerae. For all strains isolated in 2003 (n = 133), PCR analysis was run in parallel to validate the API 20E results and to check whether the strains possessed cholera-relevant or other important virulence genes. Except with three samples, the API results coincided with the results of the PCR. In two cases, a strain which was not identified as V. cholerae by the API system was identified as V. cholerae by the PCR-based approach (positive for the 16S-23S ISR). In both cases, V. alginolyticus was suggested by the API approach. For clarifying this discrepancy, parts of the 16S rRNA gene were sequenced and the strains identified as V. cholerae. In only one case was a positive detection with API not corroborated by the PCR method. By 16S rRNA gene sequence analysis, this strain was identified as Exiguobacterium sp.
All cultured V. cholerae strains were positive for the ISR and toxR, and in the case of hlyA, 132 out of 133 strains were positive. For the ompU gene, 14 out of 133 strains were negative, while all strains were negative for ctxA and tcpI. The E. coli and the V. mimicus control strains showed a negative PCR result in all cases.
Growth experiments.
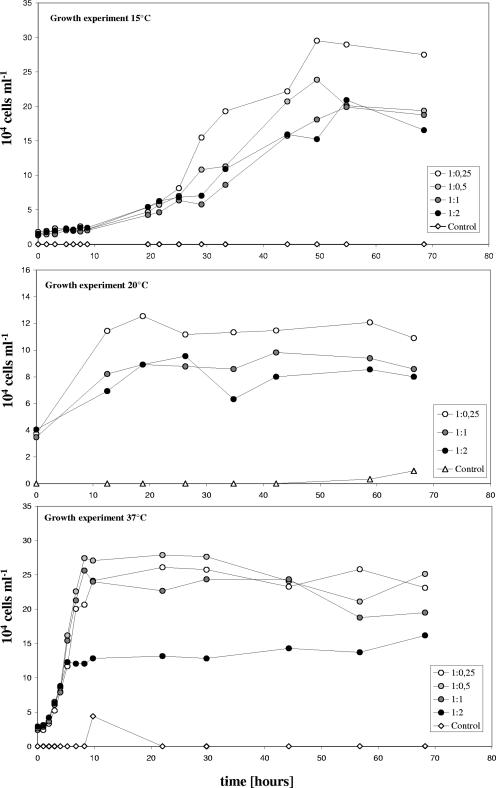
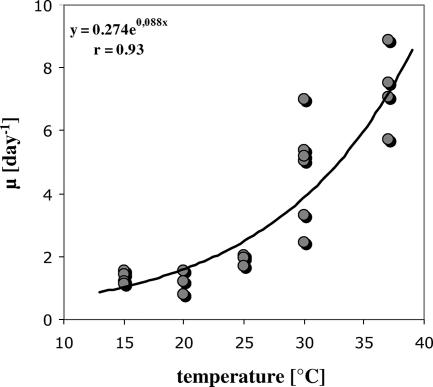
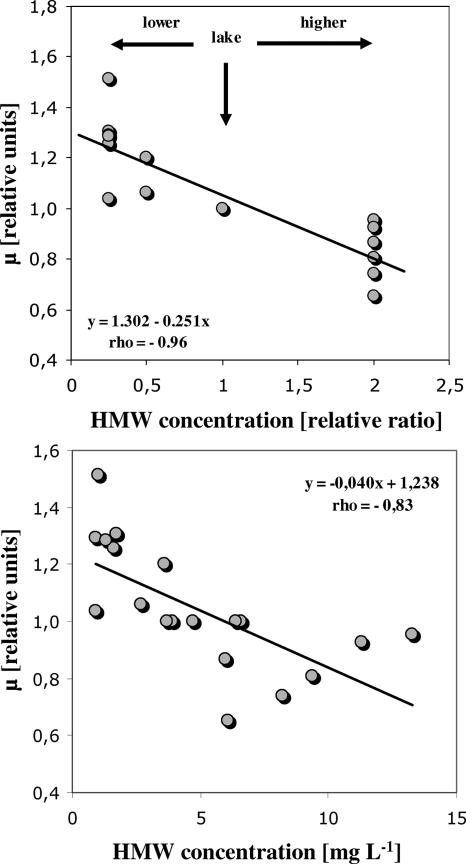
All growth experiments performed at five different temperatures showed that the selected V. cholerae strains isolated from the lake Neusiedler See could grow rapidly in sterile lake water. Figure 3 gives three representative examples of V. cholerae growth in the batch cultures run at 15, 20, and 37°C. Cell numbers increased from 1.5 × 104 to 4 × 104 cells ml−1 to maximal values of 9.0 × 104 to 2.7 × 105 cells ml−1. Calculated growth rates ranged from 0.78 to 8.9 day−1 and were significantly dependent on temperature (r = 0.93; P < 0.001), with an exponential relationship observed for the investigated temperature interval (Fig. 4). Another clear trend observed in each of the experiments was that V. cholerae growth decreased with increasing concentrations of HMW humic organic matter in the cultures. Table 3 lists the concentrations of total DOC, HMW humic organic matter, and LMW nonhumic organic matter for the six experiments. Total DOC concentrations of the growth cultures simulating that of the original water sample (1:1) ranged from 15.9 to 26.3 mg liter−1, HMW-substance concentrations from 3.0 to 6.6 mg liter−1, and LMW-substance concentrations from 12.8 to 19.9 mg liter−1. There was no significant change in these concentrations during the experiment, which was also reflected by the 250-nm/365-nm absorption ratios, where no clear trend could be observed. Concentrations in the other growth cultures were according to their mixture ratio of LMW and HMW substances. There was a highly significant negative linear relationship between the growth rate (expressed in relative units to make the experiments comparable) and the HMW-substance concentration expressed as the relative ratio between the concentration in the culture and the concentration in the lake (rho = −0.96; P < 0.001) (Fig. 5). When HMW-substance concentrations are expressed in mg liter−1, this correlation exhibits the same significance level (rho = −0.83; P < 0.001) (Fig. 5). Also, the yield in bacterial numbers (expressed in relative units for each experiment) was significantly negatively correlated with the HMW-substance concentration (rho = −0.53; P < 0.05) but positively with the LMW-substance concentration (rho = 0.73; P < 0.001). In contrast, no significant correlation of the V. cholerae growth rate or yield with the total DOC concentration was observed (rho = −0.20; P > 0.1). To predict the growth rates and the yields in the conducted experiments, multiple-step linear regression analysis was performed. The growth rate (μ) was best predicted by the formula μ (day−1) = 0.32 (temperature, °C) − 1.74 (HMW organic matter relative ratio) + 0.20 (DOC, mg liter−1) − 7.08, with an adjusted coefficient of determination (r2adj) of 0.88 (P < 0.001), while the yield (Y) was best predicted by the formula Y (104 cells liter−1) = 0.38 (temperature, °C) − 0.76 (HMW organic matter, mg liter−1) + 2.01 (LMW organic matter, mg liter−1) − 18.18, with an r2adj of 0.72 (P < 0.001).
FIG. 3.
Growth of V. cholerae in sterile lake water at 15°C (top), 20°C (middle), and 37°C (bottom) with different ratios of HMW-humic-organic-matter concentrations (expressed in ratios relative to the in situ concentrations). Results of experiments performed at 25°C and 30°C are not shown.
FIG. 4.
Exponential dependence of the growth rate of V. cholerae (μ) on temperature in the batch culture experiments.
TABLE 3.
Temperatures, V. cholerae strains used, DOC levels, HMW- and LMW-organic-matter concentrations, and 250-nm/365-nm absorbance ratios for the six growth experimentsa
| Temp (°C) | Strain | HMW-matter ratiob | DOC (mg liter−1) | HMW-matter concn (mg liter−1) | LMW-matter concn (mg liter−1) | 250-nm/365-nm absorbance ratio |
|---|---|---|---|---|---|---|
| 37 | VC030529α2 | 0.25 | 15.7 | 1.6 | 14.1 | 15.7 |
| 0.5 | 16.8 | 2.7 | 14.1 | 12.5 | ||
| 1 | 18.8 | 4.7 | 14.1 | 11.2 | ||
| 2 | 23.5 | 9.4 | 14.1 | 10.5 | ||
| 30 | VC030529α2 | 0.25 | 15.8 | 1.0 | 14.7 | 12.1 |
| 1 | 18.6 | 3.9 | 14.7 | 9.4 | ||
| 2 | 22.9 | 8.2 | 14.7 | 7.9 | ||
| 30 | VC030710γ1 | 0.25 | 15.7 | 1.7 | 14.0 | 6.5 |
| 1 | 20.6 | 6.6 | 14.0 | 5.8 | ||
| 2 | 27.3 | 13.3 | 14.0 | 5.4 | ||
| 25 | VC030529α2 | 0.25 | 13.4 | 0.9 | 12.5 | 11.8 |
| 1 | 16.2 | 3.7 | 12.5 | 8.6 | ||
| 2 | 18.5 | 6.0 | 12.5 | 7.7 | ||
| 20 | VC030529α2 | 0.25 | 13.1 | 0.9 | 12.2 | 15.3 |
| 1 | 15.9 | 3.7 | 12.2 | 11.5 | ||
| 2 | 18.3 | 6.1 | 12.2 | 9.7 | ||
| 15 | VC030529α2 | 0.25 | 21.2 | 1.3 | 19.9 | 20.8 |
| 0.5 | 23.5 | 3.6 | 19.9 | 17.5 | ||
| 1 | 26.3 | 6.4 | 19.9 | 15.7 | ||
| 2 | 31.2 | 11.3 | 19.9 | 14.1 |
Values represent the means of two measurements taken at the beginning and the end of each experiment. Differences between the two measurements were always below 0.5 mg liter−1 and a 1.0 absorbance ratio without a significant trend.
Values are expressed as ratios relative to the original concentration in the lake (see Materials and Methods).
FIG. 5.
Linear dependence of the growth rate of V. cholerae on the concentration of HMW humic organic matter in the batch culture experiments. To enable a comparison of the results of the different experiments run at different temperatures, the growth rates are expressed in relative units. (Top) HMW substances expressed as a ratio relative to the actual concentration in the lake; (bottom) HMW substances expressed in absolute concentrations (mg liter−1).
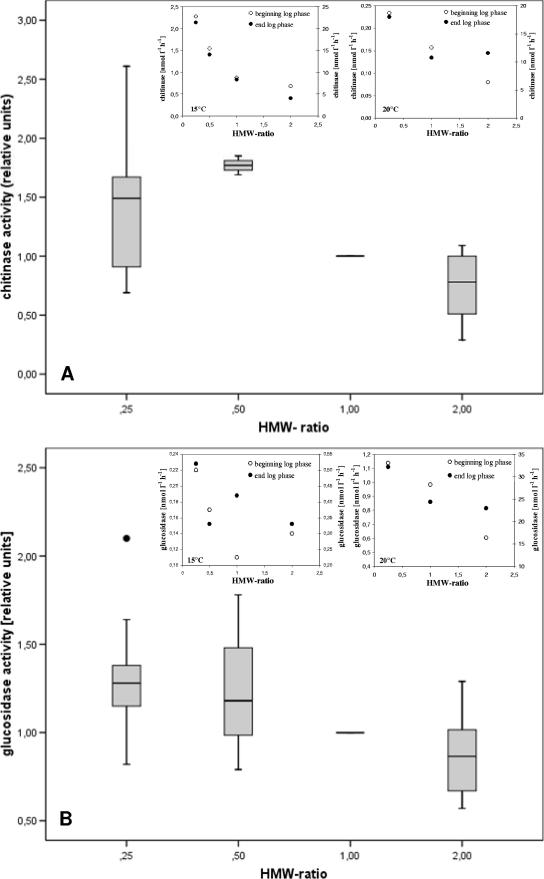
Chitinase activities varied between 0.15 and 2.44 nmol liter−1 h−1 (mean, 0.94) at the beginning and between 0.71 and 21.3 nmol liter−1 h−1 (mean, 6.49) at the end of the logarithmic phase of V. cholerae growth. In the case of beta-glucosidase, activities ranged from 0.11 to 20.7 nmol liter−1 h−1 (mean, 4.05) at the beginning and from 0.33 to 32.2 nmol liter−1 h−1 (mean, 9.25). Both enzymatic activities, measured at the beginning and at the end of logarithmic growth, showed no correlation with temperature (rho < 0.37; P > 0.1), with the exception of chitinase at the end of the logarithmic phase (rho = 0.61; P < 0.05). Slight yet significant negative correlations were found with increasing HMW-substance concentrations. The velocity of enzymatic substrate degradation, expressed in relative units to make experiments comparable, decreased on average by 45% in the case of chitinase when the HMW organic matter ratio increased from 0.25 to 2 (rho = −0.44; P < 0.05; n = 33) (Fig. 6A). In the case of beta-glucosidase, the decrease was on average 35% (rho = −0.48; P < 0.05; n = 29) (Fig. 6B). When cell-specific enzymatic degradation rates were calculated, the decrease was on average 30% for chitinase (rho = −0.40; P < 0.05; n = 33) and 34% for beta-glucosidase (rho = −0.43; P < 0.05; n = 29).
FIG. 6.
Decreasing trend of chitinase (A) and beta-glucosidase (B) activities with increasing HMW-substance concentrations, expressed as a ratio relative to the actual concentration in the lake. Activity values were expressed in relative units to make experiments comparable, and box whisker plots represent pooled data from four experiments. For both enzymes, results from two representative experiments are shown in the small insets. Data in the insets show rates measured at the beginning (left y axis) and at the end (right y axis) of the logarithmic growth phase.
DISCUSSION
Prevalence and potential pathogenicity of V. cholerae in the lake.
V. cholerae in Austria has become a matter of public interest since 2001 because of several reports of ear infections caused by this pathogen in people bathing in the lake Neusiedler See (22). Since then, the lake has been tested for the presence of V. cholerae at regular intervals. V. cholerae was detected by a qualitative cultivation-based approach in each investigated year at varying frequencies from the end of April till the end of October and was never detected during the period from November to March. All isolated strains, verified as V. cholerae (∼300 strains), were found to be non-O1/non-O139 strains by agglutination tests. In addition, PCR analyses of all strains isolated during 2003 (n = 133) verified that none of the strains was positive for the cholera toxin gene (ctx) or the toxin-coregulated pilus gene (tcp), both of which are prerequisites for the pathogen to have the ability to cause cholera. In only a very few cases has it been reported that non-O1/non-O139 strains were positive for ctx and tcp (7), and thus, it can be assumed that, at least during the study period, no cholera-toxigenic strains were present in the lake. In fact, all clinical strains isolated during that period from patients with a history of being in the lake were also non-O1/non-O139 V. cholerae (22), including the one which caused lethal septicemia. Cholera cases have been reported occasionally in Austria during the past 10 years, but all of them were imported from non-European countries (56, 58, 59). Endemic cholera cases in Europe in the last decade were all restricted to the Russian Federation (56, 57) and Italy (56). However, PCR analyses further revealed that the endemic strains in the lake Neusiedler See were positive for a variety of other virulence genes, like the hemolysin gene, the toxR gene (coregulating toxin expression), and an outer membrane protein gene involved in V. cholerae adhesion to human cells. This present gene pool underlines the potential pathogenicity of the strains in the lake.
We are aware that the presented data set provides only semiquantitative information on V. cholerae occurrence in the lake via the frequency of positive samples determined via a cultivation-based approach. No quantitative data have been available until now, which would be a prerequisite for linking the prevalence of this pathogen to public health concerns. In addition to performing quantitative, cultivation-based investigations, we are currently adopting a fluorescent in situ hybridization-based protocol to directly detect V. cholerae cells in the lake water and on planktonic organisms (18) in order to obtain quantitative information on spatial and temporal V. cholerae distribution in Neusiedler See. The infectious dose of V. cholerae O1 necessary to cause cholera has been reported to vary significantly, depending on the health state of the afflicted person, between >108 in healthy volunteers and <104 in patients with low gastric acid production (48). In the case of the V. cholerae non-O1/non-O139 strains, no information could be found in the literature on the number of cells which is needed to cause ear, wound, or blood infections.
Environmental factors influencing V. cholerae growth.
During the seasonal study, a variety of environmental variables were measured, and significant correlation of the frequency of V. cholerae detection in the lake with temperature, the zooplankton biomass, and conductivity was found. No correlation with DOC or CHLA levels was found.
(i) Temperature.
Temperature is a critical environmental determinant for V. cholerae growth (25, 35, 53). Culture-based detection of this pathogen is usually possible above a temperature of approximately 10°C to 15°C; if the temperature is below a critical value, the organism becomes viable but nonculturable, and its presence can be detected only with direct microscopic (24) or molecular biological (47) methods. Under favorable conditions (such as a rise in temperature), reversion from the viable-but-nonculturable state to the culturable state takes place (11), and the patterns found within this study suggest that the annual V. cholerae prevalence in the lake is controlled mainly by this mechanism. As expected, temperature was also the main factor influencing bacterial growth rates in the lab experiments. This relationship was based on an exponential function for the interval from 15 to 37°C, indicating that the environmental strains are well adapted to high temperatures and that global warming may lead to an advantage for microorganisms, which have a second ecological niche as pathogens in warm-blooded animals (12).
(ii) Zooplankton.
Crustacean zooplankton has frequently been reported to promote V. cholerae proliferation by serving as both a substratum and a substrate (see references 18 and 23), and the highly significant correlation of the crustacean zooplankton biomass to the frequency of V. cholerae detection found in the lake may indicate such a relationship. However, crustacean zooplankton showed a higher correlation coefficient with temperature than with the presence of V. cholerae, and thus, the correlation between V. cholerae and zooplankton may not be causally determined. As our size fractionation experiments revealed, V. cholerae was not more frequently isolated from zooplankton than in the filtrates from the <1.2-μm and even the <0.2-μm filtrations, suggesting strongly that the free-living state may be an important life strategy of this pathogen in the lake Neusiedler See. We are aware that the results from our size filtration experiments are only semiquantitative but nevertheless corroborate the conclusions obtained from our laboratory batch culture experiments of a rapid planktonic growth.
(iii) Salinity.
Also difficult to interpret was the relationship between V. cholerae and conductivity. Conductivity usually increases in the lake during the summer due to evaporation of up to 3,000 μS cm−1, corresponding to approximately 3.5‰ salinity. During winter, conductivity is markedly lower at values slightly above 1,600 μS cm−1, corresponding to approximately 1.3‰ salinity. Salinity was demonstrated to influence the abundance and growth of V. cholerae, which is regarded, like other Vibrio species, as a moderately halophilic bacterium, thriving optimally in estuarine and brackish environments at salinities between 2 and 14‰ (35). At salinities of >14‰, V. cholerae growth is delayed, caused by the necessity to produce compatible solutes to maintain osmotic pressure (29). Similarly, preliminary results from small sodium lakes east of Neusiedler See revealed that at salinities higher than 11‰, no V. cholerae organisms could be detected, whereas at salinities below 8‰, 30 to 80% of the samples (n = 14) tested as V. cholerae positive (unpublished data). Also, very low salinities are suboptimal for the growth of V. cholerae, although growth is possible when organic matter concentrations are high and Na+ is available (50). Studies have suggested (36) that strains vary greatly in survival and culturability under low-salinity conditions, and it is thus also probable that despite the moderate seasonal variations in the lake, salinity directly influenced V. cholerae growth.
(iv) DOC quality and concentration.
We found no correlation between the frequency of V. cholerae detection via cultivation with CHLA and that with DOC, both of which are parameters representing surrogates for the nutrient supply of bacteria. The CHLA level was often rather high during the spring, autumn, and winter at periods when the temperature was too low for V. cholerae growth, showing that low temperatures overruled the positive effect of LMW substances exuded by the phytoplankton. In the case of DOC, a large fraction in the lake consists of refractory HMW humic substances, derived from the extensive reed vegetation. However, it was shown by Reitner et al. (45) that this material is the main fuel for bacterial growth in the lake. Because V. cholerae is known to produce a variety of enzymes for degrading polymeric HMW substrates, we hypothesized that this bacterium would preferentially grow on these substances as its ecological niche in the water body. In contrast, our laboratory experiments showed much higher growth rates at low HMW-substance concentrations and a significant influence of the concentration of LMW substances on the growth yield. Also, enzymatic activities decreased markedly with increasing HMW-substance concentrations. As with the in situ situation, no correlation of V. cholerae growth rates with the total DOC concentration was observed, showing clearly that the quality of the DOC has a decisive influence. As the concentrations of LMW substances were kept constant, HMW substances seemed to have inhibited bacterial growth and enzyme activity. The inhibition of extracellular phosphatase activity (4) and glucosidase activity (17) by humic substances was reported to occur through the formation of complexes with the enzymes, a mechanism which could have taken place in our lake water experiments. Until now, there has been only very limited information in the literature on the role of DOC quality in V. cholerae growth. A very recent study indicated that below a threshold concentration of assimilable organic carbon (50 to 100 μg liter−1), no growth was observed in tap water (54), but these concentrations are far lower than expected for surface waters. Cyanobacterial dissolved organic matter was found to stimulate V. cholerae growth in the northern Baltic Sea, indicating a positive effect of readily utilizable LMW substrates (15). More experiments are under way to investigate the influence of DOC quality on V. cholerae growth and to consider also the possible influence of competition, as it is conceivable that V. cholerae switches to HMW substrates or growth on surfaces in the presence of competitive bacteria. For example, it was reported that antagonistic interactions among marine bacteria impede V. cholerae proliferation on particles (34).
Rapid planktonic growth of V. cholerae.
Overall, our experiments demonstrated that V. cholerae non-O1/non-O139 from lake Neusiedler See could rapidly grow in the planktonic state in the lake water. Growth rates ranged from 0.8 day−1 to 8.9 day−1, corresponding to doubling times of about 2 to 21 h. When only rates from in situ summer temperatures (20 to 30°C) and in situ DOC ratios (HMW to LMW substances, 1:1) are considered, values ranged from 1.2 to 5.4 day−1, corresponding to doubling times of about 14 and 3 h, respectively. These rates are similar in magnitude to those recently reported for V. cholerae non-O1/non-O139 growth in coastal (0.6 to 2.9 day−1) (60) and offshore (0.3 to 14.3 day−1) (39) marine waters. The growth rates of V. cholerae O1 in freshwater reported for the same temperature range (54) were 5.2 to 10.8 day−1, approximately two to five times higher than those from our experiments. Reasons for this might be that Vital et al. used autoclaving for their batch cultures, a method which significantly increases the concentration and availability of assimilable organic substrates, as they stated in their paper. Moreover, they used a 30-ml incubation volume (instead of the 800-ml volume used in this study), which might lead to severe overestimations of bacterial growth due to bottle effects (31). When the growth rates of V. cholerae are compared to the growth rates of natural bacterioplankton in the lake, it becomes obvious that this potential pathogen is able to grow at similar velocities. The growth rates of bulk bacterioplankton in the lake, measured via [3H]leucine and [3H]thymidine incorporation, ranged from 1.0 to 2.6 day−1 from April to September at in situ temperatures between 15 and 24°C (45), while growth rates of V. cholerae in our experiments at these temperatures ranged from 1.2 to 2.0 day−1. This supports the hypothesis that in addition to its biofilm mode of life at the surfaces of copepods (23), V. cholerae is able to grow in the planktonic state as an alternative ecological niche in the environment. Because all strains selected from our strain collection used in the preliminary laboratory growth experiments and both strains selected for the six main laboratory growth experiments showed the same rapid growth behavior, we believe that our findings describe a common feature of the V. cholerae strains present in the lake Neusiedler See. Whether these results are expandable also to other V. cholerae non-O1/non-O139 or to O1/O139 strains remains to be examined.
Supplementary Material
Acknowledgments
The study was financed by grants from the Arbeitsgemeinschaft Natürliche Ressourcen (AGN) to A.K.T.K. and B.R.
We thank Franz Rauchwarter for providing environmental data and Alexander Eiler (University of Uppsala, Sweden) as well as Jean Lesne and Sandrine Baron (CNSR, France) for providing isolated environmental strains of V. cholerae, V. alginolyticus, V. parahaemolyticus, and V. vulnificus. We also thank Rita Colwell and three anonymous reviewers for their valuable suggestions to improve the paper.
Supplemental material for this article may be found at http://aem.asm.org/.
Footnotes
Published ahead of print on 1 February 2008.
REFERENCES
- 1.Abd, H., A. Weintraub, and G. Sandström. 2005. Intracellular survival and replication of Vibrio cholerae O139 in aquatic free-living amoebae. Environ. Microbiol. 7:1003-1008. [DOI] [PubMed] [Google Scholar]
- 2.Aiken, G. R. 1985. Isolation and concentration techniques for aquatic humic substances, p 363-383. In G. R. Aiken, D. M. McKnight, and R. L. Wershaw (ed.), Humic substances in soil, sediment, and water. Geochemistry, isolation, and characterization. John Wiley and Sons, New York, NY.
- 3.Blake, P. A., D. T. Allegra, and J. D. Snyder. 1980. Cholera—a possible endemic focus in the United States. N. Engl. J. Med. 302:305-309. [DOI] [PubMed] [Google Scholar]
- 4.Boavida, M. J., and R. G. Wetzel. 1998. Inhibition of phosphatase activity by dissolved humic substances and hydrolytic reactivation by natural ultraviolet light. Freshwater Biol. 40:285-293. [Google Scholar]
- 5.Broza, M., and M. Halpern. 2001. Chironomid egg masses and Vibrio cholerae. Nature 412:40. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter, S. R. 1996. Microcosm experiments have limited relevance for community and ecosystem ecology. Ecology 77:677-680. [Google Scholar]
- 7.Chakraborty, S., A. K. Mukhopadhyay, R. K. Bhadra, A. N. Ghosh, R. Mitra, T. Shimada, S. Yamasaki, S. M. Faruque, Y. Takeda, R. R. Colwell, and G. B. Nair. 2000. Virulence genes in environmental strains of Vibrio cholerae. Appl. Environ. Microbiol. 66:4022-4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheasty, T., B. Said, and E. J. Threlfall. 1999. Vibrio cholerae non-O1: implications for man? Lancet 354:89-90. [DOI] [PubMed] [Google Scholar]
- 9.Chun, J., A. Huq, and R. R. Colwell. 1999. Analysis of 16S-23S rRNA intergenic spacer regions of Vibrio cholerae and Vibrio mimicus. Appl. Environ. Microbiol. 65:2202-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colwell, R. R., J. B. Kaper, and S. W. Joseph. 1977. Vibrio cholerae, Vibrio parahaemolyticus and other Vibrios: occurrence and distribution in Chesapeake Bay. Science 198:394-396. [PubMed] [Google Scholar]
- 11.Colwell, R. R., and A. Huq. 1994. Vibrios in the environment: viable but non-culturable Vibrio cholerae, p. 117-133. In I. K. Wachsmuth, P. A. Blake, and O. Olsvik (ed.), Vibrio cholerae and cholera: molecular to global perspectives. American Society for Microbiology, Washington, DC.
- 12.Colwell, R. R. 1996. Global climate and infectious disease: the cholera paradigm. Science 274:2025-2031. [DOI] [PubMed] [Google Scholar]
- 13.Cottingham, K. L., D. A. Chiavelli, and R. K. Taylor. 2003. Environmental microbe and human pathogen: the ecology and microbiology of Vibrio cholerae. Front. Ecol. Environ. 1:80-86. [Google Scholar]
- 14.Drake, J., G. Huxel, and C. Hewitt. 1996. Microcosms as models for generating and testing community theory. Ecology 77:670-677. [Google Scholar]
- 15.Eiler, A., C. Gonzalez-Rey, S. Allan, and S. Bertilsson. 2007. Growth response of Vibrio cholerae and other Vibrio spp. to cyanobacterial dissolved organic matter and temperature in brackish water. FEMS Microbiol. Ecol. 60:411-418. [DOI] [PubMed] [Google Scholar]
- 16.Epstein, P. R. 1993. Algal blooms in the spread and persistence of cholera. BioSystems 31:209-221. [DOI] [PubMed] [Google Scholar]
- 17.Espeland, E. M., and R. G. Wetzel. 2001. Complexation, stabilization, and UV photolysis of extracellular and surface-bound glucosidase and alkaline phosphatase: implications for biofilm microbiota. Microb. Ecol. 42:572-585. [DOI] [PubMed] [Google Scholar]
- 18.Heidelberg, J. F., K. B. Heidelberg, and R. R. Colwell. 2002. Bacteria of the γ-subclass Proteobacteria associated with zooplankton in Chesapeake Bay. Appl. Environ. Microbiol. 68:5498-5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herzig, A. 1974. Some population characteristics of planktonic crustaceans in Neusiedler See. Oecologia 15:127-141. [DOI] [PubMed] [Google Scholar]
- 20.Herzig, A., and M. Dokulil. 2001. Neusiedler See—a steppe lake in Europe, p. 401-415. In M. Dokulil, A. Hamm, and J. G. Kohl (ed.), Ökologie und Schutz von Seen. Facultas Universitäts Verlag, Vienna, Austria.
- 21.Hoppe, H. G. 1983. Significance of exoenzymatic activities in the ecology of brackish water: measurements by means of methylumbelliferyl substrates. Mar. Ecol. Prog. Ser. 11:299-308. [Google Scholar]
- 22.Huhulescu, S., A. Indra, A. Stoeger, W. Ruppitsch, B. Sarkar, and F. Allerberger. 2007. Occurrence of Vibrio cholerae serogroups other than O1 and O139 in Austria. Wien. Klin. Wochenschr. 119:235-241. [DOI] [PubMed] [Google Scholar]
- 23.Huq, A., E. B. Small, P. A. West, M. I. Huq, R. Rahman, and R. R. Colwell. 1983. Ecological relationships between Vibrio cholerae and planktonic crustacean copepods. Appl. Environ. Microbiol. 45:275-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huq, A., R. R. Colwell, R. Rahman, A. Ali, M. A. R. Chowdhury, S. Parveen, D. A. Sack, and E. Russek-Cohen. 1990. Detection of Vibrio cholerae O1 in the aquatic environment by fluorescent-monoclonal antibody and culture methods. Appl. Environ. Microbiol. 56:2370-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huq, A., R. B. Sack, A. Nizam, I. M. Longini, G. B. Nair, A. Ali, J. G. Morris, Jr., M. N. H. Khan, K. A. Siddique, M. Yunus, M. J. Albert, D. A. Sack, and R. R. Colwell. 2005. Critical factors influencing the occurrence of Vibrio cholerae in the environment of Bangladesh. Appl. Environ. Microbiol. 71:4645-4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Islam, M. S., B. S. Drasar, and R. B. Sack. 1994. The aquatic flora and fauna as reservoirs of Vibrio cholerae: a review. J. Diarrhoeal Dis. Res. 12:87-96. [PubMed] [Google Scholar]
- 27.Islam, M. S., Z. Rahim, M. J. Alam, S. Begum, S. Moniruzzaman, A. Umeda, K. Amako, M. J. Albert, R. B. Sack, A. Huq, and R. R. Colwell. 1999. Association of Vibrio cholerae O1 with the cyanobacterium Anabaena sp., elucidated by polymerase chain reaction and transmission electron microscopy. Trans. R. Soc. Trop. Med. Hyg. 93:36-40. [DOI] [PubMed] [Google Scholar]
- 28.Islam, M. S., S. Mahmuda, M. G. Morshed, H. B. M. Bakht, M. N. H. Khan, R. B. Sack, and D. A. Sack. 2004. Role of cyanobacteria in the persistence of V. cholerae O139 in saline microcosms. Can. J. Microbiol. 50:127-131. [DOI] [PubMed] [Google Scholar]
- 29.Kampfhammer, D., E. Karatan, K. J. Pflughoeft, and P. I. Watnick. 2005. Role for glycine betaine transport in Vibrio cholerae osmoadaptation and biofilm formation within microbial communities. Appl. Environ. Microbiol. 71:3840-3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirschner, A. K. T., T. C. Zechmeister, G. G. Kavka, C. Beiwl, A. Herzig, R. L. Mach, and A. H. Farnleitner. 2004. Integral strategy for evaluation of fecal indicator performance in bird-influenced saline inland waters. Appl. Environ. Microbiol. 70:7396-7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krammer, M., B. Velimirov, U. Fischer, A. H. Farnleitner, A. Herzig, and A. K. T. Kirschner. 2007. Growth response of soda lake bacterial communities to simulated rainfall. Microb. Ecol. doi: 10.1007/s00248-007-9267-5. [DOI] [PubMed]
- 32.Leclerc, H., A. Schwartzbrod, and E. Dei-Cas. 2004. Microbial agents associated with waterborne diseases. In T. E. Cloete, J. B. Rose, L. H. Nel, and T. Ford (ed.), Microbial waterborne pathogens. IWA Publishing, London, United Kingdom.
- 33.Löffler, H. 1979. Neusiedler See: the limnology of a shallow lake in central Europe. Dr. W. Junk Publishers, The Hague, The Netherlands.
- 34.Long, R. A., D. C. Rowley, E. Zamora, J. Liu, D. H. Bartlett, and F. Azam. 2005. Antagonistic interactions among marine bacteria impede the proliferation of Vibrio cholerae. Appl. Environ. Microbiol. 71:8531-8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Louis, V. R., E. Russek-Cohen, N. Choopun, I. N. G. Rivera, B. Gangle, S. C. Jiang, A. Rubin, J. A. Patz, A. Huq, and R. R. Colwell. 2003. Predictability of Vibrio cholerae in Chesapeake Bay. Appl. Environ. Microbiol. 69:2773-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller, C. J., B. S. Draser, and R. J. Heyes. 1984. Response of toxigenic V. cholerae O1 to physicochemical stresses in the aquatic environment. J. Hyg. 93:475-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moran, M. A., and R. E. Hodson. 1990. Bacterial production on humic and nonhumic components of dissolved organic carbon. Limnol. Oceanogr. 35:1744-1756. [Google Scholar]
- 38.Morris, J. G., Jr. 1990. Non-O group 1 Vibrio cholerae: a look at the epidemiology of an occasional pathogen. Epidemiol. Rev. 12:179-191. [DOI] [PubMed] [Google Scholar]
- 39.Mouriño-Pérez, R. R., A. Z. Worden, and F. Azam. 2003. Growth of Vibrio cholerae O1 in red tide waters off California. Appl. Environ. Microbiol. 69:6923-6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nalin, D. R. 1976. Cholera, copepods and chitin. Lancet ii:958. [DOI] [PubMed] [Google Scholar]
- 41.Nel, L. H., and W. Markotter. 2004. Emerging infectious waterborne diseases: bacterial agents. In T. E. Cloete, J. B. Rose, L. H. Nel, and T. Ford (ed.), Microbial waterborne pathogens. IWA Publishing, London, United Kingdom.
- 42.Parsons, T. R., Y. Maita, and C. Lalli. 1984. A manual of chemical and biological methods for seawater analysis. Pergamon Press, Oxford, United Kingdom.
- 43.Porter, K. G., and Y. S. Feig. 1980. The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 25:943-948. [Google Scholar]
- 44.Reitner, B., A. Herzig, and G. J. Herndl. 1997. Role of ultraviolet-B radiation on photochemical and microbial oxygen consumption in a humic-rich shallow lake. Limnol. Oceanogr. 42:950-960. [Google Scholar]
- 45.Reitner, B., A. Herzig, and G. J. Herndl. 1999. Dynamics in bacterioplankton production in a shallow, temperate lake (Lake Neusiedl, Austria): evidence for dependence on macrophyte production rather than on phytoplankton. Aquat. Microb. Ecol. 19:245-254. [Google Scholar]
- 46.Rivera, I. N. G., J. Chun, A. Huq, R. B. Sack, and R. R. Colwell. 2001. Genotypes associated with virulence in environmental isolates of Vibrio cholerae. Appl. Environ. Microbiol. 67:2421-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rivera, I. N. G., E. K. Lipp, A. Gil, N. Choopun, A. Huq, and R. R. Colwell. 2003. Method of DNA extraction and application of multiplex polymerase chain reaction to detect toxigenic V. cholerae O1 and O139 from aquatic ecosystems. Environ. Microbiol. 5:599-606. [DOI] [PubMed] [Google Scholar]
- 48.Sack, D. A., R. B. Sack, G. B. Nair, and A. K. Siddique. 2004. Cholera. Lancet 363:223-232. [DOI] [PubMed] [Google Scholar]
- 49.Schneider, D. R., and C. D. Parker. 1982. Purification and characterisation of the mucinase of Vibrio cholerae. J. Infect. Dis. 145:474-482. [DOI] [PubMed] [Google Scholar]
- 50.Singleton, F. L., R. Attwell, S. Jangi, and R. R. Colwell. 1982. Effects of temperature and salinity on Vibrio cholerae growth. Appl. Environ. Microbiol. 44:1047-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strickland, J. D., and T. R. Parsons. 1968. A practical handbook of seawater analysis. Bull. Fish. Res. Board Can. 167:77-80. [Google Scholar]
- 52.Twedt, R. M., J. M. Madden, J. M. Hunt, D. W. Francis, J. T. Peeler, A. P. Duran, W. O. Hebert, S. G. McCay, C. N. Roderick, G. T. Spite, and T. J. Wazenski. 1981. Characterization of Vibrio cholerae isolated from oysters. Appl. Environ. Microbiol. 41:1475-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Venkateswaran, K., T. Takai, I. M. Navarro, H. Nakano, H. Hashimoto, and R. J. Siebeling. 1989. Ecology of Vibrio cholerae non-O1 and Salmonella spp. and role of zooplankton in their seasonal distribution in Fukuyama coastal waters, Japan. Appl. Environ. Microbiol. 55:1591-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vital, M., H. P. Füchslin, F. Hammes, and T. Egli. 2007. Growth of Vibrio cholerae O1 Ogawa Eltor in freshwater. Microbiology 153:1993-2001. [DOI] [PubMed] [Google Scholar]
- 55.Watnick, P. I., and R. Kolter. 1999. Steps in the development of a Vibrio cholerae biofilm. Mol. Microbiol. 34:586-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.WHO. 1999. Cholera 1998. Wkly. Epidemiol. Rec. 74:257-264. [PubMed] [Google Scholar]
- 57.WHO. 2002. Cholera 2001. Wkly. Epidemiol. Rec. 77:257-268. [PubMed] [Google Scholar]
- 58.WHO. 2003. Cholera 2002. Wkly. Epidemiol. Rec. 78:269-276. [PubMed] [Google Scholar]
- 59.WHO. 2006. Cholera 2005. Wkly. Epidemiol. Rec. 81:297-308. [PubMed] [Google Scholar]
- 60.Worden, A. Z., M. Seidel, S. Smriga, A. Wick, F. Malfatti, D. Bartlett, and F. Azam. 2006. Trophic regulation of Vibrio cholerae in coastal marine waters. Environ. Microbiol. 8:21-29. [DOI] [PubMed] [Google Scholar]
- 61.Zechmeister, T. C., A. K. T. Kirschner, M. Fuchsberger, A. G. Gruber, B. Süβ, R. Rosengarten, F. Pittner, R. L. Mach, A. Herzig, and A. H. Farnleitner. 2005. Prevalence of botulinum neurotoxin C1 and its corresponding gene in environmental samples from low and high risk avian botulism areas. ALTEX 22:185-195. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.