Abstract
The water in the canals and some recreational lakes in Amsterdam is microbiologically contaminated through the discharge of raw sewage from houseboats, sewage effluent, and dog and bird feces. Exposure to these waters may have negative health effects. During two successive 1-year study periods, the water quality in two canals (2003 to 2004) and five recreational lakes (2004 to 2005) in Amsterdam was tested with regard to the presence of fecal indicators and waterborne pathogens. According to Bathing Water Directive 2006/7/EC, based on Escherichia coli and intestinal enterococcus counts, water quality in the canals was poor but was classified as excellent in the recreational lakes. Campylobacter, Salmonella, Cryptosporidium, and Giardia were detected in the canals, as was rotavirus, norovirus, and enterovirus RNA. Low numbers of Cryptosporidium oocysts and Giardia cysts were detected in the recreational lakes, despite compliance with European bathing water legislation. The estimated risk of infection with Cryptosporidium and Giardia per exposure event ranged from 0.0002 to 0.007% and 0.04 to 0.2%, respectively, for occupational divers professionally exposed to canal water. The estimated risk of infection at exposure to incidental peak concentrations of Cryptosporidium and Giardia may be up to 0.01% and 1%, respectively, for people who accidentally swallow larger volumes of the canal water than the divers. Low levels of viable waterborne pathogens, such as Cryptosporidium and Giardia, pose a possible health risk from occupational, accidental, and recreational exposure to surface waters in Amsterdam.
Exposure to microbiologically contaminated surface water may have adverse health effects and may result in gastroenteritis (GE); fever; skin, ear, and eye complaints; or more severe illnesses, such as hepatitis and meningitis (61). Protozoan parasites have frequently been the cause of water-associated outbreaks in Europe (33). The surveillance system in the United States has detected numerous outbreaks of disease associated with recreational waters over the years in which pathogens such as Cryptosporidium, Giardia, and norovirus were regularly identified as the etiological agent (20). Illness as a result of infections due to recreational water contact is difficult to detect and to attribute to water exposure (61). In The Netherlands, records of health complaints possibly related to recreational water demonstrate that each year over 50% of the authorities responsible for recreational water quality are aware of water-related health complaints, the majority comprising GE and skin conditions (48). Microorganisms in surface waters may originate from several sources. In The Netherlands, it has been demonstrated that pathogenic microorganisms may enter surface waters through discharges of raw and treated sewage and manure runoff from agricultural land (55, 37).
In Amsterdam, particularly during summer, people jump, fall, or get pushed into the canals and thus are exposed to the canal water. Other people, like professional divers, are exposed to canal water when engaged in their profession. The Amsterdam canals are not official European bathing sites, and therefore, water quality is not routinely monitored. However, Amsterdam Municipal Health Services is aware of contamination of the water in Amsterdam canals by sewage discharge from houseboats in the canals that are not connected to the sewer system, effluents from sewage treatment plants in the vicinity of Amsterdam transported into the canals by the river Amstel, runoff of dirt from the streets (including dog feces), and direct fecal droppings of birds. Several recreational lakes within the city boundaries of Amsterdam are official bathing sites at which water quality is tested as required by the European Bathing Water Directive (1, 12). Bathing water profiles (12) have indicated that water quality in five of these lakes is affected by the discharge of raw sewage from boats and houseboats, sewage effluent, dog feces on the beaches, and direct fecal droppings from birds.
The presence of waterborne pathogens in bird feces has frequently been reported. In Europe, Campylobacter jejuni has been detected in gull (Larus spp.) feces in Northern Ireland (41) and Sweden (15, 60). Birds may also contribute to the parasite load of recreational waters. Cryptosporidium and Giardia have been detected in goose feces in the United States (26) and in gull feces in Scotland (52) and the Czech Republic (44). Giardia cysts have been found in the feces of wild ducks (Anas spp.) in New Mexico (34). In Finland, Cryptosporidium oocysts and Giardia cysts were detected in dog feces (46), whereas a Canadian study showed the presence of Giardia cysts and Cryptosporidium antibodies in fecal samples from dogs (51).
Previous studies have demonstrated an increase in the number of fecal indicators in surface waters following heavy rainfall events, due to sewage overflow and surface runoff (24, 45). These data suggest that heavy rainfall events may contribute to surface water contamination in Amsterdam and may give rise to increased concentrations of pathogens in the water.
Despite the awareness of sources possibly contributing to surface water contamination in Amsterdam, no data were available on the water quality in the Amsterdam canals and the occurrence of pathogenic organisms in both the canals and recreational lakes. Therefore, this study aimed at testing surface water in Amsterdam intended for recreational and nonrecreational purposes for the presence of a range of waterborne pathogens and compliance with the standards for microbiological quality as required by the 1976 European Bathing Water Directive (1) as well as the revised directive which came into force in 2006 (12). Obtained data were used to provide a rough estimate of the risk of infection with Cryptosporidium and Giardia from occupational and accidental exposure to canal water and the recreational use of lakes in Amsterdam.
MATERIALS AND METHODS
Sampling sites.
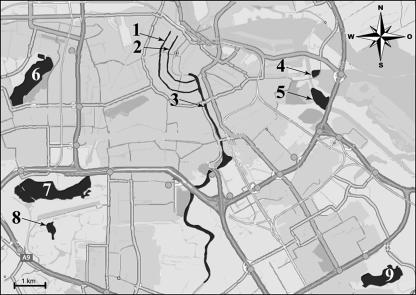
Water from a canal with houseboats (Prinsengracht), a canal without houseboats (Herengracht), and the River Amstel and the IJmeer (Fig. 1) was sampled eight times from June 2003 to June 2004. Water from the recreational lakes Sloterplas, Amsterdamse Bos, Gaasperplas, Nieuwe Meer, and Nieuwe Diep (Fig. 1) was sampled 11 times from September 2004 to September 2005. Samples were taken and handled according to ISO 5667-2 (3); sample volumes of approximately 40 liters were collected in polypropylene vessels. All samples were tested for the presence of fecal coliforms, Escherichia coli, fecal streptococci, and intestinal enterococci. Samples from the canals and the Amstel and the IJmeer were also examined for F-specific bacteriophages, somatic coliphages, Campylobacter, Salmonella, Escherichia coli O157, Cryptosporidium, Giardia, rotavirus, enterovirus, reovirus, norovirus, astrovirus, and hepatitis A and E viruses. Samples from the lakes were additionally examined for the presence of Cryptosporidium and Giardia.
FIG. 1.
Nonrecreational sampling sites (Prinsengracht [1], Herengracht [2], Amstel [3], and IJmeer [4]) and recreational sampling sites (Nieuwe Diep [5], Sloterplas [6], Nieuwe Meer [7], Amsterdamse Bos [8], and Gaasperplas [9]) in Amsterdam.
Analytical procedures. (i) Fecal indicator bacteria.
The fecal indicator parameters included in European Bathing Water Directive 76/160/EEG, fecal coliforms and fecal streptococci, were enumerated according to Dutch standards (2, 4), using lauryl sulfate agar (M500.02; Tritium Microbiology, Veldhoven, The Netherlands) and KF streptococcus agar (76.4 g KF streptoccus agar [BD Difco 249610; BD Benelux, Breda, The Netherlands], 2 ml 5% 2,3,5-triphenyltetrazolium chloride solution [SR0211; Oxoid Ltd., Basingstoke, United Kingdom], and 1 liter distilled water, prepared according to the manufacturer's instructions) as isolation media, respectively. Fecal indicator parameters included in European Bathing Water Directive 2006/7/EC, E. coli and intestinal enterococci, were enumerated by using the rapid test on tryptone soy agar (P05012A; Oxoid, Wesel, Germany) and tryptone bile agar (P05017; Oxoid) described in ISO 9308-1 (6) and according to ISO 7899-2 (7) on Slanetz and Bartley agar (P05018A; Oxoid), respectively.
(ii) Bacteriophages.
F-specific bacteriophages were enumerated according to ISO 10705-1 (5) and grown on tryptone yeast extract glucose agar (20 g Bacto Agar [BD 214010; BD Benelux], 8 g sodium chloride [1.06404; Merck KGaA, Darmstadt, Germany], 10 g trypticase peptone [BD 211921; BD Benelux], 1 g yeast extract [Lp0021; Oxoid Ltd.], and 1 liter distilled water, prepared according to ISO 10705-1). Somatic coliphages were enumerated according to ISO 10705-2 (8) and grown on modified Scholtens agar with nalidixic acid (20 g Bacto Agar [BD 214010; BD Benelux], 10 g peptone [Lp0034; Oxoid Ltd.], 12 g Lab Lemco powder [Lp0029; Oxoid Ltd.], 3 g sodium chloride [1.06404; Merck KGaA], 5 ml Na2CO3 solution at 150 g/liter [1.00392; Merck KGaA], 0.3 ml MgCl2 · 6H2O solution at 2 g/ml [1.05833; Merck KGaA], 250 mg nalidixic acid [190246; ICN Biomedicals Inc., Costa Mesa], and 1 liter distilled water, prepared according to ISO 10705-2).
(iii) Cryptosporidium and Giardia.
For enumeration of Cryptosporidium and Giardia, water samples (approximately 20 liters) were concentrated by using Envirochek HV filtration capsules (Pall Gelman Laboratory, Ann Arbor, MI) as described in ISO 15553 (11). Concentrated samples were purified by immunomagnetic separation using the Dynal GC-Combo system (Dynal Biotech ASA, Oslo, Norway) according to the manufacturer's instructions. Slides for microscopy were stained with 50 μl Cryptosporidium and Giardia staining reagent without Evans blue (Cellabs Diagnostics, Brookvale, Australia) at 37°C for 45 min in the dark; subsequently, 5 μl of a propidium iodide solution (PI) (1 mg/ml in phosphate-buffered saline [0.01 M, pH 7.2] was added and incubated for 2 min at room temperature. Slides were subsequently washed with phosphate-buffered saline, dried with a medium-warm hairdryer, mounted with DABCO-glycerol mounting medium, sealed with colorless nail polish, and examined at ×250 magnification using epifluorescence microscopy (Zeiss Axioskop; Carl Zeiss, Jena, Germany). (Oo)cysts were examined in detail by using Nomarski differential interference contrast at ×1,000 magnification to verify the presence of internal structures. (Oo)cysts taking up PI and staining red were considered dead, whereas Cryptosporidium oocysts that excluded PI and contained sporozoites (18) and Giardia cysts that excluded PI and had nongranular cytoplasm were considered viable (54).
(iv) Viruses.
For virus detection, water samples (approximately 20 liters) were concentrated by a conventional filter adsorption-elution procedure (57) and then separated by a modified two-phase method (36). Samples were further concentrated and purified by spin column gel chromatography and ultrafiltration in a Centricon microconcentrator (36). RNA extraction was performed according to Boom et al. (13) with slight modifications (36). Semiquantitative reverse transcription-PCR assays were used to detect the presence of enterovirus, rotavirus, norovirus, astrovirus, and hepatitis A and E virus RNA. Enterovirus detection was performed according to Schwab et al. (50) using primer pair Entero1 and Entero2. Rotaviruses were detected by using primer pair VP6-3 and VP6-4 (59). Norovirus detection was done according to Vennema et al. (58) using the modified primer pair JV12Y and JV13i. For detection of astrovirus, the method described by Guix et al. (27), applying primers A2 and A1, was used. Primer pairs HAV240 and HAV68 were used for hepatitis A detection (14). Detection of hepatitis E virus was performed according to van der Poel et al. (56) and used primer pair Orf2-S1 and Orf2-A1.
A fraction of the concentrated water samples, obtained as described above, was used to inoculate monolayers of buffalo green monkey cells. The assay was performed as described by Lodder and de Roda Husman (37).
(v) Campylobacter.
The presence or absence of Campylobacter in 1-liter volumes was determined by using the method described in ISO 17995 (10). The primary selective enrichment medium was Preston broth (12.5 g nutrient broth no. 2 [CM0067; Oxoid Ltd.], 475 ml distilled water, 25 ml lysed horse blood [SR0048; Oxoid Ltd.], 1 vial Campylobacter growth supplement [SR0232; Oxoid Ltd.], and 1 vial Preston selective supplement [SR0117; Oxoid Ltd.], prepared according to ISO 17995), and Karmali agar (P05041A; Oxoid) was used as the secondary growth medium. Typical colonies were examined for the characteristic spiral shape and corkscrew-like motility of Campylobacter by microscopy.
(vi) Salmonella.
The presence or absence of Salmonella in 1-liter volumes was determined according to ISO 6579 (9). Buffered peptone water (K168; bioTrading Benelux BV, Mijdrecht, The Netherlands) was used as primary enrichment broth, and Rappaport Vassiliadis soya peptone broth (26.75 g [CM0866; Oxoid Ltd.] and 1 liter distilled water, prepared according to the manufacturer's instructions) as secondary selective enrichment broth. Brilliant green agar (P05033A; Oxoid) was used as selective solid culture medium. Typical colonies were confirmed on urea agar (U010.86.0008; Tritium Microbiology) and triple sugar iron agar (T352.26.0008; Tritium Microbiology) and in lysine decarboxylation medium (L401.25.0005; Tritium Microbiology). Colonies displaying results characteristic for Salmonella were typed by the National Reference Laboratory for Salmonella at the Laboratory for Infectious Diseases and Perinatal Screening of the RIVM.
(vii) Escherichia coli O157.
For molecular detection of E. coli O157, approximately 500-ml volumes were filtered through 0.4-μm-pore-size polycarbonate membrane filters (Isopore; Millipore, Billerica, MA). DNA was extracted by using a DNeasy tissue kit (Qiagen Benelux BV, Venlo, The Netherlands) according to the manufacturer's instructions. Real-time PCR assays using a LightCycler real-time PCR device were performed to detect the rfbE gene present in E. coli O157 as described previously (47).
(viii) European Bathing Water Directive.
Compliance with the 1976 European Bathing Water Directive requires that 80% of the samples taken during a bathing season (n = 11 or 12) meet the quality standards for the fecal indicators as outlined in Table 1. Moreover, Salmonella and enteroviruses must be absent in 1 liter and 10 liters, respectively. The revised 2006 directive distinguishes separate standards for coastal and fresh waters. At least four observations obtained during the current bathing season, supplemented with observations from previous bathing seasons (total n = 16), should be tested for compliance with the standards for fecal indicators (Table 1).
TABLE 1.
Standards for fecal indicators according to European Bathing Water Directive 76/160/EEC and revised European Bathing Water Directive 2006/7/EC
| Directive | Water type | Parameter | Excellent quality (CFU/100 ml) | Good quality (CFU/100 ml) | Sufficient quality (CFU/100 ml) |
|---|---|---|---|---|---|
| 76/160/EEC | All | Total coliforms | 500 | 10,000 | |
| Fecal coliforms | 100 | 2,000 | |||
| Fecal streptococci | 100 | ||||
| 2006/7/EC | Inland waters | Escherichia coli | 500a | 1,000a | 900b |
| Intestinal enterococci | 200a | 400a | 330b | ||
| Coastal waters and transitional waters | Escherichia coli | 250a | 500a | 500b | |
| Intestinal enterococci | 100a | 200a | 185b |
Based upon a 95th-percentile evaluation.
Based upon a 90th-percentile evaluation.
(ix) Precipitation.
Rainfall and rainfall intensity data on sampling days and the 3 days preceding sampling were obtained from the Royal Netherlands Meteorological Institute (www.knmi.nl). For all microbiological parameters at all sampling sites, the correlation coefficient between the observed numbers and the amount of rainfall and rainfall intensity was calculated by using the CORREL function in Excel (Microsoft, version 2003).
(x) Risk assessment.
For both professional and accidental contact with canal water and recreational use of the studied lakes in Amsterdam, the risk of infection with Cryptosporidium and Giardia per exposure event was estimated (30). The range of estimated ingested volumes per contact event used in the calculations was based on the results obtained in a survey of diving behavior and water ingestion among occupational and sport divers (49) and the outcome of a study on water ingestion by swimmers in an indoor swimming pool (23). The risk of infection was estimated by using the exponential dose-response model (28) for which Pinf = 1 − e−rμ, where Pinf is the probability of infection, and the dose μ = CV (where C is the measured concentration of viable [oo]cysts in the water samples [n/liter] and V is individual consumption of water [liters]. Dose-response parameter values (rCryptosporidium = 0.0040 and rGiardia = 0.0199) were used (53). Calculations were done using Mathematica (Wolfram Inc., version 5.1.0).
RESULTS
Fecal indicators and bacteriophages.
E. coli, fecal coliforms, intestinal enterococci, and fecal streptococci were detected in the majority of the samples from all sites. Concentrations varied throughout the sampling years and per sampling site but were generally lower in the recreational lakes than in the canals and the Amstel and the IJmeer; arithmetic means, medians, and concentration ranges are displayed in Table 2. Somatic coliphages were detected in all samples from the canals, the Amstel, and the IJmeer, except one from the last site (Table 2). Concentrations varied throughout the year and were highest in the Amstel and lowest in the IJmeer. F-specific phages occurred at much lower concentrations (Table 2).
TABLE 2.
Fecal indicator bacteria and bacteriophages in surface waters in Amsterdama
| Sitec | Quantity | FC (no./100 ml) | FS (no./100 ml) | EC (no./100 ml) | IE (no./100 ml) | FPb (no./ml) | SCb (no./ml) |
|---|---|---|---|---|---|---|---|
| Nonrecreational sites | |||||||
| IJmeer | Mean | 575 | 112 | 1,928 | 127 | 0.06 | 2.2 |
| Median | 400 | 50 | 200 | 100 | 0 | 1.8 | |
| Range | 100-1,900 | 0-400 | 8-10,000 | 0-425 | 0-0.3 | 0-5.4 | |
| Amstel | Mean | 1,550 | 300 | 1,404 | 140 | 1.4 | 21 |
| Median | 1,350 | 100 | 450 | 100 | 0.4 | 18 | |
| Range | 400-4,200 | 0-1,500 | 28-6,300 | 35-350 | 0.1-5.3 | 2.3-54 | |
| Herengracht | Mean | 1,838 | 225 | 1,552 | 158 | 2.2 | 16 |
| Median | 550 | 100 | 338 | 155 | 0.1 | 6.3 | |
| Range | 100-9,000 | 0-1,200 | 40-8,100 | 0-350 | 0-0.94 | 0.7-54 | |
| Prinsengracht | Mean | 1,462 | 212 | 1,354 | 148 | 2.6 | 13 |
| Median | 450 | 100 | 475 | 160 | 0.2 | 5.6 | |
| Range | 300-6,000 | 0-700 | 80-7,000 | 90-200 | 0-14 | 0.9-45 | |
| Recreational sites | |||||||
| Amsterdamse Bos | Mean | 35 | 71 | 130 | 33 | ND | ND |
| Median | 18 | 33 | 50 | 2 | |||
| Range | 0-160 | 2-400 | 0-800 | 0-200 | |||
| Gaasperplas | Mean | 12 | 8 | 41 | 5 | ND | ND |
| Median | 16 | 10 | 24 | 0 | |||
| Range | 0-28 | 0-16 | 0-110 | 0-30 | |||
| Nieuwe Diep | Mean | 33 | 75 | 74 | 72 | ND | ND |
| Median | 14 | 38 | 80 | 6 | |||
| Range | 0-130 | 0-400 | 0-140 | 0-600 | |||
| Sloterplas | Mean | 45 | 105 | 122 | 42 | ND | ND |
| Median | 20 | 48 | 100 | 10 | |||
| Range | 0-230 | 8-500 | 0-400 | 0-190 | |||
| Nieuwe Meer | Mean | 8 | 27 | 34 | 3 | ND | ND |
| Median | 6 | 18 | 20 | 0 | |||
| Range | 0-30 | 0-90 | 0-160 | 0-14 |
FC, fecal coliforms; FS, fecal streptococci; EC, E. coli; IE, intestinal enterococci; FP, F-specific bacteriophages; SC, somatic coliphages.
ND, not done.
Nonrecreational sites were sampled eight times from June 2003 to June 2004; recreational sites were sampled 11 times from September 2004 to September 2005.
In the canals and the Amstel, the fecal indicator parameters, except E. coli, and both somatic and F-specific phages displayed peak concentrations on 15 December 2003, which coincided with heavy rainfall events on this sampling day and 3 days before sampling (25.5 mm in 4 days; average intensity, 1.42 mm/h). E. coli concentrations in the canals, the Amstel, and the IJmeer peaked on 27 October 2003; the total amount of rainfall on this day and the preceding 3 days was 10.7 mm; the average intensity was 1.35 mm/h. Correlations between fecal indicator and bacteriophage concentrations and rainfall intensity were average to strong, with correlation coefficients from 0.5 to 0.9.
Fecal indicator concentrations in the recreational lakes varied throughout the sampling year and showed a clear peak at all sites on 7 July 2004. The total amount of rainfall on this day and the preceding 3 days was 57.3 mm; the average rainfall intensity was 7.0 mm/h. Correlations between fecal indicator concentrations and rainfall amount and intensity varied strongly (−0.3 to 0.9), and there was no common pattern for all recreational sites.
Compliance with European bathing water legislation.
The water quality at none of the studied nonrecreational sites in Amsterdam complied with the standards for excellent water quality in Bathing Water Directive 76/160/EEC. Water quality in the IJmeer and the Prinsengracht canal was, however, good, but standards for good quality were not met in the Herengracht canal due to high numbers of fecal indicators and the presence of culturable enteroviruses and Salmonella and in the Amstel due to the presence of Salmonella. The water quality at these sites did not meet the standards for acceptable bathing water quality as required by revised European Bathing Water Directive 2006/7/EC and was therefore classified as “poor.” It should, however, be noted that compliance with European bathing water standards was assessed by using data collected throughout a year and not by using data obtained during the bathing season only. Also, fewer observations (n = 8) than required by the directives were obtained and used for compliance testing.
The water quality in the Amsterdam recreational lakes complied with the standards for excellent water quality, according to both Bathing Water Directive 76/160/EEC and 2006/7/EC. The required number of samples (n = 11) was used to test for compliance with Directive 76/160/EEC; however, this is fewer observations than required for compliance testing with Directive 2006/7/EC.
Pathogenic viruses and bacteria in canals, the Amstel, and the IJmeer.
Astroviruses and hepatitis A and E viruses were not found in any of the samples, whereas rotavirus, norovirus, and enterovirus RNA was detected in several samples from all sites (Table 3). Culturable enteroviruses were found in one sample from the canal Herengracht at a concentration of 3.2 PFU per liter. Samples taken from the Amstel and the Herengracht and Prinsengracht canals on 15 December 2003 and 9 February 2004 contained culturable reoviruses. Concentrations were 25 to 37 PFU/liter in the Amstel, 36 to 42 PFU/liter in the Herengracht canal, and 18 to 19 PFU/liter in the Prinsengracht canal. The sample taken from the Herengracht canal on 7 June 2004 contained 3.3 PFU/liter culturable reovirus. No culturable viruses were detected in water from the IJmeer.
TABLE 3.
Enteric viruses in surface water at four nonrecreational sites in Amsterdam from June 2003 to June 2004
| Site | No. of positive samples/total no. of samples
|
||||
|---|---|---|---|---|---|
| Norovirus RNA | Rotavirus RNA | Enterovirus RNA | Culturable enterovirus | Culturable reovirus | |
| IJmeer | 1/8 | 3/8 | 4/8 | 0/8 | 0/8 |
| Amstel | 4/8 | 7/8 | 7/8 | 0/8 | 2/8 |
| Herengracht | 2/8 | 7/8 | 3/8 | 1/8 | 3/8 |
| Prinsengracht | 2/8 | 5/8 | 2/8 | 0/8 | 2/8 |
Salmonella enterica serovar Newport and Salmonella enterica serovar Virchow were isolated from the Amstel on 15 December 2003 and 9 February 2004, respectively. Salmonella serovar Typhimurium phagetype 690 was isolated from the Herengracht canal on 9 February 2004. Salmonella was not detected in samples from the IJmeer or the Prinsengracht canal.
Campylobacter was found in three of seven samples from the IJmeer and the Amstel and in six of seven samples from the Herengracht and Prinsengracht canals. E. coli O157 was not detected in any of the samples.
Protozoan parasites.
Cryptosporidium was found at all nonrecreational sites, but the detection frequencies and concentrations varied (Table 4). Detection frequency was lowest in the IJmeer and highest in the Prinsengracht canal. Concentrations in positive samples were generally low, ranging from one to seven viable oocysts per 10 liters, with one outlier of 29 oocysts/10 liters (Table 4). Giardia cysts were detected in all of these samples except one from the IJmeer. The number of viable Giardia cysts in positive samples was higher than the number of viable Cryptosporidium oocysts and ranged from 1 to 157 cysts per 10 liters (Table 4). For all sites except the Prinsengracht canal, there was a moderate-to-high correlation between the number of (oo)cysts and the amount of rainfall (correlation coefficient 0.5 to 0.9); the correlation with rainfall intensity was moderate (correlation coefficient 0.4 to 0.7). Cryptosporidium oocysts were found in all studied recreational lakes in Amsterdam but with a low frequency (Table 4); in positive samples, numbers ranged from one to four viable oocysts per 10 liters. Giardia cysts were detected in all lakes except the Amsterdamse Bos. The detection frequency was also low, and in positive samples, numbers ranged from one to eight viable cysts per 10 liters (Table 4).
TABLE 4.
Protozoan parasites in surface waters in Amsterdam
| Sitea | Type of quantity |
Cryptosporidium
|
Giardia
|
||||
|---|---|---|---|---|---|---|---|
| No. of positive samples/total samples | Total count (no. of oocysts/ 10 liters) | Viable count (no. of oocysts/ 10 liters) | No. of positive samples/total samples | Total count (no. of cysts/10 liters) | Viable count (no. of cysts/10 liters) | ||
| Nonrecreational sites | |||||||
| IJmeer | 2/8 | 7/8 | |||||
| Mean | 0.4 | 0.2 | 35 | 30 | |||
| Median | 0 | 0 | 34 | 16 | |||
| Range | 0-2 | 0-1 | 0-105 | 0-84 | |||
| Amstel | 5/8 | 8/8 | |||||
| Mean | 2.4 | 1.2 | 68 | 53 | |||
| Median | 1 | 0 | 60 | 44 | |||
| Range | 0-9 | 0-5 | 8-157 | 7.2-157 | |||
| Herengracht | 5/8 | 8/8 | |||||
| Mean | 2.2 | 1 | 45 | 37 | |||
| Median | 1 | 0 | 27 | 48 | |||
| Range | 0-10 | 0-7 | 1-118 | 0-94 | |||
| Prinsengracht | 6/8 | 8/8 | |||||
| Mean | 5.7 | 4.4 | 70 | 55 | |||
| Median | 2.6 | 0.5 | 69 | 55 | |||
| Range | 0-29 | 0-29 | 2-167 | 2-134 | |||
| Recreational sites | |||||||
| Amsterdamse Bos | 4/11 | 0/11 | |||||
| Mean | 0.5 | 0 | 0 | 0 | |||
| Median | 0 | 0 | 0 | 0 | |||
| Range | 0-2 | 0 | 0 | 0 | |||
| Gaasperplas | 2/11 | 3/11 | |||||
| Mean | 0.6 | 0.6 | 0.4 | 0 | |||
| Median | 0 | 0 | 0 | 0 | |||
| Range | 0-4 | 0-4 | 0-2 | 0 | |||
| Nieuwe Diep | 1/11 | 6/11 | |||||
| Mean | 0.1 | 0 | 2.5 | 1.2 | |||
| Median | 0 | 0 | 1 | 0 | |||
| Range | 0-1 | 0 | 0-11 | 0-5 | |||
| Sloterplas | 5/11 | 6/11 | |||||
| Mean | 1.4 | 0.3 | 1.1 | 0.3 | |||
| Median | 0 | 0 | 1 | 0 | |||
| Range | 0-12 | 0-1 | 0-4 | 0-4 | |||
| Nieuwe Meer | 2/11 | 5/11 | |||||
| Mean | 0.3 | 0.2 | 2.2 | 1.2 | |||
| Median | 0 | 0 | 0 | 0 | |||
| Range | 0-2 | 0-1 | 0-8 | 0-8 | |||
Nonrecreational sites were sampled eight times from June 2003 to June 2004; recreational sites were sampled 11 times from September 2004 to September 2005.
Risk assessment.
Estimated average volumes of water ingested by occupational divers in fresh water (5.7 ml) (49) and by adult (16 ml) and nonadult (37 ml) swimmers in a swimming pool (23) were used to calculate the risk of infection with Cryptosporidium and Giardia for an exposed individual.
The infection risk per exposure event at ingestion of 5.7 to 37 ml ranges from 0.00006% to 0.006% for average detected Cryptosporidium concentrations at the four studied nonrecreational sites in Amsterdam (Table 5). For Giardia, the infection risk ranges from 0.03% to 0.4% (Table 5). For all sites and ingested volumes of 5.7 to 37 ml, maximum Cryptosporidium concentrations result in an infection risk of 0.0002 to 0.04%, whereas maximum Giardia concentrations lead to an infection risk of 0.09 to 1.2% (Table 5). At the recreational sites, the low average parasite concentrations give rise to an estimated infection risk of 0 to 0.0009% for Cryptosporidium and 0 to 0.009% for Giardia, whereas the maximum concentrations result in an infection risk of 0 to 0.006% for Cryptosporidium and 0 to 0.06% for Giardia (Table 5). Figure 2 displays estimated infection risks for ingested volumes of 0 to 100 ml.
TABLE 5.
Risk of infection with Cryptosporidium or Giardia at exposure to recreational and nonrecreational water in Amsterdam, assuming ingestion of different volumes of water and taking into account the mean and maximum detected parasite concentrations
| Site | Pathogen | Infection risk (%) at ingested vol (ml) ofd:
|
|||||
|---|---|---|---|---|---|---|---|
| 5.7a
|
16b
|
37c
|
|||||
| Mean | Max | Mean | Max | Mean | Max | ||
| Nonrecreational sites | |||||||
| IJmeer | Cryptosporidium | 0.00006 | 0.0002 | 0.0002 | 0.0006 | 0.0004 | 0.002 |
| Amstel | 0.0003 | 0.001 | 0.0008 | 0.003 | 0.002 | 0.007 | |
| Herengracht | 0.0002 | 0.002 | 0.0006 | 0.004 | 0.002 | 0.01 | |
| Prinsengracht | 0.001 | 0.007 | 0.003 | 0.02 | 0.006 | 0.04 | |
| IJmeer | Giardia | 0.03 | 0.09 | 0.09 | 0.3 | 0.2 | 0.6 |
| Amstel | 0.06 | 0.2 | 0.2 | 0.5 | 0.4 | 1.2 | |
| Herengracht | 0.04 | 0.1 | 0.1 | 0.3 | 0.3 | 0.7 | |
| Prinsengracht | 0.06 | 0.2 | 0.2 | 0.4 | 0.4 | 1.0 | |
| Recreational sites | |||||||
| Amsterdamse Bos | Cryptosporidium | 0 | 0 | 0 | 0 | 0 | 0 |
| Gaasperplas | 0.0002 | 0.0009 | 0.0004 | 0.003 | 0.0009 | 0.006 | |
| Nieuwe Diep | 0 | 0 | 0 | 0 | 0 | 0 | |
| Sloterplas | 0.00008 | 0.0002 | 0.0002 | 0.0006 | 0.0005 | 0.002 | |
| Nieuwe Meer | 0.00004 | 0.0002 | 0.0001 | 0.0006 | 0.0003 | 0.002 | |
| Amsterdamse Bos | Giardia | 0 | 0 | 0 | 0 | 0 | 0 |
| Gaasperplas | 0.0001 | 0.001 | 0.0003 | 0.003 | 0.0007 | 0.007 | |
| Nieuwe Diep | 0.001 | 0.007 | 0.004 | 0.02 | 0.009 | 0.04 | |
| Sloterplas | 0.0004 | 0.001 | 0.001 | 0.003 | 0.003 | 0.007 | |
| Nieuwe Meer | 0.001 | 0.009 | 0.004 | 0.03 | 0.009 | 0.06 | |
FIG. 2.
The risk of infection with Cryptosporidium (Cr) and Giardia (Gi) in surface water in Amsterdam for mean (a) and maximum (b) concentrations detected at nonrecreational sites (Pr, Prinsengracht; He, Herengracht; Am, Amstel; IJ, IJmeer) and mean (c) and maximum (d) concentrations detected at recreational sites (Nd, Nieuwe Diep; Sp, Sloterplas; Nm, Nieuwe Meer; Ab, Amsterdamse Bos; Ga, Gaasperplas).
DISCUSSION
Water quality.
The water in the Amsterdam canals Prinsengracht and Herengracht is fecally contaminated. Testing for compliance with the stringent standards outlined in revised European Bathing Water Directive 2006/7/EC demonstrated that water quality was poor and unsuitable for safe swimming. The water contained high numbers of fecal indicators, and the presence of the waterborne pathogens Salmonella, Campylobacter, Cryptosporidium, Giardia, rotavirus, norovirus, enterovirus, and reovirus was confirmed in several samples. International literature does not provide much information on the microbiological quality of canal water in other Western cities. In the 1980s, Aeromonas sobria was isolated from canal water in London (42). The water isolates could be related to fecal isolates from children in a children's hospital. Some other papers report the presence of pathogens in city canals in less developed countries. Large numbers of Enterobacteriaceae, including a wide range of Salmonella serotypes, were found in the city canals in Jakarta (25). In a Thai study, Campylobacter was detected in the canals of the Bangkok Metropolitan Area (22), whereas Giardia cysts were found in Mexico City canals (32). However, in these parts of the world, city canals serve different purposes than canals in cities in developed countries, such as washing, drinking, and cooking, and are subject to other contamination sources, like the disposal of raw waste by the part of the population that is not connected to the sewer systems. The Amsterdam canals are not designated for recreation, but exposure does occur through professional, accidental, and purposeful contact. In the cases of the last two, information should be provided to the public to prevent exposure, whereas in the case of the first, advice should be given on the protection of, e.g., professional divers (49).
Contamination sources.
The previously reported relation between heavy rainfall and increased numbers of fecal indicators in surface water (24, 45) was confirmed for the Amsterdam canals. High fecal indicator concentrations, the detection of elevated numbers of Cryptosporidium and Giardia (oo)cysts, and the occurrence of Salmonella and culturable enteroviruses appeared to be related to rainfall events of high intensity. During these events, dirt from the streets is washed into the canals, sewage systems may overflow, and discharged water from nearby polders containing runoff from agricultural land is transported into the canals. High fecal indicator counts and rainfall were less obviously related in the recreational lakes in Amsterdam. Only extreme rainfall intensity caused peaks in indicator levels at all recreational sites, suggesting that the lakes are subject to sewage overflow and surface runoff to a lesser extent than the nonrecreational sites.
The assay applied for the detection of culturable entero- and reoviruses detects only human viruses, indicating that the culturable enteric viruses in the canals and the Amstel were most likely of human origin (37). Enteroviruses (29) and reoviruses (31) have been demonstrated to be the cause of meningitis in humans, and therefore, their presence in the Amsterdam canals may pose a health threat to people who are exposed to this water. However, because of limited quantitative virus data and the unavailability of dose-response parameters for reoviruses, no attempts were made to estimate the risk of infection with these viruses. The molecular detection of pathogenic rota- and noroviruses indicates the possible presence of infectious virus particles. Moreover, it has been shown that at low levels of norovirus PCR-detectable units, both infection and illness may be established in human volunteers (35).
The isolated Salmonella species can cause human GE, and their presence therefore poses a health risk. Salmonella serovars Virchow and Newport, which were isolated from the Amstel, have been isolated from both animal and human samples in The Netherlands (39, 40). Increased antibiotic resistance of these Salmonella types, which was observed in France (17) and the United States (38), has not been observed in The Netherlands to date (39, 40). In The Netherlands, Salmonella serovar Typhimurium phagetype 690, isolated from the Herengracht canal, has been found mainly in doves, but also in humans (W. van Pelt, RIVM, personal communication).
The detection of Campylobacter was frequent at all nonrecreational sampling sites and was not related to heavy rainfall events, suggesting that sources such as sewage overflow and runoff from agricultural land and streets play a less profound role. Large numbers of ducks and gulls are regularly observed in the Amsterdam canals. Considering the frequent isolation of Campylobacter from various birds (15, 34, 41, 52, 60), including ducks and gulls, the direct input of bird feces may be an important source of Campylobacter contamination of the canal water. Bird types may be zoonotic and pose a potential risk for public health.
The levels of fecal indicator parameters in the canal with houseboats (Prinsengracht) and the canal without houseboats (Herengracht) were almost equal and did not suggest that houseboats contributed to the fecal contamination of canals to a larger extent than other sources did. However, Cryptosporidium and especially Giardia numbers were much higher in the Prinsengracht canal than in the Herengracht canal, suggesting that wastewater from houseboats may have been a source of these parasites. Moreover, the Prinsengracht canal was the only site for which there was no correlation between parasite concentrations and rainfall, suggesting that sources other than sewage overflow caused contamination of the water. Data on the prevalence of Giardia infections among people who live on houseboats are not available. Typing could have provided more information on the origin of the isolated Giardia cysts but was not included in the original assignment. Retrospective genotyping of (oo)cysts present in the stored remainder of the concentrated samples failed due to low (oo)cyst numbers in the concentrates and the limited sensitivity of the available molecular methods.
Risk assessment.
Our data indicate that there is a health risk for occupational divers and people who are accidentally exposed to pathogens in the water of the Amsterdam canals. For divers swallowing maximum volumes of approximately 6 ml canal water (49), the estimated infection risk per dive is generally low (0.0002 to 0.001% for Cryptosporidium; 0.04 to 0.06% for Giardia). Exposure to incidental peak concentrations that were detected in the canal Prinsengracht and the river Amstel may, however, result in higher infection risks per dive (0.007% for Cryptosporidium and 0.2% for Giardia). Most pathogens detected in the canals cause mild illness such as GE; prevention of infection can be achieved by minimizing the ingested volume of water as much as possible. Wearing a full face mask provides more protection than an ordinary diving mask (49). People who are accidentally exposed to the canal water and presumably swallow more water than divers, but at most the same volume as nonadult swimmers (37 ml) (23), are particularly at risk for an infection with Giardia. When cyst concentrations peak, like in the Prinsengracht canal and the Amstel, the infection risk is 1.0 to 1.2% per exposure event, indicating that approximately 1 in 100 exposed persons may become infected.
The recreational lakes in Amsterdam contained viable Cryptosporidium and Giardia (oo)cysts, despite their compliance with European bathing water legislation. The concentrations were lower than those in canals, and consequently, the estimated risks of infection per exposure event were generally 10- to 1,000-fold lower. The risk estimates were consistent with results reported by Coupe et al. (19), who estimated the risk of infection with Cryptosporidium and Giardia associated with swimming in surface water near Paris, France. They reported infection risks below 0.01% when (oo)cyst concentrations were less than 2 per 10 liters. These concentrations were observed both in Paris and in Amsterdam recreational lakes. (Oo)cyst concentrations of 2 or more per 10 liters, found in canal water in Amsterdam and river water near Paris, resulted in risks of infection of 0.01% or more.
From a prospective population-based cohort study, the GE incidence for the general population in The Netherlands was estimated to be 283 per 1,000 person-years, indicating an average annual GE risk of 28% (21). The case control study that was nested in this cohort study yielded estimated average risks of GE due to Giardia and Cryptosporidium infections of 1.4% and 0.6%, respectively. For Giardia, infection risks due to exposure to canal water were estimated in our study to be approximately 2 to 50 times lower. However, when cyst concentrations peak, the infection risk per exposure event is in the same order of magnitude as reported by de Wit et al. (21), namely 1.0 to 1.2%. For Cryptosporidium, the estimated infection risks due to canal water exposure are 15 to 10,000 times below the baseline level of 0.6%. For the recreational lakes, the estimated risks of infection per exposure event are 10 to 1,000 times lower than those estimated for canal water exposure. Although the frequency of exposure to canal water and recreational lakes in Amsterdam is unknown, the number of GE cases caused by Giardia or Cryptosporidium as a result of contact with these waters will most likely not exceed the baseline level of Giardia or Cryptosporidium GE cases in the general Dutch population and will go unnoticed.
The infection risks we report here may be overestimated since a fraction of the (oo)cysts that were considered viable based on the outcome of the applied viability tests may not be infectious. It has been demonstrated that the results of viability assays do not always correlate with the outcome of in vivo (43) and in vitro infectivity assays (16). Moreover, parasite genotypes could not be confirmed and a fraction of the (oo)cysts may belong to species other than those infectious to humans. It should also be noted that Cryptosporidium and Giardia analyses in the recreational lakes obtained many zero counts, resulting in increased uncertainties in infection risk estimates.
The results of this study demonstrate the presence of waterborne pathogens in surface water in Amsterdam and show that both occupational and accidental exposure to water in the Amsterdam canals may pose a health risk. Although the Amsterdam canals are unique, their microbiological status may not be very different from canals in other developed countries, and therefore, the data presented here may be of use for health care workers in other cities. The presence of Cryptosporidium and Giardia in recreational lakes may pose a possible health risk for bathers, despite fecal indicator parameters indicating safe swimming.
Acknowledgments
This study was performed by the order and for the account of the Municipal Health Services (GGD) of Amsterdam.
We appreciate the input of Harold van den Berg, Ronald Italiaander, Willemijn Lodder, Saskia Rutjes, Ria de Bruin, and Sylvain Skraber in sample analysis and the assistance of Leonard Bik in creating the figures.
Footnotes
Published ahead of print on 15 February 2008.
REFERENCES
- 1.Anonymous. 1976. Council directive 76/160/EEC of 8 December 1975 concerning the quality of bathing water. Off. J. Eur. Communities L31:1-7. [Google Scholar]
- 2.Anonymous. 1982. Bacteriologisch onderzoek van oppervlaktewater: kwantificeren van thermotolerante bacteriën van de coligroep met behulp van membraanfiltratie. NEN 6750. Nederlands Normalisatie Instituut, Delft, The Netherlands.
- 3.Anonymous. 1991. Water quality: sampling. Part 5: guidance on sampling of drinking water and water used for food and beverage processing. ISO 5667-5. International Organization for Standardization, Geneva, Switzerland.
- 4.Anonymous. 1995. Bacteriologisch onderzoek van water: kwantificeren van fecale streptococcen door membraanfiltratie. NEN 6274. Nederlands Normalisatie Instituut, Delft, The Netherlands.
- 5.Anonymous. 1995. Water quality: detection and enumeration of bacteriophages. Part 1: enumeration of F-specific RNA bacteriophages. ISO 10705-1. International Organization for Standardization, Geneva, Switzerland.
- 6.Anonymous. 2000. Water quality: detection and enumeration of Escherichia coli and coliform bacteria. Part 1: membrane filtration method. ISO 9308-1. International Organization for Standardization, Geneva, Switzerland.
- 7.Anonymous. 2000. Water quality: detection and enumeration of intestinal enterococci. Part 2: membrane filtration method. ISO 7899-2. International Organization for Standardization, Geneva, Switzerland.
- 8.Anonymous. 2000. Water quality: detection and enumeration of bacteriophages. Part 2: enumeration of somatic coliphages. ISO 10705-2. International Organization for Standardization, Geneva, Switzerland.
- 9.Anonymous. 2002. Microbiology of food and animal feeding stuffs: horizontal method for the detection of Salmonella spp. ISO 6579. International Organization for Standardization, Geneva, Switzerland.
- 10.Anonymous. 2005. Water quality: detection and enumeration of thermotolerant Campylobacter species. ISO 17995. International Organization for Standardization, Geneva, Switzerland.
- 11.Anonymous. 2006. Water quality: isolation and identification of Cryptosporidium oocysts and Giardia cysts from water. ISO 15553. International Organization for Standardization, Geneva, Switzerland.
- 12.Anonymous. 2006. Directive 2006/7/EC of the European Parliament and of the Council of 15 February 2006 concerning the management of bathing water quality and repealing directive 76/160/EEC. Off. J. Eur. Union L64:37-51. [Google Scholar]
- 13.Boom, R., C. J. Sol, M. M. Salimans, C. L. Jansen, P. M. Wertheim van Dillen, and J. S. O. van der Noordaa. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28:495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosch, A., G. Sánchez, F. Le Guyader, H. Vanaclocha, L. Haugarreau, and R. M. Pintó. 2001. Human enteric viruses in coquina clams associated with a large hepatitis A outbreak. Water Sci. Technol. 12:61-66. [PubMed] [Google Scholar]
- 15.Broman, T., H. Palmgren, S. Bergström, M. Sellin, J. Waldenström, M.-L. Danielsson-Tham, and B. Olsen. 2002. Campylobacter jejuni in black-headed gulls (Larus ridibundus): prevalence, genotypes, and influence on C. jejuni epidemiology. J. Clin. Microbiol. 40:4594-4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bukhari, Z., M. M. Marshall, D. G. Korich, C. R. Fricker, H. V. Smith, J. Rosen, and J. L. Clancy. 2000. Comparison of Cryptosporidium parvum viability and infectivity assays following ozone treatment of oocysts. Appl. Environ. Microbiol. 66:2972-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cailhol, J., R. Lailler, P. Bouvet, S. la Vieille, F. Gauchard, P. Sanders, and A. Brisabois. 2006. Trends in antimicrobial resistance phenotypes in non-typhoid Salmonellae from human and poultry origins in France. Epidemiol. Infect. 134:171-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell, A. T., L. J. Robertson, and H. V. Smith. 1992. Viability of Cryptosporidium parvum oocysts: correlation of in vitro excystation with inclusion or exclusion of fluorogenic vital dyes. Appl. Environ. Microbiol. 58:3488-3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coupe, S., K. Delabre, R. Pouillot, S. Houdart, M. Santillana-Hayat, and F. Derouin. 2006. Detection of Cryptosporidium, Giardia and Enterocytozoon bieneusi in surface water, including recreational areas: a one-year prospective study. FEMS Immunol. Med. Microbiol. 47:351-359. [DOI] [PubMed] [Google Scholar]
- 20.Craun, G. F., R. L. Calderon, and M. F. Craun. 2005. Outbreaks associated with recreational water in the United States. Int. J. Environ. Health Res. 15:243-262. [DOI] [PubMed] [Google Scholar]
- 21.de Wit, M. A. S., M. P. G. Koopmans, L. M. Kortbeek, W. J. B. Wannet, J. Vinjé, F. van Leusden, A. I. M. Bartelds, and Y. T. H. P. van Duynhoven. 2001. Sensor, a population-based cohort study on gastroenteritis in the Netherlands: incidence and etiology. Am. J. Epidemiol. 154:666-674. [DOI] [PubMed] [Google Scholar]
- 22.Dhamabutra, N., P. Kamol-Rathanakul, and K. Pienthaweechai. 1992. Isolation of Campylobacters from the canals of Bangkok metropolitan area. J. Med. Assoc. Thai. 75:350-364. [PubMed] [Google Scholar]
- 23.Dufour, A. P., O. Evans, T. D. Behymer, and R. Cantú. 2006. Water ingestion during swimming activities in a pool: a pilot study. J. Water Health 4:425-430. [PubMed] [Google Scholar]
- 24.Goyal, S. M., C. P. Gerba, and J. L. Melnick. 1977. Occurrence and distribution of bacterial indicators and pathogens in canal communities along the Texas coast. Appl. Environ. Microbiol. 34:139-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gracey, M., P. Ostergaard, S. W. Adnan, and J. B. Iveson. 1979. Faecal pollution of surface waters in Jakarta. Trans. R. Soc. Trop. Med. Hyg. 73:306-308. [DOI] [PubMed] [Google Scholar]
- 26.Graczyk, T. K., R. Fayer, J. M. Trout, E. J. Lewis, C. A. Farley, I. Sulaiman, and A. A. Lal. 1998. Giardia sp. cysts and infectious Cryptosporidium parvum oocysts in the feces of migratory Canada geese (Branta canadensis). Appl. Environ. Microbiol. 64:2736-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guix, S., S. Caballero, C. Villena, R. Bartolomé, C. Latorre, N. Rabella, M. Simó, A. Bosch, and R. M. Pintó. 2002. Molecular epidemiology of astrovirus infection in Barcelona, Spain. J. Clin. Microbiol. 40:133-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haas, C. N. 1983. Estimation of the risk due to low doses of microorganisms: a comparison of alternative methodologies. Am. J. Epidemiol. 118:573-582. [DOI] [PubMed] [Google Scholar]
- 29.Hauri, A. M., M. Schimmelpfennig, M. Walter-Domes, A. Letz, S. Diedrich, J. Lopez-Pila, and E. Schreier. 2005. An outbreak of viral meningitis associated with a public swimming pond. Epidemiol. Infect. 133:291-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.International Life Sciences Institute. 2000. Revised framework for microbial risk assessment. ILSI Press, Washington, DC.
- 31.Johansson, P. J., T. Sveger, K. Ahlfors, J. Ekstrand, and L. Svensson. 1996. Reovirus type 1 associated with meningitis. Scand. J. Infect. Dis. 28:117-120. [DOI] [PubMed] [Google Scholar]
- 32.Juárez-Figueroa, L. A., J. Silva-Sánchez, F. J. Uribe-Salas, and E. Cifuentes-García. 2003. Micrbiological indicators of water quality in the Xochimilco canals, Mexico City. Salud Pública Mex. 45:389-395. [DOI] [PubMed] [Google Scholar]
- 33.Karanis, P., C. Kourenti, and H. Smith. 2007. Waterborne transmission of protozoan parasites: a worldwide review of outbreaks and lessons learnt. J. Water Health 05:1-38. [DOI] [PubMed] [Google Scholar]
- 34.Kuhn, R. C., C. M. Rock, and K. H. Oshima. 2002. Occurrence of Cryptosporidium and Giardia in wild ducks along the Rio Grande river valley in southern New Mexico. Appl. Environ. Microbiol. 68:161-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindesmith, L., C. Moe, S. Marionneau, N. Ruvoen, X. Jiang, L. Lindblad, P. Stewart, J. LePendu, and R. Baric. 2003. Human susceptibility and resistance to Norwalk virus infection. Nat. Med. 9:548-553. [DOI] [PubMed] [Google Scholar]
- 36.Lodder, W. J., J. Vinjé, R. van de Heide, A. M. de Roda Husman, E. J. T. M. Leenen, and M. P. G. Koopmans. 1999. Molecular detection of Norwalk-like caliciviruses in sewage. Appl. Environ. Microbiol. 65:5624-5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lodder, W. J., and A. M. de Roda Husman. 2005. Presence of norovirus and other enteric viruses in sewage and surface waters in The Netherlands. Appl. Environ. Microbiol. 71:1453-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopes, V. C., S. D. Wedel, J. B. Bender, K. E. Smith, F. T. Leano, D. J. Boxrud, D. C. Lauer, B. T. Velayudhan, and K. V. Nagaraja. 2006. Emergence of multidrug-resistant Salmonella enterica serotype Newport in Minnesota. Clin. Infect. Dis. 43:210-213. [DOI] [PubMed] [Google Scholar]
- 39.Mevius, D. J., C. Pelicaan, and W. van Pelt (ed.). 2004. Maran 2004: monitoring of antimicrobial resistance and antibiotic usage in animals in The Netherlands in 2004. CIDC, Lelystad, The Netherlands.
- 40.Mevius, D. J., and W. van Pelt (ed.). 2005. Maran 2005: monitoring of antimicrobial resistance and antibiotic usage in animals in The Netherlands in 2005. CIDC, Lelystad, The Netherlands.
- 41.Moore, J. E., D. Gilpin, E. Crothers, A. Canney, A. Kaneko, and M. Matsuda. 2002. Occurence of Campylobacter spp. and Cryptosporidium spp. in seagulls (Larus spp.). Vector Borne Zoonotic Dis. 2:111-114. [DOI] [PubMed] [Google Scholar]
- 42.Nazer, H., E. Price, G. Hunt, U. Patel, and J. Walker-Smith. 1990. Isolation of Aeromonas spp. from canal water. Indian J. Pediatr. 57:115-118. [DOI] [PubMed] [Google Scholar]
- 43.Neumann, N. F., L. L. Gyürék, L. Gammie, G. R. Finch, and M. Belosevic. 2000. Comparison of animal infectivity and nucleic acid staining for assessment of Cryptosporidium parvum viability in water. Appl. Environ. Microbiol. 66:406-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pavlásek, I. 1993. The black-headed gull (Larus ridibundus L.), a new host for Cryptosporidium baileyi (Apicomplexa: Cryptosporidiidae). Vet. Med. (Praha) 38:629-638. (In Czech.) [PubMed] [Google Scholar]
- 45.Rechenburg, A., C. Koch, T. Classen, and T. Kistemann. 2006. Impact of sewage treatment plants and combined sewer overflow basins on the microbiological quality of surface water. Water Sci. Technol. 54:95-99. [DOI] [PubMed] [Google Scholar]
- 46.Rimhanen-Finne, R., H. L. Enemark, J. Kolehmainen, P. Toropainen, and M. L. Hänninen. 2007. Evaluation of immunofluorescence microscopy and enzyme-linked immunosorbent assay in detection of Cryptosporidium and Giardia infections in asymptomatic dogs. Vet. Parasitol. 145:345-348. [DOI] [PubMed] [Google Scholar]
- 47.Schets, F. M., M. During, R. Italiaander, L. Heijnen, S. A. Rutjes, W. K. van der Zwaluw, and A. M. de Roda Husman. 2005. Escherichia coli O157:H7 in drinking water from private water supplies in the Netherlands. Water Res. 39:4485-4493. [DOI] [PubMed] [Google Scholar]
- 48.Schets, F. M., and A. M. de Roda Husman. 2007. Gezondheidsklachten gerelateerd aan recreatie in oppervlaktewater, zomer 2005. Infectieziekten Bull. 18:55-60. [Google Scholar]
- 49.Schijven, J. F., and A. M. de Roda Husman. 2006. A survey of diving behavior and accidental water ingestion among Dutch occupational and sport divers to assess the risk of infection with waterborne pathogenic microorganisms. Environ. Health Perspect. 114:712-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwab, K. J., R. de Leon, and M. D. Sobsey. 1995. Concentration and purification of beef extract mock eluted from water samples for the detection of enteroviruses, hepatits A virus, and Norwalk virus by reverse transcriptase-PCR. Appl. Environ. Microbiol. 61:531-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shukla, R., P. Giraldo, A. Kraliz, M. Finnigan, and A. L. Sanchez. 2006. Cryptosporidium spp. and other zoonotic enteric parasites in a sample of domestic dogs and cats in the Niagara region of Ontario. Can. Vet. J. 47:1179-1184. [PMC free article] [PubMed] [Google Scholar]
- 52.Smith, H. V., J. Brown, J. C. Coulson, G. P. Morris, and R. W. Girdwood. 1993. Occurrence of oocysts of Cryptosporidium sp. in Larus spp. gulls. Epidemiol. Infect. 110:135-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teunis, P. F. M., O. G. van der Heijden, J. W. B. van der Giessen, and A. H. Havelaar. 1996. The dose-response relation in human volunteers for gastro-intestinal pathogens. RIVM report nr. 284550002. National Institute for Public Health and the Environment, Bilthoven, The Netherlands.
- 54.Thiriat, L., F. Sidaner, and J. Schwartzbrod. 1998. Determination of Giardia cyst viability in environmental and faecal samples by immunofluorescence, fluorogenic dye staining and differential interference contrast microscopy. Lett. Appl. Microbiol. 26:237-242. [DOI] [PubMed] [Google Scholar]
- 55.van den Berg, H.,. W. Lodder, W. van der Poel, H. Vennema, and A. M. de Roda Husman. 2005. Genetic diversity of noroviruses in raw and treated sewage water. Res. Microbiol. 156:532-540. [DOI] [PubMed] [Google Scholar]
- 56.van der Poel, W. H. M., F. Verschoor, R. van der Heide, M. I. Herrera, A. Vivo, M. Kooreman, and A. M. de Roda Husman. 2001. Hepatitis E virus sequences in swine related to sequences in humans, the Netherlands. Emerg. Infect. Dis. 7:970-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Olphen, M., J. G. Kapsenberg, E. van de Baan, and W. A. Kroon. 1984. Removal of enteric viruses from surface water at eight waterworks in The Netherlands. Appl. Environ. Microbiol. 47:927-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vennema, H., E. de Bruin, and M. Koopmans. 2002. Rational optimization of generic primers used for Norwalk-like virus detection by reverse transcriptase polymerase chain reaction. J. Clin. Virol. 25:233-235. [DOI] [PubMed] [Google Scholar]
- 59.Villena, C., W. M. El-Senousy, F. X. Abad, R. M. Pinto, and A. Bosch. 2003. Group A rotavirus in sewage samples from Barcelona and Cairo: emergence of unusual genotypes. Appl. Environ. Microbiol. 69:3919-3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Waldenström, J., T. Broman, I. Carlsson, D. Hasselquist, R. P. Achterberg, J. A. Wagenaar, and B. Olsen. 2002. Prevalence of Campylobacter jejuni, Campylobacter lari, and Campylobacter coli in different ecological guilds and taxa of migrating birds. Appl. Environ. Microbiol. 68:5911-5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.World Health Organization. 2003. Guidelines for safe recreational water environments. Volume 1: coastal and fresh waters. World Health Organization, Geneva, Switzerland.