Abstract
The ectomycorrhizal basidiomycete Tricholoma matsutake produces commercially valuable fruit bodies, matsutake, in forests. Here we report a PCR system targeting retroelement integration sites to differentiate among individual Asian isolates of T. matsutake based on their geographical origins, such as Japan, the area of South Korea through North Korea, the northeastern provinces of China, and the area of the southwestern provinces of China through Bhutan. The overall misjudgment rate of the analytical system was approximately 5% based on 95 samples of T. matsutake examined including those from cultures and from commodities. We also provide evidence that T. matsutake isolates grown throughout the Far East, including the northeastern provinces of China, are closely related to each other while distinct from those in the area of the southwestern provinces of China through Bhutan. The method allows us to trace back geographical origins of Asian matsutake, thus contributing to food safety, appropriate tariffs, and proper price setting.
Tricholoma matsutake is an ectomycorrhizal basidiomycete that produces economically important edible mushrooms, matsutake, in forests (11, 12, 24). The annual yield of matsutake is estimated to be 2,000 tons worldwide, with its total retail value amounting to approximately $500 million, most of which is consumed by the Japanese (11). In Japan, although the annual yield of matsutake had once climbed up to 12,000 tons in the year 1941, the yield in the past decade was about 50 to 100 tons per year (12, 35). Such a devastating decline in the yield of matsutake has also occurred in South Korea (35). Currently, a large portion of matsutake traded in Japan is imported from China (Ministry of Agriculture, Forestry, and Fisheries of Japan). Before the economic sanctions, North Korea was another main exporter of matsutake (35). Mediterranean and American matsutake mushrooms are also being imported (35). These non-Asian matsutake mushrooms are, however, morphologically distinct from Asian ones (12, 13, 24).
Traceability is one of the most serious issues faced in the market for matsutake in Japan. In general, domestic matsutake and South Korean matsutake are traded at a premium and at a semipremium, respectively. For example, the former products are traded at the price of approximately $500/kg and the latter are traded at $250/kg recently, while the Chinese counterpart is traded at $100/kg. Tariffs are imposed depending upon exporting countries. For example, as of the year 2007, products from China and Bhutan are free of extra charge, whereas those from South and North Korea are subjected to customs duties of 4 and 5%, respectively (Ministry of Finance of Japan). Food safety is another concern, for safety regulations vary among countries. To prevent falsification of the geographical origin of matsutake, a simple method, such as a PCR-based analytical system, to efficiently differentiate among Asian isolates of T. matsutake has been demanded.
Retroelements are retrovirus-like DNA parasites associated with host eukaryotic genomes, which replicate through an RNA intermediate and integrate into multiple genomic loci, exerting great influence on the genome structure and function (4, 5, 7, 8, 23, 26, 28, 31). Such a transposition process through copy-and-paste, rather than through cut-and-paste, leaves stable positive markers of genome evolution that are inherited by the host's descendants (4, 5, 7, 8, 23, 26, 28, 31). We previously reported that interretrotransposon amplified polymorphism (IRAP) analysis using as a genetic marker the long terminal repeat (LTR) of the gypsy-type retroelement marY1, designated σmarY1, which is accumulated in the genome of T. matsutake, could specify strains of the symbiont by exhibiting highly polymorphic fingerprints (16, 18, 20). The analysis, however, fails to inform us of subtle differences among biogeographical groups of individual Asian isolates of T. matsutake (18). The failure of biogeographical typing, or geotyping, through IRAP may be attributed to highly polymorphic fingerprints that may obscure some σmarY1-associated markers generated upon the occurrence of biogeographical diversification of T. matsutake. In the present study, we explored the structure of σmarY1 insertion sites for use as genetic markers useful in tracing the geographical origin of Asian matsutake.
MATERIALS AND METHODS
Fungal strains.
Table 1 contains descriptions of T. matsutake strains used in this study. T. matsutake mycelia were cultured after isolation from fruit bodies on slants of the modified MMN medium containing V8 juice (MMNV8) at 25°C (22). Subcultures have been transferred once a year onto the fresh slants, grown at 25°C, and maintained at 10°C.
TABLE 1.
Tricholoma matsutake strains used in this study
| Strain | Geographical origina | Culture (L) or commodity (M) | Yr of isolation | Lane no. (figure[s]) | σmarY1-based geotype | Reference(s) |
|---|---|---|---|---|---|---|
| TM-15 | Iwate, Japan | L | 1992 | 1 (Fig. 3) | A1 | 18 |
| Y1b | Ibaraki, Japan | L (ourselves)c | 1993 | 2 (Fig. 3) | A1 | 34 |
| Y4 | Ibaraki, Japan | L (ourselves) | 1996 | 3 (Fig. 3) | A1 | 18 |
| F1 | Fukushima, Japan | L (ourselves) | 1993 | 4 (Fig. 3) | A1 | 18 |
| Tm-8 | Kyoto, Japan | L | 1992 | 5 (Fig. 3) | A1 | 18 |
| MR32 | Hyogo, Japan | L | 1987 | 6 (Fig. 3) | A1 | 18 |
| Tm029 | Shiga, Japan (shiro-#S1)d | L (ourselves) | 1983 | 7/2 (Fig. 3/4) | A1 | 18, 20 |
| OK-T4 | Okayama, Japan | L | 1992 | 8 (Fig. 3) | A1 | 18 |
| Tm-T4 | Tokushima, Japan | L | 1993 | 9 (Fig. 3) | A2 | 18 |
| Tm-H507 | Hiroshima, Japan | L | 1997 | 10 (Fig. 3) | A2 | 21 |
| Tm-H102 | Hiroshima, Japan | L | 1992 | 11 (Fig. 3) | A2 | 18 |
| Tm-Y59AFB | Yamaguchi, Japan | L | 1984 | 12 (Fig. 3) | A2 | 18 |
| Tm-Y62B | Yamaguchi, Japan | L | 1984 | 13/1 (Fig. 3/5) | A2 | 18 |
| K1 | South Korea | L | 1997 | 14 (Fig. 3) | A3 | 18 |
| Tm-K2 | South Korea | L | 1992 | 15 (Fig. 3) | A3 | 18 |
| Tm-31 | Gyeongsangbuk-doe (Kyongsan North), South Korea | L (ourselves) | 1998 | 16, 37 (Fig. 3) | A3 | 18 |
| Tm-156 | Gyeongsangbuk-do, South Korea | L (ourselves) | 2004 | 17 (Fig. 3) | A3 | 18 |
| Tm-157 | Gyeongsangbuk-do, South Korea | L (ourselves) | 2004 | 18 (Fig. 3) | A3 | 18 |
| Tm-158 | Gyeongsangbuk-do, South Korea | L (ourselves) | 2004 | 19/2 (Fig. 3/5) | A3 | 18 |
| SKR02 | South Korea | M | 2006 | 20 (Fig. 3) | A3 | This study |
| SKR03 | South Korea | M | 2006 | 21 (Fig. 3) | A3 | This study |
| NK1 | North Korea | L | 1998 | 22/3 (Fig. 3/5) | A2 | 18 |
| NKR02 | North Korea | M | 2006 | 23/4 (Fig. 3/5) | A3 | This study |
| NKR03 | North Korea | M | 2006 | 24 (Fig. 3) | A1 | This study |
| NKR04 | North Korea | M | 2006 | 25 (Fig. 3) | A1 | This study |
| NKR05 | North Korea | M | 2006 | 26 (Fig. 3) | A3 | This study |
| CN01 | Yunnan, China | M | 2006 | 27/25 (Fig. 3/5) | B | This study |
| CHI1 | China | L | 1998 | 28 (Fig. 3) | B | 18 |
| Tm-9 | China | L | 1992 | 29 (Fig. 3) | B | 18 |
| CH381 | China | L | 2003 | 30 (Fig. 3) | B | 18 |
| CH382 | China | L | 2003 | 31 (Fig. 3) | B | 18 |
| CH383 | China | L | 2003 | 32 (Fig. 3) | B | 18 |
| CH384 | China | L | 2003 | 33 (Fig. 3) | B | 18 |
| CH385 | China | L | 2003 | 34 (Fig. 3) | B | 18 |
| CH387 | China | L | 2003 | 35 (Fig. 3) | B | 18 |
| BH1 | Bhutan | L | 1998 | 36 (Fig. 3) | B | 18 |
| K3 | South Korea | L | 2003 | 38 (Fig. 3) | A3 | 18 |
| K4 | South Korea | L | 2003 | 39 (Fig. 3) | A3 | 18 |
| Tm159 | Gyeongsangbuk-do, South Korea | L (ourselves) | 2004 | 40 (Fig. 3) | A3 | 18 |
| SKR01 | South Korea | M | 2006 | 41 (Fig. 3) | A3 | This study |
| SKR04 | South Korea | M | 2006 | 42 (Fig. 3) | A3 | This study |
| SKR05 | South Korea | M | 2006 | 43 (Fig. 3) | A1 | This study |
| NKR01 | North Korea | M | 2006 | 44 (Fig. 3) | A1 | This study |
| Tm027 | Shiga, Japan (shiro-#M3)f | L (ourselves) | 1983 | 1 (Fig. 4) | A2 | 20 |
| Tm040 | Shiga, Japan (shiro-#M7) | L (ourselves) | 1991 | 3 (Fig. 4) | A2 | 20 |
| Tm043 | Shiga, Japan (shiro-#M7) | L (ourselves) | 1991 | 4 (Fig. 4) | A1 | 20 |
| Tm050 | Shiga, Japan (shiro-#S6) | L (ourselves) | 1991 | 5 (Fig. 4) | A1 | 20 |
| Tm068 | Shiga, Japan (shiro-#S6) | L (ourselves) | 1993 | 6 (Fig. 4) | A1 | 20 |
| Tm077 | Shiga, Japan (shiro-#S6) | L (ourselves) | 1994 | 7 (Fig. 4) | A1 | 20 |
| Tm078 | Shiga, Japan (shiro-#S6) | L (ourselves) | 1994 | 8 (Fig. 4) | A1 | 20 |
| Tm082 | Shiga, Japan (shiro-#S6) | L (ourselves) | 1994 | 9 (Fig. 4) | A1 | 20 |
| Tm069 | Shiga, Japan (shiro-#S6) | L (ourselves) | 1994 | 10 (Fig. 4) | A1 | 20 |
| Tm072 | Shiga, Japan (shiro-#S6) | L (ourselves) | 1994 | 11 (Fig. 4) | A1 | 20 |
| Tm074 | Shiga, Japan (shiro-#S6) | L (ourselves) | 1994 | 12 (Fig. 5) | A1 | 20 |
| Tm100 | Shiga, Japan (shiro-#S4) | L (ourselves) | 1998 | 13 (Fig. 4) | A2 | 20 |
| Tm127 | Shiga, Japan (shiro-#S5) | L (ourselves) | 2003 | 14 (Fig. 4) | A1 | 20 |
| Tm172 | Shiga, Japan (shiro-#S6) | L (ourselves) | 2004 | 15 (Fig. 4) | A1 | 20 |
| Tm195 | Shiga, Japan (shiro-#S10) | L (ourselves) | 2005 | 16 (Fig. 5) | A1 | 20 |
| Tm196 | Shiga, Japan (shiro-#S10) | L (ourselves) | 2005 | 17 (Fig. 4) | A1 | 20 |
| AT-634 | Ibaraki, Japan (site #B1)g | L (ourselves) | 1997 | 18 (Fig. 4) | A1 | 20 |
| AT-636 | Ibaraki, Japan (site #B2) | L (ourselves) | 1997 | 19 (Fig. 4) | A1 | 20 |
| AT-639 | Ibaraki, Japan (site #B5) | L (ourselves) | 1997 | 20 (Fig. 4) | A1 | 20 |
| AT-640 | Ibaraki, Japan (site #B3) | L (ourselves) | 1997 | 21 (Fig. 4) | A1 | 20 |
| AT-641 | Ibaraki, Japan (site #B1) | L (ourselves) | 1997 | 22 (Fig. 4) | A1 | 20 |
| AT-642 | Ibaraki, Japan (site #B5) | L (ourselves) | 1997 | 23 (Fig. 4) | A2 | 20 |
| AT-635 | Ibaraki, Japan (site #B7) | L (ourselves) | 1997 | 24 (Fig. 4) | A2 | 20 |
| AT-637 | Ibaraki, Japan (site #B4) | L (ourselves) | 1997 | 25 (Fig. 4) | A1 | 20 |
| AT-638 | Ibaraki, Japan (site #B6) | L (ourselves) | 1997 | 26 (Fig. 4) | A1 | 20 |
| IW-92602-1 | Iwate, Japan (site #W1)h | M (ourselves) | 2002 | 27 (Fig. 4) | A1 | This study |
| IW-92602-2 | Iwate, Japan (site #W1) | L (ourselves) | 2002 | 28 (Fig. 4) | A1 | 20 |
| IW-92602-4 | Iwate, Japan (site #W1) | L (ourselves) | 2002 | 29 (Fig. 4) | A1 | 20 |
| IW-100702-2 | Iwate, Japan (site #W1) | L (ourselves) | 2002 | 30 (Fig. 4) | A1 | 20 |
| IW-100702-4 | Iwate, Japan (site #W1) | L (ourselves) | 2002 | 31 (Fig. 4) | A1 | 20 |
| I-84 | Iwate, Japan (site #W2) | L (ourselves) | 2001 | 32 (Fig. 4) | A2i | 20 |
| I-114 | Iwate, Japan (site #W2) | L (ourselves) | 2001 | 33 (Fig. 4) | A1 | 20 |
| CH-HE1 | Heilongjiang, China | M | 2007 | 5 (Fig. 5) | A4 | This study |
| CH-HE2 | Heilongjiang, China | M | 2007 | 6 (Fig. 5) | A4 | This study |
| CH-HE3 | Heilongjiang, China | M | 2007 | 7 (Fig. 5) | A4 | This study |
| CH-HE4 | Heilongjiang, China | M | 2007 | 8 (Fig. 5) | A4 | This study |
| CH-HE5 | Heilongjiang, China | M | 2007 | 9 (Fig. 5) | A4 | This study |
| CH-JI1 | Jilin, China | M | 2007 | 10 (Fig. 5) | A4 | This study |
| CH-JI2 | Jilin, China | M | 2007 | 11 (Fig. 5) | A4 | This study |
| CH-JI3 | Jilin, China | M | 2007 | 12 (Fig. 5) | A4 | This study |
| CH-JI4 | Jilin, China | M | 2007 | 13 (Fig. 5) | A4 | This study |
| CH-JI5 | Jilin, China | M | 2007 | 14 (Fig. 5) | A4 | This study |
| CH-SI1 | Sichuan, China | M | 2007 | 15 (Fig. 5) | B | This study |
| CH-SI2 | Sichuan, China | M | 2007 | 16 (Fig. 5) | B | This study |
| CH-SI3 | Sichuan, China | M | 2007 | 17 (Fig. 5) | B | This study |
| CH-SI4 | Sichuan, China | M | 2007 | 18 (Fig. 5) | B | This study |
| CH-SI5 | Sichuan, China | M | 2007 | 19 (Fig. 5) | B | This study |
| CH-YU1 | Yunnan, China | M | 2007 | 20 (Fig. 5) | B | This study |
| CH-YU2 | Yunnan, China | M | 2007 | 21 (Fig. 5) | B | This study |
| CH-YU3 | Yunnan, China | M | 2007 | 22 (Fig. 5) | B | This study |
| CH-YU4 | Yunnan, China | M | 2007 | 23 (Fig. 5) | B | This study |
| CH-YU5 | Yunnan, China | M | 2007 | 24 (Fig. 5) | B | This study |
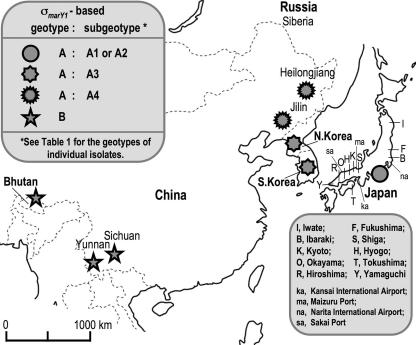
Figure 2 shows a map of Asia. Some foreign strains were obtained at local matsutake markets in Japan (K1, K3, CHI1, Tm-9, CH381 to CH385, and BH1) or obtained at Japanese Customs Service facilities (SKR01 to SKR04 and CN01 [Kansai International Airport], SKR05 [Narita International Airport], NKR02 to NKR04 [Sakai Port], and NKR05 [Maizuru Port]) or imported through a trading authority (CH-HE1 to CH-HE5, CH-JI1 to CH-JI5, CH-SI1 to CH-SI5, and CH-YU1 to CH-YU5).
Y1 (IFO33136, Institute of Fermentation [Osaka, Japan]) is a model organism that produces in vitro a rhizosphere colony that resembles naturally occurring shiro.
Isolates of matsutake that we picked by ourselves in P. densiflora woodlands are designated by “ourselves.”
Shiro is a massive persisting rhizosphere aggregate of T. matsutake mycelia and mycorrhizas. Shiro-#S is located at the Kohnan study site, Shiga prefecture, Japan.
Gyeongsangbuk-do (Kyongsan North) produces the largest amount (65%) of South Korean matsutake based on the record of the year 2004.
Shiro-#M is located at the Minakuchi study site, Shiga prefecture, Japan. The Minakuchi study site is distant from the Kohnan study site by 2.7 km.
Isolation sites B1 to B7 are located at the Morigane study site, Ibaraki prefecture, Japan.
Isolation sites W1 and W2 are located at the Yokkaichi study site, Iwate prefecture, Japan.
This isolate lacked the 493-bp DNA segment but retained the 337-bp derivative.
PCR.
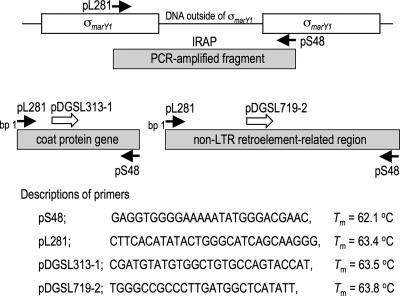
Whole genomic DNA was extracted from either fresh mycelia cultured in the MMNV8 liquid medium or mycelia surrounding gills of fruiting bodies by use of a lysis buffer containing hexadecyltrimethylammonium bromide and phenol-chloroform and stored at −20°C until use (22). PCR was carried out in 50-μl reaction mixtures containing 250 μM of deoxynucleoside triphosphates, 0.5 μM of primers, 50 ng of template DNA, and 0.5 units of Taq polymerase (Gene Taq NT; Wako Pure Chemicals, Osaka, Japan) and a universal buffer provided along with the enzyme. Cycle reactions were performed as follows: 1 cycle of 94°C for 2 min; 30 cycles of 94°C for 30 s, annealing temperature (Ta) for 30 s, and 72°C for 1 min; and 1 cycle of 72°C for 10 min in a GeneAmp 9700 (Applied Biosystems, Foster City, CA) cycler. A high-fidelity PCR was carried out using TaKaRa LA Taq (Takara Bio Inc., Otsu, Japan). The best Ta, desirably near or above melting temperature, was determined by a gradient thermal cycler using a ramp condition equivalent to that of the GeneAmp 9700 cycler (Takara Bio Inc. model TP600). Figure 1 includes descriptions of primers.
FIG. 1.
Primer design to differentiate among individual Asian isolates of T. matsutake. Locations and directions of primers are indicated by arrows: solid arrows, primers designed for IRAP previously (18); open arrows, primers designed to be used with pS48 in this study.
Gel electrophoresis.
The PCR amplicons were electrophoresed in a Tris-buffered EDTA-1.8% agarose gel containing 0.5 μg/ml ethidium bromide in a 27-cm by 42-cm apparatus (Nippon Eido, Tokyo, Japan), which was enough to run 37 samples simultaneously, at 195 V for 4.5 h (Nusieve GTG agarose-Seakem GTG agarose [3:2]; FMC BioProducts, Rockland, ME). The profiles were visualized under a long-wavelength UV light and photographed (ATTO Printgraph, Tokyo, Japan) (18, 20). DNA profiles to be used as markers of biogeographical typing, or geotyping, were reproducible based on repetition of independent PCRs for each primer set (Table 2).
TABLE 2.
Specifications of the σmarY1-based T. matsutake geotypes in Asia
| Geotype and subgeotype | Profile shown by analytical system (primer set)/size of PCR-amplified DNA (bp)a
|
Geographical distribution | ||||||
|---|---|---|---|---|---|---|---|---|
| pDGSL313-1/pS48
|
pDGSL719-2A/pS48 (457 bp) | |||||||
| 573 | 493b | 450c | 337b | 299c | 273 | |||
| A | V | + | V | + | V | V | V | Far East |
| A1 | − | + | − | + | − | V | − | Japan |
| A2 | − | + | + | + | + | V | V | Japan |
| A3 | − | + | − | + | − | V | + | Korean Peninsula |
| A4 | + | + | V | + | V | V | + | China (northeastern provinces) |
| B | − | − | − | − | − | + | − | China (southwestern provinces)-Bhutan |
DNA profiles predominant in a given geotype or subgeotype are shown (Fig. 3 to 5 show raw data). DNA profiles showing positive and negative signals of each component in gel electrophoresis are indicated by plus and minus signs, respectively. Profiles that vary depending on the isolates are indicated by V.
The nucleotide sequences of the 493-bp and the 337-bp fragments are 99% identical at bp 1 to 337 in the direction from pS48 to pDGSL313-1.
The nucleotide sequences of the 450-bp and the 299-bp fragments are 99% identical at bp 1 to 299 in the direction from pS48 to pDGSL313-1.
Evaluation of traceability.
The misjudgment rate (%) was scored as an average rate of the occurrence of confusing geotypes assigned among 95 specimens listed in Table 1. The 95% confidence interval was calculated based on binomial distribution.
Nucleotide sequencing.
Desired fragments were extracted from the agarose gels after separation by gel electrophoresis using the Wizard DNA purification system (Promega, Madison, WI), ligated with pCR2.1 vector (Invitrogen, Carlsbad, CA), and introduced into Escherichia coli JM109 competent cells. The plasmids containing the target inserts were isolated from the E. coli transformants using the Wizard Plus SV miniprep DNA purification system (Promega). After confirmation of the presence of appropriate DNA inserted in the plasmids, nucleotide sequencing was carried out in both directions using the 3130xl Genetic Analyzer (Applied Biosystems/Hitachi) with Big Dye Terminator FS core kit, version 3.1 (Applied Biosystems). Sequence data were analyzed with Genetix Mac version 13.1 and ATSQ (Software Development Inc., Tokyo, Japan), and homology search analysis was conducted with the BLASTN program provided by DDBJ.
Nucleotide sequence accession numbers.
Nucleotide sequences have been deposited in the DDBJ database under the accession numbers AB330884-96, AB360715-40, and AB363821-38.
RESULTS AND DISCUSSION
Primer design.
To design primers for a PCR system that specifies geographical origin of T. matsutake isolates, DNA segments resulting from IRAP were cloned and analyzed, making an assumption that some insertions should have occurred in the course of biogeographical diversification and are shared among individual isolates of biogeographical subgroups. A high-fidelity PCR was conducted with genomic DNA from T. matsutake Y1 or Tm-31 as a template, and a set of primers, pS48 and pL281, was designed based on the sequences of internal portions of the 426-bp σmarY1 as described previously (Fig. 1) (18). The PCR amplicons, ranging from approximately 140 bp to 700 bp, were extracted, ligated into pCR2.1, and sequenced. While the sequences associated with pS48 are conserved carrying the 5′-terminal (bp 1 to 47) sequence of σmarY1 in all the fragments analyzed, those with pL281 are diversified (AB330884-96). Therefore, we fixed pS48 for one end of the primer and designed primers to be used with pS48 for the other end based on the sequence data (Fig. 1).
Two primers, pDGSL313-1 and pDGSL719-2, were selected (Fig. 1). pDGSL313-1 was designed based on a sequence of a 313-bp DNA segment from Y1 (Fig. 1; AB330894 [bp 41 to 67]). The 313-bp DNA segment consists of sequences of a putative coat protein gene throughout (AB160897). pDGSL719-2 was designed based on a sequence of a 719-bp DNA segment from Tm-31 (Fig. 1; AB330884 [bp 263 to 288]). The 719-bp DNA segment has sequences of the non-LTR retroelement marY2N cluster at bp 101 to 596 (19) (AB047280).
pDGSL313-1/pS48 system.
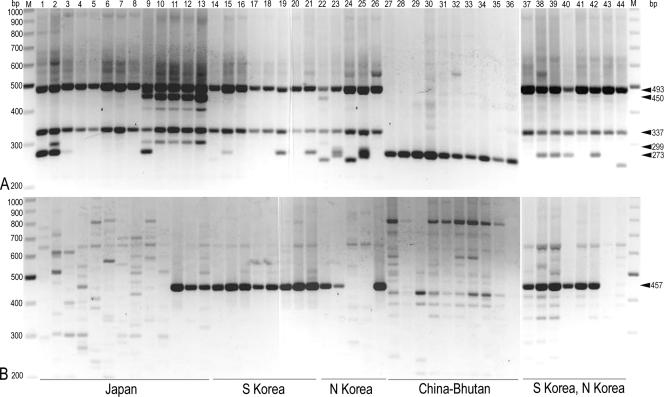
Table 1 shows the σmarY1-based geotypes of individual T. matsutake isolates, and Fig. 2 shows the relevant biogeographical map of Asian matsutake. PCR with pDGSL313-1/pS48 (Ta = 64.0°C) overall conferred two types of polymorphisms: one designated geotype A exclusively sharing the 493-bp and the 337-bp bands as the basic characteristic feature among isolates from the Far East including Japan, South Korea, and North Korea (Table 2; Fig. 3A, lanes 1 to 26 and 37 to 44) and the other designated geotype B composed solely of the 273-bp band that is specific to the isolates from the area of China through Bhutan (Table 2; Fig. 3A, lanes 27 to 36). In geotype A, subgeotype A2 was assigned to the DNA profile with the 450-bp and the 299-bp bands that are conserved among isolates from the far western part of the main island (Honshu) of Japan (Table 2; Fig. 3A, lanes 9 to 13), while subgeotype A1 and subgeotype A3, which should allow us to differentiate between Japanese and Korean isolates, were assigned to the profile based on the other analytical system with pDGSL719-2/pS48 (see below).
FIG. 2.
Biogeography of T. matsutake in Asia based on the σmarY1-based typing. The predominant distribution of T. matsutake geotypes is given. The blank map was obtained from Freemap.jp (http://www.freemap.jp/ or http://english.freemap.jp/world_paint/world_paint.html).
FIG. 3.
PCR profiles of Asian isolates of T. matsutake. After electrophoresis, the 26-cm by 30-cm agarose gel, which was enough to run 37 samples simultaneously, was cut into pieces large enough to observe the result on a 20-cm by 20-cm transilluminator. Photographs of lanes 1 to 36 and lanes 37 to 44 were taken after gel electrophoresis that was separately carried out. The molecular size (bp) of a relevant DNA band is indicated by arrowheads to the right. Geographical origins of specimens are given at the bottom. (A) pDGSL313-1/pS48 primer system. (B) pDGSL719-2/pS48 primer system. Lanes M, molecular markers (200 to 1,000 bp); lanes 1 to 44, T. matsutake strains (Table 1 shows assigned geotypes): 1, TM15; 2, Y1; 3, Y4; 4, F1; 5, Tm-8; 6, MR32; 7, Tm029; 8, OK-T4; 9, Tm-T4; 10, Tm-507; 11, Tm-H102; 12, Tm-Y59ABF; 13, Tm-Y62B; 14, K1; 15, Tm-K2; 16, Tm-31; 17, Tm-156; 18, Tm-157; 19, Tm-158; 20, SKR02; 21, SKR03; 22, NK1; 23, NKR02; 24, NKR03; 25, NKR04; 26, NKR05; 27, CN01; 28, CHI1; 29, Tm-9; 30, CH381; 31, CH382; 32, CH383; 33, CH384; 34, CH385; 35, CH387; 36, BH1; 37, Tm-31; 38, K3; 39, K4; 40, Tm-159; 41, SKR01; 42, SKR04; 43, SKR05; 44, NKR01.
pDGSL719-2/pS48 system.
PCR with pDGSL719-2/pS48 (Ta = 68.5°C) overall conferred two types of polymorphisms: one with faint polymorphic signals exhibited by most isolates from Japan and from China examined (Fig. 3B, lanes 1 to 10 and lanes 27 to 36), and the other with the intense 457-bp band distinctively exhibited by the majority of isolates from the Korean peninsula (Fig. 3B, lanes 14 to 26 and 37 to 44). The former faint signals were regarded as negative because they were unreliable nonspecific bands often encountered in PCR in general. The latter 457-bp band was solid enough to be used as a marker to differentiate between Japanese and Korean isolates, i.e., subgeotype A1 and subgeotype A3, respectively (Table 2). A few isolates from the far western part of Honshu exhibited the 457-bp band (Fig. 3B, lanes 11 to 13). These Japanese isolates, however, were regarded as subgeotype A2 and could be differentiated from Korean isolates (Fig. 3, lanes 11 to 13). In contrast, some Korean isolates were classified with the subgeotype A1-like Japanese isolates (Fig. 3, lanes 24, 25, 43, and 44), bringing about the risk of misjudgment in tracing their geographical origins (see also “Evaluation of traceability” and “Conclusion”).
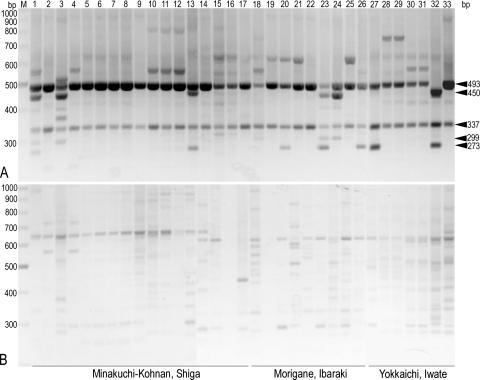
Geotyping of T. matsutake from three representative localities in Japan.
Since the Japanese commodities are the most probable target for counterfeiting, we attempted to confirm the reliability of the test to specify the Japanese subgeotypes with additional samples that we had picked by ourselves in Pinus densiflora woodlands, i.e., samples with no risk of contamination (Table 2). We assayed samples from study sites representing three major localities distantly located from each other: the Khonan-Minakuchi study site in Shiga prefecture, the Morigane site in Ibaraki, and the Yokkaichi site in Iwate (Table 1; Fig. 2) (20). The results clearly showed that isolates from all three locations in Japan, as well as domestic ones examined earlier, shared the genotypic identity of either A1 or A2 that was distinct from isolates from the continent (Fig. 4; Tables 1 and 2). It is noteworthy that Iwate and North Korea are at the same distance from Shiga, located on the same latitude and with almost similar longitudinal spans from Shiga (Fig. 2). Such is also the case with Ibaraki and South Korea (Fig. 2). Note that the Korean isolates examined earlier also include samples that we had picked by ourselves in P. densiflora woodlands in Gyeongsangbuk-do, South Korea, as well as those obtained by the Japanese Customs Service at ports and airports prior to circulation (see above) (Table 1; Fig. 3).
FIG. 4.
PCR profiles of T. matsutake from three representative localities in Japan. The molecular size (bp) of a relevant DNA band is indicated by arrowheads to the right. Geographical origins of specimens are given at the bottom. (A) pDGSL313-1/pS48 primer system. (B) pDGSL719-2/pS48 primer system. Lane M, molecular markers (300 to 1,000 bp); lanes 1 to 33, T. matsutake strains (Table 1 shows assigned geotypes): 1, Tm027; 2, Tm029; 3, Tm040; 4, Tm043; 5, Tm050; 6, Tm068; 7, Tm077; 8, Tm078; 9, Tm082; 10, Tm069; 11, Tm072; 12, Tm074; 13, Tm100; 14, Tm127; 15, Tm172; 16, Tm195; 17, Tm196; 18, AT634; 19, AT636; 20, AT639; 21, AT640; 22, AT641; 23, AT642; 24, AT635; 25, AT637; 26, AT638; 27, IW-92602-1; 28, IW-92602-2; 29, IW-92602-4; 30, IW-100702-2; 31, IW-100702-4; 32, I-84; 33, I-114.
Two distinct geotypes in Chinese isolates of T. matsutake.
In China, T. matsutake is known to grow primarily in two distantly located regions: the area of the southwestern provinces, i.e., Yunnan and Sichuan, near Bhutan, and the area of the northeastern provinces, i.e., Heilongjiang and Jilin, in the vicinity of Russian Siberia and North Korea (Fig. 2) (35). For decades, the major portion of Chinese matsutake exported to Japan has been from the southwestern provinces. We wondered if such a trend might have biased our data regarding Chinese isolates, and if we might have excluded specimens from the northeastern provinces of China from the analysis described above. In the year 2007, we obtained the fruiting bodies of T. matsutake harvested in all these four provinces through a trading authority and subjected them to geotyping.
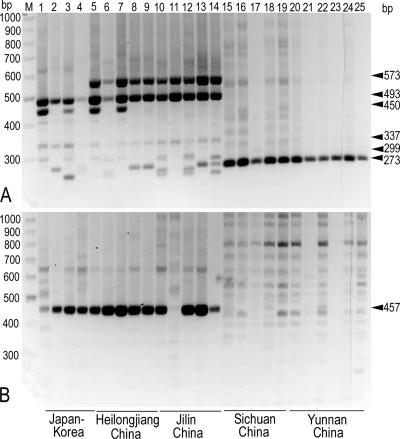
DNA was extracted from gills of the specimens at the stage prior to breaking the veil. The pDGSL313-1/pS48 system conferred polymorphisms that clearly specify T. matsutake isolates as being either from the southwestern provinces or from the northeastern provinces (Fig. 5A; Tables 1 and 2). All the isolates from the southwestern provinces examined were geotype B (Fig. 5A, lanes 15 to 25), and those that we had obtained as Chinese matsutake at local markets in Japan were proven to be from the same region (Fig. 3A, lanes 28 to 35). In contrast, the isolates from the northeastern provinces showed a 573-bp band unique to the Asian population examined while exhibiting the profile of geotype A characterizing the Far East isolates (Fig. 5A, lanes 5 to 14). These isolates also showed a close relationship with those from the Korean peninsula as revealed by the pDGSL719-2/pS48 system (Fig. 5B, lanes 5 to 14). We, therefore, assigned subgeotype A4 to the profile featuring the northeastern provinces of China (Table 2).
FIG. 5.
PCR profiles of T. matsutake from northeastern and southwestern provinces of China. The molecular size (bp) of a relevant DNA band is indicated by arrowheads in the right column. Geographical origins of specimens are given at the bottom. (A) pDGSL313-1/pS48 primer system. (B) pDGSL719-2/pS48 primer system. Lane M, molecular markers (300 to 1,000 bp); lanes 1 to 25, T. matsutake strains (Table 1 shows assigned geotypes): 1, Tm-Y62B; 2, Tm-158; 3, NK1; 4, NKR02; 5, CH-HE1; 6, CH-HE2; 7, CH-HE3; 8, CH-HE4; 9, CH-HE5; 10, CH-JI1; 11, CH-JI2; 12, CH-JI3; 13, CH-JI4; 14, CH-JI5; 15, CH-SI1; 16, CH-SI3; 17, CH-SI4; 18, CH-SI5; 19, CH-SI2; 20, CH-YU1; 21, CH-YU2; 22, CH-YU3; 23, CH-YU4; 24, CH-YU-5; 25, CN01.
Evaluation of traceability.
Thus far, only 5 out of 95 samples examined, including those from cultures and from commodities, showed equivocal typing results. Most of the confusion occurred between isolates from Japan and from the countries of the Korean peninsula (Table 1). Overall, the 95% confidence interval based on binomial distribution was 0.0526 ± 0.0449 between T. matsutake isolates from Japan and those from the rest of Asia. As for the relevancy of the sampling in our studies, the specimens analyzed must be regarded as representing their geographical origins, for extensive diversification of mushroom species generally existing in a local habitat makes the risk of reisolation of the same samples hardly possible (1, 2, 6, 9, 14, 20, 25, 27, 29, 30, 33).
Nucleotide sequence analysis of the geotype determinants.
Representative DNA segments that feature geotypes assigned in this study were cloned into pCR2.1 and analyzed by nucleotide sequencing (AB360715-40 and AB363821-38). The sequences of the 493-bp and the 337-bp fragments, which determine geotype A, are 99% identical at bp 1 to 337 in the direction from pS48 to pDGSL313-1, and so are those of the 450-bp and the 299-bp fragments, which determine subgeotype A2, at bp 1 to 299. Therefore, we classified isolates exhibiting one of the former fragments into geotype A and one of the latter into subgeotype A2 (Fig. 4, lane 32; Table 1 [I-84]). The sequence of the 573-bp fragment, which specifies subgeotype A4, is related to neither that of the geotype A determinant nor that of the A2 determinant. None of these fragments is the homolog of the 313-bp DNA segment whose sequence was used for designing the primer pDGSL313-1. Rather, the sequence of the 273-bp DNA segment, which is the marker of geotype B, is 99% identical to that of the 313-bp DNA segment at bp 1 to 273 in the direction from pS48 to pDGSL313-1. Although we found that some strains belonging to geotype A have the 273-bp fragment shared with geotype B, we dared to establish another subtype in geotype A (Fig. 3A, lanes 1 to 2, 9, 15, 19, 21, 23, 25, 38 to 40, and 42). Similarly, although some isolates from North Korea and the Jilin province of China exhibited fragments smaller than the 273-bp fragment, such as 255-bp and 265-bp fragments that are recognized as deletion derivatives of the 273-bp fragment, their significance in biogeography is yet to be clarified (Fig. 3A, lanes 22 to 25 and 44; Fig. 5A, lanes 10, 12, and 14). The sequence of the 457-bp fragment which determines subgeotype A3 is 99% identical among isolates belonging to this geotype but is not the homolog of the 719-bp DNA segment used for designing the primer pDGSL719-2. This phenomenon may be attributed to the preference of sequences for PCR amplification among repetitive sequences dispersed throughout the genome (16, 19, 33) (AB047280).
Conclusion.
We demonstrated for the first time that two PCR systems concurrently carried out differentiate among individual Asian isolates of T. matsutake by their geographical origins, allowing us to trace them back to Japan, the area of South Korea through North Korea, the northeastern provinces of China, and the area of the southwestern provinces of China through Bhutan (Table 2; Fig. 2). The results presented here are consistent with our hypothesis that the genome of T. matsutake could have dramatically evolved during a rather later evolutionary stage by the involvement of retroelements (16, 17). This study strongly suggests that some retroelement integrations have occurred during the course of biogeographical diversification.
The results are reproducible. Note that Japanese isolates were collected from various locations between 1983 and 2005, Korean isolates were collected between 1997 and 2006, and Chinese and Bhutanese isolates were collected between 1992 and 2007 (Table 1). The occurrence of local diversification at the level of isolates has been reported in T. matsutake (14, 20, 33), and diversification through mosaicism has been documented as a general phenomenon in mushroom-producing basidiomycetes (1, 2, 9, 25, 27, 29, 30). Therefore, the retroelement-based geotyping markers are recognized as highly conserved over generations. Retroelements are mobile with copy-and-paste rather than with cut-and-paste processes. This unique transposition process allows the original copy and copies once amplified and integrated to remain in the genomic loci as footprints of genome evolution (4, 5, 7, 8, 23, 26, 28, 31). In this view, it is not surprising that the retroelement-based analytical system unearthed for the first time the significant biogeographical diversification of Chinese matsutake, in which the isolates from northeastern provinces are not only distinct among the population of Asian matsutake tested but also closely related to the Korean and Japanese isolates, while isolates from the southeastern provinces of China through Bhutan, a high-altitude area distant from the Far East, are unique among the population.
In biogeographical studies of mushroom species, the sequence of ribosomal DNA (rDNA) has been regarded as a reliable genetic marker, as in the typical case with species of Pleurotus (32, 36). In T. matsutake, however, the sequences of both intergenic spacer 1 (IGS1) and the interspacer regions (ITS1 and ITS2) are highly conserved among isolates from all over the world (10, 15). We previously demonstrated that T. matsutake has megB1, an approximately 500-bp DNA segment that is the major component of IGS1 from many mushroom species including species of Pleurotus, in a genomic region out of rDNA (3) (AB304914 and AB304915). The sequence of megB1 is also too conserved as a marker to classify T. matsutake isolates (unpublished data; AB293550 and AB298297 to AB298306).
The system developed in this study offers us a simple but highly reliable polymorphism analysis system, enabling rapid assay of Asian matsutake geotypes without requiring dendrogram calculation of a number of DNA fragments (Table 2). The situation contrasts with IRAP, which generates tremendous numbers of DNA segments as the fingerprint of each strain, making their classification very difficult (18, 20). Since matsutake is a fresh food and should be consumed within a week after harvest, the availability of a rapid analytical system is extremely important. By the same token, it could be a practical method suitable for identifying the geographical origin of matsutake at the Customs and Consumer Services. Also, the demonstration presented here opens a new horizon in the scope of molecular ecology and the evolution of mushroom-producing basidiomycetes, a research area that has seldom explored mobile DNA as genetic markers that should inform us of the dynamics of molecular ecology and the evolution of eukaryotes (4, 5, 7, 8, 23, 26, 28, 31).
Like any other traceability methods designed to examine agricultural products, including the elemental composition analysis widely used, the PCR-based analytical system is not always perfectly accurate. In fact, some Korean isolates exhibited the same subgeotype A1 as Japanese isolates. However, the analytical system met a reliability standard with an average misjudgment rate of approximately 5% and, therefore, is useful in the primary screening of products traded all over Japan to be followed by tracing back the geographical origin through the in-depth examination of trading records. Such a primary screening method not only facilitates traceability but also deters counterfeiting. In the summer of 2007, Scandinavian matsutake mushrooms were imported for the first time and traded at a price of $400/kg, which is equivalent to an average price for Japanese matsutake in a high season, while early crops harvested in the northern part of Japan were traded at the record high price of $4,000/kg. We hope that the traceability method presented here contributes to mutual trust among consumers, trading authorities, and exporting countries, as well as to conservation of natural resources through rationalizing international trading in the field of ectomycorrhizal specialty mushrooms.
Acknowledgments
This work was supported by a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan.
Footnotes
Published ahead of print on 15 February 2008.
REFERENCES
- 1.Anderson, J. B., and L. M. Kohn. 2007. Dikaryons, diploids, and evolution, p. 333-348. In J. Heitman, J. W. Kronstad, J. W. Taylor, and L. A. Casselton (ed.), Sex in fungi. ASM Press, Washington, DC.
- 2.Babasaki, K., K. Masuno, and H. Murata. 2003. Interactions of heterologous mycelia colonized in the substrate govern fruit body production in the cultivated homobasidiomycete Pholiota nameko. Biosci. Biotechnol. Biochem. 67:100-106. [DOI] [PubMed] [Google Scholar]
- 3.Babasaki, K., H. Neda, and H. Murata. 2007. megB1, a novel macroevolutionary genomic marker of the fungal phylum Basidiomycota. Biosci. Biotechnol. Biochem. 71:1927-1939. [DOI] [PubMed] [Google Scholar]
- 4.Bejerano, G., C. Lowe, N. Ahituv, B. King, A. Siepel, S. Salama, E. M. Rubin, W. J. Kent, and D. Haussler. 2006. A distal enhancer and ultraconserved exon are derived from a novel retroposon. Nature 441:87-90. [DOI] [PubMed] [Google Scholar]
- 5.Bushman, F. 2002. Lateral DNA transfer: mechanisms and consequences, p. 1-448. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 6.Clark, T. A., and J. B. Anderson. 2004. Dikaryons of the basidiomycete fungus Schzophyllum commune: evolution in long-term culture. Genetics 167:1663-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deininger, P. L., and A. M. Roy-Engel. 2002. Mobile elements in animal and plant genomes, p. 1074-1092. In N. L. Craig, R. Craigie, M. Gellert, and A. M. Lambowitz (ed.), Mobile DNA II. ASM Press, Washington, DC.
- 8.Eickbush, T. H., and H. S. Malik. 2002. Origin and evolution of retrotransposons, p. 1111-1144. In N. L. Craig, R. Craigie, M. Gellert, and A. M. Lambowitz (ed.), Mobile DNA II. ASM Press, Washington, DC.
- 9.Fukuda, M., and Y. Fukumasa-Nakai. 1996. Genetic evidence supporting the multicellular origin of fruiting body formation in the basidiomycete Lentinula edodes. Mokuzai Gakkaishi 42:1025-1028. [Google Scholar]
- 10.Guerin-Laguett, A., N. Matsushita, K. Kikuchi, K. Iwase, F. Lapeyrie, and K. Suzuki. 2002. Identification of a prevalent Tricholoma matsutake ribotype in Japan by rDNA IGS1 spacer characterization. Mycol. Res. 106:435-443. [Google Scholar]
- 11.Hall, I. R., W. Yun, and A. Amicucci. 2003. Cultivation of edible ectomycorrhizal mushrooms. Trends Biotechnol. 21:433-438. [DOI] [PubMed] [Google Scholar]
- 12.Hosford, D., D. Pilz, R. Molina, and M. Amaranthus. 1997. Ecology and management of the commercially harvested American Matsutake mushrooms. USDA Forest Service PNW-GTR-412. USDA Forest Service, Washington, DC.
- 13.Intini, M., H. H. Dogan, and A. Riva. 2003. Tricholoma anatolicum spec. nov.: a new member of the matsutake group. Micol. Veget. Medit. 18:135-142. [Google Scholar]
- 14.Lian, C., M. Narimatsu, K. Nara, and T. Hogetsu. 2006. Tricholoma matsutake in a natural Pinus densiflora forest: correspondence between above- and below-ground genets, association with multiple host trees and alteration of existing ectomycorrhizal communities. New Phytol. 171:825-836. [DOI] [PubMed] [Google Scholar]
- 15.Matsushita, N., K. Kikuchi, Y. Sasaki, A. Guerin-Laguette, F. Lapeyrie, L.-M. Vaario, M. Intini, and K. Suzuki. 2005. Genetic relationship of Tricholoma matsutake and T. nauseosum from the Northern Hemisphere based on analyses of ribosomal DNA spacer regions. Mycoscience 46:90-96. [Google Scholar]
- 16.Murata, H., and K. Babasaki. 2005. Intra- and inter-specific variations in the copy number of two types of retrotransposons from the ectomycorrhizal basidiomycete Tricholoma matsutake. Mycorrhiza 15:381-386. [DOI] [PubMed] [Google Scholar]
- 17.Murata, H., K. Babasaki, Y. Miyazaki, and A. Yamada. 2002. Genetic evidence that two types of retroelements evolved through different pathways in ectomycorrhizal homobasidiomycetes Tricholoma spp. Biosci. Biotechnol. Biochem. 66:1880-1886. [DOI] [PubMed] [Google Scholar]
- 18.Murata, H., K. Babasaki, and A. Yamada. 2005. Highly polymorphic DNA markers to specify strains of the ectomycorrhizal basidiomycete Tricholoma matsutake based on σmarY1, the long terminal repeat of gypsy-type retroelement marY1. Mycorrhiza 15:179-186. [DOI] [PubMed] [Google Scholar]
- 19.Murata, H., Y. Miyazaki, and A. Yamada. 2001. marY2N, a LINE-like non-long terminal repeat (non-LTR) retroelement from the ectomycorrhizal homobasidiomycete Tricholoma matsutake. Biosci. Biotechnol. Biochem. 65:2301-2305. [DOI] [PubMed] [Google Scholar]
- 20.Murata, H., A. Ohta, A. Yamada, M. Narimatsu, and N. Futamura. 2005. Genetic mosaics in the massive persistent rhizosphere colony “shiro” of the ectomycorrhizal basidiomycete Tricholoma matsutake. Mycorrhiza 15:505-512. [DOI] [PubMed] [Google Scholar]
- 21.Murata, H., and A. Yamada. 2000. marY1, a member of the gypsy group of long terminal repeat retroelements from the ectomycorrhizal basidiomycete Tricholoma matsutake. Appl. Environ. Microbiol. 66:3642-3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murata, H., A. Yamada, and K. Babasaki. 1999. Identification of repetitive sequences containing motifs of retrotransposons in the ectomycorrhizal basidiomycete Tricholoma matsutake. Mycologia 91:766-775. [Google Scholar]
- 23.Nishihara, H., A. F. A. Smit, and N. Okada. 2006. Functional noncoding sequences derived from SINEs in the mammalian genome. Genome Res. 16:864-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogawa, M. 1978. The biology of matsutake. Tsukiji-shokan, Tokyo, Japan. (In Japanese.)
- 25.Peabody, R. B., D. C. Peabody, and K. M. Sicard. 2000. A genetic mosaic in the fruiting stage of Armillaria gallica. Fungal Genet. Biol. 29:72-80. [DOI] [PubMed] [Google Scholar]
- 26.Pennisi, E. 2007. Jumping genes hop into the evolutionary limelight. Science 317:894-895. [DOI] [PubMed] [Google Scholar]
- 27.Raper, J. R. 1966. Genetics of sexuality in higher fungi, p. 1-283. The Ronald Press Company, New York, NY.
- 28.Robertson, H. M. 2002. Evolution of DNA transposons in eukaryotes, p. 1093-1110. In N. L. Craig, R. Craigie, M. Gellert, and A. M. Lambowitz (ed.), Mobile DNA II. ASM Press, Washington, DC.
- 29.Saville, B. J., Y. Kohli, and J. B. Anderson. 1998. mtDNA recombination in a natural population. Proc. Natl. Acad. Sci. USA 95:1331-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saville, B. J., H. Yoell, and J. B. Anderson. 1996. Genetic exchange and recombination in populations of the root-infecting fungus Armillaria gallica. Mol. Ecol. 5:485-497. [DOI] [PubMed] [Google Scholar]
- 31.Shedlock, A. M., K. Takahashi, and N. Okada. 2004. SINEs of speciation: tracking lineages with retroposons. Trends Ecol. Evol. 19:545-553. [DOI] [PubMed] [Google Scholar]
- 32.Vilgalys, R., and B. L. Sun. 1994. Ancient and recent patterns of geographic speciations in the oyster mushroom Pleurotus revealed by phylogenetic analysis of ribosomal DNA sequences. Proc. Natl. Acad. Sci. USA 91:4599-4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu, X., H. Guo, and Z.-L. Yang. 2007. Single nucleotide polymorphisms in the ectomycorrhizal mushroom Tricholoma matsutake. Microbiology 153:2002-2012. [DOI] [PubMed] [Google Scholar]
- 34.Yamada, A., K. Maeda, H. Kobayashi, and H. Murata. 2006. Ectomycorrhizal symbiosis in vitro between Tricholoma matsutake and Pinus densiflora seedlings that resembles naturally occurring ‘shiro.’ Mycorrhiza 16:111-116. [DOI] [PubMed] [Google Scholar]
- 35.Yun, W., I. R. Hall, and L. A. Evans. 1997. Ectomycorrhizal fungi with edible fruiting bodies. 1. Tricholoma matsutake and related fungi. Econ. Bot. 51:311-327. [Google Scholar]
- 36.Zervakis, G. I., J.-M. Moncalvo, and R. Vilgalys. 2004. Molecular phylogeny, biogeography and speciation of the mushroom species Pleurotus cystidiosus and allied taxa. Microbiology 150:715-726. [DOI] [PubMed] [Google Scholar]