Abstract
Anaerobic microbial dechlorination is an important step in the detoxification and elimination of polychlorinated biphenyls (PCBs), but a microorganism capable of coupling its growth to PCB dechlorination has not been isolated. Here we describe the isolation from sediment of an ultramicrobacterium, strain DF-1, which is capable of dechlorinating PCBs containing double-flanked chlorines added as single congeners or as Aroclor 1260 in contaminated soil. The isolate requires Desulfovibrio spp. in coculture or cell extract for growth on hydrogen and PCB in mineral medium. This is the first microorganism in pure culture demonstrated to grow by dehalorespiration with PCBs and the first isolate shown to dechlorinate weathered commercial mixtures of PCBs in historically contaminated sediments. The ability of this isolate to grow on PCBs in contaminated sediments represents a significant breakthrough for the development of in situ treatment strategies for this class of persistent organic pollutants.
Polychlorinated biphenyls (PCBs) were manufactured between 1930 and 1978, and their widespread use in high-temperature electrical coolants, hydraulic fluids, paints, carbonless paper, and as dedusting agents has resulted in their global distribution in even the most remote regions of the planet and throughout the food chain. The 2005 Priority List of Hazardous Substances (http://www.atsdr.cdc.gov/cercla/) published by the U.S. Agency for Toxic Substances and Disease Registry ranks PCBs fifth out of 275 substances. Ranking on this list is a combined metric based on the compound's prevalence at facilities within the United States, known or suspected toxicity, and potential for human exposure. With the discovery of Desulfomonile tiedjei strain DCB1 (24) in 1984, the door was opened for the study of bacteria that can reductively dechlorinate halogenated organic compounds that were manufactured for a wide range of applications throughout the 20th century. Subsequently, it was discovered that such bacteria can couple their growth to reductive dehalogenation in a process referred to as dehalorespiration (15) or halorespiration (15, 22). There has been an explosion of discoveries in this field, resulting in the identification of dozens of different species and strains that are capable of dechlorinating compounds ranging from chlorinated ethenes (19) to dioxins (5). Most of the bacteria that reductively dechlorinate toxic halogenated industrial pollutants have turned out to be members of the genus Dehalococcoides. Although several of these microorganisms have been successfully developed for commercially viable bioremediation of soils contaminated with chlorinated solvents, a proven effective treatment for in situ treatment of PCBs does not currently exist. As a result, the only accepted treatments for PCBs are remedial technologies such as dredging and capping, which are expensive, disruptive to the environment, and impractical to implement over large areas and in remote locations.
Dehalococcoides ethenogenes strain 195, the first of the Dehalococcoides to be isolated in pure culture (19), respires with chlorinated ethenes. Since then, several other Dehalococcoides spp. capable of dechlorinating chlorinated ethenes have been isolated (13, 14, 28). Dehalococcoides sp. strain CBDB1 is capable of dehalorespiration with chlorobenzenes and chlorinated dioxins (1, 5), whereas D. ethenogenes strain 195 has been shown subsequently to dechlorinate chlorinated naphthalenes and a polychlorinated biphenyl (2,3,4,5,6-PCB) when grown with tetrachloroethene in sediment (11). In addition to D. ethenogenes strain 195, four different bacterial phylotypes are known to reductively dechlorinate PCBs (6, 10, 30). One of these, phylotype DEH10, is a member of the Dehalococcoides (10), but the remaining phylotypes belong to a phylogenetically diverse clade of bacteria that is distinct from the Dehalococcoides. Although this clade includes a number of phylotypes associated with dechlorination, most have not been cultured and isolates have not been described. One member of this group, dechlorinating strain DF-1 from Charleston Harbor (Charleston, SC), has been reported previously in a sediment-free coculture with a Desulfovibrio sp. (30). Reductive analysis of the 16S rRNA genes of this nonmethanogenic coculture determined that strain DF-1 is capable of dechlorinating PCBs, chlorobenzenes, and chlorinated ethenes (20, 29, 30), but for unknown reasons the microorganism could not be grown as a monoculture. Here we describe for the first time the growth of strain DF-1 by dehalorespiration of PCBs in pure culture and the reductive dechlorination of weathered commercial PCBs in soil by bioaugmentation with an isolate.
MATERIALS AND METHODS
Culture procedures.
Unless stated otherwise, all bacterial culture work was done under strict anaerobic conditions in E-Cl medium with 10 mM sodium formate and 173 μM 2,3,4,5-PCB as described previously (3, 30). The PCB was added in acetone (0.1% [vol/vol] final concentration of acetone added to the culture medium). The cultures containing strain DF-1 were routinely grown in 50 ml of medium in 160-ml serum bottles sealed with 20-mm Teflon-coated butyl stoppers (West Co., Lionville, PA). Sequential dilution series were conducted with 25 ml of medium in 60-ml serum bottles. l-Cysteine-HCl monohydrate (1.5 mM) or 0.5 mM titanium(III) nitrilotriacetate (TiNTA) was used as a chemical reductant to remove oxygen from the medium. The TiNTA was prepared as described previously (21). All cultures were incubated statically at 30°C in the dark. To test for growth effects of pH, organic buffers with a range of pKa values were substituted for the carbonate-phosphate buffer used in E-Cl medium as described previously (25).
A Desulfovibrio sp. isolated by Wu et al. (30) was used for preparation of cell extracts. Desulfovibrio isolation medium (DIM) containing 10 mM sodium lactate and 10 mM sodium sulfate (30) was used to grow the Desulfovibrio sp. and Desulfovibrio vulgaris strain Hildenborough (ATCC 29579). Both Desulfovibrio spp. were grown using 50 ml of DIM in 160-ml serum bottles or 10 ml of DIM in 18-ml anaerobe tubes, sealed with black butyl stoppers (Geo-Microbial Technologies, Inc., Ochelata, OK). Cultures were incubated at 30°C in the dark. Escherichia coli strain K-12 was grown aerobically at 37°C in the dark in Luria broth (LB) base (Difco, Detroit, MI) with 0.2% (wt/vol) glucose. Growth of the Desulfovibrio spp. and E. coli was monitored based on the optical density at 550 nm for the Desulfovibrio spp. in DIM and 660 nm for E. coli in LB medium.
Sterilized cell extracts of the Desulfovibrio spp., D. vulgaris, and E. coli were prepared by autoclaving 50 ml of culture (approximately 108 cells per ml) in a 165-ml glass serum bottle for 45 min. Extract cooled to room temperature was passed through a sterile 0.2-μm filter in an anaerobic glove box and subsequently added to cultures (1%, vol/vol) for growth of strain DF-1.
To test for reductive dechlorination of weathered PCB, soil contaminated with 4.62 μg/g Aroclor 1260 was collected from a drainage ditch located in Mechanicsburg, PA (40°13′54"N, 76°59′33"W). The soil sample used in the bioaugmentation experiment was untreated, black, and had a total organic content of 3,220 mg per kg soil. In total, 4 g of soil (wet weight) was inoculated (in triplicate) into 10 ml of anaerobically prepared low-saline mineral medium (26) in 25-ml anaerobe tubes sealed under N2-CO2 (80:20) with Teflon septa. The medium composition was as follows (in mmol per liter): NaCl, 49.6; MgCl2, 0.5; KCl, 10.2; CaCl2, 1.0; NH4Cl, 9.4; Na2HPO4, 4.2; cysteine, 1.4; Na2CO3, 28.3. This medium also contained vitamins and trace metals. No electron donors were added except for the components in the low-saline medium (0.0125% [wt/vol] cysteine) and residual hydrogen (≤5% [vol/vol]) present in the atmosphere of the anaerobic chamber used for inoculating and sampling of the microcosms. A DF1 culture grown to approximately 107 cells per ml with tetrachloroethene was flushed with N2-CO2 to remove perchloroethene and trichloroethene prior to inoculating 2 ml into sediment microcosms. PCB analysis was performed as described by Kjellerup et al. (16). Nonbioaugmented controls included medium and soil containing indigenous microorganisms without DF1. Controls for abiotic activity included medium, soil containing indigenous microorganisms, and DF1 and were sterilized by autoclaving for 1 h on three sequential days.
PCB analysis.
PCBs and chlorobenzenes from sediment-free cultures were extracted with ethyl acetate (1:5 [vol/vol, sample to solvent]) and analyzed by gas chromatography as described previously (3, 29). Weathered PCBs from contaminated sediments were extracted by sonication and analyzed as described by Dunnivant and Elzerman (7). Chlorinated ethenes were analyzed as described by Miller et al. (20). PCBs supplied to the cultures and used as GC standards were of the highest purity available (99%+) and were purchased from Accustandard (New Haven, CT). Chlorinated ethenes and chlorobenzenes of the highest purity available (98 to 99%+) were purchased from Sigma Aldrich (Milwaukee, WI).
Microscopy.
Cultures of the isolated strain DF-1 were examined under phase contrast (oil immersion, 1,000× magnification) with a Zeiss Axiolab phase-contrast microscope (Zeiss, Thornwood, NY). In order to disrupt clusters of cells, 1-ml samples of culture were placed in a sterile 1.5-ml microcentrifuge tube and centrifuged for 10 min at 16,000 × g. A 900-μl aliquot of the supernatant was discarded, the pellet was resuspended in the remaining 100 μl, and then the sample was exposed to mild sonication for 20 min (twice for a total of 40 min) in a Fisher Scientific FS20 sonicating water bath (Fisher Scientific Inc.). Staining of cells with 4′,6′-diamidino-2-phenylindole (DAPI) was done as previously described (17).
Transmission electron microscopy was conducted on a Hitachi H-8000 transmission electron microscope (Tokyo, Japan) at 200 kV accelerating voltage. Samples were prepared by drying 50 μl of the isolated strain DF-1 cells (grown in cysteine-reduced medium, centrifuged, and sonicated as described above) on a 150-mesh copper grid (EMS, Hatfield, PA) precoated with Collodion (nitrocellulose; EMS, Hatfield, PA) and a sputter-coated carbon film. Dried samples were then negatively stained with uranyl acetate (2%) and imaged after drying. Scanning electron microscopy was performed on a FEI Quanta 200 ESEM (Hillsboro, OR) at 30 kV accelerating voltage. Samples were prepared by dehydration of 100 μl of the isolated strain DF-1 on a conductive sample stub prepared with a sticky carbon tab (EMS, Hatfield, PA). Once dried, a Denton vacuum desk II desktop sputter coater (Moorestown, NJ) was used to deposit approximately 150 Å of gold-palladium mix onto each sample.
Competitive PCR.
Dechlorinating strain DF-1 was enumerated by a competitive PCR assay (16). DNA was extracted from 1 ml of DF-1 isolate samples by using InstaGeneMatrix protocol no. 2.3. A competitor was constructed based on the primers 348F/884R by using the DNA template supplied in the competitive DNA construction kit (RR017; TaKaRa Bio Inc., Japan). Briefly, 16S rRNA gene copies per ml of isolate culture were determined according to the manufacturer's instructions (TaKaRa Bio) and by using the PCR conditions as described. PCR was conducted in 25-μl reaction volumes using GeneAmp reagents (Applied Biosystems, CA), where the master mix contained 10 mM Tris-HCl, 75 mM KCl, 0.2 mM of each deoxynucleoside triphosphate in a mix, 1.5 mM MgCl2, 1.6% dimethyl sulfoxide, 2.5 units of AmpliTaq DNA polymerase, 50 pM of each primer, and 14.75 μl of nuclease-free water. A 0.5-μl aliquot of DNA template and 2.5 μl of competitor DNA in appropriate dilutions were added. For the PCR, an initial denaturation step at 95°C for 2 min was used, followed by 40 cycles of denaturation at 95°C for 45 s, primer annealing at 58°C for 45 s, and elongation at 72°C for 1 min. A final extension step at 72°C for 30 min was used, followed by a final holding step at 4°C. PCR products of the correct length were confirmed by electrophoresis using a 1.5% agarose gel. The intensity of the PCR products was measured by densitometry with the image analysis software Quantity One (Bio-Rad, Hercules, CA). One 16S rRNA gene copy per cell was assumed based on the genome sequences of Dehalococcoides ethenogenes (23) and strain CBDB1 (18).
DNA sequencing and analysis.
The 16S rRNA gene of bacterium DF-1 was amplified from genomic DNA with primers pA and pH as described previously (8) and sequenced using the BigDye Terminator kit v3.1 (Applied Biosystems, Foster City, CA) per the manufacturer's instructions. Sequencing of purified DNA was performed on an ABI 3130 XL automated capillary DNA sequencer (Applied Biosystems, CA).
The 16S rRNA gene sequence from DF-1 and submitted gene sequences obtained from NCBI (http://www.ncbi.nlm.nih.gov/BLAST) were compiled and aligned using the automatic nucleic acid aligner in the BioEdit sequence alignment editor. A total of 21 sequences containing from 500 to 1,500 nucleotides were unambiguously aligned and used for calculation of trees by the neighbor-joining and FITCH approaches and using default settings in the PHYLIP software (http://evolution.genetics.washington.edu/phylip.html). Bootstrap analyses (1,000 replicates) were performed using the PHYLIP package.
RESULTS AND DISCUSSION
The coculture containing strain DF-1 and the Desulfovibrio sp. described by Wu et al. (30) was used as inoculum for the isolation of strain DF-1. Strain DF-1 did not produce colonies on solid or semisolid medium (0.5 to 2.0% Noble agar in E-Cl) despite several attempts with 173 μM 2,3,4,5-PCB, 173 μM pentachlorobenzene, or tetrachloroethene volatilized within a sealed anaerobic glass jar used to store the plates or agar shake tubes. Isolation attempts by sequential dilution in a defined minimal medium (E-Cl) (3) with a single carbon and energy source (10 mM sodium formate) and a single potential electron acceptor (173 μM 2,3,4,5-PCB) and by adding 10 or 100 μg/ml of ampicillin, vancomycin, neomycin, streptomycin, or chloramphenicol to the medium, which had been used successfully for isolation of Dehalococcoides spp. (1, 14, 19), were also unsuccessful. Increasing the concentration of vitamins and minerals by fourfold did not enhance dechlorination by the culture or enable us to isolate it by the means noted above. E-Cl medium at 1× strength includes 1 μg/liter of vitamin B12, which has been shown to stimulate the growth and dechlorination of Dehalococcoides ethenogenes strain 195 (12). Finally, TiNTA was substituted as a medium reducing agent for cysteine, as the latter even in trace amounts was observed to support the growth of the Desulfovibrio sp. in pure culture. The first serial dilution of the culture in TiNTA-reduced medium resulted in the dechlorination of 2,3,4,5-PCB up to a 10−6 dilution. The Desulfovibrio sp. was no longer observed microscopically at this dilution, nor was it detected when the 10−6 dilution was inoculated into lactate-sulfate medium (DIM). When the 10−6 dilution culture was serially diluted, PCB dechlorination was observed once more to a dilution of 10−6, but this time the Desulfovibrio sp. was not observed microscopically at any dilution, nor was growth observed from any dilution transferred to lactate-sulfate medium, LB, or LB plus glucose medium (full and half-strength), which confirmed that the culture contained only the PCB-dechlorinating strain.
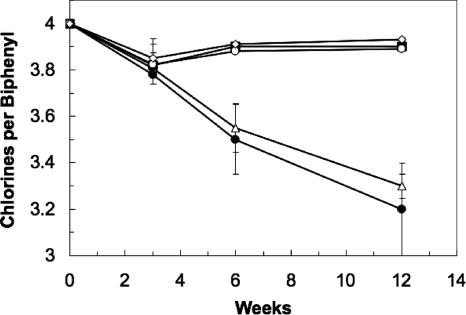
When a third sequential transfer series was made in the TiNTA-reduced medium, dechlorinating activity was no longer observed, but it could be restored when the coculture of strain DF-1 and the Desulfovibrio sp. was reconstituted by combining a 10% (vol/vol) transfer of the isolated strain DF-1 with approximately 105 cells/ml of the Desulfovibrio sp. To determine whether active Desulfovibrio sp. organisms were required to restore growth of the dechlorinator, an autoclaved cell extract prepared from the Desulfovibrio sp. was added to the inactive strain DF-1 culture. As indicated in Fig. 1, PCB dechlorination by strain DF-1 was supported in E-Cl mineral medium supplied with the Desulfovibrio extract, formate, and 2,3,4,5-PCB. Increasing the amount of extract added in a two- or fourfold excess had no further effect on dechlorination. An autoclaved cell extract prepared in the same manner from Desulfovibrio vulgaris strain Hildenborough also supported PCB dechlorination by DF-1, but autoclaved extract from yeast or Escherichia coli did not. No dechlorination occurred without DF-1, i.e., with cell extracts alone. Additionally, acetate (10 mM), lactate (10 mM), sulfate (10 mM), sulfide (0.1 and 1 mM), cysteine (1.5 mM), hydrogen (80:20 mix of H2-CO2 at 1 atm), and DIM (1%, vol/vol) when added to E-Cl medium did not support PCB dechlorination by strain DF-1 without Desulfovibrio extract. Strain DF-1 continued to grow to a 10−6 dilution after over 10 sequential transfers in medium containing Desulfovibrio sp. cell extract with 173 μM 2,3,4,5-PCB or 0.2 mM tetrachloroethene. The culture was transferred back to cysteine-reduced medium without detection of the Desulfovibrio sp. after five sequential transfers, and contaminants were not detected based on growth in DIM, LB, or LB plus glucose and microscopic examination.
FIG. 1.
Dechlorination of 2,3,4,5-PCB by strain DF-1 with sterilized culture extract from the Desulfovibrio sp. isolated with DF-1 (closed circles), Desulfovibrio vulgaris strain Hildenborough (open triangles), E. coli (closed squares), yeast extract (open diamonds), or with no addition (open circles). All data are the averages from triplicate cultures, and error bars represent the standard deviations. Each culture received 10 mM sodium formate and 173 μM 2,3,4,5-PCB. Congener 2,3,5-PCB was the only dechlorination product detected.
Although the contributing factor from the Desulfobvibrio sp. has not been identified, it appears to be specific to that genus or perhaps sulfate reducers in general. An association between microbial PCB dechlorination and sulfate reduction is not unprecedented. Several researchers have reported an inhibition or lag of PCB dechlorination by sulfate in sediments that is relieved once the sulfate has been consumed (2-4, 27). PCB dechlorination in sediment microcosms from which strain DF-1 was enriched and isolated also showed a lag in dechlorination when sulfate was present (31). Several lines of evidence support the possibility that the relationship between PCB-dechlorinating bacteria and sulfate reducers is ubiquitous in indigenous dechlorinating communities. In prior reports we discovered sulfate reducers in association with two different PCB-dechlorinating enrichment cultures (6, 30), but in neither case did the sulfate reducers prove to be directly responsible for the dechlorination. Zwiernik et al. (32) found that the addition of ferrous sulfate to sediment microcosms would stimulate PCB dechlorination after the sulfate had been consumed. Possibly, the effect that Zwiernik et al. observed was due to the delivery of a required factor from the sulfate reducers to PCB-dechlorinating bacteria. Early observations that molybdate, a specific inhibitor of sulfate reduction, also inhibited PCB dechlorination in sediment enrichment cultures (31) further suggest that sulfate reducers support the growth of PCB-dechlorinating populations.
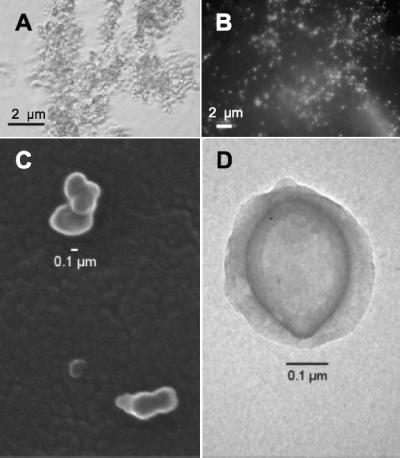
In pure culture, strain DF-1 grew as clusters of biomass, and individual cells were rarely observed (Fig. 2A). Observation of individual cells disbursed by mild sonication and followed by staining with DAPI revealed small cocci barely visible under the fluorescence microscope (Fig. 2B). Electron micrographs of strain DF-1 (Fig. 2C and D) showed clusters of small cocci, occasionally with slightly tapered ends. The individual cells were ultramicrobacteria that averaged 137 ± 51 nm (mean ± standard deviation) and ranged from 75 to 339 nm in diameter (n = 55). This is significantly smaller than the Dehalococcoides, all of which have diameters in the 0.5- to 1.0-μm range. The small size of DF-1 maximizes its surface area-to-volume ratio, which would be advantageous for a microorganism that must access a hydrophobic compound such as a PCB for its growth. Electron micrographs of the organism also revealed a structure surrounding the cells that resembled a sheath or capsule, which has not been observed with Dehalococcoides spp. A potentially adhesive extracellular matrix, possibly hydrophobic in nature, would be consistent with the tendency of the organisms to clump or cluster, and this may be another feature of the microorganism that facilitates its ability to absorb and accumulate hydrophobic compounds such as PCBs.
FIG. 2.
Microscopic examination of dechlorinating strain DF-1. (A) Phase-contrast image before mild sonication to disrupt cell aggregates. (B) DAPI stain of DF-1 after mild sonication. (C) Scanning electron micrograph. (D) Transmission electron micrographs of negatively stained cells.
Sodium formate and hydrogen (80:20 [vol/vol] mix at 1 atm) were electron donors for PCB dechlorination by bacterium DF-1; glucose (1 mM), acetate (10 mM), lactate (10 mM), pyruvate (10 mM), propionate (10 mM), butyrate (10 mM), cysteine (1.5 mM), and sulfide (1 mM) did not support PCB dechlorination. Substitution of oxygen (air), fumarate (10 mM), nitrate (10 mM), sulfate (10 mM), sulfite (10 mM), thiosulfate (10 mM), anthraquinone-2,6-disulfonate (5 mM), ferric citrate (10 mM), and amorphous Fe(III) oxide (100 mM) for organohalides as electron acceptors with 10 mM sodium formate as electron donor did not support growth, and PCB dechlorination was inhibited when these potential electron acceptors were added with PCBs to the medium. Similar to Dehalococcoides spp., growth substrates for strain DF-1 are restricted to very simple electron donors (hydrogen and formate) and halogenated compounds as electron acceptors. The use of hydrogen or formate as an electron donor indicates that in sediments strain DF-1 is dependent upon a consortium of acetogens and fermenting bacteria that generate hydrogen and formate from fermentable substrates.
PCB dechlorination (2,3,4,5-PCB to 2,3,5-PCB) by strain DF-1 was maximal at 30 to 33°C, with no dechlorination observed at 10°C or 35°C after 12 weeks of incubation. Dechlorination occurred over a wide range of NaCl concentrations (0.05 to 0.75 M) with a broad optimum (0.1 to 0.5 M) and from a pH range of 6.5 to 8.0 with an optimum at 6.8 (data not shown). Maintaining the temperature, NaCl, and pH at 30°C, 0.15 M, and 6.8, respectively, strain DF-1 in pure culture was screened for its ability to dechlorinate PCBs, chlorobenzenes, and chlorinated ethenes. The isolate reductively dechlorinated hexa- and pentachlorobenzenes, tetra- and trichloroethene, and penta- to trichlorobiphenyl congeners with double-flanked chlorines on one ring as reported previously in coculture (20, 29, 30). In the current study strain DF-1 inoculated into soil contaminated with weathered Aroclor 1260 was shown also to dechlorinate congeners ranging from octa- to pentachlorobiphenyls, further extending the range of congeners utilized by the isolate. However, the isolate consistently exhibited distinct specificity for double-flanked chlorines on one or both rings. The isolate was also tested for the ability to dechlorinate weathered Aroclor 1260 in contaminated soil (4.62 μg/g of soil). Strain DF-1 reductively dechlorinated 8.9 mol% of congeners possessing double-flanked chlorines within 145 days (Table 1), which confirms that the strain can actively transform environmentally relevant commercial mixtures of PCBs commonly associated with impacted sites. To our knowledge this is the first demonstration of a PCB-dechlorinating isolate transforming weathered Aroclor mixtures.
TABLE 1.
Reductive dechlorination of weathered Aroclor 1260-contaminated soil by bioaugmentation with bacterium DF-1a
| Congener(s) | Net reduction (ng PCB/g of soil) |
|---|---|
| PCB 63 | 0.439 |
| PCB 136 | 0.036 |
| PCB 82 + 151 | 0.083 |
| PCB 149 + 123 | 0.200 |
| PCB 153 | 0.250 |
| PCB 132 | 0.077 |
| PCB 163 + 138 | 0.339 |
| PCB 158 | 0.027 |
| PCB 183 | 0.088 |
| PCB 174 | 0.168 |
| PCB 202 + 171 + 156 | 0.077 |
| PCB 180 | 0.045 |
The results show the net reduction after subtracting activity of the indigenous microbial populations in the soil. Only congeners that were reduced are shown.
Detecting growth of strain DF-1 was difficult due to its very low yields, tendency to aggregate, and small size. Determinations of absorbance, protein, dry weight, and direct counts of DAPI-stained cells did not produce reproducible values. These observations were similar to difficulties reported with some species of Dehalococcoides (28). Growth of strain DF-1 was successfully monitored by enumeration of 16S rRNA gene copies using a competitive PCR assay recently developed for selective monitoring of dehalogenating bacteria in sediments (16). Using this method, DF-1 grown with 2,3,4,5-PCB showed an increase of more than 2 orders of magnitude in the copies of 16S rRNA genes, while a decrease in 16S gene copies was detected without PCBs present (Fig. 3). The data confirm that the growth of the organism is linked directly to reductive PCB dechlorination. The doubling time for DF-1 from day 0 to 14 was 2.0 days, and the growth during that period, assuming one 16S rRNA gene copy per cell, was 1.1 × 1014 cells per mol Cl− released with the dechlorination of 2,3,4,5-PCB. Quantitative PCR methods revealed similar doubling times and cell numbers for Dehalococcoides sp. strains FL2 (14) and BAV1 (13) grown with chlorinated ethenes. This is the first such analysis of a PCB-dechlorinating bacterium in pure culture and the first quantification of the growth of an individual microorganism in conjunction with PCB dechlorination.
FIG. 3.
Increase in 16S copy number specific to strain DF-1 in relation to dechlorination of 2,3,4,5-PCB. The open circles represent chlorines per biphenyl. The closed diamonds represent 16S copy number detected by competitive PCR from cultures grown with 2,3,4,5-PCB. The open square represents the 16S copy number detected from cultures grown without PCB at time zero (all other readings were below the detection limit of 3,500 copies). All data are the averages from triplicate cultures, and error bars represent the standard deviations. Each culture received 10 mM sodium formate and 173 μM 2,3,4,5-PCB. Congener 2,3,5-PCB was the only dechlorination product detected.
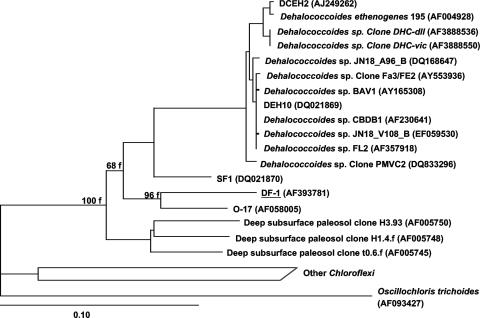
The bacterium is uniquely positioned phylogenetically based on its 16S rRNA gene sequence as the first isolate within a clade that is closely related to the Dehalococcoides but with less than 89% sequence similarity between the groups (Fig. 4). It most closely aligned with several phylotypes shown previously to be PCB-dechlorinating bacteria (6, 9, 10). However, this is the first member of that group to be isolated in pure culture and the first to be shown to exhibit a wide range of extracellular solute tolerances. Other distinctive phenotypic characteristics of DF-1 include its small size and synthesis of an extracellular polymer. The dechlorination of congeners with double-flanked chlorines in weathered PCBs following augmentation of contaminated sediment with strain DF-1 has significant implications for bioremediation. This capability supports the potential use of this microorganism in combination with PCB dechlorinators having complementary congener specificities (10) to stimulate the dechlorination and eventual degradation of these toxic compounds in situ.
FIG. 4.
Phylogenetic analysis of strain DF-1 and related 16S rRNA genes from published sequences. The tree was calculated by the neighbor-joining method and supported by FITCH. Accession numbers are shown in parentheses. Bar, 10 substitutions per 100 nucleotide positions.
Acknowledgments
We thank B. Mathis for assistance with electron microscopy and P. Paul and U. Ghosh for Aroclor 1260 analysis.
This study was funded by the Office of Naval Research, U.S. Department of Defense (grant numbers N000014-03-1-0035 [to K.R.S.] and N000014-03-1-0034 to [H.D.M.]).
Footnotes
Published ahead of print on 25 January 2008.
REFERENCES
- 1.Adrian, L., U. Szewzyk, J. Wecke, and J. Görisch. 2000. Bacterial dehalorespiration with chlorinated benzenes. Nature 408:580-583. [DOI] [PubMed] [Google Scholar]
- 2.Alder, A. C., M. M. Häggblom, S. R. Oppenheimer, and L. Y. Young. 1993. Reductive dechlorination of polychlorinated biphenyls in anaerobic sediments. Environ. Sci. Technol. 27:530-538. [Google Scholar]
- 3.Berkaw, M., K. R. Sowers, and H. D. May. 1996. Anaerobic ortho dechlorination of polychlorinated biphenyls by estuarine sediments from Baltimore Harbor. Appl. Environ. Microbiol. 62:2534-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, J. F., and R. E. Wagner. 1990. PCB movement, dechlorination, and detoxification in the Acushnet estuary. Environ. Toxicol. Chem. 9:1215-1233. [Google Scholar]
- 5.Bunge, M., L. Adrian, A. Kraus, M. Opel, W. G. Lorenz, J. R. Andreesen, H. Görisch, and U. Lechner. 2003. Reductive dehalogenation of chlorinated dioxins by an anaerobic bacterium. Nature 421:357-360. [DOI] [PubMed] [Google Scholar]
- 6.Cutter, L. A., J. E. M. Watts, K. R. Sowers, and H. D. May. 2001. Identification of a microorganism that links its growth to the reductive dechlorination of 2,3,5,6-chlorobiphenyl. Environ. Microbiol. 3:699-709. [DOI] [PubMed] [Google Scholar]
- 7.Dunnivant, F. M., and A. W. Elzerman. 1988. Determination of polychlorinated biphenyls in sediments, using sonication extraction and capillary column gas chromatography-electron capture detection with internal standard calibration. J. Assoc. Off. Anal. Chem. 71:551-556. [PubMed] [Google Scholar]
- 8.Edwards, U., T. Rogall, H. Blocker, M. Emde, and E. C. Bottger. 1989. Isolation and direct complete nucleotide determination of entire genes characterization of a gene coding for 16S-ribosomal RNA. Nucleic Acids Res. 19:7843-7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fagervold, S. K., H. D. May, and K. R. Sowers. 2007. Microbial reductive dechlorination of Aroclor 1260 in Baltimore Harbor sediment microcosms is catalyzed by three phylotypes within the Chloroflexi. Appl. Environ. Microbiol. 73:3009-3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fagervold, S. K., J. E. M. Watts, H. D. May, and K. R. Sowers. 2005. Sequential reductive dechlorination of meta-chlorinated PCB congeners in sediment microcosms by two different phylotypes of Chloroflexi. Appl. Environ. Microbiol. 71:8085-8090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fennell, D. E., I. Nijenhuis, S. F. Wilson, S. H. Zinder, and M. M. Häggblom. 2004. Dehalococcoides ethenogenes strain 195 reductively dechlorinates diverse chlorinated aromatic pollutants. Environ. Sci. Technol. 38:2075-2081. [DOI] [PubMed] [Google Scholar]
- 12.He, J., V. F. Holmes, P. K. H. Lee, and L. Alvarez-Cohen. 2007. Influence of vitamin B12 and cocultures on the growth of Dehalococcoides isolates in defined medium. Appl. Environ. Microbiol. 73:2847-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He, J., K. M. Ritalahti, K. L. Yang, S. S. Koenigsberg, and F. E. Löffler. 2003. Detoxification of vinyl chloride to ethene coupled to growth of an anaerobic bacterium. Nature 424:62-65. [DOI] [PubMed] [Google Scholar]
- 14.He, J., Y. Sung, R. Krajmalnik-Brown, K. M. Ritalahti, and F. E. Löffler. 2005. Isolation and characterization of Dehalococcoides sp. strain FL2, a trichloroethene (TCE)- and 1,2-dichloroethene-respiring anaerobe. Environ. Microbiol. 7:1442-1450. [DOI] [PubMed] [Google Scholar]
- 15.Holliger, C., G. Wohlfarth, and G. Diekert. 1998. Reductive dechlorination in the energy metabolism of anaerobic bacteria. FEMS Microbiol. Rev. 22:383-398. [Google Scholar]
- 16.Kjellerup, B. V., X. Sun, U. Ghosh, H. D. May, and K. R. Sowers. 24 February 2008. Site-specific microbial communities in three PCB-impacted sediments are associated with different in situ dechlorinating activities. Environ. Microbiol. doi: 10.1111/j.1462-2920.2007.01543.x. [DOI] [PubMed]
- 17.Kjellerup, B. V., T. R. Thomsen, J. L. Nielsen, B. H. Olesen, B. Frølund, and P. H. Nielsen. 2005. Microbial diversity in biofilms from corroding heating systems. Biofouling 21:19-29. [DOI] [PubMed] [Google Scholar]
- 18.Kube, M., A. Beck, S. H. Zinder, H. Kuhl, and R. Reinhardt. 2005. Genome sequence of the chlorinated compound-respiring bacterium Dehalococcoides species strain CBDB1. Nat. Biotechnol. 23:1269-1273. [DOI] [PubMed] [Google Scholar]
- 19.Maymo-Gatell, X., Y.-T. Chien, J. M. Gossett, and S. H. Zinder. 1997. Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethene. Science 276:1568-1571. [DOI] [PubMed] [Google Scholar]
- 20.Miller, G. S., C. E. Milliken, K. R. Sowers, and H. D. May. 2005. Reductive dechlorination of tetrachloroethene to trans-dichloroethene and cis-dichloroethene by PCB-dechlorinating bacterium DF-1. Environ. Sci. Technol. 39:2631-2635. [DOI] [PubMed] [Google Scholar]
- 21.Moench, T. T., and J. G. Zeikus. 1983. An improved preparation method for a titanium(III) media reductant. J. Microbiol. Methods 1:199-202. [Google Scholar]
- 22.Sanford, R. A., J. R. Cole, F. E. Löffler, and J. M. Tiedje. 1996. Characterization of Desulfitobacterium chlororespirans sp. nov., which grows by coupling the oxidation of lactate to the reductive dechlorination of 3-chloro-4-hydroxybenzoate. Appl. Environ. Microbiol. 62:3800-3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seshadri, R., L. Adrian, D. E. Fouts, J. A. Eisen, A. M. Phillippy, B. Methe, N. L. Ward, W. C. Nelson, R. T. Deboy, H. M. Khoudry, J. F. Kolonay, R. J. Dodson, S. C. Daugherty, L. M. Brinkac, S. A. Sullivan, R. Madupu, K. E. Nelson, K. H. Kang, M. Impraim, K. Tran, J. M. Robinson, H. A. Forberger, C. M. Fraser, S. H. Zinder, and J. F. Heidelberg. 2005. Genome sequence of the PCE-dechlorinating bacterium Dehalococcoides ethenogenes. Science 307:105-108. [DOI] [PubMed] [Google Scholar]
- 24.Shelton, D. R., and J. M. Tiedje. 1984. Isolation and partial characterization of bacteria in an anaerobic consortium that mineralizes 3-chlorobenzoic acid. Appl. Environ. Microbiol. 48:840-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sowers, K. R., S. F. Baron, and J. G. Ferry. 1984. Methanosarcina acetivorans sp. nov., an acetotrophic methane-producing bacterium isolated from marine sediments. Appl. Environ. Microbiol. 47:971-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sowers, K. R., and R. P. Gunsalus. 1988. Adaptation for growth at various saline concentrations by the archaebacterium Methanosarcina thermophila. J. Bacteriol. 170:998-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suflita, J. M., S. A. Gibson, and R. E. Beeman. 1988. Anaerobic biotransformations of pollutant chemicals in aquifers. J. Ind. Microbiol. 3:179-194. [Google Scholar]
- 28.Sung, Y., K. M. Ritalahti, R. P. Apkarian, and F. E. Löffler. 2006. Quantitative PCR confirms purity of strain GT, a novel trichloroethene-to-ethene respiring Dehalococcoides isolate. Appl. Environ. Microbiol. 72:1980-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu, Q., C. E. Milliken, G. P. Meier, J. E. Watts, K. R. Sowers, and H. D. May. 2002. Dechlorination of chlorobenzenes by a culture containing bacterium DF-1, a PCB dechlorinating microorganism. Environ. Sci. Technol. 36:3290-3294. [DOI] [PubMed] [Google Scholar]
- 30.Wu, Q., J. E. Watts, K. R. Sowers, and H. D. May. 2002. Identification of a bacterium that specifically catalyzes the reductive dechlorination of polychlorinated biphenyls with doubly flanked chlorines. Appl. Environ. Microbiol. 68:807-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu, Q. Z., K. R. Sowers, and H. D. May. 2000. Establishment of a polychlorinated biphenyl-dechlorinating microbial consortium, specific for doubly flanked chlorines, in a defined, sediment-free medium. Appl. Environ. Microbiol. 66:49-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zwiernik, M. J., I. J. F. Quensen, and S. A. Boyd. 1998. FeSO4 amendments stimulate extensive anaerobic PCB dechlorination. Environ. Sci. Technol. 32:3360-3365. [Google Scholar]