Abstract
Next to the benthic and pelagic compartments, the epiphyton of submerged macrophytes may offer an additional niche for ammonia-oxidizing bacteria in shallow freshwater lakes. In this study, we explored the potential activities and community compositions of ammonia-oxidizing bacteria of the epiphytic, benthic, and pelagic compartments of seven shallow freshwater lakes which differed in their trophic status, distribution of submerged macrophytes, and restoration history. PCR-denaturing gradient gel electrophoresis analyses demonstrated that the epiphytic compartment was inhabited by species belonging to cluster 3 of the Nitrosospira lineage and to the Nitrosomonas oligotropha lineage. Both the ammonia-oxidizing bacterial community compositions and the potential activities differed significantly between compartments. Interestingly, both the ammonia-oxidizing bacterial community composition and potential activity were influenced by the restoration status of the different lakes investigated.
By converting ammonia into nitrite, ammonia-oxidizing bacteria (AOB) are responsible for the first step of the nitrification process (57, 58). Comparative 16S rRNA gene sequence analyses revealed assemblages of AOB in two monophyletic groups (17, 59). The main group, containing the genera Nitrosomonas, Nitrosospira, Nitrosovibrio, and Nitrosolobus, is affiliated with the beta subclass of Proteobacteria. The second group is affiliated with the gamma subclass and includes two species of the genus Nitrosococcus, retrieved and isolated exclusively from marine environments.
AOB of the beta subclass have been detected in many different environments, from aquatic habitats (20, 22, 35, 36, 47, 50, 51, 54, 56) to soils (26-28, 31) and building stones (48). The distribution patterns of distinct species of AOB depend on physiological differences between distinctive representatives (23) and on environmental parameters like ammonia concentration (5, 49, 51), pH and temperature (21, 42), oxygen availability (6, 16, 43), and salinity (10, 49). Horz et al. (19) demonstrated that AOB from a grassland soil are also able to respond to multifactorial global changes. AOB are therefore considered a model group in microbial ecology studies (27). However, it remains difficult to couple results obtained from physiological experiments conducted in the laboratory with isolated strains to nitrification activities observed in the environment from which the strains were isolated (27).
AOB are known to grow either free-living or attached. For example, compared with the water column of aquatic environments, solid-phase habitats, such as sediment, particulate material, and aquatic macrophytes, seemed to be differently (35, 40, 50) and more densely populated with nitrifying bacteria (12, 33, 34). This surface-attached growth has been proposed to offer resilience against environmental constraints (41). Moreover, the formation of a biofilm on surfaces provides significant benefits for AOB of the Nitrosomonas lineages that are able to produce extracellular polymeric materials.
Hence, aquatic macrophytes may provide a habitat for attached growth of microorganisms (55). In order to create clear lakes, water managers aim for macrophytes to dominate the community of nutrient-consuming primary producers in shallow freshwater lakes. Evidence of nitrification activity on submerged macrophyte leaves was given by Eriksson et al. (13-15) in microcosm experiments. Moreover, Körner and Matulewich and Finstein (25, 34) counted nitrifying bacteria on submerged macrophytes by using the most-probable-number technique. However, to our knowledge, there is no information available on the composition of the AOB community in the epiphyton of aquatic macrophytes.
This study aims to describe in detail the AOB communities of the epiphyton of submerged macrophytes and to compare the macrophyte with the benthic and pelagic compartments in a complex hydrological system composed of interconnected, shallow, freshwater lakes.
Based on the results of previous studies conducted in the same sampling area (45-47) and on the known distribution patterns of AOB (23, 24), we hypothesized that the respective communities would differ between the compartments. Moreover, we hypothesized that colonization of the epiphyton might occur by both pelagic AOB species, which could benefit from the surface-attached conditions, and benthic AOB species resuspended from the sediment in these shallow freshwater lakes by the wind. Attempts have been made to link community structures, as well as potential activities, to several environmental variables characterizing the benthic, pelagic, and epiphytic compartments in a complex hydrological system that has been subjected to biomanipulation processes in the past.
MATERIALS AND METHODS
Description of study area and sample collection.
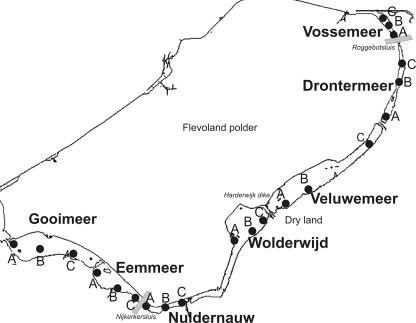
The Border Lakes are human-made, shallow, freshwater lakes, resulting from the reclamation of 1,419 km2 of land (i.e., the Flevoland polder; 52° 31′ N, 5° 29′ E) in a former brackish lagoon in the center of The Netherlands (Fig. 1). Two sluices divide the series of seven lakes into three hydrological units (from south to north): the southern lakes (Gooimeer and Eemmeer); the central lakes (Nuldernauw, Wolderwijd, Veluwemeer, and Drontermeer), and the northern lake (Vossemeer). The lakes differ in the physical (e.g., water retention time and exposure to the southwest wind) and chemical characteristics of both the sediment and the water column (e.g., nutrient content) and in the distribution and coverage of macrophyte species, as illustrated in Table 1. Interestingly, Lakes Nuldernauw, Wolderwijd, and Veluwemeer have been subjected to restoration measures in the past (e.g., dredging of phosphate-rich sediment, flushing with phosphate-free water, and removal of the stock of benthivorous fishes) (37) that resulted in the establishment of a clear-water state with Chara spp. as the dominant macrophyte species.
FIG. 1.
Schematic map of the area of the Dutch Border Lakes. In every lake, three stations were chosen (A, B, and C). The gray rectangles indicate the sluices determining the three hydrological units into which the whole area is divided. (Modified from a map courtesy of RIZA-Waterdienst.)
TABLE 1.
Summary of the environmental parameters characterizing the lakes studied
| Lake and stationa | RTb | Windc | Sampling depth (cm) | Secchi depth (cm) | Water characteristics
|
% Coverage ofe:
|
Sediment characteristics
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp (°C) | pH | O2 (mg/liter) | KjNd (mg/liter) | P (mg/liter) | Pp | Ch | Ppf | Zp | % Nf | % Organic Cf | C/Nf | P (mg/g)f | % Siltf | pHg | |||||
| GA | 150 | 45S | 120 | 100 | 20 | 8.6 | 8.0 | 2.8 | 0.3 | 30 | 5 | 0.008 | 0.082 | 11.62 | 0.05 | 0 | 8.46 | ||
| GB | 150 | 45S | 100 | 100 | 20 | 8.7 | 8.0 | 2.8 | 0.3 | 30 | 0.017 | 0.147 | 10.28 | 0.07 | 7.19 | 8.07 | |||
| GC | 150 | 45S | 80 | 80 | 20.7 | 8.4 | 8.0 | 2.8 | 0.3 | 30 | 10 | 0.086 | 1.017 | 13.91 | 0.18 | 32.34 | 7.73 | ||
| EA | 14 | 45 | 100 | 80 | 20.2 | 8.1 | 7.8 | 3 | 0.6 | 50 | 0.101 | 1.115 | 12.83 | 0.40 | 59.51 | 7.61 | |||
| EB | 14 | 45 | 70 | 70 | 20.1 | 8.2 | 7.8 | 3 | 0.6 | 50 | 0.055 | 0.914 | 19.55 | 0.19 | 32.08 | 7.67 | |||
| EC | 14 | 30 | 140 | 100 | 21.1 | 8.3 | 7.8 | 3 | 0.6 | 30 | 0.019 | 0.169 | 10.17 | 0.12 | 0 | 7.7 | |||
| NA | 45 | 20S | 170 | 100 | 18.9 | 8.4 | 9.3 | 0.9 | 0.12 | 20 | 70 | 10 | 0.017 | 0.174 | 11.73 | 0.20 | 7.05 | 8.14 | |
| NB | 45 | 20 | 200 | 120 | 19.6 | 8.6 | 9.3 | 0.9 | 0.12 | 100 | 0.019 | 0.176 | 10.72 | 0.08 | 3.44 | 8.09 | |||
| NC | 45 | 30S | 100 | 100 | 19.1 | 8.5 | 9.3 | 0.9 | 0.12 | 30 | 70 | 0.024 | 0.228 | 11.30 | 0.10 | 4.63 | 8.07 | ||
| WA | 90 | 0 | 230 | 80 | 19.3 | 8.6 | 11.3 | 0.9 | 0.07 | No plants | 0.294 | 5.03 | 19.54 | 0.57 | 36.65 | 8.72 | |||
| WB | 90 | 60S | 140 | 70 | 18.4 | 8.4 | 11.3 | 0.9 | 0.07 | 40 | 60 | 0.016 | 0.167 | 12.10 | 0.08 | 1.24 | 7.81 | ||
| WC | 90 | 60S | 220 | 70 | 18 | 9.3 | 11.3 | 0.9 | 0.07 | 40 | 60 | 0.02 | 0.158 | 9.10 | 0.08 | 0.05 | 7.79 | ||
| VeA | 60 | 60S | 50 | 50 | 18.9 | 8.5 | 9.7 | 1.4 | 0.14 | 100 | 0.023 | 0.203 | 10.41 | 0.10 | 1.94 | 7.89 | |||
| VeB | 60 | 60S | 140 | 80 | 17.8 | 9.5 | 9.7 | 1.4 | 0.14 | 100 | 0.014 | 0.128 | 10.60 | 0.07 | 0 | 8.05 | |||
| VeC | 60 | 60S | 120 | 80 | 18.1 | 9.5 | 9.7 | 1.4 | 0.14 | 100 | 0.025 | 0.233 | 11.21 | 0.09 | 2.93 | 8.01 | |||
| DA | 30 | 45 | 100 | 60 | 16.8 | 8.6 | 8.3 | 2.3 | 0.24 | 70 | 0.022 | 0.201 | 10.38 | 0.15 | 1.52 | 8.18 | |||
| DB | 30 | 45 | 100 | 50 | 18.0 | 8.7 | 8.3 | 2.3 | 0.24 | 50 | 10 | 0.029 | 0.317 | 12.64 | 0.14 | 3.72 | 7.74 | ||
| DC | 30 | 45 | 100 | 60 | 17.6 | 8.9 | 8.3 | 2.3 | 0.24 | 50 | 10 | 0.036 | 0.313 | 10.06 | 0.16 | 3.84 | 7.72 | ||
| VoA | 3 | 45 | 70 | 50 | 18.8 | 8.3 | 10.4 | 1.4 | 0.02 | 50 | 0 | 0.043 | 0.573 | 15.51 | 0.39 | 12.66 | 7.64 | ||
| VoB | 3 | 45 | 70 | 50 | 18.2 | 9.1 | 10.4 | 1.4 | 0.02 | 50 | 0 | 0.027 | 0.278 | 12.07 | 0.33 | 4.5 | 7.81 | ||
| VoC | 3 | 45 | 70 | 40 | 17.7 | 9.1 | 10.4 | 1.4 | 0.02 | 50 | 0 | 0.145 | 1.932 | 15.56 | 0.88 | 27.74 | 7.61 | ||
Lake abbreviations: G, Gooimeer; E, Eemmeer; N, Nuldernauw; W, Wolderwijd; Ve, Veluwemeer; D, Drontermeer; Vo, Vossemeer.
Water retention time (days).
Classes of exposure to the dominant southwest wind direction; the numbers indicate the degrees of the angle of the stations in relation to the southwest wind direction; “S” indicates proximity of the station to the south shore of the lake.
KjN, Kjeldahl nitrogen, i.e., organic N and N-NH3.
Pp, Potamogeton pectinatus; Ch, Chara aspera; Ppf, Potamogeton perfoliatus; Zp, Zannichellia palustris.
Values are averages of the results for five replicate samples.
pH in 1 mM KCl sediment extraction.
In June 2004, samples were collected from the sediment, the water column, and the leaves of submerged macrophytes, herein indicated as benthic, pelagic, and epiphytic samples. In every lake, three stations (A, B, and C) were chosen based on macrophyte distribution maps (44). At every station, five replicate sampling plots were determined randomly by throwing a 1-m2 polyvinyl square on the water surface. The sampling and Secchi depths, pH, and temperature of the lake water were registered on board.
Physical and chemical characterization of sediment and water samples.
Two sediment cores were collected in each of the five replicate 1-m2 plots at every station. The upper-5-cm portions of the cores were homogenized and stored in sealed glass jars to reduce oxygen diffusion. The pH, water content (%), and dry weight were determined with standard protocols (38). The percentages of organic carbon and nitrogen by weight and their molar ratios were measured from freeze-dried and ground samples combusted at 1010°C with an NA-1500 elemental analyzer equipped with a Hyasep-Q column (Carlo Erba Instruments, Milan, Italy). The phosphate content (mmol/kg) was measured with an inductively coupled plasma spectrometer (Optima 3300DV; Perkin/Elmer) after acid and microwave destruction of sediment samples. For molecular analyses of microbial communities, sediment samples were freeze-dried and stored in screw cups at −20°C until analysis.
Water samples were collected in 5-liter plastic containers prior to sediment sampling to reduce the resuspension of sediment particles. No replicate sampling plots per station were defined for the pelagic compartment. Data on oxygen, phosphate, and Kjeldahl nitrogen concentrations in the lakes were provided by K. Oostinga of the Public Service for the IJssel Lagoon of the Department of Waterways and Public Works (Table 1). Water was filtered over 0.45-μm membrane filters for the potential nitrification activity assay and over 0.2-μm membrane filters for the molecular analyses of microbial communities.
Macrophyte species composition, abundance, and sampling.
The composition and percent coverage of submerged macrophytes were visually assessed within the 1-m2 plots. Basically, two species dominated the Border Lakes, Potamogeton pectinatus and Chara aspera. Additional species retrieved and sampled were Potamogeton perfoliatus and Zannichellia palustris. The percent coverages of the macrophyte species per station are summarized in Table 1. Per macrophyte species, samples of floating leaves were collected within the five replicate plots, stored in airtight plastic bags filled with lake water, and used fresh for the activity assay and freeze-dried for the molecular analyses.
Determination of PAA.
The potential ammonia-oxidizing activity (PAA) in the benthic compartment was measured in slurries of 5 g of wet sediment in sterile 250-ml flasks containing 50 ml of 1 mM (NH4)2SO4 mineral medium and incubated on a rotary shaker at 25°C and 150 rpm according to the protocols of Belser and Mays, modified according to Verhagen and Laanbroek (4, 53). Subsamples were taken every 3 h for 24 h and then every 6 h until 160 h of incubation. The NH4+ and NO2− plus NO3− concentrations were measured colorimetrically with a Technicon autoanalyzer (TRAACS 800; Bran Luebbe, Germany). The potential activity and lag phase were determined by using the coefficient of a linear regression calculated from the amount of nitrite plus nitrate produced per hour. The results were normalized for volume loss during sampling and expressed as nmol of NO2− plus NO3− cm−3 h−1. The potential activity in the pelagic compartment was measured in triplicate from 0.45-μm-filter incubations as described above; the activity is expressed in μmol NO2− plus NO3− liter−1 h−1. The activity in the epiphytic compartment was measured from triplicate samples of 1 g of fresh macrophyte leaves, gently dried on paper. Concentrations of 4 mM HEPES and 0.04% bromothymol blue solution were added to the medium as the pH buffer and indicator, respectively. Changes of pH in the medium were restored by adding 5% NaHCO3 solution. Subsamples for the determination of inorganic nitrogen concentrations were taken after every acidification event for a maximum of 15 days. The values presented correspond to the total NO2− plus NO3− production that occurred. No potential activity except for the net NO2− plus NO3− production per dry weight of macrophytes in cm−3 was calculated. The dry weights of the macrophytes were assumed to be 8, 10, 14.0, and 15% of the total fresh weight for C. aspera, Z. palustris, P. pectinatus, and P. perfoliatus, respectively (2, 52).
Statistical analysis of potential activities.
Statistical analyses of potential activities in the benthic and pelagic compartments and of net NO2− plus NO3− production in the epiphytic compartment were conducted with the data analysis software system STATISTICA, version 7.1 (StatSoft, Inc., OK). After checking for normality and homogeneity of variances, followed by transformation if necessary, analyses of variance were conducted on the activities to test for the effects of the lake, station, and plot factors and the effects of or the correlation with the environmental parameters associated with the different compartments.
DNA extraction from sediment, water, and macrophyte samples.
Environmental DNA was extracted from 0.5 g of freeze-dried sediment or from 0.3 g of freeze-dried macrophyte leaves and from 250 ml of water samples filtered over a 0.2-μm membrane filter according to the protocol described by Henckel et al. (18), with minor modifications in the centrifugation times used. Finally, the DNA was resuspended in 100 μl of preheated (65°C) sterile purified water and concentrated in a 50-μl final volume by using an AMPure PCR purification system according to the manufacturer's instructions (Agencourt Bioscience Corporation, Beverly, MA). Quantification was done spectrophotometrically using 2-μl DNA samples (Nanodrop ND-1000; Nanodrop Technology, Wilmington, DE).
PCRs and DGGE.
The diversity of the ammonia-oxidizing community in the three compartments was analyzed with two different nested PCR-denaturing gradient gel electrophoresis (DGGE) assays based on the amplification of 16S rRNA gene sequences. Approach I used a nested PCR procedure combining sequentially primer sets βamoF and βamoR (35) and CTO189f-GC and CTO654r (28). Approach II differed in the primer set used for the first amplification step, i.e., 27f and 907r (29). The specificities and sensitivities of the primer sets have been described before (24, 28, 35). An amount of 100 ng of purified DNA was used as template for a 25-μl PCR mixture containing 1× Mg-free buffer (Invitrogen Corp., Carlsbad, CA), 0.5 μM of each primer (1.0 μM in the case of βamoF, to compensate for ambiguities), 200 μM of each deoxynucleotide triphosphate, 1.75 mM MgCl2, 400 ng/μl bovine serum albumin, 2 U Taq polymerase. The thermocycling program for the first step consisted of 5 min of denaturation at 94°C followed by 25 cycles of 30 s of denaturation at 94°C; 45 s of specific annealing at 59°C (for the βamo primer set) or, in a touchdown protocol, at 65 to 55°C (for the primer set 27f-907r); and 30 s of elongation at 72°C; 10 min of final elongation was performed for all reactions. Nested amplifications of 20 cycles were performed with the primer set CTO189f-GC and CTO 654r on 1:100 dilutions of PCR products. DNA samples of Nitrosomonas europaea and Nitrosomonas ureae were used as positive controls. All reactions were verified by UV illumination of 1.2% agarose gels stained in ethidium bromide solution. CTO amplicons were separated by electrophoresis for 17 h at 60°C in 0.5× Tris-acetate-EDTA buffer on a denaturing gradient gel of 30 to 55% as described by Kowalchuk et al. (28). The gels were stained for 1 h in a 0.05-μg/ml ethidium bromide solution and visualized with UV. Bands of interest were cut in their middle portions and, after elution from polyacrylamide overnight at 4°C, reamplified with a maximum of 20 cycles of PCR and reloaded on denaturing gradient gel until single and pure bands suitable for sequencing reactions were obtained.
Analyses of sequences of AOB-related bands.
Sequences were aligned with the fast-aligner tool of the ARB software for phylogenetic analyses (30). A 16S rRNA gene phylogenetic tree was constructed based on previously published neighbor-joining trees of the two major subgroups of the beta subclass of the AOB (i.e., Nitrosomonas spp. and Nitrosospira spp.). The band sequences were added to ammonia oxidizers’ sequence subsets using the parsimony criterion and ad hoc-created filters. Sequences that appeared to be duplicates were neither considered in the phylogenetic analyses nor submitted to the EMBL databank.
Analysis of AOB communities within and between compartments.
AOB community structures obtained by means of DGGE were analyzed with PRIMER (Plymouth routines in multivariate ecological research) software, version 5 (8). Bands related to AOB were used to build similarity matrices (Bray-Curtis coefficient) based on the presence or absence of single bands, as well as on the abundance of members in the different AOB clusters and lineages. The matrices were used as inputs for analyses of similarities (2001 PRIMER version 5, see user's manual/tutorial; PRIMER-E, Plymouth, United Kingdom), hierarchical clustering, and nonmetric multidimensional scaling to test for the effects of different factors on community composition within compartments and to compare communities between different compartments. The possible effects of environmental variables on the AOB community were tested for by combining the similarity matrices with Euclidean distance-based matrices of log-transformed environmental variables.
Nucleotide sequence accession numbers.
Sequences obtained from bands related to ammonia oxidizers were submitted to the EMBL databank under the following accession numbers: AM418429 to AM418440, AM418441 to AM418445, and AM418446 and AM418447 for bands obtained from the benthic, pelagic, and epiphytic compartments, respectively.
RESULTS
Potential activity in the benthic compartment.
The PAA in the benthic compartment varied from a minimum of 32 ± 5 (mean ± standard deviation) to a maximum of 174 ± 19 nmol NO2− plus NO3− cm−3 h−1 (Table 2). The lag-phase values ranged from a minimum of 19 h to a maximum of 51 h and were negatively correlated with the PAA values (ρ = −0.44). Samples from lakes subjected to restoration in the past, i.e., Lakes Nuldernauw, Wolderwijd, and Veluwemeer, all had lag-phase times of more than 35 h. By univariate analyses, the factor “lake” had a significant effect on both activity (df = 6, F = 3.31, P < 0.05) and lag phase (df = 6, F = 24.13, P < 0.001), the latter also being highly significantly influenced by the factor “station” (df = 2, F = 11.40, P < 0.001). The factor “plot” did not significantly affect PAA or lag phase. The water retention time and wind exposure both had a significant effect on the PAA (df = 6, F = 3.52, P < 0.05 and df = 5, F = 3.44, P < 0.05, respectively) and on the lag phase (df = 6, F = 7.02, P < 0.05 and df = 5, F = 7.61, P < 0.05, respectively). It was shown that the PAA values were significantly and positively correlated with the percentages of silt, total N, and organic C (ρ = 0.48, 0.55, and 0.32, respectively), while the lag-phase values were significantly but negatively correlated with phosphate content (ρ = −0.52).
TABLE 2.
PAA and lag phase in the benthic compartment and PAA in the pelagic compartmenta
| Lake and station | Benthic PAA (nmol NO2− plus NO3− cm−3 h−1) | Benthic lag phase (h) | Pelagic PAA (μmol NO2− plus NO3− liter−1 h−1) |
|---|---|---|---|
| GA | 129 ± 21 | 37 ± 4 | 0.18 ± 0.10 |
| GB | 86 ± 6 | 41 ± 4 | 0.07 ± 0.01 |
| GC | 80 ± 9 | 38 ± 12 | 0.92 ± 0.58 |
| EA | 132 ± 9 | 34 ± 9 | 0.31 ± 0.07 |
| EB | 110 ± 11 | 36 ± 6 | 0.70 ± 0.01 |
| EC | 124 ± 3 | 23 ± 4 | 0.15 ± 0.00 |
| NA | 71 ± 6 | 44 ± 6 | NDb |
| NB | 123 ± 8 | 43 ± 4 | ND |
| NC | 96 ± 17 | 44 ± 3 | ND |
| WA | 174 ± 19 | 41 ± 6 | ND |
| WB | 32 ± 5 | 52 ± 1 | ND |
| WC | 114 ± 8 | 35 ± 3 | ND |
| VeA | 99 ± 7 | 40 ± 4 | ND |
| VeB | 101 ± 9 | 49 ± 2 | ND |
| VeC | 122 ± 3 | 44 ± 4 | ND |
| DA | 118 ± 10 | 26 ± 6 | 0.29 ± 0.12 |
| DB | 118 ± 4 | 35 ± 3 | 0.16 ± 0.05 |
| DC | 129 ± 16 | 29 ± 6 | 0.07 ± 0.00 |
| VoA | 100 ± 12 | 19 ± 6 | 0.07 ± 0.01 |
| VoB | 155 ± 7 | 26 ± 2 | 0.04 ± 0.00 |
| VoC | 65 ± 11 | 25 ± 11 | 0.04 ± 0.01 |
Values are the averages ± standard deviations of the results for five and three replicate samples for the benthic and pelagic compartments, respectively. Lake abbreviations: G, Gooimeer; E, Eemmeer; N, Nuldernauw; W, Wolderwijd; Ve, Veluwemeer; D, Drontermeer; Vo, Vossemeer.
ND, not determined.
Potential activity in the pelagic compartment.
The PAA in the pelagic compartment varied from 0 to 1 μmol NO2− plus NO3− liter−1 h−1. It was absent in the samples from Lakes Nuldernauw, Wolderwijd, and Veluwemeer, again the lakes subjected to restoration in the past (Table 2). The responses to the addition of ammonium, expressed as the lag phase, were all around 65 h. The Kruskal-Wallis test identified a highly significant effect of the lake factor on the pelagic PAA (df = 6, H = 56.23, P < 0.001), but not of the station factor. Among the environmental variables, nonparametric univariate analyses indicated a positive effect of oxygen and phosphate concentrations (df = 6, H = 56.23, P < 0.001), as well as of water retention time (df = 6, H = 56.72, P < 0.001), on pelagic PAA.
Net NO2− plus NO3− production in the epiphytic compartment.
The net production of oxidized inorganic nitrogen compounds during the incubations of macrophyte leaves from Lakes Gooimeer and Eemmeer varied from 0 to a maximum of 175 mmol NO2− plus NO3− g dry weight−1 cm−3 but the production of oxidized inorganic nitrogen compounds was never detectable during the incubations of macrophyte leaves from Lakes Veluwemeer, Wolderwijd, Drontermeer, and Vossemeer (Table 3). Kruskal-Wallis analysis of variance indicated significant effects of the lake, macrophyte species, and station factors (df = 6, H = 98.77, P < 0.001; df = 3, H = 30.04, P < 0.001; and df = 2, H = 7.03, P < 0.05, respectively), in contrast to the plot factor that had no significant effect. Among the environmental variables tested relating to water quality, only water retention time had a highly significant effect on the activity associated with the leaves of submerged macrophytes (df = 6, H = 98.77, P < 0.001).
TABLE 3.
NO2− plus NO3− production in the epiphytic compartment of different lakes and stationsa
| Lake, station, and macrophyte species | Net epiphytic activity (mmol NO2− plus NO3− g dwt−1 cm−3) |
|---|---|
| GA P. perfoliatus | 23 ± 4.0 |
| GA P. pectinatus | 105 ± 0.8 |
| GB P. pectinatus | 105 ± 0.8 |
| GC P. pectinatus | 70 ± 3.2 |
| GC P. perfoliatus | 13 ± 2.9 |
| EA Z. palustris | 151 ± 0.3 |
| EB Z. palustris | 96 ± 0.2 |
| EC P. pectinatus | 105 ± 1.6 |
Values represent the averages ± standard deviations of the results for five replicate samples, except for sample GA Potamogeton perfoliatus for which only three replicates were available. Lake abbreviations: G, Gooimeer; E, Eemmeer. dwt, dry weight.
Ammonia-oxidizing-community composition in the benthic compartment.
The compositions of the AOB communities in the benthic, pelagic, and epiphytic compartments were assessed with two different PCR-DGGE approaches.
Table 4 gives an overview of the bands retrieved in this study together with the AOB lineages or clusters to which they were affiliated. It also presents the percentages of their recovery in the benthic, pelagic, and epiphytic samples. The large sampling effort and the use of two different molecular PCR-DGGE assays allowed a detailed comparison of the community compositions within and between compartments.
TABLE 4.
Ammonia-oxidizing community composition in the benthic, pelagic, and epiphytic compartments as obtained with approaches I and IIa
| Compartment | Approach I
|
Approach II
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Band, affiliation | % of recovered bands | % Significance of factor (global R value)
|
Band, affiliation | % of recovered bands | % Significance of factor (global R value)
|
|||||
| Lake | Station | Macrophyte sp. | Lake | Station | Macrophyte sp. | |||||
| Benthic (n = 21) | R04BS1, Nsm olig. | 100 | 0.5 (0.33) | 62 (<0) | NT | R04uS3, Nsm olig. | 100 | 0.1 (0.51) | 100 (<0) | NT |
| R04BS2, Nsp cluster 3 | 100 | R04uS8, Nsp cluster 3 | 9 | |||||||
| R04BS4, Nsp cluster 0 | 100 | R04uS2, Nsm sp. Nm 143 | 28 | |||||||
| R04BS8, Nsp cluster 0 | 47 | No AOB-related bands | 0 | |||||||
| No AOB-related bands | 0 | |||||||||
| Pelagic (n = 21) | R04BW1, Nsm olig. | 38 | 0.1 (0.45) | 90 (<0) | NT | No bands detected | 100 | |||
| R04BW7, Nsm olig. | 33 | |||||||||
| No AOB-related bands | 58 | |||||||||
| Epiphytic (n = 30) | R04BM1, Nsm olig. | 10 | 0.1 (0.40) | 94 (<0) | 4.7 (0.12) | R04uM3, Nsp cluster 3 | 60 | 5.6 (0.13) | 94 (<0) | 1.9 (0.15) |
| R04BM3, Nsp cluster 3 | 60 | R04uM5, Nsm olig. | 23 | |||||||
| R04BM5, Nsm olig. | 30 | No AOB-related | 36 | |||||||
| No AOB-related bands | 26 | bands | ||||||||
The effects of the lake, station, and macrophyte species factors on the ammonia-oxidizing communities of the three different compartments were calculated with the analysis of similarity procedure of the software package PRIMER, version 5 (9). Analyses were conducted on similarity matrices constructed separately from band patterns obtained with approaches I and II. Values in bold font are significant. Nsm, Nitrosomonas lineage; Nsm olig., Nitrosomonas oligotropha lineage; Nsp, Nitrosospira lineage; NT, not tested.
In particular in the benthic compartment, AOB-related bands were detected in all 21 samples with both approaches. A total of four AOB-related bands were detected with approach I (Table 4), of which band R04BS1 belonged to the Nitrosomonas oligotropha lineage, band R04BS2 to cluster 3 of the Nitrosospira lineage, and bands R04BS4 and R04BS8 to cluster 0 of the Nitrosospira lineage (24). A total of three bands were detected with approach II (Table 4); band R04uS3 belonged to the Nitrosomonas oligotropha lineage, band R04uS2 belonged to the Nitrosomonas sp. Nm 143 lineage, and band R04uS8 to cluster 3 of the Nitrosospira lineage. Based on single-linkage hierarchical cluster analyses, the DGGE band pattern profiles of single samples obtained with approach I showed a percentage of similarity of 87%, with the exception of sample WB, from station B from Lake Wolderwijd (only 75%). The DGGE band profiles obtained with approach II had percentages of similarity of around 70%, which was low in comparison with the percentages of similarity obtained with approach I.
The assessment of the AOB community was significantly affected by the choice of PCR-DGGE approach, as was shown also by analyses of similarity performed on matrices based on the abundance of members of AOB lineages (i.e., number of bands per lineage or cluster). With approach I, members of the N. oligotropha lineage and clusters 3 and 0 of the Nitrosospira lineage were retrieved in all samples (Table 4). In contrast, with approach II (Table 4), only members of the N. oligotropha lineage were retrieved in all samples, while members of Nitrosospira cluster 3 were retrieved in only 9% of the samples and members of Nitrosospira cluster 0 were completely absent. In addition, members of the Nitrosomonas sp. Nm 143 lineage were retrieved in 28% of the benthic samples.
Analyses of similarities showed a marginally significant and a significant effect of the lake factor on the benthic ammonia-oxidizing communities when assessed with approaches I and II, respectively (Table 4). The station factor had no effect.
Ammonia-oxidizing-community composition in the pelagic compartment.
In the pelagic compartment, approach II failed to detected AOB-related bands (Table 4). Approach I was successful in detecting AOB-related bands in 42% of the samples. A total of two bands, i.e., R04BW1 and R04BW7, were detected in 38 and 33% of the samples, respectively (Table 4). Both bands were related to AOB of the N. oligotropha lineage that apparently dominated the pelagic AOB communities. The lake factor was significant for the composition of the pelagic AOB community (Table 4).
Ammonia-oxidizing-community composition in the epiphytic compartment.
In the epiphytic compartment, AOB-related bands were detected in 74 and 64% of the 30 macrophyte samples with approaches I and II, respectively (Table 4). A total of 3 bands were detected with approach I. Bands R04BM1 and R04BM5 were affiliated with the N. oligotropha lineage and R04BM3 with cluster 3 of the Nitrosospira lineage. The latter was retrieved in 60% of the macrophyte samples. In more detail, band R04BM1 was retrieved only in epiphytic samples of Z. palustris, present at stations A and B of Lake Eemmeer, while R04BM5 was retrieved also in epiphytic samples of P. pectinatus at stations A, B, and C of Lake Gooimeer and of C. aspera at station B of Lake Wolderwijd.
With the use of approach II, two bands were detected (Table 4). Band R04uM3, belonging to cluster 3 of the Nitrosospira lineage, was detected in 60% of the samples. Band R04uM5, which belongs to the N. oligotropha lineage, was detected in 23% of samples, namely, in samples of Z. palustris collected at station A of Lake Eemmeer and of P. pectinatus gathered at stations A and B of Lake Gooimeer, at station B of Lake Wolderwijd, and at station C of Lake Vossemeer. Analyses of similarities showed no effect of the approach used on the description of epiphytic community composition, even though the detection of bands belonging to the N. oligotropha lineage was affected by the choice of PCR approaches (Table 4). In addition, the lake factor was significant for the composition of the epiphytic AOB community if assessed with approach I, but not with approach II (Table 4).
Phylogenetic analyses.
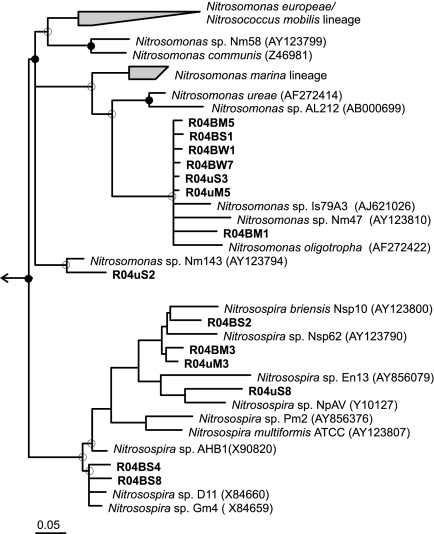
Despite the differences within the compartments, analyses of the affiliation of bands revealed percentages of similarity of above 96% between bands obtained with different PCR approaches that grouped together within the Nitrosomonas or the Nitrosospira lineages, as evident in the phylogenetic tree (Fig. 2). Moreover, bands retrieved in different compartments also showed high similarity values (>93%) when clustered together.
FIG. 2.
Neighbor-joining tree of selected 16S rRNA gene sequences of ammonia-oxidizing betaproteobacteria of the Nitrosomonas and Nitrosospira lineages. Filled and empty circles indicate bootstrap values (100 resamplings) above 90 and 70%, respectively. The tree was rooted with Gallionella ferruginea (L07897), indicated by the arrow. The gray-filled trapezoidal branches include sequences of AOB belonging to the AOB lineage indicated at the side of each trapezoidal branch. The sequences included in the branches were used to calculate the tree. The scale bar represents 5% estimated sequence divergence. Bands in bold font were retrieved from benthic, pelagic, or epiphytic compartments, represented by “S,” “W,” and “M,” respectively, in the band code. The first part of the band code (R04) corresponds to the Border Lakes sampling campaign of the year 2004 and is followed by an abbreviation for the approach, i.e., “B” for nested approach I based on the βamo primer set or “u” for nested approach II based on the 27f-907r primer set.
Comparison of AOB communities and the effects of environmental variables.
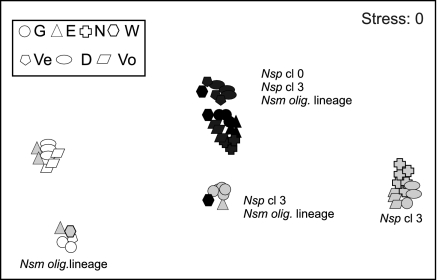
Similarity matrices based on the presence or absence of bands detected with nested PCR approach I were converted into abundance matrices of species belonging to AOB lineages and clusters and subjected to nonmetric multidimensional scaling to allow comparison between compartments (Fig. 3). The AOB communities differed highly significantly between compartments; in particular, the pelagic and epiphytic communities were slightly less dissimilar to each other than either was to the benthic compartment (Rpw = 0.53; pw indicates pairwise comparison). The Rpw value for the benthic versus the pelagic community was 0.99 and for the benthic versus the epiphytic community was 0.63. The Ppw value for all pairwise comparisons was 0.001, which indicates significant differences. The global R value for the compartment factor was 0.69 (P, 0.001; indicates significant differences). The global R value {R = rB − rW/[n(n − 1)/4], where rB is the average of all rank dissimilarities between band percentage profiles between compartments, lakes, or stations and rW is the average of all rank dissimilarities between band percentage profiles within compartments, lakes, or stations} indicates whether the difference between compartments is lower than, equal to, or higher than the difference within compartments (−1 < R > 1). Members of the N. oligotropha lineage and of Nitrosospira clusters 3 and 0 inhabited the benthic compartment, while only members of the N. oligotropha lineage dominated the pelagic zone (Fig. 3). The epiphytic compartment was populated both by members of Nitrosospira cluster 3 and by members of the N. oligotropha lineage. The majority of epiphytic samples were colonized by members of Nitrosospira cluster 3 (Fig. 3). However, the epiphytic AOB communities of all samples from Lake Eemmeer were colonized only by members of the N. oligotropha lineage, while five out of six epiphytic samples from Lake Gooimeer were colonized by members of both groups.
FIG. 3.
Multidimensional scaling plot of AOB communities of the benthic, pelagic, and epiphytic compartments based on the results of PCR approach I. Calculations were done from similarity matrices based on the abundance of AOB-related bands per AOB lineage or cluster. Different shapes are used to indicate samples from different lakes: G, Gooimeer; E, Eemmeer; N, Nuldernauw; W, Wolderwijd; Ve, Veluwemeer; D, Drontermeer; and Vo, Vossemeer. Black, white, and gray correspond to the benthic, pelagic, and epiphytic compartments, respectively. Nsm olig., Nitrosomonas oligotropha lineage; Nsp, Nitrosospira lineage; cl, cluster.
Matrices based on the abundance of species in the AOB clusters were combined with Euclidean-distance-based dissimilarity matrices of the environmental variables by using the BIOENV procedure of PRIMER. Best matches for the distribution of groups of AOB with the combined concentrations of nitrogen and phosphate (ρ = 0.65 and ρ = 0.57 for approach I- and II-based matrices, respectively) were obtained.
DISCUSSION
This study focused on the activity and composition of the communities of AOB of the beta subclass of the Proteobacteria in the benthic, pelagic, and epiphytic compartments of seven shallow freshwater lakes characterized by submerged macrophytes. The ability of the benthic AOB communities to nitrify appeared quite poor, as demonstrated by the high lag-phase values, which were all above 20 h. In particular, benthic samples from Lakes Nuldernauw, Wolderwijd, and Veluwemeer showed lower potential activities and higher lag-phase values. The significant effect of the lake factor and, additionally, the effects of nutrient concentrations (N, C, and P), silt content, water retention time, and wind exposure suggest that the potential activities of the AOB communities depend on the environmental conditions that characterize the lakes. In the pelagic compartment, the potential activities were lower than those in the benthic compartment and absent in samples from Lakes Nuldernauw, Wolderwijd, and Veluwemeer. Again, the lake factor turned out to be significant, as did the phosphate and oxygen concentrations and water retention times in the lakes themselves. The amount of nitrite plus nitrate production during the incubation of epiphytic samples was comparable to the potential nitrification activity measured by Eriksson and Andersson (14) from the litter of macrophytes. Again, the lake factor appeared to be highly significant in the analyses of variance between samples. Here, retention time was the only environmental variable with a significant effect on the activity.
The potential activity assay chosen does not allow direct comparison of actual activities between compartments. For this, the use of 15N stable isotope techniques is preferable over all other techniques (1). The potential nitrification assesses the number of potentially active cells present in the compartments and therefore reflects the environmental history of the cells more than the immediate in situ activity. It might be of interest to notice that lakes with a history of restoration (i.e., manipulation of nutrients), namely, Lakes Nuldernauw, Wolderwijd, and Veluwemeer, appeared to have lower activities in all compartments. Pauer and Auer (39) found little evidence of nitrification in the water column of hypereutrophic lakes and strongly suggested that nitrification is a sediment-based process. However, they excluded epiphytic AOB communities from their study. Bastviken et al. and Eriksson (3, 13) suggested that macrophyte litter strongly supported or restrained epiphytic nitrification depending on the macrophyte species studied. Our results confirmed their observations.
Our investigation of the compositions of AOB communities of the beta subclass of the Proteobacteria had limitations which are related to the use of molecular assays based on the PCR-DGGE procedure. When using this procedure with AOB, heteroduplexes and other artifacts are easily formed (46) and, therefore, caution is necessary when drawing conclusions on AOB community composition based on minor variations in nucleotide sequences. Bands separated on denaturing gradient gels were sequenced, and duplicates excluded from the analyses. It was shown that the approach chosen had an effect on the final assessment of the bacterial community composition. In particular, the composition of the benthic AOB communities appeared biased toward members of the Nitrosospira lineage and against members of the Nitrosomonas sp. Nm 143 lineage when assessed with approach I. This was caused by mismatches of the βamo and CTO primer sets with species belonging to the Nitrosomonas sp. Nm 143 lineage (24). On the other hand, approach II, based on a combination of universal and AOB-specific primer sets, failed to detect members of cluster 3 of the Nitrosospira lineage in the benthic compartment and was not suitable for the pelagic compartment. However, approaches I and II gave comparable results for the epiphytic community compositions. Finally, it appeared that only by applying two primer sets, both specific for AOB, i.e., βamo and CTO in approach I, could the diversity of the AOB community be assessed in all three compartments. Unfortunately, the use of several approaches is necessary when characterizing microbial communities in natural environments (32). Nevertheless, to the best of our knowledge, this is the first time AOB species were retrieved from epiphytic communities by means of molecular techniques. The presence of AOB in the epiphytic compartment of submerged macrophytes has been reported by Körner (25) on the basis of most-probable-number counts and has been assessed by Bastviken et al. and Eriksson (3, 13) by using nitrification activity assays. As hypothesized, the benthic, pelagic, and epiphytic communities differed among each other, and the epiphytic compartment appeared to be inhabited by both benthic and pelagic ammonia oxidizers.
We intentionally did not relate the diversity of AOB communities to activity measurements because of the limitation implied in the use of molecular assays that are not based on functional gene expression (7, 11). As a matter of fact, the mere retrieval of a 16S rRNA gene sequence of AOB proves neither that this organism is abundant nor that it is physiologically active (24), but it definitely indicates the presence of AOB in a particular environment.
Belonging to one or the other lake was apparently an important factor both for the potential activity and for the community composition of the AOB. Several environmental variables were tested, and the nitrogen and phosphate concentrations, as well as the water retention time, turned out to play a major role in the differences between lakes. All these factors have been subject to change according to the restoration history of the lakes. Hence, the AOB community characteristics as assessed in our study are linked to the trophic status of the lake, thereby offering the potential of being used in monitoring programs. Our sampling area, constituted of seven shallow, freshwater lakes, is representative of a typical wetland-type environment where the presence of stable stands of submerged macrophytes is desirable for the maintenance of a clear-water state against algal dominance.
The results of our study suggest that the epiphytic compartment provides a niche for the AOB community in addition to the niches offered by the benthic and pelagic compartments. The role of the epiphytic AOB community in the nitrogen turnover of shallow freshwater lakes appears to be an appealing subject for further investigation, especially from the perspective of the establishment of dense stands of macrophytes that is a target for water management to repress the growth of noxious algae and cyanobacteria.
Acknowledgments
We thank K. Swart for technical assistance on board, M. Houtekamer and W. Smant for technical assistance in the chemical analyses, and K. Oostinga for providing information on the Border Lakes.
This is publication number 4244 of The Netherlands Institute of Ecology (NIOO-KNAW).
Footnotes
Published ahead of print on 8 February 2008.
REFERENCES
- 1.Andersson, M. G. I., N. Brion, and J. J. Middelburg. 2006. Comparison of nitrifier activity versus growth in the Scheldt estuary—a turbid, tidal estuary in northern Europe. Aquat. Microb. Ecol. 42:149-158. [Google Scholar]
- 2.Anthoni, U., C. Christophersen, J. O. G. R. Madsen, S. Wium-Andersen, and N. Jacobsen. 1980. Biologically active sulphur compounds from the green alga Chara globularis. Phytochemistry 19:1228-1229. [Google Scholar]
- 3.Bastviken, S. K., P. G. Eriksson, I. Martins, J. M. Neto, L. Leonardson, and K. Tonderski. 2003. Potential nitrification and denitrification on different surfaces in a constructed treatment wetland. J. Environ. Qual. 32:2414-2420. [DOI] [PubMed] [Google Scholar]
- 4.Belser, L. W., and E. L. Mays. 1980. Specific inhibition of nitrite oxidation by chlorate and its use in assessing nitrification in soils and sediments. Appl. Environ. Microbiol. 39:505-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bollmann, A., and H. J. Laanbroek. 2001. Continuous culture enrichments of ammonia-oxidizing bacteria at low ammonium concentrations. FEMS Microbiol. Ecol. 37:211-221. [Google Scholar]
- 6.Bollmann, A., and H. J. Laanbroek. 2002. Influence of oxygen partial pressure and salinity on the community composition of ammonia-oxidizing bacteria in the Schelde estuary. Aquat. Microb. Ecol. 28:239-247. [Google Scholar]
- 7.Bollmann, A., I. Schmidt, A. M. Saunders, and M. H. Nicolaisen. 2005. Influence of starvation on potential ammonia-oxidizing activity and amoA mRNA levels of Nitrosospira briensis. Appl. Environ. Microbiol. 71:1276-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke, K. R., and R. M. Warwick. 2001. Change in marine communities: an approach to statistical analysis and interpretation, 2nd ed. PRIMER-E, Plymouth, United Kingdom.
- 9.Clarke, K. R., and R. M. Warwick. 1994. Change in marine communities: an approach to statistical analysis and interpretation, 2nd ed. Plymouth Marine Laboratory, Plymouth, United Kingdom.
- 10.Coci, M., D. Riechmann, P. L. E. Bodelier, S. Stefani, G. Zwart, and H. J. Laanbroek. 2005. Effect of salinity on temporal and spatial dynamics of ammonia-oxidising bacteria from intertidal freshwater sediment. FEMS Microbiol. Ecol. 53:359-368. [DOI] [PubMed] [Google Scholar]
- 11.Ebie, Y., N. Noda, H. Miura, M. Matsumura, S. Tsuneda, A. Hirata, and Y. Inamori. 2004. Comparative analysis of genetic diversity and expression of amoA in wastewater treatment processes. Appl. Microbiol. Biotechnol. 64:740-744. [DOI] [PubMed] [Google Scholar]
- 12.Eighmy, T. T., and P. L. Bishop. 1989. Distribution and role of bacterial nitrifying populations in nitrogen removal in aquatic treatment systems. Water Res. 23:947-955. [Google Scholar]
- 13.Eriksson, P. G. 2001. Interaction effects of flow velocity and oxygen metabolism on nitrification and denitrification in biofilms on submersed macrophytes. Biogeochemistry 55:29-44. [Google Scholar]
- 14.Eriksson, P. G., and J. L. Andersson. 1999. Potential nitrification and cation exchange on litter of emergent, freshwater macrophytes. Freshw. Biol. 42:479-486. [Google Scholar]
- 15.Eriksson, P. G., and S. E. B. Weisner. 1999. An experimental study on effects of submersed macrophytes on nitrification and denitrification in ammonium-rich aquatic systems. Limnol. Oceanogr. 44:1993-1999. [Google Scholar]
- 16.Goreau, T. J., W. A. Kaplan, S. C. Wofsy, M. B. McElroy, F. W. Valois, and S. W. Watson. 1980. Production of NO2− and N2O by nitrifying bacteria at reduced concentrations of oxygen. Appl. Environ. Microbiol. 40:526-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Head, I. M., W. D. Hiorns, T. M. Embley, A. J. McCarthy, and J. R. Saunders. 1993. The phylogeny of autotrophic ammonia-oxidizing bacteria as determined by analysis of 16S ribosomal RNA gene sequences. J. Gen. Microbiol. 139:1147-1153. [DOI] [PubMed] [Google Scholar]
- 18.Henckel, T., M. Friedrich, and R. Conrad. 1999. Molecular analyses of the methane-oxidizing microbial community in rice field soil by targeting the genes of the 16S rRNA, particulate methane monooxygenase, and methanol dehydrogenase. Appl. Environ. Microbiol. 65:1980-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horz, H. P., A. Barbrook, C. B. Field, and B. J. M. Bohannan. 2004. Ammonia-oxidizing bacteria respond to multifactorial global change. Proc. Natl. Acad. Sci. USA 101:15136-15141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hovanec, T. A., and E. F. DeLong. 1996. Comparative analysis of nitrifying bacteria associated with freshwater and marine aquaria. Appl. Environ. Microbiol. 62:2888-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang, Q. Q., and L. R. Bakken. 1999. Comparison of Nitrosospira strains isolated from terrestrial environments. FEMS Microbiol. Ecol. 30:171-186. [DOI] [PubMed] [Google Scholar]
- 22.Juretschko, S., G. Timmermann, M. Schmid, K. H. Schleifer, A. Pommerening-Röser, H. P. Koops, and M. Wagner. 1998. Combined molecular and conventional analyses of nitrifying bacterium diversity in activated sludge: Nitrosococcus mobilis and Nitrospira-like bacteria as dominant populations. Appl. Environ. Microbiol. 64:3042-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koops, H. P., and A. Pommerening-Röser. 2001. Distribution and ecophysiology of the nitrifying bacteria emphasizing cultured species. FEMS Microbiol. Ecol. 37:1-9. [Google Scholar]
- 24.Koops, H. P., U. Purkhold, A. Pommerening-Röser, G. Timmermann, and M. Wagner. 2003. The lithoautotrophic ammonia-oxidizing bacteria. In M. Dworkin et al. (ed.), The prokaryotes: an evolving electronic resource for the microbiological community, 3rd ed., release 3.13. Springer-Verlag, New York, NY.
- 25.Körner, S. 1999. Nitrifying and denitrifying bacteria in epiphytic communities of submerged macrophytes in a treated sewage channel. Acta Hydrochim. Hydrobiol. 27:27-31. [Google Scholar]
- 26.Kowalchuk, G. A., P. L. E. Bodelier, G. H. J. Heilig, J. R. Stephen, and H. J. Laanbroek. 1998. Community analysis of ammonia-oxidising bacteria, in relation to oxygen availability in soils and root-oxygenated sediments, using PCR, DGGE and oligonucleotide probe hybridisation. FEMS Microbiol. Ecol. 27:339-350. [Google Scholar]
- 27.Kowalchuk, G. A., and J. R. Stephen. 2001. Ammonia-oxidizing bacteria: a model for molecular microbial ecology. Annu. Rev. Microbiol. 55:485-529. [DOI] [PubMed] [Google Scholar]
- 28.Kowalchuk, G. A., J. R. Stephen, W. DeBoer, J. I. Prosser, T. M. Embley, and J. W. Woldendorp. 1997. Analysis of ammonia-oxidizing bacteria of the beta subdivision of the class Proteobacteria in coastal sand dunes by denaturing gradient gel electrophoresis and sequencing of PCR-amplified 16S ribosomal DNA fragments. Appl. Environ. Microbiol. 63:1489-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lane, D. J. 1991. 16S/23S rRNA sequencing, p. 115-175. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. Wiley, Chichester, United Kingdom.
- 30.Ludwig, W., O. Strunk, R. Westram, L. Richter, H. Meier, Yadhukumar, A. Buchner, T. Lai, S. Steppi, G. Jobb, W. Forster, I. Brettske, S. Gerber, A. W. Ginhart, O. Gross, S. Grumann, S. Hermann, R. Jost, A. Konig, T. Liss, R. Lussmann, M. May, B. Nonhoff, B. Reichel, R. Strehlow, A. Stamatakis, N. Stuckmann, A. Vilbig, M. Lenke, T. Ludwig, A. Bode, and K. H. Schleifer. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macdonald, R. M. 1979. Population dynamics of the nitrifying bacterium Nitrosolobus in soil. J. Appl. Ecol. 16:529-535. [Google Scholar]
- 32.Mahmood, S., T. E. Freitag, and J. I. Prosser. 2006. Comparison of PCR primer-based strategies for characterization of ammonia oxidizer communities in environmental samples. FEMS Microbiol. Ecol. 56:482-493. [DOI] [PubMed] [Google Scholar]
- 33.Matulewich, V. A., P. F. Strom, and M. S. Finstein. 1975. Length of incubation for enumerating nitrifying bacteria present in various environments. Appl. Microbiol. 29:265-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matulewich, V. A., and M. S. Finstein. 1978. Distribution of autotrophic nitrifying bacteria in a polluted river (the Passaic). Appl. Environ. Microbiol. 35:67-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCaig, A. E., T. M. Embley, and J. I. Prosser. 1994. Molecular analysis of enrichment cultures of marine ammonia oxidizers. FEMS Microbiol. Lett. 120:363-367. [DOI] [PubMed] [Google Scholar]
- 36.McCaig, A. E., L. A. Glover, and J. I. Prosser. 1999. Molecular analysis of bacterial community structure and diversity in unimproved and improved upland grass pastures. Appl. Environ. Microbiol. 65:1721-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meijer, M. L., I. de Boois, M. Scheffer, R. Portielje, and H. Hosper. 1999. Biomanipulation in shallow lakes in The Netherlands: an evaluation of 18 case studies. Hydrobiologia 409:13-30. [Google Scholar]
- 38.Mudroch, A., and J. M. Azcue. 1995. Manual of aquatic sediment sampling. CRC Press LLC, Boca Raton, FL.
- 39.Pauer, J. J., and M. T. Auer. 2000. Nitrification in the water column and sediment of a hypereutrophic lake and adjoining river system. Water Res. 34:1247-1254. [Google Scholar]
- 40.Phillips, C. J., Z. Smith, T. M. Embley, and J. I. Prosser. 1999. Phylogenetic differences between particle-associated and planktonic ammonia-oxidizing bacteria of the beta subdivision of the class Proteobacteria in the northwestern Mediterranean Sea. Appl. Environ. Microbiol. 65:779-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Powell, S. J., and J. I. Prosser. 1992. Inhibition of biofilm populations of Nitrosomonas europaea. Microb. Ecol. 24:43-50. [DOI] [PubMed] [Google Scholar]
- 42.Prinčič, A., I. Mahne, F. Megušar, E. A. Paul, and J. M. Tiedje. 1998. Effects of pH and oxygen and ammonium concentrations on the community structure of nitrifying bacteria from wastewater. Appl. Environ. Microbiol. 64:3584-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prosser, J. I. 1989. Autotrophic nitrification in bacteria. Adv. Microb. Physiol. 30:125-181. [DOI] [PubMed] [Google Scholar]
- 44.Smits, J. J., J. Postema, and W. Hulsegge. 2003. Monitoring van waterplanten en perifyton in het IJsselmeergebied. Rijkwaterstaat Directie Ijsselmeergebied (RDIJ), Lelystad, The Netherlands.
- 45.Speksnijder, A. 2000. Community analyses of beta subgroup ammonia-oxidizing bacteria in aquatic environments: a molecular approach. The Netherlands Institute of Ecology, Centre for Limnology, Nieuwersluis, The Netherlands.
- 46.Speksnijder, A., G. A. Kowalchuk, S. De Jong, E. Kline, J. R. Stephen, and H. J. Laanbroek. 2001. Microvariation artifacts introduced by PCR and cloning of closely related 16S rRNA gene sequences. Appl. Environ. Microbiol. 67:469-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Speksnijder, A., G. A. Kowalchuk, K. Roest, and H. J. Laanbroek. 1998. Recovery of a Nitrosomonas-like 16S rDNA sequence group from freshwater habitats. Syst. Appl. Microbiol. 21:321-330. [DOI] [PubMed] [Google Scholar]
- 48.Spieck, E., M. Meincke, and E. Bock. 1992. Taxonomic diversity of Nitrosovibrio strains isolated from building sandstones. FEMS Microbiol. Ecol. 102:21-26. [Google Scholar]
- 49.Stehr, G., B. Böttcher, P. Dittberner, G. Rath, and H. P. Koops. 1995. The ammonia-oxidizing nitrifying population of the river Elbe estuary. FEMS Microbiol. Ecol. 17:177-186. [Google Scholar]
- 50.Stephen, J. R., A. E. McCaig, Z. Smith, J. I. Prosser, and T. M. Embley. 1996. Molecular diversity of soil and marine 16S rRNA gene sequences related to beta-subgroup ammonia-oxidizing bacteria. Appl. Environ. Microbiol. 62:4147-4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suwa, Y., Y. Imamura, T. Suzuki, T. Tashiro, and Y. Urushigawa. 1994. Ammonia-oxidizing bacteria with different sensitivities to (NH4)2SO4 in activated sludges. Water Res. 28:1523-1532. [Google Scholar]
- 52.Van Wijk, R. J. 1988. Ecological studies on Potamogeton pectinatus L. I. General characteristics, biomass production and life cycles under field conditions. Aquat. Bot. 31:211-258. [Google Scholar]
- 53.Verhagen, F. J. M., and H. J. Laanbroek. 1991. Competition for ammonium between nitrifying and heterotrophic bacteria in dual energy-limited chemostats. Appl. Environ. Microbiol. 57:3255-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ward, B. B., D. P. Martino, M. C. Diaz, and S. B. Joye. 2000. Analysis of ammonia-oxidizing bacteria from hypersaline Mono Lake, California, on the basis of 16S rRNA sequences. Appl. Environ. Microbiol. 66:2873-2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wetzel, R. G., and M. Sondergaard. 1998. Role of submerged macrophytes for the microbial community and dynamics of dissolved organic carbon in aquatic ecosystems, p. 132-148. In E. Jeppesen, M. Sondergaard, M. Sondergaard, and K. Christoffersen (ed.), The structuring role of submerged macrophytes in lakes. Springer, New York, NY.
- 56.Whitby, C. B., J. R. Saunders, J. Rodriguez, R. W. Pickup, and A. McCarthy. 1999. Phylogenetic differentiation of two closely related Nitrosomonas spp. that inhabit different sediment environments in an oligotrophic freshwater lake. Appl. Environ. Microbiol. 65:4855-4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winogradsky, H., and G. Boutillier. 1949. Contribution aux connaissances sur le processus de la nitrification dans les eaux usees—la nitrification dans l'installation septique du type sncf. Ann. Inst. Pasteur 76:239-244. [Google Scholar]
- 58.Winogradsky, S., and H. Winogradsky. 1993. Études sur la microbiologie du sol. VII. Nouvelles recherches sur les organismes de la nitrification. Ann. Inst. Pasteur 50:350-434. [Google Scholar]
- 59.Woese, C. R., R. Gutell, R. Gupta, and H. F. Noller. 1983. Detailed analysis of the higher-order structure of 16S-like ribosomal ribonucleic acids. Microbiol. Rev. 47:621-669. [DOI] [PMC free article] [PubMed] [Google Scholar]