Abstract
Recently, we showed that the α subunit BCG1 of a heterotrimeric G protein is an upstream activator of the Ca2+/calmodulin-dependent phosphatase calcineurin in the gray mold fungus Botrytis cinerea. To identify the transcription factor acting downstream of BCG1 and calcineurin, we cloned the gene encoding the B. cinerea homologue of CRZ1 (“CRaZy,” calcineurin-responsive zinc finger transcription factor), the mediator of calcineurin function in yeast. BcCRZ1 is able to partially complement the corresponding Saccharomyces cerevisiae mutant, and the subcellular localization of the green fluorescent protein-BcCRZ1 fusion product in yeast cells depends on the calcium level and calcineurin activity. Bccrz1 deletion mutants are not able to grow on minimal media and grow slowly on media containing plant extracts. Hyphal morphology, conidiation, and sclerotium formation are impaired. The cell wall and membrane integrity, stress response (extreme pH, H2O2, Ca2+, Li+), and ability of the hyphae to penetrate the intact plant surface are affected in the mutants. However, BcCRZ1 is almost dispensable for the conidium-derived infection of bean plants. The addition of Mg2+ restores the growth rate, conidiation, and penetration and improves the cell wall integrity but has no impact on sclerotium formation or hypersensitivity to Ca2+ and H2O2. The expression of a set of recently identified BCG1- and calcineurin-dependent genes is also affected in ΔBccrz1 mutants, confirming that this transcription factor acts downstream of calcineurin in B. cinerea. Since the Bccrz1 mutants still respond to calcineurin inhibitors, we conclude that BcCRZ1 is not the only target of calcineurin.
Gray mold rot, caused by the ascomycete Botrytis cinerea Pers.:Fr. [teleomorph: Botryotinia fuckeliana (de Bary) Whetzel], is an important disease of almost all dicotyledonous plants, including vegetable and fruit crops, flowers, and greenhouse-grown crops. The fungus has developed a flexible infection strategy, including manifold tools for penetrating and overcoming plant defenses. In addition to the secretion of cell wall-lysing enzymes and the production of non-host-selective toxins, e.g., botrydial and botcinolides, B. cinerea is able to induce an oxidative burst by the production of reactive oxygen species (reviewed in references 74 and 83). All these processes must be highly regulated: the fungus needs to recognize the host plant and to find the optimum time for infection, expansion, and reproduction. It is suggested that the sensing of plant signals is managed by heterotrimeric G protein-coupled receptor systems, which transduce the external signal into an intracellular signal mediated via the dissociation of the Gα subunit from the Gβγ dimer and subsequent activation of downstream effector pathways, such as the adenylate cyclase/cyclic AMP (cAMP)/protein kinase A (PKA) cascade (reviewed in reference 25).
The functions of the three different Gα subunits (BCG1, BCG2, and BCG3) of B. cinerea, and their effects on vegetative growth and virulence, have been investigated in recent years (16, 59). The deletion of bcg1 resulted in severely reduced virulence on bean and tomato, loss of protease secretion, changed colony morphology (59), and loss of botrydial biosynthesis (65). It was shown that this Gα subunit is not only an activator of the adenylate cyclase BAC, regulating colony morphology via the cAMP level, but also the regulator of a second, cAMP-independent signaling pathway (36, 60). Recently, we demonstrated that the α subunit BCG1 acts as an activator of the Ca2+/calmodulin-dependent calcineurin phosphatase, inducing the expression of a set of genes, including those of the botrydial biosynthesis gene cluster (61).
Calcineurin is a highly conserved protein, consisting of a catalytic (CNA1/2) and a regulatory (CNB) subunit, which is activated by binding of the Ca2+/calmodulin complex when the cytosolic Ca2+ level is increased (reviewed in references 13, 19, 38, and 55). In Saccharomyces cerevisiae, calcineurin is dispensable for growth under standard culture conditions but is required for response to environmental stress conditions, such as exposure to several cations (Mn2+, Li+, and Na+), alkaline pH, high temperature, and endoplasmic reticulum stress, and incubation with mating pheromone (α-factor). In strains lacking components of the cell wall integrity mitogen-activated protein (MAP) kinase pathway, calcineurin is essential even under standard growth conditions (6, 12, 21, 42, 44, 85). In filamentous fungi, calcineurin seems to be even more important: there are only a few viable knockout mutants of calcineurin, all of them showing severely disturbed vegetative growth. Thus, in Aspergillus fumigatus cna1 deletion mutants, the growth rate, hyphal morphology, sporulation, conidial architecture, and pathogenicity are affected (15, 70). Studies of other fungi, using inducible cna1 antisense constructs or calcineurin inhibitors, such as the immunosuppressive drug cyclosporine or FK506, revealed the requirement for calcineurin in vegetative differentiation (e.g., sclerotial development in Sclerotinia sclerotiorum) (26), cell wall integrity (e.g., cell wall β-1,3-glucan content in S. sclerotiorum) (26), and virulence (e.g., infection structure formation in Magnaporthe grisea and B. cinerea) (75, 76). In the basidiomycete Cryptococcus neoformans, calcineurin is required for growth at an elevated temperature (37°C) and for virulence (18, 48, 71).
So far, the calcineurin-dependent (CND) gene expression program has been extensively characterized only in S. cerevisiae: in response to stress, calcineurin activates the transcription factor CRZ1 (“CRaZy,” calcineurin-responsive zinc finger) by docking at the PIISIQ site (the calcineurin-docking domain) and dephosphorylating the serine-rich region (SRR) motif, in a manner similar to the calcineurin-dependent regulation of members of the mammalian NFAT (nuclear factor of activated T cells) transcription factor family (reviewed in reference 2). The dephosphorylation of CRZ1 affects its subcellular localization: when calcineurin is inactive, the phosphorylated CRZ1 protein is distributed throughout the cell. After stimulation by an increase of cytosolic Ca2+, CRZ1 rapidly accumulates in the nucleus in a calcineurin-dependent manner due to its increased nuclear import and decreased nuclear export (50, 69). Antagonists of the calcineurin phosphatase are two protein kinases: the cAMP-dependent protein kinase (PKA), which negatively regulates CRZ1 activity by inhibiting its nuclear import (32), and HRR25, a casein kinase 1 homologue that affects nuclear import, export, or both (33).
CRZ1 contains a C2H2 zinc finger motif that binds to the calcineurin-dependent response element in the promoter regions of calcineurin-regulated genes and was shown to be sufficient for Ca2+ and CND gene expression in yeast (68). Analyses of global calcineurin/CRZ1-dependent gene expression by performing microarray experiments revealed 153 genes, which are involved in ion homeostasis, cell wall synthesis, vesicle transport, lipid/sterol synthesis, and protein degradation (84). The Δcrz1 phenotype is comparable to that of the calcineurin mutant, and the mutants are also defective in calcineurin-dependent induction of gene expression (42, 68). However, the loss of calcineurin is more severe than loss of CRZ1, suggesting that calcineurin has additional substrates, such as HPH1/HPH2, which are tail-anchored integral membrane proteins localized to the endoplasmic reticulum and are required to promote growth under several stress conditions (27). Signaling via calcineurin can be modulated by a conserved family of calcineurin regulators, termed calcipressins. For example, the RCN1 protein of S. cerevisiae inhibits the protein phosphatase activity of calcineurin and operates as an endogenous feedback inhibitor of calcineurin (35).
The calcineurin phosphatase and the CRZ1 transcription factors have also been investigated in the human pathogen Candida albicans. Calcineurin mutants are hypersensitive to agents that disturb cell membrane integrity, such as azoles and sodium dodecyl sulfate (SDS), and to elevated Na+, Li+, and Mn2+ concentrations and high pH and are strongly attenuated in virulence (4, 11, 57). Like the calcineurin mutants, the deletion mutants of Cacrz1 show increased sensitivity to high cation concentrations and membrane stress caused by SDS and azoles. Due to the fact that Δcna1 mutants are less virulent than Δcrz1 mutants, it is proposed that CaCRZ1 acts downstream of calcineurin but is not the only signaling effector (34, 49, 58). Furthermore, several Ca2+-, CNA-, and CRZ1-dependent genes were identified whose gene products are involved in cell wall organization, cellular organization, cellular transport and homeostasis, cell metabolism, and protein fate (34).
While in S. cerevisiae and C. albicans the calcineurin pathway and its components and functions are well characterized, little is known about the pathway and downstream targets in filamentous fungi. Recently, we showed that the Gα subunit BCG1 is an upstream regulator of the Ca2+- and calcineurin-dependent pathway, as a common set of genes were regulated by both BCG1 and calcineurin (61). Therefore, we wanted to know if the transcription factor BcCRZ1 is a potential downstream effector of calcineurin in B. cinerea.
In this work, we demonstrate that BcCRZ1 is a functional homologue of yeast CRZ1 that is able to partially complement CRZ1 function in the S. cerevisiae mutant, showing a Ca2+- and calcineurin-dependent localization pattern in yeast cells. We report that Bccrz1 deletion mutants are severely impaired in vegetative growth and differentiation, such as conidiation and sclerotium formation and cell wall and membrane integrity, as well as virulence. Interestingly, growth, conidiation, and virulence, but not the ability to form sclerotia and to grow in the presence of high H2O2 and Ca2+ concentrations, can be specifically restored by exposure to higher Mg2+ concentrations. Finally, we show that a set of previously identified CND genes are expressed in a similar manner in ΔBccrz1 mutants, suggesting that BcCRZ1 is indeed a target of the calcineurin activation pathway.
MATERIALS AND METHODS
B. cinerea.
Strain B05.10 of B. cinerea Pers.:Fr. [Botryotinia fuckeliana (de Bary) Whetz] is a putative haploid strain obtained after benomyl treatment of an isolate from Vitis (52) and is used as a host strain for gene replacement experiments. The Δbcg1 strain is a knockout mutant for the G protein α-subunit-encoding gene bcg1 (59). Wild-type and mutant strains were grown on several complex media: potato dextrose agar (Sigma-Aldrich Chemie, Steinheim, Germany) was supplemented with 10% homogenized leaves of French bean (Phaseolus vulgaris) (PDAB). Grape agar contained undiluted grape juice (100 ml contained on average 0.2 g protein, 15.2 g carbohydrates, and 0.01 g fat) supplemented with 0.1% yeast extract and was adjusted to a final pH of 5. Synthetic complete medium (CM) was made according to the method of Pontecorvo et al. (51). As minimal medium, modified Czapek-Dox (CD) medium (2% sucrose, 0.1% KH2PO4, 0.3% NaNO3, 0.05% KCl, 0.05% MgSO4·7 H2O, 0.002% FeSO4·7 H2O, pH 5.0) was used. For conidiation, the strains were incubated for 1 week at 21°C under light conditions; for sclerotium formation, they were incubated for 4 weeks at 21°C in darkness. For DNA and RNA minipreparations, mycelium was grown for 3 to 4 days at 20°C on CM agar with a cellulose acetate (cellophane) overlay. Plate assays were performed using CM agar with or without 67 mM MgCl2 (equivalent to 0.2 osmol/liter due to three osmotically active ions) supplemented with Congo red, calcofluor white, FK506, menadione, fluconazole (Sigma-Aldrich, St. Louis, MO), H2O2 (AppliChem GmbH, Darmstadt, Germany), cyclosporine (Calbiochem, Merck KGaA, Darmstadt, Germany), SDS, and Triton X-100 (MP Biomedicals Inc., Solon, OH) as indicated. Protoplasts were generated using Glucanex (Novozymes, Denmark) or β-glucanase (InterSpex Products), added to 15 μg of the linearized vector, and transformed according to the method of Siewers et al. (64). Resistant colonies were transferred to plates containing CM agar complemented with 70 μg/ml of hygromycin B (Invivogen, San Diego, CA) or 70 μg/ml of nourseothricin (Werner-Bioagents, Jena, Germany). Single conidial isolates were obtained by spreading conidial suspensions on CM plates containing 70 μg/ml of hygromycin B. The conidia were germinated, and single colonies were transferred individually to new plates containing the selection marker.
S. cerevisiae.
Wild-type strain W303-1A and its derivative MSE104 (Δcrz1) have been described previously (58, 79). Yeast cells were grown either in complete YPD medium consisting of 2% glucose, 2% peptone, and 1% yeast extract or in minimal SD medium containing 2% glucose, 0.67% yeast nitrogen base, and the amino acids, purine, and pyrimidine bases required by the strains. Solid media contained 2% agar. S. cerevisiae strains were transformed by the lithium-acetate procedure as described previously (22). For maintenance of plasmids, yeast transformants were precultured in selective media supplemented with methionine and then transferred to the experimental conditions.
E. coli.
Escherichia coli strain TOP10F′ (Invitrogen, Groningen, The Netherlands) and E. coli XL1-Blue (7) were used as hosts for plasmid construction and propagation.
Germination assays.
Analysis of nutrient-dependent germination of conidia from the B. cinerea wild-type and mutant strains was done on glass surfaces according to the method of Doehlemann et al. (16).
Pathogenicity assays.
Infection assays were performed with conidiospores from 10-day-old grape agar cultures as described previously (59). In addition, agar plugs taken from 3-day-old CM agar cultures were used to inoculate primary leaves of Phaseolus vulgaris. The infected plants were incubated in a plastic propagator box at 20°C under natural illumination conditions. Disease symptoms were scored until 12 days after inoculation.
Microscopic analyses.
To study the hyphal morphology, the B. cinerea strains were grown on microscope slides that carried an overlay of CM agar. After incubation for 2 days in a humid chamber at 20°C, the colonies were incubated for 5 min in 1% (wt/vol) calcoflour white solution and then washed with water. The stained colonies were observed by epifluorescence microscopy using a Leica DMRBE microscope with a PixelFly digital camera (PCO Computer Optics GmbH) and Leica filter set A (BP 340 to 380; RKP 400; LP425).
Standard molecular methods.
Fungal genomic DNA was isolated as described previously (9). Plasmid DNA was isolated using a plasmid DNA preparation kit (Genomed, Bad Oeynhausen, Germany). For Southern analysis, the fungal DNA was transferred to Hybond N+ filters (Amersham Biosciences, Freiburg, Germany) after digestion with restriction enzymes and size separation on a 1% agarose gel according to the method of Sambrook et al. (56). Hybridization was carried out in 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 5× Denhardt's solution, 0.1% SDS, and 50 mM phosphate buffer, pH 6.6, at 65°C in the presence of a random-primed [α-32P]dCTP-labeled probe. The membranes were washed once (2× SSPE [1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA {pH 7.7}], 0.1% SDS) before being exposed to autoradiographic film. Total RNA was isolated from mycelial samples using the Trizol procedure (Invitrogen, Groningen, The Netherlands). Samples (25 μg) of total RNA were transferred to Hybond N+ membranes after electrophoresis on a 1% agarose gel containing formaldehyde, according to the method of Sambrook et al. (56). Blot hybridizations were carried out in 0.6 M NaCl, 0.16 M Na2HPO4, 0.06 M EDTA, 1% N-lauroylsarcosine (Sigma-Aldrich, St. Louis, MO), 10% dextran sulfate (Eppendorf AG, Hamburg, Germany), 0.01% salmon sperm DNA, pH 6.2, as described for Southern blots; 1 μg of total RNA was taken for cDNA synthesis using the oligo(dT)12-18 primer and SuperScript II reverse transcriptase (Invitrogen, Groningen, The Netherlands) according to the manufacturer's instructions. PCR mixtures contained 25 ng DNA, 5 pmol of each primer, 200 nM concentrations of desoxynucleotide triphosphates, and 1 unit of BioThermDNA polymerase (GeneCraft GmbH, Lüdinghausen, Germany). The reactions started with 4 min at 94°C, followed by 35 cycles of 1 min at 94°C, 1 min at 56 to 65°C, and 1 min at 70°C, and a final 10 min at 70°C. PCR products were cloned into pCR2.1-TOPO (Invitrogen, Groningen, The Netherlands). DNA sequencing was performed with the automatic sequencer Li-Cor 4200 (MWG Biotech, Munich, Germany) using the Thermo Sequenase fluorescence-labeled primer cycle-sequencing kit (Amersham Biosciences). For sequence analysis, Lasergene v6 software (DNAStar, Madison, WI) was used.
Macroarray analysis.
The cDNA macroarrays contained B. cinerea cDNAs from three different expressed sequence tag collections. One cDNA library was created from the B. cinerea strain ATCC 58025, a nonsporulating overproducer of abscisic acid under abscisic acid biosynthesis conditions (64); the second library was derived from a suppression subtractive hybridization approach, which was used to identify genes of the wild-type B05.10, whose expression on the host plant P. vulgaris was specifically affected (60); the third library was derived from germinating conidia of B05.10 and early stages of plant infection (L. Kokkelink, unpublished data). In summary, the assembling of 16,525 cDNA sequences by means of the assembly program CAP3 (30) resulted in 1,901 contigs and 3,047 singlets. Thus, the macroarrays altogether contained 4,948 genes (including genes of plant origin). Sequence analysis for the prediction of protein function was done using BlastX at NCBI (1). Radiolabeled cDNA probes were prepared in the presence of 30 μCi [α-33P]dATP using SuperScript II reverse transcriptase as described above. Fungal cultures with the two genotypes (B05.10 and ΔBccrz1) were repeated three times. Each RNA sample was hybridized once to the macroarrays, making a total of three biological repeats and, due to the two replicates, six values per cDNA clone. The hybridization images from the Typhoon 8600 (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) were analyzed for initial data quantification using ArrayVision 8.0 (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). The Excel macro FiRe (find regulons) (http://www.unifr.ch/plantbio/FiRe/main.html) was used to select candidate genes for differential expression based upon their change ratios (5, 20), using standard parameters (lower threshold, 0.5; upper threshold, 2.0). The ratios of the genes (see Table 3) were, in at least five of six cases, below the threshold of 0.5 (down-regulation in ΔBccrz1) or above the threshold of 2.0 (up-regulation in ΔBccrz1).
TABLE 3.
BcCRZ1-dependent genes
| Contig name (EST database)b | Other name | B. cinerea B05.10 annotation | BlastX protein (organism), E value | Effecta of:
|
||
|---|---|---|---|---|---|---|
| Δbcg1 | CsA | ΔBccrz1 | ||||
| Secondary metabolism | ||||||
| Contig 867 | CND5/Bcbot1 | BC1G_16062.1 | CND5p (Botryotinia fuckeliana), 0.0 | ↓ | ↓ | ↓ |
| Contig 1410 | P450-13 | BC1G_16061.1 | Isotrichodermin C-15 hydroxylase (Fusarium sporotrichioides), 3e−42 | ↓ | ↓ | |
| Contig 1543 | CND15 | CND15p (B. fuckeliana), 8e−76 | ↓ | ↓ | ↓ | |
| Contig 1214 | CND11 | BC1G_16147.1 | CND11p (B. fuckeliana), 0.0 | ↓ | ↓ | ↓ |
| Contig 1506 | PKS6 | BC1G_16086.1 | Lovastatin nonaketide synthase (LNKS) (Aspergillus terreus), 0.0 | ↓ | ↓ | ↓ |
| OxR | Enoyl reductase (A. terreus), 5e−47 | ↓ | ↓ | ↓ | ||
| Contig 2208 | P450-1 (SSHG02) | BC1G_16085.1 | Cytochrome P450 monooxygenase (Penicillium paxilli), 5e−18 | ↓ | ↓ | ↓ |
| Contig 662 | P450-2 (SSHE10) | BC1G_16084.1 | P450 monooxygenase 1 (Phoma betae), 3e−73 | ↓ | ↓ | ↓ |
| 045_A09 | MO1 | BC1G_16083.1 | Flavin-containing monooxygenase (Burkholderia ambifaria), 5e−67 | ↓ | ↓ | ↓ |
| ORF1 | Phenylcoumaran benzylic ether reductase (Pinus taeda), 1e−04 | ↓ | ↓ | ↓ | ||
| Contig 1158 | SSHB06 | Cytochrome P450 (Gibberella fujikuroi), 4e−40 | ↓ | ↓ | ↓ | |
| Contig 2014 | CND14 | BC1G_03920.1 | Aldo-keto reductase (A. fumigatus Af293), 9e−119 | ↓ | ↓ | ↓ |
| Contig 866 | 44-B02 | BC1G_16357.1 | Short chain dehydrogenase (Rhizobium sp. strain NGR234), 6e−35 | ↓ | ↓ | ↓ |
| Contig 260 | 47-B01 | BC1G_16037.1 | Hydroxylase involved in salicylate metabolism (Acinetobacter), 9e−41 | ↓ | ↓ | ↓ |
| Contig 1489 | 53-H10 | BC1G_04230.1 | Tetrahydroxynaphthalene reductase (Chaetomium globosum), 9e−127 | ↓ | ↓ | ↓ |
| Other proteins | ||||||
| Contig 1284 | 105-F11 | BC1G_02744.1 | 6-Phosphogluconate dehydrogenase (B. fuckeliana), 0.0 | ↑ | ↑ | ↑ |
| Contig 1611 | CND6 | BC1G_05989.1 | ATP-citrate synthase subunit 1 (Sordaria macrospora), 0.0 | NA | ↓ | ↓ |
| CND16 | BC1G_05991.1 | ATP citrate lyase subunit 2 (S. macrospora), 0.0 | ↓ | ↓ | ↓ | |
| Contig 1746 | CND9 | BC1G_11550.1 | Mannitol-1-phosphate 5-dehydrogenase-like protein, | − | ↓ | ↓ |
| Contig 1123 | BcoahA | BC1G_03473.1 | Oxaloacetate acetylhydrolase OAHA (B. fuckeliana), 1e−79 | ↓ | NA | ↓ |
| Contig 1490 | Bcglyox1 | BC1G_01204.1 | Glyoxal oxidase (B. fuckeliana), 0.0 | ↓ | NA | ↓ |
| Contig 802 | BC1G_12319.1 | Formate dehydrogenase (Neurospora crassa), 0.0 | − | NA | ↓ | |
| Contig 1505 | BC1G_14217.1 | Acetyl-CoA hydrolase (B. fuckeliana),1e−137 | ↓ | NA | ↓ | |
| Contig 944 | BC1G_03468.1 | Kynureninase (Aspergillus clavatus), 5e−39 | ↓ | NA | ↓ | |
| Contig 556 | BC1G_05887.1 | Kynurenine 3-monooxygenase (A. clavatus), 3e−125 | ↓ | NA | ↓ | |
| Contig 1324 | 13-H05 | BC1G_02025.1 | NADP-dependent alcohol dehydrogenase (B. fuckeliana), 0.0 | ↑ | ↑ | ↑ |
| Contig 1337 | BC1G_14030.1 | 1,3-Beta-glucanosyltransferase gel4 (A. fumigatus), 1e−155 | ↑ | NA | ↓ | |
| 248_D11 | CND12 | BC1G_00803.1 | Calcium ion transporter VCX1 (A. fumigatus Af293), 6e−09 | − | ↓ | ↓ |
| Contig 934 | SSH26/Bcacp1 | BC1G_14153.1 | Acidic protease 1 (B. fuckeliana), 1e−120 | ↓ | NA | ↑ |
| Contig 902 | BC1G_03070.1 | Family A1 protease (Phanerochaete chrysosporium), 2e−13 | ↓ | NA | ↑ | |
| Contig 1515 | BC1G_01286.1 | Carboxypeptidase 4 (A. fumigatus), 4e−168 | ↓ | NA | ↑ | |
| Contig 783 | bcmp1 | BC1G_09180.1 | Penicillolysin (Penicillium citrinum), 5e−13 | ↓ | NA | ↑ |
| Contig 667 | CND2/bcpg1 | BC1G_11143.1 | Endopolygalacturonase 1 (B. fuckeliana), 4e−88 | − | ↓ | ↓ |
| Contig 1387 | CND7 | BC1G_00232.1 | Chitinase (A. fumigatus), 4e−8 | − | ↓ | ↓ |
| Contig 999 | 119-G07 | BC1G_02364.1 | Beta-glucosidase (A. fumigatus), 4e−71 | − | ↓ | ↓ |
| Contig 2409 | CND1 | BC1G_08931.1 | Gas1-like protein (Monacrosporium haptotylum), 1e−27 | ↓ | ↓ | ↓ |
| Contig 1290 | BC1G_13581.1 | MAS1 protein (Magnaporthe grisea), 3e−105 | ↓ | NA | ↓ | |
| Contig 1310 | BC1G_05503.1 | CipC-like antibiotic response protein (A. fumigatus), 6e−17 | ↓ | NA | ↓ | |
| Contig 1571 | BC1G_08500.1 | NUC-2 (N. crassa), 3e−128 | − | NA | ↓ | |
| Contig 2454 | BC1G_05251.1 | Cyanovirin-N family protein (Neosartorya fischeri), 3e−13 | ↓ | NA | ↓ | |
| Others of unknown function | ||||||
| Contig 1154 | CND3 | BC1G_10379.1 | Hypothetical protein SNOG_14859 (Phaeosphaeria nodorum), 5e−55 | ↓ | ↓ | ↓ |
| Contig 2449 | CND4 | BC1G_13779.1 | No significant homologue | − | ↓ | ↓ |
| Contig 1719 | BC1G_03481.1 | Hypothetical protein SNOG_00115 (P. nodorum), 5e−48 | ↑ | NA | ↓ | |
| Contig 1002 | BC1G_07825.1 | No significant homologue | ↓ | NA | ↓ | |
| Contig 905 | 104-H02 | No significant homologue | ↓ | ↑ | ↑ | |
| Contig 1236 | BC1G_16143.1 | Hypothetical protein AN3674.2 (A. nidulans), 1e−41 | ↑ | NA | ↑ | |
| Contig 1783 | BC1G_12002.1 | No significant homologue | ↑ | NA | ↓ | |
| Contig 2105 | BC1G_04368.1 | No hit found | − | NA | ↓ | |
Differential expression of the indicated genes in the Δbccrz1 mutant was found by a macroarray approach (wild-type B05.10 versus ΔBccrz1 mutant) and confirmed by Northern blot analyses. The expression patterns of the BcCRZ1-dependent genes in the Δbcg1 mutant and under cyclosporine treatment, mimicking a calcineurin deletion, are included (61, 75). Gene expression is described as follows: the gene is up-regulated (↑) or down-regulated (↓) in the mutant/after cyclosporine treatment in comparison to the wild type (control) or expression is not affected (−). CsA, cyclosporine A; NA, not applicable.
EST, expressed sequence tags (see Materials and Methods).
Complementation of S. cerevisiae Δcrz1.
The cDNA sequence of Bccrz1 for cloning a complementation vector was generated by performing PCR using total cDNA as a template and primers 1 and 2 (Table 1; see Fig. 3A) containing artificial restriction sites for XbaI or EcoRI. The PCR fragment was cloned, sequenced, isolated using XbaI and EcoRI, and inserted into the yeast expression vector pUG34 (U. Güldener and J. H. Hegemann, unpublished data), thereby replacing ygfp3. The BcCRZ1 yeast expression vector was amplified, purified, and transformed in S. cerevisiae Δcrz1.
TABLE 1.
PCR primers used in this study
| No. | Name | Sequence (5′→3′) |
|---|---|---|
| 1 | Crz1-F2 | GAAGGCGCAAACAAACTTCTAGAATG |
| 2 | Crz1-R2 | GCCGAATACCATCACAAGAATTCTATCG |
| 3 | Crz1-5′-F | GGTTAGAGAGGTACCGCATTTTGG |
| 4 | Crz1-5′-F | CATGTCGACAGTTTGTTTGCGCCTTC |
| 5 | Crz1-3′-F | CCTTGTGAAGCTTTTCGGCGGCATGC |
| 6 | Crz1-3′-R | GAACAAGACGGGAGAGCTCTTCGAGG |
| 7 | Crz1-5′-home | CGATTGTAGAACTGGACTGTCCCC |
| 8 | pLOF-oliP | GGTACTGCCCCACTTAGTGGCAGCTCGCG |
| 9 | pAN-T | ACCCAGAATGCACAGGTACAC |
| 10 | Crz1-3′-home | GTCCCGAGGACTCTTAGCGGATGG |
| 11 | Crz1-F4 | CGCCAAATGATCGATCTGGGGCAAC |
| 12 | bccrz1-prom-F1 | CCCGGGCGATTGTAGAACTGGACTGTC |
| 13 | bccrz1-prom-R1 | TCTAGAAGTTTGTTTGCGCCTTC |
| 14 | oliC-prom-ClaI | CATTCCCGATTCGGGCCGTATCGATTAAG |
| 15 | M13-universe | AGGGTTTTCCCAGTCACGACGTT |
| 16 | Tub-T2 | GGTCCTCGGAGTGCAGATGGG |
FIG. 3.
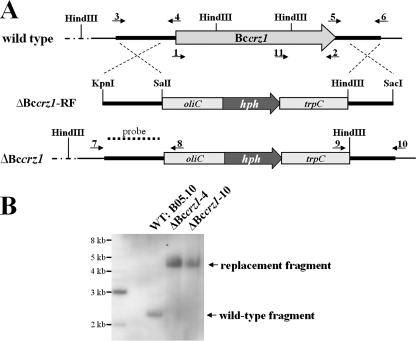
Gene replacement of Bccrz1. (A) Physical maps of Bccrz1, the gene replacement fragment ΔBccrz1-RF, and the gene locus of a Bccrz1 knockout mutant showing the Bccrz1 open reading frame (gray arrow), the components of the hygromycin resistance cassette (gray boxes), and the flanking regions of Bccrz1 (heavy lines). The small arrows indicate the positions of primers used for cloning the full-length cDNA for yeast complementation (primers 1 and 2), the replacement vector (primers 3 to 6), and the diagnostic PCR analysis of the transformants (primers 7 to 11) (see Materials and Methods). (B) For Southern blot analysis, the genomic DNAs of the wild type (WT) and the mutants were digested with HindIII, blotted, and hybridized to the 5′ flank (dotted line in panel A) of the replacement vector pΔbccrz1. In both mutants, the wild-type fragment, with a size of 2.2 kb, was replaced by a 4.5-kb fragment, resulting from the loss of HindIII restriction sites within the Bccrz1 gene.
Construction of the GFP-BcCRZ1 fusion construct for S. cerevisiae.
pUG34 was developed to improve the expression of green fluorescent protein (GFP) gene fusions in yeast. In addition to ygfp3, encoding a codon-optimized GFP, pUG34 carries an expression cassette that includes the MET25 promoter region and the CYC1 terminator sequence. A polylinker between ygfp3 and the CYC1 terminator with multiple cloning sites can be used for construction of in-frame fusions to ygfp3. When pUG34-derived vectors are transformed in yeast, the MET25 promoter-dependent expression is strongly repressed by exogenous methionine. A gfp-Bccrz1 plasmid was obtained by insertion of the above-mentioned XbaI/EcoRI Bccrz1 cDNA fragment into the SpeI/EcoRI sites of the pUG34 polylinker. The reporter plasmid was amplified, purified, and transformed in S. cerevisiae Δcrz1.
Replacement of Bccrz1.
For construction of a gene replacement vector for Bccrz1, the plasmid pOliHP (53), carrying the E. coli hygromycin B phosphotransferase gene hph under the control of the Aspergillus nidulans oliC promoter and trpC terminator, was used as a basal vector. The gene flanks were amplified by PCR with primers, derived from the genomic sequence of B. cinerea B05.10 (http://www.broad.mit.edu/annotation/genome/botrytis_cinerea/Home.html), containing artificial restriction sites for further cloning (Table 1; see Fig. 3A). An 840-bp fragment was amplified from the Bccrz1 promoter region using primers 3 and 4, and a 740-bp fragment of the 3′-untranslated region was generated as a second flank using primers 5 and 6. Both PCR products were cloned into pCR2.1-TOPO; isolated with KpnI/SalI and HindIII/SacI, respectively; and then cloned into the corresponding restriction sites of pOliHP, creating pΔbccrz1. For transformation, the replacement cassette was isolated with KpnI and SacI. The hygromycin B-resistant transformants were analyzed by PCR for homologous integration using primers 7 to 10 and were purified by single-spore isolation and screened by PCR for the absence of the crz1 wild-type allele using primers 11 and 2. For the Southern blot analysis, the genomic DNAs of the wild-type B05.10 and the Bccrz1 transformants were digested with HindIII and hybridized to the 5′ flank of Bccrz1. The approach showed that the transformants had undergone homologous integration into the Bccrz1 locus and that they did not contain additional copies of the gene replacement fragment (see Fig. 3B).
Complementation of ΔBccrz1 with Bccrz1 cDNA.
To verify that the phenotype of ΔBccrz1 was due to the deletion of Bccrz1 in B. cinerea, the mutant ΔBccrz1-4 was complemented. For this, a 1-kb promoter fragment of Bccrz1 was amplified by PCR using primers 12 and 13 (Table 1) containing artificial restriction sites for SmaI and XbaI, respectively. The promoter was fused to the XbaI/ClaI-digested Bccrz1 cDNA fragment (see “Complementation of S. cerevisiae Δcrz1” above) by insertion of both fragments into pΔbcniaD, creating pbccrz1-Com. pΔbcniaD contains the noncoding 5′ and 3′ regions of BcniaD, encoding nitrate reductase, to mediate homologous integration of complementation constructs at the BcniaD locus (61). The nourseothricin resistance cassette, containing nat1 from Streptomyces noursei under the control of the A. nidulans oliC promoter, was amplified using pNR1 as a template (40) and primers 14 (with an additional ClaI restriction site) and 15. The resulting PCR fragment was digested with ClaI (a second ClaI site within the resistance cassette) and ligated into the ClaI-digested pbccrz1-Com, creating pbccrz1-Com+. Prior to transformation, the plasmid was linearized by digestion with SacI. The nourseothricin-resistant transformants were analyzed by PCR for the presence of a complete Bccrz1 copy using primers 12 and 16. The homologous integration of the Bccrz1 complementation constructs at the BcniaD locus was confirmed by Southern analysis; after digestion of the genomic DNAs of the transformants (ΔBccrz1, Com-7, and Com-9) and B05.10 with XbaI and hybridization with the BcniaD-5′ flank, the BcniaD wild-type fragment was replaced by a smaller fragment, due to the additional XbaI restriction site within the complementation fragment (data not shown).
RESULTS
Identification of the B. cinerea CRZ1 orthologue.
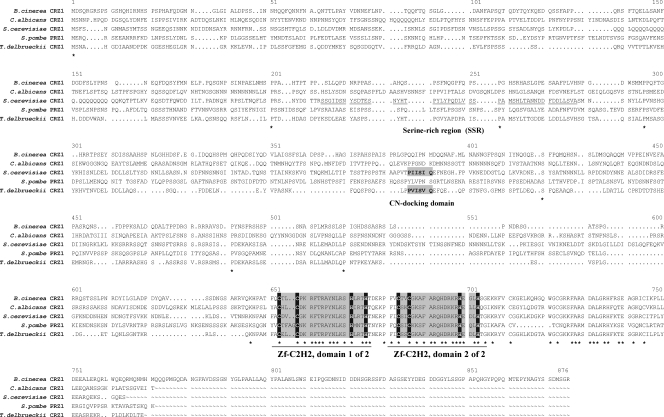
A similarity search using the CRZ1 protein sequence of S. cerevisiae and the program BlastP (1) in the protein database of the B. cinerea B05.10 genome project (http://www.broad.mit.edu/annotation/genome/botrytis_cinerea/Home.html) revealed 18 protein sequences that produced significant alignments (E values from 3e−43 to 6e−5) with a length of approximately 110 amino acids when standard parameters were used. Even though all these proteins contained two conserved zinc finger motifs (zf-C2H2 type), the respective domain of the annotated hypothetical protein BC1G_00093.1 appeared to be the best candidate, with an E value of 3e−43 and an amino acid identity of 64%, in contrast to the second hit, which displayed an E value of 5e−18 and an amino acid identity of only 41%. Thus, we investigated the first candidate gene in more detail and named it Bccrz1. The open reading frame of Bccrz1 contains 2,151 bp and one intron with a size of 51 bp, resulting in a protein with 716 amino acids. The protein sequence shows low rates of overall amino acid identity to the known CRZ1 proteins (32% to S. cerevisiae CRZ1, 30% to C. albicans CRZ1, 35% to Torulaspora delbrueckii CRZ1, and 28% to Schizosaccharomyces pombe PRZ1). An alignment of these protein sequences and of the putative CRZ1 sequence of B. cinerea is shown in Fig. 1, demonstrating the low level of similarity between the different sequences, except for the two highly conserved zinc finger domains in the C-terminal part of the protein. However, other known features of the CRZ1 protein of S. cerevisiae, such as the SRR domain in the N-terminal part or the calcineurin-docking domain (Fig. 1), were not found in the B. cinerea CRZ1 sequence. Since the similarities between the putative B. cinerea crz1 and the known yeast homologues were extremely low, we first expressed the Bccrz1 gene in the S. cerevisiae crz1 mutant to verify that we had cloned the functional homologue of yeast CRZ1.
FIG. 1.
Alignment of known CRZ1 protein sequences of different yeasts and the putative CRZ1 orthologue of B. cinerea: C. albicans (XP_716600) (49), S. cerevisiae (P53968) (42), S. pombe (Q09838) (29), T. delbrueckii (AAZ04388) (28), and B. cinerea (BC1G_00093.1) (Botrytis cinerea Database [http://www.broad.mit.edu/annotation/genome/botrytis_cinerea/Home.html]). Sequence alignment was done with ClustalW of the HUSAR sequence analysis package (http://genome.dkfz-heidelberg.de/) using standard parameters. The amino acids indicated by asterisks are conserved in all of the proteins shown. The amino acids of the zinc finger C2H2 domains are shaded gray, and the invariant cysteines and histidines within the zinc finger regions are shaded black. The SRR of S. cerevisiae CRZ1 is underlined, and the identified calcineurin (CN)-docking domains of S. cerevisiae and T. delbrueckii are indicated.
BcCRZ1 suppresses Na+ sensitivity of S. cerevisiae Δcrz1 mutants.
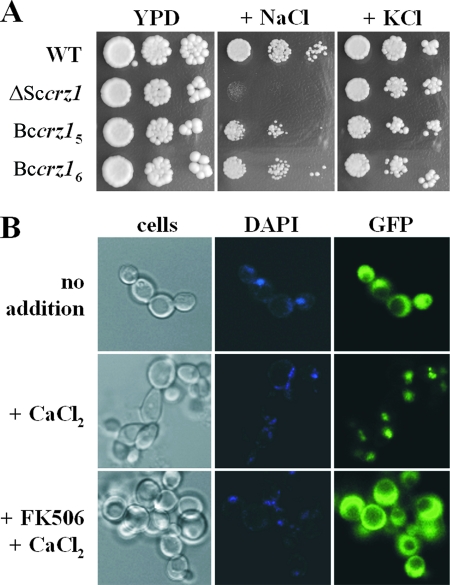
Deletion of crz1 renders S. cerevisiae more sensitive to many ions, including Na+, Li+, Mn2+, Ca2+ cations, and hydroxyl anions. As a consequence, Δcrz1 cells exhibit compromised growth when cultured in the presence of moderately high concentrations of those ions (42, 68). To find out if the Bccrz1 gene could complement crz1 gene deficiency in yeast, we transformed the Bccrz1 cDNA under the control of the yeast MET25 promoter (repressible by exogenous methionine) into the S. cerevisiae Δcrz1 strain and selected for prototrophic transformants in minimal medium. The transformants were analyzed for growth under high-Na+, -Mn2+, and -Ca2+ conditions. We found no differences with respect to the control (Δcrz1 transformed with the empty vector) when Ca2+ (0.2 to 0.3 M CaCl2) and Mn2+ (0.75 to 1 mM MnCl2) were used as selective cations (data not shown). In high Na+, however, Bccrz1-transformed cells showed improved growth, suggesting that the B. cinerea gene was able to alleviate the effects of Na+ toxicity in a Δcrz1 yeast mutant (Fig. 2A). It is notable that in contrast to the low to moderate levels of selective Mn2+ and Ca2+ concentrations used for phenotype assays, NaCl concentrations (1.2 M) were high enough to induce intense osmotic stress in yeast cells. To avoid concerns about the osmotic effects of salts in that phenotype, we performed a growth assay in 1.2 M KCl medium in parallel and found no differences in growth of the wild-type-, control-, and Bccrz1-transformed cells (Fig. 2A). These findings suggest that the B. cinerea gene indeed encodes a CRZ1-homologous protein that can partially complement the yeast crz1 deletion suppressing the Na+ sensitivity in S. cerevisiae lacking CRZ1 function.
FIG. 2.
Heterologous expression of Bccrz1 in yeast. (A) Bccrz1 complementation of a Δcrz1 mutation in S. cerevisiae. Yeast cells were grown to saturation in minimal medium without methionine and washed with sterile water, and serial 10-fold dilutions were dropped on YPD plates supplemented with 1.2 M NaCl or 1.2 M KCl. The plates were incubated at 30°C, and cell growth was recorded after 48 to 72 h. Bccrz15 and Bccrz16 are two independent yeast transformants. S. cerevisiae wild type (WT) and Δcrz1 were transformed with the empty vector. (B) BcCRZ1 localization in yeast cells is regulated by Ca2+ and calcineurin. GFP-BcCRZ1-transformed yeast cells were grown overnight at 30°C in minimal methionine-free medium with NH4Cl (5 g/liter) as a nitrogen source. Exponential-phase cells were observed in a confocal microscope (Olympus Fluoview FV500) using an argon laser (488 nm) before and immediately after addition of Ca2+ (0.2 M CaCl2) to the culture. The cells were treated with FK506 (3 μg/ml) for 45 min before Ca2+ induction. The images were processed with Fluoview software version 5.
Ca2+ and calcineurin activation lead to accumulation of BcCRZ1 in the yeast nucleus.
The molecular mechanisms by which calcineurin controls CRZ1 function are well established and involve a tight regulation of CRZ1 subcellular localization in S. cerevisiae (13). In actively growing yeasts, CRZ1 is homogeneously distributed throughout the cell, but upon calcineurin activation, the transcription factor is dephosphorylated by calcineurin, allowing fast CRZ1 import into the nucleus, where CRZ1 accumulates (69). We studied whether the putative B. cinerea CRZ1 homologue could be qualified as a target of the yeast calcineurin-dependent regulation mechanism by expressing a hybrid gfp-Bccrz1 gene in a S. cerevisiae crz1-defective strain. The GFP-BcCRZ1 chimeric protein was able to complement the growth defect of a yeast Δcrz1 mutant under high-Na+ conditions. Microscopic analysis of exponentially growing cells showed GFP-BcCRZ1 homogeneously distributed throughout the cell (Fig. 2B, top row). However, in the presence of Ca2+, GFP-BcCRZ1 accumulated in the nucleus as GFP and DAPI (4′,6′-diamidino-2-phenylindole) fluorescences colocalized in the cells (Fig. 2B, middle row). In contrast, when yeast cells were exposed to Ca2+ and the calcineurin inhibitor FK506, GFP-BcCRZ1 nuclear import was impaired and the reporter protein remained in the cytosol (Fig. 2B, bottom row). These results demonstrate that BcCRZ1 translocates to the nucleus in response to Ca2+ induction conditions and that this process is blocked by a specific inhibitor of calcineurin activity. These findings therefore confirmed that Bccrz1 encodes a bona fide calcineurin-regulated orthologue of the S. cerevisiae CRZ1 transcription factor.
Generation of ΔBccrz1 mutants.
To investigate the function of the putative BcCRZ1 transcription factor in B. cinerea, we created Bccrz1 deletion mutants using a replacement approach in which the whole open reading frame of Bccrz1 in the wild-type strain B05.10 was replaced by a hygromycin resistance cassette (Fig. 3A) (for details, see Materials and Methods). The homologous integration events at the Bccrz1 locus were detected for several transformants by diagnostic PCR. Homokaryotic knockout strains were obtained after one round of single-spore isolation and subsequent screening by PCR and Southern blot analysis for the absence of the Bccrz1 wild-type allele. Two transformants, termed ΔBccrz1-4 and ΔBccrz1-10, which had undergone homologous integration into the Bccrz1 locus without additional ectopic integrations of the replacement cassette into their genomes, were chosen for further studies (Fig. 3B).
Bccrz1 mutants are impaired in growth, morphology, conidiation, and sclerotium formation.
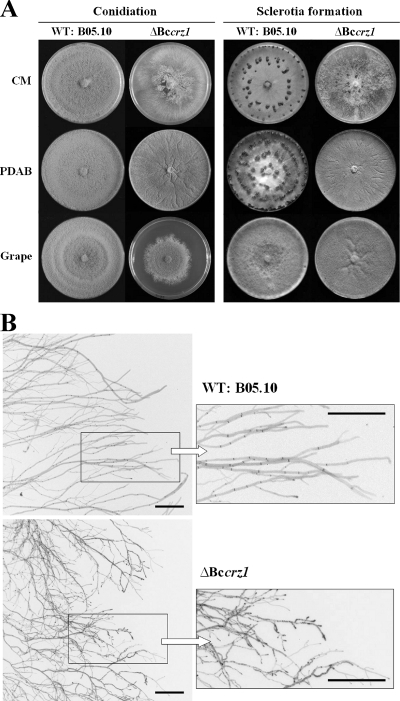
Vegetative growth of the Bccrz1 deletion mutants on CM was severely affected, associated with significantly reduced conidiation (Fig. 4A). In contrast to the wild type, only small numbers of brightly colored conidia were observed, even after extended incubation for 2 to 3 weeks on CM agar under light conditions. The spores were morphologically indistinguishable from those of the wild type and showed normal germination on glass surfaces, induced by glucose or fructose (data not shown). To overcome the strong growth defect of the Bccrz1 deletion mutants, we tested several media containing host plant-derived compounds. On PDAB, the ΔBccrz1 mutant showed an improved growth rate, similar to that of the wild type, but neither aerial hyphae, conidiophores, nor mature conidia were formed. Moreover, after a 2-week incubation period under light, the mycelium became orange (data not shown) and was no longer able to grow when transferred to fresh medium. Interestingly, conidiation, regular colony morphology, and formation of aerial hyphae were improved to similar extents on medium containing undiluted grape juice (Fig. 4A) or vegetable (V8) juice (data not shown). However, the growth rate was still significantly reduced, resulting in the formation of small colonies compared to the wild type after 7 days of incubation. On CD minimal medium, the mutants were not able to grow at all (Fig. 5A).
FIG. 4.
Impact of BcCRZ1 on growth, morphology, and development. (A) Growth, conidiation, and sclerotium formation on different media. The ΔBccrz1 mutant and the wild type (WT) were incubated for 1 week under light for conidiation and for 4 weeks at 21°C in darkness for sclerotium formation on CM, PDAB, and grape juice agar. (B) Hyphal morphology of ΔBccrz1 in comparison to the wild type. The strains were grown for 2 days on CM-overlaid microscope slides and then stained with calcoflour white and observed by epifluorescence microscopy. Negative prints are shown.
FIG. 5.
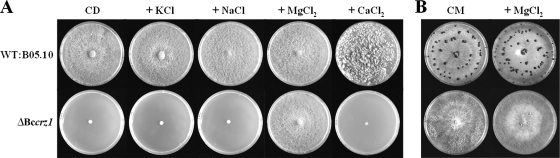
Effect of Mg2+ supplementation on growth and development of Bccrz1 mutants. (A) Addition of MgCl2 specifically restores the growth rate and conidiation of ΔBccrz1. The strains were grown for 1 week on minimal agar (CD) containing 0.2 osmol/liter KCl (0.1 M), 0.2 osmol/liter NaCl (0.1 M), 0.2 osmol/liter CaCl2 (0.07 M), and 0.2 osmol/liter MgCl2 (0.07 M). WT, wild type. (B) Addition of MgCl2 does not restore sclerotium formation of ΔBccrz1. The mutant and the wild type were incubated on CM without or with 0.2 osmol/liter (0.07 M) MgCl2 for 4 weeks at 21°C in darkness.
In addition, the deletion of Bccrz1 affected the ability to form sclerotia, a process that is usually induced by incubation in darkness. On both CM and PDAB medium, fewer or no sclerotia were formed in comparison to the wild-type strain. Interestingly, on undiluted grape juice agar, sclerotium formation was abolished in wild-type and mutant strains while conidiation was increased (Fig. 4A). Due to the fact that the Bccrz1 mutants grew with an irregular shape on CM, forming unusual hyphal extensions, we studied the hyphal morphology in more detail. The wild-type and the Bccrz1 mutant were grown for 2 days on CM-coated microscope slides and subsequently stained with calcoflour white. The hyphae of Bccrz1 mutants at the edges of the colonies were thinner and more frequently branched, and the septation was irregular, indicating an impact of BcCRZ1 on hyphal morphology (Fig. 4B).
Additional Mg2+ restores growth and conidiation, but not sclerotium formation.
In contrast to S. cerevisiae CRZ1, which is essential only under elevated stress conditions and dispensable under standard culture conditions, B. cinerea CRZ1 was necessary to promote growth and normal vegetative differentiation on minimal medium, even without exposure to environmental stresses. To identify the nutritional basis for the growth defect of Bccrz1 mutants on minimal (CD) medium, we tested different compounds, such as amino acids, vitamins, salts, and microelements, in supplementation experiments for the ability to rescue the growth defect. Although we found slightly improved growth of the Bccrz1 mutants caused by different salts and amino acids in high concentrations, only the addition of MgCl2 to the medium restored the wild-type phenotype with respect to growth rate and conidiation (Fig. 5A). To establish whether the remedial effect of MgCl2 was specifically due to the Mg2+ ions and not merely to the osmotic effect of the salt, we tested other salts consisting of monovalent (NaCl and KCl) and divalent (CaCl2) cations in concentrations leading to equivalent osmolarities (0.2 osmol/liter) in the different media. As shown in Fig. 5A, the addition of neither KCl, NaCl, nor CaCl2 had an effect comparable to that of the MgCl2 supplement on growth and conidiation, confirming the specific role of Mg2+ ions for growth of the Bccrz1 mutants. The fact that the addition of MgCl2 to the medium did not overcome the sclerotium formation defect (Fig. 5B) suggests that not all features of the ΔBccrz1 phenotype can be explained by Mg2+ starvation.
Bccrz1 mutants are affected in cell wall and membrane integrity.
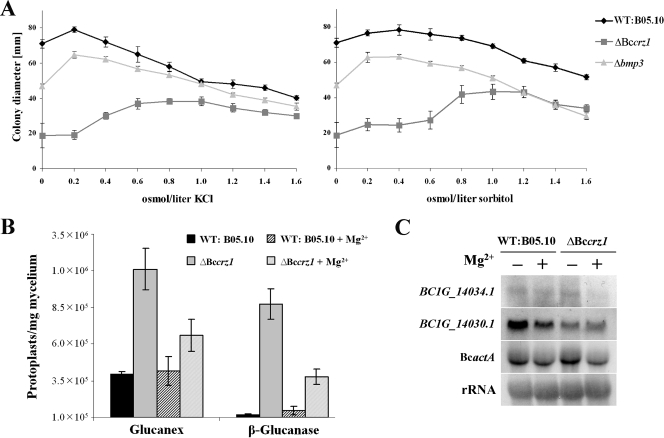
Because the growth of the Bccrz1 mutant was improved on media with high osmolarity caused by different compounds, such as salts and amino acids, we examined the impacts of different osmolarities (0 to 1.6 osmol/liter) of KCl and the osmotic protectant sorbitol on radial growth of the Bccrz1 mutants in more detail. Previously, it had been shown that the cell wall integrity SLT2-type MAP kinase (BMP3) in B. cinerea is essential for hypo-osmotic stress tolerance (54). Therefore, we included the Δbmp3 mutant in our experiment for a direct comparison. While the Δbmp3 mutants showed growth rates almost equivalent to that of the wild type on CM with slightly increased KCl concentrations (0.2 osmol/liter), the Bccrz1 mutants grew poorly at low osmolarities. The growth of the Bccrz1 mutant was improved to almost the growth rates of the wild-type and the Δbmp3 strain only by osmolarities higher than 0.8 osmol/liter. Higher osmolarities caused by sorbitol enhanced the growth rate of the Bccrz1 mutant to an extent similar to that observed for KCl (Fig. 6A). In addition to the growth rate, the conidiation of the Bccrz1 mutants was partially restored by higher osmolarities, caused by either KCl or sorbitol (data not shown).
FIG. 6.
Impact of BcCRZ1 on cell wall integrity. (A) High-osmolarity conditions caused by salts or sugars improve the growth rates of ΔBccrz1 mutants. The wild type (WT), the ΔBccrz1 mutant, and the Δbmp3 mutant (bmp3 encodes the cell wall integrity MAP kinase) were grown on CM containing different amounts of KCl or sorbitol. Colony diameters were measured after 3 days of incubation. The values are averages from at least six colonies. (B) Hypersensitivity of ΔBccrz1 to treatment with cell wall-degrading enzymes. Conidiospores of the strains were incubated in liquid CM with or without 67 mM MgCl2 for 24 h. The washed mycelia were incubated for 1.5 h in osmotically stabilized solution containing Glucanex or β-glucanase. Protoplasts were counted microscopically, and the numbers of protoplasts per mg dry weight were determined. The data are the means of four replications. The error bars indicate standard deviations. (C) Expression of genes whose products are involved in cell wall biosynthesis. Wild-type B05.10 and ΔBccrz1 mutants were cultivated as described for panel B. The Northern blot was hybridized to radioactively labeled probes of the glucan synthase (BC1G_14034.1)- and 1,3-β-glucanosyltransferase (BC1G_14030.1)-encoding genes. Actin (BcactA) and rRNA were used as loading controls.
The poor growth on media with low osmolarities was the first hint of impaired cell wall structure and/or membrane integrity of the Bccrz1 mutants, as was described for crz1 mutants of S. cerevisiae and C. albicans (34, 68). To strengthen this suggestion, we treated young conidium-derived mycelia (24 h) with different cell wall-degrading enzymes (Glucanex and β-glucanase) in osmotically stabilized solutions and determined the numbers of protoplasts per milligram dry weight. As shown in Fig. 6B, the number of protoplasts released from the ΔBccrz1 mycelium after a 1.5-h incubation was approximately threefold higher than the number of protoplasts produced from the wild-type mycelium, indicating that the Bccrz1 mutant is more susceptible to cell wall-degrading enzymes than the wild type and the bmp3 mutant (54). Interestingly, the number of protoplasts released from ΔBccrz1 mycelium was significantly decreased when the strain was grown in medium containing additional MgCl2 (67 mM), demonstrating the role of Mg2+ ions in stabilizing the mutant cell walls.
Since the ΔBccrz1 mycelium exhibited higher sensitivity only to glucanases and not to chitinases (data not shown), we suggested that the major impact of BcCRZ1 was on the glucan composition and not on the chitin content of the cell wall. In S. cerevisiae, calcineurin acts via CRZ1 as a positive regulator of the gene FKS2, encoding the key enzyme for synthesis of the β-1,3-glucan polymer, β-1,3-glucan synthase (85). In contrast to yeasts, in which two similar genes (FKS1 and FKS2) encode catalytic subunits of the glucan synthase complex and the deletion of both is lethal (31), the B. cinerea genome contains only a single gene (the protein is annotated as BC1G_14034.1). The expression of this gene was not altered in the Bccrz1 mutant under standard conditions in comparison to the wild type but was slightly down-regulated in ΔBccrz1 with MgCl2 supplementation, suggesting a deregulation of gene expression in response to Mg2+ treatment (Fig. 6C). The expression of another gene (the protein is annotated as BC1G_14030.1) encoding a protein with similarity to the 1,3-β-glucanosyltransferase Gel4 of A. fumigatus, a glucan-elongating enzyme, was slightly affected by the Bccrz1 deletion independently of Mg2+ availability (Fig. 6C). In addition, the findings that the Bccrz1 mutants were less sensitive to the cell wall-interfering compound calcoflour white and that the wild type in the presence of this compound also responded to Mg2+ supplementation with decreased sensitivity (Table 2) support the suggestion that Mg2+ ions are able to stabilize impaired cell walls.
TABLE 2.
Effects of stress conditions on growth rates of wild-type B05.10 and ΔBccrz1 strainsa
| Type of stress | Growth medium | Colony diam (mm) (% of control)
|
|||
|---|---|---|---|---|---|
| WT B05.10- MgCl2 | ΔBccrz1- MgCl2 | WT B05.10 + MgCl2 | ΔBccrz1 + MgCl2 | ||
| Control | CM | 67.0 ± 4.6 | 32.5 ± 10.8 | 70.2 ± 3.4 | 70.1 ± 4.7 |
| Cell wall | +2 mg/ml CFWb | 35.0 ± 7.8 (52) | 27.4 ± 2.3 (84) | 53.3 ± 6.3 (76) | 56.6 ± 2.8 (81) |
| +2 mg/ml Congo red | 49.1 ± 1.3 (73) | 18.8 ± 1.4 (58) | 69.0 ± 3.2 (98) | 70.6 ± 2.5 (101) | |
| Membrane | +0.02% SDS | 41.7 ± 2.1 (62) | 7.5 ± 1.4 (23) | 44.0 ± 3.1 (63) | 13.4 ± 2.9 (19) |
| +0.02% Triton X | 11.7 ± 1.1 (17) | 7.2 ± 0.5 (22) | 13.1 ± 1.3 (19) | 11.9 ± 1.4 (17) | |
| +5 μg/ml fluconazole | 46.4 ± 2.8 (69) | 9.2 ± 1.4 (28) | 48.4 ± 2.8 (69) | 56.2 ± 1.4 (80) | |
| Control | CM | 73.9 ± 1.9 | 42.6 ± 11.1 | 63.1 ± 3.4 | 62.1 ± 2.0 |
| pH | pH 3 | 54.4 ± 1.4 (74) | 6.9 ± 1.1 (16) | 31.9 ± 3.3 (51) | 8.9 ± 1.1 (14) |
| pH 9 | 45.3 ± 2.0 (61) | 8.9 ± 0.6 (21) | 34.4 ± 1.6 (55) | 34.8 ± 4.5 (56) | |
| Osmotic | +1.2 M sorbitol | 64.8 ± 1.1 (88) | 47.2 ± 1.1 (111) | 53.1 ± 1.0 (84) | 49.1 ± 2.2 (79) |
| +1 M NaCl | 25.5 ± 1.9 (35) | 13.6 ± 0.5 (32) | 29.9 ± 1.4 (47) | 24.7 ± 1.9 (40) | |
| Ionic | +30 mM LiCl | 33.4 ± 2.0 (45) | 5.0 ± 0.0 (12) | 64.3 ± 2.5 (102) | 61.5 ± 1.8 (99) |
| +20 mM MnCl2 | 17.8 ± 4.3 (24) | 42.6 ± 4.7 (100) | 46.4 ± 2.1 (74) | 47.9 ± 1.6 (77) | |
| +400 mM CaCl2 | 59.2 ± 2.4 (80) | 7.1 ± 2.7 (17) | 53.6 ± 2.0 (85) | 12.4 ± 5.7 (20) | |
| Oxidative | +10 mM H2O2 | 37.9 ± 1.8 (51) | 5.0 ± 0.0 (12) | 44.6 ± 1.0 (71) | 14.4 ± 5.6 (23) |
| +500 μM menadione | 39.1 ± 2.6 (53) | 34.8 ± 1.1 (82) | 37.4 ± 3.3 (59) | 41.4 ± 2.0 (67) | |
Strains were grown for 3 days on CM (with [+] or without [−] 67 mM MgCl2) containing the stressors indicated. The data are means of six colonies ± standard deviations. WT, wild type.
CFW, calcofluor white.
To prove whether the integrity of the membrane was also impaired in the ΔBccrz1 mutant, we examined the sensitivity of the mutants to 0.02% concentration of the anionic detergents SDS and Triton X-100, which are known to perturb plasma membranes, and to fluconazole (5 μg/ml), an inhibitor of the lanosterol 14α-demethylase (ERG11), resulting in blocking of ergosterol biosynthesis, thereby causing severe membrane stress due to the accumulation of toxic intermediates (47). To investigate the Mg2+ effect, we used CM with and without 67 mM MgCl2. As expected, the ΔBccrz1 mutants were more sensitive to SDS than the wild type in the presence, as well as in the absence, of Mg2+ ions, marked by reduced colony diameters (Table 2). In contrast to this, the hypersensitivity of the Bccrz1 mutants to fluconazole was completely restorable by Mg2+ supplementation. These data therefore demonstrate that BcCRZ1 is essential, not only for cell wall integrity, but also for the membrane stress response.
Bccrz1 mutants have altered stress responses.
S. cerevisiae and C. albicans crz1 mutants were reported to be hypersensitive to several types of stress, including exposure to high cation concentrations (58, 68). In order to examine the stress responses of the ΔBccrz1 mutants, we performed plate assays using CM and MgCl2-supplemented CM as basal media and applied different kind of stresses, such as pH stress (pH 3 and pH 9), osmotic stress (1.2 M sorbitol), oxidative stress caused by 10 mM H2O2 or 500 μM menadione, and concentrations of the cations Na+, Li+, Mn2+, and Ca2+ (1.0 M NaCl, 30 mM LiCl, 20 mM MnCl2, and 0.4 M CaCl2) that were sufficient to affect the growth rate of B. cinerea (Table 2). The responses of ΔBccrz1 mutants to osmotic stress (sorbitol and NaCl) and to oxidative stress caused by menadione, which leads to the generation of O2− ions, were not altered. However, the mutants were more sensitive to other stress conditions, such as extreme pH values and growth in the presence of high Li+ and Ca2+ concentrations, as well as oxidative stress caused by H2O2, than the wild type and the untreated mutant (CM control). Mg2+ supplementation restored wild-type-like growth at pH 9 and in the presence of Li+ but did not improve growth rates at pH 3 and during exposure to Ca2+ and H2O2. Interestingly, Mg2+ addition increased the tolerance of the wild type for Li+ and Mn2+, and the Bccrz1 mutant was less sensitive to Mn2+ than the wild type. These results indicate that BcCRZ1 is involved in oxidative-stress resistance and the regulation of ion homeostasis.
BcCRZ1 is required for full virulence on bean leaves, tomato fruits, and apricots.
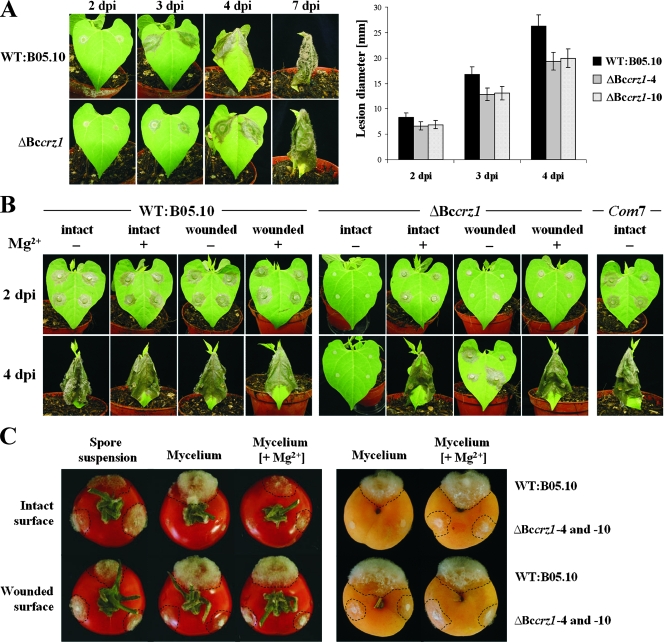
So far, no deletion mutants of the Ca2+/calcineurin signaling pathway have been generated and characterized in B. cinerea or related fungi. To study the role of BcCRZ1 in pathogenic development, we performed virulence assays on primary leaves of bean plants, using conidia or mycelia as an inoculum. Surprisingly, the conidia of the mutant germinated in a wild-type-like manner even in minimal medium, and the mutant was able to complete the whole infection cycle in planta, starting with the appearance of primary lesions after 2 days, formation of spreading lesions after 3 days, and soft rot of the whole plant after 7 days, associated with the production of numbers of conidia equivalent to the wild type (Fig. 7A). However, the lesion diameters were slightly reduced (approximately 20%) 2, 3, and 4 days postinoculation in comparison to the wild type, demonstrating that BcCRZ1 is required for full virulence.
FIG. 7.
Virulence assays on different host plants. (A) Virulence of ΔBccrz1 conidia on bean plants. Primary leaves were inoculated with 7-μl droplets of conidial suspensions (2 × 105/ml in Gamborgs B5 plus 2% glucose) of the wild type (WT) and the two ΔBccrz1 mutants. The lesion diameters were determined after 2, 3, and 4 days of incubation (dpi). Mean values were calculated from 26 lesions. The error bars indicate standard deviations. (B) Virulence of ΔBccrz1 mycelium on intact and wounded bean plants. Leaves were inoculated with agar plugs (CM with or without 67 mM MgCl2) colonized by nonsporulating, 3-day-old mycelia of the wild type, the ΔBccrz1 mutant, and the complemented mutant Com-7. The leaves were wounded with a needle prior to the inoculation. (C) Virulence of ΔBccrz1 conidia and mycelia on intact and wounded tomato fruits (left) and apricots (right). Precultivation of the wild-type strain and the two ΔBccrz1 mutants for conidium- and mycelium-derived infection (A and B). The photographs were taken 4 days postinoculation (dpi). Infected areas are highlighted by dashed lines.
Since the mycelium of the ΔBccrz1 mutant was more sensitive to environmental stress conditions (medium composition) than the conidiospores, we used agar plugs from 3-day-old nonsporulating CM cultures of the ΔBccrz1 mutant and the wild type to inoculate intact and prewounded bean leaves. The ΔBccrz1 mycelium was strongly impaired in its ability to penetrate the intact plant epidermis after 4 days, whereas the wild-type mycelium had already reached the stage of soft-rot formation by that time (Fig. 7B). It is worth noting that in very rare cases (about 8 out of 100 infection spots) the ΔBccrz1 mycelium was able to infect the bean plants, though with considerable delay of 5 days or longer. In contrast, the ΔBccrz1 mycelium caused 100% infection when the plant surface was wounded prior to inoculation with agar pieces, showing that BcCRZ1 plays a role in promoting the penetration event during mycelium-derived infection. To see whether the penetration defect was just a result of Mg2+ deprivation, we also grew the mutant on CM medium supplemented with Mg2+ (67 mM MgCl2) and used this mycelium for virulence assays. In fact, we observed 100% infection on intact and wounded bean leaves, thus demonstrating that the addition of Mg2+ compensated for the penetration defect of the ΔBccrz1 hyphae (Fig. 7B). Pathogenicity assays using detached tomato leaves (data not shown), tomato fruits, and apricots (Fig. 7C) as host systems revealed similar results for the virulence of ΔBccrz1 conidia. However, Mg2+ partially compensated for the penetration defect of the ΔBccrz1 hyphae (Fig. 7C), indicating the importance of BcCRZ1 for penetration and, to a lesser extent, for subsequent invasive growth.
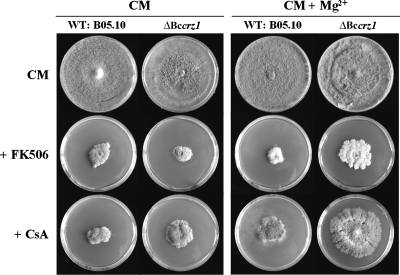
Bccrz1 mutants still respond to the calcineurin inhibitors cyclosporine and FK506.
Cyclosporine and FK506 are inhibitors of calcineurin and act in B. cinerea by binding to the specific peptidyl-prolyl isomerases cyclophilin A (BCP1) and FKBP12 (BcPIC5), respectively (23, 75), and subsequent binding of these protein-drug complexes to the active site of calcineurin. If CRZ1 is the only substrate for calcineurin, we would expect increased resistance of the Bccrz1 mutants to these inhibitors, because the substrate for calcineurin is missing. However, if there are more targets of calcineurin activity, the mutants should still respond to these inhibitors with retarded growth. In order to explore this hypothesis, we performed plate assays using the inhibitors cyclosporine and FK506 with and without Mg2+ supplementation (Fig. 8). Treatment with these inhibitors had a profound impact on the growth of both the wild type and the ΔBccrz1 mutant, resulting in the formation of small, compact, sporulating colonies after 3 weeks of incubation. Without Mg2+, the growth retardation of the mutant was similar to that of the wild type. However, when the medium was supplemented with Mg2+, the mutant appeared to be more resistant to both inhibitors but still responded with significantly reduced growth to the inhibition of calcineurin (Fig. 8). Hence, the presence of other signaling effectors of calcineurin besides BcCRZ1 is proposed for B. cinerea.
FIG. 8.
Effects of calcineurin inhibitors on growth of the wild type (WT) and the ΔBccrz1 mutants. The strains were grown on CM without or with 67 mM MgCl2 containing cyclosporine (CsA) (0.5 μg/ml) or FK506 (0.05 μg/ml). The photographs were taken after 3 weeks of incubation.
BcCRZ1 deletion affects CND gene expression.
Recently, several CND genes have been identified by macroarray approaches by treating wild-type strains with cyclosporine, mimicking the deletion of calcineurin (61, 75). To test whether the expression of the CND genes is also dependent on BcCRZ1, we compared the expression pattern in the Bccrz1 mutant with that of the wild type by differential hybridization of macroarrays and Northern blot analyses. The Bccrz1 mutant and the wild type were grown under standard culture conditions (see Material and Methods). The RNA was extracted and used for the preparation of radioactively labeled cDNA probes (macroarray hybridization) and for Northern blot analyses. Several BcCRZ1-dependent genes were identified (Table 3), including 13 of the described CND genes (75). The gene products are involved in different processes, such as secondary metabolism (e.g., botrydial biosynthesis), carbohydrate metabolism, cell wall organization, ion transport, and protein degradation.
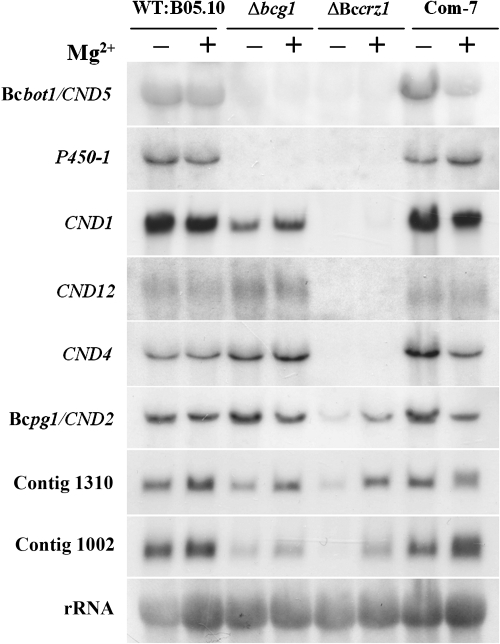
To study the effect of Mg2+on BcCRZ1-dependent gene expression, we cultivated the strains on CM supplemented with 67 mM MgCl2 and tested the expression of several chosen target genes by Northern blot analyses (Fig. 9). To confirm our recent finding that BCG1 is an upstream regulator of calcineurin signaling (61), we included the Δbcg1 mutant in our expression studies. Different expression patterns of BcCRZ1-dependent genes were found. One group of genes whose expression was dependent on calcineurin and the Gα subunit BCG1 but was unaffected by Mg2+ (Table 3 and Fig. 9) included the genes for botrydial biosynthesis (e.g., Bcbot1/CND5) and for the production of another as yet unknown secondary metabolite with polyketide structure (e.g., P450-1) (61). Another group represented genes whose expression was found to be dependent on calcineurin activity but unaffected by the bcg1 deletion, as well as Mg2+ supplementation. As examples, CND12, exhibiting similarity to the calcium ion transporter VCX1-encoding gene of S. cerevisiae, and CND4, without significant homology to known genes, are shown (Fig. 9). A third group comprised genes whose expression was affected in Δbcg1, as well as in ΔBccrz1, strains. Interestingly, the expression of these genes (e.g., contig 1310 and contig 1002) can be significantly increased in the Bccrz1 mutants through Mg2+ supplementation, confirming the impact of Mg2+ on gene expression in ΔBccrz1. Also, the expression of some genes, e.g., the endopolygalaturonase 1-encoding gene Bcpg1/CND2, is independent of BCG1 but down-regulated in ΔBccrz1 without Mg2+ and more highly expressed with Mg2+ addition. Moreover, the up-regulation of protease-encoding genes (contig 934, contig 902, contig 1515, and contig 783) was noticeable in ΔBccrz1 (Table 3).
FIG. 9.
Expression of several BcCRZ1-dependent genes in the wild-type (WT) and mutant strains. The wild-type B05.10, the Δbcg1 and ΔBccrz1 mutants, and the complemented mutant ΔBccrz1 Com-7 were grown for 3 days on CM agar with or without 67 mM MgCl2. The Northern blot was hybridized to radioactively labeled probes of several BcCRZ1-dependent genes (listed in Table 3). rRNA was used as a loading control.
Due to the fact that most of the CND genes were also altered in the ΔBccrz1 mutant, we conclude that BcCRZ1 is indeed a target of calcineurin, modulating the expression of a specific set of genes.
Complementation of ΔBccrz1 mutants restores the wild-type phenotype.
To confirm that the severe phenotype of ΔBccrz1 is exclusively caused by the inactivation of Bccrz1, a complementation approach was used. For this, the mutant ΔBccrz1-4 was transformed with the vector pBccrz1-Com+, containing the cDNA sequence of Bccrz1 under the control of the native Bccrz1 promoter, and the gene flanks of BcniaD, encoding nitrate reductase, for the targeted integration of the construct at the respective gene locus. Putative complemented transformants, selected based on their noureothricin resistance, were analyzed by PCR for the complete integration of the Bccrz1 copy. For two transformants, Com-7 and Com-9, the homologous integration event at the BcniaD locus was confirmed (data not shown). Both transformants showed full restoration of the wild-type phenotype with respect to vegetative growth, conidiation, sclerotium formation, virulence on bean plants (Fig. 7B), and gene expression (Fig. 9). Moreover, the growth rates of the complemented mutants in the presence of extreme pH values, LiCl, CaCl2, and H2O2 were comparable to those of the wild type (data not shown). These data clearly show that the described phenotype of the Bccrz1 mutant is caused by the targeted inactivation of the corresponding gene.
DISCUSSION
This study is the first to report the identification and characterization of the putative calcineurin-responsive transcription factor from a filamentous fungus and describes the involvement of the calcineurin/CRZ1 signaling pathway in vegetative growth, differentiation, expression of secondary metabolite genes, cell and membrane integrity, the response to different environmental stress conditions, and virulence in the plant pathogen B. cinerea.
BcCRZ1 acts as a downstream target of (yeast) calcineurin.
We identified one putative CRZ1 homologue in the B. cinerea genome that displayed significant similarity to the C-terminal DNA-binding domain (containing two C2H2-type zinc finger motifs) of the S. cerevisiae CRZ1 protein. Several pieces of evidence supported our suggestion that the identified B. cinerea protein BC1G_00093.1 indeed encodes the functional homologue of the yeast calcineurin-regulated transcription factor CRZ1. First of all, the transformation of the S. cerevisiae crz1 mutant with the full-length cDNA of Bccrz1 under the control of the yeast MET25 promoter showed that the B. cinerea gene functionally complemented the yeast mutation during growth under high Na+ concentrations. However, the BcCRZ1-transformed yeast cells were still sensitive to Mn2+ and Ca2+. In contrast, the complementation of Δcrz1 yeast cells with the C. albicans crz1 gene (Cacrz1) led to suppression of sensitivity to Na+, Mn2+, and Ca2+ (58), indicating that the CRZ1 transcription factor possibly has distinct, as well as common, functions in yeasts and filamentous fungi. We propose that the suppression of the Na+ sensitivity by expressing Bccrz1 in crz1-defective yeast cells is based on the BcCRZ1-mediated transcriptional activation of the gene ENA1, encoding the ATP-driven ion pump, which is induced and activated by toxic concentrations of Na+ and Li+, promoting the extrusion of these ions from the cell (41, 43). The partial restoration of the wild-type phenotype by the B. cinerea homologue suggests the potential conservation of the CRZ1-binding motifs in the respective promoter sequence(s). A key factor of CRZ1 regulation by dephosphorylation is the targeting of calcineurin to the conserved PXIXIT motif (the calcineurin-docking domain). Although the calcineurin-docking domains are highly conserved in yeast and mammalian calcineurin targets (NFAT transcription factor family), no obvious docking domain was found in the sequence of the B. cinerea CRZ1 homologue. However, the localization studies of a GFP-BcCRZ1 fusion protein in yeast cells clearly show that the subcellular distribution of BcCRZ1 is regulated by Ca2+ in a calcineurin-dependent manner: activation of calcineurin by Ca2+ addition to the cells promotes the transport of the cytosolic GFP-BcCRZ1 into the nucleus, whereas calcineurin inhibition by treatment with FK506 impairs the accumulation of GFP-BcCRZ1 in the nucleus. Similar results were reported for C. albicans CRZ1. The GFP-CaCRZ1 fusion protein in S. cerevisiae cells showed the same localization pattern, and CaCRZ1 undergoes posttranslational modifications due to calcineurin activity (34, 58). Taken together, these data confirm our suggestion that BcCRZ1 is indeed a target of the calcineurin phosphatase and the functional homologue of yeast CRZ1.
Functions of CRZ1 in B. cinerea.
The BcCRZ1 deletion caused a pleiotropic phenotype characterized by abnormal vegetative growth (reduced growth and altered hyphal morphology) and differentiation (reduced conidiation and sclerotium formation). The strength of the phenotype depends on the cultivation conditions: while almost no growth has been observed on synthetic minimal medium, supplemented media, such as CM or grape juice agar, were found to promote growth. In addition, the cell wall and membrane integrity of the mutant was affected, leading to higher susceptibility to cell wall-degrading enzymes and the anionic detergent SDS, which could be the explanation for the pleiotropic phenotype mentioned above. Interestingly, the MAP kinase mutant (Δbmp3) showed significant similarities to the ΔBccrz1 strain in regard to the reduction of conidiation and sclerotium formation and poor growth in vitro under low-osmolarity conditions (54), indicating that different signaling pathways might regulate these differentiation processes. As discussed below in more detail, almost all features of the Bccrz1 mutant could be restored by specific growth conditions, such as feeding with additional MgCl2, demonstrating that the mutant responds to its environment in a different manner than the wild type, which obviously did not respond to the MgCl2 supplementation.
Due to the sensitivity of the Bccrz1 mutants to glucanases and the decreased expression of a gene involved in the elongation of glucan chains, we suppose that mainly the glucan backbone of the fungal cell wall is affected by the Bccrz1 deletion. However, an influence of calcineurin and BcCRZ1 on chitin synthesis cannot be excluded, since four chitin synthase promoters were shown to be activated by exogenous Ca2+ in a calcineurin/CaCRZ1-dependent manner in C. albicans (46). The fact that the inhibition of calcineurin in the closely related fungus S. sclerotiorum resulted in reduction of the β-1,3-glucan content and hypersensitivity to cell wall-degrading enzymes and the glucan synthase inhibitor caspofungin (26) corroborates our hypothesis. Previous studies in S. cerevisiae and C. albicans have elucidated a role for calcineurin signaling in response to membrane stress caused by detergents and antifungal azoles, such as fluconazole (4, 10, 11, 17, 34, 49, 58). This function seems to be conserved in B. cinerea, because the Bccrz1 mutant is more sensitive to SDS in an Mg2+-independent manner and to fluconazole in an Mg2+-dependent manner.
Other characteristics of the ΔBccrz1 mutant, such as sensitivity to oxidative stress caused by H2O2, in either the presence or the absence of Mg2+, differ from the findings in S. cerevisiae, in which hypersensitivity to H2O2 was observed only for calcineurin mutants (45). A correlation between oxidative-stress response and Ca2+ signaling was demonstrated for the expression of the yeast phospholipid hydroperoxide glutathione peroxidase GPX2, a part of the antioxidant system protecting cells from oxidative stress. The H2O2-induced expression of GPX2 was found to be strictly regulated by the transcription factor YAP1 and the response regulator SKN7. In addition, the expression of GPX2 was inducible by Ca2+ in a calcineurin- and CRZ1-dependent manner (72, 73). SKN7 has been shown to be a multicopy enhancer of calcineurin- and CRZ1-dependent transcription in yeast, proposing a model in which SKN7 regulates calcineurin signaling through the stabilization of CRZ1 via a direct protein-protein interaction (82). Due to the fact that Bccrz1 mutants are hypersensitive to H2O2, it is most likely that similar interconnections between Ca2+-dependent signaling and oxidative-stress response also exist in B. cinerea.
The response of the B. cinerea crz1 mutants to the cations Mn2+ and Li+ is complex and differs from the yeast system. Although the B. cinerea mutants were more sensitive to Li+ when Mg2+ was missing, the mutants appeared to be more resistant to Mn2+ ions than the wild type, and moreover, the resistance of the wild type to Mn2+ and Li+ was increased by Mg2+ supplementation. The fact that the stress-activated MAP kinase BcSAK1 mutants were more sensitive to NaCl than the wild type (62) suggests that NaCl tolerance is mainly regulated via this MAP kinase pathway in B. cinerea. However, overexpression of the crz1 gene from the salt-tolerant yeast T. delbrueckii in S. cerevisiae enhanced tolerance for Na+ in wild-type cells and suppressed sensitivity to Mn2+, Na+, and Li+ in Δcrz1 and calcineurin mutants, but surprisingly, Tdcrz1 mutants were insensitive to high Na+ and more tolerant of Li+ than wild-type cells (28). All these data show that the calcineurin/CRZ1 signaling pathway has some conserved functions, such as the regulation of cell wall/membrane integrity and the general stress response and some fungus-specific functions, reflecting the evolution of calcineurin/CRZ1 signaling output due to the adaptation of the organisms to their environmental niches.
Pathogenicity assays with the B. cinerea mutants showed that BcCRZ1 (and probably also calcineurin) is required for full virulence of B. cinerea on several host systems, such as bean plants, detached tomato leaves, tomato fruits, and apricots. While the transcription factor seems to be almost dispensable for the conidium-derived infection program, BcCRZ1 is essential for penetration of hyphae, indicating distinct modes of penetration of the plant surface by freshly germinated spores and by growing mycelium. The regulatory systems governing the different penetration methods are still unclear, although the phenomenon of different infection properties due to inoculation with conidia or mycelium has been observed previously (61). We cannot exclude the possibility that this effect is due to the impaired cell wall integrity of Bccrz1 mutants, since bmp3 mutants (loss of cell wall integrity MAP kinase) were also defective in mycelium-derived infection (data not shown), while spores were still able to infect the plant (54). Moreover, B. cinerea chitin synthase (Bcchs1 or Bcchs3a) mutants exhibiting reduced chitin contents in their cell walls were also shown to be affected in virulence (66, 67). The fact that Mg2+ supplementation improved the cell wall stability and penetration rates of Bccrz1 mutants indicates the importance of intact cell walls for normal infection efficiencies. We suggest that the negligible role of BcCRZ1 in the conidium-derived infection of host plants is due to intracellular Mg2+ resources in the conidia, which allow them to germinate and penetrate normally. Once the fungus has invaded the plant cells, it is able to assimilate Mg2+ from the host plant. This hypothesis is supported by the finding that ΔBccrz1 conidia were able to germinate in minimal medium without Mg2+ supplementation and that the development of the germinated spores stopped after 24 h, possibly due to Mg2+ limitation.
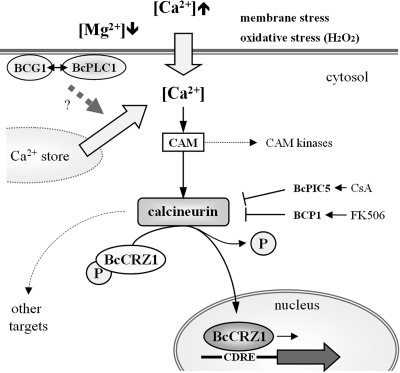
Induction of the calcineurin/BcCRZ1 pathway by Mg2+ depletion and Ca2+.
We have shown that various defects of Bccrz1 mutants, such as growth, conidiation, and virulence, could be restored by elevated Mg2+ concentrations in the culture medium. Similar observations were made for yeast mutants lacking components of Mg2+ high-affinity systems, such as the plasma membrane Mg2+ transport protein ALR1/2 (24, 78) or the mitochondrial Mg2+ channel protein MSR2 (37, 80), which could survive only when high external Mg2+ concentrations were provided. As a consequence of low mitochondrial Mg2+ concentrations caused by mutations of MSR2, group II intron RNA splicing was decreased (80), demonstrating the impact of Mg2+ on normal cell function. Preliminary results showed that the expression of two genes (whose proteins were annotated as BC1G_11674.1 and BC1G_11425.1) with similarities to the yeast ALR1/2 genes was slightly increased in the ΔBccrz1 strain (data not shown). However, the up-regulation of these genes was not sufficient to compensate for the Mg2+ deficit of the mutants, indicating the involvement of other transporters, such as MSR2 (BC1G_09979.1), whose expression could not be detected by Northern blot analyses.
Recent studies in S. cerevisiae revealed an interconnection between Mg2+- and Ca2+/calcineurin/CRZ1-dependent signaling. Mg2+ starvation conditions led to enhanced cellular Ca2+ concentrations, which subsequently activated the calcineurin pathway, leading to the translocation of CRZ1 from the cytosol into the nucleus (81). Moreover, S. cerevisiae calcineurin B or crz1 mutants were shown to be more sensitive to low Mg2+, consistent with our results for the B. cinerea crz1 mutant. Thus, we conclude that the calcineurin/CRZ1 pathway is required in B. cinerea to cope with low-Mg2+ conditions. Consistent with our suggestion that calcineurin/BcCRZ1-dependent signaling is induced by Ca2+, we found that high Ca2+ concentrations (>0.3 M CaCl2) were toxic for the Bccrz1 mutants even in the presence of additional Mg2+. We conclude, therefore, that BcCRZ1 is essential for the mediation of the transcriptional response to increased Ca2+ concentrations.
BcCRZ1 is a mediator of CND gene expression in B. cinerea.
The analysis by a macroarray approach and Northern blot analyses of gene expression in ΔBccrz1 in comparison to the wild type under standard culture conditions revealed 48 genes whose expression is dependent on BcCRZ1. Most of the BcCRZ1-dependent genes are down-regulated, suggesting that BcCRZ1 is an activator of transcription. Most importantly, the expression of a set of previously identified CND genes was also dependent on BcCRZ1, confirming the interconnection between calcineurin and BcCRZ1. Some of these genes (e.g., the secondary-metabolite genes) were also dependent on the function of BCG1 and phospholipase C (BcPLC1), confirming our finding that these signaling components are upstream regulators of the calcineurin pathway (61). At this stage, we do not know whether all the affected genes are directly regulated by BcCRZ1 binding to their promoter sequences. It seems unlikely that BcCRZ1 is the main/sole regulator of gene clusters responsible for the biosynthesis of botrydial and an unknown secondary metabolite, because the mutant was still able to produce botrydial and its derivates under certain conditions (data not shown). The restoration of the expression of several BcCRZ1-dependent genes by Mg2+ supplementation raises the question of the degree to which the Mg2+ deprivation in consequence of the Bccrz1 deletion influences the global gene expression. The identification of the regulatory regions (the calcineurin-dependent response element) of the calcineurin-CRZ1-dependent genes in B. cinerea will help to clarify these factors.
In addition, it would be interesting to gain more information about the signals and conditions that result in the activation of the calcineurin/BcCRZ1 signaling pathway in B. cinerea, e.g., by studying the cellular localization of BcCRZ1 during distinct phases of growth (e.g., germinating conidia versus growing mycelium) and when the cells are exposed to different conditions, such as Ca2+, oxidative stress, or extreme pH values. Another interesting question is whether a connection between RIM101, the yeast counterpart of PacC in filamentous fungi, and the calcineurin signal transduction pathway also exists in B. cinerea, as was described in S. cerevisiae (63, 77) and recently in C. albicans (39).
BcCRZ1 is not the sole signaling effector of calcineurin.
Several facts lead to the assumption that BcCRZ1 is not the only downstream target of the B. cinerea calcineurin signaling cascade responsible for vegetative growth and pathogenicity. Although an attempt to create a B. cinerea calcineurin mutant failed (75), the impact of calcineurin on vegetative growth and infection-related morphogenesis has been studied by using calcineurin inhibitors. Treatment of conidia with cyclosporine did not affect germination, but the conidia produced abnormal, hyperbranched hyphae, which were unable to penetrate the epidermis of onion, bean, or tomato leaves (75), indicating the requirement for calcineurin in pathogenicity. In comparison to this, the phenotype of ΔBccrz1 mutants was less impaired, their conidia being almost as pathogenic as the wild-type conidia. Moreover, Bccrz1 mutants still responded to the inhibition of calcineurin. Although the mutants seemed to be more resistant to cyclosporine and FK506 in the presence of additional Mg2+ than the wild type, there was an additive effect due to the calcineurin inhibition, indicating that other downstream targets of calcineurin that are involved in growth and pathogenicity must exist.
In S. cerevisiae, calcineurin has several substrates in addition to CRZ1 that are not transcription factors and are posttranslationally modified by calcineurin activity. Thus, the calcineurin substrate HPH1 (high-pH protein), containing a calcineurin-docking domain, localizes to the endoplasmic reticulum and promotes cell growth under conditions of high salinity, alkaline pH, and cell wall stress independently of CRZ1 (27). SLM1 and SLM2 are other calcineurin targets activated when cells were grown under nutrient limitation or environmental stress. They are plasma membrane-localized proteins required for actin cytoskeleton polarization and heat stress-induced endocytosis of nutrient permeases and interconnect calcineurin signaling with the TOR (target of rapamycin) cascade. The TOR2 kinase phosphorylates these proteins under favorable growth conditions, and therefore, calcineurin and TOR2 act in an antagonistic manner (3, 8, 14). While no obvious homologues of HPH1 could be identified in the B. cinerea genome, two putative homologues of SLM proteins showing significant similarity to the yeast proteins (E values, 6e−69 and 5e−20) were found, leading to the assumption that a similar regulatory network may also exist in B. cinerea.
Conclusions and perspectives.
In yeast cells, a negative regulation of CRZ1 activity by PKA, the downstream effector of G protein/adenylate cyclase/cAMP signaling, has been shown; the PKA directly phosphorylates the nuclear localization signal, thereby preventing the nuclear import of CRZ1 (32). The in silico search for PKA phosphorylation sites within the BcCRZ1 sequence resulted in the identification of one putative interaction motif. The study of localization patterns of GFP-BcCRZ1 fusion proteins in the respective B. cinerea PKA mutants (J. Schumacher, L. Kokkelink, C. Huesmann, I. Collado, R. Barakat, P. Tudzynski, and B. Tudzynski, unpublished data) will provide insight into the interconnection between cAMP- and Ca2+/calcineurin/BcCRZ1-dependent signal transduction pathways in B. cinerea.
Based on our data, we present a model for the supposed Ca2+/calcineurin- and BcCRZ1-dependent signaling pathway (Fig. 10). We assume that the pathway is activated during exposure to high Ca2+ concentrations and Mg2+ limitation and that it is essential to mediate the responses to membrane stress and oxidative stress caused by H2O2. As several calcineurin- and BcCRZ1-dependent genes were also dependent on the Gα subunit BCG1 and the phospholipase C BcPLC1 (61), an interconnection between calcineurin signaling and the G protein-coupled receptor system is proposed.
FIG. 10.
Model of Ca2+/calcineurin- and BcCRZ1-dependent signal transduction in B. cinerea. Calcium from the external environment or storage organelles binds to the Ca2+ sensor protein calmodulin (CAM), which then activates different kinases and the calcineurin phosphatase via interaction with the calcineurin heterodimer. Activation of calcineurin via the Gα subunit BCG1 and phospholipase C (BcPLC1), probably by modulation of the cytosolic Ca2+ level, is proposed (61). The dephosphorylation of the transcription factor BcCRZ1 by calcineurin results in its translocation from the cytoplasm to the nucleus, where it affects gene expression. The sites of action of the calcineurin inhibitors cyclosporine (CsA) and FK506 are indicated; they first bind to the specific peptidyl-prolyl isomerases cyclophilin A (BCP1) and FKBP12 (BcPIC5), respectively (23, 75). BcCRZ1 is required when the fungus is exposed to Mg2+ deprivation, Ca2+, membrane stress, and oxidative stress. Other target proteins for calcineurin activity are proposed. CDRE, calcineurin-dependent response element.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (Tu101/10-5).
We are grateful to Brian Williamson for reading the manuscript, to Isidro G. Collado (Cadiz, Spain) for analyzing the botrydial production in the B. cinerea Δcrz1 strains, and to Matthias Hahn for providing the Δbmp3 mutant.
Footnotes
Published ahead of print on 8 February 2008.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 253389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aramburu, J., J. Heitman, and G. R. Crabtree. 2004. Calcineurin: a central controller of signalling in eukaryotes; workshop on the calcium/calcineurin/NFAT pathway: regulation and function. EMBO Rep. 5343-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Audhya, A., R. Loewith, A. B. Parsons, L. Gao, M. Tabuchi, H. Zhou, C. Boone, M. N. Hall, and S. D. Emr. 2004. Genome-wide lethality screen identifies new PI4,5P2 effectors that regulate the actin cytoskeleton. EMBO J. 233747-3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bader, T., B. Bodendorfer, K. Schroppel, and J. Morschhauser. 2003. Calcineurin is essential for virulence in Candida albicans. Infect. Immun. 715344-5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckers, G. J., and U. Conrath. 2006. Microarray data analysis made easy. Trends Plant Sci. 11322-323. [DOI] [PubMed] [Google Scholar]
- 6.Bonilla, M., K. K. Nastase, and K. W. Cunningham. 2002. Essential role of calcineurin in response to endoplasmic reticulum stress. EMBO J. 212343-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bullock, W. O., J. M. Fernandez, and J. M. Short. 1987. XL1-Blue: a high efficiency plasmid transforming RecA Escherichia coli strain with beta-galactosidase selection. BioTechniques 5376-378. [Google Scholar]
- 8.Bultynck, G., V. L. Heath, A. P. Majeed, J. M. Galan, R. Haguenauer-Tsapis, and M. S. Cyert. 2006. Slm1 and Slm2 are novel substrates of the calcineurin phosphatase required for heat stress-induced endocytosis of the yeast uracil permease. Mol. Cell. Biol. 264729-4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cenis, J. L. 1992. Rapid extraction of fungal DNA for PCR amplification. Nucleic Acids Res. 202380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowen, L. E., A. E. Carpenter, O. Matangkasombut, G. R. Fink, and S. Lindquist. 2006. Genetic architecture of Hsp90-dependent drug resistance. Eukaryot. Cell 52184-2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruz, M. C., A. L. Goldstein, J. R. Blankenship, M. Del Poeta, D. Davis, M. E. Cardenas, J. R. Perfect, J. H. McCusker, and J. Heitman. 2002. Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J. 21546-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunningham, K. W., and G. R. Fink. 1996. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol. Cell. Biol. 162226-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cyert, M. S. 2003. Calcineurin signaling in Saccharomyces cerevisiae: how yeast go crazy in response to stress. Biochem. Biophys. Res. Commun. 3111143-1150. [DOI] [PubMed] [Google Scholar]
- 14.Daquinag, A., M. Fadri, S. Y. Jung, J. Qin, and J. Kunz. 2007. The yeast PH domain proteins Slm1 and Slm2 are targets of sphingolipid signaling during the response to heat stress. Mol. Cell. Biol. 27633-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Da Silva Ferreira, M. E., T. Heinekamp, A. Härtl, A. A. Brakhage, C. P. Semighini, S. D. Harris, M. Savoldi, P. F. de Gouvêa, M. H. de Souza Goldman, and G. H. Goldman. 2007. Functional characterization of the Aspergillus fumigatus calcineurin. Fungal Genet. Biol. 44219-230. [DOI] [PubMed] [Google Scholar]
- 16.Doehlemann, G., P. Berndt, and M. Hahn. 2006. Different signalling pathways involving a Gα protein, cAMP and a MAPK control germination of Botrytis cinerea conidia. Mol. Microbiol. 59821-836. [DOI] [PubMed] [Google Scholar]
- 17.Edlind, T., L. Smith, K. Henry, S. Katiyar, and J. Nickels. 2002. Antifungal activity in Saccharomyces cerevisiae is modulated by calcium signalling. Mol. Microbiol. 46257-268. [DOI] [PubMed] [Google Scholar]
- 18.Fox, D. S., M. C. Cruz, R. A. Sia, H. Ke, G. M. Cox, M. E. Cardenas, and J. Heitman. 2001. Calcineurin regulatory subunit is essential for virulence and mediates interactions with FKBP12-FK506 in Cryptococcus neoformans. Mol. Microbiol. 39835-849. [DOI] [PubMed] [Google Scholar]
- 19.Fox, D. S., and J. Heitman. 2002. Good fungi gone bad: the corruption of calcineurin. Bioassays 24894-903. [DOI] [PubMed] [Google Scholar]
- 20.Garcion, C., R. Baltensperger, T. Fournier, J. Pasquier, M. A. Schnetzer, J. P. Gabriel, and J. P. Metraux. 2006. FiRe and microarrays: a fast answer to burning questions. Trends Plant Sci. 11320-322. [DOI] [PubMed] [Google Scholar]
- 21.Garrett-Engele, P., B. Moilanen, and M. S. Cyert. 1995. Calcineurin, the Ca2+/calmodulin-dependent protein phosphatase, is essential in yeast mutants with cell integrity defects and in mutants that lack a functional vacuolar H+-ATPase. Mol. Cell. Biol. 154103-4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gietz, R. D., R. H. Schiestl, A. R. Willems, and R. A. Woods. 1995. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast 11355-360. [DOI] [PubMed] [Google Scholar]
- 23.Gioti, A., A. Simon, P. Le Pecheur, C. Giraud, J.-M. Pradier, M. Viaud, and C. Levis. 2006. Expression profiling of Botrytis cinerea genes identifies three patterns of up-regulation in planta and a FKBP12 protein affecting pathogenicity. J. Mol. Biol. 358372-386. [DOI] [PubMed] [Google Scholar]
- 24.Graschopf, A., J. A. Stadler, M. K. Hoellerer, E. Eder, M. Sieghardt, S. D. Kohlwein, and R. J. Schweyen. 2001. The yeast plasma membrane protein Alr1 controls Mg2+ homeostasis and is subject to Mg2+-dependent control of its synthesis and degradation. J. Biol. Chem. 27616216-16222. [DOI] [PubMed] [Google Scholar]
- 25.Hamm, H. E. 1998. The many faces of G protein signaling. J. Biol. Chem. 273669-672. [DOI] [PubMed] [Google Scholar]
- 26.Harel, A., S. Bercovich, and O. Yarden. 2006. Calcineurin is required for sclerotial development and pathogenicity of Sclerotinia sclerotiorum in an oxalic acid-independent manner. Mol. Plant-Microbe Interact. 19682-693. [DOI] [PubMed] [Google Scholar]
- 27.Heath, V. L., S. L. Shaw, S. Roy, and M. S. Cyert. 2004. Hph1p and Hph2p, novel components of calcineurin-mediated stress responses in Saccharomyces cerevisiae. Eukaryot. Cell 3695-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernandez-Lopez, M. J., J. Panadero, J. A. Prieto, and F. Randez-Gil. 2006. Regulation of salt tolerance by Torulaspora delbrueckii calcineurin target Crz1p. Eukaryot. Cell 5469-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirayama, S., R. Sugiura, Y. Lu, T. Maeda, K. Kawagishi, M. Yokoyama, H. Tohda, Y. Giga-Hama, H. Shuntoh, and T. Kuno. 2003. Zinc finger protein Prz1 regulates Ca2+ but not Cl− homeostasis in fission yeast. Identification of distinct branches of calcineurin signaling pathway in fission yeast. J. Biol. Chem. 27818078-18084. [DOI] [PubMed] [Google Scholar]
- 30.Huang, X., and A. Madan. 1999. CAP3: a DNA sequence assembly program. Genome Res. 9868-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inoue, S. B., H. Qadota, M. Arisawa, Y. Anraku, T. Watanabe, and Y. Ohya. 1996. Signaling toward yeast 1,3-β-glucan synthesis. Cell Struct. Funct. 21395-402. [DOI] [PubMed] [Google Scholar]
- 32.Kafadar, K. A., and M. S. Cyert. 2004. Integration of stress responses: modulation of calcineurin signaling in Saccharomyces cerevisiae by protein kinase A. Eukaryot. Cell 31147-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kafadar, K. A., H. Zhu, M. Snyder, and M. S. Cyert. 2003. Negative regulation of calcineurin signaling by Hrr25p, a yeast homolog of casein kinase I. Genes Dev. 172698-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karababa, M., E. Valentino, G. Pardini, A. T. Coste, J. Bille, and D. Sanglard. 2006. CRZ1, a target of the calcineurin pathway in Candida albicans. Mol. Microbiol. 591429-1451. [DOI] [PubMed] [Google Scholar]
- 35.Kingsbury, T. J., and K. W. Cunningham. 2000. A conserved family of calcineurin regulators. Genes Dev. 141595-1604. [PMC free article] [PubMed] [Google Scholar]
- 36.Klimpel, A., C. Schulze Gronover, B. Williamson, J. A. Stewart, and B. Tudzynski. 2002. The adenylate cyclase (BAC) in Botrytis cinerea is required for full pathogenicity. Mol. Plant Pathol. 3439-450. [DOI] [PubMed] [Google Scholar]
- 37.Kolisek, M., G. Zsurka, J. Samaj, J. Weghuber, R. J. Schweyen, and M. Schweigel. 2003. Mrs2p is an essential component of the major electrophoretic Mg2+ influx system in mitochondria. EMBO J. 221235-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kraus, P. R., and J. Heitman. 2003. Coping with stress: calmodulin and calcineurin in model and pathogenic fungi. Biochem. Biophys. Res. Commun. 3111151-1157. [DOI] [PubMed] [Google Scholar]
- 39.Kullas, A. L., S. J. Martin, and D. Davis. 2007. Adaptation to environmental pH: integrating the Rim101 and calcineurin signal transduction pathways. Mol. Microbiol. 66858-871. [DOI] [PubMed] [Google Scholar]
- 40.Malonek, S., M. C. Rojas, P. Hedden, P. Gaskin, P. Hopkins, and B. Tudzynski. 2004. The NADPH-cytochrome P450 reductase gene from Gibberella fujikuroi is essential for gibberellin biosynthesis. J. Biol. Chem. 27925075-25084. [DOI] [PubMed] [Google Scholar]
- 41.Marquez, J. A., and R. Serrano. 1996. Multiple transduction pathways regulate sodium extrusion gene PMR2/ENA1 during salt stress in yeast. FEBS Lett. 38289-92. [DOI] [PubMed] [Google Scholar]
- 42.Matheos, D. P., T. J. Kingsbury, U. S. Ahsan, and K. W. Cunningham. 1997. Tcn1p/ Crz1p, a calcineurin-dependent transcription factor that differentially regulates gene expression in Saccharomyces cerevisiae. Genes Dev. 113445-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mendizabal, I., A. Pascual-Ahuir, R. F. Serrano, and I. F. de Larrinoa. 2001. Promoter sequences regulated by the calcineurin-activated transcription factor Crz1 in the yeast ENA1 gene. Mol. Genet. Genomics 265801-811. [DOI] [PubMed] [Google Scholar]
- 44.Mendoza, I., F. J. Quintero, R. A. Bressan, P. M. Hasegawa, and J. M. Pardo. 1996. Activated calcineurin confers high tolerance to ion stress and alters the budding pattern and cell morphology of yeast cells. J. Biol. Chem. 27123061-23067. [DOI] [PubMed] [Google Scholar]
- 45.Mulet, J. M., D. E. Martin, R. Loewith, and M. N. Hall. 2006. Mutual antagonism of target of rapamycin and calcineurin signalling. J. Biol. Chem. 28133000-33007. [DOI] [PubMed] [Google Scholar]
- 46.Munro, C. A., S. Selvaggini, I. de Brujin, L. Walker, M. D. Lenardon, B. Gerssen, S. Milne, A. J. P. Brown, and N. A. R. Gow. 2007. The PKC, HOG and Ca2+ signalling pathways co-ordinately regulate chitin synthesis in Candida albicans. Mol. Microbiol. 631399-1413. [DOI] [PMC free article] [PubMed]
- 47.Odds, F. C., A. J. Brown, and N. A. Gow. 2003. Antifungal agents: mechanisms of action. Trends Microbiol. 11272-279. [DOI] [PubMed] [Google Scholar]
- 48.Odom, A., S. Muir, E. Lim, D. L. Toffaletti, J. Perfect, and J. Heitman. 1997. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 162576-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Onyewu, C., F. L. Wormley, Jr., J. R. Perfect, and J. Heitman. 2004. The calcineurin target, Crz1, functions in azole tolerance but is not required for virulence of Candida albicans. Infect. Immun. 727330-7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Polizotto, R. S., and M. S. Cyert. 2001. Calcineurin-dependent nuclear import of the transcription factor Crz1p requires Nmd5p. J. Cell Biol. 154951-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pontecorvo, G. V., J. A. Roper, L. M. Hummonns, K. D. MacDonald, and A. W. J. Buften. 1953. The genetics of Aspergillus nidulans. Adv. Genet. 5141-238. [DOI] [PubMed] [Google Scholar]
- 52.Quidde, T., P. Büttner, and P. Tudzynski. 1999. Evidence for three different specific saponin-detoxifying activities in Botrytis cinerea and cloning and functional analysis of a gene coding for a putative avenacinase. Eur. J. Plant Pathol. 105273-283. [Google Scholar]
- 53.Rolke, Y., S. Liu, T. Quidde, B. Williamson, A. Schouten, K.-M. Weltring, V. Siewers, K. B. Tenberge, B. Tudzynski, and P. Tudzynski. 2004. Functional analysis of H2O2-generating systems in Botrytis cinerea: the major Cu-Zn superoxide dismutase (BCSOD1) contributes to virulence on French bean, whereas a glucose oxidase (BCGOD1) is dispensable. Mol. Plant Pathol. 517-27. [DOI] [PubMed] [Google Scholar]
- 54.Rui, O., and M. Hahn. 2007. The Slt2-type MAP kinase Bmp3 of Botrytis cinerea is required for normal saprotrophic growth, conidiation, plant surface sensing and host tissue colonization. Mol. Plant Pathol. 8173-184. [DOI] [PubMed] [Google Scholar]
- 55.Rusnak, F., and P. Mertz. 2000. Calcineurin: form and function. Physiol. Rev. 801483-1521. [DOI] [PubMed] [Google Scholar]
- 56.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 57.Sanglard, D., F. Ischer, O. Marchetti, J. Entenza, and J. Bille. 2003. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol. Microbiol. 48959-976. [DOI] [PubMed] [Google Scholar]
- 58.Santos, M., and I. F. de Larrinoa. 2005. Functional characterization of the Candida albicans CRZ1 gene encoding a calcineurin-regulated transcription factor. Curr. Genet. 4888-100. [DOI] [PubMed] [Google Scholar]
- 59.Schulze Gronover, C., D. Kasulke, P. Tudzynski, and B. Tudzynski. 2001. The role of G protein alpha subunits in the infection process of the gray mould fungus Botrytis cinerea. Mol. Plant-Microbe Interact. 141293-1302. [DOI] [PubMed] [Google Scholar]
- 60.Schulze Gronover, C., C. Schorn, and B. Tudzynski. 2004. Identification of Botrytis cinerea genes up-regulated during infection and controlled by the G alpha subunit BCG1 using suppression subtractive hybridization (SSH). Mol. Plant-Microbe Interact. 17537-546. [DOI] [PubMed] [Google Scholar]
- 61.Schumacher, J., M. Viaud, A. Simon, and B. Tudzynski. 2008. The Gα subunit BCG1, the phospholipase C (BcPLC1) and the calcineurin phosphatase co-ordinately regulate gene expression in the grey mould fungus Botrytis cinerea. Mol. Microbiol. 671027-1050. [DOI] [PubMed] [Google Scholar]
- 62.Segmüller, N., U. Ellendorf, B. Tudzynski, and P. Tudzynski. 2007. BcSAK1, a stress-activated mitogen-activated protein kinase, is involved in vegetative differentiation and pathogenicity in Botrytis cinerea. Eukaryot. Cell 6211-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Serrano, R., A. Ruiz, D. Bernal, J. R. Chambers, and J. Ariño. 2002. The transcriptional response to alkaline pH in Saccharomyces cerevisiae: evidence for calcium mediated signalling. Mol. Microbiol. 461319-1333. [DOI] [PubMed] [Google Scholar]
- 64.Siewers, V., J. Smedsgaard, and P. Tudzynski. 2004. The P450 monooxygenase BcABA1 is essential for abscisic acid biosynthesis in Botrytis cinerea. Appl. Environ. Microbiol. 703868-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Siewers, V., M. Viaud, D. Jimez-Teja, I. G. Collado, C. Schulze Gronover, J.-M. Pradier, B. Tudzynski, and P. Tudzynski. 2005. Functional analysis of the cytochrome P450 monooxygenase gene bcbot1 of Botrytis cinerea indicates that botrydial is a strain-specific virulence factor. Mol. Plant-Microbe Interact. 18602-612. [DOI] [PubMed] [Google Scholar]
- 66.Soulié, M. C., C. Perino, A. Piffeteau, M. Choquer, P. Malfatti, A. Cimeran, C. Kunz, M. Boccara, and A. Vidal-Cros. 2006. Botrytis cinerea virulence is drastically reduced after disruption of chitin synthase class III gene (Bcchs3a). Cell Microbiol. 81310-1321. [DOI] [PubMed] [Google Scholar]
- 67.Soulié, M. C., A. Piffeteau, M. Choquer, M. Boccara, and A. Vidal-Cros. 2003. Disruption of Botrytis cinerea class I chitin synthase gene Bcchs1 results in cell wall weakening and reduced virulence. Fungal Genet. Biol. 4038-46. [DOI] [PubMed] [Google Scholar]
- 68.Stathopoulos, A. M., and M. S. Cyert. 1997. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev. 113432-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stathopoulos-Gerontides, A., J. J. Guo, and M. S. Cyert. 1999. Yeast calcineurin regulates nuclear localization of the Crz1p transcription factor through dephosphorylation. Genes Dev. 13798-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Steinbach, W. J., R. A. Cramer, Jr., B. Z. Perfect, Y. G. Asfaw, T. C. Sauer, L. K. Najvar, W. R. Kirkpatrick, T. F. Patterson, D. K. Benjamin, Jr., J. Heitman, and J. R. Perfect. 2006. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot. Cell 51091-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Steinbach, W. J., J. L. Reedy, R. A. Cramer, J. R. Perfect, and J. Heitman. 2007. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat. Rev. Microbiol. 5418-430. [DOI] [PubMed] [Google Scholar]
- 72.Tsuzi, D., K. Maeta, Y. Takatsume, S. Izawa, and Y. Inoue. 2004. Regulation of the yeast phospholipid hydroperoxide glutathione peroxidase GPX2 by oxidative stress is mediated by YAP1 and SKN7. FEBS Lett. 565148-154. [DOI] [PubMed] [Google Scholar]
- 73.Tsuzi, D., K. Maeta, Y. Takatsume, S. Izawa, and Y. Inoue. 2004. Distinct regulatory mechanism of yeast GPX2 encoding phospholipid hydroperoxide glutathione peroxidase by oxidative stress and a calcineurin/Crz1-mediated Ca2+ signaling pathway. FEBS Lett. 569301-306. [DOI] [PubMed] [Google Scholar]
- 74.Van Kan, J. A. L. 2006. Licensed to kill: the lifestyle of a necrotrophic plant pathogen. Trends Plant Sci. 11247-253. [DOI] [PubMed] [Google Scholar]
- 75.Viaud, M., A. Brunet-Simon, Y. Brygoo, J.-M. Pradier, and C. Levis. 2003. Cyclophilin A and calcineurin functions investigated by gene inactivation, cyclosporine A inhibition and cDNA arrays approaches in the phytopathogenic fungus Botrytis cinerea. Mol. Microbiol. 501451-1465. [DOI] [PubMed] [Google Scholar]
- 76.Viaud, M. C., P. V. Balhadere, and N. J. Talbot. 2002. A Magnaporthe grisea cyclophilin acts as a virulence determinant during plant infection. Plant Cell 14917-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Viladevall, L., R. Serrano, A. Ruiz, G. Domenech, J. Giraldo, A. Barceló, and J. Ariño. 2004. Characterization of the calcium-mediated response to alkaline stress in Saccharomyces cerevisiae. J. Biol. Chem. 27943614-43624. [DOI] [PubMed] [Google Scholar]
- 78.Wachek, M., M. C. Aichinger, J. A. Stadler, R. J. Schweyen, and A. Graschopf. 2006. Oligomerization of the Mg2+ transport protein Alr1p and Alr2p in yeast plasma membrane. FEBS Lett. 2734236-4249. [DOI] [PubMed] [Google Scholar]
- 79.Wallis, J. W., G. Chrebet, G. Brodsky, M. Rolfe, and R. Rothstein. 1989. A hyper-recombination mutation in S. cerevisiae identifies a novel eukaryotic topoisomerase. Cell 58409-419. [DOI] [PubMed] [Google Scholar]
- 80.Weghuber, J., F. Dieterich, E. M. Froschauer, S. Svidovà, and R. J. Schweyen. 2006. Mutational analysis of functional domains in Mrs2p, the mitochondrial Mg2+ channel protein of Saccharomyces cerevisiae. FEBS J. 2731198-1209. [DOI] [PubMed] [Google Scholar]
- 81.Wiesenberger, G., K. Steinleitner, R. Malli, W. F. Graier, J. Vormann, R. J. Schweyen, and J. A. Stadler. 2007. Mg2+ deprivation elicits rapid Ca2+ uptake and activates Ca2+/calcineurin signaling in Saccharomyces cerevisiae. Eukaryot. Cell 6592-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Williams, K., and M. S. Cyert. 2001. The eukaryotic response regulator SKN7 regulates calcineurin signalling through stabilization of Crz1p. EMBO J. 203473-3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Williamson, B., B. Tudzynski, P. Tudzynski, and J. A. L. van Kan. 2007. Pathogen profile—Botrytis cinerea: the cause of grey mould disease. Mol. Plant Pathol. 8561-580. [DOI] [PubMed] [Google Scholar]
- 84.Yoshimoto, H., K. Saltsman, A. P. Gasch, H. X. Li, N. Ogawa, D. Botstein, P. O. Brown, and M. S. Cyert. 2002. Genome-wide analysis of gene expression regulated by the calcineurin/Crz1p signaling pathway in Saccharomyces cerevisiae. J. Biol. Chem. 27731079-31088. [DOI] [PubMed] [Google Scholar]
- 85.Zhao, C., U. S. Jung, P. Garrett-Engele, T. Roe, M. S. Cyert, and D. E. Levin. 1998. Temperature-induced expression of yeast FKS2 is under the dual control of protein kinase C and calcineurin. Mol. Cell. Biol. 181013-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]