Abstract
Directional growth is a function of polarized cells such as neurites, pollen tubes, and fungal hyphae. Correct orientation of the extending cell tip depends on signaling pathways and effectors that mediate asymmetric responses to specific environmental cues. In the hyphal form of the eukaryotic fungal pathogen Candida albicans, these responses include thigmotropism and galvanotropism (hyphal turning in response to changes in substrate topography and imposed electrical fields, respectively) and penetration into semisolid substrates. During vegetative growth in C. albicans, as in the model yeast Saccharomyces cerevisiae, the Ras-like GTPase Rsr1 mediates internal cellular cues to position new buds in a prespecified pattern on the mother cell cortex. Here, we demonstrate that Rsr1 is also important for hyphal tip orientation in response to the external environmental cues that induce thigmotropic and galvanotropic growth. In addition, Rsr1 is involved in hyphal interactions with epithelial cells in vitro and its deletion diminishes the hyphal invasion of kidney tissue during systemic infection. Thus, Rsr1, an internal polarity landmark in yeast, is also involved in polarized growth responses to asymmetric environmental signals, a paradigm that is different from that described for the homologous protein in S. cerevisiae. Rsr1 may thereby contribute to the pathogenesis of C. albicans infections by influencing hyphal tip responses triggered by interaction with host tissues.
The growth and behavior of living cells is achieved by a process of vectorial metabolism in which growth is directed or restricted to specific cellular locations under the influence of endogenous and exogenous signals and stimuli. For example, in the model yeast Saccharomyces cerevisiae, budding yeast cell growth involves polarization in a direction that is prescribed by proteins that provide internal cues. These proteins include cortically localized bud-site selection proteins that recruit and activate the major polarity establishment Rho GTPase Cdc42 either adjacent to or directly opposite the site of emergence by the previous daughter cell (12, 13). In contrast to the case for budding cells in S. cerevisiae, where positional landmarks are internal, pheromones provide mating yeast cells with extracellular signals that override the internal cues so that the growth of the mating projection (shmoo) is oriented in the direction of the putative mating partner (13).
During yeast cell growth in Candida albicans, an opportunistic fungal pathogen of humans, the direction of polarity establishment is controlled, as it is in S. cerevisiae, by internal cues at the mother cell cortex (23, 30, 57, 64). C. albicans also grows in a filamentous hyphal morphology that is not exhibited by S. cerevisiae. Understanding how hyphal morphogenesis occurs and is regulated has generated a great deal of interest because the hyphal form of C. albicans has been associated with the invasion of host tissues and mortality (54, 65).
The hyphae of C. albicans and other human and plant fungal pathogens respond to a range of asymmetric environmental signals by altering their axes of growth in a regulated fashion (1, 8, 26, 49, 67). For example, in C. albicans, hyphae change their direction of growth in response to alterations in surface topography (thigmotropism) (24, 28, 56) and when exposed to electrical fields (galvanotropism) (17, 27). These responses are dependent upon the presence of external calcium ions (9, 36, 61), and mutants affected in calcium uptake and calcium signaling have reduced thigmotropic and galvanotropic responses (9). In addition to these tropisms, physical contact with semisolid substrates triggers the invasion of the substrates by C. albicans hyphae (10). Thus, C. albicans hyphal cells orient polarized growth in response to asymmetric external cues, but the molecular mechanism that aligns the growth axis remains unknown.
The C. albicans bud-site selection protein Rsr1, a Ras-like GTPase, is important for both yeast bud-site and hyphal branch-site selection: strains lacking Rsr1 or its GTPase-activating protein (GAP) Bud2 have random, disorganized budding and branching patterns (30, 64) due to the loss of the internal polarity landmark at the mother cell cortex. Interestingly, rsr1 and bud2 null strains have additional defects that are exhibited during hyphal morphogenesis and that are not predicted from the roles of similar proteins in S. cerevisiae. Hyphae lacking Rsr1 or Bud2 are wider than wild-type hyphae and have an increased tendency to bend, which is associated with disrupted actin cable polarization (30). These findings are consistent with the idea that GDP-GTP cycling of Rsr1 is involved with positioning events at the hyphal tip in addition to its role in providing an internal landmark at the yeast mother cell cortex. This raises the possibility that Rsr1 GTPase activity is part of the molecular mechanism that orients hyphal growth in response to environmental signals. To test the hypothesis that Rsr1 GTPase activity is important for hyphal tip responses to external cues in C. albicans, we analyzed the directional growth responses of hyphae in strains lacking Rsr1 or Bud2. The deletion of Rsr1 and Bud2 attenuated hyphal thigmotropism and essentially abolished two galvanotropic responses, cathodal germ tube emergence in an electrical field (germ tube emergence galvanotropism) and cathodal orientation of hyphal tips (hyphal reorientation galvanotropism). In addition, strains lacking Rsr1 and Bud2 were attenuated in their abilities to damage and penetrate epithelial cells in vitro, with the rsr1 null strain also having reduced invasion of kidney tissue during systemic infection of mice. Thus, this Ras-like GTPase is important for two polarity processes in C. albicans, positioning of new cell growth on mother cells in response to internal cortical cues and hyphal growth responses to asymmetric external signals.
MATERIALS AND METHODS
Media and growth conditions.
For yeast growth, strains were grown in synthetic dextrose complete (SDC) (55) medium at 28°C. Uridine (80 μg/ml) was added to all media, except when we selected for uridine prototrophs during strain construction.
C. albicans strains.
Yeast strains used in this study are listed in Table 1. To control for artifactual phenotypes due to differences in the genomic location of URA3 in the RSR1 reintegrant strain (30), a disrupted rsr1 plasmid sequence was integrated into the deleted rsr1 locus to create the control MG9339 (rsr1::rsr1/rsr1), as follows. The RSR1 promoter was generated by PCR using primers 1833 (5′-AAGGAAAAAAGCGGCCGCGGGAGAATTTTTGTTGGGCAACC-3′) and 1938 (5′-AACTGCAGAACCAATGCATCTGACAGAAGATTGATGTTTGTG-3′) and genomic DNA from strain BWP17 as the template and cloned into pGEM-URA3 (62) by virtue of the engineered NotI and NsiI restriction enzyme sites to generate pMG2157. pMG2157 was then digested with MluI to target integration to the RSR1 promoter locus and used to transform the Ura− rsr1 null strain CA8832 (30) to uridine prototrophy and generate the rsr1 reintegrant strain MG9339. Strain construction was verified by PCR using two different primer sets that distinguished the integration of the disrupted rsr1 plasmid sequence into the promoter region of the deleted rsr1 locus.
TABLE 1.
Yeast strains used in this study
| C. albicans strain | Relevant genotype | Source |
|---|---|---|
| BWP17 | ura3::λimm434/ura3::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG | 62 |
| JB6284 | BWP17 his1::hisG/hisG::his1::HIS1 arg4::hisG/ARG4-URA3::arg4::hisG | 7 |
| CA8832 | BWP17 rsr1::ARG4/rsr1::HIS1 | 30 |
| CA8880 | BWP17 rsr1::ARG4/rsr1::HIS1 arg4::hisG/ARG4-URA3::arg4::hisG | 30 |
| MG9215 | BWP17 rsr1::ARG4/URA3-RSR1::rsr1::HIS1 | 30 |
| MG9339 | BWP17 rsr1::ARG4/URA3-rsr1::rsr1::HIS1 | This study |
| DH7451 | BWP17 BUD2/bud2::HIS1 arg4::hisG/ARG4-URA3::arg4::hisG | 30 |
| DH7453 | BWP17 bud2::ARG4/bud2::HIS1 arg4::hisG/ARG4-URA3::arg4::hisG | 30 |
| MG9017 | BWP17 bud2::ARG4/pURA3-BUD2::bud2::HIS1 | 30 |
| MG9061 | BWP17 bud2::ARG4/pURA3-bud2::bud2::HIS1 | 30 |
Tropism assays.
The thigmotropism assay was modified from the method of Brand et al. (9). Microfabricated quartz slides with ridges of 0.79 μm and pitches of 25 μm were from Kelvin Nanotechnology (Glasgow, United Kingdom). The 0.79-μm ridge height was selected as that which gave maximal turning from the range of ridge heights tested using a wild-type strain (9). The ridged slides were incubated for 5 min in xylene, dried at 80°C, washed for 20 min in water, dried again, and UV-ozone treated for 1 min (Jelight Company, Inc., Irvine, CA). Slides were coated with poly-l-lysine for 20 min, washed, and dried. C. albicans yeast cells were adhered to the slides for 30 min, and nonadhered cells were washed off with double-distilled water. The slides were incubated for 6 h in 20 ml prewarmed 20% (vol/vol) fetal bovine serum (Biosera, Kent, United Kingdom) and 2% (vol/vol) glucose at 37°C for 6 h to induce hyphal growth. Hyphal contacts with ridges were observed by light microscopy, and the number of contacts that resulted in the reorientation of tip growth direction was expressed as a percentage of total hypha-ridge contacts. Results are givens as the mean from three independent experiments. Statistical significance was determined by Dunnett's two-tailed t test.
The galvanotropism assay has been described previously (9). Briefly, C. albicans yeast cells were adhered to poly-l-lysine-coated slides and cultured for 6 h in 500 ml modified Soll's medium at 37°C, while exposed to an electric field of 10 V/cm and a current of 33 ± 2 (expressed as mean ± standard deviation) mA. The angle of germ tube emergence and the final tip orientation relative to the cathode were determined by using Improvision Openlab 4.0 software. The percentage of cathodal orientation (p) was calculated for germ tube emergence and hyphal tips by using the expression p = Σ(−sin θ/n) × 100, for n measurements. A minimum of 100 final and emergence angles was measured in each of three independent experiments.
For thigmotropism images (Fig. 1B to H), hyphae were induced on a ridged polydimethylsiloxane mold (Sylgard 184; Dow Corning, Coventry, United Kingdom) and incubated under a coverslip in 20% (vol/vol) fetal bovine serum in SD medium (0.67% yeast nitrogen base, 2% glucose) at 37°C for 6 h. Differential interference contrast (DIC) images of tropic growth were captured with a DeltaVision RT wide-field microscope system using Applied Precision SoftWoRx 3.5.0 (Applied Precision Instruments, Seattle, WA).
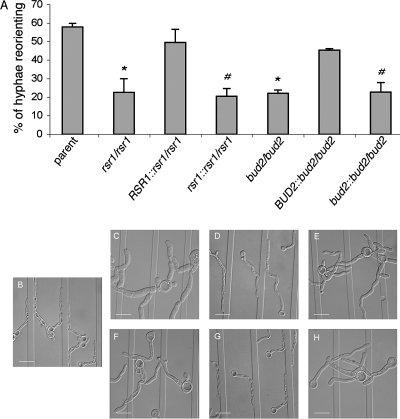
FIG. 1.
Thigmotropism responses of C. albicans strains. C. albicans strains JB6284 (wild type) (B), CA8880 (rsr1/rsr1) (C), MG9215 (RSR1::rsr1/rsr1) (D), MG9339 (rsr1::rsr1/rsr1) (E), DH7453 (bud2/bud2) (F), MG9017 (bud2/BUD2::bud2) (G), and MG9061 (bud2/bud2::bud2) (H) were incubated on ridged slides. (A) The number of hyphal contacts with ridges that resulted in a reorientation of growth direction is expressed as a percentage of total contacts (n, >100 for each strain and experiment). Error bars represent the standard deviations of the means obtained from three independent experiments. *, the P value was <0.001 based on a comparison with the parent strain; #, the P value was <0.005 based on a comparison with the RSR1-expressing reintegrant strain (for the rsr1 null strain) or the BUD2-expressing reintegrant strain (for the bud2 null strain), by analysis of variance and post hoc Dunnett's t test. (B to H) Photomicrographs of the interactions of C. albicans mutant hyphae, described above, with ridges in the substrate. Bars, 15 μm.
Epithelial cell damage assay.
The ability of C. albicans strains to invade host tissue was assessed in vitro using the CytoTox 96 cytotoxicity assay (Promega, Madison, WI), which quantifies the amount of lactate dehydrogenase (LDH) released from injured cells in vitro. OKF6/TERT-2 (TERT-2) epithelial cells (provided by James G. Rheinwald, Harvard Medical School) were cultured at a concentration of 1.5 × 104 cells per well in a 96-well tissue culture plate (Falcon) in 100 μl of keratinocyte serum-free medium (Invitrogen) supplemented with 0.4 mM CaCl2, 25 mg/ml bovine pituitary extract, and 0.2 ng/ml of human recombinant-epithelial growth factor (TERT-2 growth medium), as described previously (19). TERT-2 monolayers became approximately 80% confluent after 20 to 24 h. C. albicans yeast cells were incubated with TERT-2 monolayers at a ratio of 10:1 (Candida cells to epithelial cells) in 100 μl of TERT-2 growth medium at 37°C in 5% CO2. At 8 h postincubation, 50 ml of the culture supernatants were collected by centrifugation at 1,000 rpm for 4 min. The amount of LDH released in culture supernatants was determined spectrophotometrically according to the manufacturer's instructions. Wells containing epithelial cells alone (control 1) and C. albicans strains alone (control 2) were treated in a manner identical to that for the experimental samples and served as controls for the spontaneous release of LDH by both cell types. The theoretical maximum amount of LDH released by the epithelial cells was determined by treating uninfected monolayers with lysis buffer (9% Triton X-100) for 45 min at 37°C. The LDH released by the epithelial cells in the presence of C. albicans was quantified according to the following formula: [(experimental − control 1 − control 2)/(maximum − control 1)] × 100, with values being expressed as percent cytotoxicity. Each sample was assessed in triplicate, and the assay was repeated three times. Differences between the strains were assessed using analysis of variance, and P values of ≤0.05 were considered significant.
Immunofluorescence analysis of fungal penetration of epithelial cells.
TERT-2 cells (1 × 105 to 2 × 105 cells) were cultured on glass coverslips coated with 0.2% gelatin in a 12-well tissue-culture plate (BD Biosciences, San Jose, CA). The monolayers (approximately 70 to 90% confluent after 12 to 24 h) were rinsed three times with 1 ml Hanks' balanced salt solution (HBSS; Invitrogen, Carlsbad, CA) and incubated with 105 yeast cells suspended in 1 ml TERT-2 growth medium (prewarmed to 37°C) for 8 h at 37°C in 5% CO2. After incubation, the coverslips were rinsed three times with 1 ml of HBSS and then fixed with 4% paraformaldehyde in phosphate-buffered saline for 10 min at room temperature. C. albicans that had not penetrated the epithelial cells (and was therefore accessible for interaction with antibody) was labeled with polyclonal, biotin-conjugated rabbit anti-C. albicans immunoglobulin G (IgG) (Biodesign International, Saco, Maine) at a dilution of 1:200 in 3% bovine serum albumin in phosphate-buffered saline for 1 h. After incubation, the coverslips were washed three times with 1 ml HBSS, and then streptavidin (conjugated with Alexa 568 [Invitrogen]) at a dilution of 1:2,000 in 3% bovine serum albumin was incubated with the coverslips for 1 h to detect biotin-conjugated primary antibody. Coverslips were then washed three times with HBSS and inverted onto slides that had been overlaid with Fluoromount-G mounting medium (SouthernBiotech, Birmingham, AL). Coverslips incubated with normal rabbit IgG were included as well as coverslips incubated with streptavidin conjugated with Alexa 568, but not primary antibody, as negative controls. The coverslips were viewed by DIC and epifluorescence microscopy by using a Nikon Eclipse E600 photomicroscope equipped with a Texas Red filter set (Chroma Technology Corp., Brattleboro, VT). Digital images were collected using a CoolSnap HQ camera (Photometrics, Tucson, AZ) and MetaMorph software, version 6.2r5 (Universal Imaging Corp., Downingtown, PA). Only hyphal filaments that were in the same general plane of focus as that of the epithelial cells were included in the analysis. A total of 8 to 15 fluorescent images along the z axis, in 1-μm increments, were collected for each hyphal cell to insure that any signal present was captured throughout the diameter of the cell body. The z series stack was then collapsed into a single image by using the stack arithmetic/maximum function of MetaMorph for analysis and presentation. A cell, or a portion of a cell (i.e., the hyphal tip), was considered to be inside an epithelial cell and inaccessible to antibody staining if it was devoid of fluorescent signal. Control coverslips containing TERT-2 epithelial cells infected with C. albicans and treated with isotype control IgG as well as coverslips treated with secondary antibody alone were consistently and completely devoid of any nonspecific signal on the yeast or hyphal cells (data not shown). Three independent experiments were performed on each strain, and statistical significance between isogenic strains was assessed by Student's t test.
Mouse model of systemic candidiasis.
Female 18- to 22-g Swiss Webster mice (Harlan Sprague-Dawley, Indianapolis, IN) were allowed to acclimatize for 1 week before experiments. Male mice were not used, because they tend to fight and wound each other, confounding the origin of systemic infection. Mice were killed by cervical dislocation. Experiments were performed according to the National Institutes of Health guidelines, and the University of Minnesota Institutional Animal Care and Use Committee approved all protocols.
The classical model of intravenous (i.v.) injection of yeast cells into the tail vein was used to assess the systemic virulence of these C. albicans strains (6, 58). Mortality was observed following i.v. 0.1-ml inoculations containing 2 × 106 cells of each of the following C. albicans strains suspended in sterile saline: JB6284, CA8880, MG9215, and MG9339 (described in Table 1). Other groups of mice were inoculated with 106 cells of C. albicans JB6284 or CA8880, and surviving mice were randomly chosen to be sacrificed 1, 7, and 14 days later for quantitative culture of liver and kidneys (six to eight mice per strain per day). Separate groups of mice were also inoculated with 106 cells of C. albicans JB6284 or CA8880 and sacrificed for kidney histology when they appeared moribund (this occurred with six mice infected with 6284 and five mice infected with 8880) as described previously (5). Kidneys were excised and fixed in 3% paraformaldehyde and 3% glutaraldehyde in 0.1 M cacodylate buffer, placed overnight in acetone at 4°C, embedded in JB-4 glycol methacrylate resin (Polysciences, Inc., Warrington, PA), and cut into sections 2 μm wide, and separate sections were stained with periodic acid-Schiff and counterstained with Harris hematoxylin.
To quantify C. albicans in livers and kidneys, each tissue was weighed, homogenized, serially diluted, and plated on Sabouraud's dextrose agar for the cultivation of yeast as well as on MacConkey agar and colistin nalidixic agar supplemented with 5% sheep red blood cells. Microbes were identified by standard techniques (63). Viable colony counts from homogenized tissue represent a best estimate of fungal burden; colony counts may be influenced by the focal nature of the fungal elements within the tissue as well as the ability of hyphae to disperse, or not disperse, into single cells. Strains were enumerated as the average ± standard error viable log10/g wet weight of tissue. Lower limits of detection were 1.9 log10/g liver and 2.1 log10/g kidney. Differences in tissue concentrations of C. albicans strains were analyzed by unpaired Mann-Whitney (two groups) or Kruskal-Wallis test, followed by unpaired Mann-Whitney post hoc test (more than two groups). For statistical analysis, tissues with no detectable C. albicans were assigned a value equal to the lower limit of assay detection. Statistical significance was a P value of <0.05.
RESULTS
RSR1 and BUD2 are important for tropic responses in hyphae.
In hyphal cells of filamentous fungi, Rsr1-like GTPases are important for maintaining linear growth on agar surfaces (4, 22, 30), possibly via their ability to stabilize polarity structures, such as the polarisome, at the tips of hyphae (30). The existence of thigmotropic and galvanotropic behaviors in C. albicans (17, 27, 28, 56) predicts that the hyphal tip has a mechanism that allows it to reorient in response to altered surface topography and ionic charge, respectively. We tested whether Rsr1 and its cycling activity via the GAP Bud2 are required for hyphal tropic behaviors since they function as internal landmarks for growth direction during yeast bud and hyphal branch development.
First, we analyzed whether mutant hyphae could reorient tip growth upon contact with raised ridges in a quartz substrate (thigmotropism) (Fig. 1). In a previous study, we observed an inverse correlation between the ridge height and the thigmotropic response of wild-type hyphae (9; A. Brand and N. A. R. Gow, unpublished results). It is therefore unlikely that the wider hyphae of the rsr1 null strain (average hyphal diameter/ridge height ratio of 3.2:1) would be less sensitive to ridges on glass slides than those of the parent strain (average ratio of 2.6:1). In the Rsr1-expressing parent strain, approximately 60% of hyphae that contacted a ridge responded by reorienting the growth axis (Fig. 1), consistent with previous reports for the thigmotropic response of wild-type C. albicans hyphae (9). However, in contrast to our prediction, the deletion of either RSR1 or BUD2 or the integration of a disrupted copy of either gene into the null strains reduced the number of reorientation events by 60% (P, <0.001 or <0.005, respectively). The turning response was restored by the reintegration of a wild-type copy of the deleted gene. Taken together, these observations support the idea that it is the loss of Rsr1 activity, and not a general defect in cell shape, that causes hyphae to be less responsive to changes in surface topography.
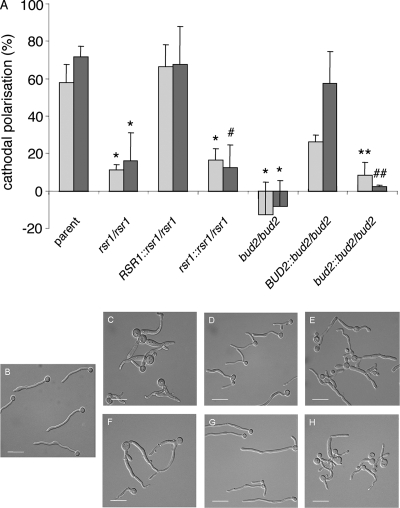
The alignment of wild-type hyphae toward the cathode in an electric field (galvanotropism) appears to be dependent on two separate responses. The emergence of new hyphae from the cathodal face of the mother cell depends on calcium influx, but the cathodal alignment of already-formed hyphal tips is calcium influx independent (9). We analyzed the rsr1 and bud2 null strains for their responses in an electric field, where cathodal polarization values close to zero indicate that tip orientation is completely random. The deletion of either RSR1 or BUD2 essentially abolished both reorientation responses (P < 0.001) (Fig. 2). A similar result was observed for the disrupted versions in each reintegrant pair, which were significantly less responsive than the reintegrants with functional gene copies. Together, these thigmotropism and galvanotropism results indicate that Rsr1 is important for the reorientation response to environmental signals. In addition, the finding that the deletion of either RSR1 or BUD2 significantly affects not just one but both types of galvanotropism responses indicates that Rsr1 GTPase activity likely functions downstream of several signaling pathways, acting either as a common effector of reorientation signals or as a more indirect component that stabilizes the function of reorientation structures at hyphal tips.
FIG. 2.
Galvanotropism responses of C. albicans strains. Control and mutant yeast cells (described in the legend for Fig. 1) were adhered to poly-l-lysine-coated glass slides. Hyphae were induced by incubation at 37°C for 6 h, and an electric field of 10 V/cm was applied for the duration of the experiment. (A) Germ tube emergence angles (light bars) and final hyphal angles (dark bars) relative to the cathode are shown where +100% cathodal polarization denotes perfect cathodal orientation, −100% denotes anodal orientation, and 0% is obtained for a randomly oriented population (n, >100 cells per strain). Error bars represent the standard deviations of the means obtained from three independent experiments. P values were <0.001 (*) and 0.016 (#) based on comparisons with the parent strain. P values were 0.002 (**) and 0.005 (##) based on comparisons with the BUD2-expressing reintegrant strain, by analysis of variance and post hoc Dunnett's t test. (B to H) Photomicrographs of the response of C. albicans mutant hyphae (described in the legend for Fig. 1) to an applied electric field. The cathodal stimulus is to the left of each image panel. Bars, 15 μm.
The addition of 1 mM calcium enhances cathodal germ tube emergence in wild-type cells (9), presumably by an enhancement of calcium influx at the depolarized face of the mother yeast cell. However, in the rsr1 null strain, neither defect in galvanotropism could be rescued by the addition of calcium (data not shown). Rather, unlike the case for wild-type cells, when 1 mM calcium was added in the presence of an electrical field, hyphal growth was completely inhibited in strains lacking Rsr1. This observation indicates that under conditions of increased extracellular calcium, Rsr1 is essential for hyphal morphogenesis.
Strains lacking Rsr1 and Bud2 have reduced abilities to damage and penetrate oral epithelial cells.
Tissue injury by C. albicans in vitro requires direct interaction and penetration of host cells by live organisms (21), and in histological specimens from patients with oropharyngeal candidiasis, disruption of the superficial epithelium is commonly seen (20). Strains lacking Rsr1 are defective in the ability to invade agar, using both embedded and surface inoculations, despite being able to generate polarized filaments (30). To test the hypothesis that Rsr1 is important for invasive interactions in response to contact with biological cues relevant to human mucosal disease, we analyzed the abilities of rsr1 null strains to injure TERT-2 oral epithelial cells by using a colorimetric assay to measure LDH released from target host cells.
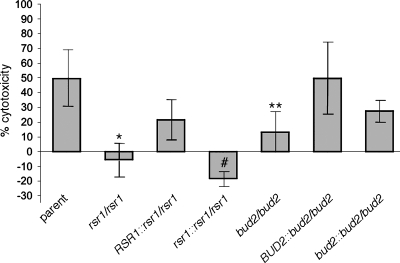
In contrast to the parent strain, strains lacking Rsr1 or Bud2 had reduced cytotoxic effects on TERT-2 cells (P values were <0.001 and 0.007, respectively) (Fig. 3). Indeed, the relative negative number obtained for cytotoxicity by the rsr1 null strain indicates that the background level of enzyme, caused by the spontaneous release of LDH by TERT-2 cells compared with that of noninoculated controls, was reduced and, thus, the rsr1 null strain may be inhibiting cell damage in some way. Reduced cytotoxicity was also observed for the RSR1 and BUD2 reintegrant strain pair analyses. Cytotoxicity of the rsr1 null reintegrant strain was significantly reduced compared to that of its isogenic RSR1 reintegrant strain (P = 0.002), but the differences between the BUD2 reintegrant and bud2 null reintegrant did not reach statistical significance (P = 0.15). This result was not due to an effect of haplo-insufficiency: the isogenic heterozygous BUD2/bud2 strain had cytotoxicity that was not significantly different from that of the isogenic parent strain (P = 0.75) (data not shown). Three additional independently generated bud2 null strains also had reduced cytotoxicity compared to that of the parent strain (the P value was ≤0.01 for each) (data not shown), supporting the conclusion that the deletion of BUD2, rather than an unintended mutation occurring during transformation, attenuates cytotoxicity. Cytotoxicity results were also not due to differences in growth rates between parent and mutant strains; rsr1 and bud2 null strains have growth and elongation rates similar to those of the isogenic parent strain (30). In fact, at early time points after hyphal induction, filaments of the rsr1 null strain grew faster than those of the parent strain (30). In addition, under the growth conditions of the assay, the rsr1 and bud2 null strains formed elongated filaments, albeit wider than those of the parent strain (Fig. 4 and data not shown), indicating that the defect in epithelial cell damage was not a result of a complete inability to elongate.
FIG. 3.
Oral epithelial cell damage caused by C. albicans strains as determined by the LDH release assay. C. albicans strains, described in the legend for Fig. 1, were grown to stationary phase in SDC medium. Equal numbers of cells were inoculated to TERT-2 epithelial cell monolayers and incubated for 8 h. The results are expressed as percent cytotoxicity as defined in Materials and Methods and are the means ± standard deviations (error bars) from at least four independent experiments, each performed in triplicate. *, the P value was 0.001 based on a comparison with the parent strain; #, the P value was 0.002 based on a comparison with the RSR1 reintegrant strain; **, the P value was 0.007 based on a comparison with the parent strain by analysis of variance and post hoc t test. Large standard deviations were due to day-to-day variation in the raw numbers obtained and not due to variations in the triplicate samples on any single day.
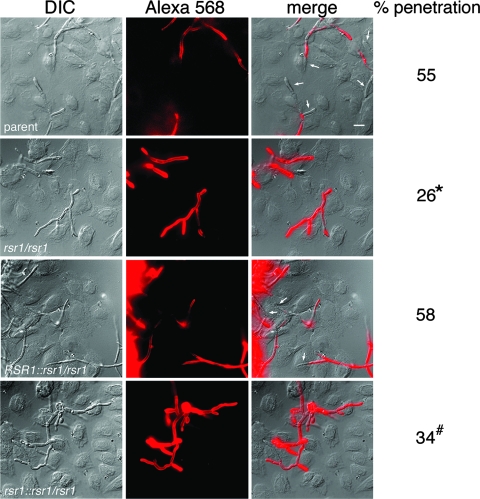
FIG. 4.
DIC and immunofluorescence (Alexa 568) analysis of C. albicans interaction with TERT-2 oral epithelial cells (strains are described in the legend for Fig. 1). DIC and Alexa 568 images were merged in MetaMorph to achieve the images in the panels on the right. Scale bar, 10 μm. Arrows, hyphal cells devoid of antibody staining indicating penetration into epithelial cells. At least 75 hyphal cells were analyzed per strain per experiment, with representative images shown. The number of penetrating hyphae is expressed as a percentage of the total number of hyphae analyzed (percent penetration; average from three experiments). *, The difference between the parent and rsr1 null strain was statistically significant, with a P value of 0.01. #, Although the difference between the rsr1 null reintegrant and the RSR1 reintegrant strain suggests a trend toward decreased penetration events for the null reintegrant, this difference did not reach statistical significance (P = 0.19).
To investigate how strains lacking Rsr1and Bud2 differed in their interactions with oral epithelial cells relative to the parent strain, we performed immunocytochemical analysis of TERT-2 cells infected with parent and mutant C. albicans strains. We hypothesized that the degree of cytotoxicity would correlate with the ability of hyphae to penetrate into epithelial cells and, thus, TERT-2 cells infected with strains lacking Rsr1 and Bud2 would exhibit fewer hyphal penetration events. To distinguish penetrating hyphae from nonpenetrating hyphae, we used an anti-Candida antibody conjugated to red-fluorescing Alexa 568; C. albicans cells that do not penetrate epithelial cells are accessible for interaction with antibody, whereas penetrating hyphae are inaccessible and thus devoid of fluorescent signal (examples of images are shown in Fig. 4). Importantly, the labeling of hyphal tips by the anti-Candida antibody was consistent: in control experiments utilizing wild-type or mutant Candida cells alone (no epithelial cells), surface staining was observed uniformly in over 99% of hyphae (n, >200 hyphae for each strain). During epithelial cell interactions, the deletion of RSR1 resulted in a 44% reduction in the number of hyphal filaments penetrating TERT-2 cells (Fig. 4). Similarly, the rsr1 null reintegrant strain exhibited a 27% reduction in penetration events compared to its isogenic RSR1 reintegrant strain (Fig. 4). The deletion of BUD2 also resulted in a reduction in penetration events (68% reduction; n, >110 cells for each strain), with the bud2 null reintegrant strain exhibiting a 44% reduction compared to the level in the BUD2 reintegrant strain (n, >160 cells for each strain). Thus, Rsr1 GTPase activity is important for the efficiency of hyphal penetration into epithelial cells and this may partially explain the defects in cytotoxicity observed in strains lacking Rsr1 and Bud2.
Rsr1 is important for the infiltration of kidney tissue during a systemic infection.
Strains lacking Rsr1 were attenuated in virulence in intravenous inoculation models of systemic candidiasis in mice (2, 64). It is unclear from these studies how Rsr1 influences the pathogenesis process, but it is unlikely to be due to differences in growth rates as parent and mutant strains have similar rates for both yeast- and hyphal-form growth (2, 30). To extend our in vitro studies of interactions between rsr1 and bud2 mutant strains and the environment, we analyzed the abilities of parent and mutant strains to invade kidney tissue during the course of a systemic infection (with a mouse intravenous inoculation model).
To study interaction with tissues during systemic infection, two groups of mice were injected with C. albicans strains: the first group was used to determine tissue fungal burdens for each Candida strain, and the second group was used for histological analysis of kidneys. Mortality was monitored in a third group of mice and was reduced in strains lacking Rsr1 (17% mortality) compared to the level for the parent strain (100% mortality) at 21 days postinoculation, consistent with previous reports of attenuated virulence for strains lacking Rsr1 (2, 64).
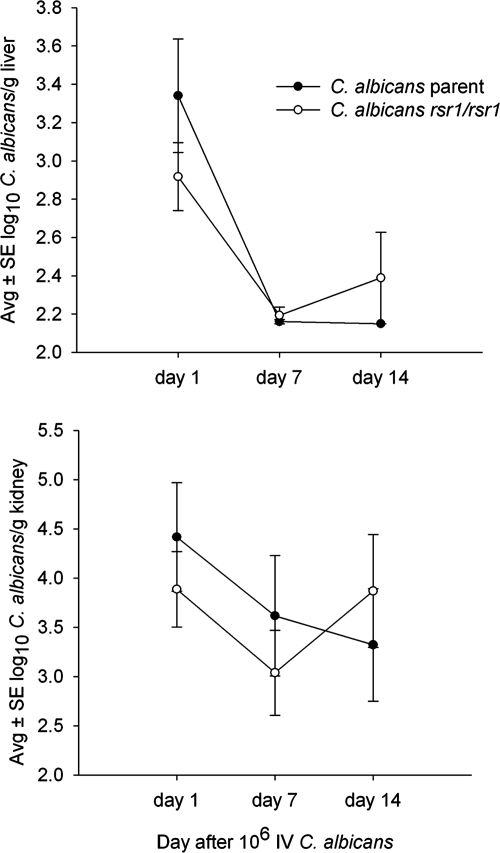
Fungal burden, assessed as the number of viable organisms in the liver and kidneys, did not differ significantly between the parent strain and the rsr1 null strain, both were cleared from the liver, with persistence in the kidney throughout the 14-day experiment (Fig. 5). The lack of a difference in fungal burdens between the strains indicates that growth rates were likely not different in vivo and that both strains were equally disseminated to organs.
FIG. 5.
Liver (top panel) and kidney (bottom panel) fungal burdens after infection with RSR1-expressing and rsr1 null strains. Numbers of viable C. albicans recovered from the livers and kidneys of mice killed 1, 7, and 14 days after i.v. inoculation with 106 cells of C. albicans JB6284 (parent) and CA8880 (rsr1/rsr1). There were no significant differences between the concentrations of C. albicans JB6284 and CA8880 at any of the time points in both livers and kidneys. Error bars indicate standard errors. Avg., average; SE, standard error.
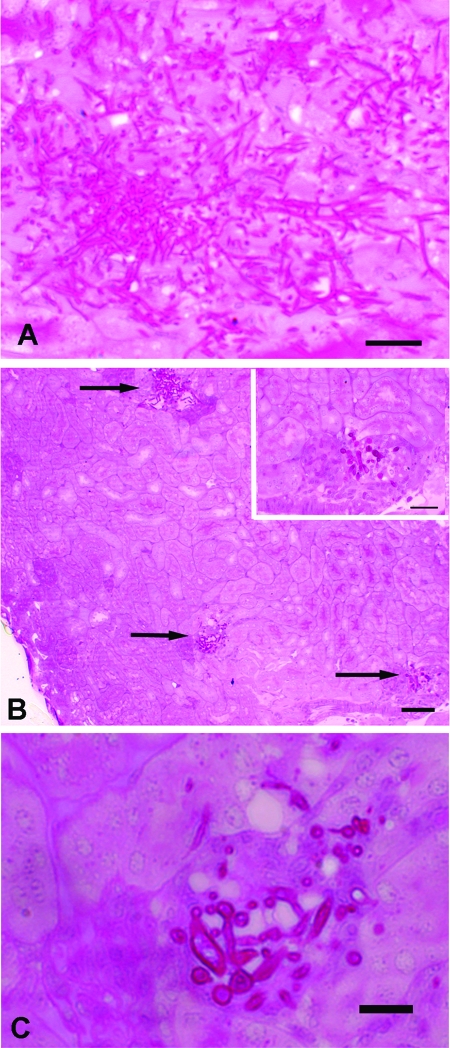
However, in comparing the kidney histology of mice inoculated with the null strain versus the parent strain, we found a dramatic difference in the pattern of infection. Infection with the parent strain resulted in diffuse and extensive infiltration of the renal parenchyma and pelvis by hyphae in all animals (an example is shown in Fig. 6A). Infection with the rsr1 null strain also resulted in seeding of the kidney, but the lesions were small and localized in three of five kidneys analyzed (an example is shown in Fig. 6B). In addition, the mutant cells appeared to be predominantly localized in glomeruli (Fig. 6B, inset, and C). In the two remaining kidneys infected with the rsr1 null strain, sections for one contained no fungal forms, and for the other, fungal forms were present but were restricted to the calyx of the kidney. Thus, Rsr1 is important for the extent of tissue invasion in vivo during a systemic infection.
FIG. 6.
Periodic acid-Schiff stains of kidney tissue from mice sacrificed one day after i.v. inoculation of 106 CFU C. albicans JB6284 (parent) and CA8880 (rsr1/rsr1). Similar results were seen for the rsr1 mutant strain in kidneys from mice sacrificed at later times after inoculation (see the text for a description). (A) JB6284 yeast cells and elongated hyphae were localized in essentially all areas of kidney tissue. (B) CA8880 was often localized as focal accumulations (arrows) containing yeast cells and abnormal hyphae. The lower two arrows highlight foci of fungal elements that appeared preferentially localized in glomeruli. Inset, higher magnification view of the area indicated by the arrow on the lower right side of the panel. (C) The focal accumulation in the middle of panel B is shown at higher magnification. Scale bars, 50 μm (A), 100 μm (B), 25 μm (inset to B), and 25 μm (C).
DISCUSSION
In this paper, we demonstrate that the Ras-like GTPase, Rsr1, and its GAP, Bud2, are involved in hyphal growth responses induced by asymmetric environmental cues. Our previous findings (30), along with those presented here, show that strains lacking Rsr1 have defects in hyphal growth responses similar to those of strains lacking Bud2. This result supports the model, initially proposed for the S. cerevisiae Rsr1 GTPase, that dynamic cycling of Rsr1 between GTP- and GDP-bound states (rather than a particular guanine nucleotide binding state) is necessary for its function (42, 52).
Hyphal morphogenesis in filamentous fungi, in contrast to budding morphogenesis in yeast, requires continuous cell-cycle-independent polarization of growth. In C. albicans, this requirement is achieved by the constitutive localization and activity of the Cdc42 Rho-type GTPase (3, 31, 32), with linear growth orientation being maintained by stable, persistent localization of tip polarity structures, such as the polarisome and Spitzenkörper (16, 66). Changes to the trajectory of linear growth, induced by extracellular signals, require a mechanism that repositions these polarity determinants, overriding the internal cortical cues, to reorient growth in the direction of the stimulus. Because Rsr1 localizes to the cell membrane and is required for the stable localization of the polarisome protein Spa2 at the hyphal tip (30), Rsr1 activity in essence provides an internal landmark for the site and, hence, the direction of polarized growth. We therefore hypothesize that in the tip reorientation response, either the positional information provided by Rsr1 is overridden (i.e., the signals act downstream of Rsr1) or the information acts upstream of Rsr1, resulting in the repositioning of its activity. Our study suggests that because environmental signals could not alter the growth direction of the rsr1 mutant strain, these signals act upstream of Rsr1, which is consistent with the idea that Rsr1 plays a role in reorienting the growth axis. Alternatively, Rsr1 may be part of a more indirect mechanism, where it is necessary for the activity or stability of tip polarity structures, but does not interact directly with upstream components that sense environmental positional cues, although this idea deviates from its function as a positional landmark in both C. albicans and S. cerevisiae (14, 30, 43, 64). For example, Rsr1 may indirectly affect the stability of the Spitzenkörper or the activity of proteins that generate a calcium ion gradient that facilitates the fusion of secretory vesicles with the cell surface. Recent reports state that reorientation responses in neuronal growth cones require the asymmetric localization and activation of Rho-type GTPases, in some cases mediated by intracellular calcium gradients (29, 47, 48). Our finding that Rsr1, a positioning factor for the Rho GTPase Cdc42 (35), is involved in hyphal reorientation mechanisms raises the possibility that similar positioning factors in vertebrates may be involved in Rho GTPase-mediated neuron guidance behaviors.
In addition, these studies show that Rsr1 is important for orientation responses to both internal cortical cues and external stimuli. This finding is different from the paradigm in S. cerevisiae shmoos; in wild-type S. cerevisiae cells, mating projection orientation does not require Rsr1 but instead depends upon the carboxyl terminus of the pheromone receptor (59), which signals to the cytoskeleton via dissociation of the Gβγ subunit from the heterotrimeric G protein and the eventual activation of Cdc42p by the Gβγ effector Far1 (11, 38). Interestingly, in S. cerevisiae cells lacking Cdc24, the guanine nucleotide exchange factor for Cdc42, Rsr1 becomes important for shmoo tip orientation to pheromone (39). Furthermore, in S. cerevisiae, Rsr1 and the polarisome protein Spa2 are not required for the formation or orientation of mating projections (59). Thus, while an externally linked landmark function for Rsr1 is uncovered in S. cerevisiae only by disruption of the pheromone signaling pathway, in C. albicans hyphae, Rsr1 appears to play a primary role in growth behavior in response to external cues.
The ability to form hyphae strongly correlates with the ability to injure host cells (44), and many of the C. albicans strains reported to have reduced cytotoxicity are unable to form hyphae (41, 51, 53). The strains lacking Rsr1 and Bud2, although able to form elongated filaments, had reduced cytotoxicity for oral epithelial cells. Although the rsr1 and bud2 null cells exhibited increased filament branching (30) with the possibility of increased contacts with epithelial cells, cytotoxicity was nevertheless reduced in these strains (Fig. 3). The inmunocytochemical finding that the mutant strains exhibited fewer penetration events than did control cells (Fig. 4) suggests that the cytotoxicity defects of the mutant strains are at least partially due to a defect in penetration. In addition, the observation that cytotoxicity was reduced more severely than was penetration indicates that mechanisms in addition to primary hyphal penetration play a role in tissue injury and LDH release in the cytotoxicity assay. For example, Chiang and colleagues (15) postulated that a postpenetration mechanism for cytotoxicity involves the secretion of lytic enzymes by C. albicans. Since tip extension is less focused in the rsr1 and bud2 null strains, it is possible that apical secretion of degradative enzymes is also less than optimal for effective tissue damage (34, 46). Reports of other mutant C. albicans strains that form hyphae yet are defective in tissue invasion and virulence (15, 33, 37, 45, 60) collectively support the idea that coordination of sustained, focused polarized growth, localized secretion of degradative enzymes, and induced endocytosis are all important for effective tissue invasion and injury.
In the mouse model of systemic candidiasis, dissemination to and seeding of the kidneys after intravenous inoculation in mice were similar for the rsr1 null and parent strains (Fig. 5), yet tissue invasion was markedly attenuated in the mice infected with the rsr1 null strain (Fig. 6). It has been proposed that tropism responses may allow the fungal hypha to identify and penetrate infectible sites (e.g., cell junctions and wounds) in the host, thus facilitating the pathogenesis process (18, 25, 34, 40, 50, 56). It is not known whether the tropic responses used to investigate Rsr1 function in this study are relevant to tissue invasion in vivo. However, the loss of orientation responses to environmental signals exhibited by strains lacking Rsr1 activity raises the possibility that the ability to focus and/or control the direction of tip growth is important for the pathogenesis process.
Acknowledgments
We thank Maryam Gerami-Nejad for technical assistance in construction of the rsr1 null reintegrant strain and Mary-Alice Johnson for expert assistance with mouse studies. We are very grateful to Judith Berman and Mark Herzberg for helpful discussions during the preparation of the manuscript and to Timothy Heisel for careful reading of the manuscript.
This work was supported by the Minnesota Medical Foundation, NIH R56AI057440 and NIH R01AI057440 awards to C.G. and an NIH R01GM059221 award to C.L.W. A.V. and K.R. were supported by NIH/NIDCR DE015056. N.G. and A.B. thank the BBRSC (BB/E008372/1), MRC, EC Signalpath Marie Curie network, and Wellcome Trust (080088) for financial support. The Nikon fluorescence microscope system was provided, in part, through a generous gift from the University of Minnesota Pediatrics Foundation.
Footnotes
Published ahead of print on 15 February 2008.
REFERENCES
- 1.Allen, E. A., H. C. Hoch, J. R. Stavely, and J. R. Steadman. 1991. Uniformity among races of Uromyces appendiculatus in response to topographical signalling for appressorium formation. Phytopathology 81883-887. [Google Scholar]
- 2.Bassilana, M., J. Blyth, and R. A. Arkowitz. 2003. Cdc24, the GDP-GTP exchange factor for Cdc42, is required for invasive hyphal growth of Candida albicans. Eukaryot. Cell 29-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassilana, M., J. Hopkins, and R. A. Arkowitz. 2005. Regulation of the Cdc42/Cdc24 GTPase module during Candida albicans hyphal growth. Eukaryot. Cell 4588-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer, Y., P. Knechtle, J. Wendland, H. Helfer, and P. Philippsen. 2004. A Ras-like GTPase is involved in hyphal growth guidance in the filamentous fungus Ashbya gossypii. Mol. Biol. Cell 154622-4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bendel, C. M., K. M. Kinneberg, R. P. Jechorek, C. A. Gale, S. L. Erlandsen, M. K. Hostetter, and C. L. Wells. 1999. Systemic infection following intravenous inoculation of mice with Candida albicans int1 mutant strains. Mol. Genet. Metab. 67343-351. [DOI] [PubMed] [Google Scholar]
- 6.Bendel, C. M., S. M. Wiesner, R. M. Garni, E. Cebelinski, and C. L. Wells. 2002. Cecal colonization and systemic spread of Candida albicans in mice treated with antibiotics and dexamethasone. Pediatr. Res. 51290-295. [DOI] [PubMed] [Google Scholar]
- 7.Bensen, E. S., S. G. Filler, and J. Berman. 2002. A forkhead transcription factor is important for true hyphal as well as yeast morphogenesis in Candida albicans. Eukaryot. Cell 1787-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowen, A. D., F. A. Davidson, R. Keatch, and G. M. Gadd. 2007. Induction of contour sensing in Aspergillus niger by stress and its relevance to fungal growth mechanics and hyphal tip structure. Fungal Genet. Biol. 44484-491. [DOI] [PubMed] [Google Scholar]
- 9.Brand, A., S. Shanks, V. M. Duncan, M. Yang, K. Mackenzie, and N. A. Gow. 2007. Hyphal orientation of Candida albicans is regulated by a calcium-dependent mechanism. Curr. Biol. 17347-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown, D. H., Jr., A. D. Giusani, X. Chen, and C. A. Kumamoto. 1999. Filamentous growth of Candida albicans in response to physical environmental cues and its regulation by the unique CZF1 gene. Mol. Microbiol. 34651-662. [DOI] [PubMed] [Google Scholar]
- 11.Butty, A. C., P. M. Pryciak, L. S. Huang, I. Herskowitz, and M. Peter. 1998. The role of Far1p in linking the heterotrimeric G protein to polarity establishment proteins during yeast mating. Science 2821511-1516. [DOI] [PubMed] [Google Scholar]
- 12.Casamayor, A., and M. Snyder. 2002. Bud-site selection and cell polarity in budding yeast. Curr. Opin. Microbiol. 5179-186. [DOI] [PubMed] [Google Scholar]
- 13.Chang, F., and M. Peter. 2003. Yeasts make their mark. Nat. Cell Biol. 5294-299. [DOI] [PubMed] [Google Scholar]
- 14.Chant, J., and I. Herskowitz. 1991. Genetic control of bud site selection in yeast by a set of gene products that constitute a morphogenetic pathway. Cell 651203-1212. [DOI] [PubMed] [Google Scholar]
- 15.Chiang, L. Y., D. C. Sheppard, V. M. Bruno, A. P. Mitchell, J. E. Edwards, Jr., and S. G. Filler. 2007. Candida albicans protein kinase CK2 governs virulence during oropharyngeal candidiasis. Cell. Microbiol. 9233-245. [DOI] [PubMed] [Google Scholar]
- 16.Crampin, H., K. Finley, M. Gerami-Nejad, H. Court, C. Gale, J. Berman, and P. Sudbery. 2005. Candida albicans hyphae have a Spitzenkörper that is distinct from the polarisome found in yeast and pseudohyphae. J. Cell Sci. 1182935-2947. [DOI] [PubMed] [Google Scholar]
- 17.Crombie, T., N. A. Gow, and G. W. Gooday. 1990. Influence of applied electrical fields on yeast and hyphal growth of Candida albicans. J. Gen. Microbiol. 136311-317. [DOI] [PubMed] [Google Scholar]
- 18.Davies, J. M., A. J. Stacey, and C. A. Gilligan. 1999. Candida albicans hyphal invasion: thigmotropism or chemotropism? FEMS Microbiol. Lett. 171245-249. [DOI] [PubMed] [Google Scholar]
- 19.Dickson, M. A., W. C. Hahn, Y. Ino, V. Ronfard, J. Y. Wu, R. A. Weinberg, D. N. Louis, F. P. Li, and J. G. Rheinwald. 2000. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol. Cell. Biol. 201436-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farah, C. S., R. B. Ashman, and S. J. Challacombe. 2000. Oral candidosis. Clin. Dermatol. 18553-562. [DOI] [PubMed] [Google Scholar]
- 21.Filler, S. G., J. N. Swerdloff, C. Hobbs, and P. M. Luckett. 1995. Penetration and damage of endothelial cells by Candida albicans. Infect. Immun. 63976-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fortwendel, J. R., W. Zhao, R. Bhabhra, S. Park, D. S. Perlin, D. S. Askew, and J. C. Rhodes. 2005. A fungus-specific Ras homolog contributes to the hyphal growth and virulence of Aspergillus fumigatus. Eukaryot. Cell 41982-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gale, C., M. Gerami-Nejad, M. McClellan, S. Vandoninck, M. S. Longtine, and J. Berman. 2001. Candida albicans Int1p interacts with the septin ring in yeast and hyphal cells. Mol. Biol. Cell 123538-3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gow, N. A. 1989. Circulating ionic currents in micro-organisms. Adv. Microb. Physiol. 3089-123. [DOI] [PubMed] [Google Scholar]
- 25.Gow, N. A. 1997. Germ tube growth of Candida albicans. Curr. Top. Med. Mycol. 843-55. [PubMed] [Google Scholar]
- 26.Gow, N. A. 2004. New angles in mycology: studies in directional growth and directional motility. Mycol. Res. 1085-13. [DOI] [PubMed] [Google Scholar]
- 27.Gow, N. A., and A. M. McGillivray. 1986. Ion currents, electrical fields and the polarized growth of fungal hyphae. Prog. Clin. Biol. Res. 21081-88. [PubMed] [Google Scholar]
- 28.Gow, N. A., T. H. Perera, J. Sherwood-Higham, G. W. Gooday, D. W. Gregory, and D. Marshall. 1994. Investigation of touch-sensitive responses by hyphae of the human pathogenic fungus Candida albicans. Scanning Microsc. 8705-710. [PubMed] [Google Scholar]
- 29.Guan, C. B., H. T. Xu, M. Jin, X. B. Yuan, and M. M. Poo. 2007. Long-range Ca2+ signaling from growth cone to soma mediates reversal of neuronal migration induced by slit-2. Cell 129385-395. [DOI] [PubMed] [Google Scholar]
- 30.Hausauer, D. L., M. Gerami-Nejad, C. Kistler-Anderson, and C. A. Gale. 2005. Hyphal guidance and invasive growth in Candida albicans require the Ras-like GTPase Rsr1p and its GTPase-activating protein Bud2p. Eukaryot. Cell 41273-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hazan, I., and H. Liu. 2002. Hyphal tip-associated localization of Cdc42 is F-actin dependent in Candida albicans. Eukaryot. Cell 1856-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hazan, I., M. Sepulveda-Becerra, and H. Liu. 2002. Hyphal elongation is regulated independently of cell cycle in Candida albicans. Mol. Biol. Cell 13134-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoyer, L. L., T. L. Payne, M. Bell, A. M. Myers, and S. Scherer. 1998. Candida albicans ALS3 and insights into the nature of the ALS gene family. Curr. Genet. 33451-459. [DOI] [PubMed] [Google Scholar]
- 34.Jayatilake, J. A., Y. H. Samaranayake, and L. P. Samaranayake. 2005. An ultrastructural and a cytochemical study of candidal invasion of reconstituted human oral epithelium. J. Oral Pathol. Med. 34240-246. [DOI] [PubMed] [Google Scholar]
- 35.Kozminski, K. G., L. Beven, E. Angerman, A. H. Tong, C. Boone, and H. O. Park. 2003. Interaction between a Ras and a Rho GTPase couples selection of a growth site to the development of cell polarity in yeast. Mol. Biol. Cell 144958-4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lever, M., B. Robertson, A. D. B. Buchan, G. W. Gooday, and N. A. R. Gow. 1994. pH and Ca2+ dependent galvanotropism of filamentous fungi: implications and mechanisms. Mycol. Res. 98301-306. [Google Scholar]
- 37.Martinez-Lopez, R., H. Park, C. L. Myers, C. Gil, and S. G. Filler. 2006. Candida albicans Ecm33p is important for normal cell wall architecture and interactions with host cells. Eukaryot. Cell 5140-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nern, A., and R. A. Arkowitz. 1999. A Cdc24p-Far1p-Gbetagamma protein complex required for yeast orientation during mating. J. Cell Biol. 1441187-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nern, A., and R. A. Arkowitz. 2000. G proteins mediate changes in cell shape by stabilizing the axis of polarity. Mol. Cell 5853-864. [DOI] [PubMed] [Google Scholar]
- 40.Odds, F. C. 1994. Pathogenesis of Candida infections. J. Am. Acad. Dermatol. 31S2-5. [DOI] [PubMed] [Google Scholar]
- 41.Park, H., C. L. Myers, D. C. Sheppard, Q. T. Phan, A. A. Sanchez, E. E. J., and S. G. Filler. 2005. Role of the fungal Ras-protein kinase A pathway in governing epithelial cell interactions during oropharyngeal candidiasis. Cell. Microbiol. 7499-510. [DOI] [PubMed] [Google Scholar]
- 42.Park, H. O., J. Chant, and I. Herskowitz. 1993. BUD2 encodes a GTPase-activating protein for Bud1/Rsr1 necessary for proper bud-site selection in yeast. Nature 365269-274. [DOI] [PubMed] [Google Scholar]
- 43.Park, H. O., P. J. Kang, and A. W. Rachfal. 2002. Localization of the Rsr1/Bud1 GTPase involved in selection of a proper growth site in yeast. J. Biol. Chem. 27726721-26724. [DOI] [PubMed] [Google Scholar]
- 44.Phan, Q. T., P. H. Belanger, and S. G. Filler. 2000. Role of hyphal formation in interactions of Candida albicans with endothelial cells. Infect. Immun. 683485-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phan, Q. T., C. L. Myers, Y. Fu, D. C. Sheppard, M. R. Yeaman, W. H. Welch, A. S. Ibrahim, J. E. Edwards, Jr., and S. G. Filler. 2007. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol. 5e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pugh, D., and R. A. Cawson. 1977. The cytochemical localization of phospholipase in Candida albicans infecting the chick chorio-allantoic membrane. Sabouraudia 1529-35. [PubMed] [Google Scholar]
- 47.Rajnicek, A. M., L. E. Foubister, and C. D. McCaig. 2006. Growth cone steering by a physiological electric field requires dynamic microtubules, microfilaments and Rac-mediated filopodial asymmetry. J. Cell Sci. 1191736-1745. [DOI] [PubMed] [Google Scholar]
- 48.Rajnicek, A. M., L. E. Foubister, and C. D. McCaig. 2006. Temporally and spatially coordinated roles for Rho, Rac, Cdc42 and their effectors in growth cone guidance by a physiological electric field. J. Cell Sci. 1191723-1735. [DOI] [PubMed] [Google Scholar]
- 49.Read, N. D., L. J. Kellock, H. Knight, and A. J. Trewavas. 1992. Contact sensing during infection by fungal pathogens, p. 137-172. In J. A. Callow and J. R. Green (ed.), Perspectives in plant cell recognition. Cambridge University Press, Cambridge, United Kingdom.
- 50.Reichart, P. A., H. P. Philipsen, A. Schmidt-Westhausen, and L. P. Samaranayake. 1995. Pseudomembranous oral candidiasis in HIV infection: ultrastructural findings. J. Oral Pathol. Med. 24276-281. [DOI] [PubMed] [Google Scholar]
- 51.Rouabhia, M., M. Schaller, C. Corbucci, A. Vecchiarelli, S. K. Prill, L. Giasson, and J. F. Ernst. 2005. Virulence of the fungal pathogen Candida albicans requires the five isoforms of protein mannosyltransferases. Infect. Immun. 734571-4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruggieri, R., A. Bender, Y. Matsui, S. Powers, Y. Takai, J. R. Pringle, and K. Matsumoto. 1992. RSR1, a ras-like gene homologous to Krev-1 (smg21A/rap1A): role in the development of cell polarity and interactions with the Ras pathway in Saccharomyces cerevisiae. Mol. Cell. Biol. 12758-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanchez, A. A., D. A. Johnston, C. Myers, J. E. Edwards, Jr., A. P. Mitchell, and S. G. Filler. 2004. Relationship between Candida albicans virulence during experimental hematogenously disseminated infection and endothelial cell damage in vitro. Infect. Immun. 72598-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saville, S. P., A. L. Lazzell, C. Monteagudo, and J. L. Lopez-Ribot. 2003. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot. Cell 21053-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sherman, F. 1991. Getting started with yeast, p. 3-20. In C. Guthrie and G. R. Fink (ed.), Methods in enzymology: guide to yeast genetics and molecular biology, vol. 194. Academic Press, Inc., San Diego, CA. [Google Scholar]
- 56.Sherwood, J., N. A. Gow, G. W. Gooday, D. W. Gregory, and D. Marshall. 1992. Contact sensing in Candida albicans: a possible aid to epithelial penetration. J. Med. Vet. Mycol. 30461-469. [DOI] [PubMed] [Google Scholar]
- 57.Song, Y., and J. Y. Kim. 2006. Role of CaBud6p in the polarized growth of Candida albicans. J. Microbiol. 44311-319. [PubMed] [Google Scholar]
- 58.Spellberg, B., A. S. Ibrahim, J. E. Edwards, Jr., and S. G. Filler. 2005. Mice with disseminated candidiasis die of progressive sepsis. J. Infect. Dis. 192336-343. [DOI] [PubMed] [Google Scholar]
- 59.Vallier, L. G., J. E. Segall, and M. Snyder. 2002. The alpha-factor receptor C terminus is important for mating projection formation and orientation in Saccharomyces cerevisiae. Cell. Motil. Cytoskelet. 53251-266. [DOI] [PubMed] [Google Scholar]
- 60.Warenda, A. J., S. Kauffman, T. P. Sherrill, J. M. Becker, and J. B. Konopka. 2003. Candida albicans septin mutants are defective for invasive growth and virulence. Infect. Immun. 714045-4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Watts, H. J., A. A. Very, T. H. Perera, J. M. Davies, and N. A. Gow. 1998. Thigmotropism and stretch-activated channels in the pathogenic fungus Candida albicans. Microbiology 144689-695. [DOI] [PubMed] [Google Scholar]
- 62.Wilson, R. B., D. Davis, and A. P. Mitchell. 1999. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J. Bacteriol. 1811868-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Winn, W., S. Allen, W. Janda, E. Koneman, G. Procop, P. Schreckenberger, and G. Woods. 2006. Koneman's color atlas and textbook of diagnostic microbiology, 6th ed. Lippincott, Williams & Wilkins, Philadelphia, PA.
- 64.Yaar, L., M. Mevarech, and Y. Koltin. 1997. A Candida albicans RAS-related gene (CaRSR1) is involved in budding, cell morphogenesis and hypha development. Microbiology 1433033-3044. [DOI] [PubMed] [Google Scholar]
- 65.Zheng, X., and Y. Wang. 2004. Hgc1, a novel hypha-specific G1 cyclin-related protein regulates Candida albicans hyphal morphogenesis. EMBO J. 231845-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng, X. D., Y. M. Wang, and Y. Wang. 2003. CaSPA2 is important for polarity establishment and maintenance in Candida albicans. Mol. Microbiol. 491391-1405. [DOI] [PubMed] [Google Scholar]
- 67.Zhou, X. L., M. A. Stumpf, H. C. Hoch, and C. Kung. 1991. A mechanosensitive channel in whole cells and in membrane patches of the fungus Uromyces. Science 2531415-1417. [DOI] [PubMed] [Google Scholar]