Abstract
Neisserial porins are potent immune adjuvants and have been demonstrated to stimulate and induce the activation of human and murine B lymphocytes. Their immunopotentiating ability is due largely to the upregulation of the surface expression of the costimulatory ligand CD86 (B7-2) on B cells and other antigen-presenting cells. Porin-induced activation is dependent on the innate immune pattern recognition receptor Toll-like receptor 2 (TLR2). These data have led us to investigate the signal transduction events induced by PorB from Neisseria meningitidis and then, using inhibitors of these pathways, to establish the mechanism by which this bacterial major outer membrane protein induces CD86 upregulation and the proliferation of murine B cells. PorB was able to induce (i) protein tyrosine kinase (PTK) activity, (ii) the phosphorylation of Erk1 and Erk2, and (iii) IκB-α phosphorylation, leading to NF-κB nuclear translocation in B cells in a TLR2-dependent manner. PorB-induced NF-κB nuclear translocation was not dependent on either PTK or Erk1/2 activities. However, B-cell proliferation and the induction of increased surface expression of CD86 by PorB were dependent on PTK activity and not Erk1/2 activation. In conclusion, PorB acts through TLR2 as a B-cell mitogen, triggering tyrosine phosphorylation of various cellular proteins that are involved in proliferation and CD86 expression, as well as the phosphorylation of Erk1/2, which is not necessary for CD86 upregulation or the proliferation of B cells.
Neisserial porins are the major outer membrane proteins of the pathogenic Neisseria meningitidis, constituting approximately 60% of the outer membrane protein content (4). Neisserial outer membrane protein complexes (OMPC) act as an adjuvant in Haemophilus influenzae type b OMPC vaccines, and porins’ being a significant component of the OMPC has led to their investigation as an immune adjuvant. Studies indicate that neisserial porins can induce an immune response in humans and animals in the absence of exogenous adjuvants (3, 47, 64, 69). This adjuvanticity is demonstrated by the ability of neisserial porins to induce an immune response to poorly immunogenic substances, such as peptides (34), as well as alter the immune response to antigens like capsular polysaccharide (CPS) from a T-cell-independent response to a T-cell-dependent response (11, 14, 32-34, 63).
The function of neisserial porins as immune adjuvants is demonstrated by the ability of porins of gram-negative bacteria to stimulate antigen-presenting cells, including B cells, and affect B-cell function (42, 54-56, 60-62, 66). Results from in vitro studies in our laboratory show that PorB from N. meningitidis can activate murine dendritic cells (DCs) and B cells by upregulating class II major histocompatibility complex (MHC) molecules and the costimulatory ligand CD86, but not CD80 (52, 66). The ability of PorB to induce DC maturation is functionally important, as further studies have demonstrated that PorB-treated DCs activate antigen-specific T cells. In addition, porins from Neisseria can induce B-cell proliferation and the secretion of immunoglobulin (Ig), which may be enhanced by coincubation with CD40L (54). The upregulation of CD86 is essential to the adjuvanticity of PorB in vivo, since the administration of anti-CD86 monoclonal antibodies (MAbs) in conjunction with porin conjugated to meningococcal CPS greatly diminishes the anti-CPS response in mice (35). The importance of CD86 in the generation of an effective immune response is further demonstrated by CD86 knockout mice, which have reduced germinal center formation and are deficient in isotype switching (7). These data indicate that PorB functions as an immune adjuvant by bridging the innate and adaptive immune responses.
The ability of microbial products to activate the immune system has long been recognized, and the identification of Toll-like receptors (TLRs) as innate immune receptors has furthered our understanding of how microbial components initiate an immune response (40). TLRs recognize microbial products such as lipopolysaccharide (LPS), lipoproteins, peptidoglycan, double-stranded RNA, and bacterial CpG DNA (19, 20, 23, 27, 30, 39, 43, 57). Researchers from our laboratory have reported previously that TLR2 is required for the CD86 and class II MHC upregulation induced by the N. meningitidis porin PorB on murine B cells and DCs (36, 52), and further analysis has demonstrated that PorB activation occurs through TLR1/TLR2 heterodimers (38, 49, 50).
B-lymphocyte stimulation by cross-linking of the Ig receptor induces a series of signal transduction events that lead to the activation of one of the most defined B-cell transcription factors, NF-κB (16, 31), and subsequently increase the surface expression of CD86 and class II MHC molecules (1, 28, 58). These signaling events also occur when antigen-presenting cells are activated upon TLR ligation. Signaling through most TLRs involves the recruitment of the adapter molecule MyD88 (41), leading to the activation of mitogen-associated protein kinases (MAPKs) and NF-κB nuclear translocation. As neisserial porins are potent activators of B cells, we set out to establish if these bacterial outer membrane proteins would induce signaling events similar to those generated by B-cell receptor (BCR) cross-linkage. Further, we address how these signaling events correlate to B-cell proliferation and the upregulation of the costimulatory molecule CD86.
MATERIALS AND METHODS
Porin preparation.
PorB was purified (65) from N. meningitidis H44/76 mutant strain H44/76Δ1Δ4, which lacks PorA and RmpM (17). This mutant strain allowed the purification of PorB without contamination from other outer membrane proteins. PorB was purified by detergent extraction and column chromatography as described previously (37, 65). Polyacrylamide gel electrophoresis and silver staining (59) (data not shown) demonstrated negligible contamination by other proteins and LPS. Furthermore, the disruption of the trimeric structure of PorB resulted in the complete inactivity of the PorB preparation, demonstrating the lack of other contaminating stimulants (37).
Murine B-cell isolation.
Naïve B cells were isolated from the spleens of LPS-hyporesponsive strain C3H/HeJ, C57BL/6, and TLR2-deficient (TLR2−/−) (57) mice according to standard methods (66) and protocols approved by the institutional review committee. The LPS hyporesponsiveness of C3H/HeJ mice is attributed to a defect in the LPS signal transducer TLR4 (46). Briefly, single-cell suspensions were obtained by the passage of the spleens through a fine wire mesh, followed by the lysis of red blood cells (in 0.15 M Tris-buffered NH4Cl). T cells were removed by T-cell-specific antibody-mediated complement lysis using anti-CD4 and anti-CD3 MAbs. Other nonspecific cells were removed by passage through a Sephadex G-10 column followed by Ficoll gradient separation. The purities of the B-cell preparations were >95% as determined by flow cytometric analysis utilizing a fluorescein isothiocyanate-conjugated MAb recognizing the B-cell marker B220 (Caltag) (8).
Porin stimulation of B cells.
Single-cell suspensions of B cells (5 × 106 cells/ml) were incubated with a 10-μg/ml concentration of PorB in the form of proteosomes, pure protein micelles lacking detergent (64). BCR cross-linkage was achieved with 10 μg of anti-IgM (Caltag)/ml combined with CD40L, at a 1:1,000 dilution, which was used as a positive control for NF-κB induction and protein tyrosine kinase (PTK) and MAPK activation (48). CD40L was generously provided by Marilyn Kehry and was generated by utilizing recombinant baculovirus-expressed multimers of CD40L (Boeringher-Ingelheim Pharmaceuticals, Inc.) (21, 24, 53, 54). In all experiments, the incubation of B cells with medium alone served as the negative control. Cells were incubated for various time periods, as indicated in Results, with or without a 30-min pretreatment with various kinase inhibitors. These inhibitors included the PTK inhibitors genistein (Sigma), at 25 μg/ml, and herbimycin (Sigma), at 1 μg/ml, and the MEK1/2 inhibitors PD98059 (Sigma), at 50 μM, and U0126 (Sigma), at 10 μM. The inhibitors require solubilization in 10 mM dimethyl sulfoxide (DMSO); therefore, 10 mM DMSO in medium was used as the negative vehicle control when appropriate. This concentration of DMSO does not stimulate B cells (data not shown). Western blot analysis confirmed the efficacy of all inhibitors.
Immunoblotting of B-cell lysates.
After incubation with PorB or the controls, the levels of phosphorylation of various proteins were determined by gel electrophoresis and immunoblotting by standard methods (8). B-cell lysates were resolved on 10% polyacrylamide gels and immunoblotted using the following antibodies: peroxidase-conjugated antiphosphotyrosine MAb (Sigma), anti-phospho-IκB-α MAb (New England Biolabs), and anti-phospho-Erk1/2 polyclonal antibody (Cell Signaling).
Flow cytometric analysis.
B cells were incubated for 24 h in the presence of 10 μg of PorB/ml, medium alone as a negative control, or CD40L plus anti-IgM as a positive control, with or without pretreatment with various kinase inhibitors as indicated in the figures. The level of surface expression of CD86 was determined by flow cytometric analysis as previously described (2, 8, 66). Fluorescein isothiocyanate-conjugated anti-CD86 was obtained from Caltag Laboratories (Burlingame, CA). Cells were evaluated using Becton Dickinson FACScan and analyzed using Cell Quest.
Detection of NF-κB nuclear translocation.
Nuclear extraction and electrophoretic mobility shift assays (EMSA) were performed to measure the extent of NF-κB nuclear translocation induced by PorB per standard methods (10). Nuclear extracts were obtained by nonionic detergent cell lysis and centrifugation. A 32P-labeled NF-κB oligonucleotide substrate [the NF-κB binding sequence from the murine Ig(κ) light-chain gene enhancer labeled by 32P Klenow end labeling (22)] was added to nuclear extracts in amounts equivalent to those of the extracts (as determined by Bradford analysis), and the samples were analyzed by nondenaturing gel electrophoresis and subsequent autoradiography. The labeled substrate bound to NF-κB migrated at a lower rate on a nondenaturing gel than the unbound substrate. A subset of B cells were treated in the presence of either the PTK inhibitor genistein, at 25 μg/ml, or the MEK1/2 inhibitor PD98059, at 50 μM, as described above to determine if PTK or Erk1/2 activities are necessary for PorB induction of NF-κB nuclear translocation. The PTK and MEK1/2 inhibitory experiments were performed using B cells treated for 4 h, as peak NF-κB translocation was observed at this time point.
Supershift assays were also performed to determine which isoform(s) of NF-κB (p50, p65, or c-Rel) PorB induced. The experiments were performed similarly to those described above, except that nuclear extracts were also treated with anti-murine p50, p65, or c-Rel MAb (Santa Cruz Biotechnology, Santa Cruz, CA). The addition of these MAbs further retards the mobility of the appropriate NF-κB subunits, indicating which isoforms are present in the NF-κB dimers.
Measurement of DNA synthesis by [3H]TdR incorporation.
B cells were cultured for 30 min with or without the indicated inhibitors and then seeded into flat-bottom 96-well tissue culture plates at a final volume of 0.1/ml. Cells were then treated with PorB for 48 h, after which the cells were pulsed with 1 μCi of [3H]thymidine ([3H]TdR) per well for an additional 18 h. Cultured cells were then harvested onto filter paper with a Packard filtermate harvester, and the [3H]TdR incorporation was measured on a 1450 MicroBeta scintillation counter (PerkinElmer, Wellesley, MA).
RESULTS
PorB induces PTK activity and Erk1/2 phosphorylation.
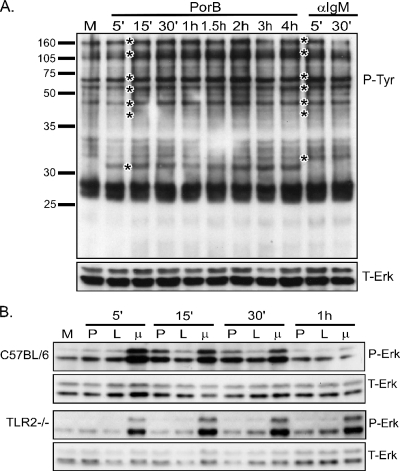
The earliest signaling events that occur in B cells after receptor engagement include PTK activation, resulting in the phosphorylation of multiple cellular proteins (9). To examine this phenomenon, we incubated B cells from C57BL/6 mice for the times indicated in the figures with PorB or anti-IgM plus CD40L to cross-link the BCR and deliver a second signal. Whole-cell lysates were obtained, and the presence of proteins containing phosphorylated tyrosines was determined by immunoblotting. PorB induced tyrosine phosphorylation beginning at 5 to 15 min after the start of incubation (Fig. 1A). The pattern of tyrosine phosphorylation was similar to that induced by anti-IgM-CD40L except for a protein of approximately 31 kDa that was phosphorylated following PorB but not anti-IgM-CD40L treatment.
FIG. 1.
PorB induces PTK activation and Erk1/2 phosphorylation. B cells were isolated from either C57BL/6 or TLR2−/− mice and treated for the indicated times with medium alone (M), 10 μg of PorB/ml (P), 100 ng of N. meningitidis LPS/ml (L), or 10 μg of anti-IgM/ml plus a 1:1,000 dilution of CD40L (μ). Whole-cell lysates were generated and subjected to Western blotting for either phosphorylated tyrosine (P-Tyr) (A) or phosphorylated Erk1/2 (P-Erk) (B). Immunoblots were stripped and reprobed for total Erk1/2 (T-Erk) as a loading control. Molecular size markers are shown on the left of panel A. 5′, 15′, and 30′, 5, 15, and 30 min.
The activation of the MAPK pathways occurs downstream of PTK activation and is a common signaling event upon TLR ligation or BCR cross-linkage (9, 18). Threonine-tyrosine phosphorylation of the MAPK Erk1/2 in B cells from wild-type C57BL/6 and TLR2-deficient mice was examined. Immunoblots of lysates from wild-type, but not TLR2−/−, B cells revealed that PorB induced Erk1/2 phosphorylation, which peaked between 15 and 30 min (Fig. 1B). As controls, B cells were treated with either N. meningitidis LPS, a TLR4 ligand, or anti-IgM-CD40L. Both agonists induced Erk1/2 phosphorylation in both wild-type and TLR2-deficient B cells. Erk1/2 phosphorylation induced by N. meningitidis LPS was not as strong as that induced by either anti-IgM-CD40L or PorB.
PorB induces NF-κB nuclear translocation in B cells.
NF-κB is one of the best-described transcription factors involved in the activation of the immune response (16). It is essential to the survival of B cells in culture (67), and NF-κB nuclear translocation is induced upon the cross-linkage of the surface Ig receptor (13, 31) in addition to TLR ligation (29). The nuclear translocation of NF-κB requires the phosphorylation of IκB-α, which in an inactivated state is bound to NF-κB, retaining NF-κB in the cytoplasm. The phosphorylation of IκB-α results in its dissociation from NF-κB and its subsequent degradation by the proteasome complex, thus exposing the nuclear localization sequence on NF-κB and allowing for the translocation of NF-κB to the nucleus, where it induces the transcription of numerous genes involved in cell activation and innate immunity (16). To establish if PorB activates the NF-κB pathway, we examined the level of IκB-α phosphorylation by immunoblot analysis. B cells were treated with PorB or CD40L, as a positive control, for 45, 90, and 120 min, cellular lysates were obtained, and immunoblots were probed with anti-phospho-IκB-α MAb. PorB and CD40L treatment induced IκB-α phosphorylation within 45 min, while incubation with medium alone did not (Fig. 2A).
FIG. 2.
PorB induces the phosphorylation of IκB-α and the nuclear translocation of NF-κB, mainly the p50 and p65 isoforms. B cells were isolated from C3H/HeJ mice and treated for the indicated times with medium alone, PorB (10 μg/ml), or CD40L (1:1,000). (A) Whole-cell lysates were generated, and Western blotting for phosphorylated IκB-α (P-IκBα) was performed. 45′, 90′, and 120′, 45, 90, and 120 min; +, present; −, absent. (B and C) Nuclear extracts were generated and subjected to EMSA using an NF-κB-specific probe. Supershift assays with results shown in panel C were performed using antibodies specific to the different NF-κB isoforms, and the antibodies retarded mobility in the gel. Ab., antibody; α-p50, anti-p50 antibody.
To discern the ability of PorB to induce NF-κB nuclear translocation, nuclear extracts from B cells treated with PorB, anti-IgM-CD40L, or medium alone were isolated and EMSA were performed. PorB treatment resulted in NF-κB nuclear translocation within 30 to 60 min, and the nuclear translocation peaked at 3 to 5 h and returned to the baseline level by 24 h (Fig. 2B). The timing of NF-κB nuclear translocation was similar to that in CD40L-activated B cells, which is consistent with the results demonstrating IκB-α phosphorylation.
NF-κB consists of different isoforms forming homo- and heterodimers. Supershift EMSAs were performed to characterize the isoforms of NF-κB present in the nuclei of PorB-activated B cells. Specific antibodies recognizing the p50, p65, and c-Rel isoforms of NF-κB were added to the nuclear extracts, and the mixtures were incubated with the NF-κB-specific probe. All cells were treated for 4 h, as the peak induction of NF-κB nuclear translocation occurred 3 to 5 h after PorB incubation. The addition of anti-p50 MAb retarded the mobility of NF-κB in the nuclear extracts from PorB-treated B cells, indicating that PorB activates the p50 isoform of NF-κB (Fig. 2C). Anti-p65 MAb and, to a lesser extent, anti-c-Rel MAb also elicited slower mobility of NF-κB but not to the same degree as anti-p50. This result suggests that PorB induces the nuclear translocation of NF-κB consisting mainly of p50/p65 dimers and occasionally the c-Rel isoforms, which is similar to the composition of NF-κB in B cells stimulated with CD40L.
PorB-induced NF-κB nuclear translocation is not dependent on PTK activity or Erk1/2 phosphorylation.
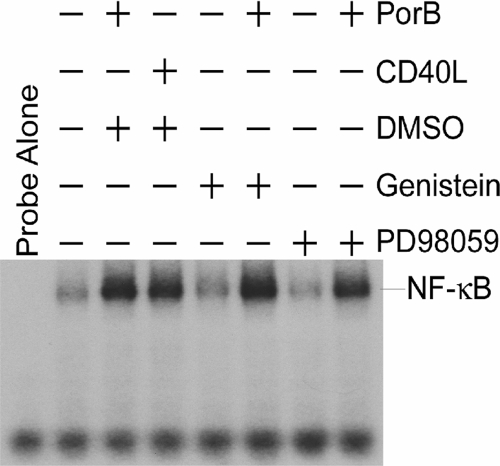
We next sought to determine if PTK activity or Erk1/2 phosphorylation is involved in PorB-induced NF-κB nuclear translocation. B cells were incubated with PorB for 4 h after pretreatment with genistein, a PTK inhibitor, or PD98059, a MEK1/2 inhibitor. EMSA of nuclear extracts demonstrated that neither PTK nor MEK1/2 inhibition prevented PorB induction of NF-κB nuclear translocation (Fig. 3). This result indicates that PorB-induced PTK and Erk1/2 activation is not an essential prerequisite for PorB induction of NF-κB nuclear translocation.
FIG. 3.
Neither PTK nor Erk1/2 activation is required for NF-κB nuclear translocation. B cells were isolated from C3H/HeJ mice and pretreated with either the PTK inhibitor genistein or the MEK1/2 inhibitor PD98059 for 30 min. Cells were then treated with medium alone, PorB (10 μg/ml), or CD40L (1:1,000) for 4 h, and nuclear extracts were isolated and subjected to EMSA using an NF-κB-specific probe. +, present; −, absent.
CD86 upregulation involves PTK activity but not Erk1/2 phosphorylation.
The interaction of CD86 on antigen-presenting cells with CD28 on T cells acts to costimulate T cells (signal 2) that recognize antigens presented by MHC molecules (signal 1) (5). Previous data demonstrate that PorB upregulates the surface expression of CD86 and class II MHC molecules on B cells in a TLR2- and MyD88-dependent manner (36, 52), indicating that TLR ligation on B cells may aid the immune response. The in vivo relevance of CD86 has also been identified previously, as blocking CD86 by using anti-CD86 MAbs prevents the antipolysaccharide antibody response to group C meningococcal CPS conjugated to PorB (35). Although B cells are not the primary antigen-presenting cells, they are capable of functioning in this capacity. For example, the porin PIB from Neisseria gonorrhoeae is capable of costimulating CD3-activated T cells in a CD86-dependent manner, as demonstrated previously using anti-CD86 blocking antibodies (66). Given the importance of CD86 in T-cell activation and the capability of B cells to fulfill this role, we were interested in elucidating the signaling pathways involved in CD86 surface upregulation on B cells.
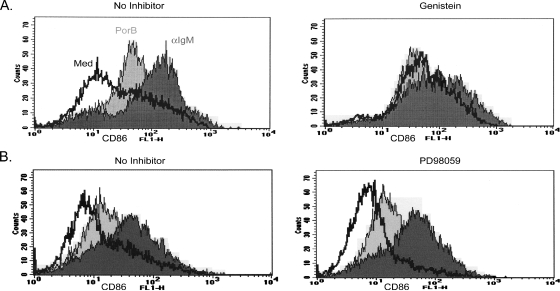
To determine if PTK activity or Erk1/2 activation by PorB is required for CD86 upregulation, we pretreated B cells for 1 h with either genistein (a PTK inhibitor) or PD98059 (a MEK1/2 inhibitor) and then treated the cells with PorB or anti-IgM-CD40L. After 24 h, the level of surface expression of CD86 was determined by flow cytometric analysis. Both PorB and anti-IgM-CD40L treatment resulted in increased CD86 surface expression. Treatment with only genistein induced the upregulation of CD86; however, CD86 was not further increased upon the addition of PorB, indicating that PTK may be involved in PorB-induced CD86 expression (Fig. 4A). Our results also show that when PTK was inhibited, the addition of anti-IgM-CD40L minimally increased CD86 expression above the level observed with genistein alone, suggesting that PTKs may partly regulate CD86 expression following anti-IgM-CD40L stimulation. These findings were not due to the induction of cell death, since degrees of cell viability in cultures of genistein-treated and untreated cells at 24 h were similar, as determined by trypan blue exclusion (data not shown). The efficacy of genistein in PTK inhibition was confirmed by Western blot analysis (data not shown).
FIG. 4.
The inhibition of PTK, but not Erk1/2, prevents PorB augmentation of CD86. (A) B cells from C3H/HeJ mice were pretreated for 30 min with a DMSO vehicle (no inhibitor) or genistein, a PTK inhibitor. Cells were then stimulated with medium alone (Med), PorB (10 μg/ml), or anti-IgM (α-IgM; 10 μg/ml) plus CD40L (1:1,000) for 24 h and subjected to flow cytometric analysis using fluorescein isothiocyanate-conjugated rat anti-mouse CD86. (B) In a separate experiment, B cells from C3H/HeJ mice were treated with a DMSO vehicle (no inhibitor) or PD98059, a MEK1/2 inhibitor, and then treated as described in the legend to panel A. Lines correspond to the treatments indicated in panel A.
The inhibition of MEK1/2 and, therefore, the prevention of Erk1/2 phosphorylation had no effect on PorB- or anti-IgM-CD40L-induced CD86 upregulation (Fig. 4B), which is consistent with our observations with macrophages (our unpublished data). As for PTK activity, the inhibition of Erk1/2 phosphorylation was confirmed by Western blot analysis (data not shown). Overall, these data indicate that PTK and not Erk1/2 activation is involved in the CD86 upregulation induced by PorB or anti-IgM-CD40L.
PorB-induced proliferation is dependent on PTK activity and not on Erk1/2 phosphorylation.
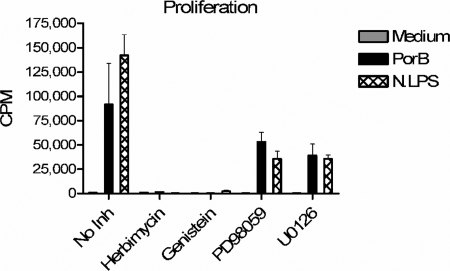
B cells undergo rapid proliferation following the engagement of the BCR (9) and treatment with PorB (54). Therefore, the ability of PorB to induce B-cell proliferation may also be related to its adjuvant activity. The BCR is made up of surface Ig that is noncovalently associated with the accessory molecules Ig(α) and Ig(β), which are responsible for signaling. The ligation of the BCR results in tyrosine phosphorylation of Ig(α) and Ig(β), which go on to activate Src tyrosine kinases (18). Therefore, the blocking of PTK activity will result in the termination of the signal through the BCR's preventing proliferation. Additionally, Erk1/2 phosphorylation is required for BCR-induced B-cell proliferation (51). However, it had yet to be determined if either PTK or Erk1/2 activities were necessary for TLR2-induced proliferation. Our results show that, like anti-IgM treatment, the inhibition of PTK by using either of the inhibitors genistein and herbimycin completely abolished PorB-induced proliferation, as well as proliferation induced by the TLR4 agonist N. meningitidis LPS (Fig. 5). These results are in contrast to those obtained with the MEK1/2 inhibitors PD98059 and U0126, which partly reduced PorB- and N. meningitidis LPS-induced proliferation (Fig. 5). These data illustrate that both BCR cross-linkage-induced proliferation and TLR2-induced proliferation require PTK activity; however, they differ in their requirement for Erk1/2 phosphorylation.
FIG. 5.
The inhibition of PTK, but not Erk1/2, prevents PorB-induced B-cell proliferation. B cells from C57BL/6 mice were pretreated for 30 min with a DMSO vehicle (no inhibitor [no Inh]), genistein or herbimycin (PTK inhibitors), or PD98059 or U0126 (MEK1/2 inhibitors). Cells were then treated with medium alone, PorB (10 μg/ml), or N. meningitidis LPS (N.LPS; 100 ng/ml) for 48 h and pulsed with [3H]TdR for an additional 18 h.
DISCUSSION
Microorganisms express a plethora of TLR ligands that upon entering the lymphatic system can potentially activate B cells expressing the appropriate TLR. Although B cells are not the major antigen-presenting cell involved in establishing an immune response, they are capable of the presentation of antigens to T cells, and this capacity is further enhanced by TLR ligation (44). In addition, previous data demonstrate that B cells treated with PorB, a TLR2 ligand, are capable of activating T cells, enhancing antibody production, and inducing B-cell proliferation (35, 54, 66). The adjuvant activity of neisserial porins is likely based on the ability of the porins to stimulate antigen-presenting cells and induce increased surface expression of the costimulatory molecule CD86 (35). Regarding B cells, neisserial porins also induce B-cell proliferation, thus linking the porins’ mitogen and adjuvant activities. To further understand the mechanism by which these bacterial outer membrane proteins stimulate B cells, the effect of the neisserial porin PorB on B-cell signal transduction events associated with TLR2 was investigated and compared to the effects of BCR cross-linkage and CD40L. We also sought to determine the signaling pathways that link the innate activation of B cells through TLR2 with B-cell proliferation and CD86 surface upregulation, which result in the induction of an adaptive immune response.
The phosphorylation of tyrosine kinases and MAPK activation are some of the earliest signaling events (9), and we needed to demonstrate that PorB treatment resulted in their induction. We established that PorB induced tyrosine phosphorylation as well as Erk1/2 phosphorylation in a TLR2-dependent manner and went on to confirm that NF-κB nuclear translocation also occurred in PorB-treated B cells. The induction of these signaling molecules was very similar to what was observed for BCR cross-linkage or CD40L stimulation, including the usage of p50/p65 NF-κB dimers. However, our data showed that PorB treatment resulted in an additional phosphorylated tyrosine protein of approximately 31 kDa, which was not observed upon anti-IgM-CD40L treatment. Taken together, these data indicate that there is some overlap in the signal transduction pathways of B cells activated through TLR2 and the BCR.
In an effort to further map the signaling pathways that occur following PorB activation, we sought to establish whether NF-κB nuclear translocation is dependent on either PTK activity or Erk1/2 phosphorylation. The inhibition of PTK and MAPK activities was achieved by using chemical inhibitors, which permeate the cell and function by competing for the activation site of a particular kinase. These inhibitors have been used extensively to identify targets of various kinases and determine the roles of kinases in cellular responses. The main caveat of using inhibitors is that they can impede the activity of other kinases, generating misleading results. The PTK inhibitor genistein can inhibit protein kinase C at the concentrations we used (15). However, similar results were obtained with herbimycin, another PTK inhibitor that does not affect protein kinase C activity, suggesting that our results were due to the inhibition of PTK activity. The Erk inhibitor PD98059 functions by binding to MEK1 and preventing the activation of MEK1, thereby inhibiting the activation of the downstream target Erk (12), and has not yet been found to affect other targets.
The inhibition of either PTK or Erk1/2 activities had no effect on NF-κB nuclear translocation, suggesting that the activation of this transcription factor is not dependent on either PTK or Erk1/2 and may involve a different set of signaling molecules entirely. It is possible that other MAPKs, p38 and Jun N-terminal protein kinase, may be involved in the activation of the NF-κB pathway. However, establishing the contribution of these MAPKs in PorB-induced activation has been difficult, as the process of isolating B cells elevates the baseline level of phosphorylation, making it complicated to determine any alterations.
It has been well-established previously that CD86 is important in the generation of an effective adaptive immune response (7, 35), and the expression of CD86 is upregulated by adjuvants. Therefore, we wanted to determine the roles of PTK and MAPK activation in the CD86 upregulation induced by PorB. Results from other groups indicate a possible role for Erk1/2 activation, as B cells stimulated through the BCR or the β-adrenergic receptor require Erk1/2 phosphorylation for CD86 upregulation (26). Further, human DCs require Erk1/2 phosphorylation for CD86 upregulation induced by the hapten nickel but not for CD86 expression induced by tumor necrosis factor alpha (6). However, the involvement of these signaling pathways in TLR2-induced CD86 surface expression on B cells has yet to be identified.
The inhibition of PTK activity with genistein resulted in an increase in the basal levels of CD86 compared to those in B cells that were untreated. This finding implies that tyrosine kinase activity may be involved in maintaining basal levels of CD86, which may be important for the maintenance of tolerance and the prevention of autoimmune disease. In B cells in which PTK activity was prevented, the addition of PorB did not alter the expression levels of CD86 compared to those in cells treated with genistein alone, suggesting that PTK inhibition prevents PorB-induced CD86 upregulation. The level of upregulation of CD86 on B cells treated with genistein followed by anti-IgM-CD40L was lower than that on B cells treated with anti-IgM-CD40L alone, suggesting that there are additional signaling molecules involved in the regulation of CD86. It is interesting to speculate that the additional tyrosine-phosphorylated protein induced by PorB and not by anti-IgM-CD40L may be involved in PorB-induced CD86 upregulation. Moreover, it may explain the differences in the roles of PTK activity in CD86 upregulation induced by PorB and that stimulated by anti-IgM-CD40L. Overall, these data illustrate that PTK activation is involved in TLR2- and, to a lesser extent, BCR/CD40L-induced CD86 surface expression, lending further evidence that there are some common signaling pathways for TLR2 and the BCR.
Previous data from our laboratory demonstrated that PorB can induce the proliferation of murine splenic B cells (54), and we were therefore interested in determining if the induction of B-cell proliferation through TLRs required PTK or Erk1/2 activation. There is some evidence indicating that PTKs may be involved in TLR-induced B-cell proliferation. Results from Kim et al. show that PTK activity is essential for B-cell proliferation induced by the TLR4 agonist LPS (25). We demonstrated a similar finding in which the inhibition of tyrosine phosphorylation prevented the PorB- and LPS-induced proliferation of B cells (Fig. 5). Our data parallel previous results from BCR-activated B cells in which PTK had been inhibited and proliferation was prevented (68), indicating that PTK activation is necessary for TLR2-, TLR4-, and BCR-induced B-cell proliferation.
It has been demonstrated previously that Erk1/2 phosphorylation is necessary for the activation of cyclin D2, which is essential for BCR-induced B-cell proliferation (45). However, it has not been determined if this same pathway is necessary for TLR-stimulated B-cell proliferation. Preventing the phosphorylation of the MAPK Erk1/2 by inhibiting MEK1/2 had little effect on PorB-induced proliferation but reduced TLR4-induced proliferation by approximately 50%. Interestingly, this result differs from those reported previously for B-cell activation by BCR cross-linking, in which Erk1/2 inhibition prevented proliferation (45). Thus, there is a divergence in the TLR2 and BCR signaling pathways after PTK activation leading to proliferation.
In summary, our data revealed that the activation of B cells by the TLR2 ligand PorB led to PTK activation, Erk1/2 phosphorylation, and NF-κB nuclear translocation. The relationship of these signaling events to CD86 upregulation and B-cell proliferation was then examined. The induction of PTK activity was necessary for both PorB upregulation of CD86 and proliferation, while in contrast, Erk1/2 activation was not, linking PTK activation to the adjuvant effect of PorB. Of interest, there is a difference in PorB- and BCR/CD40L-induced signaling events leading to proliferation, as Erk1/2 inhibition had no effect on PorB-induced proliferation but abolishes BCR-induced proliferation (45). This finding indicates that there may be a divergence in the signaling pathways between the activation of the innate immune system through TLRs and the more specific activation via the BCR that may lead to differential gene regulation.
Acknowledgments
This work is supported by the Immunology Training Program, National Institutes of Health grants AI049388 and AI040944.
We thank Lisa Ganley-Leal for critical review of the manuscript, Carol King and XiuPing Lu for technical assistance, Michael Apicella and Marilyn Kehry for reagents, and Tom Chiles for scientific discussion.
Footnotes
Published ahead of print on 20 February 2008.
REFERENCES
- 1.Barois, N., F. Forquet, and J. Davoust. 1997. Selective modulation of the major histocompatibility complex class II antigen presentation pathway following B cell receptor ligation and protein kinase C activation. J. Biol. Chem. 272:3641-3647. [DOI] [PubMed] [Google Scholar]
- 2.Bhasin, N., Y. Ho, and L. M. Wetzler. 2001. Neisseria meningitidis lipopolysaccharide modulates the specific humoral immune response to neisserial porins but has no effect on porin-induced upregulation of costimulatory ligand B7-2. Infect. Immun. 69:5031-5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjune, G., E. A. Hoiby, J. K. Gronnesby, O. Arnesen, J. H. Fredriksen, A. Halstensen, E. Holten, A.-K. Lindbak, H. Nokleby, E. Rosenquist, L. K. Solberg, O. Closs, J. Eng, L. O. Froholm, A. Lystad, L. S. Bakketeig, and B. Hareide. 1991. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 338:1093-1096. [DOI] [PubMed] [Google Scholar]
- 4.Blake, M. S., and E. C. Gotschlich. 1986. Functional and immunological properties of pathogenic neisserial surface proteins, p. 377-400. In M. Inouye (ed.), Bacterial outer membranes as model systems. John Wiley, New York, NY.
- 5.Bluestone, J. A. 1995. New perspectives of CD28-B7-mediated T cell costimulation. Immunity 2:555-559. [DOI] [PubMed] [Google Scholar]
- 6.Boisleve, F., S. Kerdine-Romer, and M. Pallardy. 2005. Implication of the MAPK pathways in the maturation of human dendritic cells induced by nickel and TNF-alpha. Toxicology 206:233-244. [DOI] [PubMed] [Google Scholar]
- 7.Borriello, F., M. P. Sethna, S. D. Boyd, A. N. Schweitzer, E. A. Tivol, D. Jacoby, T. B. Strom, E. M. Simpson, G. J. Freeman, and A. H. Sharpe. 1997. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity 6:303-313. [DOI] [PubMed] [Google Scholar]
- 8.Coligan, J. E., A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, and W. Strober (ed.). 2001. Current protocols in immunology, p. 5.0.1-5.8.8 and 11.0.1-11.10.6. John Wiley & Sons, Inc., New York, NY.
- 9.Dal Porto, J. M., S. B. Gauld, K. T. Merrell, D. Mills, A. E. Pugh-Bernard, and J. Cambier. 2004. B cell antigen receptor signaling 101. Mol. Immunol. 41:599-613. [DOI] [PubMed] [Google Scholar]
- 10.Delude, R. L., M. J. Fenton, R. J. Savedra, P. Y. Perera, S. N. Vogel, R. Thieringer, and D. T. Golenbock. 1994. CD14-mediated translocation of nuclear factor-kappa B induced by lipopolysaccharide does not require tyrosine kinase activity. J. Biol. Chem. 269:22253-22260. [PubMed] [Google Scholar]
- 11.Donnelly, J. J., R. R. Deck, and M. A. Liu. 1990. Immunogenicity of a Haemophilus influenzae polysaccharide-Neisseria meningitidis outer membrane protein vaccine. J. Immunol. 145:3071-3079. [PubMed] [Google Scholar]
- 12.Dudley, D. T., L. Pang, S. J. Decker, A. J. Bridges, and A. R. Saltiel. 1995. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc. Natl. Acad. Sci. USA 92:7686-7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francis, D. A., J. G. Karras, X. Y. Ke, R. Sen, and T. L. Rothstein. 1995. Induction of the transcription factors NF-kappa B, AP-1 and NF-AT during B cell stimulation through the CD40 receptor. Int. Immunol. 7:151-161. [DOI] [PubMed] [Google Scholar]
- 14.Fusco, P. C., F. Michon, M. Laude-Sharp, C. A. Minetti, C. H. Huang, I. Heron, and M. S. Blake. 1998. Preclinical studies on a recombinant group B meningococcal porin as a carrier for a novel Haemophilus influenzae type b conjugate vaccine. Vaccine 16:1842-1849. [DOI] [PubMed] [Google Scholar]
- 15.Geissler, J. F., P. Traxler, U. Regenass, B. J. Murray, J. L. Roesel, T. Meyer, E. McGlynn, A. Storni, and N. B. Lydon. 1990. Thiazolidine-diones. Biochemical and biological activity of a novel class of tyrosine protein kinase inhibitors. J. Biol. Chem. 265:22255-22261. [PubMed] [Google Scholar]
- 16.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225-260. [DOI] [PubMed] [Google Scholar]
- 17.Guttormsen, H. K., L. M. Wetzler, and A. Naess. 1993. Humoral immune response to the class 3 outer membrane protein during the course of meningococcal disease. Infect. Immun. 61:4734-4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harnett, M. M., E. Katz, and C. A. Ford. 2005. Differential signalling during B-cell maturation. Immunol. Lett. 98:33-44. [DOI] [PubMed] [Google Scholar]
- 19.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 20.Hertz, C. J., S. M. Kiertscher, P. J. Godowski, D. A. Bouis, M. V. Norgard, M. D. Roth, and R. L. Modlin. 2001. Microbial lipopeptides stimulate dendritic cell maturation via Toll-like receptor 2. J. Immunol. 166:2444-2450. [DOI] [PubMed] [Google Scholar]
- 21.Hsing, Y., B. S. Hostager, and G. A. Bishop. 1997. Characterization of CD40 signaling determinants regulating nuclear factor-kappa B activation in B lymphocytes. J. Immunol. 159:4898-4906. [PubMed] [Google Scholar]
- 22.Hunninghake, G. W., B. G. Monks, L. J. Geist, M. M. Monick, M. A. Monroy, M. F. Stinski, A. C. Webb, J. M. Dayer, P. E. Auron, and M. J. Fenton. 1992. The functional importance of a cap site-proximal region of the human prointerleukin 1β gene is defined by viral protein trans-activation. Mol. Cell. Biol. 12:3439-3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaisho, T., and S. Akira. 2001. Dendritic-cell function in Toll-like receptor- and MyD88-knockout mice. Trends Immunol. 22:78-83. [DOI] [PubMed] [Google Scholar]
- 24.Kehry, M. R., and B. E. Castle. 1994. Regulation of CD40 ligand expression and use of recombinant CD40 ligand for studying B cell growth and differentiation. Semin. Immunol. 6:287-294. [DOI] [PubMed] [Google Scholar]
- 25.Kim, J., H. R. Kim, J. C. Lee, and Y. S. Jang. 2002. Involvement of ERK, p38 MAP kinase, and PKC in MHC class II-mediated signal transduction in a resting B cell line. Biochem. Biophys. Res. Commun. 291:139-145. [DOI] [PubMed] [Google Scholar]
- 26.Kohm, A. P., A. Mozaffarian, and V. M. Sanders. 2002. B cell receptor- and beta 2-adrenergic receptor-induced regulation of B7-2 (CD86) expression in B cells. J. Immunol. 168:6314-6322. [DOI] [PubMed] [Google Scholar]
- 27.Krieg, A. M. 2000. Signal transduction induced by immunostimulatory CpG DNA. Springer Semin. Immunopathol. 22:97-105. [DOI] [PubMed] [Google Scholar]
- 28.Lenschow, D. J., A. I. Sperling, M. P. Cooke, G. Freeman, L. Rhee, D. C. Decker, G. Gray, L. M. Nadler, C. C. Goodnow, and J. A. Bluestone. 1994. Differential up-regulation of the B7-1 and B7-2 costimulatory molecules after Ig receptor engagement by antigen. J. Immunol. 153:1990-1997. [PubMed] [Google Scholar]
- 29.Li, Q., and I. M. Verma. 2002. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2:725-734. [DOI] [PubMed] [Google Scholar]
- 30.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 31.Liu, J. L., T. C. Chiles, R. J. Sen, and T. L. Rothstein. 1991. Inducible nuclear expression of NF-kappa B in primary B cells stimulated through the surface Ig receptor. J. Immunol. 146:1685-1691. [PubMed] [Google Scholar]
- 32.Livingston, P. O., M. J. Calves, F. Helling, W. D. Zollinger, M. S. Blake, and G. H. Lowell. 1993. GD3/proteosome vaccines induce consistent IgM antibodies against the ganglioside GD3. Vaccine 11:1199-1204. [DOI] [PubMed] [Google Scholar]
- 33.Lowell, G. H. 1990. Proteosomes, hydrophobic anchors, iscoms, and liposomes for improved presentation of peptides and protein vaccines, p. 141-160. In G. C. Woodrow and M. M. Levine (ed.), New generation vaccines. Marcel Dekker, Inc., New York, NY.
- 34.Lowell, G. H., W. R. Ballou, L. F. Smith, R. A. Wirtz, W. D. Zollinger, and W. T. Hockmeyer. 1988. Proteosome-lipopeptide vaccines: enhancement of immunogenicity for malaria CS peptides. Science 240:800-802. [DOI] [PubMed] [Google Scholar]
- 35.Mackinnon, F. G., Y. Ho, M. S. Blake, F. Michon, A. Chandraker, M. H. Sayegh, and L. M. Wetzler. 1999. The role of B/T costimulatory signals in the immunopotentiating activity of neisserial porin. J. Infect. Dis. 180:755-761. [DOI] [PubMed] [Google Scholar]
- 36.Massari, P., P. Henneke, Y. Ho, E. Latz, D. T. Golenbock, and L. M. Wetzler. 2002. Cutting edge: immune stimulation by neisserial porins is Toll-like receptor 2 and MyD88 dependent. J. Immunol. 168:1533-1537. [DOI] [PubMed] [Google Scholar]
- 37.Massari, P., C. A. King, H. MacLeod, and L. M. Wetzler. 2005. Improved purification of native meningococcal porin PorB and studies on its structure/function. Protein Expr. Purif. 44:136-146. [DOI] [PubMed] [Google Scholar]
- 38.Massari, P., A. Visintin, J. Gunawardana, K. A. Halmen, C. A. King, D. T. Golenbock, and L. M. Wetzler. 2006. Meningococcal porin PorB binds to TLR2 and requires TLR1 for signaling. J. Immunol. 176:2373-2380. [DOI] [PubMed] [Google Scholar]
- 39.Matsumoto, M., S. Kikkawa, M. Kohase, K. Miyake, and T. Seya. 2002. Establishment of a monoclonal antibody against human Toll-like receptor 3 that blocks double-stranded RNA-mediated signaling. Biochem. Biophys. Res. Commun. 293:1364-1369. [DOI] [PubMed] [Google Scholar]
- 40.Medzhitov, R., P. Preston-Hurlburt, and C. A. Janeway, Jr. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388:394-397. [DOI] [PubMed] [Google Scholar]
- 41.Medzhitov, R., P. Preston-Hurlburt, E. Kopp, A. Stadlen, C. Chen, S. Ghosh, and C. A. Janeway, Jr. 1998. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell 2:253-258. [DOI] [PubMed] [Google Scholar]
- 42.Melancon, J., R. A. Murgita, and I. W. DeVoe. 1983. Activation of murine B lymphocytes by Neisseria meningitidis and isolated meningococcal surface antigens. Infect. Immun. 42:471-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ozinsky, A., D. M. Underhill, J. D. Fontenot, A. M. Hajjar, K. D. Smith, C. B. Wilson, L. Schroeder, and A. Aderem. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc. Natl. Acad. Sci. USA 97:13766-13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pasare, C., and R. Medzhitov. 2005. Control of B-cell responses by Toll-like receptors. Nature 438:364-368. [DOI] [PubMed] [Google Scholar]
- 45.Piatelli, M. J., C. Doughty, and T. C. Chiles. 2002. Requirement for a hsp90 chaperone-dependent MEK1/2-ERK pathway for B cell antigen receptor-induced cyclin D2 expression in mature B lymphocytes. J. Biol. Chem. 277:12144-12150. [DOI] [PubMed] [Google Scholar]
- 46.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 47.Poolman, J. T. 1988. Meningococcal vaccines. J. Med. Microbiol. 26:170-172. [PubMed] [Google Scholar]
- 48.Purkerson, J. M., and D. C. Parker. 1998. Differential coupling of membrane Ig and CD40 to the extracellularly regulated kinase signaling pathway. J. Immunol. 160:2121-2129. [PubMed] [Google Scholar]
- 49.Ray, A., and T. Biswas. 2005. Porin of Shigella dysenteriae enhances Toll-like receptors 2 and 6 of mouse peritoneal B-2 cells and induces the expression of immunoglobulin M, immunoglobulin G2a and immunoglobulin A. Immunology 114:94-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ray, A., P. Karmakar, and T. Biswas. 2004. Up-regulation of CD80-CD86 and IgA on mouse peritoneal B-1 cells by porin of Shigella dysenteriae is Toll-like receptors 2 and 6 dependent. Mol. Immunol. 41:1167-1175. [DOI] [PubMed] [Google Scholar]
- 51.Richards, J. D., S. H. Dave, C. H. Chou, A. A. Mamchak, and A. L. DeFranco. 2001. Inhibition of the MEK/ERK signaling pathway blocks a subset of B cell responses to antigen. J. Immunol. 166:3855-3864. [DOI] [PubMed] [Google Scholar]
- 52.Singleton, T. E., P. Massari, and L. M. Wetzler. 2005. Neisserial porin-induced dendritic cell activation is MyD88 and TLR2 dependent. J. Immunol. 174:3545-3550. [DOI] [PubMed] [Google Scholar]
- 53.Snapper, C. M., M. R. Kehry, B. E. Castle, and J. J. Mond. 1995. Multivalent, but not divalent, antigen receptor cross-linkers synergize with CD40 ligand for induction of Ig synthesis and class switching in normal murine B cells. A redefinition of the TI-2 vs T cell-dependent antigen dichotomy. J. Immunol. 154:1177-1187. [PubMed] [Google Scholar]
- 54.Snapper, C. M., F. R. Rosas, M. R. Kehry, J. J. Mond, and L. M. Wetzler. 1997. Neisserial porins may provide critical second signals to polysaccharide-activated murine B cells for induction of immunoglobulin secretion. Infect. Immun. 65:3203-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sparkes, B. G. 1983. Dual effect of meningococcal antigens on T cell dependent immune response. Can. J. Microbiol. 29:1619-1625. [DOI] [PubMed] [Google Scholar]
- 56.Sparkes, B. G. 1983. Immunomodulating activity of meningococcal antigens. Can. J. Microbiol. 29:1611-1618. [DOI] [PubMed] [Google Scholar]
- 57.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 58.Tasker, L., and S. Marshall-Clarke. 1997. Immature B cells from neonatal mice show a selective inability to up-regulate MHC class II expression in response to antigen receptor ligation. Int. Immunol. 9:475-484. [DOI] [PubMed] [Google Scholar]
- 59.Tsai, C. M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 60.Vordermeier, H. M., and W. G. Bessler. 1987. Polyclonal activation of murine B lymphocytes in vitro by Salmonella typhimurium porins. Immunobiology 175:245-251. [DOI] [PubMed] [Google Scholar]
- 61.Vordermeier, H. M., H. Drexler, and W. G. Bessler. 1987. Polyclonal activation of human peripheral blood lymphocytes by bacterial porins and defined porin fragments. Immunol. Lett. 15:121-126. [DOI] [PubMed] [Google Scholar]
- 62.Vordermeier, H. M., K. Stab, and W. G. Bessler. 1986. A defined fragment of bacterial protein I (OmpF) is a polyclonal B-cell activator. Infect. Immun. 51:233-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wetzler, L. M. 1994. Specificity of murine humoral and T helper cell re-sponses towards neisserial porins, p. 127-134. In C. J. Conde-Glez, S. A. Morse, P. A. Rice, P. F. Sparling, and E. Calderón (ed.), Pathobiology and immunobiology of Neisseriaceae. Instituto Nacional de Salud Pública, Cuernavaca, Morelos, Mexico.
- 64.Wetzler, L. M., M. S. Blake, K. Barry, and E. C. Gotschlich. 1992. Gonococcal porin vaccine evaluation: comparison of Por proteosomes, liposomes and blebs isolated from rmp deletion mutants. J. Infect. Dis. 166:551-555. [DOI] [PubMed] [Google Scholar]
- 65.Wetzler, L. M., M. S. Blake, and E. C. Gotschlich. 1988. Characterization and specificity of antibodies to protein I of Neisseria gonorrhoeae produced by injection with various protein I-adjuvant preparations. J. Exp. Med. 168:1883-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wetzler, L. M., Y. Ho, and H. Reiser. 1996. Neisserial porins induce B lymphocytes to express costimulatory B7-2 molecules and to proliferate. J. Exp. Med. 183:1151-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu, M., H. Lee, R. E. Bellas, S. L. Schauer, M. Arsura, D. Katz, M. J. FitzGerald, T. L. Rothstein, D. H. Sherr, and G. E. Sonenshein. 1996. Inhibition of NF-kappaB/Rel induces apoptosis of murine B cells. EMBO J. 15:4682-4690. [PMC free article] [PubMed] [Google Scholar]
- 68.Yao, X. R., and D. W. Scott. 1993. Inhibition of protein tyrosine kinase activity by herbimycin A prevents anti-mu but not LPS-mediated cell cycle progression and differentiation of splenic B lymphocytes. Cell. Immunol. 149:364-375. [DOI] [PubMed] [Google Scholar]
- 69.Zollinger, W. D. 1990. New and inproved vaccines against meningococcal disease, p. 325-348. In G. C. Woodrow and M. M. Levine (ed.), New generation vaccines. Marcel Dekker, Inc., New York, NY.