Abstract
Histoplasmosis is a common endemic mycosis in the Americas, often causing severe disease in patients with AIDS. Antigen detection has become an important method for rapid diagnosis of histoplasmosis in the United States but not in Central or South America. Isolates from patients in the United States are predominantly found to be class 2 isolates when typed using the nuclear gene YPS3, while isolates from Latin America are predominantly typed as class 5 or class 6. Whether infection with these Latin American genotypes produces positive results in the Histoplasma antigen assay has not been reported. In this study, we have compared the sensitivity of antigen detection for AIDS patients from Panama who had progressive disseminated histoplasmosis to that for those in the United States. Antigenuria was detected in the MVista Histoplasma antigen enzyme immunoassay (EIA) in 95.2% of Panamanian cases versus 100% of U.S. cases. Antigenemia was detected in 94.7% of the Panamanian cases versus 92% of the U.S. cases. Two clinical isolates from Panama were typed using YPS3 and were found to be restriction fragment length polymorphism class 6. We conclude that the MVista Histoplasma antigen EIA is a sensitive method for diagnosis of histoplasmosis in Panama.
Progressive disseminated histoplasmosis (PDH) is a common and serious opportunistic disease among patients with AIDS in the United States and Latin America. In Panama, PDH occurred in 8% of hospitalized patients from 1997 to 2003 and was fatal in 12% of cases (6). The clinical picture was similar to that reported elsewhere and usually included fever, weight loss, and respiratory symptoms, often in association with diarrhea, as well as hepatosplenomegaly, hepatic enzyme elevation, and pancytopenia. Skin lesions were noted in 17% of the Panamanian cases (6), lower than the observed rate for cases from Brazil (66%) but higher than that for cases from the United States (∼3%) (7).
Disease in patients in Mexico and Latin America has been described as being caused by isolates of Histoplasma capsulatum var. capsulatum that exhibit distinct genetic profiles in comparison to those from the United States (3, 8, 9). Keath et al. (9) first described typing based upon restriction fragment length polymorphism (RFLP) in the YPS3 gene. They showed that Panamanian strains were typed as YPS3 class 3, 5, or 6, while the North American strains were predominantly class 2. Later, Kasuga et al. used the DNA sequence substitution rates in four independent protein-coding genes to identify at least eight different genotypes, or clades (8). North American class 2 was the predominant genotype in the United States and Latin American group A in Latin America. Latin American group B was found only in Columbia and Argentina. Based on only four isolates from Panama included in the study by Kasuga et al., Panamanian strains included Latin American group A and a lone lineage, designated H81.
The diagnosis of PDH relies on demonstration of the organism in clinical specimens or detection of antigen in body fluids (2). Histopathology may be falsely negative in up to 50% of patients, caused by sampling error, paucity of organisms, or inexperience of the pathologist. Culture may require several weeks to isolate and identify the organism and may be falsely negative for 15% of patients (13). Antigenuria can be detected in 95% of cases in patients with AIDS in the United States (4, 13) but has not been evaluated in Latin America. Whether genetic differences in Latin American isolates would affect the sensitivity for diagnosis by antigen detection is unknown. The prevalence of PDH in AIDS patients from Panama offered the opportunity to address this question.
MATERIALS AND METHODS
Case definition.
The definition included the presence of AIDS based upon a serologic test positive for human immunodeficiency virus infection, a CD4 cell count below 200 cells/μl and/or a previous AIDS defining condition according to CDC classification, and PDH proven by culture or histopathology.
Methods.
We collected samples of serum and urine from Panamanian patients with AIDS and clinical suspicion of PDH who were admitted to the AIDS ward of the Arnulfo Arias Madrid Hospital in Panama City, Panama, from December 2005 through November 2006. All of the samples were taken before the start of antifungal therapy, referred to as baseline, and then stored frozen at −20°C. Samples were shipped in batches to MiraVista Diagnostics on three occasions during the course of the study, according to International Air Transport Association regulations.
We included 21 patients with PDH in the study. The study was approved by the institution's review board and followed guidelines for clinical research. All patients read and signed an informed-consent form before the samples were taken. The investigators also completed a case report form for each patient with demographic and clinically relevant data.
For comparison, paired serum and urine specimens collected at enrollment or week 1 or 2 of treatment from 65 AIDS patients in the United States with PDH who participated in a fluconazole treatment study (11) were also tested. The basis for diagnosis was positive culture or histopathology in 92% of cases and positive antigen in urine in 8% of cases. These specimens were obtained from 1991 and 1992 and had been stored frozen since original collection for a study conducted by the AIDS Clinical Trials Group, in accordance with institutional guidelines for clinical research.
Genotyping of isolates using the YPS3 gene.
The isolates were identified based on both the size of the PCR-amplified YPS3 gene (3) and RFLP pattern analysis of the HaeIII-digested YPS3 locus. Briefly, DNA from H. capsulatum isolates was obtained with a MasterPure yeast DNA purification kit (Epicenter, Madison, WI). The primers used to amplify the YPS3 locus were Yps3F (5′-ATGCTGAACATCAAATCGATCTC-3′) and Yps3R (5′-TTATGCCTCGCAGTGTTTATAAAGC-3′) (3). PCR was performed using the high-fidelity polymerase Triplemaster (Eppendorf, Westbury, NY) with buffer components provided by the supplier. The amplification parameters were as follows: an initial denaturation step at 95°C for 5 min, followed by 25 cycles which consisted of denaturation at 95°C for 30 s, annealing at 53°C for 30 s, and extension at 72°C for 1 min. PCR products were separated on a 2% agarose gel, and their sizes were determined using a 50-bp DNA ladder (NEB, Ipswich, MA). The sizes of the amplified YPS3 sequences were compared with those for appropriate RFLP class-defined control strains [UCLA531S, RFLP class 1; G217B (ATCC 26032), RFLP class 2; G186B (ATCC 26030), RFLP class 3; JHMA(H502), RFLP class 5; and CFLA(H248), RFLP class 6]. In addition, PCR products were digested with HaeIII according to the manufacturer's recommendations (Boehringer, Mannheim, Germany), and digestion products were analyzed after separation on a 2% agarose gel. The unique RFLP patterns were compared with those obtained from the control strains listed above.
MVista Histoplasma antigen EIA.
This method was reported previously (4) and will be briefly described. The test is a sandwich enzyme immunoassay (EIA) using microplates coated with polyclonal rabbit anti-Histoplasma antibodies. Patient specimens and galactomannan calibrators and controls are incubated in the precoated plates, permitting binding of antigen to the capture antibody. Bound antigen is then detected with a biotinylated detector antibody. The microplates are read spectrophotometrically, and optical densities that are more than three times that of the negative control are classified as positive; antigen is quantitated in ng/ml by extrapolation from a calibration curve. Specimens with optical densities that exceed the cutoff for the assay but that are less than the 0.6-ng/ml standard are reported as positive, <0.6 ng/ml, and those with results exceeding the 39-ng/ml standard are reported as positive, >39 ng/ml; all others are read directly from the curve. All of the specimens were tested in a single batch. Testing was performed at MiraVista Diagnostics, Indianapolis, IN.
Statistical analysis.
The respective proportion of patients with positive antigenuria or antigenemia among patients from Panama was compared to that from the United States by using Fisher's exact test. Quantitative antigen results in serum or urine from both patient groups were compared using the Mann-Whitney rank sum test.
RESULTS
Clinical and laboratory findings.
Twenty-one patients with newly diagnosed cases of PDH were enrolled in the study over 12 months in 2005 and 2006. Our patients’ median age was 36.7 years (range, 24 to 60), and 90% of them were male (19/21). The most common findings were fever, weight loss, hepatosplenomegaly, hematological alterations, and diarrhea. The diagnosis of histoplasmosis was confirmed by blood culture in 12 cases and by bone marrow culture in 4, and 5 had positive results in both. Four patients died in the first 6 weeks of treatment, at days 5, 6, 14, and 35. Genotyping using YPS3 was performed on two clinical isolates from Panama, and both were typed as class 6.
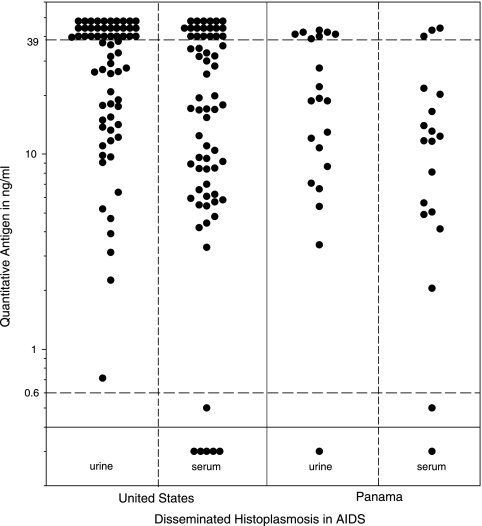
Antigen levels were similar in patients from Panama and the United States (Fig. 1). The urine antigen test was positive in 20 of 21 (95.2%) of Panamanian cases versus 65 of 65 (100%) of U.S. cases (P = 0.244). The median urinary antigen level was also similar between Panamanian and U.S. cases, 18.8 ng/ml (range, none detected to >39.0 ng/ml; 95% confidence interval [CI], 8.4 to 40.0), versus 37.0 ng/ml (range, 0.71 to >39.0 ng/ml; 95% CI, 26.0 to 40.0) (P = 0.088). Serum antigen was positive in 18 of 19 (94.7%) Panamanian cases versus 60 of 65 (92%) U.S. cases (P = 1.0). The median serum antigen level was 11.7 ng/ml (range, none detected to >39.0 ng/ml; 95% CI, 4.9 to 20.0) in the Panamanian group versus 17.0 ng/ml (range, none detected to >39 ng/ml; 95% CI, 9.2 to 31.7) in the U.S. cases (P = 0.155).
FIG. 1.
Urine and serum antigen in patients with AIDS and PDH. The solid horizontal line represents the cutoff for positivity and the dashed lines the lowest (0.6 ng/ml) or highest (39 ng/ml) calibrator. Each point represents a single case.
False-negative results with serum and urine occurred for one Panamanian patient. He was a 32-year-old with miliary infiltrates on chest radiograph, diarrhea, and vomiting, suggesting gastrointestinal involvement, and a positive blood culture for H. capsulatum.
DISCUSSION
The purpose of this study was to determine if the MVista Histoplasma antigen EIA could be used for diagnosis of PDH in patients with AIDS from Panama. The rationale for addressing this issue was that H. capsulatum strains from Panama were genetically different from those from the United States (3, 8, 9) and thus might also be antigenically different, affecting the sensitivity for diagnosis by antigen detection. Antigen was detected in specimens from 95% of the Panamanian patients, at levels comparable to that for U.S. patients. Of note is that the samples used for the comparison from the U.S. cases were stored frozen for more than 15 years and the Panamanian specimens were stored frozen for 6 to 12 months before testing. We have not observed a reduction in antigen concentration in frozen specimens, as shown by the similar individual levels and proportion that were positive in the U.S. specimens compared to the initial testing of those samples (11). The two Panamanian isolates that were typed were YPS3 RFLP class 6, belonging to Latin American group A. (8). In a separate study, we have also shown that antigen was detected in the tissues of mice infected with YPS3 RFLP class 5 or class 6, Latin American group A isolates (1, 5). These findings establish that the antigens produced by the most common Latin American genotypes of H. capsulatum are detected in the MVista Histoplasma antigen EIA.
We previously noted the sensitivity for diagnosis of PDH in AIDS patients from Panama to be 67% for blood cultures, 51% for bone marrow cultures, and only 24% for histopathology of bone marrow biopsy (6). The low sensitivity of nonculture methods and delay in diagnosis by culture supports the value of the antigen test for diagnosis of PDH in Panamanian patients. Of note is that specimens from patients with other endemic mycoses are cross-reactive in the MVista Histoplasma antigen EIA (10, 12). However, cross-reactivity does not reduce the usefulness of the test for rapid diagnosis, since treatment decisions and regimens are similar and in most cases the causative organism can be suspected based on epidemiological or clinical findings or proven by histopathology, culture, or serology.
In summary, antigenemia and antigenuria were present in similar proportions of patients from the United States and Panama, showing the potential use of the MVista Histoplasma antigen EIA for diagnosis of PDH in Panama and probably other Latin American countries.
Acknowledgments
We disclose that L. J. Wheat and P. A. Connolly are employees of MiraVista Diagnostics and MiraBella Technologies, the company that developed and performs the MVista Histoplasma antigen EIA.
Footnotes
Published ahead of print on 20 February 2008.
REFERENCES
- 1.Allton, D. R., R. G. Rivard, P. A. Connolly, L. J. Wheat, and D. R. Hospenthal. 2007. Antigen detection of Latin American strains of Histoplasma in a murine model. Abstr. 45th Conf. Infect. Dis. Soc. America, abstr. 159. [DOI] [PMC free article] [PubMed]
- 2.Ascioglu, S., J. H. Rex, B. De Pauw, J. E. Bennett, J. Bille, F. Crokaert, D. W. Denning, J. P. Donnelly, J. E. Edwards, Z. Erjavec, D. Fiere, O. Lortholary, J. Maertens, J. F. Meis, T. F. Patterson, J. Ritter, D. Selleslag, P. M. Shah, D. A. Stevens, and T. J. Walsh. 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 34:7-14. [DOI] [PubMed] [Google Scholar]
- 3.Bohse, M. L., and J. P. Woods. 2007. Expression and interstrain variability of the YPS3 gene of Histoplasma capsulatum. Eukaryot. Cell 6:609-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connolly, P. A., M. M. Durkin, A. M. LeMonte, E. J. Hackett, and L. J. Wheat. 2007. Detection of Histoplasma antigen by a quantitative enzyme immunoassay. Clin. Vaccine Immunol. 14:1587-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durkin, M. M., P. A. Connolly, K. Karimi, E. Wheat, C. Schnizlein-Bick, S. D. Allen, K. Alves, R. P. Tewari, and E. Keath. 2004. Pathogenic differences between North American and Latin American strains of Histoplasma capsulatum var. capsulatum in experimentally infected mice. J. Clin. Microbiol. 42:4370-4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutierrez, M. E., A. Canton, N. Sosa, E. Puga, and L. Talavera. 2005. Disseminated histoplasmosis in patients with AIDS in Panama: a review of 104 cases. Clin. Infect. Dis. 40:1199-1202. [DOI] [PubMed] [Google Scholar]
- 7.Karimi, K., L. J. Wheat, P. Connolly, G. Cloud, R. Hajjeh, E. Wheat, K. Alves, C. S. Lacaz Cd, and E. Keath. 2002. Differences in histoplasmosis in patients with acquired immunodeficiency syndrome in the United States and Brazil. J. Infect. Dis. 186:1655-1660. [DOI] [PubMed] [Google Scholar]
- 8.Kasuga, T., T. J. White, G. Koenig, J. McEwen, A. Restrepo, E. Castaneda, L. C. Da Silva, E. M. Heins-Vaccari, R. S. De Freitas, R. M. Zancope-Oliveira, Z. Qin, R. Negroni, D. A. Carter, Y. Mikami, M. Tamura, M. L. Taylor, G. F. Miller, N. Poonwan, and J. W. Taylor. 2003. Phylogeography of the fungal pathogen Histoplasma capsulatum. Mol. Ecol. 12:3383-3401. [DOI] [PubMed] [Google Scholar]
- 9.Keath, E. J., G. S. Kobayashi, and G. Medoff. 1992. Typing of Histoplasma capsulatum by restriction fragment length polymorphisms in a nuclear gene. J. Clin. Microbiol. 30:2104-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuberski, T., R. Myers, L. J. Wheat, B. M. Kubak, D. Bruckner, and D. Peuges. 2007. Diagnosis of coccidioidomycosis by antigen detection using cross-reaction with a Histoplasma antigen. Clin. Infect. Dis. 44:e50-e54. [DOI] [PubMed] [Google Scholar]
- 11.Wheat, J., S. MaWhinney, R. Hafner, D. McKinsey, D. Chen, A. Korzun, K. J. Shakan, P. Johnson, R. Hamill, D. Bamberger, P. Pappas, J. Stansell, S. Koletar, K. Squires, R. A. Larsen, T. Cheung, N. Hyslop, K. K. Lai, D. Schneider, C. Kauffman, M. Saag, W. Dismukes, W. Powderly, et al. 1997. Treatment of histoplasmosis with fluconazole in patients with acquired immunodeficiency syndrome. Am. J. Med. 103:223-232. [DOI] [PubMed] [Google Scholar]
- 12.Wheat, J., H. Wheat, P. Connolly, M. Kleiman, K. Supparatpinyo, K. Nelson, R. Bradsher, and A. Restrepo. 1997. Cross-reactivity in Histoplasma capsulatum variety capsulatum antigen assays of urine samples from patients with endemic mycoses. Clin. Infect. Dis. 24:1169-1171. [DOI] [PubMed] [Google Scholar]
- 13.Williams, B., M. Fojtasek, P. Connolly-Stringfield, and J. Wheat. 1994. Diagnosis of histoplasmosis by antigen detection during an outbreak in Indianapolis, Ind. Arch. Pathol. Lab. Med. 118:1205-1208. [PubMed] [Google Scholar]