Abstract
Currently, there is no routine monitoring of an immune response to the anthrax vaccine. Simple on-site tests are needed to evaluate the antibody response of anthrax-vaccinated individuals in the Armed Forces and others at high risk. Using a prototype lateral flow assay (LFA) (R. E. Biagini, D. L. Sammons, J. P. Smith, B. A. MacKenzie, C. A. F. Striley, J. E. Snawder, S. A. Robertson, and C. P. Quinn, Clin. Vaccine Immunol. 13:541-546, 2006), we investigated the agreement between a validated anthrax protective antigen (PA) immunoglobulin G (IgG) enzyme-linked immunosorbent assay (ELISA) and the LFA for 335 unvaccinated and vaccinated subjects. We also investigated the performance of the LFA under the following conditions: thermal shock (i.e., thermal cycling between temperature extremes), high temperature/high relative humidity, high temperature/low relative humidity, and low temperature/low relative humidity. With the anti-PA ELISA used as a standard, the LFA was shown to be optimally diagnostic at 11 μg/ml anti-PA-specific IgG. At this concentration, the LFA specificity and sensitivity were 98% (95% confidence interval [CI], 97% to 100%) and 92% (CI, 88% to 97%), respectively. Receiver operating characteristic curve analysis yielded an area under the curve value of 0.988 (CI, 0.976 to 1.00), suggesting that the LFA is an extremely accurate diagnostic test. For ≤4 or ≥50 μg/ml PA-specific IgG, the LFA results for each environmental condition were identical to those obtained in the laboratory. These data indicate that this rapid point-of-care test would be a feasible tool in monitoring the serological antibody responses of individuals that have been vaccinated against anthrax.
Presently, all military personnel, emergency essential Department of Defense civilians, and contractors deploying to high-risk areas receive Anthrax Vaccine Adsorbed (AVA), now known as “Biothrax” (BioPort Corp., Lansing, MI). The Food and Drug Administration (FDA)-cleared vaccination regimen consists of six subcutaneous injections, with the first three doses administered at 2-week intervals and doses four to six given at 6-month intervals. Thereafter, boosters are administered annually (Anthrax Vaccine Adsorbed package insert, 1999; ioPort Corp., Lansing, MI).
The 83-kDa protective antigen (PA) is a highly immunogenic protein that is the principal immunogen of the current vaccine (14). The vaccine elicits an anti-PA antibody response in humans (1, 4, 5, 15, 22-24, 28) as well as in several animal models (9, 11-13, 16, 17, 20, 21). The blood anti-anthrax PA immunoglobulin G (IgG) level is considered to be an effective indicator for assessing vaccine efficacy (27).
Antibody responses to AVA and its principal component of PA are not routinely monitored in vaccine recipients. In the past, scientists have used laboratory-based tests to assess the immune response evoked by the anthrax vaccine. A quantitative enzyme-linked immunosorbent assay (ELISA) (25) and a multiplexed fluorescent covalent microsphere immunoassay (3) have been developed for the serological detection of PA-specific IgG. Unfortunately, neither of these tests is practical in field settings, as they require laboratory equipment and highly trained personnel.
With advancing technology, the development and commercialization of screening tests are moving toward rapid point-of-care assays. Lateral flow assays (LFAs) are semiquantitative colorimetric tests that are well established in the public sector (e.g., to test for pregnancy, drugs of abuse, and human immunodeficiency virus). The lateral flow-style test is subdivided into cassette, card, and dipstick configurations. Although these test configurations are performed differently, they function through the same basic principle. The donor's specimen and chase buffer are applied to the sample pad. The sample flows via capillary action through a conjugate pad (i.e., impregnated with conjugated gold or latex particles) and across a nitrocellulose membrane that has a test stripe and a control stripe. For detecting disease or monitoring vaccination status, the device is usually designed to detect the presence of specific antibodies. If disease-specific antibodies are present in the specimen, they will react with the test stripe, containing the diagnostic target molecule. A control stripe, designed to test the performance of the assay, can be biotin, to which conjugated gold streptavidin migrates. The results are obtained after 15 to 20 min with the unaided eye.
To detect anti-PA antibodies in serum and whole blood, Biagini et al. developed a blood-based LFA (4). In that study, samples from 18 vaccinated individuals with relatively high concentrations (52 to 340 μg/ml) of PA-specific IgG were tested. With these samples, the diagnostic sensitivity and specificity of the LFA were 100%, with ELISA-measured anti-PA used as the standard. Additionally, a human anti-anthrax vaccine serum standard was used, which determined the detection limit of the LFA to be 2.8 μg/ml of PA-specific IgG.
The aim of this study was to determine whether the above-mentioned LFA maintains sensitivity and specificity in a cohort with a broad range of anthrax-specific antibody concentrations (from below the ELISA minimum detection concentration [MDC; 3 μg/ml PA-specific IgG] to 708 μg/ml). Also, simulation experiments were conducted to determine the stability of the LFA in environments that are commonly encountered under field conditions. The latter experiments included exposure of the devices to fluctuating temperature and humidity cycles (i.e., thermal shock, high temperature/high relative humidity [RH], high temperature/low RH, and low temperature/low RH).
MATERIALS AND METHODS
Study participants.
Samples from 335 human subjects, as described in other communications (5, 15), were used in these experiments. Recruitment and sampling of subjects were conducted under protocols (DOD30428 and NIDBR2004.0001) approved by the Naval Institute for Dental and Biomedical Research (Great Lakes, IL) Institutional Review Board and in compliance with all applicable federal regulations governing the protection of human subjects. Exclusion criteria included the inability to collect blood. Participants were classified into two groups, unvaccinated (n = 139) and AVA vaccinated (n = 187). The AVA-vaccinated group comprised personnel who received the vaccine as part of their military routine. The enrollment of human subjects occurred during a period when the Anthrax Vaccine Immunization Program was halted. Prior to the suspension, vaccinations were administered in a manner that was consistent with Department of Defense policy and FDA guidelines. The vaccination statuses of nine study participants were unavailable; nonetheless, the PA-specific IgG concentration was known for all study participants.
Specimens.
As described previously, blood was collected from the participants, using a 21-gauge needle and a serum separation Vacutainer tube (Becton Dickinson, Franklin Lakes, NJ) (5, 15). After the whole blood was allowed to clot, it was centrifuged (1,470 × g) and the supernatant collected. Serum samples used for the immunoassays were frozen at −35°C or less until needed.
As a gold standard for comparison, a quantitative ELISA was used to characterize the specimens by their amounts of PA-specific IgG (25). The authors determined that the MDC of the serum antibody ELISA using standard reference serum pool AVR414 is 3.0 μg/ml.
For environmental stability testing, 25 specimens were divided into arbitrary groups, with 0, 4 to 10, 11 to 15, 20 to 50, 150 to 180, and >250 μg/ml of PA-specific IgG. For each of these groups, the specimens were tested consecutively in quadruplicate.
Antigen source.
Recombinant PA (rPA) was used as the target molecule in the immunoassays. For the ELISA, rPA in 10 mM Tris buffer was obtained from BioPort Corp. To verify its authenticity, this preparation was tested by mass spectrometry, N-terminal sequencing, amino acid analysis, and immunoblot analysis (5, 15). The antigen used for the LFA was purchased from List Biological Laboratories, Inc. (Campbell, CA). As described below, parallel ELISA testing with the two antigen preparations was conducted to ensure that the results obtained from these assays were comparable. Specifically, with the same reagent concentrations and ELISA procedure, both rPA preparations were tested with 20 serum samples in replicate.
LFA.
The LFA device for the detection of anti-PA IgG was manufactured under contract with Arista Biologicals, Inc. (Allentown, PA), as a single-antigen direct sandwich assay. Some of the details in the preparation of the device were proprietary. The device consists of a plastic support to which a nitrocellulose membrane (thickness, 205 ± 1 μm) is mounted. Purified anthrax rPA (List Biological Laboratories, Inc.) was striped in the “test line” position (2 mg/ml), while a biotinylated bovine serum albumin conjugate was striped in the “control line” position (2 mg/ml). Gold particles (40 nm) individually conjugated to PA and streptavidin were prepared and mixed. The mixture of PA and streptavidin colloidal gold conjugate was dispensed onto a conjugate pad. The conjugate pad was then affixed to the test strip by overlapping the nitrocellulose membrane at its proximal end; the addition of a sample pad completed the assembly by overlapping onto the conjugate pad. Individual lateral flow devices were sealed in a foil pouch with a desiccant to protect the test strips.
Prior to the opening of the foil pouch and the conducting of the test, the devices were allowed to reach room temperature. To assess the antibody response to anthrax immunizations, 30 μl of serum and 3 drops of chase buffer (50 mM phosphate buffer containing 0.1% Igepal CA-720, 0.03% sodium dodecyl sulfate, 1% bovine serum albumin, and 0.1% sodium azide) were applied to the sample pad. When anthrax-specific antibodies were present in the specimen, they reacted with the test stripe, which contained rPA. Nonspecific antibodies were bound at the control line. Qualitative positive and negative results were visually obtained with the unaided eye after 20 min. Any staining visible at the test line was considered to be a positive reaction.
ELISA.
To compare the two rPA preparations, an ELISA was used according to the method of Quinn et al. (25), with modifications. Two hundred nanograms of rPA in 10 mM phosphate-buffered saline (PBS) (pH 7.4) was adsorbed onto the wells of an Immulon 2-HB polystyrene 96-well plate (Thermo Electron Corp., Milford, MA). The plates were incubated at 4°C overnight and subsequently washed with 10 mM PBS containing 0.1% Tween 20. The serum samples were diluted 1:100 in blocking buffer (PBS containing 5% skim milk and 0.1% Tween 20), added to antigen-coated wells, and incubated for 1 h at 37°C. Each serum sample (n = 20) was analyzed on each of two separate days. The plates were then washed and incubated with 100 μl of mouse anti-human PAN IgG-specific horseradish peroxidase conjugate (for all four subclasses of IgG; Hybridoma Reagent Laboratories, Baldwin, MD) (diluted 1:16,000) for 1 h at 37°C. Peroxidase activity was detected by the addition of a peroxidase substrate system (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD) that contained 2,2′-azino-di-(3-ethylbenzthiazoline-6-sulfonate) in glycine-citric acid buffer. The reactions were terminated by adding 1% sodium dodecyl sulfate, and the absorbance values were measured at 405 nm (490-nm reference filter) using a Thermo Labsystems MRX Revelation microtiter plate reader (Chantilly, VA).
Environmental exposure.
Simulated environmental exposure testing consisted of exposing the cassettes and buffer solution to conditions of thermal shock, high temperature/high RH, high temperature/low RH, and low temperature/low RH. Exposure temperatures and humidity levels were based on meteorological data for countries to which the supplies are most likely to be deployed. For each set of conditions, 112 cassettes and 12 ml of buffer solution were tested. With the exception of the thermal shock treatment, the materials were conditioned in an environmental chamber (model WP-216-THCM1-3-3; Thermotron Industries, Holland, MI) for 2 h at 23°C and 50% RH immediately prior to exposure testing. Following conditioning, the materials were subjected to one of the exposure conditions. After the completion of the exposure period, the materials were removed from the chamber and tested. A new group of devices was tested for each of the exposure conditions. Specific procedures for attaining the simulated environmental exposure conditions are provided below.
For thermal shock exposure, the materials were placed in the environmental chamber and conditioned for 2 h at 23°C and 35% RH. The temperature was then lowered to −40°C and the humidity allowed to reach in-chamber ambient levels (∼50%) over a 1.5-h period. The materials were maintained at that temperature and humidity for 4 h. The chamber temperature and humidity were then changed to 65°C and 35% RH over a 2-h period. This temperature and humidity condition was maintained for 4 h, after which the chamber temperature was adjusted to 23°C over a 1-h period and a new cycle begun. Five cycles were run without interruption. The materials were then removed from the chamber.
For the high-temperature/high-RH exposure, the chamber temperature and humidity were raised to 65°C and 75% RH over a 1-h period. The materials were then exposed to these conditions for a period of 96 h. At the end of the exposure period, the chamber conditions were returned to 23°C and 50% RH over a 1-h period. After 2 h under these conditions, the materials were removed.
For the high-temperature/low-RH exposure, the chamber temperature was adjusted to 65°C and the humidity allowed to reach in-chamber ambient levels (∼15%) over a 1-h period. The materials were exposed to these conditions for a period of 96 h. At the end of the exposure period, the chamber was returned to 23°C and 50% RH over a 1-h period. After 2 h under these conditions, the materials were removed.
For the low-temperature/low-RH exposure, the chamber temperature was adjusted to −40°C and the humidity allowed to reach in-chamber ambient levels (∼50%) over a 1.5-h period. The materials were exposed to these conditions for a period of 96 h. At the end of the exposure period, the chamber was returned to 23°C and 50% RH over a 1-h period. After 2 h under these conditions, the materials were removed.
Statistical analyses.
The diagnostic performance of the LFA was computed using the following definitions and anti-PA IgG ELISA results as the “gold standard”: TP, true-positive diagnostic test result; TN, true-negative diagnostic test result; FN, false-negative diagnostic test result; and FP, false-positive diagnostic test result. Sensitivity [TP/(TP + FN) × 100] was computed as the percentage of positive LFA test responses for subjects with positive ELISA responses. Specificity [TN/(FP + TN) × 100] was computed as the percentage of negative LFA test responses for subjects with negative ELISA responses. Diagnostic efficiency [(TP + TN)/(TP + FP + TN + FN) × 100] describes the percentage of subjects correctly classified as having positive and negative anti-PA IgG results by LFA. Receiver operating characteristic (ROC) curves were calculated (SigmaPlot 10; Systat Software, Inc., San Jose, CA) as previously described (2). An averaging method was used to minimize bias in cases in which sera had anti-PA IgG results below the limit of detection of the ELISA (10). A Kolmogorov-Smirnov test (SigmaStat; Systat Software, Inc.) was used to investigate whether the data distribution was consistent with normality. Simple linear regression was used to evaluate the correlation between log10 anti-PA IgG values, percentages of positive LFA responses, numbers of AVA injections, and levels of PA-specific IgG detected by rPA (obtained from BioPort Corp. and List Biological Laboratories, Inc.). The anti-PA IgG threshold for maximal diagnostic efficiency was determined using logistic regression, with the equation y = 0.5 (SAS 9.1; SAS Institute, Inc., Cary, NC). Differences in rates of positive test results between the laboratory condition and the combined environmental test exposures for the groups with PA-specific IgG levels of 4 to 10, 11 to 15, and 20 to 50 μg/ml were analyzed using exact chi-square methods (which are necessary when cell frequencies are at or near 0) (StatXact; Cytel Software Corp.) for differences between two independent proportions.
RESULTS
ELISA.
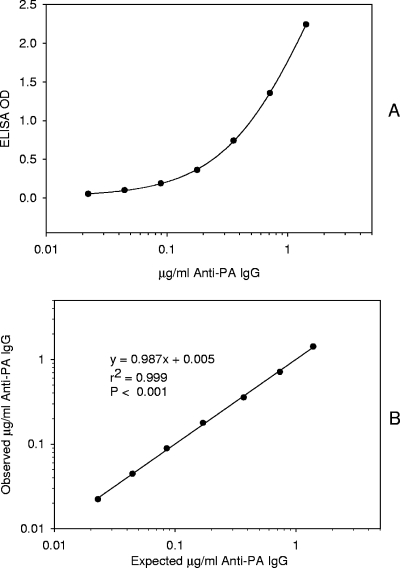
The ELISA-measured anti-PA was used to evaluate the performance of the LFA. When the four-parameter logistic-log curve fitting model was applied to the ELISA data (25), the MDC was calculated to be 3 μg/ml anti-PA IgG (for 1:100-diluted serum) (Fig. 1). At a 1:100 dilution, the linearity of the standard serum curve occurred from the MDC to 142 μg/ml anti-PA IgG.
FIG. 1.
Representative four-parameter logistic regression of μg/ml anti-PA IgG versus ELISA optical density (OD). (A) Data are the means of triplicates. (B) Linear regression of the observed interpolated results from the four-parameter logistic regression fit compared to concentrations of anti-PA IgG added (AVR414 standard sera).
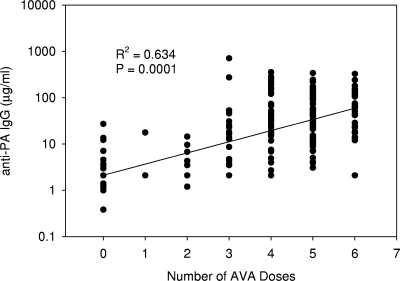
The mean anti-PA IgG concentration measured by ELISA for the unvaccinated group was below the ELISA MDC (3 μg/ml anti-PA IgG), while for the vaccinated group it was 57.2 ± 85.2 μg/ml (standard deviation) anti-PA IgG. There was a significant (P = 0.0001; R2 = 0.634) linear trend between the number of AVA injections and the log10 anti-PA IgG concentration (data were logarithmically transformed as the raw data failed the Kolmogorov-Smirnov test). This relationship was as follows: number of μg/ml anti-PA IgG = 0.326 + 0.241 × number of AVA doses (Fig. 2).
FIG. 2.
Linear regression of log10 anti-PA IgG (ELISA) concentration versus number of doses of AVA received (n = 326).
LFA.
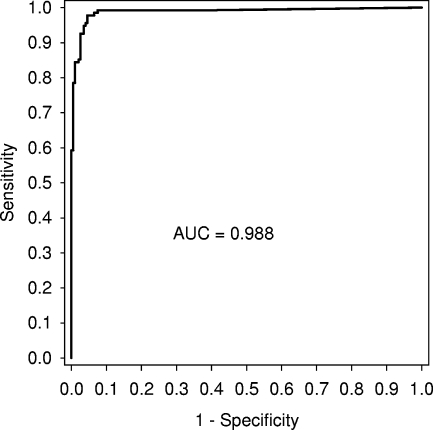
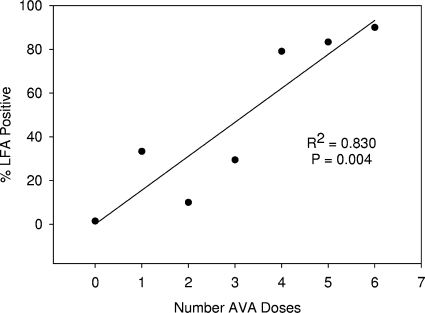
The anti-PA IgG concentration measured by ELISA yielded maximal diagnostic efficiency for the LFA of 11 μg/ml (efficiency = 96%; 95% confidence interval [CI], 94% to 98%). At this cutoff, the LFA diagnostic specificity and sensitivity (based on anti-PA IgG ELISA) were 98% (CI, 97% to 100%) and 92% (CI, 88% to 97%), respectively (Table 1). ROC analysis yielded an area under the curve of 0.988 (CI, 0.976 to 1.00) (Fig. 3). When the percentage of subjects with LFA-positive sera was interrogated to determine the percentage of individuals at a certain AVA injection having agreement between LFA and ELISA results, it was found that there was a significant linear trend (P = 0.004; R2 = 0.830), with the following relationship: percent LFA positive = 0.071 + (15.5 × number of AVA doses) (Fig. 4).
TABLE 1.
Diagnostic performance of PA-specific IgG, using vaccination status as the criterion to determine the reactivity of serum test samples
| Sample result or performance parametera | No. of samples | Value for test (%) |
|---|---|---|
| Sample results | ||
| TP | 132 | |
| TN | 189 | |
| FP | 3 | |
| FN | 11 | |
| Total | 335 | |
| Performance parameters | ||
| DSPb | 98.4 | |
| DSNc | 92.3 | |
| PPVd | 97.8 | |
| NPVe | 94.5 | |
| DEf | 95.8 |
Abbreviations: TP, true positives; TN, true negatives; FP, false positives; FN, false negatives; DSP, diagnostic specificity; DSN, diagnostic sensitivity; PPV, positive predictive value; NPV, negative predictive value; DE, diagnostic efficiency.
DSP = TN/(TN + FP).
DSN = TP/(TP + FN).
PPV = TP/(TP + FP).
NPV = TN/(TN + FN).
DE = (TP + TN)/(TP + FP + TN + FN).
FIG. 3.
ROC curve (sensitivity versus 1 − specificity) of anthrax LFA results based on ELISA anti-PA IgG concentrations of ≥3.0 μg/ml as the gold standard. The area under the ROC curve (AUC) was 0.988.
FIG. 4.
Linear regression of percentage of positive LFA results (based on ELISA) versus number of doses of AVA received (n = 326).
rPA comparison.
To determine whether the two rPA preparations yielded similar results, 20 serum samples were tested with each antigen preparation in replicate. For the ELISA results, we considered consistencies among replicate values. For each of the rPA preparations, there was a strong positive correlation (P < 0.0001; R2 > 0.996) between the replicate values. Moreover, there was a strong positive correlation (P < 0.0001; R2 = 0.983) between the mean amounts of anti-PA IgG detected by the two rPA preparations. The linear regression equation for predicting the ELISA optical densities of the two rPA preparations was y = −0.01703 + 1.1108x. The y intercept was not significantly different from 0. The slope of 1.11 (95% CI, 1.042 to 1.178) indicates that one rPA preparation may be slightly more reactive (data not shown). Taken together, these results indicate that use of ELISA data to estimate the cutoff value of the LFA is acceptable.
Environmental testing.
For all environmental conditions, the LFA results were identical for samples containing <4 or >50 μg/ml PA-specific IgG (Table 2). For sera with 4 to 10 μg/ml PA-specific IgG, there was not a significant effect of environmental exposure on the devices, as the false-positive rates were similar to those observed with devices that were maintained under laboratory conditions. Similarly, for PA-specific antibody concentrations of 11 to 15 and 20 to 50 μg/ml, there was no statistically significant evidence that environmental manipulations degraded the performance of the device.
TABLE 2.
Environmental stability testing of the anthrax lateral flow assay, using samples with ranges of concentrations of PA-specific IgG
| Environmental treatment | Valuea for indicated range of concn (μg/ml) of PA-specific IgG
|
|||||
|---|---|---|---|---|---|---|
| 0 | 4-10 | 11-15 | 20-50 | 150-180 | >250 | |
| Thermal shock | 0/20 | 3/12 | 7/8 | 20/20 | 20/20 | 20/20 |
| High temp/high humidity | 0/20 | 3/12 | 3/8 | 18/20 | 20/20 | 20/20 |
| High temp/low humidity | 0/20 | 2/12 | 3/8 | 20/20 | 20/20 | 20/20 |
| Low temp/low humidity | 0/20 | 7/12 | 8/8 | 20/20 | 20/20 | 20/20 |
| Laboratory conditions | 0/20 | 6/12 | 5/8 | 20/20 | 20/20 | 20/20 |
No. of positive reactions/no. of devices tested.
DISCUSSION
Over 7 million AVA immunizations have been administered to over 1.8 million troops. In late 2004, the U.S. courts placed an injunction against mandatory administration of AVA to military personnel. Early in 2007, all the services reimplemented the Anthrax Vaccine Immunization Program. As part of that implementation, each individual continued the vaccination series where he or she left off. Given this, the administration of the next AVA dose was delayed up to 24 months.
The level of anti-PA IgG which is protective against anthrax exposure in humans has not been established (24). Protection against cutaneous or inhalational anthrax exposure in AVA-treated individuals is inferred upon receipt of the AVA vaccine. Anti-PA antibody levels are not routinely measured either after the AVA injection series or after booster injections. The major reason for this is that anti-PA IgG measurements are laboratory based, necessitating specific equipment and trained personnel (3, 25).
Biagini et al. (4) reported on an LFA which displayed 100% sensitivity and specificity and a positive cutoff of 2.8 μg/ml compared to ELISA anti-PA IgG values. These values ranged from nondetectable to 340 μg/ml for sera from vaccinees who received the FDA-cleared AVA regimen. In the present report, anti-PA IgG values ranged from nondetectable to 708 μg/ml with the vaccinated subjects receiving between one and six AVA injections. The anthrax LFA had a 92% diagnostic sensitivity and 98% diagnostic specificity at a maximal diagnostic efficiency cutoff (based on ELISA anti-PA IgG) of 11 μg/ml. The ROC area under the curve at this concentration was 0.988, suggesting that the LFA is an extremely accurate diagnostic test.
Choosing the optimal decision cutoff is a compromise between optimizing sensitivity and specificity. The optimal decision threshold reported in the present analysis assumed that the cost of a false-positive result and the cost of a false-negative result were equal. This is not the case in outcomes of exposure to anthrax when preexisting immunity is not adequate for protection. Further complicating the analysis is that the level of immunity necessary to protect from the consequences of anthrax exposure is not known, the level of protection in a vaccinated individual is related to the absorbed dose of anthrax spores (a high enough dose of anthrax could overcome preexisting immunity), and the level of putative protective immunity is based on the number of doses of AVA received. This was shown in the present work by the orthogonal relationships between anti-PA IgG concentrations, the percentages of subjects found positive for anti-PA IgG by LFA, and the numbers of doses of AVA received by the subjects.
Like those of other diagnostic tests, the components of the LFA are susceptible to environmental conditions and gradually deteriorate, even under ideal storage. Most manufacturers recommend that their products be stored at 4 to 30°C, and the expiration dates assume that these conditions are met. For field applications, temperatures above this level are often unavoidable. Exposure of rapid diagnostic tests to constant temperatures above 45°C has been reported to result in (i) warping of the test strip, (ii) a defect in the nitrocellulose membrane that hampers sample flow, (iii) a failing control line (8), and (iv) lost diagnostic sensitivity (8, 18). For the rapid diagnosis of disease, it appears that the effect of freezing needs to be considered on a case-by-case basis. For instance, storage for 21 days at −20°C and −80°C yielded results comparable to those observed at 4°C for samples with Yersinia pestis F1 antigen (7). Conversely, freeze-thaw with a rapid test for malaria caused a reduction in sensitivity at lower parasite densities (8). Humidity has also been reported to adversely affect the performance of rapid diagnostic tests as a result of a failed control line (7) or a decrease in diagnostic sensitivity (19).
While these studies with constant temperatures are indicators of environmental effects on rapid diagnostic tests, they may not reflect test performance in the field, as the ambient temperature in the field could fluctuate greatly within a 24-hour period. With this in mind, we exposed the LFA to fluctuating temperatures and RHs. The diagnostic performance of the LFAs in the present experiment was not affected by temperatures/humidities of 65°C and 15% to 75% RH and −40°C and ∼50% RH. Nonetheless, we anticipate that subsequent field/clinical trials will need to evaluate numerous temperature and humidity cycles, as there may be poorly controlled transport conditions and infrequent resupply to remote sites.
With this rapid point-of-care test, it would be easy to screen vaccinated individuals prior to deployment to ensure that antibody levels of deferred vaccinees have achieved an appropriate concentration (i.e., a level of PA-specific IgG that is comparable to those in recipients of an uninterrupted AVA regimen). When a discrepancy is observed, additional immunizations may be recommended.
With the advancement of technology, it is probable that the anthrax vaccine regimen will change. A recent human clinical trial indicated that administration of the currently licensed vaccine may be changed from subcutaneous to intramuscular. As well, the number of priming doses may be reduced (J. Wright, A. Seright, J. Wheeling, S. Parker, M. Mulligan, J. Babcock, W. Keitel, H. E. Sahly, G. Poland, R. Jacobson, H. Keyserling, B. Plikaytis, S. Martin, C. Rose, C. P. Quinn, and N. Marano, presented at the International Conference on Bacillus anthracis, B. cereus, and B. thuringiensis, Oslo, Norway, 17 to 21 June 2007). For new anthrax vaccine candidates, it is expected that PA will be a component, as anti-PA is a major correlate of protection. Recently, Selinsky et al. have identified a positive quality control and standard reference sera and developed an ELISA to measure anthrax lethal factor (LF)-binding IgG (26). With this, they have elucidated the antibody response and anticipate an assessment of the contribution of the LF-specific immune response to protection against anthrax. As the correlates of protection against B. anthracis continue to be defined, it may be useful to develop a multiplexed LFA, such that a single sample could be simultaneously screened for anti-PA and anti-LF antibodies. Moreover, it may be possible to modify this LFA to utilize noninvasively collected specimens, because the salivary PA-specific IgG response parallels that observed in serology (5).
Acknowledgments
This work was supported in part by an interagency agreement between NIOSH and NIEHS (Y1-ES-0001; Clinical Immunotoxicity). Support from the Center for Commercialization of Advanced Technology (solicitation no. 2004-01G) also facilitated this study.
We appreciate the provision by BioPort Corp. of purified rPA and specific antibodies developed against it. We offer special thanks to M. E. Cohen and C. K. Chang for providing statistical consulting and skilled technical assistance, respectively.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or positions of the Department of the Navy, the Department of Defense, or the U.S. Government. The use of commercially available products does not imply the endorsement of these products or preferences to other, similar products on the market. We are military service members or employees of the U.S. Government. This work was prepared as part of our official duties. Mention of a product or company name does not constitute endorsement by NIOSH. The content and conclusions of this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Footnotes
Published ahead of print on 5 March 2008.
REFERENCES
- 1.Baillie, L. W. J., K. Fowler, and P. C. B. Turnbull. 1999. Human immune responses to the UK human anthrax vaccine. J. Appl. Microbiol. 87:306-308. [DOI] [PubMed] [Google Scholar]
- 2.Biagini, R. E., E. F. Krieg, L. E. Pinkerton, and R. G. Hamilton. 2001. Receiver operating characteristics analyses of Food and Drug Administration-cleared serological assays for natural rubber latex-specific immunoglobulin E antibody. Clin. Diagn. Lab. Immunol. 8:1145-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biagini, R. E., D. L. Sammons, J. P. Smith, B. A. MacKenzie, C. A. F. Striley, V. Semenova, E. Steward-Clark, K. Stamey, A. E. Freeman, C. P. Quinn, and J. E. Snawder. 2004. Comparison of a multiplexed fluorescent covalent microsphere immunoassay and an enzyme-linked immunosorbent assay for measurement of human immunoglobulin G antibodies to anthrax toxins. Clin. Diagn. Lab. Immunol. 11:50-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biagini, R. E., D. L. Sammons, J. P. Smith, B. A. MacKenzie, C. A. F. Striley, J. E. Snawder, S. A. Robertson, and C. P. Quinn. 2006. Rapid, sensitive, and specific lateral-flow immunochromatographic device to measure anti-anthrax protective antigen immunoglobulin G in serum and whole blood. Clin. Vaccine Immunol. 13:541-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bienek, D. R., C. K. Chang, and M. E. Cohen. 2007. Detection of anti-protective antigen salivary IgG antibodies in recipients of the US licensed vaccine. Vaccine 25:5978-5984. [DOI] [PubMed] [Google Scholar]
- 6.Reference deleted.
- 7.Chanteau, S., L. Rahalison, L. Ralafiarisoa, J. Foulon, M. Ratsitorahina, L. Ratsifasoamanana, E. Carniel, and F. Nato. 2003. Development and testing of a rapid diagnostic test for bubonic and pneumonic plague. Lancet 361:211-216. [DOI] [PubMed] [Google Scholar]
- 8.Chiodini, P. L., K. Bowers, P. Jorgensen, J. W. Barnwell, K. K. Grady, J. Luchavez, A. H. Moody, A. Cenizal, and D. Bell. 2007. The heat stability of Plasmodium lactate dehydrogenase-based and histidine-rich protein 2-based malaria rapid diagnostic tests. Trans. R. Soc. Trop. Med. Hyg. 101:331-337. [DOI] [PubMed] [Google Scholar]
- 9.Fellows, P. F., M. K. Linscott, B. E. Ivins, M. L. M. Pitt, C. A. Rossi, P. H. Gibbs, and A. M. Friedlander. 2001. Efficacy of a human anthrax vaccine in guinea pigs, rabbits, and rhesus macaques against challenge by Bacillus anthracis isolates of diverse geographical origin. Vaccine 19:3241-3247. [DOI] [PubMed] [Google Scholar]
- 10.Hornung, R., and L. Reed. 1990. Estimate of average concentration in the presence of non-detectable values. Appl. Occup. Environ. Hyg. 5:46-51. [Google Scholar]
- 11.Ivins, B. E., P. F. Fellows, and G. O. Nelson. 1994. Efficacy of a standard human anthrax vaccine against Bacillus anthracis spore challenge in guinea-pigs. Vaccine 12:872-874. [DOI] [PubMed] [Google Scholar]
- 12.Ivins, B. E., M. L. M. Pitt, P. F. Fellows, J. W. Farchaus, G. E. Benner, D. M. Waag, S. F. Little, G. W. Anderson, P. H. Gibbs, and A. M. Friedlander. 1998. Comparative efficacy of experimental anthrax vaccine candidates against inhalation anthrax in rhesus macaques. Vaccine 16:1141-1148. [DOI] [PubMed] [Google Scholar]
- 13.Ivins, B. E., S. L. Welkos, S. F. Little, M. H. Crumrine, and G. O. Nelson. 1992. Immunization against anthrax with Bacillus anthracis protective antigen combined with adjuvants. Infect. Immun. 60:662-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joellenbeck, L. M., L. L. Zwanziger, J. S. Durch, and B. L. Strom (ed.). 2002. The anthrax vaccine. Is it safe? Does it work? National Academy Press, Washington, DC. [PubMed]
- 15.Lininger, L. A., M. E. Cullum, M. B. Lyles, and D. R. Bienek. 2007. The impact of incomplete vaccination schedules on the magnitude and duration of protective antigen-specific IgG responses in recipients of the US licensed anthrax vaccine. Vaccine 25:1619-1625. [DOI] [PubMed] [Google Scholar]
- 16.Little, S. F., B. E. Ivins, P. F. Fellows, M. L. M. Pitt, S. L. W. Norris, and G. P. Andrews. 2004. Defining a serological correlate of protection in rabbits for a recombinant anthrax vaccine. Vaccine 22:422-430. [DOI] [PubMed] [Google Scholar]
- 17.Little, S. F., and G. B. Knudson. 1986. Comparative efficacy of Bacillus anthracis live spore vaccine and protective antigen vaccine against anthrax in the guinea pig. Infect. Immun. 52:509-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lon, C. T., S. Alcantara, J. Luchavez, R. Tsuyuoka, and D. Bell. 2005. Positive control wells: a potential answer to remote-area quality assurance of malaria rapid diagnostic tests. Trans. R. Soc. Trop. Med. Hyg. 99:493-498. [DOI] [PubMed] [Google Scholar]
- 19.Nato, F., A. Phalipon, L. P. T. Nguyen, T. T. Diep, P. Sansonetti, and Y. Germani. 2007. Dipstick for rapid diagnosis of Shigella flexneri 2a in stool. PLoS ONE 2:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pitt, M. L., S. F. Little, B. E. Ivins, P. Fellows, J. Barth, J. Hewetson, P. Gibbs, M. Dertzbaugh, and A. M. Friedlander. 2001. In vitro correlate of immunity in a rabbit model of inhalational anthrax. Vaccine 19:4768-4773. [DOI] [PubMed] [Google Scholar]
- 21.Pitt, M. L. M., S. Little, B. E. Ivins, P. Fellows, J. Boles, J. Barth, J. Hewetson, and A. M. Friedlander. 1999. In vitro correlate of immunity in an animal model of inhalational anthrax. J. Appl. Microbiol. 87:304. [DOI] [PubMed] [Google Scholar]
- 22.Pittman, P. R., D. Hack, J. Mangiafico, P. Gibbs, K. T. McKee, A. M. Friedlander, and M. H. Sjogren. 2002. Antibody response to a delayed booster dose of anthrax vaccine and botulinum toxoid. Vaccine 20:2107-2115. [DOI] [PubMed] [Google Scholar]
- 23.Pittman, P. R., J. A. Mangiafico, C. A. Rossi, T. L. Cannon, P. H. Gibbs, G. W. Parker, and A. M. Friedlander. 2000. Anthrax vaccine: increasing intervals between the first two doses enhances antibody response in humans. Vaccine 19:213-216. [DOI] [PubMed] [Google Scholar]
- 24.Pittman, P. R., S. L. Norris, J. G. Barrera Oro, D. Bedwell, T. L. Cannon, and K. T. McKee. 2006. Patterns of antibody response in humans to the anthrax vaccine adsorbed (AVA) primary (six-dose) series. Vaccine 24:3654-3660. [DOI] [PubMed] [Google Scholar]
- 25.Quinn, C. P., V. A. Semenova, C. M. Elie, S. Romero-Steiner, C. Greene, H. Li, K. Stamey, E. Steward-Clark, D. S. Schmidt, E. Mothershed, J. Pruckler, S. Schwartz, R. F. Benson, L. O. Helsel, P. F. Holder, S. E. Johnson, M. Kellum, T. Messmer, W. L. Thacker, L. Besser, B. D. Plikaytis, T. H. Taylor, A. E. Freeman, K. J. Wallace, P. Dull, J. Sejvar, E. Bruce, R. Moreno, A. Schuchat, J. R. Lingappa, N. Marano, S. K. Martin, J. Walls, M. Bronsdon, G. M. Carlone, M. Bajani-Ari, D. A. Ashford, D. S. Stephens, and B. A. Perkins. 2002. Specific, sensitive, and quantitative enzyme-linked immunosorbent assay for human immunoglobulin G antibodies to anthrax toxin protective antigen. Emerg. Infect. Dis. 8:1103-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selinsky, C. L., V. D. Whitlow, L. R. Smith, D. C. Kaslow, and H. M. Horton. 2007. Qualification and performance characteristics of a quantitative enzyme-linked immunosorbent assay for human IgG antibodies to anthrax lethal factor antigen. Biologicals 35:123-129. [DOI] [PubMed] [Google Scholar]
- 27.Semenova, V. A., E. Steward-Clark, K. L. Stamey, T. H. Taylor, D. S. Schmidt, S. K. Martin, N. Marano, and C. P. Quinn. 2004. Mass value assignment of total and subclass immunoglobulin G in a human standard anthrax reference serum. Clin. Diagn. Lab. Immunol. 11:919-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turnbull, P. C. B., M. G. Broster, J. A. Carman, R. J. Manchee, and J. Melling. 1986. Development of antibodies to protective antigen and lethal factor components of anthrax toxin in humans and guinea pigs and their relevance to protective immunity. Infect. Immun. 52:356-363. [DOI] [PMC free article] [PubMed] [Google Scholar]