Abstract
CpG oligodeoxynucleotides (CpG ODN) have been shown to have potent adjuvant activity for a wide range of antigens. The purpose of this study was to determine the potential benefit of using liposomes as a delivery vehicle to enhance the adjuvant activity of CpG ODN with Leishmania major stress-inducible protein 1 (LmSTI1) antigen in induction of the Th1 response in a murine model of leishmaniasis. BALB/c mice were immunized subcutaneously three times in 3-week intervals with liposomal recombinant LmSTI1 (Lip-rLmSTI1), rLmSTI1 coencapsulated with CpG ODN in a liposome (Lip-rLmSTI1-CpG ODN), rLmSTI1 plus CpG ODN in phosphate-buffered saline (PBS), rLmSTI1 plus non-CpG ODN in PBS, rLmSTI1 in PBS, empty liposome, or PBS. The intensity of infection induced by L. major promastigote challenge was measured by footpad swelling. A significant (P < 0.001) inhibition of infection in mice immunized with Lip-rLmSTI1-CpG ODN was shown compared to the other groups, and no parasite was detected in the spleens of this group 14 weeks after challenge. The highest immunoglobulin G2a (IgG2a) titer and the highest IgG2a/IgG1 ratio were also shown in the sera of mice immunized with Lip-rLmSTI1-CpG ODN before and 14 weeks after challenge. The results indicated the superiority of CpG ODN in its liposomal form over its soluble form to induce the Th1 response when used in association with rLmSTI1 antigen. It seems that using a liposome delivery system carrying CpG ODN as an adjuvant coencapsulated with Leishmania antigen plays an important role in vaccine development strategies against leishmaniasis.
Leishmaniasis is caused by different species of Leishmania, and leishmaniasis control measures are not always successful. Available drugs are toxic and show limited efficacy (10). In leishmaniasis, recovery and protection against further infection are mainly dependent on induction of a Th1-type immune response (36), which justifies the search for an effective vaccine against leishmaniasis. Development of an effective anti-Leishmania vaccine is theoretically possible due to the fact that recovery from cutaneous leishmaniasis or leishmanization induces long-lasting protection against further infection, yet there is no vaccine available against any form of human leishmaniasis (18, 25, 29, 45).
In recent years, the results of phase 3 clinical trials of first-generation Leishmania vaccines showed limited efficacy in some trials (17, 27, 29), possibly due to the lack of a suitable adjuvant. Recombinant Leishmania major stress-inducible protein 1 (rLmSTI1), alone or combined with thiol-specific antioxidant or Leishmania elongation initiation factor and delivered as a DNA-based vaccine or mixed with different adjuvants, showed protection against leishmaniasis in murine and nonhuman primate models (4, 9, 28, 37). However, Leish-111f (a tandem-linked polyprotein composed of a thiol-specific antioxidant, Leishmania elongation initiation factor, and LmSTI1) formulated in either monophosphoryl lipid A with squalene or Adjuprime failed to protect vaccinated dogs against experimental canine leishmaniasis (12). It seems that using an appropriate adjuvant is a key factor in induction of the immune response required for protection.
Liposomes are artificial closed vesicles composed of concentric lipid bilayers separated by aqueous domains and are utilized as delivery systems for drugs, peptides, proteins, and DNA. Liposomes are used as immunoadjuvants to induce immune responses to various antigens (3, 32). Liposomes are safe, biodegradable, FDA-approved compounds. The composition of a liposome might be designed to preferentially induce a humoral or cellular immune response against a specific antigen (3).
Unmethylated CpG motifs are present at a high frequency in bacterial but not in vertebrate DNA. CpG motifs are recognized by Toll-like receptor 9 expressed by antigen-presenting cells, plasmacytoid dendritic cells, and B cells (22). Synthetic oligodeoxynucleotides (ODN) expressing CpG motifs mimic the immunostimulatory activity of bacterial DNA and mediate a variety of immunological functions, including stimulation of a Th1 or Th2 immune response, based on their sequence; therefore, CpG ODN are able to act as immunoadjuvants to accelerate and boost antigen-specific immune responses 5- to 500-fold (21). The innate immune response elicited by CpG ODN peaks within days and is maintained for about 2 weeks (20). The immunoadjuvant effect of CpG ODN is optimized by maintaining close physical contact between the antigen and CpG ODN (21). Considering the difficulty in the chemical conjugation procedure and the possibility of altering the three-dimensional structure of an antigen, thereby changing its immunogenicity, it is of particular interest to explore other means of CpG ODN-antigen association (23). One strategy to extend the duration of CpG activity is the encapsulation of CpG ODN in liposomes (20). Liposomes have been used as a delivery vehicle to provide a close association of CpG ODN with antigen, and they enhanced the immune response (21).
In the current study, the extent of protection and type of immune response generated in susceptible BALB/c mice immunized with a liposomal form of rLmSTI1 coencapsulated with CpG ODN were assessed and compared with those for the free form of rLmSTI1 and CpG ODN.
MATERIALS AND METHODS
Animals, parasites, soluble Leishmania antigen (SLA), and ODN.
Female BALB/c mice aged 6 to 8 weeks were purchased from Pasteur Institute (Tehran, Iran). The mice were maintained in the animal house of the Biotechnology Research Center and fed with tap water and laboratory pellet chow (Khorassan Javane Co., Mashhad, Iran). Animals were housed in a colony room with a 12-h-12-h light-dark cycle at 21°C and had free access to water and food. Experiments were carried out according to the Mashhad University of Medical Sciences Ethical Committee Acts.
The L. major strain (MRHO/IR/75/ER) and preparation used in this experiment were the same as those described previously (15).
SLA was prepared according to a previously described method (14). Briefly, promastigotes were washed three times in cold phosphate-buffered saline (PBS) (pH 7.5). The suspension was freeze-thawed three times and then sonicated at 4°C with 20 cycles of 2-s blasts, followed by centrifugation at 100,000 × g for 2 h at 4°C. The supernatant containing SLA was passed through a 0.22-μm filter and stored at −70°C until used. The protein concentration was determined using the Lowry protein assay method (24).
The CpG ODN (Microsynth, Balgach, Switzerland) used in this study, termed CpG ODN 1826, was a 20-mer (5′-TCC ATG ACG TTC CTG ACG TT-3′) with a nuclease-resistant phosphorothioate backbone containing two CpG motifs (in bold) known to have effective immunostimulatory properties on the murine immune system (6, 44). A non-CpG ODN (5′-TCC AGG ACT TCT CTC AGG TT-3′) was used as a control.
Encapsulation of rLmSTI1 and CpG ODN into liposomes.
Liposomes containing rLmSTI1 and CpG ODN (Lip-rLmSTI1-CpG ODN) were prepared by the dehydration-rehydration vesicle method (19), with some modifications, as described previously (14). Briefly, the lipid phase, consisting of distearoylphosphatidylcholine (16 μM; Avanti Polar Lipids) and cholesterol (Avanti Polar Lipids) (2:1 molar ratio), was dissolved in chloroform-methanol (2:1 [vol/vol]) in a round-bottomed flask. The solvent was removed by rotary evaporation, and then the lipid film was freeze-dried overnight to ensure total removal of the solvent. Empty multilamellar vesicles were prepared by lipid film hydration and converted to 100-nm small unilamellar vesicles by use of a mini extruder (Avestin, Canada). rLmSTI1 and CpG ODN were then added to empty small unilamellar vesicle liposomes, dried with a freeze-drier overnight, and rehydrated as described before (14). Unentrapped rLmSTI1 and CpG ODN were separated from entrapped ones by centrifugation at 14,000 × g for 15 min at 4°C and subsequently washed three times with PBS, and the pellet was resuspended in PBS. To prepare liposomes containing only rLmSTI1 (Lip-rLmSTI1) and control empty liposomes, the same procedure was followed, except that the CpG ODN was omitted for Lip-rLmSTI1 and CpG ODN and rLmSTI1 were omitted for control empty liposomes.
An optical microscope (Olympus, Germany) was used to check the morphological features of liposomes. The mean diameter and particle size distribution of liposomes were determined by a particle size analyzer (Klotz, Germany).
Encapsulation efficiency of rLmSTI1 and CpG ODN into liposomes.
The efficiencies of incorporation (percent entrapment) of rLmSTI1 and CpG ODN into liposomes were determined using the Bradford protein assay and UV absorption at 260 nm, respectively. The analysis was performed on supernatants following PBS washes. The percentage of entrapment was calculated as described before (15). The concentrations of rLmSTI1 and CpG ODN in the liposomes were adjusted to 2 μg and 10 μg/50 μl, respectively, after purification and calculation of percent entrapment.
SDS-PAGE analysis of rLmSTI1 and liposomal formulations.
Analytical sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out to characterize the purified rLmSTI1 and to determine qualitatively whether Lip-rLmSTI1-CpG ODN and Lip-rLmSTI1 contained rLmSTI1 after purification. The gel consisted of a running gel (12% [wt/vol] acrylamide) and a stacking gel (3% [wt/vol] acrylamide). The gel thickness was 1 mm. The electrophoresis buffer was 25 mM Tris, 192 mM glycine, 0.1% SDS, pH 8.3. Electrophoresis was carried out at a 150-V constant voltage for 45 min. The gels were stained by the silver staining method for protein detection.
Mouse immunization.
Different groups of female BALB/c mice (10 mice per group) were immunized subcutaneously (s.c.) three times in 3-week intervals with one of the following: Lip-rLmSTI1-CpG ODN (2 μg rLmSTI1 and 10 μg CpG ODN/50 μl liposome/mouse), Lip-rLmSTI1 (2 μg rLmSTI1/50 μl liposome/mouse), rLmSTI1 in PBS (2 μg rLmSTI1/50 μl PBS/mouse), rLmSTI1 in PBS (2 μg rLmSTI1/50 μl PBS/mouse) plus CpG ODN in PBS (10 μg CpG ODN/50 μl PBS/mouse), rLmSTI1 in PBS (2 μg rLmSTI1/50 μl PBS/mouse) plus non-CpG ODN in PBS (10 μg non-CpG ODN/50 μl PBS/mouse), PBS, or control empty liposomes.
Challenge with L. major promastigotes.
The immunized mice (seven per group) were challenged s.c. in the left footpad 3 weeks after the last booster injection with L. major promastigotes (MRHO/IR/75/ER) harvested at stationary phase (1.5 × 106 in a 50-μl volume), and as a control, right footpads were injected with the same volume of PBS. The development of lesions was recorded by weekly measurement of footpad thickness using a metric caliper (Mitutoyo Measuring Instruments, Japan). Grading of lesion size was done by subtracting the thickness of the uninfected contralateral footpad from that of the infected one.
Quantitative parasite burden after challenge.
The numbers of viable L. major parasites in the spleens of mice were enumerated by a limiting dilution assay (41) as described previously (14). Briefly, the mice were sacrificed 14 weeks after challenge; the spleens were aseptically removed and homogenized in RPMI medium-fetal calf serum. The homogenate was diluted with the medium in eight serial 10-fold dilutions, and then 150 μl of each dilution was placed in each well of 96-well microtiter plates containing a solid layer of rabbit blood agar and incubated at 25°C for 10 days. The number of viable parasites per spleen was determined by ELIDA software, a statistical method for limiting dilution assay (40).
Antibody isotype assay.
Blood samples were collected from the mice before and 14 weeks after challenge, and the sera were separated and kept at −20°C. Sera were used to titrate anti-SLA immunoglobulin G (IgG), IgG1, and IgG2a by using an enzyme-linked immunosorbent assay (ELISA) method as described before (14).
Statistical analysis.
A one-way analysis of variance statistical test was used to assess the significance of the differences among various groups. In cases of significant F values, the multiple-comparison Tukey test was used to compare the means of different treatment groups. P values of <0.05 were considered statistically significant.
RESULTS
Liposome characterization.
The liposomes were morphologically multilamellar vesicles, as observed under an optical microscope. The mean diameters calculated by a particle size analyzer were 1.11 ± 0.84, 1.07 ± 0.75, and 1.23 ± 0.9 μm (n = 3) for Lip-rLmSTI1, Lip-rLmSTI1-CpG ODN, and empty liposomes, respectively. The entrapment of rLmSTI1 in Lip-rLmSTI1 was 79% ± 4%. The entrapment of rLmSTI1 and CpG ODN in Lip-rLmSTI1-CpG ODN was estimated to be 74% ± 4% and 44% ± 5%, respectively.
Characterization of rLmSTI1 in liposomes by SDS-PAGE.
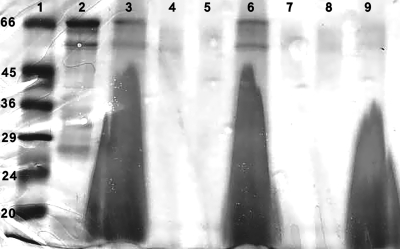
SDS-PAGE analysis of rLmSTI1 revealed a single protein band of the expected size (43) (Fig. 1, lane 2). SDS-PAGE analysis of Lip-rLmSTI1 and Lip-rLmSTI1-CpG ODN after removal of free rLmSTI1 in the supernatant revealed rLmSTI1 incorporation into the liposomes (Fig. 1, lanes 3 and 6). The amounts of rLmSTI1 in supernatants of Lip-rLmSTI1 and Lip-rLmSTI1-CpG ODN after two and three washes with PBS are shown in Fig. 1, lanes 4, 5, 7, and 8. A number of faint, lower-molecular-weight bands assumed to be proteolytic breakdown products of rLmSTI1 were also seen, similar to what was described before (43).
FIG. 1.
SDS-PAGE analysis of purified rLmSTI1, Lip-rLmSTI1, Lip-rLmSTI1-CpG ODN, and empty liposome. Lane 1, low-range protein standard (Sigma); lane 2, purified rLmSTI1; lane 3, Lip-rLmSTI1; lane 4, supernatant of Lip-rLmSTI1 after two washes with PBS; lane 5, supernatant of Lip-rLmSTI1 after three washes with PBS; lane 6, Lip-rLmSTI1-CpG ODN; lane 7, supernatant of Lip-rLmSTI1-CpG ODN after two washes with PBS; lane 8, supernatant of Lip-rLmSTI1-CpG ODN after three washes with PBS; lane 9, empty liposome.
Challenge infection results.
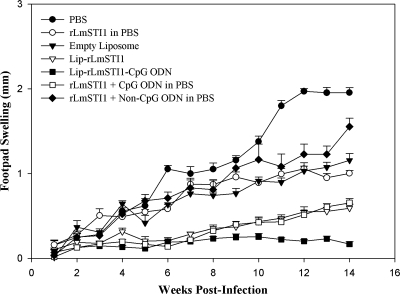
To assess the rate of protection, the immunized mice were challenged with L. major promastigotes inoculated s.c. into the footpad, and lesion development was checked and recorded weekly (Fig. 2). The amounts of footpad swelling in immunized and control groups of mice were similar for 3 weeks after challenge, but thereafter, lesion progress was faster for control groups, as the footpad thickness was significantly greater in control mice than in mice immunized with rLmSTI1 plus CpG ODN (P < 0.01), Lip-rLmSTI1 (P < 0.01), or Lip-rLmSTI1-CpG ODN (P < 0.001). At week 14 after challenge, the lesion size was the smallest (P < 0.001) in mice immunized with Lip-rLmSTI1-CpG ODN compared with all other groups. There was no significant difference in lesion size between the group of mice immunized with rLmSTI1 plus CpG ODN in free form and the group of mice immunized with Lip-rLmSTI1 at week 14 after challenge, but significantly (P < 0.05), the average lesion size observed for the group of mice immunized with rLmSTI1 plus CpG ODN or Lip-rLmSTI1 was smaller than that observed for the group of mice immunized with either rLmSTI1 or rLmSTI1 plus non-CpG ODN. There was no significant difference in lesion size in mice immunized with rLmSTI1, rLmSTI1 plus non-CpG ODN, or empty liposomes at week 14 after challenge. The lesion size reached a plateau for mice immunized with PBS after week 12 (Fig. 2), but progression of the disease was seen by metastasis to other organs.
FIG. 2.
Footpad swelling in BALB/c mice immunized s.c. three times in 3-week intervals with Lip-rLmSTI1-CpG ODN, Lip-rLmSTI1, rLmSTI1 plus CpG ODN in PBS, rLmSTI1 plus non-CpG ODN in PBS, empty liposomes, or PBS after challenge with L. major promastigotes. Three weeks after the last booster, the mice (n = 7) were challenged in the left footpad with 1.5 × 106 L. major promastigotes. The footpad thicknesses of mice were measured for both footpads for 14 weeks. Each point represents the average increase in footpad thickness ± standard error of the mean.
Splenic parasite burden.
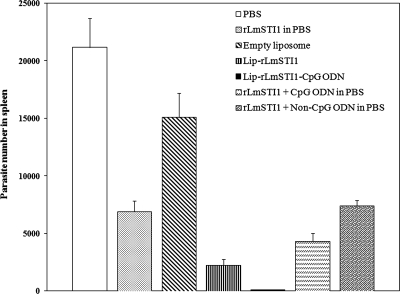
The numbers of viable L. major parasites were quantified in the spleens of different groups of mice at week 14 after challenge (Fig. 3). The significantly (P < 0.001) smallest number of live parasites was seen in the spleens of mice immunized with Lip-rLmSTI1-CpG ODN compared with all other groups. Mice immunized with Lip-rLmSTI1 or rLmSTI1 plus CpG ODN in free form showed significantly (P < 0.001) smaller parasite numbers in spleens than did the control groups; however, the parasite numbers in these groups were significantly (P < 0.05) higher than those for the group of mice immunized with Lip-rLmSTI1-CpG ODN. There was no significant difference in parasite number between mice which received rLmSTI1 alone and mice immunized with rLmSTI1 plus non-CpG ODN.
FIG. 3.
Splenic parasite burden in BALB/c mice immunized s.c. three times in 3-week intervals with Lip-rLmSTI1-CpG ODN, Lip-rLmSTI1, rLmSTI1 plus CpG ODN in PBS, rLmSTI1 plus non-CpG ODN in PBS, empty liposomes, or PBS after challenge with L. major promastigotes. A limiting dilution analysis was performed 14 weeks after challenge on cells isolated from the spleens of individual mice (n = 4) and cultured in triplicate for 10 days at 25°C in serial eightfold dilutions. The number of viable parasites per spleen was determined by ELIDA software, based on the limiting dilution assay method. Each bar represents the average score plus the standard error of the mean.
Antibody response.
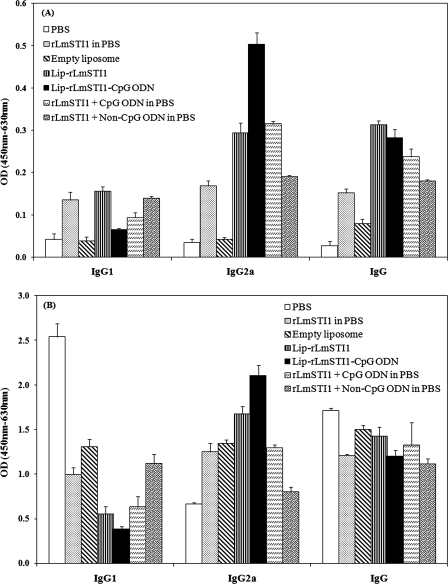
The anti-SLA-specific IgG, IgG1, and IgG2a antibodies were titrated before (Fig. 4A) and at week 14 after challenge (Fig. 4B) to determine the type of immune response generated in immunized mice.
FIG. 4.
Levels of SLA-specific IgG, IgG1, and IgG2a in sera of BALB/c mice immunized s.c. three times in 3-week intervals with Lip-rLmSTI1-CpG ODN, Lip-rLmSTI1, rLmSTI1 plus CpG ODN in PBS, rLmSTI1 plus non-CpG ODN in PBS, empty liposomes, or PBS. Blood samples were collected from mice 3 weeks after the last booster (A) and 14 weeks after challenge (B). The SLA-specific IgG, IgG1, and IgG2a levels were assessed using an ELISA method. The assays were performed in triplicate at a 200-fold dilution for each serum sample. Values are means plus standard deviations (n = 7). OD, optical density.
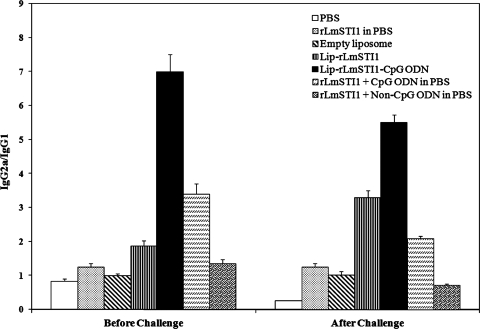
As shown in Fig. 4A, the sera of mice immunized with Lip-rLmSTI1-CpG ODN, Lip-rLmSTI1, or rLmSTI1 plus CpG ODN showed significantly (P < 0.001) higher levels of specific IgG2a antibody than did sera from the other groups before challenge. However, the level of IgG1 in mice immunized with Lip-rLmSTI1 was significantly (P < 0.001) higher than that in the group of mice immunized with Lip-rLmSTI1-CpG ODN or rLmSTI1 plus CpG ODN in free form. The significantly (P < 0.001) highest ratio of IgG2a to IgG1 was seen in the sera of mice immunized with Lip-rLmSTI1-CpG ODN compared to all other groups (Fig. 5). The levels of IgG1 and IgG2a in mice immunized with rLmSTI1 alone or rLmSTI1 plus non-CpG ODN in free form were also significantly (P < 0.05) higher than those in the control groups. The ratio of IgG2a to IgG1 in mice immunized with rLmSTI1 plus CpG ODN was significantly (P < 0.05) higher than that for the group of mice immunized with Lip-rLmSTI1, rLmSTI1 plus non-CpG ODN, or rLmSTI1 alone (Fig. 5).
FIG. 5.
Ratios of IgG2a to IgG1 in BALB/c mice immunized s.c. three times in 3-week intervals with Lip-rLmSTI1-CpG ODN, Lip-rLmSTI1, rLmSTI1 plus CpG ODN in PBS, rLmSTI1 plus non-CpG ODN in PBS, empty liposomes, or PBS. Blood samples were collected 3 weeks after the last booster (before challenge) and 14 weeks after challenge. The SLA-specific IgG1 and IgG2a levels were assessed using an ELISA method. Values are means plus standard deviations (n = 7).
Upon challenge with L. major promastigotes, elevations of IgG, IgG1, and IgG2a antibodies were seen in all groups of mice compared with the antibody titers before challenge (Fig. 4). There was no significant difference between the levels of total IgG antibodies in the sera of different groups of mice (Fig. 4B), although the sera of mice immunized with Lip-rLmSTI1-CpG ODN or Lip-rLmSTI1 showed significantly (P < 0.05) elevated levels of IgG2a antibody compared with the other groups. Similar to the antibody response before challenge, sera of mice immunized with Lip-rLmSTI1-CpG ODN showed, significantly (P < 0.001), the highest ratio of IgG2a to IgG1 compared to all other groups. The ratio of IgG2a to IgG1 in mice immunized with rLmSTI1 plus CpG ODN was significantly (P < 0.05) lower than that in mice immunized with Lip-rLmSTI1 (Fig. 5).
DISCUSSION
As a result of more than a decade of global efforts, a few preparations of first-generation Leishmania vaccines reached phase 3 clinical trials. The results of clinical trials showed an acceptable level of safety with a limited level of efficacy, and the efficacy was not improved with multiple injections (17). The reason for this failure was possibly due to the lack of an appropriate adjuvant. New vaccines against leishmaniasis, particularly those based on recombinant proteins and DNA, are likely to be less immunogenic than first-generation vaccines (8). Therefore, there is a need for the development of new and improved adjuvants to enhance the immunogenicity of vaccines against leishmaniasis. The most effective adjuvant used with Leishmania antigens in the murine model of leishmaniasis was interleukin-12 (4, 34, 46), but problems related to its manufacturing, cost, and availability, as well as safety issues, currently preclude this adjuvant from the list of adjuvant candidates to be used in humans (37).
In the current study, liposomes consisting of distearoylphosphatidylcholine, which has a high transition temperature (Tc) (54°C), were used to optimize the adjuvanticity of liposomes. Liposomes with high Tc may favor heightened in vivo stability and enhanced adjuvant activity for the entrapped antigens because of their greater membrane rigidity (26). Liposomes by themselves act as an adjuvant (13), but to further increase the adjuvanticity, immunostimulatory adjuvants might be incorporated into liposomes (31). Therefore, CpG ODN 1826, containing two CpG motifs with a phosphorothioate backbone, was encapsulated with rLmSTI1 into liposomes to stimulate a potent Th1-type immune response (6, 44).
To evaluate the rate of protection, footpad swelling after challenge with L. major was measured in immunized mice and compared with that of the control groups. The results showed that the sizes of lesions in mice immunized with Lip-rLmSTI1-CpG ODN at week 14 were significantly (P < 0.001) smaller than those of the other groups. Interestingly, the mice immunized with Lip-rLmSTI1 had no significant difference in footpad swelling compared with the group of mice immunized with rLmSTI1 plus CpG ODN in free form. Moreover, mice immunized with rLmSTI1 plus non-CpG ODN (lacking the critical CpG dinucleotide) showed a significantly (P < 0.001) larger footpad size than did mice which received rLmSTI1 plus CpG ODN, demonstrating that the adjuvant effect was CpG dependent (Fig. 2). The ability of free CpG ODN to induce protection against leishmaniasis was previously reported by others (35, 38, 42). BALB/c mice vaccinated with autoclaved Leishmania major plus CpG ODN in free form were protected against L. major challenge for up to 6 weeks after immunization (35). A significantly smaller lesion size was seen in L. major-infected BALB/c mice immunized with SLA plus CpG ODN (38), and in another study, 40% of BALB/c mice vaccinated with intact freeze-thawed L. major plus CpG ODN were protected against L. major challenge (42). The results of this study showed that CpG ODN alone is an effective adjuvant to induce protection against the murine model of leishmaniasis, but stronger immune responses were induced when liposomes were used to deliver CpG ODN. An improvement of adjuvanticity of CpG ODN was previously reported for encapsulation of CpG ODN in liposomes (16, 23, 39).
The numbers of viable L. major parasites in the spleens of different groups of mice after challenge were quantitated as another indication of the rate of protection. The smallest number of live parasites was seen for the group of mice immunized with Lip-rLmSTI1-CpG ODN, which paralleled the footpad swelling results. It seems that CpG ODN in free form induces protection for a shorter time and that liposomal CpG ODN induces protection for a longer time, as described by others (20). On the other hand, CpG ODN as an immunoadjuvant needs some delivery system to maintain and enhance the immunoadjuvant activity.
IgG1 and IgG2a antibody titers are used as markers of Th2 and Th1 immune responses, respectively (7). The significantly (P < 0.001) highest ratio of IgG2a to IgG1 was seen in the sera of mice immunized with Lip-rLmSTI1-CpG ODN, before as well as after challenge, compared with all other groups. Interestingly, the highest ratio of IgG2a to IgG1 was seen in the sera of mice immunized with liposomal rLmSTI1, especially after challenge, compared with the group of mice receiving rLmSTI1 plus CpG ODN in free form. This might be an indication that using the liposomal form of CpG ODN induces a longer immune response than that obtained with free CpG ODN. The enhanced ratio of IgG2a to IgG1 in mice, particularly after challenge, is correlated with a Th1 response and is consistent with studies showing that antigens plus CpG ODN induces a potent Th1-type immune response (5, 6).
There is plenty of evidence that CpG ODN have potent immunostimulatory effects, but this activity is often transient because of the rapid degradation and absorption of CpG ODN, and hence, repeated administration or a much higher dose of CpG ODN is required to achieve the desired effects (30). Moreover, the use of CpG ODN might be limited by the difficulty of delivery to the intracellular compartments because CpG ODN are recognized by Toll-like receptor 9 as a synthetic ligand (2) and must be delivered to the endosomes before signaling is possible (1). This severely limits the potential clinical application of the CpG ODN technology (30). In this regard, some investigators have focused on searching for formulations and delivery systems to improve the stability and stimulatory effect of CpG ODN (30).
In this study, liposomes were used as a delivery system to enhance the biological activity of CpG ODN. Liposomes may mediate this enhancement of activity in a number of ways. Liposomes protect CpG ODN from nuclease activity and hamper the distribution of CpG ODN to tissue, consequently increasing the CpG ODN half-life (11). Liposomes also facilitate the intracellular delivery of CpG ODN into the cytoplasm (47). In addition, phagocytosis of liposomes by antigen-presenting cells as well as localization of liposomes in the draining lymph nodes occurs following s.c. administration (33). Since the draining lymph nodes may contain a greater number of cells that express Toll-like receptor 9 than do other tissues, such as the skin and muscles, the concentration of CpG ODN in the draining lymph nodes may be an important mechanism by which liposomes enhance the biological activity of CpG ODN (30). This capacity of liposomes to enhance the activity of CpG ODN through numerous mechanisms makes them potentially useful for the delivery of CpG ODN. Moreover, the coencapsulation of CpG ODN and antigen in the same liposomes presumably allows antigen presentation and adjuvant stimulation to occur in the same cell, which is critical for the induction of immune responses (30).
In summary, the results of the present study suggest that coencapsulation of CpG ODN with rLmSTI1 in liposomes might be a suitable strategy to enhance the Th1 type of immune response and the induction of protection against leishmaniasis, at least in a murine model.
Acknowledgments
The financial support of the Biotechnology Research Center and Pharmaceutical Research Center, Mashhad University of Medical Sciences (MUMS), and the Center for Research and Training in Skin Diseases and Leprosy, Tehran University of Medical Sciences (TUMS), is gratefully acknowledged.
We thank J. R. Webb for the gift of rLmSTI1 antigen and R. G. Titus for the gift of ELIDA software.
Footnotes
Published ahead of print on 30 January 2008.
REFERENCES
- 1.Ahmad-Nejad, P., H. Hacker, M. Rutz, S. Bauer, R. M. Vabulas, and H. Wagner. 2002. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur. J. Immunol. 32:1958-1968. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124:783-801. [DOI] [PubMed] [Google Scholar]
- 3.Alving, C. R. 1995. Liposomal vaccines: clinical status and immunological presentation for humoral and cellular immunity. Ann. N. Y. Acad. Sci. 754:143-152. [DOI] [PubMed] [Google Scholar]
- 4.Campos-Neto, A., R. Porrozzi, K. Greeson, R. N. Coler, J. R. Webb, Y. A. W. Seiky, S. G. Reed, and G. Grimaldi. 2001. Protection against cutaneous leishmaniasis induced by recombinant antigens in murine and nonhuman primate models of the human disease. Infect. Immun. 69:4103-4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu, R. S., D. Askew, and C. V. Harding. 2000. CpG DNA switches on Th1 immunity and modulates antigen-presenting cell function. Curr. Top. Microbiol. Immunol. 247:199-210. [DOI] [PubMed] [Google Scholar]
- 6.Chu, R. S., O. S. Targoni, A. M. Krieg, P. V. Lehmann, and C. V. Harding. 1997. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J. Exp. Med. 186:1623-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coffman, R. L., D. A. Lebman, and P. Rothman. 1993. Mechanism and regulation of immunoglobulin isotype switching. Adv. Immunol. 54:229-270. [DOI] [PubMed] [Google Scholar]
- 8.Coler, R. N., and S. G. Reed. 2005. Second-generation vaccines against leishmaniasis. Trends Parasitol. 21:244-249. [DOI] [PubMed] [Google Scholar]
- 9.Coler, R. N., Y. A. Skeiky, K. Bernards, K. Greeson, D. Carter, C. D. Cornellison, F. Modabber, A. Campos-Neto, and S. G. Reed. 2002. Immunization with a polyprotein vaccination consisting of the T-cell antigens thiol-specific antioxidant, Leishmania major stress-inducible protein 1, and Leishmania elongation initiation factor protects against leishmaniasis. Infect. Immun. 70:4215-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Croft, S. L., and G. H. Coombs. 2003. Leishmaniasis—current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 19:502-508. [DOI] [PubMed] [Google Scholar]
- 11.De Oliveira, M. C., V. Boutet, E. Fattal, D. Boquet, J. M. Grognet, P. Couvreur, and J. R. Deverre. 2000. Improvement of in vivo stability of phosphodiester oligonucleotide using anionic liposomes in mice. Life Sci. 67:1625-1637. [DOI] [PubMed] [Google Scholar]
- 12.Gradoni, L., F. V. Manzillo, A. Pagano, D. Piantedosi, R. De Luna, M. Gramiccia, A. Scalone, T. Di Muccio, and G. Oliva. 2005. Failure of a multi-subunit recombinant leishmanial vaccine (MML) to protect dogs from Leishmania infantum infection and to prevent disease progression in infected animals. Vaccine 23:5245-5251. [DOI] [PubMed] [Google Scholar]
- 13.Gregoriadis, G. 1990. Immunological adjuvant: a role for liposomes. Immunol. Today 11:89-97. [DOI] [PubMed] [Google Scholar]
- 14.Jaafari, M. R., A. Badiee, A. Khamesipour, A. Samiei, D. Soroush, M. T. Kheiri, F. Barkhordari, W. R. McMasterd, and F. Mahboudi. 2007. The role of CpG ODN in enhancement of immune response and protection in BALB/c mice immunized with recombinant major surface glycoprotein of Leishmania (rgp63) encapsulated in cationic liposome. Vaccine 25:6107-6117. [DOI] [PubMed] [Google Scholar]
- 15.Jaafari, M. R., A. Ghafarian, A. Farrokh-Gisour, A. Samiei, M. T. Kheiri, F. Mahboudi, F. Barkhordari, A. Khamesipour, and W. R. McMaster. 2006. Immune response and protection assay of recombinant major surface glycoprotein of Leishmania (rgp63) reconstituted with liposomes in BALB/c mice. Vaccine 24:5708-5717. [DOI] [PubMed] [Google Scholar]
- 16.Jiao, X., R. Y. Wang, Q. Qiu, H. J. Alter, and J. W. Shih. 2004. Enhanced hepatitis C virus NS3 specific Th1 immune responses induced by co-delivery of protein antigen and CpG with cationic liposomes. J. Gen. Virol. 85:1545-1553. [DOI] [PubMed] [Google Scholar]
- 17.Khamesipour, A., S. Rafati, N. Davoudi, F. Maboudi, and F. Modabber. 2006. Leishmaniasis vaccine candidates for development: a global overview. Indian J. Med. Res. 123:423-438. [PubMed] [Google Scholar]
- 18.Khamesipour, A., Y. Dowlati, A. Asilian, R. Hashemi-Fesharki, A. Javadi, S. Noazin, and F. Modabber. 2005. Leishmanization: use of an old method for evaluation of candidate vaccines against leishmaniasis. Vaccine 23:3642-3648. [DOI] [PubMed] [Google Scholar]
- 19.Kirby, C., and G. Gregoriadis. 1984. Dehydration-rehydration vesicles: a simple method for high yield drug entrapment in liposomes. Biotechnology 2:979-984. [Google Scholar]
- 20.Klinman, D. M. 2004. Use of CpG oligodeoxynucleotides as immunoprotective agents. Expert Opin. Biol. Ther. 4:937-946. [DOI] [PubMed] [Google Scholar]
- 21.Klinman, D. M., D. Currie, I. Gurse, and D. Verthelyi. 2004. Use of CpG oligodeoxynucleotides as immune adjuvants. Immunol. Rev. 199:201-216. [DOI] [PubMed] [Google Scholar]
- 22.Krieg, A. M. 2002. CpG motif in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20:709-760. [DOI] [PubMed] [Google Scholar]
- 23.Li, W. M., W. H. Dragowska, M. B. Bally, and M. P. Schutze-Redelmeier. 2003. Effective induction of CD8+ T-cell response using CpG oligodeoxynucleotides and HER-2/neu-derived peptide co-encapsulated in liposomes. Vaccine 21:3319-3329. [DOI] [PubMed] [Google Scholar]
- 24.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 25.Mauel, J. 2002. Vaccination against Leishmania infections. Curr. Drug Targets Immune Endocr. Metab. Disord. 2:201-226. [DOI] [PubMed] [Google Scholar]
- 26.Mazumdar, T., K. Anam, and N. Ali. 2005. Influence of phospholipid composition on the adjuvanticity and protective efficacy of liposome-encapsulated Leishmania donovani antigens. J. Parasitol. 91:269-274. [DOI] [PubMed] [Google Scholar]
- 27.Melby, P. C. 2002. Vaccination against cutaneous leishmaniasis. Curr. Status Am. J. Clin. Dermatol. 3:557-570. [DOI] [PubMed] [Google Scholar]
- 28.Mendez, S., S. Gurunathan, S. Kamhawi, Y. Belkaid, M. A. Moga, Y. A. Skeiky, A. Campos-Neto, S. Reed, R. A. Seder, and D. Sacks. 2001. The potency and durability of DNA- and protein-based vaccines against Leishmania major evaluated using low-dose, intradermal challenge. J. Immunol. 166:5122-5128. [DOI] [PubMed] [Google Scholar]
- 29.Modabber, F. 1995. Vaccines against leishmaniasis. Ann. Trop. Med. Parasitol. 89:83-88. [DOI] [PubMed] [Google Scholar]
- 30.Mutwiri, G. K., A. K. Nichani, S. Babiuk, and L. A. Babiuk. 2004. Strategies for enhancing the immunostimulatory effects of CpG oligodeoxynucleotides. J. Control. Release 97:1-17. [DOI] [PubMed] [Google Scholar]
- 31.O'Hagan, D. T., M. L. MacKichan, and M. Singh. 2001. Recent developments in adjuvants for vaccines against infectious diseases. Biomol. Eng. 18:69-85. [DOI] [PubMed] [Google Scholar]
- 32.O'Hagan, D. T., and M. Singh. 2003. Microparticles as vaccine adjuvants and delivery systems. Expert Rev. Vaccines 2:269-283. [DOI] [PubMed] [Google Scholar]
- 33.Oussoren, C., and G. Storm. 2001. Liposomes to target the lymphatics by subcutaneous administration. Adv. Drug Deliv. 50:143-156. [DOI] [PubMed] [Google Scholar]
- 34.Park, A. Y., and P. Scott. 2001. IL-12: keeping cell-mediated immunity alive. Scand. J. Immunol. 53:529-532. [DOI] [PubMed] [Google Scholar]
- 35.Rhee, E. G., S. Mendez, J. A. Shah, C. Y. Wu, J. R. Kirman, T. N. Turon, D. F. Davey, H. Davis, D. M. Klinman, R. N. Coler, D. L. Sacks, and R. A. Seder. 2002. Vaccination with heat-killed Leishmania antigen or recombinant leishmanial protein and CpG oligodeoxynucleotides induces long-term memory CD4+ and CD8+ T cell responses and protection against Leishmania major infection. J. Exp. Med. 195:1565-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sacks, D., and N. Noben-Trauth. 2002. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2:845-858. [DOI] [PubMed] [Google Scholar]
- 37.Skeiky, Y. A., R. N. Coler, M. Branon, E. Stromberg, K. Greeson, R. T. Crane, A. Campos-Neto, and S. G. Reed. 2002. Protective efficacy of a tandemly linked, multi-subunit recombinant leishmanial vaccine (leish-111f) formulated in MPL adjuvant. Vaccine 20:3292-3303. [DOI] [PubMed] [Google Scholar]
- 38.Stacey, K. J., and J. M. Blackwell. 1999. Immunostimulatory DNA as an adjuvant in vaccination against Leishmania major. Infect. Immun. 67:3719-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki, Y., D. Wakita, K. Chamoto, Y. Narita, T. Tsuji, T. Takeshima, H. Gyobu, Y. Kawarada, S. Kondo, S. Akira, H. Katoh, H. Ikeda, and T. Nishimura. 2004. Liposome-encapsulated CpG oligodeoxynucleotides as a potent adjuvant for inducing type 1 innate immunity. Cancer Res. 64:8754-8760. [DOI] [PubMed] [Google Scholar]
- 40.Taswell, C. 1981. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J. Immunol. 126:1614-1619. [PubMed] [Google Scholar]
- 41.Titus, R. G., M. Marchand, T. Boon, and J. A. Louis. 1985. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 7:545-555. [DOI] [PubMed] [Google Scholar]
- 42.Walker, P. S., T. Scharton-Kersten, A. M. Krieg, L. Love-Homan, E. D. Rowton, M. C. Udey, and J. C. Vogel. 1999. Immunostimulatory oligodeoxynucleotides promote protective immunity and provide systemic therapy for leishmaniasis via IL-12- and IFN-γ-dependent mechanisms. Proc. Natl. Acad. Sci. USA 96:6970-6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Webb, J. R., W. Kaufmann, A. Campos-Neto, and S. G. Reed. 1996. Molecular cloning of a novel protein antigen of Leishmania major that elicits a potent immune response in experimental murine leishmaniasis. J. Immunol. 157:5034-5041. [PubMed] [Google Scholar]
- 44.Weeratna, R. D., C. L. Brazolot Millan, M. J. McCluskie, and H. L. Davis. 2001. CpG ODN can re-direct the Th bias of established Th2 immune responses in adult and young mice. FEMS Immunol. Med. Microbiol. 32:65-71. [DOI] [PubMed] [Google Scholar]
- 45.World Health Organization. 2004. Leishmaniasis—disease information. World Health Organization, Geneva, Switzerland. http://www.who.int/tdr/diseases/leish/diseaseinfo.htm.
- 46.Yamakami, K., S. Akao, M. Sato, Y. Nitta, J. Miyazaki, and T. Tadakuma. 2001. A single intradermal administration of soluble leishmanial antigen and plasmid expressing interleukin-12 protects BALB/c mice from Leishmania major infection. Parasitol. Int. 50:81-91. [DOI] [PubMed] [Google Scholar]
- 47.Zhang, Y. M., M. Rusckowski, N. Liu, C. Liu, and D. J. Hnatowich. 2001. Cationic liposomes enhance cellular/nuclear localization of 99mTc-antisense oligonucleotides in target tumor cells. Cancer Biother. Radiopharm. 16:411-419. [DOI] [PubMed] [Google Scholar]