Abstract
Infection of cattle with Neospora caninum protozoa, the causative agent of bovine protozoal abortion, results in robust cellular and humoral immune responses, particularly CD4+ T-lymphocyte activation and gamma interferon (IFN-γ) secretion. In the present study, N. caninum SRS2 (NcSRS2) T-lymphocyte-epitope-bearing subunits were incorporated into DNA and peptide preparations to assess CD4+ cell proliferation and IFN-γ T-lymphocyte-secretion immune responses in cattle with predetermined major histocompatibility complex (MHC) genotypes. In order to optimize dendritic-cell processing, NcSRS2 DNA vaccine was delivered with granulocyte macrophage-colony-stimulating factor and Flt3 ligand adjuvant. The synthesized NcSRS2 peptides were coupled with a palmitic acid molecule (lipopeptide) and delivered with Freund's adjuvant. Cattle vaccinated with NcSRS2 DNA vaccine alone did not induce T-lymphocyte activation or IFN-γ secretion, whereas subsequent booster inoculation with NcSRS2-lipopeptides induced robust NcSRS2-specific immune responses. Compared to the response in control animals, NcSRS2-lipopeptide-immunized cattle had significantly increased NcSRS2-specific T-lymphocyte proliferation, numbers of IFN-γ-secreting peripheral blood mononuclear cells, and immunoglobulin G1 (IgG1) and IgG2a antibody levels. The findings show that N. caninum NcSRS2 subunits bearing T-lymphocyte epitopes induced cell-mediated immune responses similar to the protective immune responses previously described against live parasite infection, namely T-lymphocyte activation and IFN-γ secretion. The findings support the investigation of NcSRS2 immunogens for protection against N. caninum-induced fetal infection and abortion in cattle.
Neosporosis, caused by the protozoan parasite Neospora caninum, is a major cause of infectious abortion and congenital disease in cattle, persisting in cattle herds via transplacental transmission (10). To date, practical measures to reduce losses from neosporosis in cattle have not been achieved. There is no chemotherapy available and, although progress has been made toward understanding immunity to N. caninum infections, no consistent, highly efficacious vaccine is available to limit fetal infection or prevent abortions. Two field efficacy studies of a commercially available N. caninum vaccine, based upon lysates of whole tachyzoites, show low efficacy in lowering the overall crude abortion rate (46%) or efficacy that varied greatly from farm to farm because precise causes of abortion were not determined, resulting in variability from other infectious agents or noninfectious causes of abortion (25, 35). In cattle, both cellular and humoral responses, particularly CD4+ T-lymphocyte activation and gamma interferon (IFN-γ) secretion, are important for immune protection against fetal infection and abortion (1, 18, 22-24, 40, 42, 43). The development of an effective vaccine based upon molecularly defined immunogens targeting T-lymphocyte activation and IFN-γ secretion could be of great value for the control of neosporosis.
The development of effective vaccines based upon molecularly defined, immunogenic-subunit molecules would increase the repertoire of vaccines available against infection by intracellular pathogens. Although live attenuated vaccines can induce the cell-mediated immune responses often necessary for protection against intracellular infectious agents, live vaccines may be limited by the potential for reversion to virulence and shedding into the environment and the requirements for consistent quality assurance during production. Furthermore, unlike live attenuated vaccines, subunit vaccines can be more easily designed to retain the ability to differentiate between infected and immunized animals (for example, by serological diagnosis) and to include antigens that target protective immune responses rather than antigens that induce immune responses that may exacerbate disease (4, 27, 34, 38) or be positively associated with N. caninum abortion (17). A potential obstacle to developing epitope-based vaccines stimulating effective T-lymphocyte responses in cattle is major histocompatibility complex (MHC) molecule polymorphism.
N. caninum SRS2 (NcSRS2), a surface antigen of N. caninum, is a good subunit vaccine candidate because it is highly conserved (15), is associated with immunity to transplacental parasite transmission in mice (15, 30) and to infection of sheep placental cells (16), and is an immunodominant antigen in N. caninum-infected cattle (11, 39). T-lymphocyte cell lines of cattle infected with N. caninum readily proliferate and secrete IFN-γ when stimulated with recombinant NcSRS2 (rNcSRS2), and T-lymphocyte epitopes of NcSRS2 have been mapped in cattle infected with N. caninum (39). Peripheral blood mononuclear cells (PBMC) of N. caninum-infected outbred cattle with diverse MHC class II (MHC-II) haplotypes show marked parasite-specific secretion of IFN-γ and expanded numbers of IFN-γ-secreting T-lymphocytes and cytotoxic T-lymphocytes when stimulated ex vivo with NcSRS2 peptides bearing T-lymphocyte epitopes. This led to the rationale that epitope clusters within NcSRS2 could be included in peptide-based or DNA-based vaccines. Herein we describe dendritic-cell-targeting DNA and lipopeptide immunization of cattle, showing that the T-lymphocyte responses were similar to live parasite infection responses shown by previously published work in cattle bearing similar MHC haplotypes. Importantly, lipopeptide subunits containing T-lymphocyte-epitope-bearing subunits of NcSRS2 induced the activation of IFN-γ secretion, crucially important in immune protection against fetal infection and abortion.
MATERIALS AND METHODS
NcSRS2 DNA vaccine and lipopeptides.
NcSRS2 subunits containing cytotoxic T-lymphocyte epitopes, namely, peptides 20 and 21 (containing NcSRS2 amino acids 77 to 95) and peptides 34 to 36 (containing NcSRS2 amino acids 133 to 155) (39), were designed for inclusion in both a DNA-based immunogen for initial immunization and a lipopeptide-based immunogen for final booster immunizations. The DNA immunization was designed to include the NcSRS2 sequence spanning amino acids 77 to 155 (SRS2PI). The gene encoding SRS2PI was amplified by overlap extension PCR with two forward primers (5′ ACCTTGTACCTGCTGGGGATGCTGGTCGCTTCCTGCCTCGGACTGCAGATGGAGTGGG TGACTGGAACTCTTC3′ and 5′ATAGATATCACCATGCCCATGGGGTCTCTGCAACCGCTGGCCACCTTGTACCTGCTGGGGATGCTG 3′) that also incorporated a sequence encoding a CD5 secretory signal (28). The second primer introduced an EcoRV restriction site (in bold) at the 5′ end of the PCR product. The SRS2PI reverse primer (5′ATAGGATCCTTACTTATCGTCATCGTCCTTGTAGTCTGTCACTCCGTTGTTTTCTGG 3′) was extended to include the complementary sequence (in bold) of the codons encoding the FLAG tag (DYKDDDDK) and a BamHI restriction site (in italics) at the 3′ end of the PCR product. The resultant chimeric gene, designated cd5srs2piflag, was EcoRV-BamHI digested and subcloned into the EcoRV-BamHI-cut VR-1055 eukaryotic expression vector (Vical, San Diego, CA) to generate a construct designated VR-NcSRS2PI. NcSRS2PI was expressed in COS-7L cells (Life Technologies) as previously described (28). Both the pVR-NcSRS2PI-transfected and pVR-1055-transfected COS-7L cell monolayers were incubated with a 1/1,000 dilution of a mouse anti-FLAG M2-alkaline phosphatase conjugate (Sigma-Aldrich, St. Louis, MO) in blocking buffer. Duplicate transfected cells were reacted with an isotype control monoclonal antibody (MAb) followed by alkaline phosphatase-conjugated goat anti-mouse MAb (Tropix, Bedford, MA) in blocking buffer. The alkaline phosphatase activity was detected by using Fast Red TR-naphthol AS-MX substrate (Sigma-Aldrich, St. Louis, MO). Stained cells were visualized under a microscope, and pVR-NcSRS2PI-transfected COS-7L cells expressing the encoded antigen counted to calculate expression efficiency.
Lipopeptides were synthesized at the Washington State University Laboratory of Biotechnology and Bioanalysis by a solid-phase method based on standard 9-fluorenylmethoxy carbonyl chemistry as described previously (13). The purity of the lipopeptides was determined by high-pressure liquid chromatography to be >90%. Peptide stock solutions (2 mg/liter) were dissolved in RPMI 1640 with 10% dimethyl sulfoxide and stored at −20°C. Lipopeptides were constructed by coupling a palmitic acid to the NH2-terminal amino acid of each peptide and contained <5% free peptide. Two lipopeptides, one encompassing NcSRS2 amino acids 133 to 155 (LP 34-36) and a second encompassing amino acids 77 to 95 (LP 20-21), were constructed. Lipopeptide stocks (20 mg/ml) were dissolved in 100% dimethyl sulfoxide and stored at −20°C.
Cattle.
Twenty-four nonpregnant female or castrated male yearling Friesian-Holstein cattle (Bos taurus) were selected for MHC-I and MHC-II NcSRS2 responder haplotypes as identified previously (39) (Table 1). The bovine lymphocyte antigen class I haplotypes and DRB3 alleles were characterized by microarray typing as previously described (31, 39). Animals were housed and cared for in accordance with the animal care and use regulations of Washington State University, Pullman, WA, and the animal welfare committee guidelines of the Kimron Veterinary Institute, Bet-Dagan, Israel. Two experimental groups (one immunized and one negative control [mock immunized]) consisted of six cattle each with disparate MHC-I and MHC-II haplotypes. The experiments were duplicated at two institutions, Washington State University, Pullman, WA, and Kimron Veterinary Institute, Bet-Dagan, Israel. All cattle were determined to be free of N. caninum infection by serology using either a commercial competitive N. caninum antibody enzyme-linked immunosorbent assay (ELISA) kit (VMRD, Inc., Pullman, WA) or immunofluorescent antibody test (37). VR-NcSRS2PI DNA was inoculated intradermally three times at 4-week intervals with DNA encoding fetal liver tyrosine kinase 3 (Flt3L) and granulocyte-macrophage colony-stimulating factor (GM-CSF) as adjuvant to increase dendritic-cell recruitment as previously described (28). The negative-control group received empty vector (VR-1055) and the same adjuvants. Briefly, individual cattle were inoculated with 1 mg of each DNA construct (NcSRS2, GM-CSF, or Flt3ligand) or empty vector. For each DNA dose, multiple intradermal injections (200 μl per site) were administered in the right flank region within a circular area approximately 10 cm in diameter using a 25-gauge needle. The NcSRS2 DNA and adjuvant GM-CSF and Flt3 ligand DNA were administered at the same time but as separate injections within the defined right flank area. Four weeks following the last DNA immunization, groups were immunized with LP 20-21 and LP 34-36 intramuscularly (two times at 2 week intervals) together with complete Freund's adjuvant for the first immunization and incomplete Freund's adjuvant for the second immunization (7). Control group cattle for the lipopeptide portion of the immunization received Freund's adjuvant mixed with phosphate-buffered saline (PBS) (adjuvant control). Immune responses in PBMC and blood serum of both immunized and control cattle were measured before immunization and 2 weeks after each booster DNA and lipopeptide immunization, using enzyme-linked immunospot assay (ELISPOT) for antigen-specific T-lymphocyte proliferation and IFN-γ secretion and ELISA for specific antibody production.
TABLE 1.
MHC haplotypes of experimental Holstein cattle used in immunization trials at Washington State University and Kimron Veterinary Institutea
| Institution and group | Animal | MHC-I haplotypeb | DRB3 typec | MHC-II haplotyped |
|---|---|---|---|---|
| WSU | ||||
| Vaccinate | 3719 | AH20/AH44 | 1201/14011 | DH08A/DH27A |
| 3694 | AH12/AH14 | 1501/0902 | DH16A/DH11A | |
| 3731 | AH12/AH14 | 1501/14011 | DH16A/DH27A | |
| 3752 | AH15/AH11 | 1101/0101 | DH22H/DH24A | |
| 3755 | AH12/AH13 | 1501/2703 | DH16A/DH23A | |
| 3765 | AH12/AH14 | 1501/0902 | DH16A/DH11A | |
| Control | 3717 | AH20/AH14 | 1201/14011 | DH08A/DH27A |
| 3736 | AH19/AH15 | 0902/1101 | DH11A/DH22H | |
| 3766 | AH11/AH44 | 0101/14011 | DH24A/DH27A | |
| 3773 | AH12/AH13 | 1501/2703 | DH16A/DH23A | |
| 3784 | AH12/AH14 | 1501/0902 | DH16A/DH11A | |
| 3786 | AH12/AH12 | 1501/1501 | DH16A/DH16A | |
| KVI | ||||
| Vaccinate | 8251 | AH15/AH19 | 1101/0101 | DH22H/DH24A |
| 6286 | AH15/AH44 | 0201/1101 | DH22H/DH07A | |
| 6280 | ND | 0201/1101 | ND | |
| 6293 | AH14/AH15 | 0902/1101 | DH11A/DH22H | |
| 6289 | AH15/AH15 | 1101/1101 | DH22H/DH22H | |
| 6300 | AH12/AH20 | 1201/1501 | DH16A/DH08A | |
| Control | 8235 | AH11/AH15 | 0101/1101 | DH24A/DH22H |
| 3249 | AH15/AH20 | 1201/1101 | DH22H/DH08A | |
| 3217 | AH12/AH15 | 0201/1101 | DH07A/DH22H | |
| 3240 | AH14/AH15 | 0902/1101 | DH11A/DH22H | |
| 3207 | AH15/AH15 | 1101/14011 | DH22H/DH27A | |
| 8236 | AH12/AH12 | 1501/1501 | DH16A/DH16A |
WSU, Washington State University, Pullman, WA; KVI, Kimron Veterinary Institute, Israel; ND, not determined (animal 6280 was excluded from the study).
Class I haplotypes were determined by microarray typing with exon 2 and 3 arrays.
DRB3 typing was performed with a DRB3 microarray.
Class II haplotypes were inferred from the class I and DRB3 typing. The DRB3, DQA, and DQB alleles associated with these haplotypes in American Holstein cattle have been confirmed by exon 2 cloning and sequencing (31).
Lymphocyte proliferation assays.
PBMC were plated in round-bottomed 96-well plates at a density of 2 × 106 per ml and were stimulated with 10 μg per ml of LP 20-21 or LP 34-36, lysate of COS cells transfected with VR-NcSRS2PI, lysate of COS cells transfected with VR-1055 (empty plasmid negative control), medium only (antigen negative control), 5 μg per ml of concanavalin A (positive control), 10 μg per ml of NcSRS2 peptide pool consisting of 81 overlapping 15-mer peptides (39), 10 μg per ml of rNcSRS2 (39), or 10 μg per ml of whole-N. caninum-tachyzoite lysate (NSo) (3). PBMC were cultured for 5 days, and then 0.5 μCi of [3H]thymidine per well was added and incubated for 18 h at 37C; supernatants were harvested on day 6 to measure counts per minute (cpm) determined in a beta scintillation counter. The results are presented as the stimulation index, calculated by dividing the mean cpm of treated wells by the mean cpm of wells containing medium alone. Proliferation was considered significant if the stimulation index was >3.0, the mean cpm was >1,000, and an unpaired Student's t test comparing experimental and control groups gave a P value of ≤0.05.
IFN-γ ELISPOT assays.
To detect NcSRS2 subunit-specific T lymphocytes from PBMC without in vitro expansion, the IFN-γ-expressing cells in PBMC were quantified by using an ELISPOT specific for bovine IFN-γ as previously described (39). Lipopeptides (LP 20-21 and LP 34-36) or lysates of COS cells transfected with DNA vaccine constructs (VR-NcSRS2PI and VR-1055) were added to freshly isolated PBMC (1 × 106 cells in 100 μl complete RPMI medium) at final concentrations of 10 μg per ml. The positive controls were concanavalin A at 5 μg per ml, NcSRS2 peptide pool at 10 μg per ml, or NSo at 10 μg per ml. The negative-control wells contained only PBMC in complete RPMI medium. The ELISPOT assays were conducted in triplicate wells of MultiScreen-Immobilon-P plates (Millipore) coated with mouse anti-bovine IFN-γ MAb CC330 (8 μg/ml) that was incubated for 72 h at 37°C with 5% CO2 and detected with biotinylated mouse anti-bovine IFN-γ MAb CC302 (5 μg/ml).
For each animal, the mean number of spots in the negative-control wells was subtracted from the mean number of spots in the test wells to determine the number of NcSRS2 peptide-specific spot-forming cells. The results are presented as the number of IFN-γ spot-forming cells per 1 × 106 PBMC. The PBMC response to NcSRS2 peptides was considered positive if two criteria were met: (i) the number of spot-forming cells following peptide stimulation was significantly different from the number in the medium as analyzed by unpaired Student's t test with the control group (P ≤ 0.05) and (ii) the number of IFN-γ spot-forming cells per million was more than 2.5 times the background number. The background IFN-γ values for lymphocytes stimulated with individual NcSRS2 peptides were calculated as the mean number of IFN-γ-secreting spot-forming cells per million from triplicate wells containing medium alone for each 96-well plate.
Serum antibody analysis.
Three types of serum antibody analyses were conducted: one for NcSRS2-lipopeptide-specific immunoglobulin G (IgG), one for NcSRS2-lipopeptide-specific IgG1 and IgG2a, and a commercially available competitive ELISA based upon an MAb to a non-NcSRS2 immunodominant surface antigen (VMRD, Inc.) (2). NcSRS2-lipopeptide-specific serum antibody was measured in cattle after both NcSRS2 DNA and NcSRS2-lipopeptide immunization. Briefly, 1.0 μg per ml of lipopeptide (LP 20-21 and LP 34-36), NcSRS2 peptide pool, or NSo at 10 μg per ml was coated on 96-well plates overnight at 4°C. After being blocked in PBS with 3% bovine serum albumin for 60 min at 25°C, each test serum sample was run as twofold serial dilutions. Negative controls consisted of wells incubated with pooled sera from N. caninum-negative cattle (2). Positive controls consisted of serum from cattle infected with live N. caninum (40). Samples were incubated at 25°C for 60 min. After being washed with 0.05% Tween in PBS, peroxidase-conjugated anti-bovine IgG was added to each well at a dilution of 1:1,000 and, after incubation for 30 min, the substrate OPD (Sigma) was added. The color was allowed to develop for 20 min, and then the reaction was stopped with 1 N HCl. An electronic plate reader at a wavelength of 450 nm determined the optical density (OD450) of each well and provided arbitary OD450 units. The background cutoff in OD450 units was determined from wells containing negative-control sera. The OD450 units were compared between experimental groups by using the unpaired Student's t test (P ≤ 0.05).
NcSRS2-lipopeptide-specific IgG1 and IgG2a were measured by ELISA as described previously, with some modifications (15). Briefly, pooled lipopeptides LP 20-21 and LP 34-36 were used to coat 96-well plates (Immulon) at 1.0 μg per ml in carbonate buffer, pH 9.6, overnight at 4°C. Following washing with PBS-0.05% Tween 20 and blocking with PBS-Tween 20 containing 3% bovine serum albumin, the plates were incubated for 60 min with twofold serial dilutions (1:100 to 1:6,400) of serum from individual calves. Following washing with the blocking buffer, the wells were incubated for an additional 1 h at room temperature with peroxidase-conjugated murine anti-bovine IgG1 or IgG2 MAb (Serotec Ltd., Oxford, United Kingdom) diluted 1:5,000 in the blocking buffer and washed in buffer. Then, OPD substrate (Sigma) was added and color was allowed to develop for 20 min and stopped with 1 N HCl. An electronic plate reader determined the OD450 of each well. Negative controls consisted of wells incubated with pooled sera from N. caninum-negative cattle (2). Positive controls consisted of serum from cattle infected with live N. caninum (40). The background cutoff in OD450 units was determined from wells containing negative-control sera. OD450 units were compared between experimental groups by using unpaired Student's t tests (P ≤ 0.05).
The induction of cross-reactive antibody responses in NcSRS2 DNA- and peptide-immunized cattle to immunodominant antigens commonly used in N. caninum diagnostic serology was analyzed by using a commercially available competitive ELISA targeting an immunodominant N. caninum tachyzoite surface antigen (2) (cELISA N. caninum antibody test kit; VMRD, Inc., United States). The test was conducted according to the manufacturer's instructions.
RESULTS
Plasmid DNA expression of NcSRS2PI in vitro.
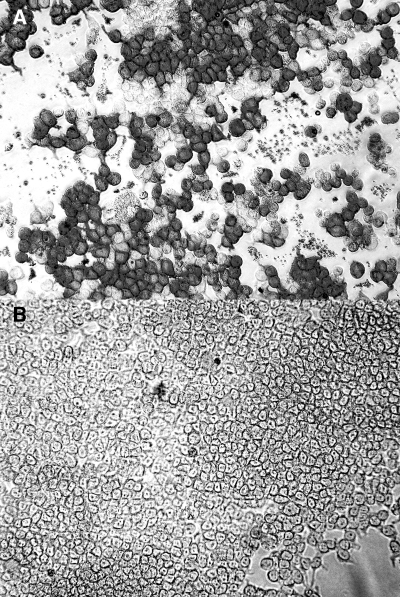
The protein expression of DNA constructs was determined by immunocytochemistry in transfected COS-7L cells using anti-FLAG antibody. Protein expression was detected in the pVR-NcSRS2PI COS-7L cell transfectants, but not in the COS-7L cells transfected with empty parent pVR-1055 (Fig. 1). Immunoreactivity was not identified in either construct by using irrelevant isotype control MAb (data not shown). The transfection efficiency of pVR-NcSRS2PI in various experiments ranged from 30 to 50% (data not shown).
FIG. 1.
Expression of NcSRS2PI in COS7L cells. (A) Abundant NcSRS2PI construct immunoreactivity (dark gray) in COS-7L cells transfected with pVR-NcSRS2PI encoding N. caninum NcSRS2PI. (B) No NcSRS2 construct immunoreactivity in COS-7L cells transfected with empty vector VR-1055. Indirect immunoalkaline phosphatase immunohistochemistry using anti-Flag MAb. Fast Red chromogen. Magnification, ×200.
Proliferation and expansion of IFN-γ-secreting T-lymphocytes following immunization with DNA and NcSRS2-lipopeptides.
To determine if an NcSRS2 subunit vaccine containing T-lymphocyte epitopes could mimic previously published results for the induction of T-lymphocyte immune responses similarly to live N. caninum infection, groups of cattle were immunized with both DNA and lipopeptides containing T-lymphocyte epitopes, followed by analysis for antigen-specific T-lymphocyte activation. A DNA construct containing the CD5 secretory signal inoculated intradermally simultaneously with separate DNA constructs containing Flt3L and GM-CSF as adjuvant was previously shown to induce Anaplasma-specific CD4+ T-lymphocyte responses in outbred cattle (28). To increase the robustness of the analysis, the experiments were carried out in duplicate in two separate laboratories (Washington State University, WA, and Kimron Veterinary Institute, Israel) using identical methods but different sets of cattle. Immunization with pVR-NcSRS2PI with GM-CSF and Flt3L, following primary immunization and two booster inoculations (4 weeks apart), did not induce antigen-specific T-cell activation (data not shown). Similar to the preimmunization responses, there was no significant difference in T-cell proliferation or the number of IFN-γ-secreting T-lymphocytes between immunized and control groups of cattle (P ≥ 0.05; Student's t test). Concanavalin A mitogen-treated PBMC in cattle from both immunized and control groups had robust T-cell proliferation and a substantial number of IFN-γ-secreting cells, indicating that the negative results were not due to inability of the PBMCs to become activated (data not shown).
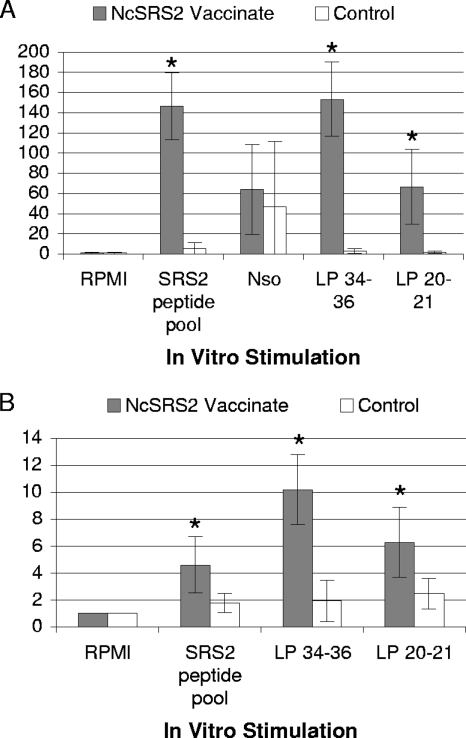
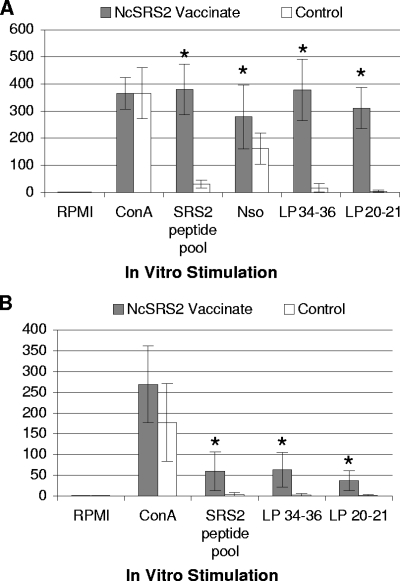
Following the first and the second NcSRS2-lipopeptide immunizations with LP 20-21 and LP 34-36, together with complete or incomplete Freund's adjuvant, respectively, there was robust and statistically significant antigen-specific T-cell proliferation and IFN-γ secretion by NcSRS2 antigen-stimulated PBMC; the data from analyses following the second immunization are shown in Fig. 2 and 3 and Tables 2 and 3. For T-cell proliferation, there were statistically significant differences between experimental and control groups (P ≤ 0.05; Student's t test) when PBMC were stimulated in vitro with immunogen lipopeptide (LP 34-36 and LP 20-21) or with non-lipid-modified NcSRS2 peptide pool but no significant differences when PBMC were stimulated with NSo (Fig. 2 and Table 2). For IFN-γ secretion, cattle in the NcSRS2-lipopeptide-immunized group had statistically significant increases over the level in the control group when PBMC were stimulated with immunogen lipopeptides (LP 34-36 and LP 20-21), non-lipid-modified NcSRS2 peptides, or NSo (Fig. 3 and Table 3). The immune responses induced by NcSRS2-lipopeptides persisted for up to 4 weeks after the last booster inoculation. Of the MHC-II genotypes selected for the study, namely DH16A, DH22H, DH24A, and DH27A, no particular MHC-II genotype was associated with consistently greater or lesser T-lymphocyte-activation responses within individual animals. All MHC haplotypes responded to both LP 34-36 and LP 20-21 (Tables 2 and 3).
FIG. 2.
T-cell proliferation in PBMC of cattle following NcSRS2 DNA and NcSRS2-lipopeptide immunization. There were significant differences between immunized and control groups (*, P ≤ 0.05; Student's t test) for LP 20-21, LP 34-36, and NcSRS2 peptide pool antigens. (A) Results from Washington State University. NSo was used only at WSU. (B) Results from Kimron Veterinary Institute. NcSRS2 Vaccinate, experimental group received VR-NcSRS2PI plus LP 20-21 and LP 34-36 plus adjuvant; Control, VR-1055 vector alone plus adjuvant alone; RPMI, medium alone (negative control); SRS2 peptide pool, NcSRS2 peptide pool (Staska et al. [39]). Proliferation index (y axis), cpm in wells treated with [3H]thymidine/cpm in RPMI medium-only wells. Error bars, 1 standard deviation. For data on individual animals, see Table 2.
FIG. 3.
Enumeration of NcSRS2-specific PBMC by IFN-γ ELISPOT in cattle following NcSRS2 DNA and NcSRS2-lipopeptide immunization. There were significant differences between immunized and control groups (*, P ≤ 0.05; Student's t test) for LP 20-21, LP 34-36, NSo (WSU), and NcSRS2 peptide pool. (A) Results from Washington State University. NSo was used only at WSU. (B) Results from Kimron Veterinary Institute. NcSRS2 Vaccinate, experimental group received VR-NcSRS2PI plus LP 20-21 and LP 34-36 plus adjuvant; Control, group received VR-1055 vector alone plus adjuvant alone; RPMI, medium alone (negative control); ConA, concanavalin A mitogen (positive control); SRS2 peptide pool, NcSRS2 peptide pool (Staska et al. [39]). ELISPOT values (y axis), number of IFN-γ-secreting cells per 1 × 106 PBMC. Error bars, 1 standard deviation. For data on individual animals, see Table 3.
TABLE 2.
Stimulation index for T-cell proliferation in PBMC following NcSRS2 DNA and lipopeptide immunizationa
| Institution, group, and animal | Haplotype
|
Stimulation index for:
|
||||
|---|---|---|---|---|---|---|
| MHC-II | MHC-I | ConA | LP 34-36 | LP 20-21 | NcSRS2 peptide pool | |
| WSU | ||||||
| Vaccinate | ||||||
| 3719 | DH08A/DH27A | AH20/AH44 | 538.8 | 165.8 | 20.2 | 135.6 |
| 3694 | DH16A/DH11A | AH12/AH14 | 498.2 | 107.8 | 62.1 | 108.8 |
| 3731 | DH16A/DH27A | AH12/AH14 | 371.5 | 172.2 | 67.6 | 148.1 |
| 3752 | DH22H/DH24A | AH15/AH11 | 276.5 | 178.1 | 32.4 | 128.2 |
| 3755 | DH16A/DH23A | AH12/AH13 | 772.6 | 189.2 | 97.0 | 205.8 |
| 3765 | DH16A/DH11A | AH12/AH14 | 250.5 | 106.2 | 20.8 | 152.7 |
| Control | ||||||
| 3717 | DH08A/DH27A | AH20/AH14 | 653.5 | 2.2 | 2.1 | 0.5 |
| 3736 | DH11A/DH22H | AH19/AH15 | 416.7 | 1.3 | 1.3 | 12.9 |
| 3766 | DH24A/DH27A | AH11/AH44 | 898.7 | 4.7 | 4.3 | 15.4 |
| 3773 | DH16A/DH23A | AH12/AH13 | 531.4 | 1.2 | 1.0 | 0.7 |
| 3784 | DH16A/DH11A | AH12/AH14 | 528.4 | 1.0 | 1.1 | 1.1 |
| 3786 | DH16A/DH16A | AH12/AH12 | 367.4 | 7.1 | 1.3 | 1.2 |
| KVI | ||||||
| Vaccinate | ||||||
| 8251 | DH22H/DH24A | AH15/AH19 | 35.5 | 8.9 | 9.3 | 2.3 |
| 6286 | DH22H/DH07A | AH15/AH44 | 110.3 | 4.1 | 2.5 | 5.9 |
| 6293 | DH11A/DH22H | AH14/AH15 | 126.3 | 18.7 | 6.5 | 7.5 |
| 6289 | DH22H/DH22H | AH15/AH15 | 167.2 | 5.4 | 5.2 | 3.4 |
| 6300 | DH16A/DH08A | AH12/AH20 | 92.5 | 14.0 | 7.9 | 3.9 |
| Control | ||||||
| 8235 | DH24A/DH22H | AH11/AH15 | 54.5 | 1.0 | 1.5 | 1.5 |
| 3249 | DH22H/DH08A | AH15/AH20 | 181.5 | 2.2 | 1.7 | 1.3 |
| 3217 | DH07A/DH22H | AH12/AH15 | 48.7 | 2.5 | 2.8 | 2.5 |
| 3240 | DH11A/DH22H | AH14/AH15 | 148.6 | 4.6 | 4.6 | 2.8 |
| 3207 | DH22H/DH27A | AH15/AH15 | 128.8 | 0.6 | 2.2 | 1.1 |
| 8236 | DH16A/DH16A | AH12/AH12 | 46.5 | 0.7 | 2.2 | 1.6 |
Individual animal data. Overall results are shown in Fig. 2. The stimulation index was calculated by dividing the mean cpm of wells treated with [3H]thymidine by the mean cpm of wells containing medium alone. ConA, concanavalin A.
TABLE 3.
ELISPOT results for IFN-γ-secreting cells in PBMC following NcSRS2 DNA and lipopeptide immunizationa
| Institution, group, and animal | Haplotype
|
ELISPOT values (no. of spot-forming cells per 1 × 106 PBMC) for:
|
||||
|---|---|---|---|---|---|---|
| MHC-II | MHC-I | ConA | LP 34-36 | LP 20-21 | NcSRS2 peptide pool | |
| WSU | ||||||
| Vaccinate | ||||||
| 3719 | DH08A/DH27A | AH20/AH44 | 394.0 | 201.3 | 5.3 | 200.3 |
| 3694 | DH16A/DH11A | AH12/AH14 | 326.3 | 355.0 | 303.7 | 347.3 |
| 3731 | DH16A/DH27A | AH12/AH14 | 375.0 | 348.3 | 260.3 | 341.0 |
| 3752 | DH22H/DH24A | AH15/AH11 | 398.7 | 494.7 | 298.0 | 478.7 |
| 3755 | DH16A/DH23A | AH12/AH13 | 345.7 | 458.3 | 485.7 | 449.3 |
| 3765 | DH16A/DH11A | AH12/AH14 | 379.0 | 232.7 | 209.0 | 283.3 |
| Control | ||||||
| 3717 | DH08A/DH27A | AH20/AH14 | 296.0 | 0.7 | 10.7 | 0.3 |
| 3736 | DH11A/DH22H | AH19/AH15 | 174.7 | 87.0 | 13.7 | 64.7 |
| 3766 | DH24A/DH27A | AH11/AH44 | 127.9 | 2.0 | 0.7 | 103.0 |
| 3773 | DH16A/DH23A | AH12/AH13 | 327.7 | 0.0 | 3.7 | 1.0 |
| 3784 | DH16A/DH11A | AH12/AH14 | 340.3 | 0.3 | 0.7 | 0.3 |
| 3786 | DH16A/DH16A | AH12/AH12 | 346.0 | 17.3 | 0.7 | 13.3 |
| KVI | ||||||
| Vaccinate | ||||||
| 8251 | DH22H/DH24A | AH15/AH19 | 405.0 | 80.7 | 28.7 | 135.0 |
| 6286 | DH22H/DH07A | AH15/AH44 | 190.4 | 21.3 | 18.5 | 32.3 |
| 6293 | DH11A/DH22H | AH14/AH15 | 175.7 | 35.4 | 24.3 | 27.0 |
| 6289 | DH22H/DH22H | AH15/AH15 | 291.0 | 126.0 | 77.3 | 72.3 |
| 6300 | DH16A/DH08A | AH12/AH20 | 283.0 | 53.0 | 34.0 | 29.3 |
| Control | ||||||
| 8235 | DH24A/DH22H | AH11/AH15 | 185.6 | 1.0 | 1.0 | 2.0 |
| 3249 | DH22H/DH08A | AH15/AH20 | 300.0 | 1.0 | 1.0 | 1.0 |
| 3217 | DH07A/DH22H | AH12/AH15 | 28.3 | 1.0 | 1.0 | 1.5 |
| 3240 | DH11A/DH22H | AH14/AH15 | 183.3 | 10.5 | 5.7 | 13.3 |
| 3207 | DH22H/DH27A | AH15/AH15 | 124.2 | 1.0 | 1.0 | 1.6 |
| 8236 | DH16A/DH16A | AH12/AH12 | 242.0 | 1.2 | 1.4 | 2.2 |
Individual animal data. Overall results are shown in Fig. 3. ConA, concanavalin A.
NcSRS2-specific serum antibody in cattle immunized with NcSRS2-lipopeptides.
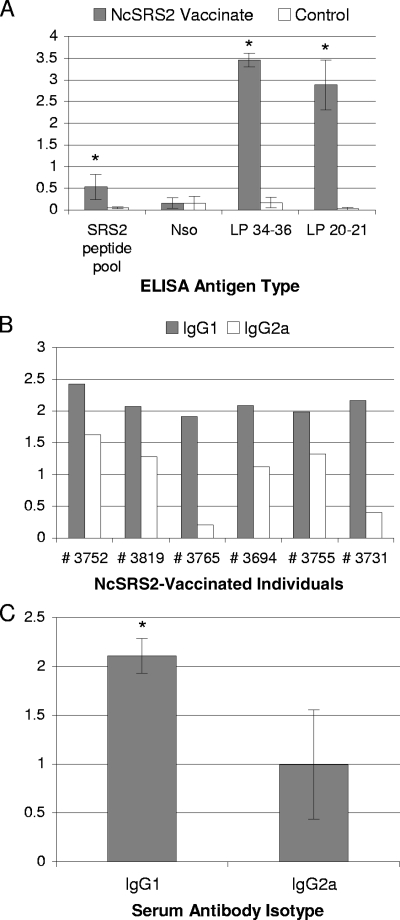
To determine if immunization with NcSRS2 subunits resulted in B-lymphocyte activation, an indirect ELISA measured NcSRS2-specific serum antibodies. The data was analyzed only from cattle immunized at Washington State University. NcSRS2-specific serum antibody was identified only following NcSRS2-lipopeptide immunization boost; no antibodies were produced following DNA immunization with pVR-NcSRS2PI and adjuvant (data not shown). Following NcSRS2-lipopeptide immunization, there were statistically significant differences between experimental and control groups of cattle (P ≤ 0.05; Student's t test) specifically for lipopeptides (LP 34-36 and LP 20-21) and non-lipid-modified NcSRS2 peptide epitopes (NcSRS2 peptide pool) (Fig. 4A). There was no significant difference between experimental and control groups in serum antibody specific to NSo in the ELISA or immunofluorescent antibody assay.
FIG. 4.
Antigen-specific serum antibody responses in cattle following NcSRS2 DNA and NcSRS2-lipopeptide immunization. Specificity of NcSRS2 antibody following NcSRS2-lipopeptide immunization using indirect ELISA was determined for Washington State University cattle only. (A) NcSRS2-specific IgG in NcSRS2-immunized and negative-control groups. Test serum dilution, 1:200. x axis, type of coating antigen used for indirect ELISA. SRS2 peptide pool, NcSRS2 peptide pool. Error bars, 1 standard deviation. *, significant difference between immunized and control groups (P ≤ 0.05; Student's t test). (B) NcSRS2-specific serum IgG1 and IgG2a levels for individual cattle from the NcSRS2 vaccinate group. Test serum dilution, 1:200. Coating antigen for indirect ELISA was LP 20-21/LP 34-36 pool. (C) Mean NcSRS2-specific serum IgG1 and IgG2a levels from the NcSRS2 vaccinate group. Test serum dilution, 1:200. Coating antigen for indirect ELISA was LP 20-21/LP 34-36 pool. Error bars, 1 standard deviation.
The type 1 or type 2 immune response bias induced by NcSRS2-lipopeptide immunization was evaluated by measuring specific serum IgG1 and IgG2a isotypes from cattle in the NcSRS2-immunized group. All cattle in the NcSRS2-immunized group produced NcSRS2-lipopeptide-specific serum antibodies of both IgG1 and IgG2a isotypes (Fig. 4B). The mean levels of lipopeptide-specific IgG1 were significantly higher than the mean levels of lipopeptide-specific IgG2a (P ≤ 0.05) (Fig. 4C). No calves in the NcSRS2-lipopeptide-immunized group were seropositive according to the results of a commercial competitive ELISA for the detection of N. caninum-infected cattle (VMRD, Inc.) that recognizes serum antibodies against an immunodominant tachyzoite surface protein unrelated to NcSRS2 (data not shown).
DISCUSSION
Neosporosis is a major cause of infectious abortion and congenital disease in cattle. Because no effective treatment is available, vaccine development to prevent N. caninum abortion remains a high research priority. Epitope sequences determine the specificity of immune responses and are an appealing target for vaccine development against infectious agents. We show here that palmitic acid-modified peptides of N. caninum tachyzoite surface antigen NcSRS2 containing T-lymphocyte-bearing epitopes (39) stimulated both T-lymphocyte and B-lymphocyte immune responses in cattle following immunization. The T-lymphocyte activation and IFN-γ secretion induced by NcSRS2-lipopeptide vaccination in this study mimic previously reported cell-mediated immune responses following experimental infection with live N. caninum parasites (18, 39, 43), as well as immune responses in field studies investigating their correlation with protection from N. caninum-induced abortion (22). The NcSRS2 DNA subunit immunization alone did not stimulate either T-lymphocyte or B-lymphocyte immune responses following DNA immunization, despite using GM-CSF and Flt3 ligand, adjuvants known to assist strong immune stimulation in cattle immunized for other pathogens (28, 29). However, booster immunization with lipopeptides containing the same T-lymphocyte-bearing epitopes along with Freund's adjuvant stimulated strong, statistically significant increases of NcSRS2-specific lymphocyte proliferation, numbers of IFN-γ-secreting cells, and levels of specific IgG1 and IgG2a antibodies in serum. The specific cell-mediated immune responses were induced not only to specific lipopeptide immunogen but also to non-lipid-modified synthetic peptide pools of the entire NcSRS2 antigen and to native antigen in NSo (for IFN-γ ELISPOT). Thus, the observed T-lymphocyte activation was not induced by an artificially modified lipid epitope but by peptide sequences in the NcSRS2 antigen. Furthermore, the T-lymphocyte immune responses induced by specific subunits within NcSRS2 (LP 34-36 and LP 20-21) were sufficiently robust to be detected when the stimulating epitopes were diluted with all peptides in the NcSRS2 antigen (NcSRS2 peptide pool) or with NSo.
In contrast to specific IFN-γ induced by NcSRS2 subunit immunization, specific antibody responses to the lipopeptide immunogen were very robust, those to the non-lipid-modified NcSRS2 peptide pool were weak but statistically significant, and those against NSo were not detected. This could be an actual effect of the NcSRS2-lipopeptide immunogen, where B-lymphocyte-stimulating portions of the antigen were primarily lipid-modified epitopes. Alternatively, the failure to find robust antibody responses in vaccinated cattle against NcSRS2 peptide pools and NSo could be related to the ELISA detection methods. Antigen bound onto plastic may not provide an optimal matrix for the detection of all NcSRS2-specific antibody responses, either because the NcSRS2 epitopes in the mixed antigen preparations were too highly diluted or because NcSRS2 epitopes were conformationally altered by binding to plastic. Regardless, the studies do show that a robust and specific, mixed IgG1 and IgG2a antibody response was induced by NcSRS2 subunit vaccination, which along with the data showing the induction of strong T-lymphocyte responses, warrants investigation of the efficacy against N. caninum-induced abortion.
The contribution of initial immunization with NcSRS2 DNA to the eventual immune stimulation following boosting with NcSRS2-lipopeptide preparation is unclear from the data presented herein. Even though no specific T-lymphocyte activation or IFN-γ secretion was detected following DNA immunization, it is possible that there was an enhancing or priming effect induced by the NcSRS2 DNA, GM-CSF, and Flt3 ligand primary immunization. A group of cattle immunized with the NcSRS2-lipopeptide preparation only (no NcSRS2 DNA vaccination) could have determined a possible enhancing mechanism for DNA vaccination but was not included in the original experimental design. The primary purpose of the experiments as designed was not to determine a mechanism of immunity to N. caninum subunits nor compare types of immunization parameters, such as different adjuvants, routes of immunization, or specific immunogen types. Before moving forward with efficacy trials in pregnant cattle, we simply were asking, “Can NcSRS2 subunits be designed and administered that will induce T-lymphocyte activation and IFN-γ secretion similar to what we and others had observed when experimentally infecting cattle with N. caninum tachyzoites (36, 39, 43)?” Previous studies with collaborators showed that the DNA immunization procedure used in the present study could induce antigen-specific T-cell responses, optimally detected in short-term T-lymphocyte lines (28). In the current study, it was expected that boosting with lipopeptides would expand the DNA vaccine-primed T-cell responses in vivo, allowing the detection and quantification of NcSRS2-specific T-lymphocytes directly from PBMC. At the conclusion of the studies, we had determined that parasite-specific T-lymphocyte activation and IFN-γ secretion could be induced with small NcSRS2 subunits after sequential inoculation with both NcSRS2 DNA and NcSRS2-lipopeptide preparations. Determining the mechanism of the successful NcSRS2 subunit immunization, including the overall contributions of specific adjuvants or immunogen types, would need to be addressed in another study.
Lipopeptides are reported to be more-efficient immunogens than conventional synthetic peptides, which alone, without modification or haptens, are not particularly immunogenic. Possibly, the lipid component enables rapid access to antigen-presenting cells, particularly dendritic cells, resulting in the activation of Toll-like receptors (TLR); cytosolic uptake of antigens and access to both class I and class II presentation pathways; and prolonged functional antigen presentation (6). In particular, synthetic palmitic acid moieties of lipopeptides, as used in the present study, bind TLR 2 present in dendritic cells and induce their maturation, resulting in more-effective type 1 protective immunity (44). Homologues of human TLR family genes and their expression were shown for TLR 1 to 5 and TLR 7 to 10 in bovine skin (26). Lipopeptide subunits of various infectious agents stimulate protective T-lymphocyte immune responses in outbred species; examples include responses to Plasmodium falciparum in chimpanzees (5), human immunodeficiency virus type 1 in humans (14), and equine infectious anemia virus in horses (33). The palmitic acid-based NcSRS2-lipopeptides inducing T-lymphocyte responses in the current study likely involved similar enhancement of dendritic-cell antigen presentation through TLR-based mechanisms, and the responses occurred in cattle with diverse genetic backgrounds.
N. caninum antigen subunits have previously been shown to be effective in inducing protective immune responses in murine models of neosporosis (8, 15, 20, 21, 30, 32). The present study is the first report of the induction of potentially protective cell-mediated and humoral immune responses in cattle by using subunits of N. caninum antigens. The immune responses induced by NcSRS2-lipopeptides shown in the present study, namely, T-lymphocyte proliferation in and IFN-γ secretion by PBMC, were similar to the immune responses induced by live N. caninum infection in previous studies (22, 39, 43). The induction of CD4+ cytotoxic T-lymphocytes, T-lymphocyte proliferation in PBMC, and IFN-γ-secreting cells in PBMC are evident in cattle experimentally infected with N. caninum, including pregnant cattle (1, 36, 40). More importantly, parasite-specific immune responses involving T-lymphocyte activation and IFN-γ secretion in PBMC are associated with protection against N. caninum-induced fetal transmission and death (19, 22, 43). Because the induction of cell-mediated immune responses and IFN-γ secretion appear crucial to fetal protection and NcSRS subunits induce those same immune responses, it is logical to extend the study toward testing the efficacy of NcSRS2-lipopeptide immunization against fetal infection and death in pregnant cattle.
In the present study, the cattle were at least half-matched for MHC-II haplotypes shown previously to respond to NcSRS2 epitope clusters by the binding of multiple MHC types to activate CD4+ T-lymphocytes and IFN-γ in N. caninum-infected cattle (39). Although limiting, the selected NcSRS2-presenting MHC-II haplotypes (DH08A, DH16A, DH22H, DH24A, and DH27A) are widely represented in the Holstein cattle population (31). The robustness of the current study was further increased by using Holstein cattle from different genetic stocks (herds from the U.S. Pacific Northwest and Israel). An obstacle to developing T-lymphocyte-inducing subunit vaccines effective in cattle is the genetic polymorphism of the major MHC molecules. However, as shown here, cattle with disparate MHC haplotypes responded to immunization with NcSRS2-lipopeptides with immune responses involving T-lymphocytes and IFN-γ. Within all vaccinated groups at WSU and the KVI, there was no apparent pattern in the PBMC proliferation, PBCM IFN-γ ELISPOT results, or IgG isotype responses of individual cattle with either MHC-I or MHC-II haplotypes. Thus, it appears that including cross-reactive epitope clusters or supertype motifs in the design of T-lymphocyte-inducing vaccines makes the use of subunit vaccines in large cattle populations plausible.
Field observations suggest that natural immunity to N. caninum-induced abortion develops (22). Critical parameters to consider with vaccine development for bovine neosporosis were recently recommended by the World Association for the Advancement of Veterinary Parasitology (9). Information for the development of vaccines for bovine neosporosis should include efficacy for both exogenous and endogenous transplacental transmission (41), safety, ability to induce maternal CD4+ T-lymphocytes and IFN-γ, and compatibility with current diagnostic techniques so that vaccinated and infected cattle can be distinguished (12). A subunit vaccine based upon NcSRS2-lipopeptide could fulfill many of these parameters, including safety (compared to whole-N. caninum isolates, which could contain bovine spongiform encephalopathy prions), targeting of relevant immune responses rather than broad immune responses that may in some cases exacerbate disease (4, 38), and the ability to easily distinguish infected and immunized cattle, as was demonstrated in the current study. Although there was strong induction of NcSRS2-specific antibody in cattle immunized with NcSRS2-lipopeptides, the immunization did not induce antibodies to an unrelated immunodominant N. caninum tachyzoite surface glycoprotein used in a commercial serology ELISA kit (3). Thus, NcSRS2-lipopeptide-immunized cattle could be easily distinguished from naturally infected cattle when using an NcSRS2-lipopeptide ELISA and some commercially available N. caninum diagnostic tests, allowing seroepidemiological approaches to continue to be used in vaccinated herds. Firstly, efficacy studies of an NcSRS2 subunit vaccine in cattle showing protection of vaccinated cows against N. caninum-induced reproductive loss would need to be successfully completed.
Acknowledgments
This research was supported by research grant no. US-3538-04CR from BARD, the United States-Israel Binational Agricultural Research and Development Fund.
Footnotes
Published ahead of print on 27 February 2008.
REFERENCES
- 1.Andrianarivo, A. G., B. C. Barr, M. L. Anderson, J. D. Rowe, A. E. Packham, K. W. Sverlow, and P. A. Conrad. 2001. Immune responses in pregnant cattle and bovine fetuses following experimental infection with Neospora caninum. Parasitol. Res. 87:817-825. [DOI] [PubMed] [Google Scholar]
- 2.Baszler, T. V., S. Adams, J. Vander-Schalie, B. A. Mathison, and M. Kostovic. 2001. Validation of a commercially available monoclonal antibody-based competitive-inhibition enzyme-linked immunosorbent assay for detection of serum antibodies to Neospora caninum in cattle. J. Clin. Microbiol. 39:3851-3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baszler, T. V., D. P. Knowles, J. P. Dubey, J. M. Gay, B. A. Mathison, and T. F. McElwain. 1996. Serological diagnosis of bovine neosporosis by Neospora caninum monoclonal antibody-based competitive inhibition enzyme-linked immunosorbent assay. J. Clin. Microbiol. 34:1423-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baszler, T. V., T. F. McElwain, and B. A. Mathison. 2000. Immunization of BALB/c mice with killed Neospora caninum tachyzoite antigen induces a type 2 immune response and exacerbates encephalitis and neurological disease. Clin. Diagn. Lab. Immunol. 7:893-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.BenMohamed, L., A. Thomas, and P. Druilhe. 2004. Long-term multiepitopic cytotoxic-T-lymphocyte responses induced in chimpanzees by combinations of Plasmodium falciparum liver-stage peptides and lipopeptides. Infect. Immun. 72:4376-4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, L. E., and D. C. Jackson. 2005. Lipid-based self-adjuvanting vaccines. Curr. Drug Deliv. 2:383-393. [DOI] [PubMed] [Google Scholar]
- 7.Brown, W. C., G. H. Palmer, H. A. Lewin, and T. C. McGuire. 2001. CD4+ T lymphocytes from calves immunized with Anaplasma marginale major surface protein 1 (MSP1), a heteromeric complex of MSP1a and MSP1b, preferentially recognize the MSP1a carboxyl terminus that is conserved among strains. Infect. Immun. 69:6853-6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cannas, A., A. Naguleswaran, N. Muller, S. Eperon, B. Gottstein, and A. Hemphill. 2003. Vaccination of mice against experimental Neospora caninum infection using NcSAG1- and NcSRS2-based recombinant antigens and DNA vaccines. Parasitology 126:303-312. [DOI] [PubMed] [Google Scholar]
- 9.Conraths, F. J., and L. M. Ortega Mora. 2005. Options for control of protozoal abortion in ruminants: practical experience, conclusions. Workshop session T, p. 229. 20th Int. Conf. World Assoc. Adv. Vet. Parasitol., Christchurch, New Zealand, 16 to 20 October 2005.
- 10.Dubey, J. P., D. Buxton, and W. Wouda. 2006. Pathogenesis of bovine neosporosis. J. Comp. Pathol. 134:267-289. [DOI] [PubMed] [Google Scholar]
- 11.Dubey, J. P., and G. Schares. 2006. Diagnosis of bovine neosporosis. Vet. Parasitol. 140:1-34. [DOI] [PubMed] [Google Scholar]
- 12.Dubey, J. P., G. Schares, and L. M. Ortega-Mora. 2007. Epidemiology and control of neosporosis and Neospora caninum. Clin. Microbiol. Rev. 20:323-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraser, D. G., S. R. Leib, B. S. Zhang, R. H. Mealey, W. C. Brown, and T. C. McGuire. 2005. Lymphocyte proliferation responses induced to broadly reactive Th peptides did not protect against equine infectious anemia virus challenge. Clin. Diagn. Lab. Immunol. 12:983-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gahery, H., N. Daniel, B. Charmeteau, L. Ourth, A. Jackson, M. Andrieu, J. Choppin, D. Salmon, G. Pialoux, and J. G. Guillet. 2006. New CD4+ and CD8+ T cell responses induced in chronically HIV type-1-infected patients after immunizations with an HIV type 1 lipopeptide vaccine. AIDS Res. Hum. Retrovir. 22:684-694. [DOI] [PubMed] [Google Scholar]
- 15.Haldorson, G. J., B. A. Mathison, K. Wenberg, P. A. Conrad, J. P. Dubey, A. J. Trees, I. Yamane, and T. V. Baszler. 2005. Immunization with native surface protein NcSRS2 induces a Th2 immune response and reduces congenital Neospora caninum transmission in mice. Int. J. Parasitol. 35:1407-1415. [DOI] [PubMed] [Google Scholar]
- 16.Haldorson, G. J., J. B. Stanton, B. A. Mathison, C. E. Suarez, and T. V. Baszler. 2006. Neospora caninum: antibodies directed against tachyzoite surface protein NcSRS2 inhibit parasite attachment and invasion of placental trophoblasts in vitro. Exp. Parasitol. 112:172-178. [DOI] [PubMed] [Google Scholar]
- 17.Huang, P., M. Liao, H. Zhang, E. G. Lee, Y. Nishikawa, and X. Xuan. 2007. The dense granule protein NcGRA7, a new marker for the serodiagnosis of Neospora caninum infection in aborting cows. Clin. Vaccine Immunol. 14:1640-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Innes, E. A., A. G. Andrianarivo, C. Bjorkman, D. J. Williams, and P. A. Conrad. 2002. Immune responses to Neospora caninum and prospects for vaccination. Trends Parasitol. 18:497-504. [DOI] [PubMed] [Google Scholar]
- 19.Innes, E. A., S. E. Wright, S. Maley, A. Rae, A. Schock, E. Kirvar, P. Bartley, C. Hamilton, I. M. Carey, and D. Buxton. 2001. Protection against vertical transmission in bovine neosporosis. Int. J. Parasitol. 31:1523-1534. [DOI] [PubMed] [Google Scholar]
- 20.Jenkins, M., C. Parker, W. Tuo, B. Vinyard, and J. P. Dubey. 2004. Inclusion of CpG adjuvant with plasmid DNA coding for NcGRA7 improves protection against congenital neosporosis. Infect. Immun. 72:1817-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liddell, S., C. Parker, B. Vinyard, M. Jenkins, and J. P. Dubey. 2003. Immunization of mice with plasmid DNA coding for NcGRA7 or NcsHSP33 confers partial protection against vertical transmission of Neospora caninum. J. Parasitol. 89:496-500. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Gatius, F., S. Almeria, G. Donofrio, C. Nogareda, I. Garcia-Ispierto, G. Bech-Sabat, P. Santolaria, J. L. Yaniz, M. Pabon, N. M. de Sousa, and J. F. Beckers. 2007. Protection against abortion linked to gamma interferon production in pregnant dairy cows naturally infected with Neospora caninum. Theriogenology 68:1067-1073. [DOI] [PubMed] [Google Scholar]
- 23.Lunden, A., J. Marks, S. W. Maley, and E. A. Innes. 1998. Cellular immune responses in cattle experimentally infected with Neospora caninum. Parasite Immunol. 20:519-526. [DOI] [PubMed] [Google Scholar]
- 24.Marks, J., A. Lunden, D. Harkins, and E. Innes. 1998. Identification of Neospora antigens recognized by CD4+ T cells and immune sera from experimentally infected cattle. Parasite Immunol. 20:303-309. [DOI] [PubMed] [Google Scholar]
- 25.Meeusen, E. N., J. Walker, A. Peters, P. P. Pastoret, and G. Jungersen. 2007. Current status of veterinary vaccines. Clin. Microbiol. Rev. 20:489-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menzies, M., and A. Ingham. 2006. Identification and expression of Toll-like receptors 1-10 in selected bovine and ovine tissues. Vet. Immunol. Immunopathol. 109:23-30. [DOI] [PubMed] [Google Scholar]
- 27.Moreira, A. L., L. Tsenova, M. H. Aman, L. G. Bekker, S. Freeman, B. Mangaliso, U. Schroder, J. Jagirdar, W. N. Rom, M. G. Tovey, V. H. Freedman, and G. Kaplan. 2002. Mycobacterial antigens exacerbate disease manifestations in Mycobacterium tuberculosis-infected mice. Infect. Immun. 70:2100-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mwangi, W., W. C. Brown, H. A. Lewin, C. J. Howard, J. C. Hope, T. V. Baszler, P. Caplazi, J. Abbott, and G. H. Palmer. 2002. DNA-encoded fetal liver tyrosine kinase 3 ligand and granulocyte macrophage-colony-stimulating factor increase dendritic cell recruitment to the inoculation site and enhance antigen-specific CD4+ T cell responses induced by DNA vaccination of outbred animals. J. Immunol. 169:3837-3846. [DOI] [PubMed] [Google Scholar]
- 29.Mwangi, W., W. C. Brown, G. A. Splitter, C. J. Davies, C. J. Howard, J. C. Hope, Y. Aida, Y. Zhuang, B. J. Hunter, and G. H. Palmer. 2007. DNA vaccine construct incorporating intercellular trafficking and intracellular targeting motifs effectively primes and induces memory B- and T-cell responses in outbred animals. Clin. Vaccine Immunol. 14:304-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishikawa, Y., X. Xuan, H. Nagasawa, I. Igarashi, K. Fujisaki, H. Otsuka, and T. Mikami. 2001. Prevention of vertical transmission of Neospora caninum in BALB/c mice by recombinant vaccinia virus carrying NcSRS2 gene. Vaccine 19:1710-1716. [DOI] [PubMed] [Google Scholar]
- 31.Park, Y. H., Y. S. Joo, J. Y. Park, J. S. Moon, S. H. Kim, N. H. Kwon, J. S. Ahn, and C. J. Davies. 2004. Characterization of lymphocyte subpopulations and the major histocompatibility complex haplotypes of mastitis-resistant and susceptible cows. J. Vet. Sci. 5:29-39. [PubMed] [Google Scholar]
- 32.Pinitkiatisakul, S., J. G. Mattsson, M. Wikman, M. Friedman, K. L. Bengtsson, S. Stahl, and A. Lunden. 2005. Immunisation of mice against neosporosis with recombinant NcSRS2 iscoms. Vet. Parasitol. 129:25-34. [DOI] [PubMed] [Google Scholar]
- 33.Ridgely, S. L., B. Zhang, and T. C. McGuire. 2003. Response of ELA-A1 horses immunized with lipopeptide containing an equine infectious anemia virus ELA-A1-restricted CTL epitope to virus challenge. Vaccine 21:491-506. [DOI] [PubMed] [Google Scholar]
- 34.Roberts, M. T., C. B. Stober, A. N. McKenzie, and J. M. Blackwell. 2005. Interleukin-4 (IL-4) and IL-10 collude in vaccine failure for novel exacerbatory antigens in murine Leishmania major infection. Infect. Immun. 73:7620-7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romero, J. J., E. Perez, and K. Frankena. 2004. Effect of a killed whole Neospora caninum tachyzoite vaccine on the crude abortion rate of Costa Rican dairy cows under field conditions. Vet. Parasitol. 123:149-159. [DOI] [PubMed] [Google Scholar]
- 36.Rosbottom, A., C. S. Guy, E. H. Gibney, R. F. Smith, J. F. Valarcher, G. Taylor, and D. J. Williams. 2007. Peripheral immune responses in pregnant cattle following Neospora caninum infection. Parasite Immunol. 29:219-228. [DOI] [PubMed] [Google Scholar]
- 37.Shkap, V., A. Reske, E. Pipano, L. Fish, and T. Baszler. 2002. Immunological relationship between Neospora caninum and Besnoitia besnoiti. Vet. Parasitol. 106:35-43. [DOI] [PubMed] [Google Scholar]
- 38.Srinivasan, S., J. Mueller, A. Suana, and A. Hemphill. 2007. Vaccination with microneme protein NcMIC4 increases mortality in mice inoculated with Neospora caninum. J. Parasitol. 93:1046-1055. [DOI] [PubMed] [Google Scholar]
- 39.Staska, L. M., C. J. Davies, W. C. Brown, T. C. McGuire, C. E. Suarez, J. Y. Park, B. A. Mathison, J. R. Abbott, and T. V. Baszler. 2005. Identification of vaccine candidate peptides in the NcSRS2 surface protein of Neospora caninum by using CD4+ cytotoxic T lymphocytes and gamma interferon-secreting T lymphocytes of infected Holstein cattle. Infect. Immun. 73:1321-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Staska, L. M., T. C. McGuire, C. J. Davies, H. A. Lewin, and T. V. Baszler. 2003. Neospora caninum-infected cattle develop parasite-specific CD4+ cytotoxic T lymphocytes. Infect. Immun. 71:3272-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trees, A. J., and D. J. Williams. 2005. Endogenous and exogenous transplacental infection in Neospora caninum and Toxoplasma gondii. Trends Parasitol. 21:558-561. [DOI] [PubMed] [Google Scholar]
- 42.Williams, D. J., C. S. Guy, J. W. McGarry, F. Guy, L. Tasker, R. F. Smith, K. MacEachern, P. J. Cripps, D. F. Kelly, and A. J. Trees. 2000. Neospora caninum-associated abortion in cattle: the time of experimentally-induced parasitaemia during gestation determines foetal survival. Parasitology 121:347-358. [DOI] [PubMed] [Google Scholar]
- 43.Williams, D. J., C. S. Guy, R. F. Smith, J. Ellis, C. Bjorkman, M. P. Reichel, and A. J. Trees. 2007. Immunization of cattle with live tachyzoites of Neospora caninum confers protection against fetal death. Infect. Immun. 75:1343-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu, X., T. V. Ramos, H. Gras-Masse, B. E. Kaplan, and L. BenMohamed. 2004. Lipopeptide epitopes extended by an Nɛ-palmitoyl-lysine moiety increase uptake and maturation of dendritic cells through a Toll-like receptor-2 pathway and trigger a Th1-dependent protective immunity. Eur. J. Immunol. 34:3102-3114. [DOI] [PubMed] [Google Scholar]