Abstract
The nontoxic mutant lethal factor (mLF; which has the E687C substitution) and functional protective antigen (PA63) of Bacillus anthracis were evaluated for their use as mucosal vaccines against anthrax in A/J mice. Intranasal vaccination of three doses of 30 μg of mLF or 60 μg of PA63 elicited significant serum and mucosal antibody responses, with anthrax lethal toxin-neutralizing titers of 40 and 60 in immune sera, respectively. However, only 30% and 60% of the vaccinated animals in the two groups could survive a challenge with 100 times the 50% lethal dose of B. anthracis Sterne spores, respectively. In contrast, vaccination with three doses of the combination of 30 μg of mLF and 60 μg of PA63, the detoxified lethal toxin, elicited antibody responses against LF and PA significantly higher than those elicited after vaccination with mLF or PA63 individually by use of the same dose and schedule. Vaccination with the detoxified lethal toxin resulted in significantly higher lethal toxin-neutralizing antibody titers in sera (titer, 90). Animals vaccinated with three doses of the detoxified lethal toxin were completely protected against the spore challenge. The data suggest that mLF and PA63 have a mutual enhancement effect for evoking systemic and mucosal immune responses and that the detoxified lethal toxin can be used as an efficient mucosal vaccine against anthrax.
Anthrax, a severe infectious disease in human and animals, is caused by the gram-positive, spore-forming organism Bacillus anthracis (22). Recently, the intentional dissemination of B. anthracis Ames spores by bioterrorists caused 11 cases of cutaneous anthrax and 11 cases of inhalational anthrax, which resulted in five deaths in the United States (14). Thus, a program of immunization against anthrax is needed for selective military, emergency response, and medical personnel for biodefense preparedness.
The disease-causing B. anthracis strains are characterized by a poly-γ-d-glutamic acid capsule and the production of the three-component toxins: protective antigen (PA), lethal factor (LF), and edema factor (EF). Virulent strains of B. anthracis carry two plasmids, pX01 and pX02, which encode the three toxin components and enzymes for capsule biosynthesis. The 83-kDa PA, 85-kDa LF, and 89-kDa EF toxin components are encoded by the pag, lef, and cya genes in pX01, respectively (6). PA binds to host cell anthrax toxin receptors (ANTXRs), such as tumor endothelial marker 8 (TEM8/ANTXR1) and human capillary morphogenesis protein 2 (CMG2/ANTXR2), and is cleaved by cell surface furin to produce a 63-kDa fragment, PA63 (4). After PA63 binds to ANTXRs, it oligomerizes to a heptamer and acts to translocate the catalytic moieties of LF and EF from the endosomes to the cytosol (3). The combination of PA and LF is named anthrax lethal toxin (Letx). LF is a zinc-dependent endopeptidase which removes specifically the N-terminal tail of mitogen-activated protein kinase kinases, leading to macrophage lysis (1). EF is an adenylate cyclase that generates cyclic AMP. The combination of PA and EF, named edema toxin, disables phagocytes and other cells due to the intracellular adenylate cyclase activity of EF (21). Recently, we found that the expression of ANTXR mRNA in murine macrophage J774A.1 cells was upregulated by edema toxin (32).
The current U.S. human anthrax vaccine, BioThrax, produced by BioPort Corporation (Lansing, MI), consists of aluminum hydroxide-adsorbed supernatant material, primarily PA and undefined quantities of LF and EF, from fermentor cultures of a toxigenic, nonencapsulated strain of B. anthracis. Human vaccination with the BioThrax vaccine requires six immunizations, followed by annual boosters (anthrax vaccine adsorbed [BioThrax] product insert, Bioport Corporation, 2002). This underscores the need to develop more efficacious vaccines or alternative vaccination regimens (18).
PA has been shown to be an essential component of an anthrax vaccine (5). There have been intensive efforts to improve the safety profile and immunogenicity of the anthrax vaccine by using PA as an antigen, including the formulation of PA in adjuvants (19), conjugation of capsular poly-γ-d-glutamic acid to PA (26), the use of purified PA (27) and its C-terminal domain 4 (PA-D4) (10), the development of PA-based DNA vaccines (13), and the expression of PA in viral and bacterial vectors (12, 29). PA-D4 (residues 596 to 735 in PA) is responsible for binding to cellular receptors (ANTXRs) and has been shown to contain the dominant protective epitopes of PA (10). However, studies of spore vaccines suggested that some other B. anthracis antigens may contribute to protective immunity in a significant manner (7).
It has been suggested that in addition to PA, LF and EF also play an important role in providing immunity (24). Recent research indicated that immunization with plasmid expression vectors in a combination of PA and N-terminal region-truncated LF (LFn; residues 10 to 254 of the mature LF protein) provides better protection than PA alone (11). In addition, we have shown that immunization with an adenoviral vector expressing the N-terminal fragment of EF (EFn; residues 1 to 254) elicited neutralizing antibody responses against both EF and LF and provided partially protective immunity against anthrax (33). Unfortunately, vaccination with solely somatic components of B. anthracis, such as surface polysaccharides and cell-associated antigens EA1 and EA2, has not been shown to provide protective immunity (9).
Mutation at amino acid 687 of LF has been shown to eliminate its enzymatic activity (20). Although the detoxified mutant LF (mLF; which has the E687C substitution), which has a Glu-to-Cys substitution in the zinc binding site, was made in research conducted more than a decade ago, the feasibility of using it as a candidate anthrax vaccine has not been shown to date. In this report, we provide experimental data that demonstrate that detoxified Letx can be used as a mucosal vaccine against anthrax.
MATERIALS AND METHODS
Materials.
Recombinant full-length PA, functional PA (PA63), LF, and mLF were purchased from List Biological Laboratories, Inc. (Campbell, CA). mLF (E687C) has a Glu-to-Cys substitution in the zinc binding site. The mutation at amino acid 687 has been shown to eliminate enzymatic activity (20). B. anthracis Sterne strain spores were from an anthrax spore vaccine, which is a viable suspension of the Sterne strain 34F2 spores in saponin (Colorado Serum Company, Denver, CO). Mouse monocyte macrophage cell line J774A.1 was purchased from the American Type Culture Collection (Manassas, VA).
Animal vaccination and sample collection.
Six- to eight-week-old female A/J mice were purchased from Jackson Laboratory (Bar Harbor, ME). They were housed in a biosafety level 2 pathogen-free animal facility at the University of Rochester Medical Center (four mice per cage) and were maintained in a controlled environment (22 ± 2°C; 12 h-light/12 h-dark cycles). The animals were provided Laboratory Rodent Diet 5001 with ad libitum access to food and water. The research was conducted in compliance with the Animal Welfare Act and other federal and state statutes and regulations relating to animals and experiments involving animals and adhered to the principles stated in the Guide for the Care and Use of Laboratory Animals (23).
Evaluation of immunogenicity and efficacy.
Thirty-two animals were allotted into four groups (eight mice per group). After anesthesia with ketamine and xylazine, they were intranasally administered 60 μg of PA63, 30 μg of mLF, 60 μg of PA63 plus 30 μg of mLF in 30 μl of physiological saline, or 30 μl of physiological saline only (negative control) at weeks 0, 2, and 4. Serum samples were obtained by retro-orbital bleeding at weeks 0, 2, 4, and 6 for determination of anti-PA and anti-LF antibody concentrations and Letx neutralization. The animals were subcutaneously challenged with 100 times the 50% lethal dose (LD50) of B. anthracis Sterne spores at week 7, as described previously (34).
Assessment of mucosal immune responses.
Sixteen mice were divided into four groups (four mice per group) and were intranasally administered 60 μg of PA63, 30 μg of mLF, 60 μg of PA63 plus 30 μg of mLF, or 30 μl of physiological saline (negative control) at weeks 0, 2, and 4. After the animals were killed at week 6, mucosal secretions, such as saliva, nasal wash, and vaginal wash samples, were collected to measure the anti-PA and anti-LF antibody concentrations by a quantitative enzyme-linked immunosorbent assay (ELISA), as described previously (34).
Quantitative ELISA for measurement of antigen-specific antibody responses.
Anti-PA and anti-LF immunoglobulin G (IgG), IgG1, and IgG2a concentrations in sera and anti-PA and anti-LF IgG and IgA concentrations in saliva, nasal wash, and vaginal wash samples were determined by a modified procedure with an ELISA quantitation kit (Bethel Laboratories Inc., Montgomery, TX). Briefly, 96-well flat-bottom immunoplates (Nalge Nunc International, Rochester, NY) were coated with recombinant PA (100 ng/well) in 100 μl coating buffer (0.05 M carbonate-bicarbonate buffer, pH 9.6) and incubated overnight at 4°C. After the wells were washed five times with washing buffer (0.05% Tween 20 in phosphate-buffered saline [PBS]), the wells were blocked with 200 μl of blocking buffer (PBS containing 1.0% bovine serum albumin) for 1 h at room temperature. After another five washes, 1 μl (serum) or 10 μl of a saliva, nasal wash, or vaginal wash sample was mixed with 100 μl of sample buffer (PBS, pH 7.4, containing 0.05% Tween 20 and 1% bovine serum albumin) prior to addition to a well, followed by 2 h of incubation at 37°C. The plates were washed with washing buffer five times and were then incubated with 100 μl (per well) of a 1:10,000 dilution of goat anti-mouse IgG, IgG1, or IgG2a conjugated to alkaline phosphatase for 1 h at room temperature. Unbound antibodies were removed by washing the plates five times with washing buffer, and the bound antibodies were detected following incubation with a p-nitrophenylphosphate phosphatase substrate system (Kirkegaard & Perry Laboratories, Gaithersburg, MD) for 30 min. The reaction was stopped by addition of 0.5 M EDTA, and the absorbance values were obtained with a Dynatech microplate reader (model MR4000) at 405 nm. Each sample was analyzed in duplicate, and measurement of the antibody subclasses was performed in parallel. A standard curve was generated in parallel for each set of samples by using capture antibodies (affinity-purified goat anti-mouse IgG, IgG1, IgG2a, or IgA) and mouse reference serum containing the given amount of antibodies, according to the manufacturer's instruction. Antibody concentrations were calculated by comparison with the standard curve.
Measurement of Letx-neutralizing antibody titer.
The titers of neutralizing antibodies to Letx were determined by measuring the ability of sera to neutralize the cytotoxicity of Letx (LF plus PA) in murine macrophage J774A.1 cells, as described previously (34). Briefly, 5 × 104 J774A.1 cells in 100 μl of Dulbecco's modified Eagle's medium containing 10% fetal bovine serum were seeded into each well of 96-well culture plates and were grown to 90% confluence at 37°C. Twofold serial dilutions of the serum samples were incubated at 37°C for 1 h with recombinant PA and LF at final concentrations of 60 and 40 ng/ml, respectively, prior to addition to the wells. After a 7-h incubation at 37°C, 20 μl of 3-(4,5-dimethlthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Sigma-Aldrich, St. Louis, MO) at 5 mg/ml in PBS was added to each well, and then the plates were incubated for an additional 2 h. Then, 50 μl of 20% sodium dodecyl sulfate in 50% dimethyl formamide was added to each well. The optical densities were measured at 570 nm, with a reference wavelength of 690 nm, by using a microplate reader (model MR4000; Dynatech). The ratio of LF plus serum and PA versus medium alone, expressed as percent cell viability, was calculated for each dilution. The Letx-neutralizing antibody titers of the serum samples, calculated by linear regression analysis, were expressed as the reciprocal antibody dilution that prevented the death of 50% of the cells.
Challenge with B. anthracis Sterne spores.
The vaccinated animals were challenged by subcutaneous injection of a dose of approximately 100 times the LD50 (31), which consisted of 2 × 105 spores of B. anthracis Sterne, 3 weeks after the last vaccination. The B. anthracis Sterne spores were from an anthrax spore vaccine, which is a viable suspension of the Sterne strain 34F2 spores in saponin (Colorado Serum Company). The animals were monitored twice a day for 1 week after spore challenge and daily thereafter.
Statistical analysis.
The antibody concentrations among the different groups at different time points were compared and analyzed by using the Fisher least-significant-difference test and analysis of variance. For comparisons of the groups, P values of <0.05 were considered significantly different. All statistical analyses were performed with Statistica (version 7.1) software from StatSoft, Inc. (Tulsa, OK).
RESULTS
Serum antibody responses after intranasal vaccination with detoxified Letx.
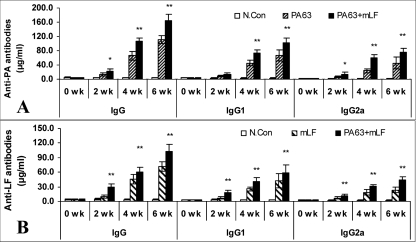
To determine if detoxified Letx could be used as a candidate mucosal vaccine, groups of A/J mice were inoculated intranasally with 60 μg of PA63, 30 μg of mLF, or 60 μg of PA 63 plus 30 μg of mLF three times at weeks 0, 2, and 4. Serum samples were collected at weeks 0, 2, 4, and 6 to determine the anti-PA and anti-LF antibody concentrations by quantitative ELISA. Figure 1A and B (n = 8) show the anti-LF and anti-PA antibody titers in sera from the experimental groups, respectively.
FIG. 1.
Antigen-specific serum antibody responses in mice after mucosal vaccination with detoxified Letx. (A) Anti-PA antibody concentrations; (B) anti-LF antibody concentrations. Six- to 8-week-old A/J mice were intranasally vaccinated with a dose of 60 μg of PA63 and/or 30 μg of mLF at weeks 0, 2, and 4. Serum samples were collected at weeks 0, 2, 4, and 6. Anti-PA and anti-LF IgG, IgG1, and IgG2a antibody concentrations were measured with a quantitative ELISA kit (Bethel Laboratories). N.Con (control), animals were intranasally inoculated with physiological saline. Values are means ± standard errors (n = 8). *, P < 0.05 compared with the results obtained with PA63 alone (A) or mLF alone (B); **, P < 0.01 compared with the results obtained with PA63 alone (A) or mLF alone (B).
Animals vaccinated with three doses of the single antigen mLF or PA63 had significant serum antibody responses against LF or PA, including total IgG, IgG1, and IgG2a responses, in comparison with the responses of the control group (P < 0.05). Clearly, both Th2 and Th1 immune responses against PA and LF, with predominant Th2 responses, were elicited (IgG2a and IgG1 titers, <1.0). In addition, the data show that booster vaccination (the second and third doses) significantly increased the serum antibody responses against LF and PA.
Interestingly, intranasal vaccination with the combination of 60 μg of PA63 and 30 μg of mLF, the detoxified Letx, elicited significantly higher antibody responses against LF (Fig. 1A) and PA (Fig. 1B) than vaccination with the same amount of PA63 or mLF individually at all time points at which the responses were measured (P < 0.05). The data suggest that PA63 and mLF had a mutual enhancement effect in evoking the host immune response.
Mucosal antibody responses after intranasal vaccination with detoxified Letx.
To determine if intranasal mucosal vaccination with detoxified Letx was able to elicit antigen-specific mucosal immunity, we measured secretory anti-PA and anti-LF antibody levels in saliva, nasal wash, and vaginal wash samples from vaccinated animals. The anti-PA IgA and IgG concentrations in mucosal secretions after vaccination with three doses are shown in Fig. 2 (n = 4). Significant mucosal anti-PA IgG and IgA responses were observed after intranasal vaccination with three doses of 60 μg of PA63 (P < 0.05) (Fig. 2A). Similarly, vaccination with three doses of 30 μg of mLF elicited significant mucosal anti-LF IgG and IgA responses (P < 0.05) (Fig. 2A). In contrast, three doses of detoxified Letx consisting of 60 μg of PA63 and 30 μg of mLF resulted in considerably higher mucosal anti-PA and anti-LF IgG and IgA responses than vaccination with PA63 or mLF alone. The data are consist with the serum antibody response results, which showed that PA63 and mLF had a mutual enhancement effect in evoking a host immune response when they were combined in the mucosal vaccine.
FIG. 2.
Antigen-specific mucosal antibody responses in mice after mucosal vaccination with detoxified Letx. (A) Anti-PA antibody concentrations; (B) anti-LF antibody concentrations. Six- to 8-week-old A/J mice were intranasally vaccinated with a dose of 60 μg of PA63 and/or 30 μg of mLF at weeks 0, 2, and 4. Saliva, nasal wash, and vaginal wash samples were collected at week 6. Anti-PA and anti-LF IgG and IgA concentrations were measured with a quantitative ELISA kit (Bethel Laboratories). Control animals were intranasally inoculated with physiological saline. Values are means ± standard errors (n = 4). *, P < 0.05 compared with the results obtained with PA63 (A) or mLF (B); **, P < 0.01 compared with the results obtained with PA63 (A) or mLF (B).
In vitro protection against Letx.
In order to determine whether anti-PA and anti-LF antibodies in the immune sera were capable of neutralizing Letx, an in vitro protection assay was performed with J774A.1 mouse macrophage cells, which are sensitive to Letx. As shown in Fig. 3 (n = 8), intranasal vaccination with three doses of 60 μg of PA63, 30 μg of mLF, or detoxified Letx (60 μg of PA63 plus 30 μg of mLF) elicited significant Letx-neutralizing antibody responses, with titers of 40, 60, and 90, respectively. Again, a mutual enhancement effect in the elicitation of an Letx-neutralizing antibody response was observed after administration of the combination of PA63 and mLF compared to the effect achieved after vaccination with PA63 or mLF alone.
FIG. 3.
In vitro neutralization of Letx by sera from vaccinated animals. Mice were intranasally vaccinated with 60 μg of PA63 or/and 30 μg of mLF at weeks 0, 2, and 4. Serum samples were obtained at weeks 0, 2, 4, and 6. Serial twofold dilutions of sera were preincubated with recombinant LF and PA, and the mixtures were added to J774A.1 mouse macrophage cells. Cell viability was measured by a colorimetric toxin neutralization assay. The Letx-neutralizing antibody titers are expressed as the reciprocal of the antibody dilutions that prevented the death of 50% of the cells. Control animals were intranasally inoculated with physiological saline. Values are means ± standard errors (n = 8). The values in columns without the same letters (a, b, c, or d) differ significantly between different treatments (P < 0.05).
Protective immunity elicited by intranasal vaccination with detoxified Letx.
After the A/J mice were intranasally vaccinated with three doses of PA63 (60 μg), mLF (30 μg), or detoxified Letx (60 μg of PA63 plus 30 μg of mLF), they were subcutaneously challenged with 100 times the LD50 of B. anthracis Sterne spores. Figure 4 shows that only 60% and 30% of the animals vaccinated with PA63 and with mLF survived the spore challenge, respectively. In contrast, animals vaccinated with detoxified Letx were completely protected against the spore challenge, while the control animals inoculated with physiological saline died 5 days after the spore challenge (Fig. 4, n = 8).
FIG. 4.
Protection of vaccinated mice against lethal challenge with B. anthraces Sterne spores. A/J mice were intranasally vaccinated with 60 μg of PA63 or/and 30 μg of mLF at weeks 0, 2, and 4 and were then subcutaneously challenged with B. anthracis Sterne spores at 100 times the LD50 at week 7 (n = 8). Con (control) animals were intranasally inoculated with physiological saline.
DISCUSSION
In this research, we provide evidence that intranasal vaccination with PA63, mLF, and detoxified Letx is capable of eliciting significant systemic immune responses (Fig. 1, n = 8) and mucosal immune responses (Fig. 1, n = 4) against PA or/and LF, as well as protective immunity against lethal challenge with B. anthracis spores (Fig. 4, n = 8). Letx neutralization assays also demonstrated that the anti-PA and anti-LF antibodies inhibit the toxicity of Letx by blocking the incorporation of LF into macrophage cells (Fig. 3, n = 8). Most interestingly, a significant synergistic enhancement effect of the host immune response was shown when PA63 and mLF were coadministered as a detoxified Letx. This mutual enhancement effect was demonstrated not only in serum and by the mucosal antibody responses but also as protective immunity against B. anthracis spore challenge.
Hepler et al. have shown that recombinant PA63 could be used for vaccination of rabbits and primates to prevent inhalational anthrax (16). In this study, we chose to use PA63 because PA63 is the biologically active form of PA in anthrax toxins. The reason for the mutual enhancement effect of PA63 and mLF in eliciting the host immune response, as shown in the current study, remains to be explored. One explanation is that the active entry of mLF into the host cells triggers the intracellular antigen presentation machinery for the efficient processing of intracellular antigens through the major histocompatibility complex class I pathway and augments the host immune response. In addition, the formation of the mLF-PA63 complex may also contribute to the boost of the host immune defense and accelerate antigen presentation on host antigen-presenting cells through the major histocompatibility complex class II pathway. Clearly, it is a significant finding that intranasal vaccination with detoxified Letx may have a strong potential to become an effective needle-free protocol for vaccination against anthrax. Since PA63 tends to aggregate, whereas the uncleaved PA83 protein does not, we will need to determine the optimal formulation for the candidate vaccine in order to avoid the aggregation of PA63 in future studies.
Immunization with the currently licensed anthrax vaccine (BioThrax) requires multiple injections. This highlights the inefficiency of the vaccination program. Apparently, well-characterized subunit vaccine candidates are needed, and perhaps more effective anthrax vaccines would contain multiple antigens. Other antigens, such as anthrax toxin components PA, the N-terminal fragments (amino acids 10 to 254) of LF (LFn) (25) and EF (EFn) (33), and B. anthracis spore-associated collagen-like glycoprotein BclA (15), have been evaluated and have been shown to be promising candidate antigens for use in vaccines against anthrax. Vaccination with a plasmid vector encoding LFn confers protection against Letx challenge, and the combination of LFn and PA provides better protection against anthrax than the use of LFn or PA alone does (11, 25). Similarly, vaccination with a combination of PA- and BclA-encoding plasmid DNA led to significantly better survival than vaccination with only PA-encoding or only BclA-encoding plasmids (15).
In addition, the alternative mucosal method of vaccination would be a significant advantage over the needle injection method of vaccination. It not only is a painless and convenient vaccination protocol but also is a highly efficient way to elicit mucosal immunity. B. anthracis spores may invade the host via the mucosal route and cause inhalational anthrax, which is the major threat when B. anthracis spores are used as a biological weapon. Therefore, host immunity at the mucosal sites of entry may be effective for protection against inhalational anthrax. Previous studies by Welkos and colleagues have demonstrated that anti-PA antibodies stimulate spore uptake and interfere with germination in macrophage culture and animal models (8, 30). This suggests a potentially protective role of anti-PA antibodies at mucosal surfaces against anthrax spore infection. Mucosal immunization but not parenteral immunization with PA induces immune responses in both the systemic and the secretory immune compartments (2). Our study demonstrated that intranasal vaccination with detoxified Letx elicited robust mucosal and systemic antibody responses against both PA and LF and subsequently protected the host against anthrax. Although previous studies have shown that PA is the candidate antigen of choice for systemic and intranasal vaccination against anthrax, an appropriate adjuvant is required for a PA-based vaccine for augmentation of the host immune response (2, 17, 28). However, the currently available vaccine adjuvunts (such as aluminum and microbial extraction adjuvunts) have not been licensed for nasal administration in humans. The advantage from the use of detoxified Letx for vaccination, as shown in the current study, is that our candidate mucosal anthrax vaccine can also elicit a host immune response against LF and it provides a more robust protective immune response than the use of PA alone. This adds another mechanism of protective immunity from vaccination. However, future experiments with additional animal models (murine, rabbit, and nonhuman primate models) with virulent B. anthracis spore challenge are needed to determine if this candidate mucosal vaccine could protect against inhalational anthrax. In addition, the use of new mucosal vaccine adjuvunts should also be explored in future studies so that one dose of the mucosal vaccine may be used to provide protection against anthrax.
In summary, the data reported here are the first to demonstrate that detoxified Letx could be used as an effective mucosal anthrax vaccine without the addition of any adjuvant. This candidate mucosal vaccine has merit for further studies in the search for a highly effective and easily administered new generation of anthrax vaccine.
Acknowledgments
This work was supported by U.S. Public Health Service research grant AI053598 (to M.Z.) from the National Institute of Allergy and Infectious Diseases.
We thank Mary C. Zeng for carefully editing the manuscript. We greatly appreciate the support of Michael E. Pichichero and Barbara H. Iglewski.
Footnotes
Published ahead of print on 6 February 2008.
REFERENCES
- 1.Ascenzi, P., P. Visca, G. Ippolito, A. Spallarossa, M. Bolognesi, and C. Montecucco. 2002. Anthrax toxin: a tripartite lethal combination. FEBS Lett. 531:384-388. [DOI] [PubMed] [Google Scholar]
- 2.Boyaka, P. N., A. Tafaro, R. Fischer, S. H. Leppla, K. Fujihashi, and J. R. McGhee. 2003. Effective mucosal immunity to anthrax: neutralizing antibodies and Th cell responses following nasal immunization with protective antigen. J. Immunol. 170:5636-5643. [DOI] [PubMed] [Google Scholar]
- 3.Bradley, K. A., J. Mogridge, M. Mourez, R. J. Collier, and J. A. Young. 2001. Identification of the cellular receptor for anthrax toxin. Nature 414:225-229. [DOI] [PubMed] [Google Scholar]
- 4.Bradley, K. A., and J. A. Young. 2003. Anthrax toxin receptor proteins. Biochem. Pharmacol. 65:309-314. [DOI] [PubMed] [Google Scholar]
- 5.Brey, R. N. 2005. Molecular basis for improved anthrax vaccines. Adv. Drug Deliv. Rev. 57:1266-1292. [DOI] [PubMed] [Google Scholar]
- 6.Brossier, F., and M. Mock. 2001. Toxins of Bacillus anthracis. Toxicon 39:1747-1755. [DOI] [PubMed] [Google Scholar]
- 7.Cohen, S., I. Mendelson, Z. Altboum, D. Kobiler, E. Elhanany, T. Bino, M. Leitner, I. Inbar, H. Rosenberg, Y. Gozes, R. Barak, M. Fisher, C. Kronman, B. Velan, and A. Shafferman. 2000. Attenuated nontoxinogenic and nonencapsulated recombinant Bacillus anthracis spore vaccines protect against anthrax. Infect. Immun. 68:4549-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cote, C. K., C. A. Rossi, A. S. Kang, P. R. Morrow, J. S. Lee, and S. L. Welkos. 2005. The detection of protective antigen (PA) associated with spores of Bacillus anthracis and the effects of anti-PA antibodies on spore germination and macrophage interactions. Microb. Pathog. 38:209-225. [DOI] [PubMed] [Google Scholar]
- 9.Ezzell, J. W., Jr., and T. G. Abshire. 1988. Immunological analysis of cell-associated antigens of Bacillus anthracis. Infect. Immun. 56:349-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flick-Smith, H. C., N. J. Walker, P. Gibson, H. Bullifent, S. Hayward, J. Miller, R. W. Titball, and E. D. Williamson. 2002. A recombinant carboxy-terminal domain of the protective antigen of Bacillus anthracis protects mice against anthrax infection. Infect. Immun. 70:1653-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galloway, D., A. Liner, J. Legutki, A. Mateczun, R. Barnewall, and J. Estep. 2004. Genetic immunization against anthrax. Vaccine 22:1604-1608. [DOI] [PubMed] [Google Scholar]
- 12.Garmory, H. S., R. W. Titball, K. F. Griffin, U. Hahn, R. Bohm, and W. Beyer. 2003. Salmonella enterica serovar Typhimurium expressing a chromosomally integrated copy of the Bacillus anthracis protective antigen gene protects mice against an anthrax spore challenge. Infect. Immun. 71:3831-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu, M. L., S. H. Leppla, and D. M. Klinman. 1999. Protection against anthrax toxin by vaccination with a DNA plasmid encoding anthrax protective antigen. Vaccine 17:340-344. [DOI] [PubMed] [Google Scholar]
- 14.Guarner, J., J. A. Jernigan, W. J. Shieh, K. Tatti, L. M. Flannagan, D. S. Stephens, T. Popovic, D. A. Ashford, B. A. Perkins, and S. R. Zaki. 2003. Pathology and pathogenesis of bioterrorism-related inhalational anthrax. Am. J. Pathol. 163:701-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn, U. K., R. Boehm, and W. Beyer. 2006. DNA vaccination against anthrax in mice-combination of anti-spore and anti-toxin components. Vaccine 24:4569-4571. [DOI] [PubMed] [Google Scholar]
- 16.Hepler, R. W., R. Kelly, T. B. McNeely, H. Fan, M. C. Losada, H. A. George, A. Woods, L. D. Cope, A. Bansal, J. C. Cook, G. Zang, S. L. Cohen, X. Wei, P. M. Keller, E. Leffel, J. G. Joyce, L. Pitt, L. D. Schultz, K. U. Jansen, and M. Kurtz. 2006. A recombinant 63-kDa form of Bacillus anthracis protective antigen produced in the yeast Saccharomyces cerevisiae provides protection in rabbit and primate inhalational challenge models of anthrax infection. Vaccine 24:1501-1514. [DOI] [PubMed] [Google Scholar]
- 17.Ivins, B. E., S. L. Welkos, S. F. Little, M. H. Crumrine, and G. O. Nelson. 1992. Immunization against anthrax with Bacillus anthracis protective antigen combined with adjuvants. Infect. Immun. 60:662-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joellenbeck, L. M., L. L. Zwanziger, J. S. Durch, and B. L. Strom. 2002. The anthrax vaccine. Is it safe? Does it work? National Academy Press, Washington, DC. [PubMed]
- 19.Kenney, R. T., J. Yu, M. Guebre-Xabier, S. A. Frech, A. Lambert, B. A. Heller, L. R. Ellingsworth, J. E. Eyles, E. D. Williamson, and G. M. Glenn. 2004. Induction of protective immunity against lethal anthrax challenge with a patch. J. Infect. Dis. 190:774-782. [DOI] [PubMed] [Google Scholar]
- 20.Klimpel, K. R., N. Arora, and S. H. Leppla. 1994. Anthrax toxin lethal factor contains a zinc metalloprotease consensus sequence which is required for lethal toxin activity. Mol. Microbiol. 13:1093-1100. [DOI] [PubMed] [Google Scholar]
- 21.Leppla, S. H. 2000. Anthrax toxin, p. 445-472. In K. Aktories and I. Just (ed.), Bacterial protein toxins, vol. 145. Springer-Verlag, Berlin, Germany. [Google Scholar]
- 22.Mock, M., and A. Fouet. 2001. Anthrax. Annu. Rev. Microbiol. 55:647-671. [DOI] [PubMed] [Google Scholar]
- 23.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 24.Pezard, C., M. Weber, J. C. Sirard, P. Berche, and M. Mock. 1995. Protective immunity induced by Bacillus anthracis toxin-deficient strains. Infect. Immun. 63:1369-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price, B. M., A. L. Liner, S. Park, S. H. Leppla, A. Mateczun, and D. R. Galloway. 2001. Protection against anthrax lethal toxin challenge by genetic immunization with a plasmid encoding the lethal factor protein. Infect. Immun. 69:4509-4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhie, G. E., M. H. Roehrl, M. Mourez, R. J. Collier, J. J. Mekalanos, and J. Y. Wang. 2003. A dually active anthrax vaccine that confers protection against both bacilli and toxins. Proc. Natl. Acad. Sci. USA 100:10925-10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh, Y., B. E. Ivins, and S. H. Leppla. 1998. Study of immunization against anthrax with the purified recombinant protective antigen of Bacillus anthracis. Infect. Immun. 66:3447-3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sloat, B. R., and Z. Cui. 2006. Nasal immunization with a dual antigen anthrax vaccine induced strong mucosal and systemic immune responses against toxins and bacilli. Vaccine 24:6405-6413. [DOI] [PubMed] [Google Scholar]
- 29.Tan, Y., N. R. Hackett, J. L. Boyer, and R. G. Crystal. 2003. Protective immunity evoked against anthrax lethal toxin after a single intramuscular administration of an adenovirus-based vaccine encoding humanized protective antigen. Hum. Gene Ther. 14:1673-1682. [DOI] [PubMed] [Google Scholar]
- 30.Welkos, S., S. Little, A. Friedlander, D. Fritz, and P. Fellows. 2001. The role of antibodies to Bacillus anthracis and anthrax toxin components in inhibiting the early stages of infection by anthrax spores. Microbiology 147:1677-1685. [DOI] [PubMed] [Google Scholar]
- 31.Welkos, S. L., T. J. Keener, and P. H. Gibbs. 1986. Differences in susceptibility of inbred mice to Bacillus anthracis. Infect. Immun. 51:795-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu, Q., E. D. Hesek, and M. Zeng. 2007. Transcriptional stimulation of anthrax toxin receptors by anthrax edema toxin and Bacillus anthracis Sterne spore. Microb. Pathog. 43:37-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng, M., Q. Xu, E. D. Hesek, and M. E. Pichichero. 2006. N-fragment of edema factor as a candidate antigen for immunization against anthrax. Vaccine 24:662-670 [DOI] [PubMed] [Google Scholar]
- 34.Zeng, M., Q. Xu, and M. E. Pichichero. 2007. Protection against anthrax by needle-free mucosal immunization with human anthrax vaccine. Vaccine 25:3558-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]