Abstract
Rickettsia typhi, an obligate intracellular bacterium that causes murine typhus, possesses a heavily methylated outer membrane protein B (OmpB) antigen. This immunodominant antigen is responsible for serological reactions and is capable of eliciting protective immune responses with a guinea pig model. Western blot analysis of partially digested OmpB with patient sera revealed that most of the reactive fragments are larger than 20 kDa. One of these fragments, which is located at the N terminus (amino acids 33 to 273), fragment A (At), has been expressed in Escherichia coli. The expressed protein (rAt) was purified by chromatography and properly refolded by sequential dialysis. The refolded rAt protein was recognized by at least 87% of the typhus group patient sera as determined by enzyme-linked immunosorbent assay (ELISA). However, the titers were lower than those obtained with OmpB of R. typhi. Since native OmpB is hypermethylated at lysine residues, we chemically methylated the lysine residues in rAt. The methylation was confirmed by amino acid composition analysis, and the methylation pattern of the methylated rAt (mrAt) protein was similar to that of native At from OmpB, as revealed by liquid chromatography-mass spectrometry analysis. Both rAt and mrAt were evaluated in an ELISA for their serological reactivity with patient sera. Among patient sera tested, 83% exhibited higher titers with mrAt than with rAt. These results suggest that rAt, with or without methylation, can potentially replace rickettsia-derived OmpB or whole-cell antigen for the diagnosis of R. typhi infection.
Rickettsiae are classified into two groups: the spotted fever group and the typhus group. The typhus group of rickettsiae includes Rickettsia typhi and R. prowazekii. R. typhi, a gram-negative, obligate intracellular bacterium, is the causative agent of murine typhus (endemic typhus). While R. typhi can be transmitted to the mammalian host by the bite of an infected flea or louse (the rat flea Xenopsylla cheopis, the cat flea Ctenocephalides felis, or the rat louse Polyplax spinulosa), the more important mechanism of transmission is by inoculation of feces from the vector. Organisms in the feces enter the host through irritated, abraded skin. The bacterium then hematogenously spreads and ultimately invades endothelial cells (1). Transmission can also occur via inhalation of aerosolized fecal particles. To invade the host cell, R. typhi induces phagocytosis by an unknown mechanism. Once within the cell, the organisms rapidly escape the phagosome, multiply within the cytoplasm, and then exit the host cell by burst lysis, allowing subsequent spreading to other cells (18).
Infection with R. typhi causes fever, headache, and myalgia and if not treated in time will lead to disseminated, multisystem disease, including infection of the brain, lung, liver, kidney, and heart endothelia, lymphohistiocytic vasculitis of the central nervous system, diffuse alveolar damage and hemorrhage, interstitial pneumonia, pulmonary edema, interstitial myocarditis and nephritis, portal triaditis, and cutaneous, mucosal, and serosal hemorrhages (16, 17). The nonspecificity and nonuniformity of symptoms and the lack of specific diagnostic tests during the acute stage of the illness often lead to misdiagnosis and delaying of appropriate treatment. Although the mortality rate is low (1% of reported cases), the illness can be severe (1). Without specific treatment, 99% of those infected will clear the disease within weeks, making a proper accounting of R. typhi infections difficult (8).
The bacteria possess a heavily methylated outer membrane protein B (OmpB), an immunodominant antigen responsible for serological reactions as determined by enzyme-linked immunosorbent assay (ELISA) and Western blot analysis (5). OmpB is capable of eliciting protective immune responses in animal models, making it a good candidate for a diagnostic antigen and vaccine. However, due to the intracellular nature of the organism, mass production of the organism for downstream purification of the OmpB protein is not practical. We have previously shown that all reactive fragments of partially digested OmpB were larger than 20 kDa in Western blot analysis with patient sera (see Fig. 1A) (5). One of the fragments (At) is located at the N terminus of OmpB. We cloned, expressed, and purified the recombinant OmpB fragment A (amino acids [aa] 33 to 273) from R. typhi (rAt). rAt was chemically methylated (mrAt) under the condition that methylation occurs predominantly at the lysine residues. Methylation of rAt resulted in a significant change of the secondary structure as measured by circular dichroism (CD) spectroscopy. The presence of multiple methylated lysine residues was confirmed by amino acid composition analysis and by liquid chromatography-mass spectrometry (LC-MS). The methylation pattern is similar to that of the native OmpB protein in the same region of aa 33 to 273, suggesting that chemical methylation resulted in a hypermethylated rAt fragment that mimicks the same fragment within the native OmpB protein. The titers against mrAt were higher than those against rAt but were not as high as those against the native OmpB. The results showed that rAt or mrAt may be a potential reagent to be used for the diagnosis of R. typhi infection.
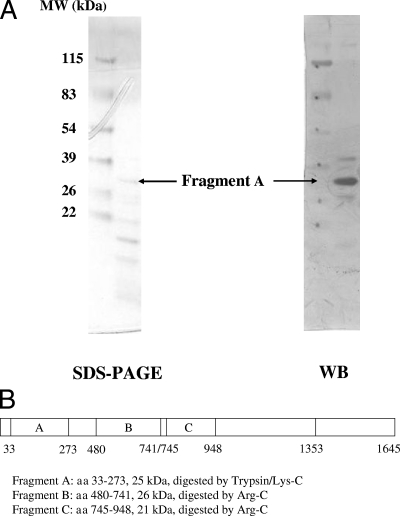
FIG. 1.
(A) SDS-PAGE and Western blotting (WB) of trypsin-digested OmpB fragments. Trypsin digest of OmpB was separated on SDS-PAGE and transferred onto a polyvinylidene fluoride membrane for Western blot analysis using a standard procedure. Fragment A was the most prominent band on the WB and is indicated by the arrow. (B) Diagram of fragments of full-length OmpB reactive with patient sera. The full-length OmpB and different fragments which reacted with patient sera were labeled A, B, and C. The proteases used to partially digest OmpB and the molecular sizes (MW) of the resultant fragments are also shown.
MATERIALS AND METHODS
Cloning, expression, and purification of fragment A from R. typhi.
Specific primers for cloning the gene of protein At [forward, 5′-TCTGGTGTACATATGGGTGCTG(T/C)TATGCAATATAATAG-3′; reverse, 5′-ACTGACGGATCCTTATTAACCAGTACCGTCT(C/A)TTCCATTAAAAT-3′] were designed and synthesized by Life Technology (Gaithersburg, MD). The genomic DNA of R. typhi was used as a template in PCR to amplify the desired fragment A (At). The amplified fragment was ligated into the plasmid pET11a (Novagen, Madison, WI), and Escherichia coli BL21 (Novagen) was subsequently transformed by the plasmid. Cells were grown in 2YT medium followed by induction of protein expression with 1 mM isopropyl-β-d-thiogalactopyranoside (Sigma-Aldrich, St. Louis, MO) for 3 h. Following centrifugation at 4,000 rpm in a GSA rotor (Beckman) for 20 min, the cell pellet was resuspended in buffer A (20 mM Tris-HCl, pH 8.0; Sigma-Aldrich), containing 5 mM EDTA and 1 mM phenylmethylsulfonyl fluoride (Sigma-Aldrich), and disrupted by sonication. The overexpressed rAt in inclusion bodies was pelleted and washed sequentially with 2 M urea (Acros, Pittsburgh, PA) and 2% deoxycholate (Sigma-Aldrich) in buffer A. The washed inclusion bodies dissolved in 8 M urea were purified by DEAE anion-exchange chromatography with a linear NaCl (Sigma-Aldrich) gradient of 0.70 to 0.86 M in 6 M urea, 20 mM Tris-HCl, pH 8.0 (buffer B). The rAt preparation in the peak fractions was >95% pure as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Invitrogen, Carlsbad, CA). The N-terminal sequence of rAt was confirmed by using a Procise 491 protein sequencer (Applied Biosystems, Foster City, CA).
Refolding of purified rAt.
The purified rAt in 6 M urea was refolded by sequential dialysis in decreasing concentrations of urea as described by Ching et al. (4). The purified polypeptides at approximately 0.5 mg/ml in buffer B were transferred into a dialysis bag (24 mm, molecular mass cutoff at 12,000 Da) and dialyzed sequentially against 4 M, 2 M, and 0 M urea in buffer A for 30 min twice at each concentration of urea with gentle stirring. All dialysis procedures except the last step without urea were done at room temperature. Usually three dialysis bags with 10 ml each in a 500-ml beaker or 20 ml each in a 1,000-ml beaker were dialyzed against buffer at a ratio of 1:15. The dialysis was continued overnight without urea in large excess of buffer A at 4°C to remove traces of urea.
Chemical methylation of rAt.
Refolded rAt was lyophilized in buffer A in a protein lyophilizer (Lyph-Lock 6; Labconco, Kansas City, MO) and used for chemical methylation. The methylation reaction with CH3I (Sigma-Aldrich) was carried out under vacuum at 37°C for 18 h as described by Taralp and Kaplan (14).
Determination of secondary structures of folded rAt and mrAt using CD.
The secondary structures of folded rAt (0.24 mg/ml) and mrAt (0.25 mg/ml) were measured using a Jasco (Easton, MD) 700 CD spectrophotometer at room temperature. CD spectra were deconvoluted using convex constraint analysis to resolve the five fundamental component CD spectra for various secondary structures, using the software provided by the manufacturer.
Identification of methylated lysines in mrAt by quantitative amino acid composition analysis using high-pressure liquid chromatography (HPLC).
The amino acid composition of rAt before and after methylation was determined by both the precolumn AccQ.Tag method (Waters, Milford, MA) and the postcolumn derivatization (Beckman Coulter, Fullerton, CA) method for mono-, di-, and trimethylated lysines with modified gradient as described previously (9, 12).
Characterization of methylated lysines in mrAt by integrated LC-MS (ProteomeX; Thermo Electron, San Jose, CA).
One milligram of rAt or mrAt was denatured in 1 ml of 6 M guanidine HCl (Sigma-Aldrich), reduced by 10 μl of 1 M dithiothreitol (Sigma-Aldrich) first, and then alkylated by 25 μl of 1 M iodoacetamide (Sigma-Aldrich) at room temperature in the dark. The sample was washed three times in a centricon (10,000-molecular-weight cutoff; Millipore, Billerica, MA) with 100 mM ammonia bicarbonate (Sigma-Aldrich). After the final wash, each sample was collected and adjusted to a total volume of 1 ml with 100 mM ammonia bicarbonate. The 1 mg/ml of alkylated protein was digested with 20 μg trypsin (Promega, Madison, WI) overnight at 37°C. A new aliquot of 20 μg trypsin was added, and the digestion was continued for an additional 6 h at 37°C. The digested protein sample (1 μg) was loaded onto a capillary reversed-phased column (75-μm inner diameter by 10 cm; Biobasic-C18; ThermoHypersil, Bellefonte, PA), and peptides were differentially eluted with a linear gradient of 5 to 65% acetonitrile (VWR, Bridgeport, NJ) in 60 min. The eluted peptides were introduced into an electrospray ionization, ion trap mass spectrometer (Deca XP Plus; Thermo Electron) for peptide sequence analysis and accurate mass measurement. The Sequest search results were initially assessed by examination of the cross-correlation (Xcorr) and delta normalized correlation (ΔCn) scores. The Xcorr function measured the similarity of mass-to-charge ratios (m/z) for the fragment ions between the predicted value from published amino acid sequences and the observed value from the experimental spectra. The ΔCn score was obtained by normalizing the Xcorr values to 1.0 and calculating the difference between the first- and second-ranked amino acid sequences (15). Thus, the ΔCn score discriminated high-quality spectra from noisy spectra even when both spectra may have matched a theoretical spectrum. The Sp value is the preliminary score assigned after initial comparison between the theoretical and the experimental spectra. As a general rule, an Xcorr value of greater than 2.5 for triply, 2.0 for doubly, or 1.5 for singly charged ions and a ΔCn value of greater than 0.1 were accepted as a positive identification (6, 13). Manual inspections of key spectra were performed to confirm the Sequest result. The Bioworks 3.2 software program was used to construct a unified ranking score based on the three matching factors (Sp, Xcorr, and ΔCn) (15, 19).
Comparison of serological reactivities of rAt, mrAt, and native OmpB in ELISA.
Purified rAt with or without chemical methylation and native OmpB from pure R. typhi were diluted to 0.3 μg/100 μl and 0.15 μg/100 μl in 1× phosphate-buffered saline (PBS), respectively. Microtiter plates were coated with different antigens for 2 days at 4°C and 100 μl/well, washed with PBS containing 0.1% Tween 20 (PBST), and blocked with 200 μl/well of blocking solution (5% milk in PBST) for 1 h at room temperature with gentle rocking. The plates were then rinsed with PBST three times. Patient sera were provided by James G. Olson while he was affiliated with the Centers for Disease Control and Prevention (Atlanta, GA). The patient sera (both acute and convalescent phase) included in this study were selected based on the ELISA titers (all 31 sera had titers of at least 100 and were considered positive) against the lipopolysaccharide (LPS) component of R. typhi. Such an ELISA did not differentiate the infection caused by R. prowazekii or R. typhi due to the cross-reactivity of LPS. Serial dilutions of patient sera from 1:100 up to 1:51,200 in blocking buffer were added to each well at 100 μl/well and incubated at room temperature for 1 h with gentle rocking. After incubation, the plates were washed with PBST three times. The peroxidase-conjugated second antibody (goat anti-human immunoglobulin G [IgG] or goat anti-human IgM) was diluted in blocking buffer at 1:2,000, added into each well at 100 μl/well, and incubated at room temperature for 1 h with gentle rocking. The plates were washed three times as before, and freshly prepared substrate solution [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) and H2O2 at 1:1; Kirkegaard & Perry Laboratories, Gaithersburg, MD] was added to each well at 100 μl/well. The absorbance at 405 nm (minus a reference value at 650 nm) was measured after a 30-min incubation at room temperature in the dark. The optical densities (ODs) from the wells without antigen were used for background subtraction. The positive cutoff used was the average OD at 405 nm (OD405) of negative controls (normal human sera) plus 2 standard deviations.
RESULTS AND DISCUSSION
Purification of rAt.
It has been shown previously that OmpB is the immunodominant protein in mice, guinea pigs, rabbits, and humans following infection by R. typhi (10). Further analysis of partially digested OmpB using various proteases revealed that multiple fragments were recognized by patient sera (5). Figure 1B depicts all of the fragments recognized by patient sera. These fragments were identified by SDS-PAGE separation followed by transfer onto a polyvinylidene fluoride membrane and confirmed by N-terminal sequence analysis of the fragments using a Procise 491 N-terminal protein sequencer. A fragment from the N terminus of OmpB, aa 33 to 273, was readily recognized by patient sera (At). rAt, which encompasses the region of aa 33 to 273, was cloned and expressed in E. coli as inclusion bodies and successfully purified by anion exchange (DEAE) chromatography as evidenced by SDS-PAGE analysis (Fig. 2). The purified rAt was dialyzed to remove salt and urea through a stepwise decrease in the urea concentration in the dialysis buffer to allow proper refolding. By gradually removing urea from the dialysis buffer, we did not observe any insoluble rAt during dialysis.
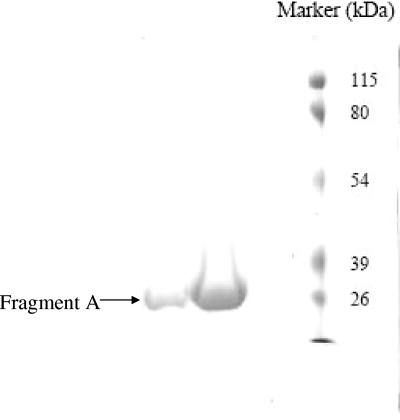
FIG. 2.
The purity of fragment A eluted from the DEAE column. The fragment was purified using the DEAE column as described in Materials and Methods. The fractions were collected and analyzed by SDS-PAGE to determine the purity of each fraction. The fractions containing fragment A are more than 95% pure, as indicated by a single band.
Methylation of rAt affected its secondary structure.
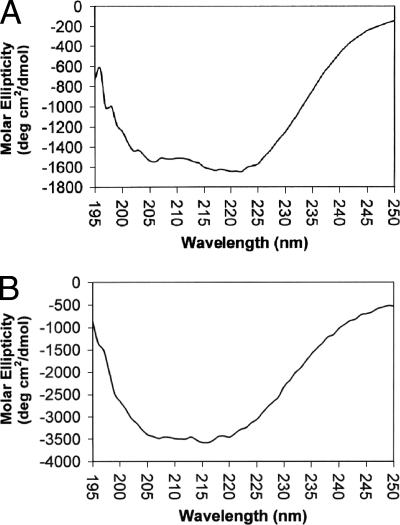
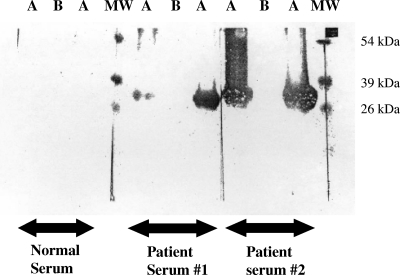
The CD spectra of refolded rAt before and after methylation were measured in a Jasco 700 CD spectrophotometer as shown in Fig. 3. The deconvolution of CD spectra was performed using software provided by the manufacturer in order to estimate the contents of different secondary structures. The results are presented in Table 1. The secondary structure predicted based on the primary amino acid sequence is also listed in Table 1. When the secondary structure predicted based on primary amino acid sequence and that based on CD analysis of refolded rAt were compared, the percentages of various secondary structures differed (Table 1), especially the content of β-sheets. Despite the differences, both showed that the secondary structure primarily consisted of random coil with very little α-helix and β-turn. It is noted that the folding of rAt may not be the same as the folding of fragment A in the native, full-length OmpB protein. Once the protein was chemically methylated under vacuum with the volatile reagent CH3I, the percentages of different types of secondary structures changed significantly. The β-sheet and chiral component changed from 13.4% in rAt to 7% in mrAt (a 45% decrease) and from 19.5% in rAt to 8.7% in mrAt (a 55% decrease), respectively. The α-helix and random coil changed from 10.3% to 8.9% (a 14% decrease) and from 43.6% to 35.0% (a 20% decrease), respectively. The change in percentage of β-turn was the most dramatic. In fact, the β-turn increased from 13.1% in rAt to 39.9% in mrAt, a more than 200% increase. This is the only secondary structure that was increased after methylation and may be the only significant change. These results suggest that methylation changed the secondary structure of rAt, which is consistent with observations by Brubaker et al. (2) that specific lysine modifications alter apoliprotein AI's secondary and tertiary structure. Methylation of the ɛ-amino group of lysine may have a stabilizing effect due to the capability of the methyl group, which acts as an electron inducer. This could stabilize the partial positive charges of their side-chain nitrogen atoms, leading to stronger ionic interactions than those with unmethylated lysyl residues (7). When analyzed using SDS-PAGE, the migration of mrAt was slightly slower than that of rAt (data not shown), indicating that no significant difference existed between the mobility of rAt and that of mrAt. mrAt reacted with patient sera much more strongly than rAt in Western blot analysis. Two typical examples are shown in Fig. 4.
FIG. 3.
CD spectra of rAt (A) and mrAt (B). Approximately equal amounts of rAt (0.24 mg/ml) and mrAt (0.25 mg/ml) were used for the measurement of secondary structures using a Jasco 700 CD spectrophotometer. The molar ellipticity (degrees cm2/dmol) was plotted on the x axis and wavelength (nm) on the y axis. The spectra were then deconvoluted into five individual components of secondary structure as described in Materials and Methods.
TABLE 1.
Secondary structures of rAt and mrAt
| Secondary structure | Content (%)
|
||
|---|---|---|---|
| Predicted by SOPMAa | In rAt | In mrAt | |
| α-Helix | 9.1 | 10.3 | 8.9 |
| β-Sheet | 41.5 | 13.4 | 7.4 |
| Random coil | 37.3 | 43.6 | 35.0 |
| β-Turn | 12.1 | 13.1 | 39.9 |
| Chiral component | NPb | 19.5 | 8.7 |
SOPMA was the software program used to predict secondary structure (http://npsa-pbil.ibcp.fr/cgi-bin/npsa_automat.pl?page=npsa_sopma.html).
NP, not predicted.
FIG. 4.
Western blot analysis of rAt and mrAt using patient sera. rAt and mrAt were loaded on an SDS-PAGE gel, and Western blotting was performed using normal serum, patient serum no. 1, and patient serum no. 2. Pure mrAt was loaded in lanes labeled A, and rAt was loaded in lanes labeled B. Two lanes, labeled MW, were loaded with molecular size markers.
Identification of methylated lysines by quantitative amino acid composition analysis.
Table 2 shows the amino acid composition analysis results. Both analysis methods (pre- and postcolumn derivatization) resulted in the identification of trimethylated lysine residues (four and three lysines by pre- and postcolumn derivatization, respectively) and unmethylated lysine residues (three and five lysines by pre- and postcolumn derivatization, respectively). The precolumn derivatizations resulted in the identification of one mono- and one dimethylated lysine. It appeared that most of the modified lysines were trimethylated and several lysine residues were not methylated. The amino acid composition analysis is a quantitative measurement. In general, our experimental data are in good agreement with the predicted values.
TABLE 2.
Amino acid composition analysis of fragment At before and after methylation
| Amino acida | No. of occurrencesc
|
||
|---|---|---|---|
| Based on DNA sequence | In rAt | In mrAt | |
| Asp/Asn | 47 | 38 | 41 |
| Thr | 33 | 33 | 25 |
| Ser | 10 | 13 | 8 |
| Glu/Gln | 12 | 15 | 14 |
| Gly | 28 | 25 | 28 |
| Ala | 28 | 26 | 31 |
| Val | 16 | 16 | 16 |
| Met | 3 | 1 | 2 |
| Ile | 16 | 16 | 16 |
| Leu | 16 | 18 | 17 |
| Tyr | 1 | 2 | 2 |
| Phe | 13 | 12 | 12 |
| Pro | 6 | 6 | 7 |
| His | 1 | 2 | 1 |
| Cys | 0 | NDb | ND |
| Arg | 2 | 3 | 3 |
| Trp | 0 | ND | ND |
| Lys | 9 | 9 | 5 (3) |
| ɛ-N-Me3-Lys | 0 | 0 | 3 (4) |
| ɛ-N-Me2-Lys | 0 | 0 | 0 (1) |
| ɛ-N-Me1-Lys | 0 | 0 | 0 (1) |
Me1, Me2, and Me3 indicate mono-, di-, and trimethyl groups, respectively.
ND, not determined.
The presence of mono- and dimethylated lysines was monitored by precolumn derivatization using the AccQ.Tag method (Waters) with a modified HPLC gradient (numbers in parentheses are data obtained from precolumn derivatization). The presence of trimethylated lysine was monitored by both pre- and postcolumn derivatization methods (Beckman).
LC-MS characterization of methylated lysine residues.
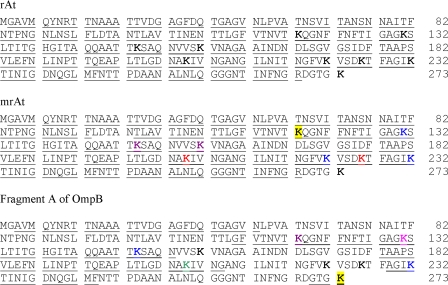
LC-MS analysis was performed (3) to identify the locations of methylated lysine residues and the type of methylation on a particular lysine residue. The underlined peptides in Fig. 5 were identified by LC-MS, and lysine residues (K) are labeled with different colors for different types of methylation. All amino acids except the initial methionine and the last six amino acids (RDGTGK) were detected in rAt (97.1% coverage means that 97.1% of the rAt sequence was detected). In addition to the initial methionine and the last six amino acids, another five amino acids (LDTAN) were not detected in mrAt (95% coverage). The N-terminal methionine was also not detected when rAt was analyzed using the N-terminal sequencer (data not shown), in agreement with LC-MS data. All lysine residues were detected, with the exception of the last one. When the data for mrAt were examined specifically, we found that among the eight detected lysines, only K118 was not methylated. K131, K222, and K232 were trimethylated. Both K205 and K226 were either mono- or trimethylated. K149 and K157 were either mono-, di-, or trimethylated (Table 3). The results are consistent with prederivatization amino acid composition analysis in that about half of the lysines were methylated. The LC-MS analysis revealed additional information which could not be obtained by amino acid composition analysis about the location of modified lysine residues. Since the HPLC quantitation method has intrinsic experimental variations and the sensitivity of detection is not as good as that with LC-MS, the results from these two methods are not in perfect accord. Nevertheless, both methods confirmed that methylation of lysine had occurred after rAt was chemically modified with CH3I under our current conditions. It has been shown that all methylated lysines are trimethylated under reaction conditions used by Taralp and Kaplan (14). Although our conditions were similar to theirs, we used a different reaction temperature for the methylation reaction. The difference in reaction temperature has been attributed to the presence of all mono-, di-, and trimethylated lysines in our previous work (3). Similarly, this may explain our current observation of mono-, di-, and trimethylated lysines in mrAt. In addition, when we evaluated the results of the methylation pattern of the native fragment from OmpB using LC-MS, we found that several lysines are multiply methylated (Fig. 5; Table 3), suggesting that the chemical methylation resulted in multiple lysine methylation in rAt similar to that in native OmpB regarding the complexity and different types of lysine methylation.
FIG. 5.
Identification of peptides from rAt, mrAt, and OmpB and the methylated lysine residues in mrAt and OmpB by LC-MS. The sequence of fragment A (amino acids 33 to 273) of R. typhi OmpB is shown with all nine lysine residues in bold. The upper panel shows the identified sequence of rAt (underlined, 97.1% coverage). The middle panel shows the identified sequence of mrAt (underlined, 95% coverage). Among the eight lysine residues detected, K118 of mrAt (black lettering on yellow background) was not methylated. Both K149 and K157 of mrAt (magenta) were either tri-, di-, or monomethylated. K205 and K226 of mrAt (red) were either mono- or trimethylated. K131, K222, and K232 of mrAt (blue) were trimethylated. The lower panel represents fragment A of OmpB, with identified peptides underlined (51.5% coverage). Among the six identified lysine residues, K273 (black lettering on yellow background) was not methylated. K118 (magenta) was either tri-, di-, or monomethylated. Both K149 and K232 (blue) were trimethylated. K205 (green) was monomethylated, and K131 (magenta) was either mono- or dimethylated.
TABLE 3.
Methylated lysine residues in mrAt and in fragment A of OmpB, determined by LC-MSa
| Position no. of lysine residue | Methylation in:
|
|
|---|---|---|
| mrAt | Fragment A of OmpB | |
| 118b | Not methylated | M, D, T |
| 131 | T | M, D |
| 149b | M, D, T | T |
| 157b | M, D, T | Not detected |
| 205 | M, T | M |
| 222b | T | Not detected |
| 226b | M, T | Not detected |
| 232 | T | T |
| 237b | Not detected | Not methylated |
M, monomethylated; D, dimethylated; T, trimethylated.
This lysine residue is predicted to be in the epitopes of fragment A (http://www.imtech.res.in/raghava/bcepred/).
Serological reactivities of rAt, mrAt, and native OmpB in ELISA.
The serological reactivities of OmpB, rAt, and mrAt with patient sera were evaluated, and titers are presented in Table 4. The patient sera included in this study were selected based on the ELISA titers against the LPS component that did not differentiate between R. prowazekii and R. typhi. Therefore, we could not know which sera were infected by R. typhi or R. prowazekii. However, sequence analysis of OmpB from R. typhi and R. prowazekii has revealed 87% identity for the full-length molecule and 98% identity for fragment A. Thus, it is possible that fragment A and OmpB from R. typhi can be recognized by sera from patients infected by either agent, suggesting the possibility of using this recombinant protein for diagnosis of typhus group rickettsia infection (11). For IgG detection, one serum (5507s1) did not react with OmpB and one serum (21s2) did not react with either rAt or mrAt. Most patient sera reacted equally well or better with mrAt than with rAt, as revealed by ELISA titers (Table 4). Specifically, for IgG detection, 25 sera (83%) exhibited either higher or equal titers using mrAt. For IgM detection, two sera (5507s1 and 5507s2) did not react with either OmpB, rAt, or mrAt. Among the sera reacted with rAt or mrAt, 26 sera (90%) exhibited titers with mrAt either higher than or equal to those with rAt. These results suggest that mrAt reacts more strongly than rAt with antibodies in sera from known typhus group rickettsia-infected patients. These results are also consistent with Western blot results showing a stronger interaction of mrAt with patient sera. In fact, an epitope prediction algorithm using physicochemical properties of the peptide (http://www.imtech.res.in/raghava/bcepred/) indicated that six of the nine lysines are located within different epitopes (Table 3). Among these six lysines, four of them were shown to be chemically methylated (Table 3). Therefore, the increased titers with patient sera for mrAt relative to those for rAt indicated that chemical methylation of lysine residues resulted in an antigen having stronger reactivity with patient sera. This is expected, since the native OmpB protein is hypermethylated and chemical methylation would allow mrAt epitopes to mimic those on the native OmpB more closely. The comparison of IgG titers of patient sera using rAt, mrAt, and full-length OmpB showed that more than 68% of tested sera exhibited higher titers against OmpB than against either rAt or mrAt (Table 4). In contrast, only 7% (or two sera) exhibited higher IgM titers against OmpB than against either rAt or mrAt (Table 4). Taken together, these results suggested that rAt or mrAt was a better antigen for IgM detection than OmpB, yet neither of them was a better antigen than OmpB for IgG detection. Although the titers for a subunit antigen (rAt and mrAt) are not as high as those for OmpB for IgG detection, use of combinations of different fragments of OmpB may result in higher titers than use of rAt or mrAt only, since the combination will include more epitopes of OmpB to be recognized by antibodies in patient sera. We are currently exploring the option of combining several fragments (A [aa 33 to 273], AN [aa 33 to 741], and K [aa 745 to 1364]) derived from OmpB to evaluate their reactivities with patient sera (H.-W. Chen, C.-C. Chao, E. Mutumanje, and W.-M. Ching, presented at the 56th American Society of Tropical Medicine and Hygiene Annual Meeting, Philadelphia, PA, 4 to 8 November 2007). Nevertheless, the use of rAt and/or mrAt provides the first step toward the use of a recombinant protein to replace the hard-to-get OmpB protein or whole-cell antigen for the diagnosis of typhus group rickettsia infection. The increased titers due to chemical methylation also indicate that steps leading to a closer mimicking of the biological antigens are beneficial in our design of recombinant protein antigens to replace native antigens. More sera, including positive sera, negative sera, and sera from other infectious-disease patients, are needed to evaluate the sensitivity and specificity necessary for rAt or mrAt to be used as a diagnostic agent. Although the reactivity of rAt or mrAt alone for IgG detection is still not as good as that of OmpB, this recombinant protein antigen approach would still offer an attractive substitution for OmpB in the quick and simple diagnosis of R. typhi infection if the necessary sensitivity and specificity can be achieved in the future. Furthermore, our previous experience of developing a scrub typhus rapid test cassette as a point-of-care assay using recombinant antigens will enable us to develop a similar point-of-care diagnostic apparatus for typhus group rickettsia infection (W.-M. Ching et al., unpublished data).
TABLE 4.
ELISA titers for recombinant fragment At before and after methylation
| Patient serum samplea | Titer of antibody against Atb
|
|||
|---|---|---|---|---|
| IgG
|
IgM
|
|||
| rAt | mrAt | rAt | mrAt | |
| 21s1 | 800 (1,600) | 400 | 1,600 (400) | 400 |
| 21s2 | 0 (400) | 0 | 100 (0) | 100 |
| 40s1 | 800 (6,400) | 800 | 100 (200) | 100 |
| 55s1 | 800 (200) | 800 | 800 (0) | 800 |
| 61s1 | 800 (400) | 1,600 | 200 (200) | 200 |
| 88s1 | 800 (3,200) | 800 | 400 (1600) | 1,600 |
| 158s1 | 6,400 (25,600) | 3,200 | 100 (0) | 100 |
| 158s2 | 6,400 (12,800) | 1,600 | 200 (200) | 200 |
| 1935s2 | 200 (6,400) | 12,800 | 6,400 (12,800) | 51,200 |
| 1936s1 | 1,600 (6,400) | 800 | 800 (100) | 100 |
| 1936s2 | 1,600 (6,400) | 1,600 | 800 (200) | 100 |
| 3210s1 | 3,200 (800) | 6,400 | 6,400 (400) | 6,400 |
| 3210s2 | 1,600 (800) | 1,600 | 800 (400) | 800 |
| 4425s3 | 3,200 (25,600) | 51,200 | 200 (NDc) | 800 |
| 5206s1 | 6,400 (12,800) | 6,400 | 100 (200) | 1,600 |
| 5206s2 | 6,400 (12,800) | 6,400 | 0 (100) | 1,600 |
| 5507s1 | 400 (0) | 400 | 0 (0) | 0 |
| 5507s2 | 200 (400) | 800 | 0 (0) | 0 |
| 6358s1 | 800 (6,400) | 1,600 | 800 (800) | 6,400 |
| 9557s1 | 400 (12,800) | 400 | 100 (200) | 100 |
| 9557s2 | 1,600 (12,800) | 800 | 100 (200) | 100 |
| 78-009241s1 | 800 (6,400) | 800 | 100 (100) | 800 |
| 78-009241s2 | 1,600 (12,800) | 3,200 | 200 (400) | 1,600 |
| 016733s2 | 1,600 (12,800) | 1,600 | 100 (800) | 100 |
| 016733s3 | 1,600 (51,200) | 1,600 | 0 (400) | 100 |
| 80-062062s2 | 6,400 (800) | 6,400 | 800 (1,600) | 1,600 |
| 81-062767s1 | 3,200 (800) | 6,400 | 400 (200) | 1,600 |
| 81-062767s2 | 3,200 (6,400) | 3,200 | 800 (400) | 1,600 |
| 77-083661 | 800 (6,400) | 3,200 | 800 (ND) | 3,200 |
| 80-053680 | 3,200 (51,200) | 12,800 | 1,600 (ND) | 51,200 |
| 82-038629 | 1,600 (25,600) | 6,400 | 800 (ND) | 12,800 |
Samples with the same prefix followed by s1 and s2, or s2 and s3 were the acute- and convalescent-phase sera from the same patient. 77-083661, 80-053680, and 82-038629 are acute-phase sera.
Numbers in parentheses are titers obtained using full-length OmpB. The titer 0 indicates that the OD was below positive cutoff (average OD405 of negatives [normal human sera] plus 2 standard deviations, 0.266) at a 1:100 dilution and was considered negative.
ND, not determined.
Conclusions.
We have cloned, purified, refolded, and methylated a recombinant protein from fragment A of OmpB, the immunodominant antigen of R. typhi. This rAt fragment was recognized by at least 87% of patient sera tested, and the titers increased after chemical methylation. mrAt appeared to have a methylation pattern similar to that of the same region in native OmpB. Although the titers of IgG against rAt or mrAt were not as high as those against OmpB, our results still suggest that a recombinant protein fragment may be able to replace the native OmpB protein as a diagnostic reagent for not only R. typhi infection but typhus group rickettsia infection in general. It is also possible that a combination of several fragments of OmpB will offer increased reactivity with patient sera that eventually will provide sufficient sensitivity and specificity to serve as a diagnostic agent and may also serve as a substitute for OmpB as a vaccine candidate for R. typhi infection.
Acknowledgments
We thank Gregory Dasch (CDC) for his help in the preparation of OmpB from R. typhi. Patient serum samples were kindly provided by James Olson (human use protocol number DOD#30556; the study protocol was approved by the Naval Medical Research Center Institutional Review Board in compliance with all applicable federal regulations governing the protection of human subjects.). Harvey Kaplan (University of Ottawa) performed the methylation of rAt, and this mrAt protein was used in Western blot analysis. We thank Elissa A. Mutumanje for her review of the manuscript.
This work was supported by Work Unit Number (WUN) 6000.RAD1.J.A0310.
The opinions and assertions contained herein are the private ones of the authors and are not to be construed as official or as reflecting the views of the Department of the Navy, the naval service at large, the Department of Defense, or the U.S. Government. Authors C. C. Chao and W. M. Ching are employees of the U.S. Government. This work was prepared as part of official duties.
Footnotes
Published ahead of print on 20 February 2008.
REFERENCES
- 1.Baxter, J. D. 1996. The typhus group. Clin. Dermatol. 14:271-278. [DOI] [PubMed] [Google Scholar]
- 2.Brubaker, G., D. Q. Peng, B. Somerlot, D. J. Abdollahian, and J. D. Smith. 2006. Apolipoprotein A-I lysine modification: effects on helical content, lipid binding and cholesterol acceptor activity. Biochem. Biophys. Acta 1761:64-72. [DOI] [PubMed] [Google Scholar]
- 3.Chao, C.-C., S.-L. Wu, and W.-M. Ching. 2004. Using LC-MS with de novo software to fully characterize the multiple methylations of lysine residues in a recombinant fragment of an outer membrane protein from a virulent strain of Rickettsia prowazekii. Biochem. Biophys. Acta 1702:145-152. [DOI] [PubMed] [Google Scholar]
- 4.Ching, W.-M., H. Wang, C. Eamsila, D. J. Kelly, and G. A. Dasch. 1998. Expression and refolding of truncated recombinant major outer membrane protein antigen (r56) of Orientia tsutsugamushi and its use in enzyme-linked immunosorbent assays. Clin. Diagn. Lab. Immunol. 5:519-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ching, W.-M., Y. S. Ni, H. Kaplan, Z. Zhang, and G. A. Dasch. 1997. Chemical methylation of E. coli expressed Rickettsial typhi protein increases its seroreactivity, abstr. 40. 13th Sesqui-Annu. Meet. Am. Soc. Rickettsiol., Champion, PA.
- 6.Ducret, A., I. Van Oostveen, J. K. Eng, J. R. Yates III, and R. Aebersold. 1998. High throughput protein characterization by automated reverse-phase chromatography/electrospray tandem mass spectrometry. Protein Sci. 7:706-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Febbraio, F., A. Andolfo, F. Tanfani, R. Briante, F. Gentile, S. Formisano, C. Vaccaro, A. Scire, E. Bertoli, P. Pucci, and R. Nucci. 2004. Thermal stability and aggregation of Sulfolobus solfataricus beta-glycosidase are dependent upon the N-epsilon-methylation of specific lysyl residues: critical role of in vivo post-translational modifications. J. Biol. Chem. 279:10185-10194. [DOI] [PubMed] [Google Scholar]
- 8.Fergie, J. E., K. Purcell, and D. Wanat. 2000. Murine typhus in South Texas children. Pediatr. Infect. Dis. J. 19:535-538. [DOI] [PubMed] [Google Scholar]
- 9.Garcia, R., W. R. Hiatt, S. Jasinski, and P. S. Sypherd. 1986. Identification of ɛ-N-mono-, ɛ-N-di-, and ɛ-N-trimethyllysine by high-performance liquid chromatography. Appl. Environ. Microbiol. 51:1355-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halle, S., D. A. Dasch, and E. Weiss. 1977. Sensitive enzyme-linked immunosorbent assay for detection of antibodies against typhus rickettsiae, Rickettsia prowazekii and Rickettsia typhi. J. Clin. Microbiol. 6:101-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.La Scola, B., L. Rydkina, J. B. Ndihokubwayo, S. Vene, and D. Raoult. 2000. Serological differentiation of murine typhus and epidemic typhus using cross-adsorption and Western blotting. Clin. Diagn. Lab. Immunol. 7:612-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paik, W. K., and S. Kim. 1980. Separation and identification methods of methylated proteins, peptides and amino acids, p. 63. In A. Meister (ed.), Protein methylation. John Wiley & Sons, Inc., New York, NY.
- 13.Tabb, D. L., W. H. McDonald, and J. R. Yates III. 2002. DTASelect and Contrast: tools for assembling and comparing protein identifications from shotgun proteomics. J. Proteome Res. 1:21-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taralp, A., and H. Kaplan. 1997. Chemical modification of lyophilized proteins in nonaqueous environments. J. Protein Chem. 16:183-193. [DOI] [PubMed] [Google Scholar]
- 15.Thermo Scientific. 2002. Xcalibur BioWorks 3.0. Thermo Finnigan product bulletin B-1033. Thermo Scientific, Waltham, MA.
- 16.Walker, D. H., H. M. Feng, S. Ladner, A. N. Billings, S. R. Zaki, D. J. Wear, and B. Hightower. 1997. Immunohistochemical diagnosis of typhus rickettsioses using an anti-lipopolysaccharide monoclonal antibody. Mod. Pathol. 10:1038-1042. [PubMed] [Google Scholar]
- 17.Walker, D. H., F. M. Parks, T. G. Betz, J. P. Taylor, and J. W. Muehlberger. 1989. Histopathology and immunohistologic demonstration of the distribution of Rickettsia typhi in fatal murine typhus. Am. J. Clin. Pathol. 91:720-724. [DOI] [PubMed] [Google Scholar]
- 18.Walker, D. H., G. A. Valbuena, and J. P. Olano. 2003. Pathogenic mechanisms of diseases caused by Rickettsia. Ann. N. Y. Acad. Sci. 990:1-11. [DOI] [PubMed] [Google Scholar]
- 19.Yates, J. R., III, J. K. Eng, and A. L. McCormack. 1995. Mining genomes: correlating tandem mass spectra of modified and unmodified peptides to sequences in nucleotide databases. Anal. Chem. 67:3202-3210. [DOI] [PubMed] [Google Scholar]