Abstract
Determination of antigen-specific T-cell responses is an important part of vaccine assessment. High levels of recovery, viability, and functionality of peripheral blood mononuclear cells (PBMCs) are essential for reliable assessment of cell-mediated immune responses. Here, we sought to find the cell preparation technique best suited for two clinical vaccine trial sites: Stockholm, Sweden, and Dar es Salaam, Tanzania. Standard Ficoll-Paque gradient centrifugation, BD Vacutainer cell preparation tube (CPT), and Greiner Bio-One LeucoSep tube techniques were tested. Cell yield and viability were recorded. Gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) testing was used to assess cell functionality. No differences in mean recovery or mean viability of fresh PBMCs were observed between Ficoll-Paque gradient centrifugation and CPT techniques as used in Stockholm. In Dar es Salaam, recovery of PBMCs isolated by use of the Ficoll-Paque gradient technique was higher than that seen with CPT (1.58 ± 0.6 versus 1.34 ± 0.4 million cells/ml of blood [P = 0.0469]), and the viability of PBMCs processed by Ficoll-Paque gradient was higher than that seen with CPT-purified cells (95.8% ± 2.3% versus 92.6% ± 4.8% [P = 0.0081]). Furthermore, LeucoSep cell separation gave higher levels of yield (1.10 ± 0.3 versus 0.92 ± 0.3 million cells/ml of blood [P = 0.0022]) and viability (95.7% ± 2.0% versus 93.4% ± 3.2% [P = 0.0012]) than Ficoll-Paque cell separation. The cells purified by the different techniques at the two sites performed equally well in IFN-γ ELISPOT assays. Both techniques generated cell preparations with excellent yield, viability, and functionality in Stockholm. In Dar es Salaam, CPT did not perform as well as Ficoll-Paque separation. In a subsequent comparison, LeucoSep performed better than Ficoll-Paque separation. Our findings emphasize the need for on-site assessment of PBMC purification techniques for optimal evaluation of cell-mediated immune responses.
Several experimental vaccines are being developed for diseases where cellular immunity is likely to contribute to protection. Vaccines for control of chronic persisting or recurrent diseases, such as those for treatment of human immunodeficiency virus (HIV), malaria, human papillomavirus, hepatitis C virus, and tuberculosis, are being designed to induce cellular immune responses (1, 2, 12, 15, 19). Determination of antigen-specific T-cell responses has therefore become an important part of vaccine assessment.
Cellular immune responses were traditionally assessed in bulk assays, such as the T-cell proliferation assay using measurement of [3H]thymidine incorporation or the cell cytotoxicity assay using measurement of 51Cr release. These methods are today being replaced by single-cell assays, including major histocompatibility complex-peptide tetramer staining, intracellular cytokine staining, and enzyme-linked immunospot (ELISPOT) assays (4, 10, 16). Multiparameter flow cytometric examination of phenotypic and functional profiles of antigen-specific cells is likely to be needed for a more comprehensive analysis of vaccine-induced cellular immune responses (3, 20). However, the flow cytometry techniques require expensive equipment and large numbers of peripheral blood mononuclear cells (PBMCs). The ELISPOT assay is a rapid, quantitative, and highly sensitive method that requires comparatively few cells. ELISPOT detection of one or more cytokines is the recommended first step for screening of antigen-specific T-cell immunity ex vivo in large clinical studies or vaccine trials (6, 7).
Critical to performing cellular immunology assays is the integrity of the PBMCs. A good cellular separation process yields a pure, highly viable population of mononuclear cells with minimal red blood cell and platelet contamination while maintaining optimum functional capacity. The standard method for purification of PBMCs is the use of Ficoll-Paque gradient centrifugation (5). Two new types of PBMC collection devices that are technically easier to handle are available commercially. The BD Vacutainer cell preparation tube (CPT) is an evacuated tube containing an anticoagulant and a cell separation medium comprising a polyester gel and a density gradient liquid. The Greiner Bio-One LeucoSep and Sigma-Aldrich Biotechnology LP Accuspin tubes have a porous membrane frit that separates the density gradient from the blood sample. This barrier eliminates the laborious overlay of blood samples over Ficoll-Paque and thus may allow reduced interpersonnel variability and higher cell yields. Both techniques may be applicable in clinical vaccine trial settings.
A phase I/II HIV type 1 vaccine trial using a multigene, multiclade HIV type 1 plasmid DNA prime modified vaccinia virus Ankara boost is ongoing in Stockholm, Sweden, and in Dar es Salaam, Tanzania. Here, we describe pretrial evaluations of standard Ficoll-Paque gradient PBMC separation, CPT, and LeucoSep tube techniques for purification of PBMCs used in assessment of cellular immune responses. This study was performed with the objective of finding the cell preparation technique best suited for each of the two clinical vaccine trial sites. Cell yield, cell viability, and gamma interferon (IFN-γ) ELISPOT reactivity were determined using PBMC samples purified by the different techniques.
MATERIALS AND METHODS
Study design and samples.
Experiments using paired blood samples were performed to establish cell separation techniques that enabled maximal throughput capacity and yet maintained optimal yield, viability, and function at the two different clinical vaccine trial sites. Standard operation procedures were established for each of the techniques used. Thereafter, we tested CPT versus standard Ficoll-Paque cell separation techniques at the Swedish Institute for Infectious Disease Control, Stockholm, Sweden. We next tested CPT versus standard Ficoll-Paque gradient separation and LeucoSep versus Ficoll-Paque gradient separation at the Department of Microbiology and Immunology laboratory, Muhimbili University of Health and Allied Sciences, in Dar es Salaam, Tanzania.
Sodium-heparinized blood was collected from Swedish (n = 50) and Tanzanian (n = 45) healthy blood donors. The samples were processed, and PBMCs were frozen, within 6 h of blood collection. For comparison of CPT versus standard Ficoll-Paque cell separation, a Vacutainer CPT (8 ml) and a heparin tube (10 ml) were used. Thus, the total blood volume collected from each donor was 18 ml. For comparison of Ficoll-Paque separation and LeucoSep separation, blood was drawn into two sodium heparin Vacutainer tubes, giving a maximal volume of 20 ml. On arrival at the laboratory, the contents of the two tubes were pooled, mixed, and divided into two aliquots—one for each of the procedures used.
Three Swedish laboratory technologists participated in the paired sample processing in Stockholm. The first group of 20 Tanzanian samples was processed by a single Tanzanian laboratory technologist, and the second group of 25 samples was processed by three laboratory technologists. In addition to determinations of yield and viability, the functionality of the PBMCs purified by the different techniques was assessed by IFN-γ ELISPOT assays performed on 10 donor samples for each of the cell purification techniques being compared. The ELISPOT assays were performed with batches of frozen/thawed cells by a single laboratory technologist in Stockholm. In Dar es Salaam, ELISPOT assays using fresh cells were run by the same laboratory technologist throughout the study period.
Isolation of PBMCs by standard density gradient technique.
PBMCs were purified using standard Ficoll-Paque gradient centrifugation according to the instructions of the manufacturer (Amersham Pharmacia, Uppsala, Sweden). Briefly, 4 ml of Ficoll-Paque gradient was pipetted into two 15-ml centrifuge tubes. The heparinized blood was diluted 1:1 in phosphate-buffered saline (PBS) and carefully layered over the Ficoll-Paque gradient (9 to 10 ml/tube). The tubes were centrifuged for 20 min at 1,020 × g. The cell interface layer was harvested carefully, and the cells were washed twice in PBS (for 10 min at 640 × g followed by 10 min at 470 × g) and resuspended in RPMI 1640 medium with Glutamax supplemented with penicillin (50 U/ml)-streptomycin (50 μg/ml) and 10 mM HEPES (complete RPMI medium) before counting.
Isolation of PBMCs by using BD CPT.
Whole blood collected in BD Vacutainer CPTs containing sodium heparin as an anticoagulant was processed according to the manufacturer's instructions. After blood drawing and inversion of the CPTs, the tubes were transported within 3 h to the laboratory, where they were inverted eight times before centrifugation (for 15 min at 1,500 × g) at room temperature. The cell suspension was collected, and the cells were washed twice in PBS (for 10 min at 640 and 470 × g, respectively, for the two successive washes) and resuspended in complete RPMI medium before counting.
Isolation of PBMCs by using LeucoSep tubes.
PBMCs were purified using LeucoSep tubes according to the instructions of the manufacturer (Greiner Bio-One). In brief, 3 ml of Ficoll-Paque was preloaded in a 14 ml LeucoSep tube by centrifugation for 30 s at 1,000 × g. The heparinized whole-blood samples were diluted with equal volumes of PBS, and 6 ml of the diluted blood was added to a LeucoSep tube. The cell separation tubes were centrifuged for 15 min at 800 × g without braking at room temperature. The cell suspension was collected, and the cells were washed twice in PBS (for 10 min at 640 and 470 × g, respectively, for the two successive washes) and resuspended in complete RPMI medium before counting.
Determination of cell yield and cell viability.
PBMC yield and viability were determined using a NucleoCounter (ChemoMetec A/S, Allerød, Denmark). The NucleoCounter detects nonviable cells by use of propidium iodide staining of the cell nuclei and determines cell viability of a sample by using the total cell count and the count of nonviable cells.
Cryopreservation and thawing of PBMCs.
Aliquots of purified PBMCs were cryopreserved and stored in liquid nitrogen. A freezing medium containing 20% fetal calf serum (FCS), 10% dimethyl sulfoxide, and 70% complete RPMI medium was prepared daily and stored at room temperature. Following centrifugation, the cells were gently resuspended in freezing medium to give at least 8 million cells/cryovial, depending on the number of PBMCs recovered after processing. The cryovials were immediately transferred to a Mr. Frosty freezing container (Nalgene) and stored at −80°C overnight. The following day the cryovials were transferred to a liquid nitrogen container for storage until assayed.
On the day of IFN-γ ELISPOT testing, the cryovials were transferred from liquid nitrogen to −80°C for an hour prior to thawing. The cryopreserved cells were thawed in a 37°C water bath until the cell suspension was almost completely melted. Each cell suspension was transferred to a 15-ml tube, and prewarmed (37°C) complete RPMI medium supplemented with 20% FCS was slowly added. The tubes were centrifuged for 10 min at 330 × g, and the cells were washed once in complete RPMI medium supplemented with 10% FCS before counting. The cell volume was adjusted to give a cell concentration of 4 million/ml in complete RPMI medium supplemented with 20% FCS for use in the IFN-γ ELISPOT assay.
IFN-γ ELISPOT assay.
IFN-γ ELISPOT assays were performed using an h-IFN-γ ELISPOTPLUS kit and a two-step detection system according to the instructions of the manufacturer (Mabtech, Nacka, Sweden). Briefly, precoated IFN-γ ELISPOT plates were washed. Phytohemagglutinin (the positive control), purified protein derivative, and a peptide pool composed of a panel of 23 peptides from cytomegalovirus, Epstein-Barr virus, and influenza virus (CEF) isolates (9) were diluted in complete RPMI medium and added to triplicate wells (at 50 μl/well) to give a final concentration of 5 μg/ml. Triplicate medium wells were used as a background control. A 50-μl cell suspension was added to each well, giving 200,000 cells/well. Empty wells were filled with 100 μl of medium, and the plates were wrapped in aluminum foil and incubated 20 h under conditions of 37°C and 7.5% CO2. After washing, IFN-γ secretion was detected by addition of biotinylated anti-IFN-γ 7-B6-1 monoclonal antibody followed by alkaline phosphatase-conjugated streptavidin. The enzyme complexes were visualized by addition of BCIP (5-bromo-4-chloro-3-indolylphosphate)-nitroblue tetrazolium substrate. Frequencies of antigen-specific spot-forming cells (SFC) were measured with an automated microscope (Zeiss, Göttingen, Germany). Results were expressed as the number of SFCs per million PBMCs and were calculated for each pool of peptides using the equation 5 × mean number of SFC/2 × 105 cells (from three stimulated wells), without subtracting background. IFN-γ ELISPOT responses were considered positive when the number of SFCs was at least four times the background (medium only) and was >55 SFCs per million PBMCs.
Ethical clearance.
The study received ethical clearances from the Regional Ethics Committee in Stockholm, Sweden, and from the Muhimbili University College of Health Sciences, Dar es Salaam, Tanzania. Informed consent was obtained from subjects, and the human experimentation guidelines of Karolinska Institutet, Stockholm, and Muhimbili University College of Health Sciences, Dar es Salaam, were followed.
Statistical analysis.
Data on PBMC yield and viability were reported as mean cell counts or percentages of viable cells ± standard deviations. Differences in the means were calculated using the Student's t test.
RESULTS
Recovery and viability of PBMCs isolated from Swedish donors.
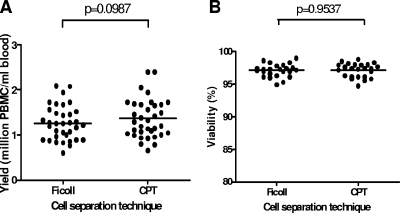
No differences in mean recovery or mean viability of fresh PBMCs were observed between the two cell preparation techniques used in the Swedish setting. The mean recovery values for fresh PBMCs isolated from 35 Swedish blood donors by use of heparinized blood and standard Ficoll-Paque gradient separation and those determined by use of CPTs containing heparin as an anticoagulant were 1.27 ± 0.4 and 1.36 ± 0.4 million cells/ml of blood, respectively (P = 0.0987) (Fig. 1A). The mean viability values for freshly recovered cells from 24 Swedish blood donors were 97.1% ± 1.0% for standard Ficoll-Paque gradient-separated cells and 97.1% ± 1.1% for CPT-purified cells (P = 0.9537) (Fig. 1B). The overall cell recovery results from Swedish donor samples ranged from 0.77 to 2.40 million cells/ml of blood.
FIG. 1.
Mean recovery (A) and viability (B) of paired fresh PBMCs isolated from Swedish blood donors obtained using heparinized blood and standard Ficoll-Paque gradient separation or CPTs containing heparin as an anticoagulant.
Recovery and viability of PBMCs isolated from Tanzanian donors.
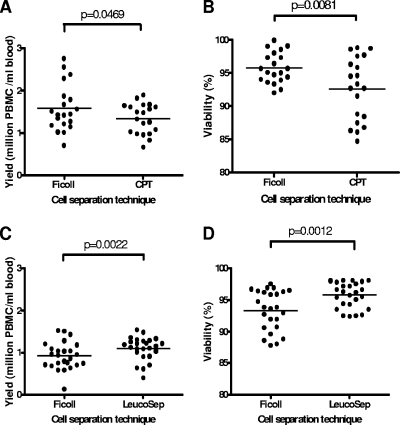
The recovery of cells isolated from 20 Tanzanian blood donors was measured at 1.58 ± 0.6 million cells/ml of blood when standard Ficoll-Paque separation was used. Fewer cells were recovered when the CPT technique was used (1.34 ± 0.4 million cells/ml of blood [P = 0.0469]) (Fig. 2A). Additionally, the viability of PBMCs processed by standard Ficoll-Paque separation (95.8% ± 2.3%) was higher than that seen with CPT-purified cells (92.6% ± 4.8% [P = 0.0081]) (Fig. 2B).
FIG. 2.
Mean recovery (A) and viability (B) of paired fresh PBMCs isolated from 20 Tanzanian blood donors obtained using heparinized blood and standard Ficoll-Paque gradient separation or CPTs containing heparin as anticoagulant. Mean recovery (C) and viability (D) of fresh PBMCs isolated from 25 Tanzanian blood donors obtained using heparinized blood and standard Ficoll-Paque gradient separation or LeucoSep tubes.
Parallel LeucoSep tube and standard Ficoll-Paque gradient cell separations were also performed on blood collected from another group of 25 Tanzanian blood donors. The mean recovery values for fresh cells isolated from these donors were 0.92 ± 0.3 million cells/ml of blood when standard Ficoll-Paque separation was used and 1.10 ± 0.3 million cells/ml when LeucoSep tube separation was used (P = 0.0022) (Fig. 2C). Mean viability values for fresh recovered cells were 93.4% ± 3.2% for standard Ficoll-Paque gradient-separated cells and 95.7% ± 2.0% for LeucoSep-separated cells (P = 0.0012) (Fig. 2D).
The overall rate of cell recovery from the first group of 20 Tanzanian donor samples ranged from 0.66 to 2.76 million cells/ml of blood. A lower overall recovery rate was recorded for the second group of 25 donors, with yields ranging from 0.13 to 1.54 million cells/ml of blood. The mean rate of recovery of cells from Ficoll-Paque gradient separations performed using blood from all 45 Tanzanian blood donors was 1.21 ± 0.54 million cells/ml of blood, while the viability was 94.4% ± 3.0%.
Functionality of purified PBMCs.
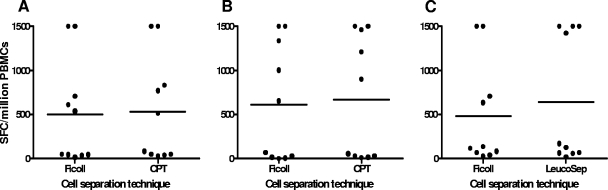
To test the functionality of purified PBMCs, the IFN-γ ELISPOT reactivity to CEF was determined using cells prepared from 10 blood donors for each of the cell separation techniques. At the Stockholm vaccine trial site, cryopreserved cells were used to assess functionality of the cells prepared by Ficoll-Paque gradient separations and CPTs. The viability of frozen/thawed PBMCs ranged from 93.2% to 99.1% and 84.9% to 97.8% for Ficoll-Paque- and CPT-separated PBMCs, respectively. Similar levels of IFN-γ ELISPOT reactivity to CEF were recorded irrespective of the PBMC isolation procedure used (Fig. 3A). At the Dar es Salaam vaccine trial site, IFN-γ ELISPOT assays were performed using fresh cells. The viability of fresh cells ranged from 88.6% to 98.0%, 84.7% to 98.6%, and 92.5% to 98.1% for Ficoll-Paque-, CPT-, and LeucoSep-separated PBMCs, respectively. No differences in rates of IFN-γ ELISPOT reactivity to CEF were seen when Ficoll-Paque- versus CPT-separated PBMCs and Ficoll-Paque- versus LeucoSep-separated PBMCs were compared (Fig. 3B and C). However, for two blood donors, LeucoSep-separated cells exhibited a rate of reactivity that was more than twice that seen with Ficoll-Paque-separated cells (Fig. 3C). The rates of response to the CEF peptide pool in IFN-γ ELISPOT assays were similar for the pair-wise comparisons of cell separation techniques (Table 1).
FIG. 3.
IFN-γ ELISPOT responses to CEF stimulation obtained using PBMCs separated by Ficoll-Paque or CPT in samples collected from 10 Swedish blood donors (A), using PBMCs separated by Ficoll-Paque or CPT from 10 Tanzanian blood donors (B), and using PBMCs separated by Ficoll-Paque or LeucoSep from another 10 Tanzanian blood donors (C).
TABLE 1.
Number of CEF-positive responders by IFN-γ ELISPOT assays using PBMCs prepared by three different cell separation techniques
| Donors (n)a | No. of responders by:
|
||
|---|---|---|---|
| Ficoll-Paque | CPT | LeucoSep | |
| SWE (10) | 5 | 6 | NDb |
| TZC (10) | 6 | 5 | ND |
| TZD (10) | 8 | ND | 9 |
SWE, Swedish blood donors tested using frozen/thawed PBMCs; TZC and TZD, two groups of Tanzanian blood donors tested using fresh PBMCs.
ND, not done.
DISCUSSION
The viability of fresh PBMCs is critical for optimal assessment of T-cell function irrespective of whether the cells are used fresh or after cryopreservation. Here, we report equivalent levels of cell viability for Ficoll-Paque separation and CPT processing when preparing PBMCs from Swedish blood donors but a significantly higher viability rate for Ficoll-Paque-separated PBMCs compared to that seen with CPT-processed PBMCs from Tanzanian blood donors. Ruitenberg et al. recently reported that Ficoll-Paque-processed fresh PBMCs exhibited significantly greater viability than CPT-processed PBMCs when samples from HIV-seropositive donors were tested (21). Additionally, in Dar es Salaam, we found that the mean rate of viability of LeucoSep-separated PBMCs was higher than that of Ficoll-Paque-purified cells.
For comprehensive assessments of immune responses in clinical vaccine trials, high cell yields are required. In Stockholm, we found similar high levels of cell recovery irrespective of the technique used. In Dar es Salaam, recovery of PBMCs isolated by Ficoll-Paque was higher than the cell yields obtained using the CPT technique. The LeucoSep tube cell separations gave higher cell yields than the Ficoll-Paque cell separations. Cox et al. (8) reported comparable rates of cell viability for citrate CPT processing and for acid citrate dextrose-treated blood separated using LeucoSep tubes but a marginally higher percentage of lymphocytes obtained with LeucoSep tubes. In contrast to our findings, others have previously found higher yields by use of CPTs compared to yields obtained using a standard gradient technique (22). The difference in our findings could be due to the separation medium used (Lymphoflot versus Ficoll-Paque) and the skill level of laboratory personnel in harvesting cells from the Ficoll-Paque gradient.
In Dar es Salaam, the overall cell yields differed between the two groups of blood donors sampled. Samples collected for comparison of the Ficoll-Paque and CPT techniques exhibited overall higher cell yields (range, 0.66 to 2.76 million cells/ml of blood) compared to the yields obtained with samples collected for comparison of Ficoll-Paque and LeucoSep tubes (range, 0.13 to 1.54 million cells/ml of blood). The differences in yield between the two groups could be due to biological influences as well as technical factors. The Ficoll-Paque separation technique is highly dependent on the skill of the laboratory personnel. The CPT and LeucoSep tubes are designed to avoid such variations. An overall lower recovery rate was seen with the second group of Tanzanian donors irrespective of separation technique used. Thus, factors other than interlaboratory personnel variations must be considered. Cell counts have been shown to vary with age, gender, and diurnal variation (13, 14, 17). Age, gender, and exact time of collection were not recorded for any of the donors whose blood was sampled in this study. A recent seroprevalence study of blood-borne pathogens among blood donors at the Muhimbili National Hospital in Dar es Salaam showed that the ages of blood donors ranged from 16 to 69 years but that most (72%) were between 20 and 39 years of age. The majority were males (18). Of note, the first group of 20 Tanzanian blood donors were sampled during January to March 2006 while the other 25 individuals were sampled during August to November 2006. To minimize the influence of biological factors, paired cell sampling and processing were performed.
To our knowledge, this is the first report applying CPT processing for PBMC purification in an African setting. The CPTs have been reported to be sensitive to excessive temperature fluctuations, resulting in the deterioration of the gel (8). Therefore, for the purpose of this study, CPTs from a batch tested in Sweden were hand carried to Tanzania and the tubes were kept at room temperature (20 to 25°C) until dispatched to the blood bank for specimen collection. Despite these precautions, CPT separation did not perform as well as standard Ficoll-Paque separation with respect to cell recovery or cell viability.
In the limited number of IFN-γ ELISPOT tests performed, the results showing the functionality of the frozen PBMCs used in Stockholm or of the fresh cells used in Dar es Salaam were similar regardless of the cell separation technique used. Others have shown that there is a relationship between viability postthawing and a capacity for functional responses. For lymphocyte proliferation, a viability rate of >70% has been suggested to be critical (11, 23). Here, the rate of viability of cells used after cryopreservation was above 84.9% and the rate of viability of fresh cells always exceeded 84.7% when used in the IFN-γ ELISPOT assay.
In summary, we have assessed three cell purification techniques with the intent of identifying mononuclear cell isolation procedures giving maximal yield and good cell quality for use in testing cell-mediated immune responses at two geographically distinct clinical trial sites. In Stockholm, the CPT separation and Ficoll-Paque gradient cell separation techniques performed equivalently. In Dar es Salaam, we first compared the Ficoll-Paque gradient cell separation with CPT separation. However, we found that the CPT technique did not perform as well as the Ficoll-Paque gradient cell separation technique, with the CPTs giving lower yield and cell viability results. Because the Ficoll-Paque gradient cell separation technique is laborious, demands a highly skilled operator to give maximal cell yields, and is difficult to standardize, the LeucoSep cell preparation technique was assessed. The LeucoSep cell separation tubes performed better than standard Ficoll-Paque gradient cell separation. The laboratory work in the ongoing phase I/II HIV DNA modified vaccinia virus Ankara vaccine trial in Dar es Salaam, Tanzania, was designed on the basis of the data from the present study, and cell separations are being performed using LeucoSep cell separation tubes.
Acknowledgments
We thank Ulrika Edbäck, Emanuel Salala, Nasra Said, and Dotto Kalovya for expert technical assistance.
The study was supported by the Swedish International Development Cooperation Agency, Department of Research Cooperation, SAREC.
Footnotes
Published ahead of print on 20 February 2008.
REFERENCES
- 1.Aidoo, I., and I. Udhayakumar. 2000. Field studies of cytotoxic T lymphocytes in malaria infections: implications for malaria vaccine development. Parasitol. Today 16:50-56. [DOI] [PubMed] [Google Scholar]
- 2.Berzofsky, J. A., J. D. Ahlers, J. Janik, J. Morris, S. Oh, M. Terabe, and I. M. Belyakov. 2004. Progress on new vaccine strategies against chronic viral infections. J. Clin. Investig. 114:450-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betts, M., M. C. Nason, S. M. West, S. C. De Rosa, S. A. Migueles, J. Abraham, M. M. Lederman, J. M. Benito, P. A. Goepfert, M. Connors, M. Roederer, and R. A. Koup. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T-cells. Blood 107:4781-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betts, M. R., D. R. Ambrozak, D. C. Douek, S. Bonhoeffer, J. M. Brenchley, J. P. Casazza, R. A. Koup, and L. J. Picker. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4 and CD8 T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 75:11983-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyum, A. 1968. Isolation of mononuclear cells and granulocytes from human blood. Scand. J. Clin. Lab. Investig. 21:77-89. [PubMed] [Google Scholar]
- 6.Bull, M., D. Lee, J. Stucky, Y.-L. Chiu, A. Rubin, H. Horton, and M. J. McElrath. 2007. Defining blood processing parameters for optimal detection of cryopreserved antigen-specific responses for HIV vaccine trials. J. Immunol. Methods 322:57-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox, J. H., G. Ferrari, and S. Janetzki. 2006. Measurement of cytokine release at the single cell level using the ELISPOT assay. Methods 38:274-282. [DOI] [PubMed] [Google Scholar]
- 8.Cox, J. H., M. DeSouza, S. Ratto-Kim, G. Ferrari, K. Weinhold, and D. L. Birx. 2006. Cellular immunity assays for evaluation of vaccine efficacy, p. 301-314. In B. Detrick, R. G. Hamilton, and J. D. Folds (ed.), Manual of clinical laboratory immunology, 7th ed. ASM Press, Washington, DC.
- 9.Currier, J. R., E. G. Kuta, E. Turk, L. B. Earhart, L. Loomis-Price, S. Janetzki, G. Ferrari, D. L. Birx, and J. H. Cox. 2002. A panel of MHC class I restricted viral peptides for use as a quality control for vaccine trial ELISPOT assays. J. Immunol. Methods 260:157-172. [DOI] [PubMed] [Google Scholar]
- 10.Czerkinsky, C. C., A Tarkowski, L. A. Nilsson, O. Ouchterlony, H. Nygren, and C. Gretzer. 1984. Reverse enzyme-linked immunospot assay (RELISPOT) for the detection of cells secreting immunoreactive substances. J. Immunol. Methods 72:489-496. [DOI] [PubMed] [Google Scholar]
- 11.Disis, M. L., C. dela Rosa, V. Goodell, L.-Y. Kuan, J. C. C. Chang, K. Kruus-Reichel, T. M. Clay, H. K. Lyerly, S. Bhatia, S. A. Ghanekar, V. C. Maino, and H. T. Maecker. 2006. Maximizing the retention of antigen specific lymphocyte function after cryopreservation. J. Immunol. Methods 308:13-18. [DOI] [PubMed] [Google Scholar]
- 12.Girard, M. P., S. K. Osmanov, and M. Paule Kieny. 2006. A review of vaccine research and development: the human immunodeficiency virus (HIV). Vaccine 24:4062-4081. [DOI] [PubMed] [Google Scholar]
- 13.Hulstaert, F., I. Hannet, V. Deneys, V. Munhyeshuli, T. Reichert, M. De Bryere, and K. Strauss. 1994. Age-related changes in human blood lymphocyte populations. II. Varying kinetics of percentage and absolute cell count measurements. Clin. Immunol. Immunopathol. 70:152-158. [DOI] [PubMed] [Google Scholar]
- 14.Jones, A. R., D. Twedt, W. Swain, and E. Gottfried. 1996. Diurnal change of blood count analytes in normal subjects. Am. J. Clin. Pathol. 106:723-727. [DOI] [PubMed] [Google Scholar]
- 15.Kaufmann, S. H. 2002. Protection against tuberculosis: cytokines, T cells and macrophages. Ann. Rheum. Dis. 61(Suppl. 2):54-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kern, F., G. LiPira, J. W. Gratama, F. Manca, and M. Roederer. 2005. Measuring Ag-specific immune responses: understanding immunepathogenesis and improving diagnostics in infectious disease, autoimmunity and cancer. Trends Immunol. 26:477-484. [DOI] [PubMed] [Google Scholar]
- 17.Malone, J. L., T. E. Simms, G. C. Gray, K. F. Wagner, J. R. Burge, and D. S. Burke. 1990. Sources of variability in repeated T-lymphocyte counts for human immunodeficiency virus type 1-infected patients: total lymphocyte count fluctuations and diurnal cycle are important. J. Aquir. Immune Defic. Syndr. 3:144-151. [PubMed] [Google Scholar]
- 18.Matee, M. I. N., P. M. Magesa, and E. F. Lyamuya. 2006. Seroprevalence of human immunodeficiency virus, hepatitis B and C viruses and syphilis infection among blood donors at Muhimbili National Hospital in Dar Es Salaam, Tanzania. BMC Public Health 6:21-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMichael, A. J. 2006. HIV vaccines. Annu. Rev. Immunol. 24:227-255. [DOI] [PubMed] [Google Scholar]
- 20.Roederer, M., J. M. Brenchley, M. R. Betts, and S. C. De Rosa. 2004. Flow cytometric analysis of vaccine responses: how many colors are enough? Clin. Immunol. 110:199-205. [DOI] [PubMed] [Google Scholar]
- 21.Ruitenberg, J. J., C. B. Mulder, V. C. Maino, A. L. Landay, and S. A. Ghanekar. 2006. VACUTAINER CPT and Ficoll density gradient separation perform equivalently in maintaining the quality and function of PBMC from HIV seropositive blood samples. BMC Immunol. 7:11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlenke, P., H. Klünter, M. Müller-Steinhardt, H.-J. Hammers, K. Borchert, and G. Bein. 1998. Evaluation of a novel mononuclear cell isolation procedure for serological HIV typing. Clin. Diagn. Lab. Immunol. 5:808-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinberg, A., L. Zhang, D. Brown, A. Erice, B. Polsky, M. S. Hirsch, S. Owens, and K. Lamb. 2000. Viability and functional activity of cryopreserved mononuclear cells. Clin. Diagn. Lab. Immunol. 7:714-716. [DOI] [PMC free article] [PubMed] [Google Scholar]