Abstract
The mechanisms of malarial anemia induction are poorly understood, but cytokines and autoantibodies are considered to play important roles. This work aimed at evaluating the degree of anemia and the plasmatic profile of the cytokines tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), interleukin-12 (IL-12), migration inhibitory factor (MIF), and IL-10 and the monocyte chemotactic protein-1 (MCP-1) chemokine, as well as evaluating the presence of antibodies directed to components of the normal erythrocyte membrane and to cardiolipin in individuals with malaria from the Brazilian Amazon. No difference was observed in the frequency of anemia between patients infected by Plasmodium vivax and those infected by Plasmodium falciparum, and there was no relationship between the levels of parasitemia and the manifestations of anemia in P. vivax and P. falciparum patients. Significant increases in the concentrations of TNF-α, IFN-γ, MIF, and MCP-1 were observed in patients with P. falciparum and P. vivax malaria, whereas the concentrations of IL-10 was increased only in patients with P. vivax infection. Higher concentrations of IL-12 and IL-10 were observed in the P. falciparum anemic patients, while for TNF-α this profile was observed in the nonanemic ones. P. vivax-infected and P. falciparum-infected patients with positive immunoglobulin M (IgM) or IgM and IgG responses, respectively, against blood-stage forms of the parasites had significantly lower hemoglobin levels than did those with negative responses. There was no correlation between the presence of anti-erythrocyte and anti-cardiolipin antibodies and the presence or intensity of the anemia. Our data suggest that in areas of low endemicity and unstable transmission of malaria, P. vivax and P. falciparum infections present similar characteristics in terms of the induction of anemia and cytokine responses.
Severe malarial anemia and cerebral malaria are the main complications of Plasmodium falciparum infection. They are responsible for most of the estimated one to three million malaria-related deaths every year in the world, mainly among children below 5 years of age in sub-Saharan Africa (49). Severe malarial anemia is reported to be the earliest complication, usually affecting children below 2 years of age (57). Although severe anemia is a major concern in malaria pathology due to its high mortality rates, milder forms of anemia also are important, since this manifestation is responsible for considerable morbidity and is one of the major factors for the high disability-adjusted life years attributed to malaria (50, 51, 66). Iron deficiency, intestinal helminths, and human immunodeficiency virus infection make significant contributions to the pathogenesis of anemia in many African countries, but now there is substantial evidence suggesting that malaria is indeed a major underlying factor (28, 48).
Although it has been estimated, the real impact of malarial anemia on the affected populations is unknown. The few available data mostly are restricted to studies conducted in Africa (20, 45, 52), where malaria is hyperendemic, and P. falciparum is by far the most prevalent species. The characteristics of malarial anemia in Brazil, where malaria is predominantly hypo- or mesoendemic and Plasmodium vivax is responsible for more than 75% of the cases, are largely unknown. Due to the low endemicity of the organism in this region, the population is constituted mainly of nonimmune individuals, and complications are expected to occur in all individuals, regardless of age. Premunition is, however, a recently observed phenomenon, and the prevalence of asymptomatic infection has been recorded with increasing frequency in several Amazonian localities (2, 43, 65). Nevertheless, the incidence of complications and mortality due to malaria infection is very low in Brazil and Latin America in general, and this is mainly a result of effective malaria control programs, which provide rapid microscopic diagnosis and prompt treatment (36, 39, 63) free of charge in countries like Brazil (18).
However, while mortality is low, morbidity is more difficult to assess. Specifically concerning the frequency of malarial anemia, very few data are available to allow us to estimate its impact on the health status and on the quality of life of the population in the area of Brazil in which malaria is endemic (8, 9, 14, 26). In a large study focused on malaria during pregnancy in Coari, a locality in the state of Amazonas with more than 6,000 pregnant women, Martínez-Espinosa (44) found that more than 90% of patients infected by P. vivax and P. falciparum were anemic.
The mechanisms of severe malarial anemia are the subject of intense study (11, 46, 47). Many factors have been reported to influence its pathogenesis, but the mechanisms themselves remain controversial (17, 21, 53, 72). The increased destruction and phagocytosis of infected and uninfected erythrocytes, the suppression of erythropoiesis by relatively impaired erythropoietin production, the autoimmune lysis of both parasitized and normal erythrocytes, and reticuloendothelial hyperfunction seem to be important causative factors (1, 21, 58), but they do not adequately explain the severity and extent of anemia. Furthermore, anemia can persist for weeks after effective antimalarial treatment (4, 6, 22, 62, 70). Although the pathological basis for the development of malarial anemia is not yet well understood, the participation of cytokines (5, 12, 21) and of autoantibodies (14, 15, 59, 62, 69) has been considered. Some works have suggested that severe anemia is associated with predominant T-helper 1 (Th1) responses, characterized by high levels of tumor necrosis factor alpha (TNF-α) in relation to interleukin-10 (IL-10) levels, and conversely, protection from this complication was associated with an inverse relationship, i.e., with a balance toward a high IL-10/TNF-α ratio (37, 55). Other cytokines and chemokines, particularly those involved in macrophage migration and activity, such as migration inhibitory factor (MIF) and monocyte chemotactic protein-1 (MCP-1), also can be involved. Since high TNF-α production by macrophages can be influenced by phospholipids such as the Plasmodium-derived glycosylphosphatidylinositol (16, 71, 73), anti-phospholipid antibodies, which are common in malaria infections (31, 56), could play a role in this system. Antibodies directed to erythrocyte membrane antigens, which also are present in malaria infections, could act by destroying parasitized and nonparasitized erythrocytes (13, 22, 23, 24).
In milder forms of malarial anemia, one could consider that the destruction of parasitized erythrocytes per se would explain the phenomenon, but there are no substantial data supporting this conclusion. Taking these facts together, we designed this work to evaluate the frequency of malarial anemia (mild, moderate, and severe) in patients infected by P. falciparum, P. vivax, or both in two localities in the Brazilian Amazon. In addition, we studied the plasmatic profiles of a number of cytokines and autoantibodies from the patients in order to identify the potential associations of these factors with the different degrees of anemia observed.
MATERIALS AND METHODS
Study area and population.
This study was carried out in two locations of the Brazilian Amazon, in Belém and Paragominas in the state of Pará, in 2001, 2002, and 2003. Patients treated at the Instituto Evandro Chagas and the Municipal Hospital of Paragominas and presenting a positive thick blood smear for P. falciparum, P. vivax, or both were invited to participate in the study. Patients were classified into two age groups according to the World Health Organization classification (54): adults (individuals over 19 years of age) and adolescents/children (individuals up to 19 years of age). A total of 199 patients with P. vivax (n = 125), P. falciparum (n = 72), or mixed (n = 2) malaria infections were enrolled in the study. The patients (median age of 30 years) presented symptoms during a median time of 4 days (range, 0 to 30 days) before diagnosis, and 75% of them mentioned that they had had at least one episode of malaria in the past. Fifty-six healthy asymptomatic individuals (median age of 27 years) living in the studied areas with negative thick blood smears and no previous history of malaria were enrolled as controls. Most of the patients (51%) and healthy individuals (53%) reported living in the studied areas for fewer than 10 years. Since this is a low-transmission area traditionally composed of migrant individuals, there is no major difference in the ethnic composition of experimental and control groups. In addition, apart from an increased proportion of public employees among the control group members, there was a similar profile of persons with professional activities in both groups. All individuals (patients and controls) signed an informed consent to participate in the study and filled out a questionnaire in which relevant epidemiological information was collected. Ethical clearance for the study was given by the Fiocruz and Instituto Evandro Chagas ethical committees.
Blood samples.
On the admission of each patient, a thick blood film was prepared and Giemsa stained for the identification and quantification of Plasmodium species, and three venous blood samples were taken: the first was put into EDTA-containing tubes for hemogram analysis (automated hematological analyzer; Coulter STKS system), erythrocyte morphology evaluation, sickle cell disease evaluation, and a Coombs gel test (DiaMed, Belo Horizonte, Brazil); the second sample was put into a tube without anticoagulant for the bilirubin-automated dosage (Cobas Mira Plus system); and the third sample was put into heparin-containing tubes for the evaluation of immunohematological and biochemical parameters and cytokine/chemokine analysis.
Evaluation of anemia.
The degree of anemia was evaluated through blood hemoglobin (Hb) quantification. The following parameters were used: (i) male adult individuals were considered anemic when the Hb level was less than or equal to 13 g/dl of blood, and (ii) adult women, adolescents, and children (more than 2 years of age) of both sexes were considered anemic when the Hb level was less than or equal to 12 g/dl of blood. Anemia was classified as mild (Hb level between 10 and 12 or 13 g/dl of blood depending on the sex and on the age), moderate (Hb level equal to or above 7 and below 10 g/dl), and severe (Hb level lower than 7 g/dl). To exclude eventual cases of anemia associated with sickle cell disease, hemoglobin electrophoresis was performed for all studied individuals.
Cytokine assays.
The plasmatic concentrations of cytokines and chemokine were measured by enzyme-linked immunosorbent assay (ELISA) using reagents from R&D Systems (Minneapolis, MN). Briefly, the capture monoclonal anti-human TNF-α (clone 28401.111), gamma interferon (IFN-γ) (clone 25718.111), IL-10 (clone 23738.111), IL-12 (clone 24945.11), MIF (clone 12302.2), and MCP-1 (clone 23007.111) antibodies (R&D Systems) were used to coat 96-well plates (Maxisorp, Nunc, CA) for 14 h at 4°C. After being washed and blocked, plasma samples diluted 1:2 were added to duplicated wells and incubated for 14 h at 4°C. After the samples were washed, biotinylated anti-human cytokine and chemokine antibodies (R&D Systems) were added. The presence of bound antibodies was detected using streptavidin-peroxidase (Sigma) for 30 min at room temperature (RT), followed by the addition of 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS; Sigma) and 30% hydrogen peroxide (Merck, Darmstadt, Germany) as the substrate. The reaction was stopped with 20% sodium dodecyl sulfate (Merck, Darmstadt, Germany), and the absorbance was read at 405 nm in a spectrophotometer (Spectra Max 250; Molecular Devices, Sunnyvale, CA). A standard curve was constructed for each cytokine and chemokine by using different dilutions of human recombinant cytokines and chemokines. The sensitivities for the assays were the following: for TNF-α and IFN-γ, 2 pg/ml; IL-10, IL-12, and MCP-1, 4 pg/ml; and MIF, 80 pg/ml.
Serological test.
All plasma specimens were tested against the asexual blood stages of P. vivax (PRG strain, from Paragominas, Brazil) and P. falciparum (PSS1 strain, from Rondonia, Brazil) by the indirect fluorescent antibody test (IFAT) according to the method described by Ferreira and Sanches (25). Briefly, slides containing 20 parasitized red blood cells per microscopic field were prepared. Following incubation of plasma at serial dilutions in phosphate-buffered saline (PBS), fluorescein isothiocyanate (FITC)-conjugated anti-human immunoglobulin G (IgG) and IgM (Sigma) diluted in PBS-Evans blue solution were added and incubated for 30 min at 37°C. Following the final wash, slides were mounted with coverslips using buffered glycerol (pH 7.8) and read in a Zeiss fluorescence microscope. The last spot with distinct parasite outline fluorescence was used for the reported titer. For the purposes of the present study, a response at or above the 1:40 dilution was considered positive. All completed IFAT slides were assessed by the same observer to ensure the consistency of interpretation.
ELISA for detection of anti-phospholipid (cardiolipin) antibodies.
Plates (Maxisorp) were coated with 50 μl of 12-μg/ml cardiolipin (Sigma) per well and diluted in ethanol (Merck KGaA, Darmstadt, Germany) at 4°C until evaporation. After that, 200 μl of PBS containing 2% bovine serum albumin (BSA; Sigma) was added to each well, and the contents were incubated for 2 h at RT. After being washed three times with PBS, the samples were diluted at 1:100 in PBS containing 0.05% Tween 20 (Sigma) (PBS-T20) and 2% BSA. Plates were incubated for 1 h at 4°C, washed with PBS, and then incubated for 1 h at RT with anti-human IgG peroxidase-conjugated antibody (Sigma) (100 μl/well). After the samples were washed, 100 μl of 0.1 M citrate buffer (Sigma), pH 5.0, containing o-phenylenediamine (OPD; Sigma) and 30% hydrogen peroxide (Merck) at 1:3,000 were added to the wells. The reaction was stopped with 6 N sulfuric acid (Merck). The optical density at 492 nm (OD492) was measured using a Spectra Max 250 microplate reader (Molecular Devices). Cutoff values were defined as the mean OD of negative control wells plus two standard deviations (with the value for the blank well subtracted).
ELISA for detection of antibodies directed to components of the normal erythrocyte membrane.
The antigen for ELISA was prepared according to Schetters and coworkers (64), with modifications. For the red blood cells, 20 ml per plate were lysed with 6 mM hypotonic phosphate buffer, pH 8.0 (lysis buffer). Ghosts were pelleted and washed with lysis buffer until the supernatant was clear. Membrane proteins were extracted by adding 3.5 ml of PBS containing 1% (vol/vol) Triton X-100 (Sigma) to the ghost pellet. The mixture was incubated for 15 min on ice. Finally, the antigen preparation was diluted with PBS-Triton X-100 to 10 ml. Microplates (Maxisorp) were filled with antigen solution (100 μl/well) and incubated overnight at 4°C. After incubation, plates were washed with PBS-T20, and the wells were blocked with 200 μl of PBS/well containing 1% BSA for 2 h at 37°C. Serum samples were diluted 1:100 in PBS-T20 and 1% BSA, and a 100-μl/well concentration of the solution was added to coated plates, which then were incubated for 1 h at 37°C. After the samples were washed with tap water, anti-human IgG conjugated to peroxidase (Sigma) was used at a 1:1,000 dilution in PBS-T20 and 1% BSA, a 100-μl/well concentration of the solution was added, and the samples were incubated for 1 h at 37°C. After the samples were washed, 100 μl of 0.1 M citrate buffer (pH 5.0) containing OPD and 30% H2O2 at a 1:3,000 dilution was added to the wells. The reaction was stopped with 6 N H2SO4. The OD492 was measured using a Spectra Max 250 microplate reader (Molecular Devices). Cutoff values were defined as the mean OD of negative control wells plus two standard deviations (with the value for the blank well subtracted).
Statistical analysis.
For unpaired analyses, the nonparametric Mann-Whitney test was used to determine the significance of differences between plasmatic concentrations of cytokines and chemokines in patients with acute malaria infection and in healthy control individuals and also between antibody responses and hematological characteristics of the patients. The Spearman rank correlation coefficient test was used to evaluate the correlation of laboratory and immunological data among malaria patients. The chi-square test (with Yates’ correction) was used to evaluate the proportions of antibodies to components of the normal erythrocyte membrane and to cardiolipin among patients and among healthy control individuals. For all analyses, P values of less than 0.05 were considered significant.
RESULTS
Malaria and anemia.
Out of the 199 studied patients, 72 (36%) were anemic. Sixty-six patients (91.7%) presented mild anemia, five presented (6.9%) moderate anemia, and one (1.4%) had severe anemia (Table 1). None of the healthy control individuals showed Hb levels below the normal threshold. In patients with mild anemia, 98% presented as normocytic and normochromic, whereas of the patients with moderate anemia, three of the five cases were microcytic and hypochromic (Table 1). All patients had a normal Hb standard, and most of them were negative on direct (99.5%) and indirect (97%) Coombs tests (data not shown).
TABLE 1.
Morphological classification of the anemia in malaria patients according to the degree of anemia and the infecting plasmodial species
| Morphology of anemia | No. (%) with the indicated degree of anemia by infection typea
|
Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mild
|
Moderate
|
Severe
|
|||||||||||
| P. vivax | P. falciparum | Mixed | Total | P. vivax | P. falciparum | Mixed | Total | P. vivax | P. falciparum | Mixed | Total | ||
| Macrocytic and normochromic | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Normocytic and normochromic | 37 (97) | 26 (100) | 2 (100) | 65 (98) | 2 (50) | 0 | 0 | 2 (40) | 1 (100) | 0 | 0 | 1 (100) | 68 (94) |
| Microcytic and hypochromic | 1 (3) | 0 | 0 | 1 (2) | 2 (50) | 1 (100) | 0 | 3 (60) | 0 | 0 | 0 | 0 | 4 (6) |
| Total (100%) | 38 | 26 | 2 | 66 | 4 | 1 | 0 | 5 | 1 | 0 | 0 | 1 | 72 |
Mild anemia, Hb between 10 and 12 or 13 g/dl; moderate anemia, Hb equal to or above 7 and below 10 g/dl; and severe anemia, Hb < 7 g/dl.
The frequency of anemia was similar between patients infected with P. falciparum (37.5%) and P. vivax (34.4%). The two patients with a mixed infection presented mild anemia. Surprisingly, the frequency of moderate anemia was higher among P. vivax (9.3%) than among P. falciparum (3.7%) patients, and the only patient presenting severe anemia was infected by P. vivax. However, the small number of patients with moderate and severe anemia did not allow for the establishment of the significance of this finding.
The time elapsed between the appearance of the first symptoms and malaria diagnosis was similar between P. falciparum-infected (4.76 ± 3.9 days) and P. vivax-infected (4.78 ± 3.8 days) patients. In the case of P. vivax-infected patients only, there was a positive relationship between the time of the diagnosis and of the manifestation, but not the severity, of anemia (P = 0.001).
Although the mean parasitemia was higher for P. falciparum-infected (16,862 ± 47,327 parasites/μl) than for P. vivax-infected (4,308 ± 6,521 parasites/μl) patients, this difference was not statistically significant. When divided in two groups according to a threshold of parasitemia (>10,000 or ≤10,000 parasites/μl, which is the threshold used by the World Health Organization to help define severe anemia in P. falciparum-infected patients), the frequency of patients with >10,000 parasites/μl was twice as high among P. falciparum-infected patients as it was among P. vivax-infected patients, but again this difference was not significant. In any case, the rates of parasitemia at admission were low, with most P. vivax (91.2%) and P. falciparum (80.6%) patients falling into the ≤10,000-parasites/μl group. There was no relationship between the level of parasitemia and the manifestation of anemia for either group.
Cytokine levels and correlations.
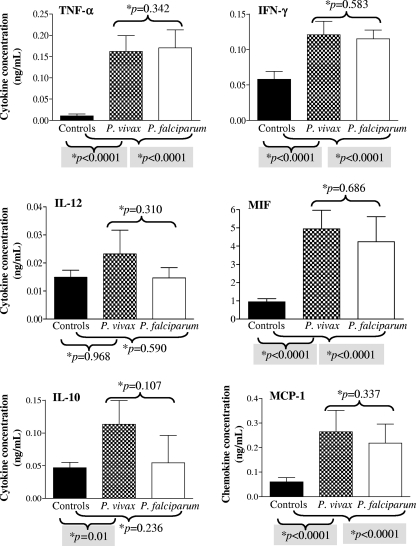
Malarious (P. vivax and P. falciparum) patients had significantly higher TNF-α, IFN-γ, MIF, and MCP-1 (P < 0.0001) plasmatic concentrations than control healthy individuals (Fig. 1). Only P. vivax-infected patients had significantly higher IL-10 (P = 0.01) levels than control healthy individuals. The levels of the cytokines and chemokines were not different between P. vivax and P. falciparum patients.
FIG. 1.
Plasmatic concentration of cytokines and a chemokines in patients with acute uncomplicated malaria infection and healthy control individuals from the Brazilian Amazon. The results shown are the means and 95% confidence intervals. *, P values were determined by nonparametric Mann-Whitney tests.
The acute-phase plasma concentrations of MCP-1 positively correlated with parasitemia in P. vivax- and P. falciparum-infected patients. The same was true for IL-12 in P. vivax-infected patients, but this was not the case for P. falciparum-infected patients. No relationship was found between parasitemia and the levels of the other cytokines assayed (data not shown).
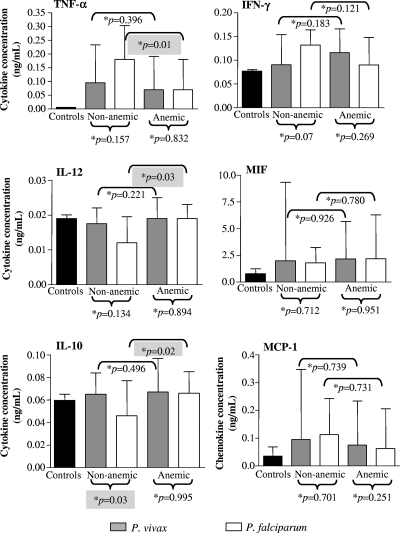
In the case of P. vivax-infected patients, there was no difference in the levels of any of the cytokines/chemokines assayed between anemic and nonanemic patients. However, in P. falciparum patients, anemic individuals presented lower concentrations of TNF-α and higher concentrations of IL-12 and IL-10 than patients with normal Hb levels (Fig. 2).
FIG. 2.
Plasmatic concentration of cytokines and chemokines in patients with uncomplicated malaria according to the presence of anemia and in healthy control individuals from the Brazilian Amazon. The results shown are the median values and interquartile ranges. *, P values were determined by nonparametric Mann-Whitney tests.
Antibody response and associations.
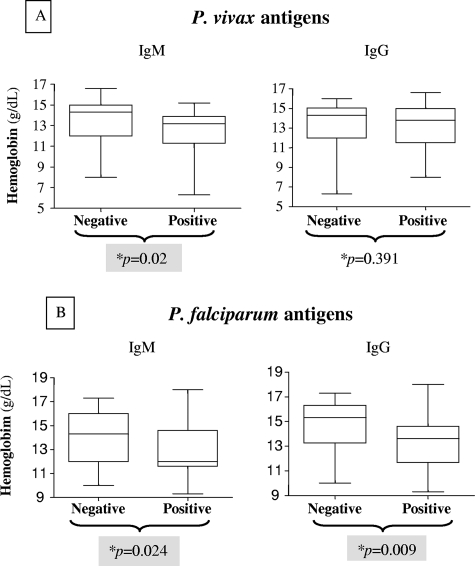
For Plasmodium vivax-infected patients with positive IgM levels (n = 25), but not those presenting IgG (n = 93), responses against the blood stages of P. vivax had significantly lower Hb levels than patients with negative IgM responses (P = 0.02) (Fig. 3A). Similarly, P. falciparum-infected patients with positive responses to both IgM and IgG against blood-stage forms of P. falciparum had significantly lower Hb levels than patients with negative responses (P = 0.024 and 0.009, respectively) (Fig. 3B).
FIG. 3.
Hb levels of malaria patients according to the presence of antibodies (IgM and IgG) against blood-stage forms of P. vivax and P. falciparum, as detected by IFAT. (A) P. vivax patients; (B) P. falciparum patients. *, P values were determined by nonparametric Mann-Whitney tests.
Significantly higher concentrations of TNF-α (P < 0.0001) and IFN-γ (P = 0.033) were observed among P. vivax-infected patients negative for IgM against P. vivax. Conversely, the concentrations of MIF (P < 0.0001) and IL-10 (P < 0.0001) were significantly increased in those with positive IgM responses for P. vivax antigen (Table 2). Significantly higher concentrations of TNF-α (P = 0.004) and IFN-γ were observed among P. vivax-infected patients positive for IgG against P. vivax. Conversely, the concentrations of IL-10 (P = 0.021) were significantly increased in those with negative responses for P. vivax antigen.
TABLE 2.
Plasmatic concentrations of TNF-α, IFN-γ, IL-12, MIF, and IL-10 cytokines and MCP-1 chemokine in malaria patients according to the IgG and IgM antibody responses against blood-stage forms of P. vivax and P. falciparum
| Species and antibody response | Median cytokine concn (interquartile range) (P value)a
|
|||||
|---|---|---|---|---|---|---|
| TNF-α | IFN-γ | IL-12 | MIF | IL-10 | MCP-1 | |
| P. vivax | ||||||
| IgM | ||||||
| Negative | 177 (3-237) | 139 (58-160) | 18 (0-23) | 1,684 (1,104-2,624) | 60 (11-80) | 81 (60-237) |
| Positive | 0 (0-52) (<0.0001) | 88 (69-102) (0.033) | 17 (16-21) (0.447) | 10,311 (4,301-12,565) (<0.0001) | 84 (75-123) (<0.0001) | 105 (66-365) (0.326) |
| IgG | ||||||
| Negative | 26 (0-72) | 88 (55-106) | 18 (16-21) | 2,369 (1,213-10,518) | 75 (56-91) | 80 (67-367) |
| Positive | 154 (36-232) (0.004) | 134 (60-156) (0.061) | 17 (0-23) (0.191) | 1,980 (1,217-4,820) (0.657) | 60 (8-83) (0.021) | 93 (58-196) (0.320) |
| P. falciparum | ||||||
| IgM | ||||||
| Negative | 132 (39-232) | 124 (61-164) | 18 (0-225) | 1,676 (1,369-2,959) | 52 (10-72) | 112 (55-323) |
| Positive | 146 (42-254) (0.941) | 101 (62-148) (0.591) | 16 (0-20) (0.663) | 2,358 (1,392-6,288) (0.156) | 63 (36-85) (0.141) | 60 (31-182) (0.149) |
| IgG | ||||||
| Negative | 170 (40-346) | 150 (93-170) | 0 (0-21) | 1,786 (1,334-2,994) | 45 (14-83) | 63 (25-149) |
| Positive | 132 (40-212) (0.392) | 100 (58-156) (0.097) | 17 (0-21) (0.349) | 2,000 (1,418-4,169) (0.329) | 62 (14-78) (0.730) | 98 (59-206) (0.159) |
Concentrations are given as nanograms/milliliter. P values were determined by nonparametric Mann-Whitney tests.
Autoantibody responses.
Antibodies directed to components of the normal erythrocyte membrane were detected in 98 (49%) of the studied patients (Table 3), and this frequency was significantly higher than that for healthy control individuals (P < 0.0001). The frequency of anti-erythrocyte membrane antibodies in P. vivax-infected patients (56%) was significantly higher than that in P. falciparum-infected patients (36%) (P = 0.01) (Table 3).
TABLE 3.
Frequencies of antibodies to components of normal erythrocyte membranes in patients with acute malaria and in healthy control individuals
| Infection type | No. of individuals positive for anti-erythrocyte antibodies/total no. infected (%)
|
|
|---|---|---|
| Infected | Control | |
| P. vivax | 70/125 (56) | |
| P. falciparum | 26/72 (36) | |
| Mixed | 2/2 (100) | |
| No infection | 4/56 (7) | |
| Total | 98/199 (49) | 4/56 (7) |
Antibodies to cardiolipin were detected in 41 (21%) patients (Table 4). No difference was observed in the frequency of antibodies among patients infected with P. vivax (22%) and those infected with P. falciparum (19%). Antibody responses to cardiolipin were detected in three (5%) of the healthy control individuals, but again, the frequency of positivity among patients was significantly higher than that in healthy control individuals (P = 0.01) (Table 4).
TABLE 4.
Frequencies of antibodies to cardiolipin in patients with acute malaria and in healthy control individuals
| Infection type | No. of individuals positive for anti-cardiolipin antibodies/total no. infected (%)
|
|
|---|---|---|
| Infected | Control | |
| P. vivax | 27/125 (22) | |
| P. falciparum | 14/72 (19) | |
| Mixed | 0/2 (0) | |
| No infection | 3/56 (5) | |
| Total | 41/199 (21) | 3/56 (5) |
The presence of antibodies against components of the normal erythrocyte membrane detected in P. vivax-infected patients was positively correlated with MIF (P < 0.0001) concentrations, while in P. falciparum-infected patients such a correlation was observed with IL-12 (P = 0.005) concentrations (data not shown). No correlation was observed between the presence of anti-cardiolipin antibodies and the concentrations of the analyzed cytokines/chemokines for P. vivax- and P. falciparum-infected patients (data not shown).
DISCUSSION
In the present work, we report and analyze the prevalence and potential intervenient mechanisms of anemia in malaria patients in an area in which the coexisting transmission of P. falciparum and P. vivax is hypoendemic. The area in which the study was conducted, in the eastern Brazilian Amazon region, has a predominant transmission of P. vivax, a species known to cause considerable morbidity but rarely deadly complications. Since transmission levels are low, the population is predominantly nonimmune, and malaria is expected to affect individuals of all ages. In addition, contrary to the infection patterns of high-transmission areas in Africa, malaria is observed with more frequency in adults, particularly males, due to the professional rural activities that subject them to increased exposure to mosquito bites (7, 18). This distribution was confirmed in the present study, in which 84% of the patients were adults, 15.5% were adolescents, and only one (0.5%) was a child. In addition, 90% of the patients were male, and 96% worked in wood extraction, agriculture, or mining.
Despite the known vulnerability of nonimmune individuals to complicated forms of malaria, only one case of severe anemia was reported. No other malaria patient enrolled in the study was hospitalized due to anemia or any other reason. Although 3.3% of all malaria patients were hospitalized in the study locations in the year 2000 (R. M. Braz and C. J. M. Silva, personal communication), the low frequency of hospitalization observed during the period of this study was in accordance with the overall picture seen in the period of 2001 to 2003, when there was a sharp reduction in the total number of malaria cases (279,303 in 2000 and 123,490 in 2003) and in the frequency of hospitalization (3.3% in 2000 and 2.6% in 2003) in the state of Pará (R. M. Braz and C. J. M. Silva, personal communication) as a result of the more intensive malaria control measures adopted by the Ministry of Health (38). The number of hospitalized patients dropped drastically from the year 2000 to the year 2003 in Belém (140 hospitalized patients in 2000 and 49 in 2003) and particularly in Paragominas (275 in 2000 and only 6 in 2003) (R. M. Braz and C. J. M. Silva, personal communication). Mortality due to malaria in 2001 to 2003 was low, with a total of 119 fatal cases out of 458,305 malaria cases (0.26 fatal outcomes per 1,000 malaria cases, or 10.2 deaths per 1,000 hospitalized patients) (R. M. Braz and C. J. M. Silva, personal communication).
The most important factor contributing to the low prevalence of severe malaria cases probably was the rapid diagnosis/treatment provided by the extensive malaria diagnosis network (around 3,000 stations) existing in the Brazilian Amazon at the time of the study. Indeed, individuals in these areas are perfectly aware that fever in the region can be malaria, a potentially lethal disease, and the resulting early diagnosis allows the establishment of prompt treatment, free of charge, which positively affects the clinical outcome. In fact, in the case of P. vivax infections, there was a relationship between the time for diagnosis and the manifestation of anemia. In addition, the characteristics of the population at risk of acquiring malaria in the region, with a large predominance of adult males (known to be less susceptible to anemia and to severe malaria in general [see below]), may help explain the data.
Even considering the low frequency of severe and fatal cases, it is noteworthy that the only reported case of severe anemia and four of the five cases of moderate anemia in the present work occurred in patients infected by P. vivax. Severe complications of P. vivax infections have been reported consistently in the past years in South America and in other regions of the globe (35, 40, 60, 61). A study focused on children (0 to 14 years old) infected by P. vivax conducted in Belém, the same locality in which we performed part of this study, showed that the vast majority (82.7%) of the patients were anemic (68). These studies referred to hospitalized patients and/or specific groups such as children and provide no information on the prevalence of such complications in the populations in which they occur.
In a community-based study in Thailand, in an area with the coexisting transmission of P. falciparum and P. vivax, 7% of P. falciparum malaria patients were hospitalized, and the incidence of severe falciparum malaria was reported to be 5%; that of severe anemia was 1.7% (41). In contrast, in this community, only 0.4% (10 out of 2,573) of the P. vivax malaria patients were hospitalized, mostly young children, and half presented with severe anemia. One study in Colombia with 104 P. vivax outpatients aged 2 to 75 years old showed that mild and moderate anemia was present in 39% of women and 51% of men (19). Both the frequency and the intensity of anemia were greater in children than in adults. In male adults, the frequency of anemia was 40% (which is similar to the frequency found in the present study), and no severe anemia was reported. These studies, together with ours, show that although anemia is a common finding in P. vivax malaria, it may not be a major player in morbidity. Besides that, although the absence of a relationship between the degrees of anemia and parasitemia in malarious patients has been widely documented (1, 58), this may result from the fact that the great majority of patients in the study presented at admission with low levels of parasitemia.
In this work, we have tried to identify possible immune mechanisms or components involved in the manifestation and severity of anemia in malaria patients in the specific epidemiological context of the Amazonia. Both P. vivax and P. falciparum patients showed elevated levels of most of the cytokines assayed, with the exception of IL-12, the levels of which were similar between malaria patients and control individuals. Interestingly, there was no difference between the levels of each of the cytokines for P. vivax and P. falciparum patients. Cytokines are considered to play important roles not only in immunity but also in the pathology of malaria, especially for P. falciparum infections (3, 30, 34, 42). High levels of TNF-α, for instance, have been incriminated in the genesis of both severe anemia and cerebral malaria (27, 67). Of course, the pathogenesis of these complications is multifactorial, but the finding here that cytokine levels are similar in P. falciparum and P. vivax patients in this specific epidemiological context may help explain the very low incidence of complicated malaria.
Levels of cytokines were not associated with the development of anemia during P. vivax infections. An interesting finding was, however, observed in P. falciparum-infected patients: significantly lower levels of TNF-α were found in anemic P. falciparum patients, while significantly higher levels of IL-10 were found in nonanemic ones. Since the opposite situation (high TNF-α and low IL-10 levels) has been classically associated with severe malarial anemia in Africa (29, 37, 55), our data suggest that the mechanisms involved in the determinism of the different degrees (mild, moderate, and severe) of anemia are different, i.e., the different degrees may not just be a consequence of intensity but of the quality of the operating mechanisms. If this is true, mild and severe anemia would represent truly different entities instead of different quantitative expressions of the same one.
We have analyzed the levels of cytokines in anemic patients that we divided in two groups, those with mild and those with moderate/severe anemia. Even considering the small number of moderate and severe cases of anemia in our study, no difference was observed for any of the cytokines assayed, but the levels of the chemokine MCP-1 were significantly higher in the moderate/severe anemia group for P. vivax as well as for P. falciparum patients. MCP-1 is involved in the attraction and activation of macrophages, and some studies have shown that hemozoin is capable of inducing the expression of a number of cytokines and chemokines, including MCP-1, and attracting macrophages and other leukocytes to specific sites (32, 33). Macrophage hyperactivation is thought to play a role in severe malaria through the secretion of high levels of cytokines such as TNF-α and also by their actions on erythrocyte destruction through phagocytosis and bone marrow inhibition (4, 10). It also is noteworthy that, in the present study, the levels of MCP-1 positively correlated with parasitemia, which could be giving origin to increased hemozoin production.
Erythrocyte destruction during malaria also can be caused by antibody-mediated lysis or opsonization. In this sense, P. vivax patients showing IgM antibody responses and P. falciparum patients showing IgM and IgG antibody responses presented significantly lower levels of hemoglobin than nonresponders to plasmodial antigens. The presence of IgM antibodies against P. vivax was associated with IL-10 and MIF responses, and conversely, TNF-α and IFN-γ responses were associated with the lack of an antibody response. A Th2-biased cytokine response seems necessary for a first-line antibody response during P. vivax infections. Therefore, although specific cytokine responses were not directly associated with anemia in P. vivax infections, an indirect action through the induction of an antiplasmodial IgM antibody response can be important.
We also have looked at the possible role of antibodies directed against host-specific components, cardiolipin and erythrocyte membrane antigens, in malarial anemia. Although a high frequency of patients presented such antibodies, no association with the development of anemia was found.
In conclusion, this work confirms that mild anemia is a common outcome of malarial infections in the epidemiological setting of the eastern Amazon region, which is characterized by low levels of transmission of both P. vivax and P. falciparum (with a predominance of the former), a population at risk with a marked predominance of nonimmune adult males, and easy and rapid access to malaria diagnosis and treatment. One of the most interesting findings is that infections by P. vivax and P. falciparum present similar characteristics, with similar levels of parasitemia, frequencies and intensities of anemia, and levels of different cytokines and antibody responses. Although infections by these two species have been considered to be very different, with quite different biological and pathophysiological characteristics, the leveling factor likely is the early phase of the infection at which patients were enrolled in the study, when they looked for health care soon after the first symptoms appeared. In addition, age must have a strong influence, since we dealt mostly with adults, and previous works in this region and in regions with similar epidemiological characteristics showed that children are unequivocally more vulnerable to malaria complications, whether from P. vivax or P. falciparum infection (19, 41, 68).
Acknowledgments
This work was supported by Fiocruz and by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Ministry of Education of Brazil.
We thank Rui Moreira Braz and Carlos José Mangabeira da Silva of Secretaria de Vigilância em Saúde (SVS) of the Ministry of Health of Brazil for providing the malaria epidemiological data of Belém and Paragominas, Pará State, Brazil.
Footnotes
Published ahead of print on 6 February 2008.
REFERENCES
- 1.Abdalla, S., D. J. Weatherall, S. N. Wickramassinghe, and M. Hughes. 1980. The anemia of P. falciparum malaria. Br. J. Haematol. 46:71-183. [DOI] [PubMed] [Google Scholar]
- 2.Alves, F. P., L. H. Gil, M. T. Marrelli, P. E. Ribolla, E. P. Camargo, and L. H. da Silva. 2005. Asymptomatic carriers of Plasmodium spp. as infection source for malaria vector mosquitoes in the Brazilian Amazon. J. Med. Entomol. 42:777-779. [DOI] [PubMed] [Google Scholar]
- 3.Angulo, I., and M. Fresno. 2002. Cytokines in the pathogenesis of and protection against malaria. Clin. Diagn. Lab. Immunol. 9:1145-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biemba, G., V. R. Gordeuk, P. E. Thuma, G. F. Mabeza, and G. Weiss. 1998. Prolonged macrophage activation and persistent anaemia in children with complicated malaria. Trop. Med. Int. Health 3:60-65. [DOI] [PubMed] [Google Scholar]
- 5.Biemba, G., V. R. Gordeuk, P. Thuma, and G. Weiss. 2000. Markers of inflammation in children with severe malarial anaemia. Trop. Med. Int. Health 5:256-262. [PubMed] [Google Scholar]
- 6.Camacho, L. H., V. R. Gordeuk, P. Wilairatana, P. Pootrakul, G. M. Brittenham, and S. Looareesuwan. 1998. The course of anemia after the treatment of acute falciparum malaria. Ann. Trop. Med. Parasitol. 92:525-537. [DOI] [PubMed] [Google Scholar]
- 7.Camargo, L. M., M. U. Ferreira, H. Krieger, E. P. de Camargo, and L. P. da Silva. 1994. Unstable hypoendemic malaria in Rondonia (western Amazon region, Brazil): epidemic outbreaks and work-associated incidence in an agro-industrial rural settlement. Am. J. Trop. Med. Hyg. 51:16-25. [DOI] [PubMed] [Google Scholar]
- 8.Cardoso, M. A., M. U. Ferreira, L. M. Camargo, and S. C. Szarfac. 1992. Anemia in a population from an endemic area of malaria, Rondonia (Brazil). Rev. Saude Publica 26:161-166. [DOI] [PubMed] [Google Scholar]
- 9.Cardoso, M. A., M. U. Ferreira, L. M. Camargo, and S. C. Szarfac. 1994. Anaemia, iron deficiency and malaria in a rural community in Brazilian Amazon. Eur. J. Clin. Nutr. 48:326-332. [PubMed] [Google Scholar]
- 10.Carvalho, L. J., H. L. Lenzi, M. Pelajo-Machado, D. N. Oliveira, C. T. Daniel-Ribeiro, and M. F. Ferreira-da-Cruz. 2000. Plasmodium berghei: cerebral malaria in CBA mice is not clearly related to plasma TNF levels or intensity of histopathological changes. Exp. Parasitol. 95:1-7. [DOI] [PubMed] [Google Scholar]
- 11.Casals-Pascual, C., O. Kai, J. O. Cheung, S. Williams, B. Lowe, M. Nyanoti, T. N. Williams, K. Maitland, M. Molyneux, C. Newton, N. Peshu, S. M. Watt, and D. J. Roberts. 2006. Suppression of erythropoiesis in malarial anemia is associated with hemozoin in vitro and in vivo. Blood 108:2569-2577. [DOI] [PubMed] [Google Scholar]
- 12.Chang, K. H., and M. M. Stevenson. 2004. Effect of anemia and renal cytokine production on erythropoietin production during blood-stage malaria. Kidney Int. 65:1640-1646. [DOI] [PubMed] [Google Scholar]
- 13.Clark, I. A., F. M. Al Yaman, and L. S. Jacobson. 1997. The biological basis of malarial disease. Int. J. Parasitol. 27:1237-1249. [DOI] [PubMed] [Google Scholar]
- 14.Daniel-Ribeiro, C. T., and G. Zanini. 2000. Autoimmunity and malaria: what are they doing together? Acta Trop. 76:205-221. [DOI] [PubMed] [Google Scholar]
- 15.Daniel-Ribeiro, C. T., D. M. Banic, I. Issa-Ahmed, and B. Galvão-Castro. 1986. Polyclonal B-lymphocyte activation and sensitization of erythrocytes by IgG in human malaria: relevance to the development of anaemia in a holoendemic area in northwestern Brazil (Ariquemes - Rondônia). Mem. Inst. Oswaldo Cruz. 81(Suppl. 2):169-176. [Google Scholar]
- 16.Debierre-Grockiego, F., L. Schofield, N. Azzouz, J. Schmidt, C. Santos de Macedo, M. A. Ferguson, and R. T. Schwarz. 2006. Fatty acids from Plasmodium falciparum down-regulate the toxic activity of malaria glycosylphosphatidylinositols. Infect. Immun. 74:5487-5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dondorp, A. M., B. J. Angus, K. Chotivanich, K. Silamut, R. Ruangveerayuth, M. R. Hardeman, P. A. Kager, J. Vreeken, and N. J. White. 1999. Red blood cell deformability as a predictor of anemia in severe falciparum malaria. Am. J. Trop. Med. Hyg. 60:733-737. [DOI] [PubMed] [Google Scholar]
- 18.Duarte, E. C., T. W. Gyorkos, L. Pang, and M. Abrahamowicz. 2004. Epidemiology of malaria in a hypoendemic Brazilian Amazon migrant population: a cohort study. Am. J. Trop. Med. Hyg. 70:229-237. [PubMed] [Google Scholar]
- 19.Echeverri, M., A. Tobon, G. Alvarez, J. Carmona, and S. Blair. 2003. Clinical and laboratory findings of Plasmodium vivax malaria in Colombia, 2001. Rev. Inst. Med. Trop. São Paulo 45:29-34. [DOI] [PubMed] [Google Scholar]
- 20.Ehrhardt, S., G. D. Burchard, C. Mantel, J. P. Cramer, S. Kaiser, M. Kubo, R. N. Otchwemah, U. Bienzle, and F. P. Mockenhaupt. 2006. Malaria, anemia, and malnutrition in African children—defining intervention priorities. J. Infect. Dis. 194:108-114. [DOI] [PubMed] [Google Scholar]
- 21.Ekvall, H. 2003. Malaria and anemia. Curr. Opin. Hematol. 10:108-114. [DOI] [PubMed] [Google Scholar]
- 22.Facer, C. A., R. S. Bray, and J. Brown. 1979. Direct Coombs antiglobulin reactions in Gambian children with Plasmodium falciparum malaria: incidence and class specificity. Clin. Exp. Immunol. 35:119-127. [PMC free article] [PubMed] [Google Scholar]
- 23.Facer, C. A. 1980. Direct Coombs antiglobulin reactions in Gambian children with Plasmodium falciparum malaria. II. Specificity of erythrocyte-bound IgG. Clin. Exp. Immunol. 39:279-288. [PMC free article] [PubMed] [Google Scholar]
- 24.Facer, C. A. 1980. Direct antiglobulin reactions in Gambian children with P. falciparum malaria. III. Expression of IgG subclass determinants and genetic markers and association with anemia. Clin. Exp. Immunol. 41:81-90. [PMC free article] [PubMed] [Google Scholar]
- 25.Ferreira, A. W., and M. C. A. Sanches. 1988. Malária humana. Padronização e optimização de testes sorológicos para diagnóstico individual e inquéritos soroepidemiológicos. Rev. Inst. Med. Trop. São Paulo 30:137-146. [DOI] [PubMed]
- 26.Ferreira, M. U., N. D. Karunaweera, M. Silva-Nunes, N. S. Silva, D. F. Wirth, and D. L. Hartl. 2007. Population structure and transmission dynamics of Plasmodium vivax in rural Amazonia. J. Infect. Dis. 195:1218-1226. [DOI] [PubMed] [Google Scholar]
- 27.Gimenez, F., S. Barraud de Lagerie, C. Fernandez, P. Pino, and D. Mazier. 2003. Tumor necrosis factor alpha in the pathogenesis of cerebral malaria. Cell. Mol. Life Sci. 60:1623-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomes, M., J. Crawley, P. Duclos, and M. Young. 2002. Malaria intermittent treatment in infants. TDR News 68:12-13. [Google Scholar]
- 29.Issifou, S., E. Mavoungou, S. Borrmann, M. K. Bouyou-Akotet, P. B. Matsiegui, P. G. Kremsner, and F. Ntoumi. 2003. Severe malarial anemia associated with increased soluble Fas ligand (sFasL) concentrations in Gabonese children. Eur. Cytokine Netw. 14:238-241. [PubMed] [Google Scholar]
- 30.Jacobs, P., D. Radzioch, and M. M. Stevenson. 1996. A Th1-associated increase in tumor necrosis factor alpha expression in the spleen correlates with resistance to blood-stage malaria in mice. Infect. Immun. 64:535-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jakobsen, P. H., S. D. Morris-Jones, L. Hviid, T. G. Theander, M. Hoier-Madsen, R. A. Bayoumi, and B. M. Greenwood. 1993. Anti-phospholipid antibodies in patients with Plasmodium falciparum malaria. Immunology 79:653-657. [PMC free article] [PubMed] [Google Scholar]
- 32.Jaramillo, M., I. Plante, N. Ouellet, K. Vandal, P. A. Tessier, and M. Olivier. 2004. Hemozoin-inducible proinflammatory events in vivo: potential role in malaria infection. J. Immunol. 172:3101-3110. [DOI] [PubMed] [Google Scholar]
- 33.Jaramillo, M., M. Godbout, and M. Olivier. 2005. Hemozoin induces macrophage chemokine expression through oxidative stress-dependent and -independent mechanisms. J. Immunol. 174:475-484. [DOI] [PubMed] [Google Scholar]
- 34.Jason, J., L. K. Archibald, O. C. Nwanyanwu, M. Bell, I. Buchanan, J. Larned, P. N. Kazembe, H. Dobbie, B. Parekh, M. G. Byrd, A. Eick, A. Han, and W. R. Jarvis. 2001. Cytokines and malaria parasitemia. Clin. Immunol. 100:208-218. [DOI] [PubMed] [Google Scholar]
- 35.Kochar, D. K., V. Saxena, N. Singh, S. K. Kochar, S. V. Kumar, and A. Das. 2005. Plasmodium vivax malaria. Emerg. Infect. Dis. 11:132-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kroeger, A., A. Avinna, J. Ordonnez-Gonzalez, and C. Escandon. 2002. Community cooperatives and insecticide-treated materials for malaria control: a new experience in Latin America. Malar. J. 1:15. [DOI] [PMC free article] [PubMed]
- 37.Kurtzhals, J. A., V. Adabayeri, B. Q. Goka, B. D. Akanmori, J. O. Oliver-Commey, F. K. Nkrumah, C. Behr, and L. Hviid. 1998. Low plasma concentrations of interleukin 10 in severe malarial anaemia compared with cerebral and uncomplicated malaria. Lancet 351:1768-1772. [DOI] [PubMed] [Google Scholar]
- 38.Ladislau, J. L. B., M. C. Leal, and P. L. Tauil. 2006. Avaliação do Plano de Intensificação das Ações de Controle da Malária na região da Amazônia Legal, Brasil, no contexto da descentralização. Epidemiol. Serviços Saúde 15:9-20.
- 39.Loiola, C. C., C. J. da Silva, and P. L. Tauil. 2002. Malaria control in Brazil: 1965 to 2001. Rev. Panam. Salud Publica 11:235-244. [DOI] [PubMed] [Google Scholar]
- 40.Lomar, A. V., J. E. Vidal, F. P. Lomar, C. V. Barbas, G. J. Matos, and M. Boulos. 2005. Acute respiratory distress syndrome due to vivax malaria: case report and literature review. Braz. J. Infect. Dis. 9:425-430. [DOI] [PubMed] [Google Scholar]
- 41.Luxemburger, C., F. Ricci, F. Nosten, D. Raimond, S. Bathet, and N. J. White. 1997. The epidemiology of severe malaria in an area of low transmission in Thailand. Trans. R. Soc. Trop. Med. Hyg. 91:256-262. [DOI] [PubMed] [Google Scholar]
- 42.Malaguarnera, L., and S. Musumeci. 2002. The immune response to Plasmodium falciparum malaria. Lancet Infect. Dis. 2:472-478. [DOI] [PubMed] [Google Scholar]
- 43.Marcano, T. J., A. Morgado, C. E. Tosta, and J. R. Coura. 2004. Cross-sectional study defines difference in malaria morbidity in two Yanomami communities on Amazonian boundary between Brazil and Venezuela. Mem. Inst. Oswaldo Cruz. 99:369-376. [DOI] [PubMed] [Google Scholar]
- 44.Martínez-Espinosa, F. 1998. Malária na gravidez: estudo de pacientes do Instituto de Medicina Tropical do Amazonas, Brasil, 1990-1997. M. S. thesis. Mestrado em Medicina Tropical, Departamento de Medicina Tropical, Fundação Instituto Oswaldo Cruz, Rio de Janeiro, Brazil.
- 45.Mayor, A., J. J. Aponte, C. Fogg, F. Saute, B. Greenwood, M. Dgedge, C. Menendez, and P. L. Alonso. 2007. The epidemiology of malaria in adults in a rural area of southern Mozambique. Malar. J. 6:3. [DOI] [PMC free article] [PubMed]
- 46.McDevitt, M. A., J. Xie, G. Shanmugasundaram, J. Griffith, A. Liu, C. McDonald, P. Thuma, V. R. Gordeuk, C. N. Metz, R. Mitchell, J. Keefer, J. David, L. Leng, and R. Bucala. 2006. A critical role for the host mediator macrophage migration inhibitory factor in the pathogenesis of malarial anemia. J. Exp. Med. 203:1185-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mishra, S. K., S. Mohanty, A. Mohanty, and B. S. Das. 2006. Management of severe and complicated malaria. J. Postgrad. Med. 52:281-287. [PubMed] [Google Scholar]
- 48.Muhangi, L., P. Woodburn, M. Omara, N. Omoding, D. Kizito, H. Mpairwe, J. Nabulime, C. Ameke, L. A. Morison, and A. M. Elliott. 2007. Associations between mild-to-moderate anaemia in pregnancy and helminth, malaria and HIV infection in Entebbe, Uganda. Trans. R. Soc. Trop. Med. Hyg. 101:899-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murphy, S. C., and J. G. Breman. 2001. Gaps in the childhood malaria burden in Africa: cerebral malaria, neurological sequelae, anemia, respiratory distress, hypoglycaemia, and complications of pregnancy. Am. J. Trop. Med. Hyg. 64(Suppl. 1-2):57-67. [DOI] [PubMed] [Google Scholar]
- 50.Murray, C. J. L., and A. D. Lopez. 1996. The global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. Global burden of disease and injury series, vol. 1. Harvard University Press, Cambridge, MA.
- 51.Murray, C. J. L., and A. D. Lopez. 1997. Mortality by cause for eight regions of the world: global burden of disease study. Lancet 349:1269-1276. [DOI] [PubMed] [Google Scholar]
- 52.Nchinda, T. C. 1998. Malaria: a reemerging disease in Africa. Emerg. Infect. Dis. 4:398-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nussenblatt, V., G. Mukasa, A. Metzger, G. Ndeezi, E. Garrett, and R. D. Semba. 2001. Anemia and interleukin-10, tumor necrosis factor alpha, and erythropoietin levels among children with acute, uncomplicated Plasmodium falciparum malaria. Clin. Diagn. Lab. Immunol. 8:1164-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Organización Mundial de la Salud. 1995. La salud de los Jóvenes: un reto y una esperanza. Organización Mundial de la Salud, Geneva, Switzerland.
- 55.Othoro, C., A. A. Lal, B. Nahlen, D. Koech, A. S. S. Orago, and V. Udhayakumar. 1999. A low interleukin-10 tumor necrosis factor-α ratio is associated with malaria anemia in children residing in a holoendemic malaria region in western Kenya. J. Infect. Dis. 179:279-282. [DOI] [PubMed] [Google Scholar]
- 56.Owens, S., L. W. Chamley, J. Ordi, B. J. Brabin, and P. M. Johnson. 2005. The association of anti-phospholipid antibodies with parity in placental malaria. Clin. Exp. Immunol. 142:512-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Owusu-Agyei, S., D. J. Fryauff, D. Chandramohan, K. A. Koram, F. N. Binka, F. K. Nkrumah, G. C. Utz, and S. L. Hoffman. 2002. Characteristics of severe anemia and its association with malaria in young children of the Kassena-Nankana District of northern Ghana. Am. J. Trop. Med. Hyg. 67:371-377. [DOI] [PubMed] [Google Scholar]
- 58.Phillips, R. E., S. Looareesuwan, D. A. Warrell, S. H. Lee, J. Karbwang, M. J. Warrell, N. J. White, C. Swasdichai, and D. J. Weatherall. 1986. The importance of anaemia in cerebral and uncomplicated falciparum malaria: role of complications, dyserythropoiesis and iron sequestration. QJM 58:305-323. [PubMed] [Google Scholar]
- 59.Ritter, K., A. Kuhlencord, R. Thomssen, and W. Bommer. 1993. Prolonged haemolytic anaemia in malaria and autoantibodies against triosephosphate isomerase. Lancet 342:1333-1334. [DOI] [PubMed] [Google Scholar]
- 60.Rodriguez-Morales, A. J., E. Sanchez, M. Vargas, C. Piccolo, R. Colina, M. Arria, and C. Franco-Paredes. 2006. Is anemia in Plasmodium vivax malaria more frequent and severe than in Plasmodium falciparum? Am. J. Med. 119:e9-e10. [DOI] [PubMed] [Google Scholar]
- 61.Rodríguez-Morales, A. J., E. Sanchez, M. Vargas, C. Piccolo, R. Colina, and M. Arria. 2006. Anemia and thrombocytopenia in children with Plasmodium vivax malaria. J. Trop. Pediatr. 52:49-51. [DOI] [PubMed] [Google Scholar]
- 62.Rosenberg, E. B., G. T. Strickland, S. L. Yang, and G. E. Whalen. 1973. IgM antibodies to red cells and autoimmune anemia in patients with malaria. Am. J. Trop. Med. Hyg. 22:146-152. [DOI] [PubMed] [Google Scholar]
- 63.Roshanravan, B., E. Kari, R. H. Gilman, L. Cabrera, E. Lee, J. Metcalfe, M. Calderon, A. G. Lescano, S. H. Montenegro, C. Calampa, and J. M. Vinetz. 2003. Endemic malaria in the Peruvian Amazon region of Iquitos. Am. J. Trop. Med. Hyg. 69:45-52. [PubMed] [Google Scholar]
- 64.Schetters, T. P., J. H. Van Run van Breda, C. Hermsen, J. Curfs, and W. M. Ling. 1989. Protective and pathological activity in serum of mice developing resistance to Plasmodium berghei infection. Parasite Immunol. 11:413-423. [DOI] [PubMed] [Google Scholar]
- 65.Scopel, K. K., C. J. Fontes, M. U. Ferreira, and E. M. Braga. 2005. Plasmodium falciparum: IgG subclass antibody response to merozoite surface protein-1 among Amazonian gold miners, in relation to infection status and disease expression. Exp. Parasitol. 109:124-134. [DOI] [PubMed] [Google Scholar]
- 66.Snow, R. W., M. H. Craig, C. R. Newton, and R. W. Steketee. 2003. The public health burden of Plasmodium falciparum malaria in Africa: deriving the numbers. Working paper 11. Disease Control Priorities Project, Bethesda, MD.
- 67.Tchinda, V. H., A. D. Tadem, E. A. Tako, G. Tene, J. Fogako, P. Nyonglema, G. Sama, A. Zhou, and R. G. Leke. 2007. Severe malaria in Cameroonian children: correlation between plasma levels of three soluble inducible adhesion molecules and TNF-alpha. Acta Trop. 102:20-28. [DOI] [PubMed] [Google Scholar]
- 68.Ventura, A. M. R., A. Y. Pinto, R. S. Silva, V. S. Calvosa, M. G. Silva-Filho, and J. M. Souza. 1999. Plasmodium vivax malaria in children and adolescents—epidemiological, clinical and laboratory features. J. Pediatr. (Rio de Janeiro) 75:187-194. [DOI] [PubMed]
- 69.Voller, A. 1974. Immunopathology of malaria. Bull. W. H. O. 50:177-186. [PMC free article] [PubMed] [Google Scholar]
- 70.Weatherall, D. J., and S. Abdalla. 1982. The anemia of Plasmodium falciparum malaria. Br. Med. Bull. 38:147-151. [DOI] [PubMed] [Google Scholar]
- 71.Weatherall, D. J., L. H. Miller, D. I. Baruch, K. Marsh, O. K. Doumbo, C. Casals-Pascual, and D. J. Roberts. 2002. Malaria and the red cell. Hematology 35-57. [DOI] [PubMed]
- 72.Wickramasinghe, S. N., and S. H. Abdalla. 2000. Blood and bone marrow changes in malaria. Baillieres Best Pract. Res. Clin. Haematol. 13:277-299. [DOI] [PubMed] [Google Scholar]
- 73.Zhu, J., G. Krishnegowda, and D. C. Gowda. 2005. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: the requirement of extracellular signal-regulated kinase p38, c-Jun N-terminal kinase and NF-κB pathways for the expression of proinflammatory cytokines and nitric oxide. J. Biol. Chem. 280:8617-8627. [DOI] [PMC free article] [PubMed] [Google Scholar]