Abstract
Patients with histoplasmosis may test falsely negative for Histoplasma capsulatum antigenuria. In some cases antigen is present at levels below the assay's detection limit, and ultrafiltration could improve sensitivity. Antigen was detected following ultrafiltration in 73.8% of falsely negative specimens versus 2% of controls. Ultrafiltration improved sensitivity with a small reduction in specificity.
When the third-generation MVista Histoplasma capsulatum antigen enzyme immunoassay (EIA) was implemented, the cutoff for a positive result was set at three times the negative-control value, based upon receiver operating characteristic curve analysis (1). The cutoff in the second-generation assay was two times the negative-control value, and results were reported as units, dividing the optical density of the specimen by two times the optical density of the negative control. Positive results between 1.0 and 2.0 units were regarded as “weak positive.” Upon recognizing that results from patients with proven histoplasmosis sometimes were between two and three times the negative-control value in the third-generation assay, we initiated an investigation to determine if ultrafiltration of the urine would increase the antigen concentration above the assay cutoff. As a proof of principle, urine specimens from patients with probable histoplasmosis, controls with other diseases, and healthy subjects were tested before and following ultrafiltration.
Residual urine specimens from 95 patients with probable or proven histoplasmosis had been stored frozen for up to 2 years before testing. The diagnosis of probable histoplasmosis was based upon the presence of at least 2 specimens with positive tests for Histoplasma antigen for 93 patients, and proven histoplasmosis was based on positive histopathology or culture for 1 patient each. Demonstration of repeated antigenuria increases the probability that the patient has histoplasmosis (L. J. Wheat, unpublished observation). Other clinical or laboratory information was not available. The histoplasmosis specimens were divided into two groups based upon the Histoplasma antigen result: low positive and false negative. Specimens with results between 1.0 and 2.0 units in the second-generation MVista Histoplasma antigen EIA (n = 15 specimens from 14 patients) were considered positive, and those with results of <0.6 ng/ml in the third-generation MVista EIA (n = 20 specimens from 20 patients) were classified as low positive. Specimens with a result of <0.6 ng/ml were classified as “positive, low” in the third-generation assay, because they were above the assay cutoff, at three times the negative-control value, but below the lowest assay calibrator, at 0.6 ng/ml. False-negative specimens included 65 specimens from 56 patients in the second-generation assay and 19 specimens from 17 patients in the third-generation assay. Patients could be represented in more than one of the above groups, accounting for a total of 95 patients.
Controls included 50 patients for whom histoplasmosis was excluded based upon clinical and laboratory findings in a study approved by the institutional review board at Clarian Hospital, Indianapolis, IN, and 50 healthy subjects, who were office and laboratory volunteers.
Specimens were concentrated using 5,000-molecular-weight-cutoff Amicon Ultra centrifugal filter tubes, according to the manufacturer's instructions (Millipore, Billerica, MA). Four milliliters of urine was centrifuged at 3,656 × g for 16 min. If the amount of retentate was less than 0.4 ml, the filtrate was added back to adjust the final volume to 0.4 ml, resulting in a final 10-fold concentration. If the amount of retentate was greater than 1 ml, centrifugation was repeated for 8 min, yielding a final volume of 0.4 to 0.8 ml, representing a 5- to 10-fold concentration. Approximately 15% of the specimens required repeat centrifugation. Unconcentrated specimens and the retentate following ultrafiltration were tested in the MVista Histoplasma antigen EIA as described previously (1). Probabilities for the observed frequencies were compared using Fisher's exact test.
Among the 35 specimens exhibiting low-level antigenuria, the median number of previously positive specimens was 5 and 72% of the patients had at least 3 prior positive specimens. Antigen levels in the low-positive group increased at least twofold in 26 of 35 specimens (74.3%) following ultrafiltration (P < 0.001). Three were negative following ultrafiltration, however. The mean increase was 3.10-fold; the standard deviation was 1.80-fold, and the range was 0.32- to 8.97-fold.
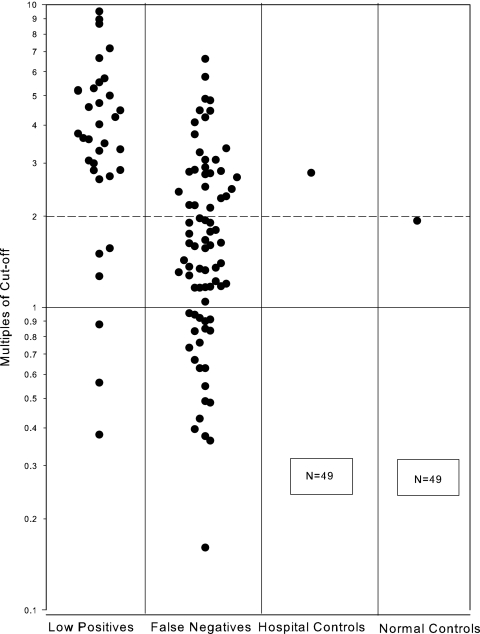
Among the 84 false-negative specimens, the median number of prior positive specimens was 5 and 83% of the patients had at least three prior positive specimens. None were positive before ultrafiltration, compared to 62 of 84 specimens (73.8%) after ultrafiltration (P < 0.001) (Fig. 1). The mean increase was 3.31-fold, the standard deviation was 1.86-fold, and the range was 0.56- to 10.07-fold. False-positive results occurred for 1 of 50 hospital controls (2%) and 1 of 50 healthy subjects (2%).
FIG. 1.
Antigen levels following ultrafiltration. Data are expressed as a multiple of the cutoff. Results above the solid horizontal line are positive at ≥1 times the cutoff. The broken horizontal line at 2.0 times the cutoff indicates the upper level for the low-positive group tested unconcentrated. The boxes in the control-group columns represent specimens with negative results before and following ultrafiltration.
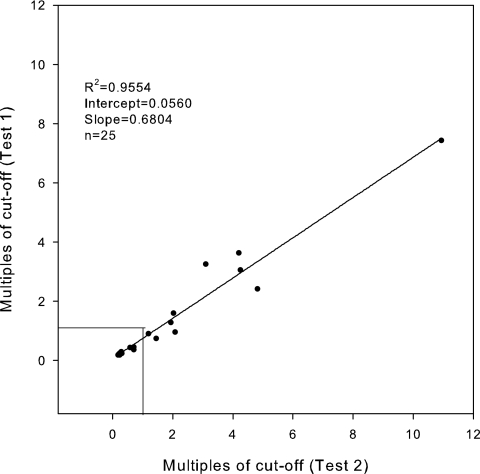
Reproducibility was assessed by performing ultrafiltration and testing on 25 specimens, of which 15 were initially negative and 10 positive, in two separate experiments. Twenty-two of the 25 (88%) were reproducibly positive or negative (Fig. 2). Seven of 10 (70%) that were initially positive were positive upon retesting, and 15 of the 15 (100%) that were initially negative were negative upon retesting. For the three that were initially positive but were negative upon repeat testing, the repeat results were 0.74, 0.90, and 0.94 times the cutoff. By linear regression analysis, the results of initial and repeat testing correlated well: r2 = 0.9554; slope = 0.684; y intercept = 0.056.
FIG. 2.
Reproducibility of antigen levels following ultrafiltration. Twenty-five specimens were concentrated and tested on consecutive days, and the results were compared by linear regression analysis. Data points below the 1.0 cutoff are clumped together for the negative controls, preventing identification of 15 different points. Data for the 10 positive specimens can be differentiated, showing that 7 of the 10 were reproducibly above the 1.0 cutoff.
Ultrafiltration improved the sensitivity for detection of antigen in false-negative specimens by 74%. Although we sought to concentrate specimens 10-fold, that goal was not achieved for about 15% of specimens because the retentate could not be adjusted to that exact ratio. Accordingly, we chose not to express data on concentrated specimens quantitatively in ng/ml. Specificity was 98%, based upon results with specimens from healthy volunteers and hospital controls without histoplasmosis. Reproducibility was 88%.
Previously we demonstrated detection of antigen in a bronchoalveolar lavage specimen from a patient with histoplasmosis (2) and in urine from a patient with coccidioidomycosis (3) only following ultrafiltration. In addition to showing that ultrafiltration improves the sensitivity of the assay for detection of false-negative specimens, the study establishes the significance of low-level antigenuria. Of patients with histoplasmosis who had low-level antigenuria (1 to 2 units in the second-generation assay and <0.6 ng/ml in the third-generation assay), results increased at least twofold following ultrafiltration for 74%. Positive results at <0.6 ng/ml in the MVista assay may indicate the presence of histoplasmosis and alert the physician to perform additional testing, including serology, culture, and repeat antigen testing. Ultrafiltration of specimens with negative results at between two and three times the negative control level in the MVista assay may assist physicians in diagnosis of histoplasmosis in patients for whom results are falsely negative.
Acknowledgments
This work was supported by MiraVista Diagnostics, Indiana- polis, IN.
L.E., P.A.C., J.W., and L.J.W. are employees of MiraVista Diagnostics.
Footnotes
Published ahead of print on 27 February 2008.
REFERENCES
- 1.Connolly, P. A., M. M. Durkin, A. M. LeMonte, E. J. Hackett, and L. J. Wheat. 2007. Detection of histoplasma antigen by a quantitative enzyme immunoassay. Clin. Vaccine Immunol. 14:1587-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hage, C. A., T. E. Davis, L. Egan, M. Parker, D. Fuller, A. M. LeMonte, M. Durkin, P. Connelly, L. J. Wheat, D. Blue-Hnidy, and K. S. Knox. 2007. Diagnosis of pulmonary histoplasmosis and blastomycosis by detection of antigen in bronchoalveolar lavage fluid using an improved second-generation enzyme-linked immunoassay. Respir. Med. 101:43-47. [DOI] [PubMed] [Google Scholar]
- 3.Kuberski, T., R. Myers, L. J. Wheat, B. M. Kubak, D. Bruckner, and D. Peuges. 2007. Diagnosis of coccidioidomycosis by antigen detection using cross-reaction with a Histoplasma antigen. Clin. Infect. Dis. 44:e50-e54. [DOI] [PubMed] [Google Scholar]