Abstract
Angiogenesis, the growth and remodeling of a vascular network, is an essential process during development, growth and disease. Here we studied the role of the vascular endothelial growth factor receptor (VEGFR) in experimentally-induced angiogenesis in the colonial ascidian Botryllus schlosseri (Tunicata, Ascidiacea). The circulatory system of B. schlosseri is composed of two distinct, but interconnected regions: a plot of sinuses and lacunae which line the body, and a transparent, macroscopic extracorporeal vascular network. The vessels of the extracorporeal vasculature are morphologically inverted in comparison to the vasculature in vertebrates: they consist of a single layer of ectodermally-derived cells with the basal lamina lining the lumen of the vessel. We found that when the peripheral circulatory system of a colony is surgically removed, it can completely regenerate within 24 to 48 hours and this regeneration is dependent on proper function of the VEGF pathway: siRNA-mediated knockdown of the VEGFR blocked vascular regeneration, and interfered with vascular homeostasis. In addition, a small molecule, the VEGFR kinase inhibitorPTK787/ZK222584, phenocopied the siRNA knockdown in a reversible manner. Despite the disparate germ layer origins and morphology of the vasculature, the developmental program of branching morphogenesis during angiogenesis is controlled by similar molecular mechanisms, suggesting that the function of the VEGF pathway may be co-opted during the regeneration of an ectoderm-derived tubular structure.
Keywords: Angiogenesis, Ascidians, Botryllus schlosseri, Tubular Branching, Regeneration, Vascular System, VEGFR
INTRODUCTION
Development of the vascular system and tubular organs such as the lungs and endocrine glands, involves common elements of cell behavior, such as proliferation, migration and adhesion, which lead to the formation and branching of epithelial tubules (Davies, 2002, 2005). The formation of tubules starts as a simple epithelial pouch from which new branches successively bud, giving rise to a tree-like network of interconnected tubes. Failure of tube formation often leads to organ failure and major disorders (i.e. spina bifida and polycystic kidney disease; Karner et al., 2006). Despite the variability in size, shape, and tissue of origin, these tubular structures are invariably composed of a wrapped epithelium where the apical surface of the cells lines the lumen. In addition, a tube is a universal structure used in both transitory developmental stages as well as in the final morphology of multiple tissues and organs, and can be found throughout many metazoan phyla. Taken together, it might be expected that a canonical genetic pathway controls this conserved process, however, this is not the case: it is known that during vertebrate development tube formation in different tissues is initiated by diverse genetic pathways (reviewed in Lubarsky and Krasnow, 2003, Davies, 2002). In addition, while these vertebrate genes and genetic pathways are conserved and found in multiple phyla, they are not evolutionarily linked to tube formation and can play diverse roles, thus it appears that the genetic control is not fixed (discussed below).
One example of this is the control of vascular formation in the vertebrates, where tubular sprouting of mesodermally-derived tissues requires an orchestrated activation of several growth factors. The most important of these occurs via activation of the VEGF (vascular endothelial growth factor) pathway (Cao et al., 2004), which has been established to play a primary role during development of the circulatory system (Tammela et al., 2005; Ferrara et al., 2003). VEGFs show different affinities for specific coupled cell surface tyrosine kinase receptors (Auguste et al., 2005). After binding of the receptor, VEGF initiates a signaling cascade that regulates the proliferation, differentiation and migration of endothelial cells (Tammela et al., 2005; Olsson et al., 2006). In mammals, the three known VEGF receptors (VEGF-R1, VEGF-R2 and VEGF-R3) differ considerably in signaling properties (Tammela et al., 2005), but are all essential for the homeostasis of lymphatic endothelial cells and may also play a role in orchestrating the recruitment of hematopoietic precursors, and the migration of monocytes and macrophages (Holmes and Zachary, 2005). VEGF-like proteins and their receptors evolved relatively early in eumetazoans as indicated by the presence of homologs in several invertebrate phyla, where they seem to be present in a single isoform (Seipel et al., 2004; Tettamanti et al., 2006).
Botryllus schlosseri (Subphylum: Tunicata Class: Ascidiacea) is a primitive chordate organism which has a large, experimentally accessible vasculature and represents a new model to study angiogenesis. Ascidians inhabit shallow waters and harbors throughout the world (Kott, 1986) and are considered to be the closest non-vertebrate relatives of vertebrates: an individual begins its life as a tadpole larva, which after a short swimming phase settles to a substratum and metamorphoses into a non-vertebrate body plan (Berrill, 1975). The resulting juvenile, called an oozooid, immediately begins a lifelong, recurring asexual budding process (blastogenesis) which eventually results in a colony of genetically identical individuals, called zooids, (Burighel and Brunetti, 1971; Fig. 1). Zooids are arranged into a star shaped “system” of between 2-16 individuals, and a single genetically identical colony may consist of hundreds of systems. (Fig. 1; Manni et al., 2007; Tiozzo et al., 2006). The entire colony is connected by a common vasculature embedded within an extracellular matrix made of both cellulose and protein components, called the tunic (De Santo, 1968; Matthysse et al., 2004; Nakashima et al., 2004).
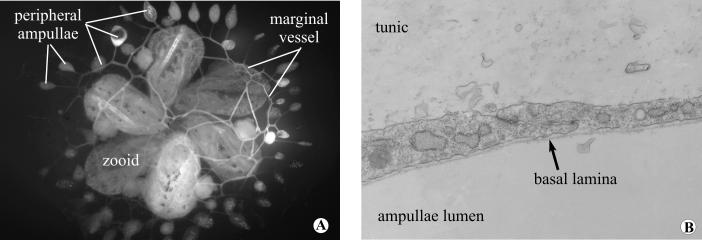
Figure 1.
(A), Colony of Botryllus schlosseri seen in vivo from the ventral side. The vasculature has been injected with fluoresceine in order to highlight the peripheral vessels and the ampullae. (B), Electron micrograph showing the baso-apical structure of a regenerated peripheral vessel after surgery. Magnification 14,000X.
The circulatory system of Botryllus consists of two vastly different structures. Within each zooid body there is an open circulatory system consisting of an internal plot of sinuses and lacunae of mesenchymal cells which surrounds the major organs and tissues. In addition there is a large extracorporeal vasculature consisting of ramified monolayered vessels running throughout the tunic and ending at the periphery of the colony in sac-like structures called ampullae (Fig. 1). Interestingly, the morphology of these extracorporeal vessels is inverted in comparison to other known tubular structures: a single layer of ectodermally-derived cells forms a tube with the basal lamina lining the lumen with, and the apical side of the cells facing outwards (Fig. 1 B; Burighel and Brunetti, 1971; Gasparini et al., 2007). In contrast, the canonical metazoan vessel morphology contains the apical cell surface lining the lumen and basal lamina forming the exterior structure. (Lubarsky and Krasnow, 2003).
The ampullae (Fig. 1) are the sight of an allorecognition reaction that occurs when two colonies grow into proximity and has been the focus of our laboratory for many years (De Tomaso, et. al., 2005). In previous experiments we developed a vasculature regeneration assay which provided us with a way to knockdown allorecognition proteins expressed in the ampullae during regeneration, bypassing issues of protein turnover (Nyholm et al., 2006; Laird et al., 2005). However, the - fast regeneration of the peripheral vessels, and the inverted morphology of the vessel epithelia led us to further investigate the cellular and molecular mechanisms which underlie angiogenesis in Botryllus. Here, using time-lapse microscopy we describe the morphology of vascular regeneration and define the molecular mechanisms underlying angiogenesis. We have cloned the homolog of VEGFR in B. schlosseri and analyzed its role during vascular regeneration by siRNA. Homology between signalling pathways in vertebrates and Botryllus has also been compared using PTK787/ZK222584, a mammalian VEGFR inhibitor currently in clinical trials for cancer treatment.
MATERIALS AND METHODS
Animals
Botryllus schlosseri colonies were raised at 18-20°C according to Boyd et al. (1986). Clonal replicates were developmentally staged from A to D before the surgery (define “ampullectmy”, see below) according to Watanabe (1953): Genetically identical synchronized systems (groups of zooids sharing a common atrial siphon) were used for the siRNA and drug treatment experiments as well as respective controls.
Electron microscopy
Colonies at various stages (A-D) were fixed in 1.5% glutaraldehyde in 0.2M sodium cacodylate, pH 7.4, plus 1.6% NaCl. After washing and postfixation in 1% osmium tetroxide (OsO4) in 0.2M sodium cacodylate, specimens were dehydrated and embedded in Epon resin. Sections (60 nm) were stained with uranyl acetate and lead citrate. Micrographs were taken with a Jeol JEM-1230 transmission electron microscope.
Vasculature removal and Imaging
Peripheral ampullae and parts of the marginal vessels were surgically removed using a wheeler dissection knife (Miltex, PA, USA). All surgical manipulations were performed under a stereomicroscope (Wild Heerbrugg M7A, Gais, Switzerland). The removal of vasculature was visually confirmed by using an inverted microscope (Diaphot 200, Nikon, NY, USA). Time lapse imaging of young colonies (n=7 different colonies, 1-3 zooids each, 1-3 weeks old) was performed by automated microscopy (ImageXpress, Molecular devices Corp., Palo Alto, CA) as described in Voskoboynik et al. (2006). Phase contrast images at varying magnifications were captured for 6 days following the ampullectomy: every 20-60 minutes during the first 4 daysand every 12 hours during the 5thand 6th day. Time lapse imaging of large colonies (n=4 colonies, different genotypes 8-15 zooids each) was performed using a stereomicroscope (Wild Heerbrugg M7A) and a microscope (Diaphot 200, Nikon), coupled to a CCD camera (coolpix995 Nikon). Pictures were taken every 0.5-5 hours during the first 2 days following vasculature removal and once a day thereafter.
BsVEGFR cloning and sequencing
Two pools of cDNA were synthesized from total RNA extracted from mixed developmental stages (A-D) of fresh B. schlosseri colonies and were screened by nested PCR using degenerate primers designed to amplify the tyrosine kinase domain of VEGF receptors (Christiaen et al., 2002; Tiozzo et al., 2005). A 152bp fragment was used to design homologous primers for 5′ and 3′ RACE. The primers used to amplify BsVEGFR cDNA extremities were as follows: VEGFrace5 5′-tcaacggtagtctcgcctct-3′ and VEGFrace5N 5′-gcctctccgtttctgacgta-3′ (external and nested primer respectively) for 5′-RACE; and VEGFrace3 5′-catctaaaaagtgtattcaccgaga-3′ and VEGFrace3N 5′-agacgtggctgccagaaata-3′ (external and nested primer respectively) for 3′-RACE. Overlapping 5′ and 3′ fragments of approximately 1.7kb and 2.1kb, respectively, were gel purified (Qiaquick gel purification kit, Qiagen), cloned into pGEM-T vector (Promega) and combined with an ori- transprimer donor (GPS-1 Genome Priming System, NEB) for complete sequencing. The putative protein obtained has been analyzed using the Simple Modular Architecture Research Tool (Letunic et al., 2006).
Molecular Phylogenetics
BsVEGFR nucleotide and amino acid sequences were analyzed with Lasergene (DNAStar). Multiple Alignment of the tyrosine kinase domains was constructed using ClustalW (v. 1.83) algorithm (Thompson et al., 1994) and the distance trees were built using both the neighbor-joining (Saitou and Nei, 1987) and maximum parsimony (data not shown) in MEGA3 (v. 3.1). Bootstrap analysis was carried for each phylogenetic analysis (1,000 interactions; Felsenstein, 1992).
Quantitative PCR (q-PCR)
For quantitative real time-PCR (q-PCR), systems from a single genotype were collected at different stages (A-D), weighed, flash frozen in liquid nitrogen and maintained at -80°C. RNA was extracted using Nucleospin RNA columns (BD Biosciences Clontech) and reverse transcribed with 200 U of Superscript II (Invitrogen), using a mix of poly-T and random hexamers as primers. Q-PCR was carried out in an iCycler (Bio-Rad) using SYBR Green detection (Bio Rad) with the following BsVEGFR primers: 5′-gaagctttgatggatcgtaagatagcacct-3′ and 5′-agtcataatgcaactcgtttatctcaaagt-3′, which amplified a fragment of 234bp. BsVEGFR expression was normalized to that of alpha-actin as described in Laird et al. (2005). Thermocycling was carried out for 2 minutes at 95°C followed by 43 cycles of 95°C for 30 sec, 58°C for 1 min, and 72°C for 1 min. Analysis of q-PCR was performed using the 2-ΔΔCt method according to Livak and Schmittgen (2001). Each experiment was performed in triplicate, i.e. three different systems from three different genotypes per stage analyzed, and duplicate measurements were reported for each experiment.
In situ hybridization (ISH)
Whole-mount in situ hybridization was performed with digoxigenin (DIG)-labeled probes as described by Brown and Swalla (2007). Sense and antisense probes were synthesized from PCR products using BsVEGFR clones coding for a 348bp specific region, according to the protocols supplied with the DIG RNA Labelling kit (Roche Molecular Biochemicals). For fluorescent ISH, the samples were photographed with a Leica MZ16-FA dissecting microscope. Alkaline phosphatase (AP) treated samples were embedded in paraplast (Tiozzo et al., 2006) sectioned at various orientations (7-8 μm), cleared from paraplast with xylene, counterstained with 1% Eosin Y, dehydrated and mounted with Eukitt medium (Electronic Microscopy Sciences), and photographed with a Leica light compound microscope.
siRNA delivery
siRNAs to BsVEGFR were generated using a commercially available kit (Silencer siRNA cocktail kit, Ambion) as previously described (Nyholm et al., 2006). Briefly, B.schlosseri cDNA was amplified with PCR primers modified adding a 20bp T7 region at the 5′ end (5′-taatacgactcactataggggaagctttgatggatcgtaagatagcacct-3′ and 5′-taatacgactcactatagggagtcataatgcaactcgtttatctcaaagt-3′; T7 promoter sequence underlined), which generated a 234bp sequence product. A control dsRNA was created according to Laird et al. 2005. For knockdown experiments, colonies were microinjected into the blood vasculature with 0.5-1.0 μl of siRNA 40-60 μM in B.shlosseri Buffer (25 mM HEPES, 10 mM cysteine, 50 mM EDTA in filtered sea water). Microinjections were repeated once a day between 2 and 7 days, and the injected colonies were subsequently soaked in 250-500 μl of filtered sea water with100pmoles of siRNA. This was repeated for up to 7 days, changing the solution every second day. Ampullaectomy were performed 7 days after the beginning of the treatment (Fig. 3 M). The controls were performed injecting the same volume of Botryllus Buffer and soaking in filtered sea water. To test potential non-specific effects of exogenous RNA, controls colonies were treated also with siRNA designed to GFP, these colonies did not show any abnormal phenotype when compared to untreated systems (data not shown). The same set of experiments was done on colonies at all the four blastogenetic stages (A-D), with no deviation in results.
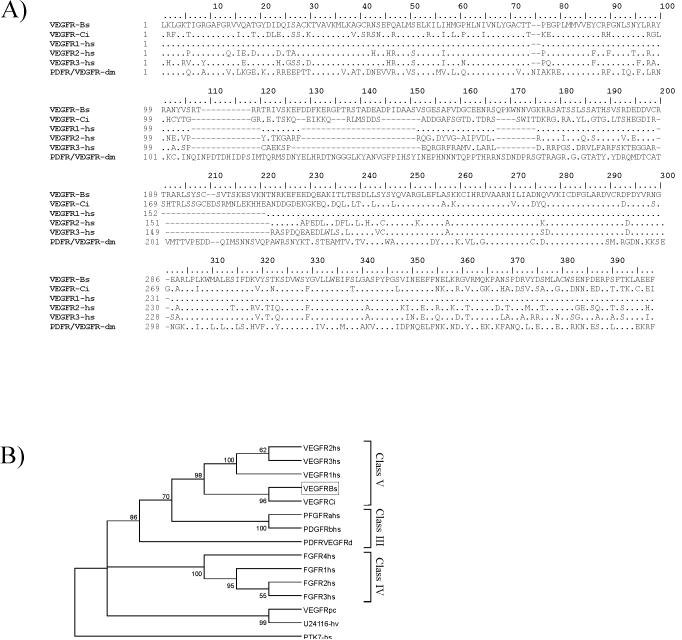
Figure 3.
Conservation of the Botryllus schlosseri vascular endothelial growth factor (BsVEGFR). (A), Sequence conservation in the BsVEGFR tyrosine kinase (TK) domain: amino acid sequence alignment of the Botryllus schlosseri VEGFR TK (VEGF-Bs) with Ciona intestinalis (VEGFR-Ci), Homo sapiens (VEGFR1-hs, VEGFR2-hs and VEGFR3-hs) and Drosophila melanogaster (PDFR.VEGFR-dm). The alignment has been carried with ClustalX and manually. Dots (.) indicate conserved amino acid residues and bars (-) indicate gaps. (B), neighbor-joining tree calculated using TK domain amino acid sequences. Only the topology is displayed. The tree has been rooted using the human colon carcinoma TK 8 (Q13308). Numbers at branching points are bootstrap values after 1,000 interactions (only numbers over 50 are shown). Species, encompassing elements of class III, IV and V of TK receptors, are abbreviated as follows: hs, Homo sapiens; Bs, Botryllus schlosseri; Ci, Ciona intestinalis; pc, Podocoryne carnea and hv and Hydra vulgaris; dm, Drosophila melanogaster. BsVERGFR TK domain is squared.
PTK787/ZK222584 treatment
PTK787/ZK222584 was kindly provided by Novartis. The powder was dissolved in DMSO to a stock concentration of 100mg/ml and then diluted to working concentrations of 5, 10 and 20μM in DMSO. Each system (5/9 blastozooid each) was microinjected with 0.5-0.9 μl of PTK787/ZK222584, then after 3 hours the peripheral vascular system was dissected and angiogenesis was monitored by microscope. Controls systems were injected with the same volume of DMSO without observing any effect.
RESULTS
Regeneration of the colonial circulatory system of Botryllus schlosseri
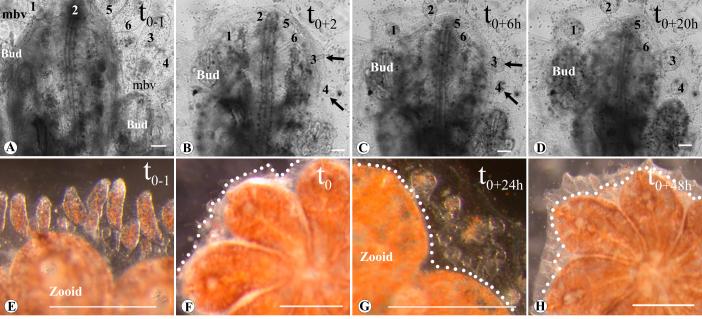
We studied vascular regeneration by surgically removing most of the peripheral vasculature consisting of the peripheral ampullae and the colony marginal vessels,- which herein will be called an ampullaectomy. A portion of the peripheral vasculature cannot be removed in our experimental assays, as it is located underneath the individual zooids and is not accessible to manipulation (Fig. 1A). In small, young colonies (1-3 blastozooids, 1-3 weeks old; n=7), when ampullae and small parts of the marginal blood vessel were removed, new ampullae regenerated within 18 hours. Regeneration occurred from the wounds in the blood vessels at the surgical sites (where the ampullae had been connected to the colony marginal vessel; Fig. 2 B, regeneration points 1-4); and where blood vessels had been torn apart (Fig. 2 B regeneration points 5 and 6). We defined five stages of regeneration during the process of angiogenesis: (1) 1-2 hours following ampullaectomy the wound in the blood vessel at the surgical site is sealed and a small bulb forms in the tip of each anastomized vessel (Fig. 2 B; regeneration point 2); (2) after 4-8 hours the bulb becomes roundish (Fig. 2 C; regeneration site 2-5); (3) then oval (Fig. 2 D); (4), within 17±2.4 hours following surgery (25°C) the ampullae and the marginal blood vessel have completed regeneration (Fig. 2 D), and (5) after 17.8±1.6 hours the ampullae recover their original average size (n=7 different genotypes; Fig. 2 A-D; supplemental video 1). The same regeneration patterns and stages were observed in large colonies, although it took longer to complete the regeneration process. Ampullae returned to their original size and shape on average after 42±5.7 hours following surgery (n=4 colonies, different genotypes, 8-15 zooids each, 2-6 months old; Fig. 1 E-H). Whether this time difference is due to size or age differences is unknown.
Figure 2.
Time lapse images of vasculature regeneration in small (single zooid) (A-D) versus large (E-H) colonies. (A), Single zooid colony, 1 hour before ampullae and parts of the marginal vessel were removed (t0-1). (B), Same zooid 2 hours following vasculature removal (t0+2), the wound in the blood vessel at the surgical site is sealed and a small bulb (arrows) forms in the tip of the anastomized vessel (regeneration site1-6). (C) Same site, 6 hours later (t0+6); the bulbs became roundish (arrows), the marginal vessels that were removed (the vessel between regeneration sites 5 and 6, and the vessel above the right bud) have regenerated. (D) Same site, 20 hours later (t0+20); the regenerating ampullae became oval and recover their original size. (E), Ampullae and marginal blood vessel 1 hour before vasculature removal in a larger colony consisting of 8 zooids (t0-1). (F), Same colony after vasculature removal (t0), and (G) 24 hours later (t+24h). (H), 48 hours following vasculature removal (t+48h) full size regenerating ampullae can be seen above zooids and buds. Dots: edge of the cut, mbv: marginal blood vessel, 1-6: regenerating sites as compared to original ampullae number. Scale bar A-D=100μm, E-H=1mm
Isolation and characterization of Botryllus schlosseri VEGFR
This rapid regeneration suggested that Botryllus represents a novel model to study the molecular mechanisms underlying angiogenesis. To assess conservation of the genetic pathways we isolated the Botryllus schlosseri VEGFR cDNA (BsVEGFR, described in the Methods). A contig of 2926bp coding for a product of 919 amino acid residues was isolated and is closely related to the single VEGFR (Flt1 / Flt4 / Kdr / Pvr) reported in Ciona intestinalis, with an overall amino acid sequence identity of 41%.
In the cloned cDNA sequence, the putative extracellular region is characterized by three immunoglobulin like domains, followed by a transmembrane domain and a split tyrosine kinase domain (TK) (Fig. 3). We have not found an N-terminal signal peptide by standard protein analysis software. The amino acid alignment of the TK domains shows 56% identity with Ciona intestinalis VEGFR (Flt1 / Flt4 / Kdr / Pvr) and 50% with Danio rerio flk1 (DrVEGFR2, Fig. 3 A). The cDNA sequence obtained was deposited into GenBank under accession no. (submission after review).
Phylogenetic analysis was done using the conserved TK domains from three families of receptors: VEGFR, fibroblast growth factors (FGFs) and platelet derived growth factors (PDGFs), with the colon carcinoma kinase 4 receptor (PTK7) used as outgroup (Seipel et al., 2004). As shown in Figure 3 B, the BsVEGFR diverged considerably from the VEGFR homologs in the jellyfish Podocoryne carnea and the TK receptor in Hydra vulgaris, and tightly clusters with the three human VEGFRs (98% NJ bootstrap).
Pattern of expression of BsVEGFR mRNAs
As an adult, B.schlosseri has a unique life history which is characterized by constant and synchronized asexual development punctuated by massive turnover of adult individuals via an apoptotic process called takeover (supplemental video 2). This cycle, called blastogenesis, is organized into four stages (A through D; Watanabe, 1953; Fig. 4 A), each stage characterizing the developmental state of a system during asexual reproduction. Asexual development, from the initiation of a bud to its maturation into an adult zooid lasts 14-days, after which it becomes a filter-feeding adult, with a lifespan of 1 week (18-20°C). At the end of that week, all zooids in a colony synchronously die in a process known as takeover that involves the simultaneous regression and resorption of parent zooids and its replacement by another asexual generation (Lauzon et al., 1993; Lauzon et al., 1992) (Fig. 4 A; supplemental video 2). Stages A through C are designated as periods of high growth, during which adult zooids are filter feeding and buds are undergoing organogenesis: takeover occurs in stage D (Fig. 4 A). Expression analysis by quantitative real time polymerase chain reaction (Q-PCR) shows that BsVEGFR is expressed in all blastogenic stages. The relative abundance of BsVEGFR mRNAs is not highly variable during the blastogenetic development, with a 1.5 fold peak at stage C (Fig. 4 A), when the most growth and morphogenesis is occurring.
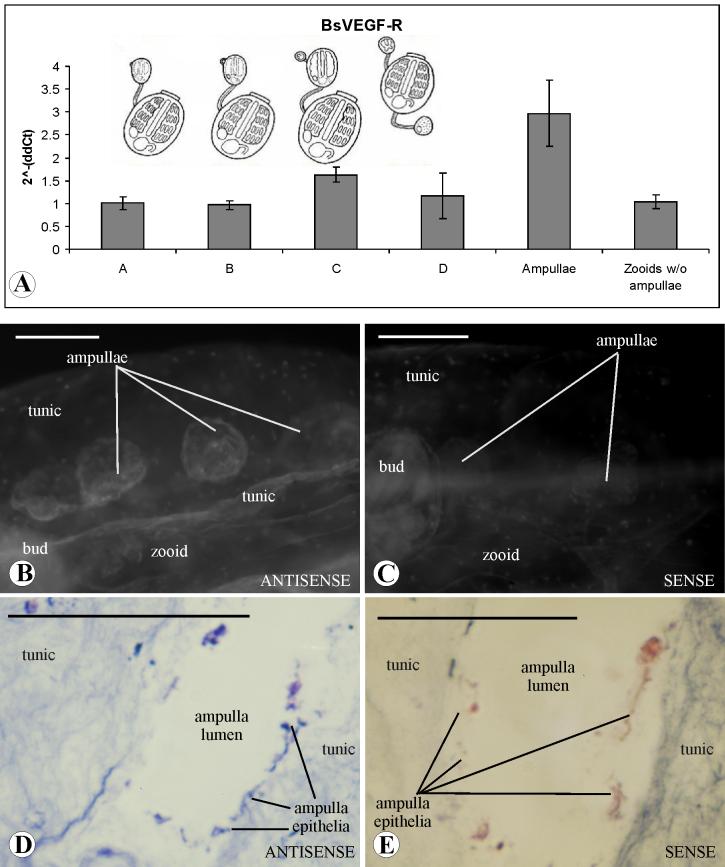
Figure 4.
Expression of BsVEGFR genes in the asexual developmental stages of Botryllus schlosseri. (A), quantitative reverse transcriptase PCR (RT-PCR) expression analysis of BsVEGFR in all stages of the blastogenetic life cycle (A, B, C and D), in the ampullae (taken from colonies at mixed stage), in a colony deprived of ampullae and marginal blood vessel (stage A). The ordinate values are relative expression levels normalize to the expression level of alpha-actin according to the 2-ΔΔCt and displayed in arbitrary units. (B, C), expression of BsVEGFR mRNAs in the epithelium of the ampullae of a zooid of B.schlosseri by fluorescein labeled in situ hybridization, antisense and sense probe respectively. Scale bar 0.5mm. Background staining due to natural fluorescence appears in the bud but not in the ampullae (C).(D, E), DIG-AP labeled in situ hybridization on frontal section of an ampullae: BsVEGFR transcripts are expressed in the monolayer epithelia. Background staining, due to the nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP) treatment, appears the sourrounding tunic. Scale bar 50μm.
As described above, the B. schlosseri circulatory system can be subdivided into two distinct segments: that within the zooid body, which consists of sinuses and lacunae, and the extracorporeal vascular system made up of epithelial vessels that connect the zooids and the buds (Fig. 1). As shown in Figure 4 A, BsVEGFR mRNA expression is higher in the isolated peripheral vasculature (marginal vessel and peripheral ampullae), than in the remaining portion of the ampullectomized colonies, consisting of the zooid bodies and the extracorporeal vasculature underneath and between the blastozooids (Fig. 1 A).
BsVEGFR patterns of expression were also studied by fluorescence whole mount in situ hybridization in young colonies (n=15): BsVEGFR transcripts were detected in the epithelial layer of the peripheral ampullae (Fig. 4 B, C) and in some scattered blood cells but not in the lacunae and sinuses (data not shown). DIG-AP labeled in situ hybridization performed in frontal and transverse sections of the ampullae confirm the presence of BsVEGFR mRNAs in the epithelial layer (Fig. 4 D, E). No expression was seen within the zooid body.
Functional analysis of BsVEGFR during vascular regeneration
The function of BsVEGFR has been studied by genetic knockdowns using short interfering double stranded RNA (siRNA), delivered by injection and soaking (Laird et al., 2005). In all experiments presented here (a series of 24 independent experiments using different genotypes), BsVEGFR expression was knocked down as shown by RT-PCR analysis (Fig. 5M). Then, the peripheral ampullae and the marginal vessel were surgically removed and the individuals visually monitored. Complete BsVEGFR knockdown, occurs after 7 days from the beginning of the treatment (Fig. 5M). Therefore, the ampullectomy was performed a week after the beginning of the siRNA delivery. Expression of housekeeping genes alpha-tubulin and beta-actin were not effected by the treatment (data not shown).
Figure 5.
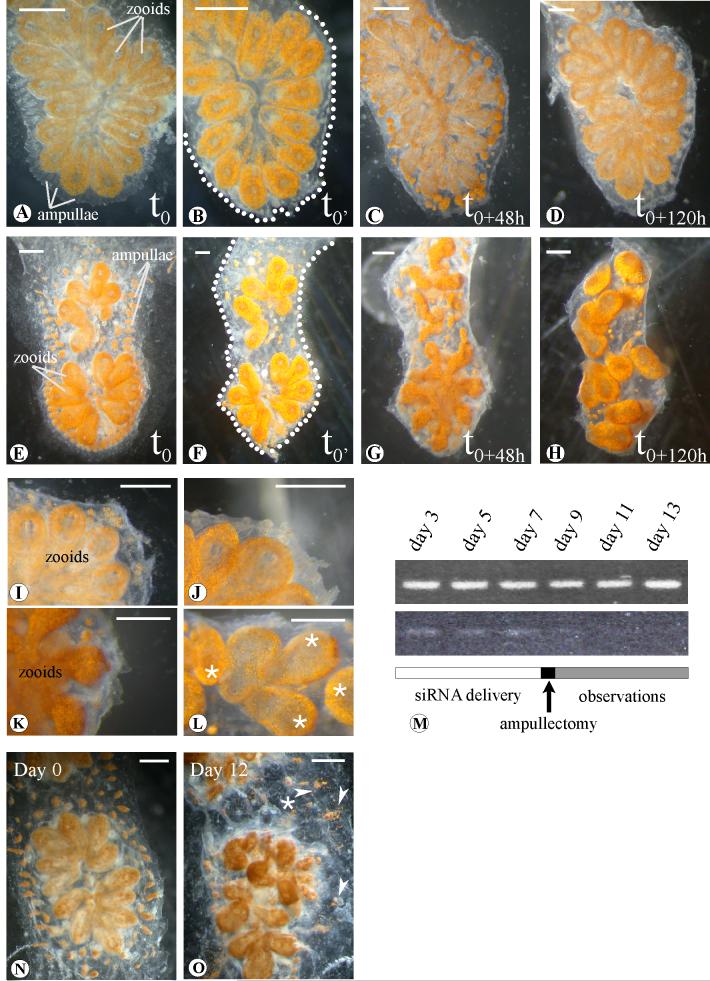
Morphological analysis of the effects induced by BsVEGFR siRNA treatment. (A-D), Botryllus schlosseri systems injected with Botryllus buffer only; (E-H), systems of the same genotype treated with BsVEGFR siRNA (in Botryllus buffer). (B) and (F) samples immediately after the complete ablation of ampullae and marginal vessel (t0’). (C), (G) (D) and (H), snapshots after 48 hours (t0+48h) and 120 hours (t0+120h) respectively: treated samples do not show vascular regrowth in comparison to the controls, after the takeover (C, G) the treated samples bud chaotically and loose the characteristic star-shaped form.Close-up view of untreated samples 48 hours (I) and a week (J) after the complete ampullaectomy. (K) and (L), magnification of marginal tunic in silenced systems after 48 and 120 hours ampullae surgical ablation (oral siphons indicated by asterisks). (M), RT-PCR at different days after the first siRNA injection: BsVEGFR siRNAs treated samples show reduction of the transcript while controls (top), injected with Botryllus buffer, maintain appreciatively the same transcript levels. Detail of a colony before treatment with BsVEGFR siRNA. Day 0 (N) and 12 days (O) after daily injections and/or soaking. Arrowheads show exploded ampullae and vessels leaking pigmented cells in the tunic. Dots underline the edge of the cut. Scale bar 1mm.
Differences between the control and BsVEGFR knockdown experiments were visible 48 hours after the ampullaectomy: the treated systems (n=13) were not capable to initiate angiogenesis, while the controls, injected with either GFP siRNA or Botryllus buffer (see Materials), did not present any evident phenotype (Fig. 5 C, G). Nevertheless, the zooids did not present any morphological aberration, the blood in the internal circulatory system kept flowing, and the development of the new zooids continued normally (Fig. 5 G, K).
However, there is a higher order morphological affect of BsVEGFR knockdown. In control colonies, after the takeover phase, the newly developed adult zooids, which were separated by the region where the previous generation was located and had been resorbed, migrated together to form the typical star shaped system (Fig. 5 H, L, Supplemental video 2). In contrast, in the BsVEGFR knockdown phenotype, the new adult zooids migrated chaotically, losing their characteristic star-shaped morphology. Specifically, they did not organize into the wild-type multi-zooid systems (Fig. 5 H, L): each blastozooid maintained an independent atrial siphon (which usually fuses into a common siphon in wild types) and came to rest in a random orientation. Despite this unusual orientation, the zooids continued their blastogenetic cycle; buds developed, and emerged without any obvious alteration in morphology or function. This was surprising, as each zooid had several connections to the peripheral vasculature that had to be reestablished after takeover-associated migration. These results suggests that the peripheral vasculature provides mechanical or chemical signals that ultimately modulate the spatial organization, and therefore shape the colony.
A recent report suggested that the VEGF pathway may also participate in homeostasis of the vasculature (Lee et al., 2007). To test this, and further assess if the disorganized system phenotype described above was the result of a lack of peripheral vasculature or an effect of BsVEGFR knockdown, similar treatments were performed in colonies where no ampullaectomy had been carried out. Seven systems from two different genotypes were injected and soaked as described previously. Phenotypes were observed and documented on a daily basis and showed no alterations of the blastogenetic cycle, thus BsVEGR did not appear to be required for asexual generation of the zooids. However, after approximately 2 weeks of BsVEGFR knockdown, the circulatory system of the treated colonies began to break down: the vasculature became engulfed with pigmented cells, the epithelia of the peripheral vessels and the ampullae degenerated and became leaky, and blood cells were released into the tunic, which gradually became opaque and lost elasticity, and the blastozooids grew chaotically (Fig. 5 O). This data suggests a role of the VEGF pathway in vascular homeostasis, as well as involvement in the maintenance of higher order organization and architecture of the colony.
Specific inhibition of BsVEGFR by PTK787/ZK222584 during angiogenesis
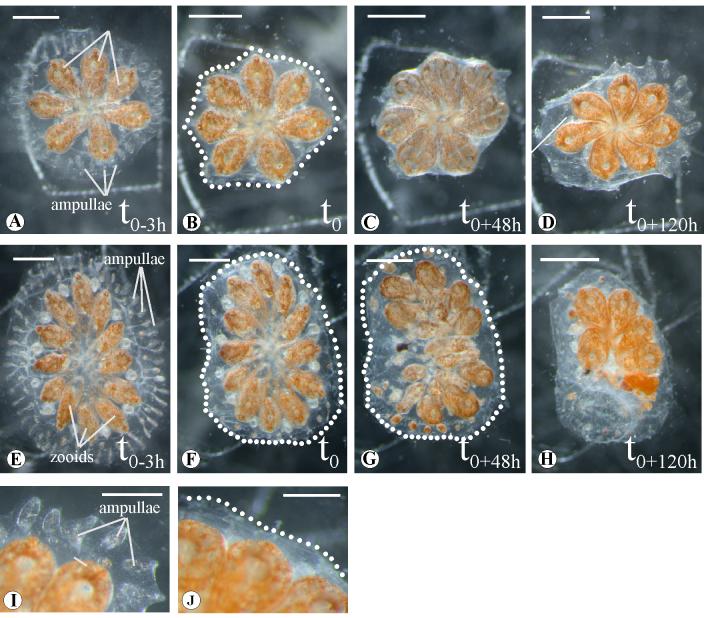
PTK787/ZK222584, also known as Vatalanib, is an anilinophthalazine compound and a high affinity inhibitor for the vertebrate VEGF receptors, in particular VEGFR-2/KDR (Wood et al., 2000). It also can inhibit PDGF, Flt-4 and c-Kit receptors, at significantly higher working concentrations (Bold et al., 2000; Hogan and Kolodziej, 2002). As a cultured, marine organism, with rapid regenerative abilities, Botryllus would be a good candidate for chemical screens, and therefore we decided to test this potent VEGF signaling inhibitor. After testing the different concentration (i.e. 5, 10 and 15 μM), eight systems composed of 5-9 blastozooids were microinjected with 0.5-0.9 μl of 20 μM PTK787/ZK222584 in DMSO three hours before (t0-3) the surgical ablation of the peripheral ampullae and the marginal vessel (t0). As shown, a single injection completely inhibited vessel and ampullae regeneration for 120 hours following the surgery (Fig. 6 H). This effect is reversible, and the systems eventually recover, initiating angiogenesis with a delay of 3-4 days in comparison to the controls injected with only DMSO (n=9).
Figure 6.
PTK787/ZK222588 treatment of B.schlosseri systems. Different systems from the same genotype were treated at diverse blastogenetic stages with PTK787/ZK222588 dissolved in DMSO (E) and with only DMSO (A) 3 hours before the complete ampullaectomy (t0-3h). (B) and (F), surgical ablation of the ampullae and marginal vessel in the untreated and treated samples respectively. Morphological analysis after 48 (t+48h) and 120 hours (t+120h) of the vascular regeneration in the controls (C), (D) and in the treated (G) and (H). Close-up of untreated (I) and treated (J) samples 120 hours past the ampullaectomy: after a week the system injected with 0.5/0.9 μl of 20μM PTK787/ZK222588 did not show vascular regeneration. Dots underline the edge of the cut. Scale bar 1mm.
These experiments were also done in colonies where only a portion of the peripheral ampullae and vessels were removed (hemiampullectomy, Fig. 7 B, F). In the PTK787/ZK222584 treated systems (n= 5), the vascular regeneration was blocked for 120 hours, with the same consequences observed in the previous set of experiments. Recapitulating the VEGFR knockdown, the remaining peripheral vasculature and ampullae became engulfed with pigmented cells and the colony lost its spatial organization (Fig. 7 F). These data further suggest that deletion of VEGF signaling affects homeostasis of the vasculature.
Figure 7.
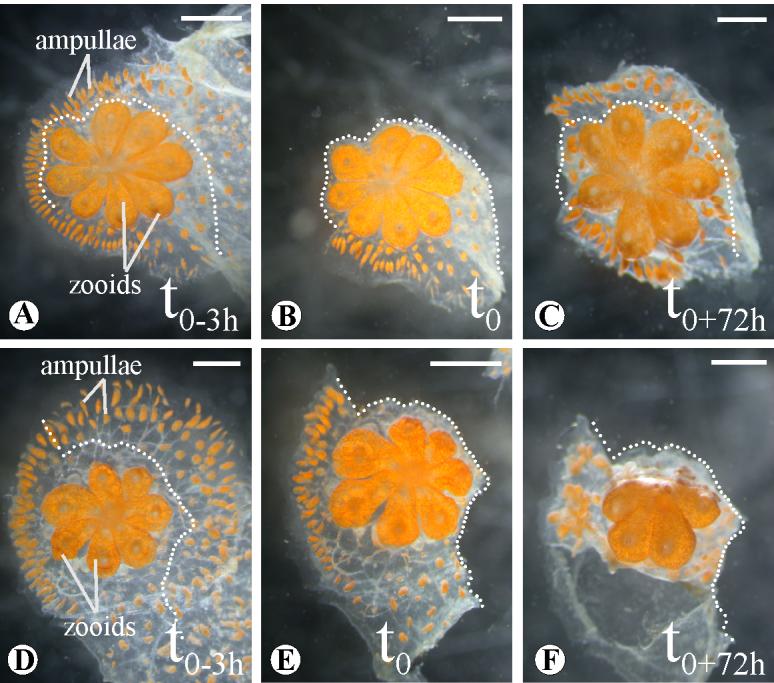
PTK787/ZK222588 treatment of B.schlosseri systems. DMSO (A, B, C) and PTK787/ZK222588 (E, F, G) injection in single genotype systems. Three hours after injection (t0), part of the ampullae and the marginal have been surgically removed (B, F; dotted line) and the grade of regrowth monitored for 72 hours (t0+72h, C, G). Scale bar 1mm.
DISCUSSION
The colonial ascidians are the closest relative to vertebrates known to use asexual propagation. Every week Botryllus schlosseri regenerates its entire body, including all somatic tissues and the germline (Manni et al., 2007). These regenerative abilities are not limited to this natural process, and will also respond after experimentally-induced injury, (Voskoboynik et al., 2007; Oka and Watababe, 1957; Sabbadin et al., 1975). This regenerative potential makes B. schlosseri a promising model to investigate the molecular basis of regeneration.
Vascular regeneration is a dynamic process; therefore we first examined branching morphogenesis of the vasculature by time-lapse microscopy in order to characterize the morphological changes during regeneration, and characterized five distinct stages which occur in both small and large individuals.
BsVEGFR in vascular regeneration
The B. schlosseri circulatory system is characterized by: (1) open lacunae and sinuses in the body of the zooid, as found in solitary ascidians and most non-vertebrate metazoans, and (2) a peripheral, or colonial, circulatory system composed of vessels which develop via mechanisms comparable to vertebrate angiogenetic sprouting (Burighel and Brunetti, 1971; Gasparini et al., 2007). In order to evaluate the genetic conservation of angiogenesis, we cloned and functionally analyzed the role of the vascular endothelial growth factor receptor (VEGFR) using RNAi-mediated knockdown as well as a mammalian VEGFR inhibitor, PTK787/ZK222584. The VEGF signaling pathway was discovered in vertebrates due to its effects on vascular endothelial cells during vasculogenesis and angiogenesis. However, VEGF and VEGFR homologs have been identified and studied in organisms that lack canonical blood vessels, such as cnidarians (Seipel et al., 2004), annelids (Tettamanti et al., 2003) and arthropods (Duchek et al., 2001). In Drosophila melanogaster the VEGF signaling pathways regulates hematopoiesis and blood cell migration (Heino et al., 2001; Choi et al., 1998) but has not been reported to be involved in typical branching morphogenesis of tubular structures like the tracheal network. In contrast, in the jellyfish Podocoryne carnea, homologs of the VEGF and VEGFR are expressed in the endoderm during the branching of the tentacles and gastrovascular canals (Seipel et al., 2004). In this case, an epithelium interacts with the extracellular matrix and smooth muscle in a process reminiscent of the blood vessel formation in vertebrates, suggesting that the VEGF signaling had an early origin controlling tube formation. Recently, Gasparini et al. (2007) used immunhistochemistry to show the presence of VEGF and VEGFR-1 during tubular sprouting of the vascular vessels during normal blastogenetic cycles of B.schlosseri. Using cross reacting polyclonal antibodies generated to mammalian proteins, they found that co-localization of VEGF and its receptor in the apex of forming vessels as they begin to sprout and elongate, suggesting a role of these angiogenetic factors in the development of the ectodermal vascular network (Gasparini et al., 2007).
In this report we confirm the presence of VEGF ligand by partial cloning and characterization of the VEGFR ortholog in B. schlosseri. Given the limits of the procedure, we cannot rule out the possibility that alternative isoforms might be present in Botryllus as in vertebrates (Ferrara et al., 2003; Olsson et al., 2006). However, only one isoform of VEGF receptor has been reported in Ciona intestinalis (Imai et al., 2004), and in Botryllus a number of RACE and RT-PCR experiments from the conserved kinase domain only resulted in a single gene. Moreover, analysis of a 32,000 clone EST library recently completed revealed only the single VEGR gene (unpublished). The phylogenetic comparisons (Fig. 3B) show that the split tyrosine kinase domain, which strongly clusters with the Homo sapiens VEGF receptors, is more closely related to human PDGF ligands than VEGF-R from cnidarians (Hydra vulgaris and Podocoryne carnea; Fig. 3 B).
Quantification by qPCR of the mRNA transcripts showed a uniform presence of BsVEGFR throughout the colonial blastogenic stages with significantly higher expression in the external ampullae and blood vessels when compared with the rest of the colony (Fig. 4 A), even though we could not surgically remove the entire peripheral vasculature (i.e. ampullae and vessels underneath the colony and between the zooids. (Fig. 1A). Similar amounts of VEGF-R transcript found throughout the colony and our ISH results (Fig. 4 B-E) concur with the presence of VEGF-R in the epithelial layer of the vasculature. The same pattern was also seen for protein localization by Gasparini et al. using anti-mammalian antibodies (2007). Taken together, this spatio-temporal expression suggests a role for VEGF signaling pathway in both normal physiological homeostasis and during regeneration of the peripheral vasculature, which was confirmed by our siRNA mediated knockdown results. However, there was no effect on the formation of the blood lacunae within the newly developed body of the zooids. This confirms the critical role of VEGF signaling pathways during the regeneration of Botryllus tubular vessels.
The Botryllus external vasculature is a dynamic tissue that remodels itself according to the stage of the system with a well-defined and consistent pattern, suggesting an essential developmental role in the colony during asexual propagation (Burighel and Brunetti, 1971). After takeover, the zooids are spatially distant from one another, but within a few hours they migrate and cluster, reconstituting the classical star shaped feature (supplemental video 2). Depletion of the ampullae and the peripheral vessels via ampullaectomy, BsVEGFR knockdown affected the organization of the newly developed zooids in the colony without altering the morphogenesis of the zooid itself, suggesting that the extracorporeal vasculature provides spatial information to mechanically drive and/or orient the zooids after each blastogenetic cycle, dictating the shape of the colony. The contribution of blood vessels to morphogenesis is not surprising; analogous interactions have been shown to occur during organogenesis of the pancreas (Lammert et al., 2001) and in the development of the liver (Matsumoto et al., 2001).
We observed that the prolonged inhibition of BsVEGFR expression in surgically unaltered colonies produced a clear perturbation of the integrity of the vessels and the ampullae, suggesting an effect on the homeostasis of the vascular system (Fig. 5 N, O). In recent work, Lee et al. (2007) reported that, besides the well know role as a paracrine signal for the formation of blood vessels in mice, VEGF also had an unexpected autocrine role in the maintenance of the endothelium. In both cases the receptor involved is VEGFR2. The co-localization of both VEGF (Gasparini et al., 2007) and BsVEGFR in the epithelia of the ampullae, coupled with the siRNA- mediated knockdown phenotype suggests a conserved functional role of the VEGF receptor in blood vessel maintenance, and would imply an ancestry of the autocrine function of the VEGF pathway.
PTK787/ZK222584 phenocopies functional loss of BsVEGFR
PTK787/ZK 222584 (1-[4-chloroanilino]-4-[4-pyridylmethyl] phthalazine succinate) is a potent inhibitor of all human vascular endothelial growth factor (VEGF) receptor tyrosine kinases, and active in a submicromolar range. The specificity of PTK787/ZK222584 across species boundaries has previously been tested in vivo and in vitro in non mammalian models like zebrafish (Chan et al., 2002; Lee et al., 2006) but never in non-vertebrate chordates. Given the level of overall homology (49% amino acid identity) between the BsVEGFR tyrosine kinase domain and the human counterpart and the potential ease of doing high throughput chemical screens in B.schlosseri during regeneration, we did a proof-of-principle experiment using this compound.
After calibrating the minimal effective dose (20μM), we observed that the PTK787/ZK222584-treated colonies responded in an identical manner to short-term VEGFR knockdown, including affects on regeneration and homeostasis. However, due to the fact that the drug had to be dissolved in DMSO, we could not perform long-term PTK treatments as repeated injection of the solvent adversely affected the colony.
BsVEGFR in vascular regeneration: homologies and analogies
Mammalian endothelial vasculogenesis and angiogenesis are morphologically analogous to ectodermal sprouting, for example morphogenesis of the tracheas in Drosophila melanogaster (Ghabrial and Krasnow, 2006; Myat, 2005) or development of mammary glands in mammals (Mailleux et al., 2002;.Radisky et al., 2003). In both cases, these processes lead to the formation of tubular structures. To our knowledge, VEGF has not been shown to play a role in ectodermally derived, tubular structures in other organisms, and to our knowledge this is the first report of VEGF pathway involvement in development of ectodermally-derived epithelia. However, these features suggest that, despite the conserved machinery that seems to control the morphogenetic events that lead to tubular structures in divergent animal clades, i.e. vertebrates, insects and tunicates, the tubular branching program may have been reinvented many times.
As discussed above, the peripheral vasculature of B.schlosseri has two peculiar dissimilarities with the vertebrates: the inverted basal-apical polarity of vessel epithelia, and its ectodermal origin. In this context the data suggests that VEGF signaling may not function directly upstream of the establishment of cell polarity in the epithelia during angiogenesis, nor is it restricted to stimulation of cells of similar ontogenetic origin.
Conclusions
Formation of branching tubular epithelia is a common process in metazoan organogenesis, where tubular systems regenerate using strategies that are based on common elements of cell behavior and variations of genetic pathways (Davies, 2002; Hogan and Kolodziej, 2002). Here we take advantage of the high regenerative potential of the extracorporeal vascular system of Botryllus schlosseri to investigate the functional role of the VEGF pathway, a key component of vertebrate angiogenesis. While the cellular organization and embryonic germ layer origin in the Botryllus tubular network is different than that of the vertebrate vasculature, both molecular and chemical approaches established a conserved function of the VEGF pathway, which controls the tubular branching in the ectodermally-derived Botryllus vascular epithelia. The rapid regeneration, transparency and experimental accessibility of the B. schlosseri extracorporeal vasculature make it a potentially insightful model for in vivo studies of angiogenesis using both reverse genetic and chemical screening approaches.
Supplementary Material
Supplemental video 1: Botryllus schlosseri vasculature regeneration following ampullaectomy. Time laps images were taken every 30 minutes for a period of 30 hours following ampullae removal. Amp: ampullae, bv: blood vessel, numbers regeneration spots, t: time, h; hours.
Supplemental video 2: Botryllus schlosseri young generation takes over its parents place. In these time lapse images, we followed the synchronous death (mainly via apoptosis) of the parent zooids (during blastogenesis stage D) and its replacement by the asexually derived buds of the next generation. Time lapse images were taken every 30 minutes during a period of 27 hours. res. Zooid: resorbing zooid, sb: secondary bud, bv: blood vessel.
ACKNOWLEDGEMENTS
We thank Kathi Ishizuka, Karla Palmeri and Randy Will for technical support and Billie Swalla, Ulrich Kurn and Michelle Roux for their comments on the manuscripts. The research was supported by NIH (RO1A104588/R01DK405762), the Stanford School of Medicine Dean’s fellowship to ST, the Lerner Gray Fund for Marine Research and the American Heart Association Fellowship Grant to FDB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Auguste P, Lemiere S, Larrieu-Lahargue F, Bikfalvi A. Molecular mechanisms of tumor vascularization. Crit Rev Oncol Hematol. 2005;54(1):53–61. doi: 10.1016/j.critrevonc.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Berrill NJ. Chordata: Tunicata. In: Giese AC, Pearse JS, editors. Reproduction ofMarine Invertebrates II. AcademicPress; N,ew York: 1975. pp. 241–282. [Google Scholar]
- Bold G, Altmann KH, Frei J, Lang M, Manley PW, Traxler P, Wietfeld B, Bruggen J, Buchdunger E, Cozens R, Ferrari S, Furet P, Hofmann F, Martiny-Baron G, Mestan J, Rosel J, Sills M, Stover D, Acemoglu F, Boss E, Emmenegger R, Lasser L, Masso E, Roth R, Schlachter C, Vetterli W. New anilinophthalazines as potent and orally well absorbed inhibitors of the VEGF receptor tyrosine kinases useful as antagonists of tumor-driven angiogenesis. J Med Chem. 2000;43(16):3200. doi: 10.1021/jm001010d. [DOI] [PubMed] [Google Scholar]
- Boyd HC, Brown SK, Harp JA, Weissman IL. Growth and sexual maturation of laboratory-cultured Monterey Botryllus schlosseri. Biol.Bull. 1986;1986;170:91–109. [Google Scholar]
- Brown FD, Swalla BJ. Vasa expression in a colonial ascidian, Botrylloides violaceus. Evol Dev. 2007;9(2):165–177. doi: 10.1111/j.1525-142X.2007.00147.x. [DOI] [PubMed] [Google Scholar]
- Burighel P, Brunetti R. The circulatory system in the blastozooid of the colonial ascidian Botryllus schlosseri (Pallas) Boll Zool. 1971;38:273–289. [Google Scholar]
- Cao R, Eriksson A, Kubo H, Alitalo K, Cao Y, Thyberg J. Comparative evaluation of FGF-2-, VEGF-A-, and VEGF-C-induced angiogenesis, lymphangiogenesis, vascular fenestrations, and permeability. Circ Res. 2004;94(5):664–670. doi: 10.1161/01.RES.0000118600.91698.BB. [DOI] [PubMed] [Google Scholar]
- Chan J, Bayliss PE, Wood JM, Roberts TM. Dissection of angiogenic signaling in zebrafish using a chemical genetic approach. Cancer Cell. 2002;1(3):257–267. doi: 10.1016/s1535-6108(02)00042-9. [DOI] [PubMed] [Google Scholar]
- Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125(4):725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- Christiaen L, Burighel P, Smith WC, Vernier P, Bourrat F, Joly JS. Pitx genes in Tunicates provide new molecular insight into the evolutionary origin of pituitary. Gene. 2002;287(12):107–113. doi: 10.1016/s0378-1119(01)00865-4. [DOI] [PubMed] [Google Scholar]
- Davies JA. Do different branching epithelia use a conserved developmental mechanism? Bioessays. 2002;24(10):937–948. doi: 10.1002/bies.10161. [DOI] [PubMed] [Google Scholar]
- Davies JA. Watching tubules glow and branch. Curr Opin Genet Dev. 2005;15(4):364–370. doi: 10.1016/j.gde.2005.06.003. [DOI] [PubMed] [Google Scholar]
- De Santo RS. Ph.D. Dissertation. Columbia University; 1968. 1968. The histochemistry, the fine structure, and the ecology of the synthesis of the test in Botryllus schlosseri (Pallas) Savigny. [Google Scholar]
- De Tomaso AW, Nyholm SV, Palmeri KJ, Ishizuka KJ, Ludington WB, Mitchel K, Weissman IL. Isolation and characterization of a protochordate histocompatibility locus. Nature. 2005;438(7067):454–459. doi: 10.1038/nature04150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchek P, Somogyi K, Jekely G, Beccari S, Rorth P. Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell. 2001;107(1):17–26. doi: 10.1016/s0092-8674(01)00502-5. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Estimating effective population size from samples of sequences: a bootstrap Monte Carlo integration method. Genet Res. 1992;60(3):209–220. doi: 10.1017/s0016672300030962. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Gasparini F, Longo F, Manni L, Burighel P, Zaniolo G. Tubular sprouting as a mode of vascular formation in a colonial ascidian (Tunicata) Dev Dyn. 2007;236(3):719–731. doi: 10.1002/dvdy.21073. [DOI] [PubMed] [Google Scholar]
- Ghabrial AS, Krasnow MA. Social interactions among epithelial cells during tracheal branching morphogenesis. Nature. 2006;441(7094):746–749. doi: 10.1038/nature04829. [DOI] [PubMed] [Google Scholar]
- Heino TI, Karpanen T, Wahlstrom G, Pulkkinen M, Eriksson U, Alitalo K, Roos C. The Drosophila VEGF receptor homolog is expressed in hemocytes. Mech Dev. 2001;109(1):69–77. doi: 10.1016/s0925-4773(01)00510-x. [DOI] [PubMed] [Google Scholar]
- Hogan BL, Kolodziej PA. Organogenesis: molecular mechanisms of tubulogenesis. Nat Rev Genet. 2002;3(7):513–523. doi: 10.1038/nrg840. [DOI] [PubMed] [Google Scholar]
- Holmes DI, Zachary I. The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease. Genome Biol. 2005;6(2):209–212. doi: 10.1186/gb-2005-6-2-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai KS, Hino K, Yagi K, Satoh N, Satou Y. Gene expression profiles of transcription factors and signaling molecules in the ascidian embryo: towards a comprehensive understanding of gene networks. Development. 2004;131(16):4047–4058. doi: 10.1242/dev.01270. [DOI] [PubMed] [Google Scholar]
- Karner C, Wharton KA, Carroll TJ. Apical-basal polarity, Wnt signaling and vertebrate organogenesis. Semin Cell Dev Biol. 2006;17(2):214–222. doi: 10.1016/j.semcdb.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Kott P. Form and function in the Ascidiacea. Bulletin of Marine Sciences. 1986;45:253–276. [Google Scholar]
- Laird DJ, Chang WT, Weissman IL, Lauzon RJ. Identification of a novel gene involved in asexual organogenesis in the budding ascidian Botryllus schlosseri. Dev Dyn. 2005;234(4):997–1005. doi: 10.1002/dvdy.20583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294(5542):564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- Lauzon RJ, Ishizuka KJ, Weissman IL. A cyclical, developmentally-regulated death phenomenon in a colonial urochordate. Dev Dyn. 1992;194(1):71–83. doi: 10.1002/aja.1001940109. [DOI] [PubMed] [Google Scholar]
- Lauzon RJ, Patton CW, Weissman IL. A morphological and immunohistochemical study of programmed cell death in Botryllus schlosseri (Tunicata, Ascidiacea) Cell Tissue Res. 1993;272(1):115–127. doi: 10.1007/BF00323577. [DOI] [PubMed] [Google Scholar]
- Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF Signaling Is Required for Vascular Homeostasis. Cell. 2007;130(4):691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YM, Cope JJ, Ackermann GE, Goishi K, Armstrong EJ, Paw BH, Bischoff J. Vascular endothelial growth factor receptor signaling is required for cardiac valve formation in zebrafish. Dev Dyn. 2006;235(1):29–37. doi: 10.1002/dvdy.20559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I, Copley RR, Pils B, Pinkert S, Schultz J, Bork P. SMART 5: domains in the context of genomes and networks. Nucleic Acids Res. 2006;34:D257–260. doi: 10.1093/nar/gkj079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lubarsky B, Krasnow MA. Tube morphogenesis: making and shaping biological tubes. Cell. 2003;112(1):19–28. doi: 10.1016/s0092-8674(02)01283-7. [DOI] [PubMed] [Google Scholar]
- Mailleux AA, Spencer-Dene B, Dillon C, Ndiaye D, Savona-Baron C, Itoh N, Kato S, Dickson C, Thiery JP, Bellusci S. Role of FGF10/FGFR2b signaling during mammary gland development in the mouse embryo. Development. 2002;129(1):53–60. doi: 10.1242/dev.129.1.53. [DOI] [PubMed] [Google Scholar]
- Manni L, Zaniolo G, Cima F, Burighel P, Ballarin L. Botryllus schlosseri: a model ascidian for the study of asexual reproduction. Dev Dyn. 2007;236(2):335–352. doi: 10.1002/dvdy.21037. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294(5542):559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- Matthysse AG, Deschet K, Williams M, Marry M, White AR, Smith WC. A functional cellulose synthase from ascidian epidermis. Proc Natl Acad Sci U S A. 2004;101:986–991. doi: 10.1073/pnas.0303623101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myat MM. Making tubes in the Drosophila embryo. Dev Dyn. 2005;232(3):617–632. doi: 10.1002/dvdy.20293. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Yamada L, Satou Y, Azuma J, Satoh N. The evolutionary origin of animal cellulose synthase. Dev Genes Evol. 2004;214:81–88. doi: 10.1007/s00427-003-0379-8. [DOI] [PubMed] [Google Scholar]
- Nyholm SV, Passegue E, Ludington WB, Voskoboynik A, Mitchel K, Weissman IL, De Tomaso AW. fester, A candidate allorecognition receptor from a primitive chordate. Immunity. 2006;25(1):163–173. doi: 10.1016/j.immuni.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Oka H, Watanabe H. Vascular budding, a new type of budding in Botryllus. Biol. Bull. 1957;112:225–240. [Google Scholar]
- Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7(5):359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- Radisky DC, Hirai Y, Bissell MJ. Delivering the message: epimorphin and mammary epithelial morphogenesis. Trends Cell Biol. 2003;13(8):426–34. doi: 10.1016/s0962-8924(03)00146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbadin A, Zaniolo G, Majone F. Determination of polarity and bilateral asymmetry in palleal and vascular buds of the Ascidian Botryllus schlosseri. Dev. Biol. 1975;46:79–87. doi: 10.1016/0012-1606(75)90088-3. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Seipel K, Eberhardt M, Muller P, Pescia E, Yanze N, Schmid V. Homologs of vascular endothelial growth factor and receptor, VEGF and VEGFR, in the jellyfish Podocoryne carnea. Dev Dyn. 2004;231(2):303–312. doi: 10.1002/dvdy.20139. [DOI] [PubMed] [Google Scholar]
- Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res. 2005;65(3):550–563. doi: 10.1016/j.cardiores.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Tarsitano M, De Falco S, Colonna V, McGhee JD, Persico MG. The C. elegans pvf-1 gene encodes a PDGF/VEGF-like factor able to bind mammalian VEGF receptors and to induce angiogenesis. FASEB J. 2006;20(2):227–233. doi: 10.1096/fj.05-4147com. [DOI] [PubMed] [Google Scholar]
- Tettamanti G, Malagoli D, Benelli R, Albini A, Grimaldi A, Perletti G, Noonan DM, de Eguileor M, Ottaviani E. Growth factors and chemokines: a comparative functional approach between invertebrates and vertebrates. Curr Med Chem. 2006;13(23):2737–2750. doi: 10.2174/092986706778521986. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiozzo S, Ballarin L, Burighel P, Zaniolo G. Programmed cell death in vegetative development: apoptosis during the colonial life cycle of the ascidian Botryllus schlosseri. Tissue Cell. 2006;38(3):193–201. doi: 10.1016/j.tice.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Tiozzo S, Christiaen L, Deyts C, Manni L, Joly JS, Burighel P. Embryonic versus blastogenetic development in the compound ascidian Botryllus schlosseri: insights from Pitx expression patterns. Dev Dyn. 2005;232(2):468–478. doi: 10.1002/dvdy.20250. [DOI] [PubMed] [Google Scholar]
- Voskoboynik A, Simon-Blecher N, Soen Y, Rinkevich B, De Tomaso AW, Ishizuka KJ, Weissman IL. Striving for normality: whole body regeneration through a series of abnormal generations. FASEB J. 2007;21(7):1335–1344. doi: 10.1096/fj.06-7337com. 2007. [DOI] [PubMed] [Google Scholar]
- Watanabe H. Studies on the regulation in fused colonies in Botryllus primigenus (Ascidiae Compositae) Sci Rep Tokyo Bunrika Daigaku B. 1953;7:183–198. [Google Scholar]
- Wood JM, Bold G, Buchdunger E, Cozens R, Ferrari S, Frei J, Hofmann F, Mestan J, Mett H, O’Reilly T, Persohn E, Rosel J, Schnell C, Stover D, Theuer A, Towbin H, Wenger F, Woods-Cook K, Menrad A, Siemeister G, Schirner M, Thierauch KH, Schneider MR, Drevs J, Martiny-Baron G, Totzke F. PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res. 2000;60(8):2178–2189. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental video 1: Botryllus schlosseri vasculature regeneration following ampullaectomy. Time laps images were taken every 30 minutes for a period of 30 hours following ampullae removal. Amp: ampullae, bv: blood vessel, numbers regeneration spots, t: time, h; hours.
Supplemental video 2: Botryllus schlosseri young generation takes over its parents place. In these time lapse images, we followed the synchronous death (mainly via apoptosis) of the parent zooids (during blastogenesis stage D) and its replacement by the asexually derived buds of the next generation. Time lapse images were taken every 30 minutes during a period of 27 hours. res. Zooid: resorbing zooid, sb: secondary bud, bv: blood vessel.