Abstract
Long-lasting protective antibody is not normally generated in children following primary respiratory syncytial virus (RSV) infection, frequently leading to reinfection. We used the BALB/c mouse model to examine the role of the nasal-associated lymphoid tissue and the bone marrow in the generation of RSV-specific long-lasting plasma cells, with a view to further understanding the mechanisms responsible for the poorly sustained RSV antibody levels following primary infection. We show here that substantial numbers of RSV-specific plasma cells were generated in the bone marrow following challenge, which were maintained thereafter. In contrast, in the nasal-associated lymphoid tissue, RSV-specific plasma cell numbers waned quickly both after primary infection and after challenge and were not maintained at a higher level after boosting. These data indicate that the inability to generate a robust local mucosal response in the nasal tissues may contribute substantially to the likelihood of subsequent reinfection and that the presence of serum anti-RSV antibody without local protection is not enough to protect against reinfection.
Infection with respiratory syncytial virus (RSV) is the most common cause of serious respiratory illness in infants and young children. More than 50% of children are infected within the first year of life, with up to 2% requiring hospitalization (7, 10). Although reinfection with RSV occurs in all age groups, it is generally only in the young, with about half of all infants reinfected by 2 years of age (17), and in the elderly (32), in whom serious disease can result.
RSV replicates predominantly in the epithelial cells of the nasopharynx, and viremia does not occur. Disease may, however, spread to the lower respiratory tract, with potentially serious complications (41). Clinical manifestations of RSV infection range from mild upper respiratory symptoms to bronchiolitis and pneumonia. First infections are generally more severe, frequently causing lower respiratory tract disease, but milder symptoms are observed on reinfection. Significant morbidity occurs in infants less than 6 months old (7), children with existing risk factors (11, 13), and the elderly (6, 15).
Almost all children are born with neutralizing maternally derived RSV-specific serum antibody at levels similar to those in the mother's serum. Studies have shown that infants who have the highest levels of maternal serum antibody (commonly those less than 2 months old) tend to have less serious disease than those with lower serum antibody levels (commonly 2 to 4 months old) (7, 33, 36). These studies suggest that the relative resistance of very young infants to severe RSV disease is due to maternally derived antibody. RSV-specific maternal antibody levels, however, gradually decrease as the infant ages and are almost undetectable by 6 to 8 months of age (2). The presence of maternal antibody may be responsible for the lower convalescent-phase serum and nasal-wash neutralizing antibody titers in children less than 8 months old compared to those 9 to 21 months old (28).
The importance of antibody to RSV has been demonstrated both in humans and in animal models. In the absence of antibody, mice have more severe illness, greater lung pathology, and only partial immunity against rechallenge with RSV compared with intact mice (8). Both mucosal and serum antibodies are generated following natural infection with RSV (25, 26) and are predominantly directed against the F and G proteins (28, 29). Unfortunately, naturally acquired immunity is not complete, and even high serum antibody titers fail to protect a considerable proportion of adults (14) and some infants (13, 14) against reinfection. Some success has nevertheless been achieved by treatment with high titers of passively acquired neutralizing antibody. In the cotton rat, virus replication during a primary infection was reduced (38), and protection against lower respiratory tract infection was observed (39), and administration of a monoclonal anti-RSV immunoglobulin A (IgA) antibody protected rhesus monkeys against upper and lower respiratory tract infection (47). In humans, treatment of high-risk children with high-titer neutralizing antibody preparations against RSV (16) and with a virus-neutralizing anti-F monoclonal antibody (12) has also been very successful.
Previously we have shown that following one intranasal exposure to influenza virus, long-lived specific plasma cells are generated and maintained both locally in the nasal-associated lymphoid tissues (NALT) and in the bone marrow (19, 23). Mice are subsequently protected from an otherwise lethal challenge with influenza virus. Here we used the BALB/c mouse model to examine the ability of the upper respiratory tract, specifically the NALT, to generate RSV-specific long-lasting plasma cells, with a view to further understanding whether the nasal tissues play a significant role in immunity to RSV infection. In addition, we hoped to further elucidate the mechanisms responsible for the poorly sustained RSV antibody levels following primary infection.
MATERIALS AND METHODS
Infection of mice and sampling.
Inbred female BALB/c mice were obtained from Charles River (United Kingdom) Ltd., held under specific-pathogen-free conditions, and used at 8 to 12 weeks of age. All animal studies were approved by the relevant ethics authority. Mice were anesthetized by intraperitoneal injection of ketamine xylazine and infected intranasally with 50 μl of RSV-A2 containing 107 PFU/ml. At certain time points postinfection, mice were sacrificed, and various tissues were removed. The nasally-associated lymphoid tissues (NALT) in the mouse are composed of a pair of organized lymphoid aggregates (O-NALT) located on the palate at the entrance to the nasopharyngeal duct and the less well organized diffuse lymphoid tissue lining the nasal passages (D-NALT) (22). These nasal tissues appear to be functionally equivalent to the Waldeyer's ring of tonsils and adenoids in humans and are most likely responsible for the local immune responses generated following intranasal immunization in the mouse (42). The O-NALT and the D-NALT were extracted by a previously described method (1). Cell suspensions of mediastinal lymph nodes and spleen were prepared by gently pressing between frosted slides, followed by filtration through gauze. Bone marrow cells were collected from femurs and tibias by flushing with Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (FCS). For Elispot assays, spleen and bone marrow erythrocytes were lysed with NH4Cl lysis buffer.
Virus.
RSV-A2 strain (a kind gift from R. Tripp, Centers for Disease Control and Prevention, Atlanta, Ga.) was grown in BS-C-1 or Vero cells (American Type Culture Collection, Manassas, Va.) in T150 tissue culture flasks (TPP Techno Plastic Products AG, Geneva, Switzerland). After removal of medium from the monolayer, the cells were infected at a multiplicity of infection of 0.05 to 0.01 PFU/cell in serum-free medium for 2 h at 33°C in a 10% CO2 atmosphere. Then 30 ml of Dulbecco's modified Eagle's medium containing 2% FCS was then added to each flask, and the cells were incubated for a further 4 to 6 days or until cytopathic effect was observed, at which time the medium was removed. For infectious virus, 5 ml of serum-free medium was added to the flask, the adherent cells were harvested, and the cell suspension was stored at −70°C until use. For purified RSV to use in antigen-specific Elispot and enzyme-linked immunosorbent assay (ELISA) assays, 5 ml of serum-free medium containing 10% stabilization buffer (1 M MgSO4, 50 mM HEPES, 150 mM NaCl, pH 7.5) was added to each flask after cytopathic effect was observed. The cells were stored at −70°C until virus was purified by sucrose gradient centrifugation as previously described (24).
RSV-specific Elispot and ELISA assays.
Virus-specific antibody secretion was measured by the Elispot assay as described previously (18). Sucrose-purified RSV at a predetermined concentration was coated on Millititre-HA 96 (Millipore) plates at 50 μl/well and left overnight at 4°C. Plaques were detected with goat anti-mouse Ig isotype-specific reagents conjugated to alkaline phosphatase (Southern Biotechnology Associates, Birmingham, Ala.) and developed with 5-bromo-4-chloro-3-indolylphosphate (BCIP; Sigma, St. Louis, Mo.). In all experiments, cells from at least four mice were pooled for each time point, and the time course was repeated three times. For ELISAs, Nunc-Immuno MaxiSorp plates (Nunc, Roskilde, Denmark) were coated overnight with sucrose-purified RSV diluted 1:30 in carbonate buffer, pH 9.5. RSV-specific serum antibody was detected with alkaline phosphatase-coupled goat anti-mouse IgG subclass-specific reagents and developed with 1 mg of p-nitrophenyl phosphate per ml (Sigma). Antibody was quantitated after 20 to 30 min by reading A405 on a SpectraMax 340 microplate reader (Molecular Devices, Sunnyvale, Calif.). Fragment culture reactions were amplified with the AmpliQ kit (Dako Ltd., Ely, United Kingdom) prior to reading at A492 with correction from values read at A650. Titers are expressed as the reciprocal of the highest dilution giving an optical density value greater than three times the value for a simultaneously titrated negative sample.
Macrotitration of RSV and virus neutralization assays.
For macrotitration assays, lungs were homogenized on ice, and stock RSV was grown and prepared as described above. Samples were centrifuged at 2,600 × g for 5 min at 4°C, and sequential dilutions of the supernatants were incubated on BS-C-1 cell monolayers previously plated in 24-well tissue culture dishes for 2 h at 33°C before 2 ml of Dulbecco's modified Eagle's medium containing 2% FCS was added. After 48 h, 2 ml of 4% paraformaldehyde was added for 1 h. The plates were then washed and blocked with 4% milk powder at 37°C for 1 h, after which goat anti-RSV antibody (Chemicon International Ltd.) was added for 2 h. The plates were washed and incubated for 2 h with rabbit anti-goat IgG-alkaline phosphatase and then developed with 1 mg of BCIP per ml. Virus neutralization assays were performed by a method similar to that described for macrotitration of RSV with the following changes. A pretitered RSV stock diluted in 100 μl of serum-free medium was used as a standard. A 100-μl volume of each sequential dilution of serum was incubated with a 100-μl volume of the standard RSV for 5 min at room temperature. The samples were then added to BS-C-1 cells previously seeded into the wells of a 24-well plate, and the assay was carried out as for the macrotitration described above. The 50% effective dose (ED50) was calculated by plotting the dilution of antibody against the number of plaques, and the antibody dilution value which gave a reduction in the number of plaques by 50% was determined.
Lung fragment cultures.
RSV-specific antibody secreted from lung tissue was measured by a method adapted from that described by Moser et al. (27). Briefly, duplicate 1-mm slices of lung tissue were placed in adjacent wells of a 24-well plate in a 0.5-ml volume of RPMI containing 10% FCS. The plates were incubated at 37°C for 5 days in a 95% O2 atmosphere, at which time the supernatant was removed and frozen at −20°C for subsequent determination of RSV-specific antibody levels by ELISA.
RESULTS
Generation of virus-specific AFCs in local and peripheral lymphoid organs following primary infection with RSV.
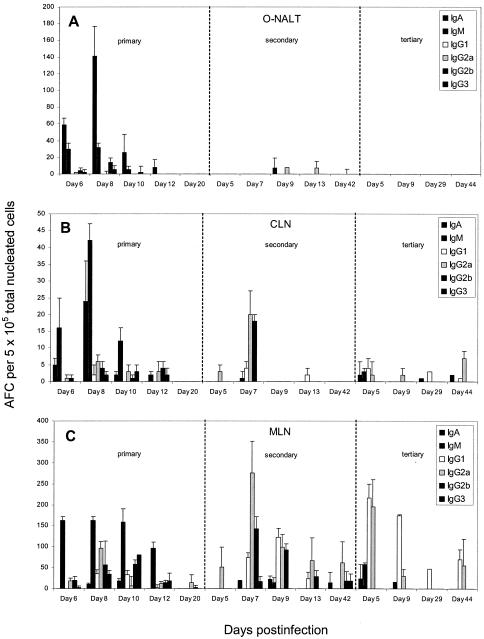
Infection of BALB/c mice with RSV evoked the expected biphasic response of IgM followed by IgG in both the mediastinal lymph node, which drains the lung, and the cervical lymph node, which drains the nasal region (Fig. 1). As expected with a respiratory pathogen, low numbers, approximately 10 to 15 antibody-forming cells (AFCs)/5 × 105 nucleated cells, of virus-specific AFCs were observed in the spleen throughout the primary immune response to RSV (data not shown). In the bone marrow, frequencies of virus-specific AFCs were very low throughout the acute response (<5AFCs/5 × 105 nucleated cells) and at some time points were indistinguishable from background levels (Fig. 2B).
FIG. 1.
Frequency of RSV-specific AFCs in the O-NALT (A), cervical lymph node (B), and mediastinal lymph node (C) following primary, secondary, and tertiary intranasal infection with respiratory syncytial virus. A minimum of four mice were pooled for each time point. The data show a representative experiment.
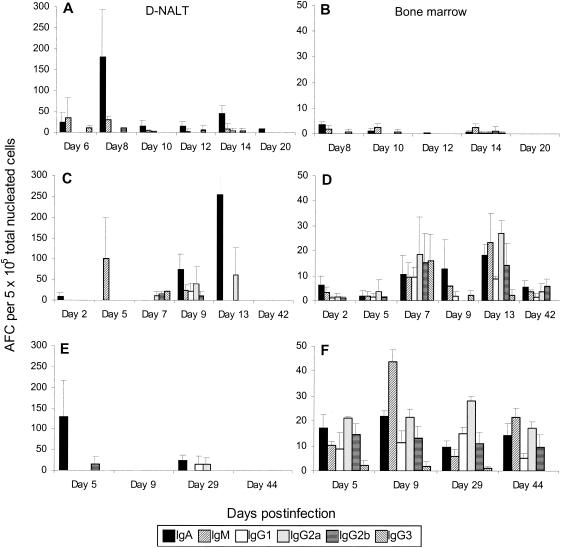
FIG. 2.
Frequency of RSV-specific AFCs in the D-NALT (A, C, and E) and the bone marrow (B, D, and F) following primary (A and B), secondary (C and D), and tertiary (E and F) intranasal infection with respiratory syncytial virus. A minimum of four mice were pooled for each time point. The data show a representative experiment.
As we have previously shown with influenza virus, both the O-NALT and the D-NALT were capable of generating RSV-specific AFCs which were predominantly of the IgA isotype throughout the primary response (Fig. 1A and 2A). Interestingly, the kinetics of the antibody response in the O-NALT closely followed that observed after influenza virus infection (23), peaking at approximately 200/5 × 105 RSV-specific AFCs on day 8 postinfection and thereafter declining quickly. In the D-NALT the kinetics of the specific AFC response following RSV infection was very similar to that seen in the O-NALT, with the peak again occurring at day 8 postinfection and with an AFC frequency similar to that observed in the O-NALT. However, the D-NALT response declined thereafter, starkly in contrast to the situation observed in the D-NALT following influenza virus infection, where robust responses were generated by day 8 postinfection but frequencies continued to rise thereafter and were maintained (23).
Importantly, very few RSV-specific AFCs were observed following primary immunization with beta-propiolactone inactivated RSV either in the nasal tissues or the draining lymph nodes, and low numbers of IgM plaques were noted in the spleen (data not shown). Following a challenge with live RSV, the kinetics of the antibody response were those of a primary infection, with the peak of RSV-specific AFCs in the mediastinal lymph node and cervical lymph node occurring at day 9 (data not shown). Moreover RSV-specific serum IgG2a and IgG2b antibody titers were similar to those observed following a primary RSV infection, but titers were approximately 10-fold less following administration of inactivated RSV (data not shown). This implies that replicating virus is actually responsible for generating the antibody response elicited during the live virus challenge.
In order to determine whether the virus-specific antibody response is maintained in the bone marrow and the D-NALT following one exposure to the virus, as occurs following influenza virus infection in C57BL/6 mice, we assessed the RSV-specific AFC frequencies in mice up to 18 months after primary infection with RSV. We found that in the bone marrow, although specific plaques were detectable at these time points, the frequencies were very low and were only just at detectable levels, being one to four plaques of IgA or IgG per 5 × 105 nucleated cells (data not shown). In the D-NALT we found that either no or low numbers (not more than 20/5 × 105 nucleated cells) of RSV-specific AFC persisted at time points up to and including 18 months after primary infection (data not shown).
Virus-specific AFC frequencies following secondary and tertiary challenge with RSV.
At 7 to 9 weeks after either a primary or a secondary infection with RSV, mice were again infected with RSV at a dose equal to that administered for the primary infection to assess secondary and tertiary RSV-specific AFC responses, respectively. Following secondary challenge with RSV, larger numbers of virus-specific IgG AFC subclasses were detected earlier than following primary infection in both the mediastinal lymph node and cervical lymph node, as would be expected. Peak RSV-specific total AFC numbers occurred on day 7 post-secondary infection and day 5 post-tertiary infection in both the cervical lymph node and mediastinal lymph node (Fig. 1B and C). Although we detected very few virus-specific AFCs in the O-NALT after either secondary or tertiary RSV infection (Fig. 1A), acute responses to both secondary and tertiary challenge could be detected in the D-NALT, although these responses also declined quickly even after such boosting (Fig. 2C and E). No virus-specific AFCs could be detected either 42 days post-secondary challenge or at 44 days post-tertiary challenge (Fig. 2C and E). In contrast, in the bone marrow, both secondary and tertiary challenge generated a quicker response and a larger number of RSV-specific AFCs of various isotypes than that observed in the primary response. Moreover, a robust response was still apparent 44 days post-tertiary challenge (Fig. 2F).
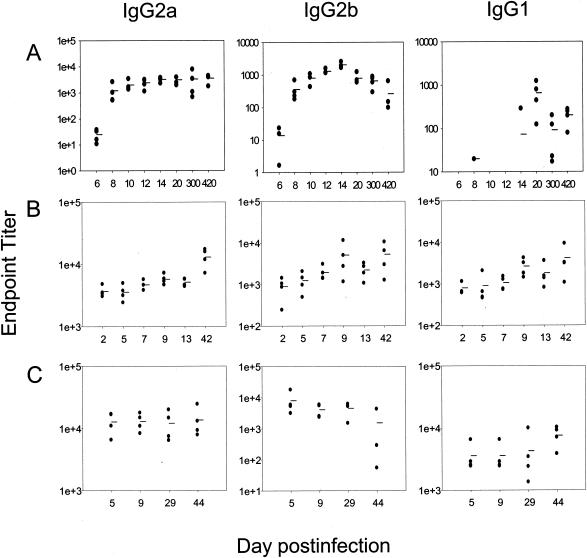
Generation of robust serum antibody titers following primary infection with RSV is boosted following secondary challenge.
Serum levels of RSV-specific IgG were assessed following primary, secondary, and tertiary intranasal administration of RSV. Significant titers of serum antibody were generated during the course of the primary infection, predominantly of the IgG2a and IgG2b isotypes. After days 12 to 14 postinfection, IgG2a titers reached a plateau and remained at this level until at least 420 days postinfection, whereas IgG2b titers declined approximately 10-fold over time (Fig. 3). Negligible amounts of virus-specific IgG1 were detected before day 14 post-primary infection. Subsequent secondary infection resulted in increased serum titers of RSV-specific antibody, which remained high 6 to 7 weeks after both secondary and tertiary infection (Fig. 3B and C). RSV-specific serum neutralizing antibody was found after primary infection (mean reciprocal ED50 at day 14 postinfection = 4.25 ± 0.5; mean reciprocal ED50 at day 300 postinfection = 3.5 ± 0.57) and was boosted approximately threefold after secondary infection (mean reciprocal ED50 at day 13 post-secondary infection = 9.67 ± 1.5). This titer did not increase further after tertiary challenge.
FIG. 3.
RSV-specific serum IgG subclass responses following primary (A), secondary (B), and tertiary (C) infection with respiratory syncytial virus. Titers were assessed from a minimum of four individual mice per time point. Due to the different titers of the RSV-specific isotypes detected, scaling of the ordinates differs for each isotype.
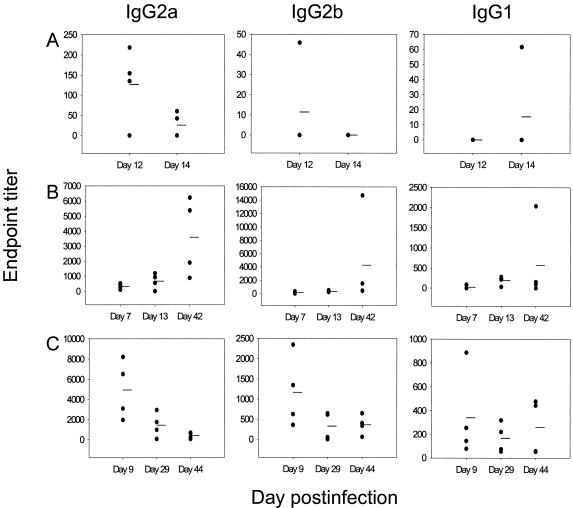
Fragments of lung were also assessed for secreted antibody in vitro. We found low levels of antibody in the supernatants of lung fragments taken from mice during the primary infection (Fig. 4A). Although antibody levels rose transiently following secondary and then tertiary challenge, 44 days after a tertiary challenge with RSV we could detect only minimal levels of RSV-specific antibody secreted from lung fragments (Fig. 4C). These fragment culture data do not reflect the serum antibody levels, which remained high on reinfection. Nevertheless, we cannot exclude the possibility that some contribution to the fragment culture supernatants was provided by transudation of serum antibody into lungs. However, other studies have shown that in passively immunized mice, serum antibody does not seem to contribute to the antibody recovered from lung fragment cultures (Julia Tree, personal communication).
FIG. 4.
RSV-specific IgG secreted from lung fragments is not long lasting. Secreted antibody from lung fragments was measured at certain time points after primary (A), secondary (B), and tertiary (C) infection with RSV. Four individual mice were assessed per time point. Due to the different titers of the RSV-specific isotypes detected, scaling of the ordinates differs for each isotype.
Reinfection of the lungs occurs following secondary and tertiary challenge with RSV.
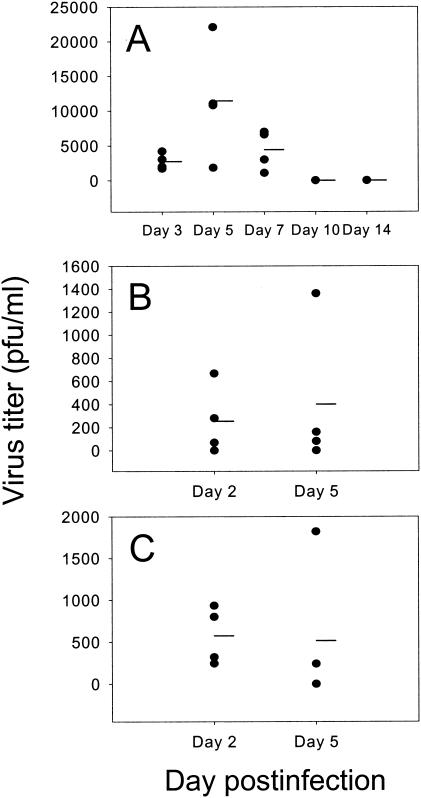
During a primary infection with RSV, peak lung virus titers occurred on day 5 postinfection and were cleared by day 10 postinfection (Fig. 5A). However, following secondary challenge, RSV titers of 250 PFU/ml could be detected on day 2 postinfection, with three out of four mice positive for virus (Fig. 5B). After a tertiary challenge, RSV titers were >500 PFU/ml on day 2 postinfection, with all four mice tested being positive for virus (Fig. 5C). Three out of four mice on day 5 post-secondary infection and two out of four mice on day 5 post-tertiary infection also tested positive for virus.
FIG. 5.
Existing RSV-specific antibody does not protect against secondary or tertiary reinfection of the lung. Lung virus titers were assessed during primary (A), secondary (B), and tertiary (C) infection with RSV. Four individual mice were assessed per time point.
DISCUSSION
Efforts to develop a safe and effective vaccine for RSV have met with difficulty. As most RSV infections occur in infants less than 6 months of age and cases of RSV peak at less than 2 months of age (4), vaccination would have to take place at a very young age. An additional problem is the relatively poor immune response evoked by immunization in neonates. This is due in part to the immunological immaturity of the neonate and in part to the well-documented inhibitory effect of passively acquired maternal antibody both on subsequent primary immune responses to respiratory infection (5) and following immunization with subunit (31) or live virus vector (30) vaccine candidates. Development of a safe vaccine must also consider any predisposition for enhanced pulmonary disease after natural RSV infection which may potentially occur in vaccinated children (21). In addition, although significant antibody titers were generated in children following a formalin-inactivated RSV vaccine, the antibody was poorly neutralizing (21).
Protection from respiratory viruses is largely antibody mediated. In children, RSV-specific serum antibody has been shown to wane considerably after primary natural infection, and although higher titers of virus-specific antibody are detected when measured shortly after rechallenge, these levels decline sharply thereafter (17). Specific serum antibody titers do however remain at a higher level after reinfection than after primary infection, implying a gradual accrual of RSV-specific antibody and resistance to reinfection (17, 20, 46). Some studies have shown that in adults, resistance to reinfection correlated better with levels of RSV-specific neutralizing nasal IgA rather than RSV-specific neutralizing serum antibody levels, and those with low nasal antibody levels developed more extensive infection compared to those with high levels of nasal antibody (26, 45). In contrast, other studies have shown no correlation between specific nasal IgA antibody titers and protection from reinfection (14). Nevertheless, despite such contradictory data, as RSV is restricted to the respiratory tract, secretory antibody responses must be considered a crucially important factor in protection against challenge.
The major source of serum specific antibody long after exposure to respiratory viruses such as influenza virus and Sendai virus is thought to be the bone marrow. In the mouse, virus-specific AFC frequencies in the bone marrow remain at a relatively constant level (approximately 40 AFCs/5 × 105 total nucleated bone marrow cells) permanently after a single exposure to influenza virus (19, 23). We show here that although the bone marrow is capable of generating antibodies against RSV after primary intranasal infection, the frequency of specific plasma cells is very low compared to that observed after intranasal influenza virus infection and does not appear to last. A frequency of bone marrow virus-specific AFCs similar to that attained after one exposure to influenza virus was only achieved after three exposures to live RSV.
Given the fact that most virus-specific serum antibody produced long after infection originates in the bone marrow (19), we compared the serum levels of RSV-specific antibody with the kinetics of RSV-specific AFC generation in the bone marrow and found that the increase in RSV-specific antibody titers detected in serum after secondary or tertiary boosting with RSV reflect the RSV-specific bone marrow AFC frequencies detected at the same time points. (Fig. 2 and 3). These data agree with previous human work showing that, in general, long-lived serum antibody responses are elicited after second or third infections with RSV (44). Furthermore, although higher serum antibody titers were present following secondary infection than following primary infection, we found that virus was still able to infect the lungs, implying that serum antibody alone is insufficient for protection against infection.
We investigated this further by examining the ability to generate a local nasal response to RSV. We have recently identified the D-NALT which lines the nasal passages as the source of the virus-specific antibody following influenza virus infection (23). In that study we showed that 12 to 18 months after one exposure to influenza virus, the frequency of virus-specific AFCs is approximately 150 total AFCs per 5 × 105 D-NALT cells, suggesting that antibody is produced in locally significant amounts. In contrast, we show here that, compared to influenza virus, the number of RSV-specific AFCs 14 to 18 months after RSV infection is less than 20 total AFCs per 5 × 105 D-NALT cells. Moreover, the anti-RSV D-NALT response is not maintained after two further challenges with live RSV (Fig. 2).
As mentioned above, although substantial serum responses were found after secondary and tertiary exposure to live RSV, virus was still found in the lungs of mice 2 to 5 days postinfection, demonstrating the potential importance of local antibody production at the site of infection. Furthermore, although low levels of RSV-specific antibody were secreted from the lung acutely after infection, these responses were short-lived, implying that local contributions from the lung itself do not play a role in protection from reinfection with RSV. These data agree with other studies which have also found RSV in the lungs after secondary challenge in BALB/c mice (9).
Nasal IgA has previously been shown to directly mediate local anti-influenza virus immunity in the mouse model, implying an important role for antibody in protection in the upper respiratory tract (40). As RSV and influenza virus are not natural pathogens of mice, it is difficult to extrapolate to the human situation. However, an indication of the importance of the nasopharyngeal lymphoid tissue in humans is the demonstration that levels of IgA specific to poliovirus in the nasopharynx do not decline over a 34-month period after immunization with live attenuated polio vaccine, and these levels of poliovirus-specific antibody are diminished or absent from children following tonsillectomy and adenoidectomy (34).
Our data shows that much lower frequencies of virus-specific AFCs are generated following RSV infection than following influenza virus infection both in the bone marrow and in the NALT. The fact that a single intranasal infection with influenza virus results in protection against reinfection but one intranasal exposure to RSV does not suggests that infection with RSV may not evoke sufficient numbers of specific AFCs in either the bone marrow or locally in the NALT to contribute to protection, at least in the mouse model. A similar situation may occur in humans, given that specific serum antibody wanes following primary infection and that increased antibody titers are found following subsequent infections. Moreover, the fact that RSV D-NALT AFC numbers remained low even after three exposures to live virus suggests that defective local responses may be a significant factor in the inability to generate protective immunity to RSV.
The mechanisms that result in the inability of RSV to evoke protective immune responses in the nasal tissues are unknown at present. Although we recognize that mice may have a low permissiveness for RSV and it is unknown to what extent RSV replicates in mouse mucosal tissues, we and others (9) have shown that RSV is able to replicate within the mouse lung to over 104 PFU of virus. In addition we have shown that inactivated RSV produces a negligible RSV-specific mucosal antibody response after multiple intranasal immunizations, indicating that replicating virus is required to stimulate local responses (unpublished data).
Interestingly, it has recently been shown that pulmonary infection with RSV impairs not only the RSV-specific CD8+ T effector cell response in the lung but also the development of pulmonary CD8+ T-cell memory (3). It is possible that the suppressive effect of RSV on CD8+ T cells or any potentially similar effect on CD4+ T cells may influence the development of the humoral immune response to RSV and subsequent maintenance of B-cell memory. Alternatively, infection of the respiratory tract with RSV may suppress B-cell effector functions through the secretion of inhibitory host soluble factors released following infection (35, 37, 43). It should also be noted that this study used adult mice. If the data shown here reflect the situation in adult humans, in whom reinfection is also common, one could speculate that the generation of effective immunity in neonates must overcome not only the problems of an immature immune system and maternal antibody-mediated suppression of immunity, but also a potentially suppressive effect of the virus itself on the development of B-cell memory.
In summary, these data suggest that, in the absence of significant levels of locally produced nasal antibody to RSV, the virus is able to replicate to higher titers or for a longer period in the nasopharyngeal area after challenge, potentially resulting in reinfection. Furthermore, multiple boosts with live virus do not increase local humoral immunity to RSV in the nasal tissues.
Acknowledgments
S. Hou ad L. Hyland contributed equally to the work.
This work was supported by the Edward Jenner Institute for Vaccine Research.
REFERENCES
- 1.Asanuma, H., A. H. Thompson, T. Iwasaki, Y. Sato, Y. Inaba, C. Aizawa, T. Kurata, and S. Tamura. 1997. Isolation and characterization of mouse nasal-associated lymphoid tissue. J. Immunol. Methods 202:123-131. [DOI] [PubMed] [Google Scholar]
- 2.Brandenburg, A. H., J. Groen, H. A. van Steensel-Moll, E. C. Claas, P. H. Rothbarth, H. J. Neijens, and A. D. Osterhaus. 1997. Respiratory syncytial virus specific serum antibodies in infants under six months of age: limited serological response upon infection. J. Med. Virol. 52:97-104. [DOI] [PubMed] [Google Scholar]
- 3.Chang, J., and T. J. Braciale. 2002. Respiratory syncytial virus infection suppresses lung CD8+ T-cell effector activity and peripheral CD8+ T-cell memory in the respiratory tract. Nat. Med. 8:54-60. [DOI] [PubMed] [Google Scholar]
- 4.Crowe, J. E., Jr. 2001. Influence of maternal antibodies on neonatal immunization against respiratory viruses. Clin. Infect. Dis. 33:1720-1727. [DOI] [PubMed] [Google Scholar]
- 5.Crowe, J. E., Jr. 1998. Immune responses of infants to infection with respiratory viruses and live attenuated respiratory virus candidate vaccines. Vaccine 16:1423-1432. [DOI] [PubMed] [Google Scholar]
- 6.Englund, J. A., C. J. Sullivan, M. C. Jordan, L. P. Dehner, G. M. Vercellotti, and H. H. Balfour, Jr. 1988. Respiratory syncytial virus infection in immunocompromised adults. Ann. Intern. Med. 109:203-208. [DOI] [PubMed] [Google Scholar]
- 7.Glezen, W. P., A. Paredes, J. E. Allison, L. H. Taber, and A. L. Frank. 1981. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J. Pediatr. 98:708-715. [DOI] [PubMed] [Google Scholar]
- 8.Graham, B. S., L. A. Bunton, J. Rowland, P. F. Wright, and D. T. Karzon. 1991. Respiratory syncytial virus infection in anti-μ-treated mice. J. Virol. 65:4936-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham, B. S., L. A. Bunton, P. F. Wright, and D. T. Karzon. 1991. Reinfection of mice with respiratory syncytial virus. J. Med. Virol. 34:7-13. [DOI] [PubMed] [Google Scholar]
- 10.Green, M., A. F. Brayer, K. A. Schenkman, and E. R. Wald. 1989. Duration of hospitalization in previously well infants with respiratory syncytial virus infection. Pediatr. Infect. Dis. J. 8:601-605. [DOI] [PubMed] [Google Scholar]
- 11.Groothuis, J. R., K. M. Gutierrez, and B. A. Lauer. 1988. Respiratory syncytial virus infection in children with bronchopulmonary dysplasia. Pediatrics 82:199-203. [PubMed] [Google Scholar]
- 12.Groothuis, J. R., and H. Nishida. 2002. Prevention of respiratory syncytial virus infections in high-risk infants by monoclonal antibody (palivizumab). Pediatr. Int. 44:235-241. [DOI] [PubMed] [Google Scholar]
- 13.Hall, C. B., K. R. Powell, N. E. MacDonald, C. L. Gala, M. E. Menegus, S. C. Suffin, and H. J. Cohen. 1986. Respiratory syncytial viral infection in children with compromised immune function. N. Engl. J. Med. 315:77-81. [DOI] [PubMed] [Google Scholar]
- 14.Hall, C. B., E. E. Walsh, C. E. Long, and K. C. Schnabel. 1991. Immunity to and frequency of reinfection with respiratory syncytial virus. J. Infect. Dis. 163:693-698. [DOI] [PubMed] [Google Scholar]
- 15.Han, L. L., J. P. Alexander, and L. J. Anderson. 1999. Respiratory syncytial virus pneumonia among the elderly: an assessment of disease burden. J. Infect. Dis. 179:25-30. [DOI] [PubMed] [Google Scholar]
- 16.Hemming, V. G., G. A. Prince, J. R. Groothuis, and G. R. Siber. 1995. Hyperimmune globulins in prevention and treatment of respiratory syncytial virus infections. Clin. Microbiol. Rev. 8:22-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson, F. W., W. A. Clyde, Jr., A. M. Collier, F. W. Denny, R. J. Senior, C. I. Sheaffer, W. G. Conley III, and R. M. Christian. 1979. The etiologic and epidemiologic spectrum of bronchiolitis in pediatric practice. J. Pediatr. 95:183-190. [DOI] [PubMed] [Google Scholar]
- 18.Hyland, L., S. Hou, C. Coleclough, T. Takimoto, and P. C. Doherty. 1994. Mice lacking CD8+ T cells develop greater numbers of IgA-producing cells in response to a respiratory virus infection. Virology 204:234-241. [DOI] [PubMed] [Google Scholar]
- 19.Hyland, L., M. Sangster, R. Sealy, and C. Coleclough. 1994. Respiratory virus infection of mice provokes a permanent humoral immune response. J. Virol. 68:6083-6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaul, T. N., R. C. Welliver, D. T. Wong, R. A. Udwadia, K. Riddlesberger, and P. L. Ogra. 1981. Secretory antibody response to respiratory syncytial virus infection. Am. J. Dis. Child. 135:1013-1016. [DOI] [PubMed] [Google Scholar]
- 21.Kim, H. W., J. G. Canchola, C. D. Brandt, G. Pyles, R. M. Chanock, K. Jensen, and R. H. Parrott. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 89:422-434. [DOI] [PubMed] [Google Scholar]
- 22.Kuper, C. F., P. J. Koornstra, D. M. H. Hameleers, J. Biewenga, B. J. Spit, A. M. Duijvestijn, P. J. C. van Breda Vriesman, and T. Sminia. 1992. The role of nasopharyngeal lymphoid tissue. Immunol. Today 13:219-224. [DOI] [PubMed] [Google Scholar]
- 23.Liang, B., L. Hyland, and S. Hou. 2001. Nasal-associated lymphoid tissue is a site of long-term virus-specific antibody production following respiratory virus infection of mice. J. Virol. 75:5416-5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mbiguino, A., and J. Menezes. 1991. Purification of human respiratory syncytial virus: superiority of sucrose gradient over percoll, renografin, and metrizamide gradients. J. Virol. Methods 31:161-170. [DOI] [PubMed] [Google Scholar]
- 25.McIntosh, K., J. McQuillin, and P. S. Gardner. 1979. Cell-free and cell-bound antibody in nasal secretions from infants with respiratory syncytial virus infection. Infect. Immun. 23:276-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mills, J., V. J. E. Van Kirk, P. F. Wright, and R. M. Chanock. 1971. Experimental respiratory syncytial virus infection of adults. Possible mechanisms of resistance to infection and illness. J. Immunol. 107:123-130. [PubMed] [Google Scholar]
- 27.Moser, C. A., S. Cookinham, S. E. Coffin, H. F. Clark, and P. A. Offit. 1998. Relative importance of rotavirus-specific effector and memory B cells in protection against challenge. J. Virol. 72:1108-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy, B. R., D. W. Alling, M. H. Snyder, E. E. Walsh, G. A. Prince, R. M. Chanock, V. G. Hemming, W. J. Rodriguez, H. W. Kim, B. S. Graham, and P. F. Wright. 1986. Effect of age and preexisting antibody on serum antibody response of infants and children to the F and G glycoproteins during respiratory syncytial virus infection. J. Clin. Microbiol. 24:894-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy, B. R., B. S. Graham, G. A. Prince, E. E. Walsh, R. M. Chanock, D. T. Karzon, and P. F. Wright. 1986. Serum and nasal-wash immunoglobulin G and A antibody response of infants and children to respiratory syncytial virus F and G glycoproteins following primary infection. J. Clin. Microbiol. 23:1009-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy, B. R., R. A. Olmsted, P. L. Collins, R. M. Chanock, and G. A. Prince. 1988. Passive transfer of respiratory syncytial virus (RSV) antiserum suppresses the immune response to the RSV fusion (F) and large (G) glycoproteins expressed by recombinant vaccinia viruses. J. Virol. 62:3907-3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy, B. R., G. A. Prince, P. L. Collins, S. W. Hildreth, and P. R. Paradiso. 1991. Effect of passive antibody on the immune response of cotton rats to purified F and G glycoproteins of respiratory syncytial virus (RSV). Vaccine 9:185-189. [DOI] [PubMed] [Google Scholar]
- 32.Nicholson, K. G., J. Kent, V. Hammersley, and E. Cancio. 1997. Acute viral infections of upper respiratory tract in elderly people living in the community: comparative, prospective, population based study of disease burden. Br. Med. J. 315:1060-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogilvie, M. M., A. S. Vathenen, M. Radford, J. Codd, and S. Key. 1981. Maternal antibody and respiratory syncytial virus infection in infancy. J. Med. Virol. 7:263-271. [DOI] [PubMed] [Google Scholar]
- 34.Ogra, P. L. 1971. Effect of tonsillectomy and adenoidectomy on nasopharyngeal antibody response to poliovirus. N. Engl. J. Med. 284:59-64. [DOI] [PubMed] [Google Scholar]
- 35.Olszewska-Pazdrak, B., A. Casola, T. Saito, R. Alam, S. E. Crowe, F. Mei, P. L. Ogra, and R. P. Garofalo. 1998. Cell-specific expression of RANTES, MCP-1, and MIP-1α by lower airway epithelial cells and eosinophils infected with respiratory syncytial virus. J. Virol. 72:4756-4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parrott, R. H., H. W. Kim, J. O. Arrobio, D. S. Hodes, B. R. Murphy, C. D. Brandt, E. Camargo, and R. M. Chanock. 1973. Epidemiology of respiratory syncytial virus infection in Washington, D.C. II. Infection and disease with respect to age, immunologic status, race and sex. Am. J. Epidemiol. 98:289-300. [DOI] [PubMed] [Google Scholar]
- 37.Preston, F. M., P. L. Beier, and J. H. Pope. 1995. Identification of the respiratory syncytial virus-induced immunosuppressive factor produced by human peripheral blood mononuclear cells in vitro as interferon-alpha. J. Infect. Dis. 172:919-926. [DOI] [PubMed] [Google Scholar]
- 38.Prince, G. A., V. G. Hemming, R. L. Horswood, P. A. Baron, and R. M. Chanock. 1987. Effectiveness of topically administered neutralizing antibodies in experimental immunotherapy of respiratory syncytial virus infection in cotton rats. J. Virol. 61:1851-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prince, G. A., V. G. Hemming, R. L. Horswood, and R. M. Chanock. 1985. Immunoprophylaxis and immunotherapy of respiratory syncytial virus infection in the cotton rat. Virus Res. 3:193-206. [DOI] [PubMed] [Google Scholar]
- 40.Renegar, K. B., and P. A. Small, Jr. 1991. Immunoglobulin A mediation of murine nasal anti-influenza virus immunity. J. Virol. 65:2146-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Staat, M. A. 2002. Respiratory syncytial virus infections in children. Semin. Respir. Infect. 17:15-20. [DOI] [PubMed] [Google Scholar]
- 42.Tamura, S., T. Iwasaki, A. Thompson, H. Asanuma, Z. Chen, Y. Suzuki, C. Aizawa, and T. Kurata. 1998. Antibody-forming cells in the nasal-associated lymphoid tissue during primary influenza virus infection. J. Gen. Virol. 79:291-299. [DOI] [PubMed] [Google Scholar]
- 43.Thomas, L. H., M. I. Wickremasinghe, M. Sharland, and J. S. Friedland. 2000. Synergistic upregulation of interleukin-8 secretion from pulmonary epithelial cells by direct and monocyte-dependent effects of respiratory syncytial virus infection. J. Virol. 74:8425-8433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner, D. K., P. Muelenaer, F. W. Henderson, M. H. Snyder, C. B. Reimer, E. E. Walsh, L. J. Anderson, D. L. Nelson, and B. R. Murphy. 1989. Serum immunoglobulin G antibody subclass response to respiratory syncytial virus F and G glycoproteins after first, second, and third infections. J. Clin. Microbiol. 27:589-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watt, P. J., B. S. Robinson, C. R. Pringle, and D. A. Tyrrell. 1990. Determinants of susceptibility to challenge and the antibody response of adult volunteers given experimental respiratory syncytial virus vaccines. Vaccine 8:231-236. [DOI] [PubMed] [Google Scholar]
- 46.Welliver, R. C., T. N. Kaul, T. I. Putnam, M. Sun, K. Riddlesberger, and P. L. Ogra. 1980. The antibody response to primary and secondary infection with respiratory syncytial virus: kinetics of class-specific responses. J. Pediatr. 96:808-813. [DOI] [PubMed] [Google Scholar]
- 47.Weltzin, R., V. Traina-Dorge, K. Soike, J. Y. Zhang, P. Mack, G. Soman, G. Drabik, and T. P. Monath. 1996. Intranasal monoclonal IgA antibody to respiratory syncytial virus protects rhesus monkeys against upper and lower respiratory tract infection. J. Infect. Dis. 174:256-261. [DOI] [PubMed] [Google Scholar]