Abstract
Arsenic-based compounds have become accepted agents for cancer therapy providing high rates of remission of some cancers such as acute promyelocytic leukemia (APL). The mechanisms by which arsenic-containing compounds kill cells and reasons for selective killing of only certain types of cancer cells such as APLs have recently been delineated. This knowledge was gained in parallel with increasing understanding and awareness of the importance of intracellular redox systems and regulation of the production of reactive oxygen species (ROS) by controlling mitochondrial function. Many of the targets for the arsenic-containing compounds are mitochondrial proteins involved in regulating the production of ROS. Inhibition of these proteins by disulfide linkage of vicinal thiol groups often leads to increased production of ROS and induction of apoptotic signalling pathways. Sensitivity or resistance to the actions of arsenic-containing compounds on cancer cells and normal cells depends on the levels of transport systems for their uptake or efflux from the cells as well as their redox defence mechanisms. The exact mechanisms of arsenic toxicity as well as its anticancer properties are likely to be related and these aspects of arsenic metabolism are covered in this review. Greater understanding of the mechanisms of action of arsenic will help determine the risks of human exposure to this chemical. Novel organic arsenic-containing compounds and the lessons learned from studying their selective sensitivity in targeting dividing endothelial cells to inhibit angiogenesis raise the future possibility for designing better targeted antineoplastic arsenic-containing compounds with less toxicity to normal cells.
1. ARSENIC AND ARSENIC-CONTAINING COMPOUNDS IN THE NATURAL ENVIRONMENT OR AS A RESULT OF HUMAN APPLICATION
Arsenic is a toxic metalloid [1, 2] that exists throughout nature in organic and inorganic forms. It is commonly present in soils on average at several mg/kg, as well as in marine sediments, and is enriched in mineral deposits as oxides and sulfides. The basic element, arsenic, exists in either of three allotropic forms: yellow, black, or grey with the stable, semimetallic form having a silver/steely-grey colour as a brittle, crystalline solid. The semimetallic form oxidises rapidly in air, and at high temperatures produces a white cloud of arsenic trioxide (As2O3). Arsenic, with its variety of chemical forms and oxidation states, is listed in Table 1. The current IUPAC nomenclature is listed in [3] as well as the more common names of the arsenic-based compounds which are mainly used throughout this review.
Table 1.
Common names and fully systematic (additive) names for Arsenic oxoacid and related structures. Some organic derivative names still contain the word “acid,'' as in the following derivatives of arsonic acid = H2AsHO3 = [AsHO(OH)2], for example, PhAsO(OH)2 phenylarsonic acid.
| Example of alternative names for Arsenic species used | |||
|---|---|---|---|
| Common name | Abbreviation | IUPAC Fully systematic additive name | Chemical formula |
|
| |||
| Arsenite, Arsenous acid, Arsorous acid | As(III), AsIII | Trihydroxidoarsenic | H3AsO3 = [As(OH)3] |
| Arsinious acid | As(III), AsIII | Dihydrohydroxidoarsenic | HAsH2O = [AsH2(OH)] |
| Arsonous acid | As(III), AsIII | Hydridodihydroxidoarsenic | H2AsHO2 = [AsH(OH)2] |
| Arsenate, Arsenic acid, Arsoric acid | As(V), AsV | Trihydroxidooxidoarsenic | H3AsO4 = AsO(OH)3 |
| Arsinic acid | As(V), AsV | Dihydridohydroxidooxidoarsenic | HAsH2O2 = [AsH2O(OH)] |
| Arsonic acid | As(V), AsV | Hydridodihydroxidooxidoarsenic | H2AsHO3 = [AsHO(OH)2] |
| Monomethylarsonic acid | MMA(V), MMAV | Methanedihydroxidooxidoarsenic | CH3AsO(OH)2 |
| Monomethylarsonous acid | MMA(III), MMAIII | Methanedihydroxidoarsenic | CH3As(OH)2 |
| Dimethylarsinic acid | DMA(V), DMAV | Dimethanehydroxidooxidoarsenic | (CH3)2AsO(OH) |
| Dimethylarsinous acid | DMA(III), DMAIII | Dimethanehydroxidoarsenic | (CH3)2AsOH |
When absorbed at toxic levels, arsenic causes severe health problems, including cancer. Acceptable levels were lowered from 50 μg/L to 10 μg/L in drinking water by the WHO [4]. Arsenic-containing compounds are applied in plant pesticides and insecticides and arsenic environmental contamination represents a global health problem, particularly from leaching into ground water. When the soluble levels exceed 50 μg/L in drinking water as in many regions of Bangladesh, arsenic becomes a particular health concern as it has recently been associated with increased cancer rates appearing after consumption with a lag time up to 10–20 years [5].
Whilst the carcinogenic aspects of arsenic compounds are not the focus of this review, nevertheless this undesirable aspect needs to be raised. Thus, arsenic and its methylated species are known carcinogens but this description is probably inaccurate as they act more as cocarcinogens by facilitating/promoting the induction of tumors of the skin, urinary bladder, and lung rather than directly inducing cell transformation and oncogenesis [2, 6]. As cocarcinogens, mechanisms include indirect effects such as DNA damaging genotoxicity by altered DNA methylation as well as inducing high levels of oxidative stress leading to altered cell proliferation and tumor promotion (detailed in [7]).
In terms of chronic toxicity, the 50% lethal dose of arsenic as arsenic trioxide in mice received by the oral route varies from 15–48 mg/kg, whereas the acute lethal dose in humans varies from 1–3 mg/kg body weight [4]. Despite its toxicity, arsenic has been applied in Chinese medicines for thousands of years as refined preparations of realgar (As4S4) or orpiment (As2S3) [8] which are two rare mineralised forms. Realgar, easily discerned by its bright red and orpiment with a browny yellow appearance are ores often occurring close to each other in hydrothermal veins of precious metal sulfide ores or hot spring deposits as volcanic sublimate products (crystallized from gases). Hence, arsenic is a common waste product from the mining of metal ores and the name realgar is possibly derived from Arabic words for “powder of the mine” (rahj al ghar).
The long history of medical applications has included treating many diseases such as cancers administering micromolar levels in patients and, in particular, arsenic compounds have proven to be very effective against certain hematological malignancies (reviewed in [9, 10]). Thus, arsenic oxides and derivatives have been established as effective treatments for acute promyelocytic leukemia (APL), and they are being tested as therapies for a range of other hematological cancers including myelodysplastic syndromes, multiple myeloma, and chronic myelogenous leukemia (CML). The results of clinical trails using arsenic-based drugs in cancer therapy have been extensively reviewed [8–12]. Hence, this review mainly concerns those factors that explain the antineoplastic mechanism of action of arsenic-based drugs, distinguishing their selectivity for killing certain types of cancer cells from their potential for toxicity to normal cells in the body. The reason for their efficacy and selectivity in treating certain hematological malignancies is covered in the last section of this review. However, the mechanisms of action of arsenic-basedcompounds on cells must first be described in detail to provide sufficient understanding to enable their selective targeting of specific types of cancer cells to be duly evaluated.
Water distributes the majority of inorganic arsenic in the biosphere either as pentavalent arsenate (As5+, As(V)) or trivalent arsenic (As3+, As(III)) [2]. In solution, the pH and redox conditions affect the chemical state of arsenic that predominates. Thus, in highly oxidised environments, the arsenate (As5+) form predominates as one of four major species of arsenic acid; H3AsO4, H2AsO4 −, HAsO4 2−, and AsO4 3− [13]. However, mildly reducing conditions such as those generally present inside all mammalian cells [14] will favour the reduction of the pentavalent arsenate (As5+) to the trivalent arsenic (As3+). Arsenous acid with the formula As(OH)3 results after the slow hydrolysis of arsenic trioxide in water. As the pH increases, arsenous acid is converted to the arsenic oxide ions [AsO(OH)2]−, [AsO2(OH)]−2, and [AsO3]−3 [13].
2. METHYLATED FORMS OF ARSENIC SHOW INCREASED CYTOTOXICITY INSIDE CELLS
Arsenic combines readily with many elements; and bacteria have evolved systems to detoxify inorganic arsenic to form organic arsenic-containing compounds such as methylated forms. Plant and insect pesticides have been made using organic arsenic derivatives; and monomethylarsonic acid (MMA(V)) and dimethylarsinic acid (DMA(V)) are used in products for weed control. Also, MMA(V) and DMA(V) are metabolites of inorganic arsenic, formed intracellularly in mammals, primarily in the liver. The metabolic process of inorganic arsenic conversion is known as biotransformation and appears to enhance the excretion of arsenic from the body, involving formation of methylated compounds of trivalent arsenic as intermediates. The metabolism involves reduction to a trivalent state and oxidative methylation to a pentavalent state. In addition, reductases present in cells and other reactions facilitate the reduction of arsenic acid [As(V)] to the arsenous [As(III)] form including methylation of the arsenous form, mainly via the liver, to produce mono-, di-, and trimethylated species [2]. The main enzyme involved in methylation of As(III) is arsenic methyltransferase (As3MT) that requires glutathione (GSH) to promote the reaction and a cycling redox system such as thioredoxin (reviewed in [15]) to detoxify arsenic-containing compounds. Many animal species convert inorganic arsenic-containing compounds into mono-, di- and tri-methylated arsenic species which are mostly then secreted in the urine [16]. This is because the methylated forms are not as well absorbed by cells compared to the inorganic forms, although they have much greater cytotoxicity if they do enter cells [15, 17].
The reductive metabolism of arsenic has an important role in its toxicity. The trivalent arsenic-containing compounds, including the methylated organic forms have much greater potency than the pentavalent arsenic-containing compounds as cytotoxics and carcinogens [18–23]. For example, in cytotoxicity assays, the IC50 values for cultures of primary hepatocytes, keratinocytes, and epithelial cells ranged from 3 μM to well over 20 μM for trihydroxidoarsenic salts whereas for monomethylarsonous acid [MMA(III)], the values were consistently much lower at only several μM for the equivalent normal cell types [24–26]. Organic arsenic-based ingredients are commonly used as feed additives in poultry farming to increase weight gain by preventing bacterial and parasitic infections, thereby increasing feed efficiency and improving pigmentation. The three major arsenic-containing compounds used in this manner are arsenilic acid (p-aminophenyl arsonic acid), roxarsone (4-hydroxy-3-nitrophenylarsonic acid), and nitarsone (4-nitro-phenylarsonic acid) [22, 23]. The metabolism of these arsenic-containing compounds and waste products produced by birds and mammals consuming them is still uncertain at present and what the global environmental impact might be.
3. CELLULAR ACTIONS OF ARSENIC-CONTAINING COMPOUNDS
3.1. Inhibitors of energy metabolism: effects on glycolysis and oxidative phosphorylation
At the biochemical level, inorganic arsenic in the pentavalent state (As(V), arsenate, AsO4) resembles a phosphate (PO4) group in structure and it can replace phosphate in many reactions. The mitochondria is a major intracellular site where arsenate is metabolised, taken up As(V), rapidly reducing it, and exporting the As(III) product back into the cytosol [27]. The specific location of the site(s) for arsenic reduction in the mitochondria has not yet been defined. However, arsenate can affect oxidative phosphorylation by binding to the Fo/F1 ATP synthase [28]. Arsenate can be used by the ATP synthase more efficiently than phosphate depending on the Ca2+ levels [29], producing ADP-arsenate which unlike ATP becomes rapidly hydrolysed and unable to form stable high-energy compounds [30]. It is suggested that the structure and charge similarities of PO4 3−, AsO4 3−, and SO4 2− result in indiscriminate binding to at least two sites located in the mitochondrial matrix [31]. At one site, occupation by any of these three anions results in protection against uncoupling of the mitochondrial proton gradient by K+; at the second site, in the Fo/F1 ATP synthase, AsO4 3−, and SO4 2− compete for binding against PO4 3−, leading to the inhibition of ATP production [31].
Intriguingly, As2O3, whilst shown to have no effect on oxidative phosphorylation levels in HeLa [10 μM] and AS-30D [100 μM] hepatoma cancer cells, significantly inhibited glycolysis, particularly during the exponential growth phase of these cells when they were actively respiring and producing 70% of their ATP from mitochondria [32]. Several other studies have also shown that arsenic compounds do not affect oxidative phosphorylation. Thus, with submitochondrial particles in the presence of an ATP regenerating system, 20 mM arsenate had no effect on NADH formation, ATP hydrolysis, and Pi ↔ H20 exchange [33]. In a related study with isolated liver mitochondria, As2O3 was again shown to have no effect on oxygen consumption, or the respiratory control ratio at a concentration (10 μM) found to be maximally effective in promoting apoptosis in whole cells [34]. However, at higher concentrations (> 50 μM), pyruvate/malate-supported respiration (via complex I) became blocked, but there was no effect on either complex II or IV. The inhibitory effect on complex I was reversed by the addition of the reducing agent, dithiothreitol, indicating that direct oxidative damage was involved. In addition, it was shown that even with the block in complex I, the cells continued to maintain cellular ATP levels through glycolysis, and hence, depletion of cellular ATP was not the cause for the cytotoxicity of As2O3 [34]. Probably the most definitive evidence for the importance of mitochondria in mediating As2O3 killing of cancer cells comes from studying cells lacking mitochondrial function [35]. A subclone of mitochondrial respiration deficient cells was derived from the HL-60 human leukaemia cell line by growth in the presence of ethidium bromide to mutate the mitochondrial genome, and these cells are known as HL-60 “ρ 0” cells [35]. Due to the lack of mitochondrial respiration, ρ 0cells depend on glycolysis for their energy source and, as would be expected, produced substantially less superoxide radicals (∼20% of the control cells). When these ρ 0 cells were incubated with ∼10 μM As2O3, they were resistant to the drug, revealing that mitochondrial respiratory function is required for the cytotoxic actions of As2O3 [35].
The inhibition of glycolysis by arsenic-based drugs appears unlikely to be a significant factor involved in the drug-induced killing of cancer cells. Thus, incubating cells in glucose-deficient medium to block glycolysis had no significant effect on the As2O3 − [30 μM] mediated levels of cell death in the Jurkat cell line [36]. However, when glycolysis was blocked and mitochondrial respiration inhibited using oligomycin A, the cells became very sensitive to As2O3− mediated cell death. In this regard, it is also worth noting that studies of individual glycolytic enzymes analysed in purified form in vitro have shown arsenite and arsenate to be relatively weak inhibitors. Hence, for hexokinase (IC50: 15mM for arsenate [37, 38]), phosphofructokinase (IC50 > 5 mM for arsenite; [39]) and pyruvate dehydrogenase (PDH IC50: 80–120 μM arsenite; [40]), relatively large concentrations were required to inhibit these enzymes. In fact, arsenate has been shown to stimulate the activities of the two important glycolytic enzymes, hexokinase [37] and GAPDH [41], by overcoming product inhibition in these reactions. In the case of GAPDH, arsenate acts catalytically to promote the oxidation of phosphoglycerate [42] and the reaction involved the formation of an arsenate analogue of the phosphate ester as an intermediate which rapidly hydrolysed, helping to drive the reaction forward [33, 43]. This process has been commonly described in relation to the effects of arsenate on numerous enzymatic reactions involving phosphate and has been termed “arsenolysis” [33].
Given the relative insensitivity to the direct effects of arsenic compounds shown by the glycolytic pathway, it follows that it is unlikely that glycolytic inhibition results from direct binding and modification of the enzymes in this pathway by arsenic-based drugs. More likely, the inhibition of glycolysis results from an indirect effect, caused by the actions of arsenic compounds in modifying mitochondrial respiration leading to production of ROS which then acts to inhibit the glycolytic enzymes. Additional support for the mitochondrial-mediated ROS involvement in the action of arsenic to inhibit glycolysis comes from studies where As2O3 was found to be ∼38 times more potent in cells than in the pure preparation at inhibiting pyruvate dehydrogenase [40]. Also, inhibiting mitochondrial respiration suppressed the resulting inhibition of pyruvate dehydrogenase activity and H2O2 production by this drug. Furthermore, the inhibition of pyruvate dehydrogenase by As2O3 was shown to require the Fenton reaction occurring via hydroxyl radical intermediates [40]. The mitochondrial effects of arsenic compounds are detailed later in this review and to reiterate at this point, the evidence indicates that the actions of arsenic compounds on glycolysis are not the main cause for the cytotoxic effects of these drugs at clinically relevant concentrations (1–6 μM) required in plasma for the killing of cancer cells in APL patients [8, 44].
3.2. Interconversion of As(III) ↔ As(V) in cells
Under conditions of high mitochondrial respiration inside cells, it is possible that trivalent arsenicals inducing significant production of ROS as superoxide, peroxide, and hydroxyl radicals can also result in oxidation to produce arsenate (AsO4 3−) ionic species [45, 46]. Consequently, the impact that arsenic compounds will have on any given cell will most likely depend on the state of cellular respiration and production of ROS affecting the arsenic speciation and whether the cell is dependant on glycolysis versus mitochondrial respiration for its ATP synthesis. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), the glycolytic enzyme abundantly found in all cells and especially blood cells and liver, is a major intracellular arsenate reductase [47] requiring GSH, NAD, and glycolytic substrate [48, 49]. Given that the levels and specific activity of GAPDH is much higher in malignant cells than in normal cells [50], this could contribute to the rapid reduction of As(V) species in their cytosol into more toxic As(III) forms.
In the GAPDH reaction, As(V) reduction may take place during, or as a consequence of the arsenolytic cleavage of the thioester bond formed between the enzyme's Cys149 residue and the 3-phosphoglyceroyl moiety of the substrate. Hydrolysis of 1-arseno-3-phosphoglycerate is at least 2000 times faster than hydrolysis of the normal substrate 1,3-diphosphoglycerate under the same conditions [51]. Hence, GAPDH is proposed as one of the key cellular converting enzymes for reducing As(V) to As(III). Although purine nucleoside phosphorylase was proposed to be an arsenate reductase [52], this was later refuted [53]. The other major class of As(V) reductases in cells are the glutathione S transferases and of these, the omega form or GSTO1 appears to be most important. Thus, GSTO1 can reduce arsenate to arsenite, MMA(V) to MMA(III), and DMA(V) to DMA(III) and deletion of the GSTO1 gene in mice reduced the extent of biotransformation by 30–80% in most tissues examined [54].
3.3. Structure and reactivity of arsenic-containing compounds with reduced thiols
Arsenic has a high affinity for sulfur and hence, reactive sulfur-containing molecules such as reduced thiols with an available sulfur atom have a significant propensity for binding to arsenic [55, 56]. As(III)-containing compounds exist as trigonal pyramidal structures and this is also the structure formed upon binding of arsenic ions to cellular proteins in vivo where the sulphur atoms of thiolate groups act as coordinating ligands. The resulting arsenic-thiol linkages are mainly responsible for the ability of arsenic to modulate the function of various key molecules, enzymes, and ion transporters inside cells and this intracellular action of arsenic is discussed in detail in this section. Arsenic-containing compounds react with mono- and dithiols, particularly the latter when two thiols are located in close proximity, acting to cross-link the thiols together.
Some debate exists about the structures and speciation of arsenic-containing compounds (both inorganic and organic forms) in solution. In the absence of sulfide, As(III) hydroxide complexes are the major arsenic-containing species and these structures probably adopt a trigonal pyramidal structure with the arsenic atom at the apex [57–59]. This trigonal pyramidal structure provides the potential for As(III) to coordinate linkages with several proximal thiol groups. This is believed to be the case in bacterial enzyme systems such as the ArsR repressor protein where it is likely to bind three Cys atoms [60]. Thus, in the more toxic form as the trivalent state (As(III)) inorganic and organic (methylated) arsenic reacts with critical thiols in proteins, inhibiting their function, as is the case with the bacterial ArsR protein. For example, As(III) was shown to target the reactive sulfhydryl group at the active site of thiolase(s) involved in ketogenesis from acetyl Coenzyme A [61].
The pentavalent species of inorganic arsenate (AsO4 3−) favoured to exist in oxidised environments, as well as organic forms of As(V) have a different structure with a trigonal-bipyramidal shape where the As(V) atom is located at the centre, co-ordinating to three equatorial and two polar atoms [62, 63]. In comparing the two different structural states of As(III) and As(V), it is not yet clear why the trivalent methylarsenic-containing compounds show a much higher toxicity than their pentavalent analogues [24, 64]. However, it could relate to cellular uptake as trivalent organoarsenic compounds are more membrane permeable than the pentavalent species [65]. Also, trivalent arsenic bonded at a phenyl ring is able to form much more stable covalent cross-links to cysteine residues compared to arsenic in small molecules such as arsenious acid or arsenite [66]. Furthermore, the organic trivalent arsenic-containing compound, phenylarsine oxide [0.1–0.5 μM], is much more potent than the simple arsenite [1–10 μM] in its cytotoxic activity in APL cells [67].
One major drawback with phenylarsine oxide as a potential cancer therapy is its high toxicity in vivo and its nonselectivity for cancer versus normal cells, resulting in cytotoxicity in normal endothelial cells in the same concentration range (0.2 μM) [68]. Thus, phenylarsine oxide, is precluded from application in clinical cancer therapy, without being further modified as a drug. The greater reactivity of phenylarsene oxide and associated cytotoxicity is in agreement with the results outlined above [66] where mass spectrometric analysis of different complexes of peptides and proteins with arsenic-containing species revealed that inorganic arsenite or arsenates did not interact well with cysteine or glutathione, whereas the organic phenylarsine (3+) oxide did. In addition, three different phenyl arsenic acids and dimethylarsinic acid that all contained As(V) also formed complexes with glutathione [66]. Hence, the bulky hydrophobic groups with electron withdrawing π orbitals (in the case of phenyl groups) may promote more stable bonds between the arsenic atom and sulfur groups inside cells, modulating a larger range of enzymes and proteins with important functions for maintaining cell viability.
In cells, the most common reactive species that are available for interaction with arsenic are the abundant free thiol moieties in the tripeptide glutathione (γGlu-Cys-Gly, GSH) and the free amino acid, cysteine. Thus, arsenic-based drugs can react by coordinating binding to free (reduced) thiol groups such as those on cysteine, particularly those of thioredoxin and glutathione as the major intracellular thiol species important in cellular redox regulation. It was observed early on that arsenite and phenylarsine oxide in particular, but not arsenate, reacted with vicinal thiol groups on proteins [69, 70]. Since demonstrating that phenylarsine oxide was particularly effective at cross-linking vicinal thiols in the active site of tyrosine phosphatases [71], the range of proteins -containing vicinal cysteine residues with which phenylarsine oxide reacts is increasing. Recent examples include the small GTP binding Rho protein family [72] and the mitochondrial carnitine/acylcarnitine transporter [73]. Phenylarsine oxide, as a strong inhibitor of tyrosine phosphatases would increase tyrosine phosphorylation levels of enzymes in cellular growth signalling pathways. Whether the inhibition of tyrosine phosphatases and other enzymes contributes to the toxic effects of arsenic-containing compounds in normal cells is not clear, but is likely an important contributing factor to its general cytotoxicity making phenylarsine oxide unsuitable for cancer therapy.
Analysis of the interactions of As(III) with glutathione or cysteine in vitro in aqueous solutions by equilibrium binding and use of biophysical techniques including NMR, electronic spectroscopy, and potentiometry revealed that As(III) binds either of glutathione or cysteine with similar equilibrium constants [74]. However, several analytical studies by mass spectroscopy have revealed that arsenate and arsenite do not complex readily with glutathione or cysteine, but prefer to react with the thiols on reduced thioredoxin molecules [reviewed in [66]]. This was confirmed by analysis of more biologically relevant samples from the intracellular environment of HeLa cells where cytosolic thioredoxin 1 (TRX1) and, in particular thioredoxin 2 (TRX2) in the mitochondria, was shown to be highly reactive with arsenite (10 μM) whereas little reactivity was detected with cellular GSH/GSSG [75].
Studies with thioredoxin reductase (TxR) purified from mouse liver showed that arsenic-containing compounds with As(III) and arsinothiols (complexes of As(III) with GSH or L-cysteine) were extremely potent inhibitors of this enzyme [24]. Methylarsenic(III)oxide was most potent with a Ki∼100 nM, as an irreversible competitive inhibitor. The effects on purified glutathione reductase (GR) showed that the levels of inhibition were not as marked with inorganic As(III) and As(V) oxides showing IC50s in the 10–50 mM range, whereas for methylarsinic(III)oxide it was ∼9 μM. Studies on this enzyme in whole cells as hepatocytes showed the IC50 to be reduced to ∼3 μM for methylarsinic(III) oxide and for As2O3 > 100 μM [24]. Hence, these observations strongly support a role for the components thioredoxins and the thioredoxin reductase system as providing cellular targets that are very sensitive to inhibition by arsenic-based drugs in the low micromolar range. It also explains the importance of studying the chemistry of arsenic-containing compounds in the context of both purified enzymes as well as whole cells as biological systems in order to obtain meaningful results. Thus, although the GSH/glutathione transferase system undoubtedly plays an important role in arsenic sensitivity of cells (see below in Section 4 for details), it would appear that the thioredoxin system might represent the most immediate point of sensitive reactivity in relation to cytotoxicity.
3.4. The major mechanism of action of arsenic-containing compounds: modifying mitochondrial function and redox regulation of the production of reactive oxygen species
Mitochondria are a main source of ROS in cells (reviewed in [76]). Thioredoxin (TRX), NADPH, and thioredoxin reductase (TxR) comprise the thioredoxin system that has multiple functions in cells including in redox signalling via interactions with other proteins, in transcriptional regulation, control of the reduced intracellular redox environment, cell growth, defense against oxidative stress and control of apoptosis (reviewed in [77]). As outlined in the previous section, the thioredoxin system is very sensitive to arsenic-based drugs and may well be the basis for one of the important mechanisms for their actions in inducing cancer cell death. The TRX system operates as a thiol-disulfide exchange reaction (see Figure 1). TRX1 and TRX2 are key regulatory isozymes that catalyse the reduction of protein disulfide bonds. They are cofactors of the apoptosis signal-regulating kinase 1 (ASK1) that mediates TNF cytokine and oxidative stress-induced apoptosis via the mitochondrial dependent pathway [78].
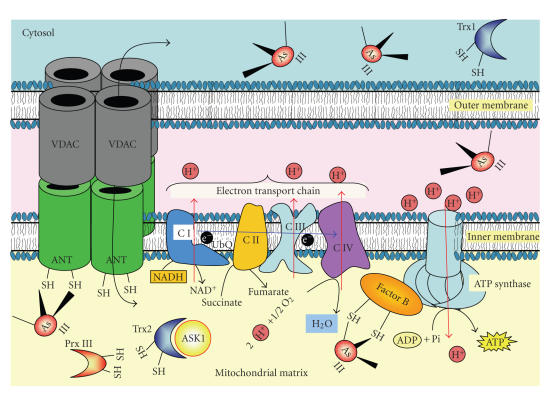
Figure 1.
Many vicinal thiol-containing redox proteins are major mitochondrial targets for binding of arsenic-containing compounds. Arsenite and particularly organic arsenites will disrupt the normal redox systems functioning in the mitochondrial matrix and intermembrane space by targeting vicinal thiols in proteins and enzymes that regulate these systems. Such enzymes include Peroxiredoxin III (Prx III), Thioredoxin 2 (Trx2). In addition, several key mitochondrial functions are affected, including Factor B regulating the ATP synthetase activity, the adenine nucleotide transporter, ANT, amongst others. See text for further detail.
In their reduced forms, cytosolic TRX1 and mitochondrial TRX2 each contain two vicinal thiol groups in their active site sequence as –C–G–P–C–. TRX 1 in the cytosol and TRX2 in mitochondria bind to Cys 250 and Cys 30, respectively, in the regulatory N-terminal domain of ASK1 and can cooperatively maintain the enzyme in the inhibited state. However, activation by TNF resulting in increased production of ROS and leads to the oxidation of the TRX dithiol group to a disulfide. Under these conditions, the thioredoxins no longer bind to ASK1 and loss of TRX2 binding to mitochondrial-located ASK1 can lead to apoptosis in a JNK-independent manner, whereas cytosolic ASK1 upon loss of TRX1 binding then becomes activated as a MAPKKK resulting in JNK activation, Bid cleavage and Bax translocation to the mitochondria [78]. Since it is known that the thioredoxins are major targets of arsenic-containing compounds (see above and [75]), it can be predicted that arsenite-mediated oxidative binding to thioredoxins will induce a similar outcome as TNF signalling, leading to the release and activation of ASK1 and induction of apoptosis.
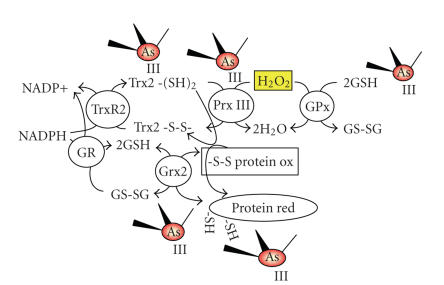
Another thioredoxin-associated protein of importance in thiol-mediated redox regulation in mitochondria is thioredoxin peroxidase II (TPX-II, also known as peroxiredoxin III, Prx-III), an enzyme abundantly expressed in the mitochondria of cancer cells that protects the cells from oxidative stress [79, 80]. PrxIII is an important antioxidant that acts in conjunction with TRX2/TXR (Figure 1) in the mitochondria to remove peroxides such as H2O2 and offset the apoptosis inducing effects of increased levels of H2O2. However, PrxIII contains three Cys residues, two of which are involved as redox-active sites in the formation of a stable intersubunit disulfide-bonded dimer, which is then reduced by thioredoxin to the monomer. PrxIII was a more abundantly expressed arsenic-binding protein when comparing arsenic resistant cells to normal cells by phenylarsine oxide affinity chromatography [81]. Hence, PrxIII is very likely to be another protein whose function is inhibited by arsenic-containing compounds leading to the promotion of apoptosis.
Increasingly, it is becoming apparent that dithiols-containing redox proteins, particularly those present in the mitochondria, act as controlling sensors during responses to changes in cellular redox. Many thiol redox proteins contain a vicinal pair or more of reactive thiol groups [82, 83] capable of binding with arsenic-containing compounds in a similar manner to those in the thioredoxin system. The most important of these are located in the mitochondria, a point of extreme sensitivity to arsenic-containing compounds whose actions culminate in triggering the apoptotic pathways via the induction of reactive oxygen species, leading to the killing of the cancer cells (Figures 1, 2). For example, two members of the glutaredoxin (GRX) family, including GRX2 located primarily in the mitochondria, catalyse GSH-dependent TRX-disulfide redox and protein thiol-disulfide redox reactions, particularly reversible glutathionylation of protein sulfhydryl groups [84]. Human Grx1 and Grx2 contain C–P–Y–C and C–S–Y–C active sites ; have three and two additional structural Cys residues, respectively; and are therefore likely to react with arsenic-containing compounds, although no reports of this could be found.
Figure 2.
Mitochondrial redox systems regulating ROS levels via thiol-disulfide exchange/coupling reactions. The mitochondrial form of Thioredoxin (Trx2) is likely to play the major role in reducing disulfides formed by vicinal thiols in both the mitochondrial Peroxiredoxin III (Prx III) and other proteins. Prx III is one of the main ways by which cancer cells can reduce their levels of H2O2 built up during active respiration. The glutathione redox system comprising GSH/GSSG, glutathione reductase, glutaredoxin, and glutathione peroxidase, although present in the mitochondria, is more likely to only become of major importance during the more extreme conditions of oxidative stress. Both of these systems are targets for inhibition by arsenic-containing compounds. See text for further detail.
Another mitochondrial protein targeted by arsenic-containing compounds with a vicinal dithiol group is regulatory protein “Factor B.” Addition of recombinant factor B back to bovine submitochondrial particles depleted of this protein restored energy coupling activity. Thus, reverse electron transfer from succinate to complex I enabling NAD+ reduction, electron transport chain function and oxidative phosphorylation/32Pi-ATP exchange of the ATP synthetase complex were reactivated [85] as was increased exchange activity of complex V [86]. Thus, the F0-F1 ATPase activity requires Factor B coupled to it for full activation. However, factor B contains six thiols and Cys 92 and Cys 94 in the bovine form were shown to bind phenylarsine oxide [87] and phenylarsine oxide or arsenite inhibit factor B coupling activity [88]. From all of these studies, it is becoming clear that redox changes to vicinal thiols affect the regulation of mitochondrial function and that these thiols are also major targets for inhibition by the arsenic-containing compounds (Figures 1, 2).
4. THE ADENINE NUCLEOTIDE TRANSPORTER (ANT): A CRITICAL TARGET OF ARSENIC-CONTAINING COMPOUNDS IN THE MITOCHONDRIA
A channel formed by the association of two proteins, the voltage dependent anion channel (VDAC) in the outer mitochondrial membrane and the adenine nucleotide transporter (ANT) in the inner mitochondrial membrane (Figure 1), is a complex involved in the induction of apoptosis activated via the mitochondrial pathway (see [89], Figure 3). The two components of this complex form part of the mitochondrial permeability transition pore (MPTP), a megachannel mediating release of molecules from the mitochondria activating apoptosis. A rapidly increased permeability of the inner mitochondrial membrane (mitochondrial permeability transition) leads to apoptosis that is mediated by the MPTP.
Arsenite induces apoptosis by a direct effect on the MPTP [90, 91] and VDAC has been shown to play an essential role in opening of the permeability pore and cytochrome c release induced by arsenic trioxide, which also caused VDAC to homodimerise [92]. In addition, the thiol-reactive compound 4,4’-diisothiocyanostilbene-2,2’-disulfonate (DIDS) has been shown to block the VDAC channel [93] and inhibit ROS-mediated cytochrome c release by VDAC [94]. Hence, the data indicates that VDAC contains critical Cys residues that can undergo intermolecular cross-links mediated by reaction with arsenic.
Analysis of ion channel activity of purified ANT-containing lipid bilayers also revealed that As2O3 treatment [30 μM] altered the ANT channel electrophysiological properties [36]. Interestingly, glutathione depletion leading to increased ROS may play an important role in the action of arsenic trioxide [91]. Whereas in both normal and cancer cells, glutathione S transferase (GST) is found to interact with ANT, during the induction phase of apoptosis, GST dissociates from ANT suggesting that GST/GSH may act as a repressor of MPTP and ANT pore opening [91, 95]. This is supported by the observation that increasing the expression of the enzyme GSTP1 in Jurkat and Raji leukemic cells renders them more resistant to arsenic trioxide-induced apoptosis at clinically relevant levels [1-2 μM]. GSTP1 expression in these cells is also accompanied by accumulation of lower levels of H2O2 production [95].
Both of the mammalian proteins, VDAC and ANT, contain two or more Cys residues in their structure thereby providing reactive thiol groups whose modification affects their function. It has been established that the redox state of thiol reactive groups are important for activation of the mitochondrial permeability transition [94, 96, 97]. Consequently, thiol cross-linkers such as DIDS, diamide, and phenylarsine oxide [20–100 μM] affect VDAC and ANT channel function and activation of mitochondrial permeability transition [93, 94, 96–98]. Many published results, such as cross-linking experiments, protein/inhibitor stoichiometry, chimeric dimers, analytical ultracentrifugation, or neutron scattering, indicate that the ANT carrier acts as a dimer and this or higher oligomers are involved in membrane permeability transition [99, 100].
Facing out into the mitochondrial matrix, ANT has three exposed loop regions containing a conserved repeat structure with one Cys residue in each loop. These Cys residues are important to the process of ANT dimerisation, but it is not clear how this operates and whether the Cys residues form intermolecular disulfide bonds or not [101]. Nevertheless, copper-o-phenanthroline is able to dimerise ANT by intermolecular cross-linking of Cys 56 (in the first matrix loop) [98]. In addition, phenylarsine oxide, eosin 5-maleimide, and diamide form intramolecular cross-links between Cys 160 and Cys 257 on the other two matrix loops, restricting ANT in the open conformation, promoting mitochondrial permeability transition [98]. Arsenic trioxide is much weaker than phenylarsine oxide at binding to the ANT Cys residues [96, 97] and this may explain the greater sensitivity exhibited by APL cells to phenylarsine oxide [IC50: 0.1 μM] than to As2O3 [IC50: 4 μM] [67].
Single thiol interacting compounds such as N-ethylmaleimide (NEM) can inhibit the mitochondrial permeability transition and this could be either the result of direct interaction with the key Cys residues on the matrix loops of ANT or indirectly via reaction with GSH and thereby preventing GSH from being oxidized and catalysing disulfide bridging between the adjacent thiol groups in the ANT loops [98]. NEM or monobromobimane, in the 25–50 μM range, preferentially react with GSH, leading to its modification in mitochondria and thereby prevents GSH from being oxidised. As a result, NEM inhibits mitochondrial permeability transition activation by the thiol reactive compounds, diamide or t-butylhydroperoxide, implying a role for GSSG in the action of these agents on the permeability transition [96, 97]. Arsenites, albeit that much higher concentrations would be required given their lower affinity for glutathione interaction, could have a similar action. Thus, high levels of arsenites could modify and inhibit glutathione redox control such that glutathione-based enzymes are unable to function, as well as directly mediating disulfide cross-linking of ANT, leading to increases in cellular ROS production, MPTP, and apoptosis. However, given their low reactivity with glutathione systems, this appears to be unlikely as opposed to the indirect action via the mitochondrial effects leading to increased ROS production which then reduces cellular GSH levels.
The multidrug resistance (MDR) protein MRP1/ABCC1 has been shown to transport AsIII out of cells as a tri-GSH conjugate (As-(GSH)3), and glutathione S-transferase (GST) probably facilitates the process [102]. This is likely to be part of the normal cell and cancer cell resistance mechanisms against the cytotoxic effects of arsenic-based compounds. GSH-depleted cells are more sensitive to killing by arsenic-containing compounds [103] and transfection of cells to express glutathione S transferase protects them from arsenite inducing death by promoting arsenite transport from the cells and decreasing ROS levels [104, 105]. In support of this proposal, the long-term exposure of cells to arsenic-containing compounds induced increased expression of glutathione S transferase and MRPs [106]. Arsenic levels do not attain very high levels in blood plasma of patients, rapidly becoming eliminated [8, 44, 107]. This removal probably results from efficient uptake of As-(GSH)3 via MRP2 in the proximal tubules of the kidneys as part of the detoxification process during the excretion of arsenic-based drugs predominantly into the urine [108, 109]. The remainder is mostly removed via uptake in the liver and secretion as bile [110–112]. As-(GSH)3 may be an important part of the metabolic process for converting inorganic As(III) to methylated species during detoxification in the liver by ASMT1/Cyt19 [113].
5. MODIFIED ORGANIC ARSENIC STRUCTURES WITH INCREASED POTENCY AS ANTINEOPLASTIC AGENTS
Arsenic-containing compounds substituted with organic groups such as modified phenylarsine oxides have been synthesized and examined for their cytotoxic effect on human leukemic cells and breast cancer cells in culture. Some of these compounds were found to exhibit potent cytotoxic anticancer activity, particularly against human breast cancer and leukemic cell lines, including primary leukemia cells, at micromolar concentrations. One of these compounds, the novel glutathionyl peptide trivalent arsenic-containing compound para 4-[N-(S-glutathionylacetyl)amino]phenylarsenoxide (p-GSAO) shows promise as a novel antineoplastic drug and is now in clinical trials. P-GSAO, like phenylarsine oxide, inactivates ANT-mediated ATP/ADP transport and triggers Ca2+-dependent MPTP opening by cross-linking the critical Cys residues of ANT. This leads to increased production of cellular ROS, ATP depletion, mitochondrial depolarization, and apoptosis of angiogenic endothelial cells and inhibition of tumor growth in mice with no apparent toxicity [114]. However, the action of p-GSAO was indirect, and did not appear to be as a result of selective tumor cell toxicity. Rather, p-GSAO inhibited the proliferating, but not growth-quiescent endothelial cells in vitro and angiogenesis in vivo and thus acted to eliminate tumors by blocking their blood supply [114, 115]. The trivalent arsenic-containing moiety of p-GSAO was shown to cross-link the matrix facing Cys160 and Cys257 thiols of ANT [114] and effectively locks ANT into the open configuration. Inactivation of ANT by p-GSAO causes an increase in superoxide levels, proliferation arrest, ATP depletion, mitochondrial depolarization, and apoptosis in the dividing endothelial cells.
It is likely that the arsenic-containing moiety of p-GSAO reacts similarly as does arsenite (see above) with one or two molecules of glutathione before it is removed from the cell by MRPs [116]. Tumor cells export p-GSAO much more efficiently than endothelial cells because they have higher MRP1 or MRP2 activity and cellular glutathione levels [116] and this may explain why p-GSAO is not highly effective at inhibiting tumor cell growth in vivo. In addition, the greater water soluble properties of p-GSAO than other arsenic-containing compounds, particularly organic species should help to retain p-GSAO in the intravascular system where it is more likely to affect endothelial cells and inhibit tumor angiogenesis.
Interestingly, although the para form of GSAO revealed no apparent toxicity in treated animals and inhibited tumor growth leading to phase I clinical trials as an anticancer agent [114], the ortho form (o-GSAO) was toxic and this was proposed to result from increased accumulation of the drug in cells, including normal cells due to loss of multidrug resistance efflux [116]. Consequently, o-GSAO is unlikely to be of much further interest as an antineoplastic agent, whereas the clinical efficacy of p-GSAO is eagerly awaited.
6. TARGETING OF CANCER CELLS: SELECTIVE UPTAKE AND DELIVERY INTO SPECIFIC TYPES OF CANCER CELLS
As(III) as the anhydrous form of As(OH)3 (Trisenox, Cell Therapeutics, Seattle, Wash, USA) received FDA approval in 2000 as a chemotherapeutic agent for the treatment of APL [117]. Acute promyelocytic leukemia (APL) is associated with reciprocal and balanced chromosomal translocations always involving the retinoic acid receptor alpha (RARalpha) gene on chromosome 17 and variable partner genes on distinct chromosomes. RARalpha fuses to the promyeloctyic leukemia (PML) gene in the majority of APL cases (reviewed in [118]). Arsenic trioxide is particularly effective at killing APL cells and this was proposed to be the direct result of its ability to induce the relocalization and degradation of the nuclear body protein PML, as well as the degradation of PML-RARalpha in APL cells [119–122]. However, this seems unlikely to be the main mechanism of action for arsenic trioxide given that no differences in sensitivity to growth inhibition and killing by apoptosis have been observed between wild-type and PML−/− cells [123].
Arsenic trioxide as a single agent has provided 86% complete hematologic remission with minimal toxicity in APL patients [124], equal to any of the current standards of care for treating APL, including the combination of all-trans retinoic acid (ATRA) plus chemotherapy [125]. This raises the question why APL cells are very sensitive to arsenic-containing compounds like arsenic trioxide. It would appear that the reason is because APL cells express the transmembrane transporter protein, aquaglyceroporin 9 (AQP9) involved in arsenic uptake [126] at much higher levels in APL cells than in other leukemic cell types and that correlates with arsenite sensitivity [127]. In this regard, it is worth noting that aquaglyceroporins AQP7 and AQP9 are present in normal cell types. Interestingly, AQP9 is primarily expressed in human lung, liver, and leukocytes [128] and this may help explain arsenic toxicity, given that liver is one of the main organs affected. The fact that AQP9 provides APL cancer cell specificity with high response rates suggests that if arsenic-containing compounds could be targeted for specific delivery into cancer cells, then they would represent outstanding agents for killing these cells. However, further modifications will be required to provide suitable drug targeting for improved delivery of arsenic-containing compounds to cancer cells.
7. CONCLUSIONS
Vicinal thiols located in key enzymes and proteins provide targets for reaction with arsenic-containing compounds, particularly organic derivatives such as phenylarsenic-containing compounds that favour intramolecular cross-linking between adjacent thiols. Intriguingly, most of the key intracellular targets for this reaction have been identified to include the main REDOX regulatory systems in the mitochondria, including thioredoxin and peroxiredoxin systems and the adenine nucleotide transporter, all of whose function is adversely affected. The net result is the activation of several independent pathways including ROS production to facilitate the induction of apoptosis. One main pathway operates via the opening of the MPTP, the other via activation of ASK1 kinase, and the JNK/Bid/Bax pathway of channel formation in MOM. In the case of APL, cell selectivity for sensitive responses to these drugs is facilitated by selective transport systems such as provided by the AQP9 transporter. Low MDR levels present in dividing endothelial cells also provides selective targeting by specially substituted phenylarsenic-containing compounds like p-GSAO, leading to decreased blood supply into tumors, with some toxicity to cancer cells, but little toxicity on normal cells. Hence, a combination of selective delivery and retention provides the necessary targeting of arsenic-containing compounds to tumors and provides scope for additional modifications to be made to enhance the antineoplastic activity of arsenic-containing compounds, given their range of actions and efficiency in killing cancer cells.
ACKNOWLEDGMENT
The author would like to thank Professor R. K. Ralph for helpful comments and editing of the manuscript.
References
- 1.Saha JC, Dikshit AK, Bandyopadhyay M, Saha KC. A review of arsenic poisoning and its effects on human health. Critical Reviews in Environmental Science and Technology. 1999;29:281–313. [Google Scholar]
- 2.Roy P, Saha A. Metabolism and toxicity of arsenic: a human carcinogen. Current Science. 2002;82(1):38–45. [Google Scholar]
- 3.Connelly NG, Damhus T, Hartshorn RM, Hutton AT, editors. Nomenclature of Inorganic Chemistry—IUPAC Recommendations. Cambridge, UK: The Royal Society of Chemistry; 2005. [Google Scholar]
- 4.International Agency for Research on Cancer IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Some Drinking-water Disinfectants and Contaminants, Including Arsenic. 2004. vol. 84. [PMC free article] [PubMed]
- 5.Lubin JH, Beane Freeman LE, Cantor KP. Inorganic arsenic in drinking water: an evolving public health concern. Journal of the National Cancer Institute. 2007;99(12):906–907. doi: 10.1093/jnci/djm012. [DOI] [PubMed] [Google Scholar]
- 6.Bredfeldt TG, Jagadish B, Eblin KE, Mash EA, Gandolfi AJ. Monomethylarsonous acid induces transformation of human bladder cells. Toxicology and Applied Pharmacology. 2006;216(1):69–79. doi: 10.1016/j.taap.2006.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-Biological Interactions. 2006;160(1):1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Lu D-P, Qiu J-Y, Jiang B, et al. Tetra-arsenic tetra-sulfide for the treatment of acute promyelocytic leukemia: a pilot report. Blood. 2002;99(9):3136–3143. doi: 10.1182/blood.v99.9.3136. [DOI] [PubMed] [Google Scholar]
- 9.Chou W-C, Dang CV. Acute promyelocytic leukemia: recent advances in therapy and molecular basis of response to arsenic therapies. Current Opinion in Hematology. 2005;12(1):1–6. doi: 10.1097/01.moh.0000148552.93303.45. [DOI] [PubMed] [Google Scholar]
- 10.Hu J, Fang J, Dong Y, Chen SJ, Chen Z. Arsenic in cancer therapy. Anti-Cancer Drugs. 2005;16(2):119–127. doi: 10.1097/00001813-200502000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Amadori S, Fenaux P, Ludwig H, O'Dwyer M, Sanz M. Use of arsenic trioxide in haematological malignancies: insight into the clinical development of a novel agent. Current Medical Research and Opinion. 2005;21(3):403–411. doi: 10.1185/030079904X20349. [DOI] [PubMed] [Google Scholar]
- 12.Douer D, Tallman MS. Arsenic trioxide: new clinical experience with an old medication in hematologic malignancies. Journal of Clinical Oncology. 2005;23(10):2396–2410. doi: 10.1200/JCO.2005.10.217. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson JF, Gavis J. Review of the arsenic cycle in natural waters. Water Research. 1972;6(11):1259–1274. [Google Scholar]
- 14.Dooley CT, Dore TM, Hanson GT, Jackson WC, Remington SJ, Tsien RY. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. The Journal of Biological Chemistry. 2004;279(21):22284–22293. doi: 10.1074/jbc.M312847200. [DOI] [PubMed] [Google Scholar]
- 15.Thomas DJ, Li J, Waters SB, et al. Arsenic (+3 oxidation state) methyltransferase and the methylation of arsenicals. Experimental Biology and Medicine. 2007;232(1):3–13. [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen SM, Arnold LL, Eldan M, Lewis AS, Beck BD. Methylated arsenicals: the implications of metabolism and carcinogenicity studies in rodents to human risk assessment. Critical Reviews in Toxicology. 2006;36(2):99–133. doi: 10.1080/10408440500534230. [DOI] [PubMed] [Google Scholar]
- 17.Vahter M, Marafante E. Intracellular interaction and metabolic fate of arsenite and arsenate in mice and rabbits. Chemico-Biological Interactions. 1983;47(1):29–44. doi: 10.1016/0009-2797(83)90145-x. [DOI] [PubMed] [Google Scholar]
- 18.Wang T-C, Jan K-Y, Wang ASS, Gurr J-R. Trivalent arsenicals induce lipid peroxidation, protein carbonylation, and oxidative DNA damage in human urothelial cells. Mutation Research. 2007;615(1-2):75–86. doi: 10.1016/j.mrfmmm.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Sakurai T, Kojima C, Kobayashi Y, et al. Toxicity of a trivalent organic arsenic compound, dimethylarsinous glutathione in a rat liver cell line (TRL 1215) British Journal of Pharmacology. 2006;149(7):888–897. doi: 10.1038/sj.bjp.0706899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su P-F, Hu Y-J, Ho I-C, Cheng Y-M, Lee T-C. Distinct gene expression profiles in immortalized human urothelial cells exposed to inorganic arsenite and its methylated trivalent metabolites. Environmental Health Perspectives. 2006;114(3):394–403. doi: 10.1289/ehp.8174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eblin KE, Bredfeldt TG, Buffington S, Gandolfi AJ. Mitogenic signal transduction caused by monomethylarsonous acid in human bladder cells: role in arsenic-induced carcinogenesis. Toxicological Sciences. 2007;95(2):321–330. doi: 10.1093/toxsci/kfl160. [DOI] [PubMed] [Google Scholar]
- 22.Jackson BP, Bertsch PM, Cabrera ML, Camberato JJ, Seaman JC, Wood CW. Trace element speciation in poultry litter. Journal of Environmental Quality. 2003;32(2):535–540. doi: 10.2134/jeq2003.5350. [DOI] [PubMed] [Google Scholar]
- 23.Jones FT. A broad view of arsenic. Poultry Science. 2007;86(1):2–14. doi: 10.1093/ps/86.1.2. [DOI] [PubMed] [Google Scholar]
- 24.Thomas DJ, Styblo M, Lin S. The cellular metabolism and systemic toxicity of arsenic. Toxicology and Applied Pharmacology. 2001;176(2):127–144. doi: 10.1006/taap.2001.9258. [DOI] [PubMed] [Google Scholar]
- 25.Petrick JS, Ayala-Fierro F, Cullen WR, Carter DE, Vasken Aposhian H. Monomethylarsonous acid (MMAIII) is more toxic than arsenite in Chang human hepatocytes. Toxicology and Applied Pharmacology. 2000;163(2):203–207. doi: 10.1006/taap.1999.8872. [DOI] [PubMed] [Google Scholar]
- 26.Petrick JS, Jagadish B, Mash EA, Vasken Aposhian H. Monomethylarsonous acid ((MMAIII) and arsenite: LD50 in hamsters and in vitro inhibition of pyruvate dehydrogenase. Chemical Research in Toxicology. 2001;14(6):651–656. doi: 10.1021/tx000264z. [DOI] [PubMed] [Google Scholar]
- 27.Németi B, Gregus Z. Mitochondria work as reactors in reducing arsenate to arsenite. Toxicology and Applied Pharmacology. 2002;182(3):208–218. doi: 10.1006/taap.2002.9443. [DOI] [PubMed] [Google Scholar]
- 28.Zharova TV, Vinogradov AD. Energy-linked binding of Pi is required for continuous steady-state proton-translocating ATP hydrolysis catalyzed by F0.F1 ATP synthase. Biochemistry. 2006;45(48):14552–14558. doi: 10.1021/bi061520v. [DOI] [PubMed] [Google Scholar]
- 29.Moreno-Sanchez R. Contribution of the translocator of adenine nucleotides and the ATP synthase to the control of oxidative phosphorylation and arsenylation in liver mitochondria. The Journal of Biological Chemistry. 1985;260(23):12554–12560. [PubMed] [Google Scholar]
- 30.Moore SA, Moennich DM, Gresser MJ. Synthesis and hydrolysis of ADP-arsenate by beef heart submitochondrial particles. The Journal of Biological Chemistry. 1983;258(10):6266–6271. [PubMed] [Google Scholar]
- 31.Cortés P, Castrejón V, Sampedro JG, Uribe S. Interactions of arsenate, sulfate and phosphate with yeast mitochondria. Biochimica et Biophysica Acta. 2000;1456(2-3):67–76. doi: 10.1016/s0005-2728(99)00109-7. [DOI] [PubMed] [Google Scholar]
- 32.Rodríguez-Enríquez S, Vital-González PA, Flores-Rodríguez FL, Marín-Hernández A, Ruiz-Azuara L, Moreno-Sánchez R. Control of cellular proliferation by modulation of oxidative phosphorylation in human and rodent fast-growing tumor cells. Toxicology and Applied Pharmacology. 2006;215(2):208–217. doi: 10.1016/j.taap.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 33.DeMaster EG, Mitchell RA. A comparison of arsenate and vanadate as inhibitors or uncouplers of mitochondrial and glycolytic energy metabolism. Biochemistry. 1973;12(19):3616–3621. doi: 10.1021/bi00743a007. [DOI] [PubMed] [Google Scholar]
- 34.Nutt LK, Gogvadze V, Uthaisang W, Mirnikjoo B, McConkey DJ, Orrenius S. Indirect effects of Bax and Bak initiate the mitochondrial alterations that lead to cytochrome c release during arsenic trioxide-induced apoptosis. Cancer Biology and Therapy. 2005;4(4):459–467. doi: 10.4161/cbt.4.4.1652. [DOI] [PubMed] [Google Scholar]
- 35.Pelicano H, Feng L, Zhou Y, et al. Inhibition of mitochondrial respiration: a novel strategy to enhance drug-induced apoptosis in human leukemia cells by a reactive oxygen species-mediated mechanism. The Journal of Biological Chemistry. 2003;278(39):37832–37839. doi: 10.1074/jbc.M301546200. [DOI] [PubMed] [Google Scholar]
- 36.Belzacq A-S, El Hamel C, Vieira HLA, et al. Adenine nucleotide translocator mediates the mitochondrial membrane permeabilization induced by lonidamine, arsenite and CD437. Oncogene. 2001;20(52):7579–7587. doi: 10.1038/sj.onc.1204953. [DOI] [PubMed] [Google Scholar]
- 37.White TK, Wilson JE. Isolation and characterization of the discrete N- and C-terminal halves of rat brain hexokinase: retention of full catalytic activity in the isolated C-terminal half. Archives of Biochemistry and Biophysics. 1989;274(2):375–393. doi: 10.1016/0003-9861(89)90451-7. [DOI] [PubMed] [Google Scholar]
- 38.White TK, Wilson JE. Binding of nucleoside triphosphates, inorganic phosphate, and other polyanionic ligands to the N-terminal region of rat brain hexokinase: relationship to regulation of hexokinase activity by antagonistic interactions between glucose 6-phosphate and inorganic phosphate. Archives of Biochemistry and Biophysics. 1990;277(1):26–34. doi: 10.1016/0003-9861(90)90545-a. [DOI] [PubMed] [Google Scholar]
- 39.Poon R, Chu I. Effects of potassium antimony tartrate on rat erythrocyte phosphofructokinase activity. Journal of Biochemical and Molecular Toxicology. 1998;12(4):227–233. doi: 10.1002/(sici)1099-0461(1998)12:4<227::aid-jbt5>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 40.Samikkannu T, Chen C-H, Yih L-H, et al. Reactive oxygen species are involved in arsenic trioxide inhibition of pyruvate dehydrogenase activity. Chemical Research in Toxicology. 2003;16(3):409–414. doi: 10.1021/tx025615j. [DOI] [PubMed] [Google Scholar]
- 41.Dagher SM, Deal WC., Jr. Glyceraldehyde-3-phosphate dehydrogenase from pig liver. Methods in Enzymology. 1982;89, part D:310–316. doi: 10.1016/s0076-6879(82)89056-3. [DOI] [PubMed] [Google Scholar]
- 42.Warburg O, Christian W. Isolierung und Kristallisation des Proteins des oxydierenden Garungsferments. Biochemis-. che Zeitschrift. 1939;303:40–68. [Google Scholar]
- 43.Slocum DH, Varner JE. Transfer of O18 in arsenolysis reactions. The Journal of Biological Chemistry. 1960;235(2):492–495. [PubMed] [Google Scholar]
- 44.Shen Z-X, Chen G-Q, Ni J-H, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (AFL)—II: clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89(9):3354–3360. [PubMed] [Google Scholar]
- 45.Eblin KE, Bowen ME, Cromey DW, et al. Arsenite and monomethylarsonous acid generate oxidative stress response in human bladder cell culture. Toxicology and Applied Pharmacology. 2006;217(1):7–14. doi: 10.1016/j.taap.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Pettine M, Campanella L, Millero FJ. Arsenite oxidation by H2O2 in aqueous solutions. Geochimica et Cosmochimica Acta. 1999;63(18):2727–2735. [Google Scholar]
- 47.Németi B, Csanaky I, Gregus Z. Effect of an inactivator of glyceraldehyde-3-phosphate dehydrogenase, a fortuitous arsenate reductase, on disposition of arsenate in rats. Toxicological Sciences. 2006;90(1):49–60. doi: 10.1093/toxsci/kfj058. [DOI] [PubMed] [Google Scholar]
- 48.Németi B, Gregus Z. Reduction of arsenate to arsenite by human erythrocyte lysate and rat liver cytosol—characterization of a glutathione- and NAD-dependent arsenate reduction linked to glycolysis. Toxicological Sciences. 2005;85(2):847–858. doi: 10.1093/toxsci/kfi157. [DOI] [PubMed] [Google Scholar]
- 49.Gregus Z, Németi B. The glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase works as an arsenate reductase in human red blood cells and rat liver cytosol. Toxicological Sciences. 2005;85(2):859–869. doi: 10.1093/toxsci/kfi158. [DOI] [PubMed] [Google Scholar]
- 50.Bagui S, Ray M, Ray S. Glyceraldehyde-3-phosphate dehydrogenase from Ehrlich ascites carcinoma cells: its possible role in the high glycolysis of malignant cells. European Journal of Biochemistry. 1999;262(2):386–395. doi: 10.1046/j.1432-1327.1999.00384.x. [DOI] [PubMed] [Google Scholar]
- 51.Byers LD, She HS, Alayoff A. Interaction of phosphate analogues with glyceraldehyde-3-phosphate dehydrogenase. Biochemistry. 1979;18(12):2471–2480. doi: 10.1021/bi00579a006. [DOI] [PubMed] [Google Scholar]
- 52.Gregus Z, Németi B. Purine nucleoside phosphorylase as a cytosolic arsenate reductase. Toxicological Sciences. 2002;70(1):13–19. doi: 10.1093/toxsci/70.1.13. [DOI] [PubMed] [Google Scholar]
- 53.Németi B, Gregus Z. Glutathione-dependent reduction of arsenate in human erythrocytes—a process independent of purine nucleoside phosphorylase. Toxicological Sciences. 2004;82(2):419–428. doi: 10.1093/toxsci/kfh301. [DOI] [PubMed] [Google Scholar]
- 54.Chowdhury UK, Zakharyan RA, Hernandez A, Avram MD, Kopplin MJ, Vasken Aposhian H. Glutathione-S-transferase-omega [MMA(V) reductase] knockout mice: enzyme and arsenic species concentrations in tissues after arsenate administration. Toxicology and Applied Pharmacology. 2006;216(3):446–457. doi: 10.1016/j.taap.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 55.Miller WH, Jr., Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Research. 2002;62(14):3893–3903. [PubMed] [Google Scholar]
- 56.Stauder S, Raue B, Sacher F. Thioarsenates in sulfidic waters. Environmental Science and Technology. 2005;39(16):5933–5939. doi: 10.1021/es048034k. [DOI] [PubMed] [Google Scholar]
- 57.Pokrovski G, Gout R, Schott J, Zotov A, Harrichoury J-C. Thermodynamic properties and stoichiometry of As (III) hydroxide complexes at hydrothermal conditions. Geochimica et Cosmochimica Acta. 1996;60(5):737–749. [Google Scholar]
- 58.Tossell JA. Theoretical studies on arsenic oxide and hydroxide species in minerals and in aqueous solution. Geochimica et Cosmochimica Acta. 1997;61(8):1613–1623. [Google Scholar]
- 59.Minyaev RM, Minkin VI. Unusual low-barrier inversion of the trigonal-pyramidal bond configuration of the arsenic atom. Doklady Chemistry. 2000;375(4–6):277–280. [Google Scholar]
- 60.Shi W, Dong J, Scott RA, Ksenzenko MY, Rosen BP. The role of arsenic-thiol interactions in metalloregulation of the ars operon. The Journal of Biological Chemistry. 1996;271(16):9291–9297. doi: 10.1074/jbc.271.16.9291. [DOI] [PubMed] [Google Scholar]
- 61.Rein KA, Borrebaek B, Bremer J. Arsenite inhibits β-oxidation in isolated rat liver mitochondria. Biochimica et Biophysica Acta. 1979;574(3):487–494. doi: 10.1016/0005-2760(79)90245-5. [DOI] [PubMed] [Google Scholar]
- 62.Brill TB, Long GG. Studies of pentavalent organoarsenic, -antimony, and -bismuth halide compounds by nuclear quadrupole resonance spectroscopy. Inorganic Chemistry. 1970;9(9):1980–1985. [Google Scholar]
- 63.Myneni SCB, Traina SJ, Waychunas GA, Logan TJ. Experimental and theoretical vibrational spectroscopic evaluation of arsenate coordination in aqueous solutions, solids, and at mineral-water interfaces. Geochimica et Cosmochimica Acta. 1998;62(19-20):3285–3300. [Google Scholar]
- 64.Hughes MF. Arsenic toxicity and potential mechanisms of action. Toxicology Letters. 2002;133(1):1–16. doi: 10.1016/s0378-4274(02)00084-x. [DOI] [PubMed] [Google Scholar]
- 65.Dopp E, Hartmann LM, Florea A-M, et al. Uptake of inorganic and organic derivatives of arsenic associated with induced cytotoxic and genotoxic effects in Chinese hamster ovary (CHO) cells. Toxicology and Applied Pharmacology. 2004;201(2):156–165. doi: 10.1016/j.taap.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 66.Schmidt A-C, Koppelt J, Neustadt M, Otto M. Mass spectrometric evidence for different complexes of peptides and proteins with arsenic(III), arsenic(V), copper(II), and zinc(II) species. Rapid Communications in Mass Spectrometry. 2006;21(2):153–163. doi: 10.1002/rcm.2823. [DOI] [PubMed] [Google Scholar]
- 67.Sahara N, Takeshita A, Kobayashi M, et al. Phenylarsine oxide (PAO) more intensely induces apoptosis in acute promyelocytic leukemia and As2O3-resistant APL cell lines than As2O3 by activating the mitochondrial pathway. Leukemia and Lymphoma. 2004;45(5):987–995. doi: 10.1080/10428190310001617222. [DOI] [PubMed] [Google Scholar]
- 68.Hirano S, Kobayashi Y, Hayakawa T, et al. Accumulation and toxicity of monophenyl arsenicals in rat endothelial cells. Archives of Toxicology. 2005;79(1):54–61. doi: 10.1007/s00204-004-0598-4. [DOI] [PubMed] [Google Scholar]
- 69.Robert Adamson S, Robinson JA, Stevenson KJ. Inhibition of pyruvate dehydrogenase multienzyme complex from Escherichia coli with a radiolabeled bifunctional arsenoxide: evidence for an essential histidine residue at the active site of lipoamide dehydrogenase. Biochemistry. 1984;23(6):1269–1274. doi: 10.1021/bi00301a039. [DOI] [PubMed] [Google Scholar]
- 70.Knowles FC. Reactions of lipoamide dehydrogenase and glutathione reductase with arsonic acids and arsonous acids. Archives of Biochemistry and Biophysics. 1985;242(1):1–10. doi: 10.1016/0003-9861(85)90472-2. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Z-Y, Davis JP, Van Etten RL. Covalent modification and active site-directed inactivation of a low molecular weight phosphotyrosyl protein phosphatase. Biochemistry. 1992;31(6):1701–1711. doi: 10.1021/bi00121a018. [DOI] [PubMed] [Google Scholar]
- 72.Gerhard R, John H, Aktories K, Just I. Thiol-modifying phenylarsine oxide inhibits guanine nucleotide binding of Rho but not of Rac GTPases. Molecular Pharmacology. 2003;63(6):1349–1355. doi: 10.1124/mol.63.6.1349. [DOI] [PubMed] [Google Scholar]
- 73.Tonazzi A, Giangregorio N, Indiveri C, Palmieri F. Identification by site-directed mutagenesis and chemical modification of three vicinal cysteine residues in rat mitochondrial carnitine/acylcarnitine transporter. The Journal of Biological Chemistry. 2005;280(20):19607–19612. doi: 10.1074/jbc.M411181200. [DOI] [PubMed] [Google Scholar]
- 74.Rey NA, Howarth OW, Pereira-Maia EC. Equilibrium characterization of the As(III)-cysteine and the As(III)-glutathione systems in aqueous solution. Journal of Inorganic Biochemistry. 2004;98(6):1151–1159. doi: 10.1016/j.jinorgbio.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 75.Hansen JM, Zhang H, Jones DP. Differential oxidation of thioredoxin-1, thioredoxin-2, and glutathione by metal ions. Free Radical Biology and Medicine. 2006;40(1):138–145. doi: 10.1016/j.freeradbiomed.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 76.Andreyev AYu, Kushnareva YuE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry. 2005;70(2):200–214. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- 77.Arnér ES, Holmgren A. The thioredoxin system in cancer. Seminars in Cancer Biology. 2006;16(6):420–426. doi: 10.1016/j.semcancer.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 78.Zhang R, Al-Lamki R, Bai L, et al. Thioredoxin-2 inhibits mitochondria-located ASK1-mediated apoptosis in a JNK-independent manner. Circulation Research. 2004;94(11):1483–1491. doi: 10.1161/01.RES.0000130525.37646.a7. [DOI] [PubMed] [Google Scholar]
- 79.Nonn L, Berggren M, Powis G. Increased expression of mitochondrial peroxiredoxin-3 (thioredoxin peroxidase-2) protects cancer cells against hypoxia and drug-induced hydrogen peroxide-dependent apoptosis. Molecular Cancer Research. 2003;1(9):682–689. [PubMed] [Google Scholar]
- 80.Chang T-S, Cho C-S, Park S, Yu S, Kang SW, Rhee SG. Peroxiredoxin III, a mitochondrion-specific peroxidase, regulates apoptotic signaling by mitochondria. The Journal of Biological Chemistry. 2004;279(40):41975–41984. doi: 10.1074/jbc.M407707200. [DOI] [PubMed] [Google Scholar]
- 81.Chang KN, Lee TC, Tam MF, et al. Identification of galectin I and thioredoxin peroxidase II as two arsenic-binding proteins in Chinese hamster ovary cells. Biochemical Journal. 2003;371(2):495–503. doi: 10.1042/BJ20021354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fomenko DE, Gladyshev VN. Identity and functions of CxxC-derived motifs. Biochemistry. 2003;42(38):11214–11225. doi: 10.1021/bi034459s. [DOI] [PubMed] [Google Scholar]
- 83.Fomenko DE, Xing W, Adair BM, Thomas DJ, Gladyshev VN. High-throughput identification of catalytic redox-active cystein residues. Science. 2007;315(5810):387–389. doi: 10.1126/science.1133114. [DOI] [PubMed] [Google Scholar]
- 84.Berndt C, Lillig CH, Holmgren A. Thiol-based mechanisms of the thioredoxin and glutaredoxin systems: implications for diseases in the cardiovascular system. American Journal of Physiology—Heart and Circulatory Physiology. 2007;292(3):H1227–H1236. doi: 10.1152/ajpheart.01162.2006. [DOI] [PubMed] [Google Scholar]
- 85.Belogrudov GI, Hatefi Y. Factor B and the mitochondrial ATP synthase complex. The Journal of Biological Chemistry. 2002;277(8):6097–6103. doi: 10.1074/jbc.M111256200. [DOI] [PubMed] [Google Scholar]
- 86.Belogrudov GI. Factor B is essential for ATP synthesis by mitochondria. Archives of Biochemistry and Biophysics. 2002;406(2):271–274. doi: 10.1016/s0003-9861(02)00431-9. [DOI] [PubMed] [Google Scholar]
- 87.Belogrudov GI. Bovine factor B: cloning, expression, and characterization. Archives of Biochemistry and Biophysics. 2006;451(1):68–78. doi: 10.1016/j.abb.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 88.Joshi S, Hughes JB. Inhibition of coupling factor B activity by cadmium ion, arsenite-2,3-dimercaptopropanol, and phenylarsine oxide, and preferential reactivation by dithiols. The Journal of Biological Chemistry. 1981;256(21):11112–11116. [PubMed] [Google Scholar]
- 89.Ralph SJ, Low P, Dong L, Lawen A, Neuzil J. Mitocans: mitochondrial targeted anti-cancer drugs as improved therapies and related patent documents. Recent Patents on Anti-Cancer Drug Discovery. 2006;1(3):327–346. doi: 10.2174/157489206778776952. [DOI] [PubMed] [Google Scholar]
- 90.Larochette N, Decaudin D, Jacotot E, et al. Arsenite induces apoptosis via a direct effect on the mitochondrial permeability transition pore. Experimental Cell Research. 1999;249(2):413–421. doi: 10.1006/excr.1999.4519. [DOI] [PubMed] [Google Scholar]
- 91.Verrier F, Deniaud A, LeBras M, et al. Dynamic evolution of the adenine nucleotide translocase interactome during chemotherapy-induced apoptosis. Oncogene. 2004;23(49):8049–8064. doi: 10.1038/sj.onc.1208001. [DOI] [PubMed] [Google Scholar]
- 92.Zheng Y, Shi Y, Tian C, et al. Essential role of the voltage-dependent anion channel (VDAC) in mitochondrial permeability transition pore opening and cytochrome c release induced by arsenic trioxide. Oncogene. 2004;23(6):1239–1247. doi: 10.1038/sj.onc.1207205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shafir I, Feng W, Shoshan-Barmataz V. Voltage-dependent anion channel proteins in synaptosomes of the Torpedo electric organ: immunolocalization, purification, and characterization. Journal of Bioenergetics and Biomembranes. 1998;30(5):499–510. doi: 10.1023/a:1020598315287. [DOI] [PubMed] [Google Scholar]
- 94.Madesh M, Hajnóczky G. VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. The Journal of Cell Biology. 2001;155(6):1003–1016. doi: 10.1083/jcb.200105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou L, Jing Y, Styblo M, Chen Z, Waxman S. Glutathione-S-transferase π inhibits As2O3-induced apoptosis in lymphoma cells: involvement of hydrogen peroxide catabolism. Blood. 2005;105(3):1198–1203. doi: 10.1182/blood-2003-12-4299. [DOI] [PubMed] [Google Scholar]
- 96.Costantini P, Chernyak BV, Petronilli V, Bernardi P. Modulation of the mitochondrial permeability transition pore by pyridine nucleotides and dithiol oxidation at two separate sites. The Journal of Biological Chemistry. 1996;271(12):6746–6751. doi: 10.1074/jbc.271.12.6746. [DOI] [PubMed] [Google Scholar]
- 97.Costantini P, Belzacq A-S, Vieira HLA, et al. Oxidation of a critical thiol residue of the adenine nucleotide translocator enforces Bcl-2-independent permeability transition pore opening and apoptosis. Oncogene. 2000;19(2):307–314. doi: 10.1038/sj.onc.1203299. [DOI] [PubMed] [Google Scholar]
- 98.McStay GP, Clarke SJ, Halestrap AP. Role of critical thiol groups on the matrix surface of the adenine nucleotide translocase in the mechanism of the mitochondrial permeability transition pore. Biochemical Journal. 2002;367(2):541–548. doi: 10.1042/BJ20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nury H, Dahout-Gonzalez C, Trézéguet V, Lauquin GJ-M, Brandolin G, Pebay-Peyroula E. Relations between structure and function of the mitochondrial ADP/ATP carrier. Annual Review of Biochemistry. 2006;75:713–741. doi: 10.1146/annurev.biochem.75.103004.142747. [DOI] [PubMed] [Google Scholar]
- 100.Dahout-Gonzalez C, Nury H, Trézéguet V, Lauquin GJ-M, Pebay-Peyroula E, Brandolin G. Molecular, functional, and pathological aspects of the mitochondrial ADP/ATP carrier. Physiology. 2006;21(4):242–249. doi: 10.1152/physiol.00005.2006. [DOI] [PubMed] [Google Scholar]
- 101.Dyall SD, Agius SC, De Marcos Lousa C, Trézéguet V, Tokatlidis K. The dynamic dimerization of the yeast ADP/ATP carrier in the inner mitochondrial membrane is affected by conserved cysteine residues. The Journal of Biological Chemistry. 2003;278(29):26757–26764. doi: 10.1074/jbc.M302700200. [DOI] [PubMed] [Google Scholar]
- 102.Leslie EM, Haimeur A, Waalkes MP. Arsenic transport by the human multidrug resistance protein 1 (MRP1/ABCC1): evidence that a tri-glutathione conjugate is required. The Journal of Biological Chemistry. 2004;279(31):32700–32708. doi: 10.1074/jbc.M404912200. [DOI] [PubMed] [Google Scholar]
- 103.Sakurai T, Ochiai M, Kojima C, et al. Preventive mechanism of cellular glutathione in monomethylarsonic acid-induced cytolethality. Toxicology and Applied Pharmacology. 2005;206(1):54–65. doi: 10.1016/j.taap.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 104.Wang H-F, Lee T-C. Glutathione-S-transferase π facilitates the excretion of arsenic from arsenic-resistant Chinese hamster ovary cells. Biochemical and Biophysical Research Communications. 1993;192(3):1093–1099. doi: 10.1006/bbrc.1993.1529. [DOI] [PubMed] [Google Scholar]
- 105.Zhou L, Jing Y, Styblo M, Chen Z, Waxman S. Glutathione-S-transferase π inhibits As2O3-induced apoptosis in lymphoma cells: involvement of hydrogen peroxide catabolism. Blood. 2005;105(3):1198–1203. doi: 10.1182/blood-2003-12-4299. [DOI] [PubMed] [Google Scholar]
- 106.Kojima C, Qu W, Waalkes MP, Himeno S, Sakurai T. Chronic exposure to methylated arsenicals stimulates arsenic excretion pathways and induces arsenic tolerance in rat liver cells. Toxicological Sciences. 2006;91(1):70–81. doi: 10.1093/toxsci/kfj117. [DOI] [PubMed] [Google Scholar]
- 107.Hughes MF. Biomarkers of exposure: a case study with inorganic arsenic. Environmental Health Perspectives. 2006;114(11):1790–1796. doi: 10.1289/ehp.9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kala SV, Kala G, Prater CI, Sartorelli AC, Lieberman MW. Formation and urinary excretion of arsenic triglutathione and methylarsenic diglutathione. Chemical Research in Toxicology. 2004;17(2):243–249. doi: 10.1021/tx0342060. [DOI] [PubMed] [Google Scholar]
- 109.Miller DS, Shaw JR, Stanton CR, et al. MRP2 and acquired tolerance to inorganic arsenic in the kidney of killifish (Fundulus heteroclitus) Toxicological Sciences. 2007;97(1):103–110. doi: 10.1093/toxsci/kfm030. [DOI] [PubMed] [Google Scholar]
- 110.Dietrich CG, Ottenhoff R, de Waart DR, Oude Elferink RPJ. Role of MRP2 and GSH in intrahepatic cycling of toxins. Toxicology. 2001;167(1):73–81. doi: 10.1016/s0300-483x(01)00459-0. [DOI] [PubMed] [Google Scholar]
- 111.Li G-X, Pei Q-L, Gao Y, et al. Protective effects of hepatocellular canalicular conjugate export pump (MRP2) on sodium arsenite-induced hepatic dysfunction in rats. Experimental and Toxicologic Pathology. 2007;58(6):447–453. doi: 10.1016/j.etp.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 112.Kobayashi Y, Cui X, Hirano S. Stability of arsenic metabolites, arsenic triglutathione [As(GS)3] and methylarsenic diglutathione [CH3As(GS)2], in rat bile. Toxicology. 2005;211(1-2):115–123. doi: 10.1016/j.tox.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 113.Hayakawa T, Kobayashi Y, Cui X, Hirano S. A new metabolic pathway of arsenite: arsenic-glutathione complexes are substrates for human arsenic methyltransferase Cyt19. Archives of Toxicology. 2005;79(4):183–191. doi: 10.1007/s00204-004-0620-x. [DOI] [PubMed] [Google Scholar]
- 114.Don AS, Kisker O, Dilda P, et al. A peptide trivalent arsenical inhibits tumor angiogenesis by perturbing mitochondrial function in angiogenic endothelial cells. Cancer Cell. 2003;3(5):497–509. doi: 10.1016/s1535-6108(03)00109-0. [DOI] [PubMed] [Google Scholar]
- 115.Folkman J. Fundamental concepts of the angiogenic process. Current Molecular Medicine. 2003;3(7):643–651. doi: 10.2174/1566524033479465. [DOI] [PubMed] [Google Scholar]
- 116.Dilda PJ, Don AS, Tanabe KM, et al. Mechanism of selectivity of an angiogenesis inhibitor from screening a genome-wide set of Saccharomyces cerevisiae deletion strains. Journal of the National Cancer Institute. 2005;97(20):1539–1547. doi: 10.1093/jnci/dji316. [DOI] [PubMed] [Google Scholar]
- 117.Antman KH. Introduction: the history of arsenic trioxide in cancer therapy. The Oncologist. 2001;6(supplement 02):1–2. doi: 10.1634/theoncologist.6-suppl_2-1. [DOI] [PubMed] [Google Scholar]
- 118.Scaglioni PP, Pandolfi PP. The theory of APL revisited. Current Topics in Microbiology and Immunology. 2007;313:85–100. doi: 10.1007/978-3-540-34594-7_6. [DOI] [PubMed] [Google Scholar]
- 119.Chen G-Q, Zhu J, Shi X-G, et al. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RARα/PML proteins. Blood. 1996;88(3):1052–1061. [PubMed] [Google Scholar]
- 120.Zhu J, Koken MHM, Quignon F, et al. Arsenic-induced PML targeting onto nuclear bodies: implications for the treatment of acute promyelocytic leukemia. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(8):3978–3983. doi: 10.1073/pnas.94.8.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shao W, Fanelli M, Ferrara FF, et al. Arsenic trioxide as an inducer of apoptosis and loss of PML/RARα protein in acute promyelocytic leukemia cells. Journal of the National Cancer Institute. 1998;90(2):124–133. doi: 10.1093/jnci/90.2.124. [DOI] [PubMed] [Google Scholar]
- 122.Müller S, Miller WH, Jr., Dejean A. Trivalent antimonials induce degradation of the PML-RARα oncoprotein and reorganization of the promyelocytic leukemia nuclear bodies in acute promyelocytic leukemia NB4 cells. Blood. 1998;92(11):4308–4316. [PubMed] [Google Scholar]
- 123.Wang Z-G, Rivi R, Delva L, et al. Arsenic trioxide and melarsoprol induce programmed cell death in myeloid leukemia cell lines and function in a PML and PML-RARα independent manner. Blood. 1998;92(5):1497–1504. [PubMed] [Google Scholar]
- 124.Mathews V, George B, Lakshmi KM, et al. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: durable remissions with minimal toxicity. Blood. 2006;107(7):2627–2632. doi: 10.1182/blood-2005-08-3532. [DOI] [PubMed] [Google Scholar]
- 125.Douer D. ATO: the forefront of APL treatment? Blood. 2006;107(7):2588–2589. [Google Scholar]
- 126.Liu Z, Carbrey JM, Agre P, Rosen BP. Arsenic trioxide uptake by human and rat aquaglyceroporins. Biochemical and Biophysical Research Communications. 2004;316(4):1178–1185. doi: 10.1016/j.bbrc.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 127.Leung J, Pang A, Yuen W-H, Kwong Y-L, Tse EWC. Relationship of expression of aquaglyceroporin 9 with arsenic uptake and sensitivity in leukemia cells. Blood. 2007;109(2):740–746. doi: 10.1182/blood-2006-04-019588. [DOI] [PubMed] [Google Scholar]
- 128.Tsukaguchi H, Weremowicz S, Morton CC, Hediger MA. Functional and molecular characterization of the human neutral solute channel aquaporin-9. American Journal of Physiology. 1999;277(5):F685–F696. doi: 10.1152/ajprenal.1999.277.5.F685. [DOI] [PubMed] [Google Scholar]