Abstract
Background
In 2002, a poster alerted the French health authorities to the possibility that the risk of childhood leukaemia might be increased by hepatitis B vaccination. Elucidating the role of vaccination in the etiology of childhood acute leukaemia was therefore included in the objectives of an ongoing national study.
Methods
The ESCALE study was a French national population-based case-control study conducted in France in 2003 and 2004 in order to investigate the role of infectious, environmental and genetic factors in 4 childhood neoplastic diseases (leukaemia, lymphoma, neuroblastoma, and brain tumor). The controls were randomly selected from the French population and age and gender frequency matched with the cases. A total of 776 cases of acute leukaemia (91% of the eligible cases) and 1681 controls (69% of the eligible controls) were included. In a specific standardized telephone interview, which was the same for both the cases and controls, each mother was asked to read out her child’s complete vaccination record.
Results
No association between vaccination and the risk of childhood acute leukaemia (ALL or AML) was observed. No relationship between the risk of leukaemia and the type of vaccine, number of doses of each vaccine, total number of injections, total number of vaccine doses or number of early vaccinations was evidenced. No confounding factor was observed.
Conclusion
The study did not show any evidence of a role of vaccination in the etiology of childhood leukaemia.
Keywords: Adolescent; Age Distribution; Case-Control Studies; Child; Child, Preschool; Drug Administration Schedule; Female; France; epidemiology; Hepatitis B Vaccines; administration & dosage; adverse effects; immunology; Humans; Infant; Leukemia; epidemiology; immunology; virology; Leukemia, Myeloid, Acute; epidemiology; immunology; virology; Male; Precursor Cell Lymphoblastic Leukemia-Lymphoma; epidemiology; immunology; virology; Risk Assessment; methods; Sex Distribution; Vaccination; adverse effects
Introduction
In 2002, a poster alerted the French health authorities to the possibility that the risk of childhood leukaemia might be increased by hepatitis B vaccination1. The potential public health implications of such an association provided the rationale for detailed investigation in the context of the Escale national population-based case-control study of the etiology of childhood cancer. Childhood leukaemia is the most common childhood cancer worldwide. In France, the incidence is 43 cases per million people and per year, equivalent to about 470 new cases diagnosed each year1. Very few risk factors, such as high dose ionizing radiation, some chemotherapeutic regimens and some genetic syndromes (Down syndrome, Fanconi syndrome), have been identified. Current research is focusing on infectious, environmental and genetic factors which may influence the risk of childhood leukaemia. Since leukaemia is a neoplastic disease of immune cells, a relationship between immune system events, such as infection, atopic disease or vaccination, and the risk of childhood leukaemia has been suggested. This paper investigates the relationship between vaccination and the risk of the immunologic subtypes of childhood acute leukaemia (AL): acute lymphoblastic leukaemia (ALL) and acute myeloblastic leukaemia (AML).
Methods
The ESCALE study, a national, comprehensive, case-control study conducted in 2003 and 2004, addressed 4 childhood neoplastic diseases (leukaemia, lymphoma, neuroblastoma and brain tumor) and infectious, environmental and genetic potential risk factors.
Study population
Each case of acute leukaemia incident in 2003–2004 in a child aged less than 15 years, residing in France at the time of diagnosis and with no previous history of malignancy was eligible. All the childhood leukaemia cases were confirmed by bone marrow analysis. Children whose mother did not speak French or who had been adopted were not eligible. The cases’ mothers were interviewed at least 2 months after diagnosis, within 5 months on average. For ethical reasons, the mothers of children who died before the scheduled interview date were not interviewed. The leukaemia cases were recruited directly by investigators assigned to each French pediatric oncology hospital department, with the support of the French National Registry of Childhood Hematopoietic Malignancies. Out of the 948 cases of childhood acute leukaemia diagnosed in France from January 1, 2003, to December 31, 2004, 860 cases were eligible. The reasons for exclusion consisted in: absence of a biological mother (10 cases); non-French-speaking mother (29 cases); serious psychological disorders (14 cases); physician’s refusal (1 case); and death (34 cases). Finally, 776 (91%) case mothers have given their consent and been interviewed.
The controls were randomly selected from the French population using quotas, a priori determined to make the control group representative of all cancer cases in terms of age and gender. Additional quotas constrained the control group to have the same distribution as the national population in terms of number of children living in the household, conditionally to the age group. Random selection was based on a representative sample of 60,000 addresses from the French national telephone directory plus unlisted numbers, which have been randomly retrieved before dialling. Among the 50,217 phone numbers dialled, 27,633 belonged to a household and 8,500 (30.8%) to a target household. Of them, 862 hanged up before eligibility could have been checked and 5,277 suited to an already completed quota. Finally, among the 2361 eligible control mothers, 679 refused the interview and 1682 (71.2%) gave their consent and were interviewed. We then excluded one control who had a prior history of neuroblastoma, to end with a total number of 1681 controls.
Data collection
Each of the case and control biological mothers responded to a personal and standardized telephone interview lasting 40 minutes. The interview elicited data on demographic and socio-economic characteristics, parental occupational history, childhood environment, familial and personal medical history, and history of the pregnancy.
In France, the vaccination section of a child’s medical record contains a separate page for each vaccine. The healthcare professional reports the proprietary name of the vaccine and the date of vaccination on the appropriate page. For the study, each mother was asked to read out each page of the vaccination record, line by line. Each interviewer’s computer screen displayed a vaccination record template and an exhaustive list, by record page, of the proprietary names of the vaccines available. Each vaccine administered and the vaccination date were thus obtained. The mother’s history of hepatitis B (disease and vaccination) was reported in the same way.
Since combined vaccines are frequently administered, four vaccination parameters were considered: vaccine type; the number of doses of each vaccine (vaccine doses); the total number of injections; and the total number of vaccine doses received (all vaccines taken together). These variables were all treated as categorical variables. For example, 2 DTP (diphtheria + tetanus + poliomyelitis) vaccinations and 1 BCG vaccination are equivalent to 4 vaccines; 2 diphtheria vaccine doses + 2 polio vaccine doses + 2 tetanus vaccine doses + 1 BCG vaccine dose; 3 injections; and a total of 7 vaccine doses. In France, during the 6 first months of life, children usually receive 4 vaccines: BCG, diphtheria, tetanus and poliomyelitis (DTP: usually in one injection). If parents wish, additional recommended vaccines such as Haemophilus influenzae B (Hib) and pertussis vaccines (both combined with the DTP injection) and Hepatitis B vaccine may also be administered. In some contexts, such as chronic disease, the child’s physician may also recommend pneumococcal and meningococcal immunization. Pneumococcal immunization has recently been recommended for infants aged less than 2 years attending daycare. During the first 6 months of life, a child may thus receive up to 9 different vaccines, 12 injections and 24 vaccine doses (Figure 1). Vaccination is usually not scheduled for infants aged 6 months to 1 year. Even though the French immunization calendar recommends early vaccination, only four vaccines are mandatory (BCG, diphtheria, tetanus and poliomyelitis) and required for starting school at age 6 years.
Figure 1.
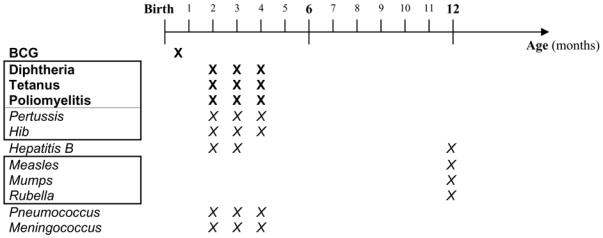
French infantile vaccination calendar (1st year of life)
Statistical analysis
The SAS® software package (version 8, Gary, North Carolina) was used for all the analyses. Non-conditional logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (CI), with adjustment for age and gender. In order to compare the different types of leukaemia, analyses were carried out using polytomous logistic regression. Age adjustment for children aged less than 2 years was conducted at intervals of 3 months. For the other age groups, adjustment complied with the stratification categories.
When the exact day of injection was not reported, a default value of 15 was allocated. When the month was not reported, the date was considered missing. Only 25 case mothers (3.2%), consisting in 18 ALL (2.9%), 5 AML (5.5%) and 2 other types of AL, and 34 control mothers (2.1%) did not manage to have their child’s immunization records for the interview.
Pre-diagnosis symptoms of childhood leukaemia such as fever, extreme fatigue or bone pain develop, at the latest, 2 or 3 months before diagnosis. As such symptoms may result in the child’s physician postponing a scheduled vaccination, the vaccination data were censored at 6 months before the reference date for cases and controls. Similarly, surgery or disease related to a malformation (38 cases and 56 controls) or genetic abnormality (14 cases and 5 controls) may result in a scheduled vaccination date being modified. In consequence, those cases and controls were excluded from analysis. Thus, out of the 776 cases and 1681 controls who were eligible, 726 cases (3 cases presented with both a malformation and a genetic abnormality) and 1620 controls were included in the analysis. When analyses concerned a given step of the vaccination calendar, only children having reached at least the corresponding age plus 6 months were included, so that both cases and controls could have completed this step. Therefore, analyses made for vaccinations before 6 months and 18 months were carried out among children at least 1 year and 2 years old, respectively.
Results
Among the 726 cases, 620 (85%) presented with acute lymphoblastic leukaemia, 91 (13%) with myeloblastic leukaemia, 11 (2%) with biphenotypic leukaemia and 4 with acute leukaemia with no immunophenotyping at the date of interview.
The cases and controls did not differ with respect to gender or the number of children living in the household (table 1). There was a small age discrepancy between the cases and controls, particularly for very young children, who were more strongly represented in the control group. The discrepancy simply reflects the fact that the cases were only a fraction of the total case population of the ESCALE study, since only the leukaemia cases are considered herein. There was at least one control for each case in each age group. Cases and controls were similar with respect to age (mean age 6,0 years for controls and 5,6 years for cases), maternal education or degree of urbanization. The paternal educational level was, however, higher for the controls.
Table 1.
Characteristics of the cases and controls
| Cases (n = 726) | Controls (n = 1620) | Control/Case ratio | p | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Gender | ns | |||||
| Male | 395 | 54.4 | 892 | 55.1 | 2.3 | |
| Age at the reference date (years) | <0.01 | |||||
| <2 | 108 | 14.9 | 362 | 22.4 | 3.4 | |
| 2 | 98 | 13.5 | 146 | 9.0 | 1.5 | |
| 3–4 | 185 | 25.5 | 293 | 18.1 | 1.6 | |
| 5–6 | 114 | 15.7 | 218 | 13.5 | 1.9 | |
| 7–8 | 74 | 10.2 | 160 | 9.9 | 2.2 | |
| 9–11 | 88 | 12.1 | 217 | 13.4 | 2.5 | |
| 12–14 | 59 | 8.1 | 224 | 13.8 | 3.8 | |
| Number of children (0–14 years) living in the household | ns | |||||
| 1 | 226 | 31.1 | 528 | 32.6 | 2.3 | |
| 2 | 321 | 44.2 | 688 | 42.5 | 2.1 | |
| ≥3 | 175 | 24.1 | 404 | 24.9 | 2.3 | |
| Missing data | 4 | 0.6 | 0 | 0.0 | ||
| Birth order | <0.01 | |||||
| 1 | 362 | 49.9 | 685 | 42.3 | 1.9 | |
| 2 | 232 | 32.0 | 589 | 36.4 | 2.5 | |
| ≥3 | 132 | 18.2 | 346 | 21.4 | 2.6 | |
| Daycare | ns | |||||
| No | 634 | 87.3 | 1418 | 87.5 | 2.2 | |
| Yes | 92 | 12.7 | 202 | 12.5 | 2.2 | |
| Maternal educational level | ns | |||||
| ≤ High school | 438 | 60.3 | 939 | 58.0 | 2.1 | |
| > High school | 288 | 39.7 | 681 | 42.0 | 2.4 | |
| Paternal educational level | 0.02 | |||||
| ≤ High school | 497 | 68.5 | 1021 | 63.0 | 2.1 | |
| > High school | 222 | 30.6 | 584 | 36.1 | 2.6 | |
| Missing data | 7 | 1.0 | 15 | 0.9 | ||
| Degree of urbanization | ns | |||||
| Rural | 226 | 31.1 | 569 | 35.1 | 2.5 | |
| Mixed | 171 | 23.6 | 367 | 22.7 | 2.1 | |
| Urban | 315 | 43.4 | 658 | 40.6 | 2.1 | |
| Missing data | 14 | 1.9 | 26 | 1.6 | ||
About 40% of the mothers reported hepatitis B immunization before pregnancy (301 case and 719 control mothers) while only 25 mothers (6 case and 19 control mothers) reported having had hepatitis B.
At age 6 months, most of the children had been immunized against tuberculosis, diphtheria, tetanus, polio, pertussis and Hib (Table 2). About one third of the children had been immunized against hepatitis B and less than 10% against Meningococcus or Pneumococcus. At age 18 months, most of the children had also been immunized against measles, mumps and rubella (Table 3).
Table 2.
Vaccines usually administered before age 6 months (children’s age > 1 year)
| Controls (n = 1434) | AL (n = 696) | ALL (n = 606) | AML (n = 76) | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| BCG | ||||
| Yes | 1309 (91.3) | 624 (89.7) | 548 (90.4) | 63 (82.9) |
| No | 94 (6.6) | 48 (6.9) | 39 (6.4) | 8 (10.5) |
| Missing data | 31 (2.2) | 24 (3.5) | 19 (3.1) | 5 (6.6) |
| Diphtheria | ||||
| Yes | 1365 (95.2) | 663 (95.3) | 578 (95.4) | 71 (93.4) |
| No | 38 (2.7) | 9 (1.3) | 9 (1.5) | 0 (0.0) |
| Missing data | 31 (2.2) | 24 (3.5) | 19 (3.1) | 5 (6.6) |
| Tetanus | ||||
| Yes | 1368 (95.4) | 664 (95.4) | 579 (95.5) | 71 (93.4) |
| No | 35 (2.4) | 8 (1.2) | 8 (1.3) | 0 (0.0) |
| Missing data | 31 (2.2) | 24 (3.5) | 19 (3.1) | 5 (6.6) |
| Poliomyelitis | ||||
| Yes | 1366 (95.3) | 665 (95.6) | 580 (95.7) | 71 (93.4) |
| No | 37 (2.6) | 7 (1.0) | 7 (1.2) | 0 (0.0) |
| Missing data | 31 (2.2) | 24 (3.5) | 19 (3.1) | 5 (6.6) |
| Pertussis | ||||
| Yes | 1325 (92.4) | 646 (92.8) | 565 (93.2) | 67 (88.2) |
| No | 78 (5.4) | 26 (3.7) | 22 (3.6) | 4 (5.3) |
| Missing data | 31 (2.2) | 24 (3.5) | 19 (3.1) | 5 (6.6) |
| Hepatitis B | ||||
| Yes | 510 (35.6) | 250 (35.9) | 211 (34.8) | 31 (40.8) |
| No | 893 (62.3) | 422 (60.6) | 376 (62.1) | 40 (52.6) |
| Missing data | 31 (2.2) | 24 (3.5) | 19 (3.1) | 5 (6.6) |
| Hib | ||||
| Yes | 1132 (78.9) | 582 (83.6) | 512 (84.5) | 58 (76.3) |
| No | 271 (18.9) | 90 (12.9) | 75 (12.4) | 13 (17.1) |
| Unknown | 31 (2.2) | 24 (3.5) | 19 (3.1) | 5 (6.6) |
| Pneumococcus | ||||
| Yes | 101 (7.0) | 30 (4.3) | 24 (4.0) | 5 (6.6) |
| No | 1302 (90.8) | 642 (92.2) | 563 (92.9) | 66 (86.8) |
| Missing data | 31 (2.2) | 24 (3.5) | 19 (3.1) | 5 (6.6) |
| Meningococcus | ||||
| Yes | 71 (5.0) | 31 (4.5) | 28 (4.6) | 2 (2.6) |
| No | 1332 (92.9) | 641 (92.1) | 559 (92.2) | 69 (90.8) |
| Missing data | 31 (2.2) | 24 (3.5) | 19 (3.1) | 5 (6.6) |
Table 3.
Vaccines usually administered before age 18 months (children’s age > 2 years)
| Controls (n = 1258) | AL (n = 618) | ALL (n = 544) | AML (n = 62) | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| Measles | ||||
| Yes | 1110 (88.2) | 541 (87.5) | 480 (88.2) | 50 (80.7) |
| No | 119 (9.5) | 55 (8.9) | 46 (8.5) | 8 (12.9) |
| Missing data | 29 (2.3) | 22 (3.6) | 18 (3.3) | 4 (6.5) |
| Mumps | ||||
| Yes | 1103 (87.7) | 539 (87.2) | 478 (87.9) | 50 (80.7) |
| No | 126 (10.0) | 57 (9.2) | 48 (8.8) | 8 (12.9) |
| Missing data | 29 (2.3) | 22 (3.6) | 18 (3.3) | 4 (6.5) |
| Rubella | ||||
| Yes | 1104 (87.8) | 540 (87.4) | 479 (88.1) | 50 (80.7) |
| No | 125 (9.9) | 56 (9.1) | 47 (8.6) | 8 (12.9) |
| Missing data | 29 (2.3) | 22 (3.6) | 18 (3.3) | 4 (6.5) |
The distribution of hepatitis B immunization was similar for the cases and controls (35.9% vs 35.6%: OR=1,0 [0.8–1.3]), for ALL (34.8% vs 35.6%: OR=1,0 [0.8–1.2]) and for AML (40.8% vs 35.6%: OR=1,3 [0.8–2.1]). The results were unchanged after adjustment for the mothers’ hepatitis B status (disease and immunization).
No association between the number of injections received and the risk of childhood leukaemia was observed (table 4). The results were the same for ALL alone and for AML alone. There was no association between the total number of vaccine doses received and the risk of acute leukaemia, ALL or AML.
Table 4.
Total number of injections and vaccine doses (children’s age > 1 year)
| Controls (n = 1434) | AL (n = 696) | OR [95%CI](*) | ALL (n = 606) | OR [95%CI] (*) | AML (n = 76) | OR [95%CI] (*) | |
|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | ||||
| Total number of injections | |||||||
| ≥10 | 446 (31.1) | 176 (25.3) | ref | 148 (24.4) | ref | 21 (27.6) | ref |
| 9, 8 or 7 | 507 (35.4) | 229 (32.9) | 1.0 [0.8–1.3] | 197 (32.5) | 1.0 [0.7–1.3] | 30 (39.5) | 1.3 [0.7–2.3] |
| 6, 5 or 4 | 380 (26.5) | 246 (35.3) | 1.3 [1.0–1.7] | 223 (36.8) | 1.4 [1.0–1.8] | 18 (23.7) | 0.9 [0.4–1.9] |
| 3. 2 or 1 | 57 (4.0) | 17 (2.4) | 0.7 [0.4–1.3] | 15 (2.5) | 0.8 [0.4–1.5] | 2 (2.6) | 0.5 [0.1–2.5] |
| 0 | 13 (0.9) | 4 (0.6) | 0.7 [0.2–2.2] | 4 (0.7) | 0.8 [0.3–2.7] | 0 (0.0) | |
| Missing data | 31 (2.2) | 24 (3.5) | 19 (3.1) | 5 (6.6) | |||
| Total number of vaccine doses | |||||||
| ≥25 | 797 (55.6) | 352 (50.6) | ref | 302 (49.8) | ref | 42 (55.3) | ref |
| 24-20 | 322 (22.5) | 197 (28.3) | 1.1 [0.9–1.5] | 181 (29.9) | 1.2 [0.9–1.5] | 12 (15.8) | 0.7 [0.4–1.5] |
| 19-15 | 180 (12.6) | 85 (12.2) | 1.1 [0.8–1.6] | 72 (11.9) | 1.2 [0.8–1.7] | 12 (15.8) | 0.9 [0.3–2.2] |
| 14-0 | 104 (7.3) | 38 (5.5) | 0.8 [0.5–1.2] | 32 (5.3) | 0.8 [0.5–1.2] | 5 (6.6) | 0.7 [0.3–2.1] |
| Missing data | 31 (2.2) | 24 (3.5) | 19 (3.1) | 5 (6.6) | |||
Non-conditional logistic regression adjusted on age and gender
A child aged 6 months should have received at least 4 injections (1 BCG and 3 DTP). An investigation to determine whether an unvaccinated or less-vaccinated status was associated with a risk of childhood leukaemia was therefore conducted. For all types of acute leukaemia taken together, there was no association between the number of injections or doses received before age 6 months and the risk of childhood leukaemia (table 5). The results were the same for ALL. An unvaccinated or less-vaccinated (1 or 2 injections) status was positively associated with a risk of acute myeloblastic leukaemia. Most of the less-vaccinated children (17/21) had not received BCG vaccine. However, this association observed with injections before 6 months was unstable for AML: the proportion of children with AML unvaccinated or less vaccinated no more differed to that of the controls when we set the threshold for early injections to 4 (31% of children with 3 or more injections in cases and 33% in controls before 4 months) or 8 months (82% of children with 3 or more injections in cases and 86% in controls before 8 months) instead of 6 months (58% of children with 3 or more injections in cases and 74% in controls before 6 months).
Table 5.
Total number of injections and vaccine doses before age 6 months (children’s age > 1 year)
| Controls (n=1434) | AL (n=696) | OR [95%CI] (*) | ALL (n=606) | OR [95%CI] (*) | AML (n=76) | OR [95%CI] (*) | |
|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | ||||
| Total number of injections before age 6 months | |||||||
| ≥4b | 684 (47.7) | 310 (44.5) | ref | 278 (45.9) | ref | 26 (34.2) | ref |
| 3 | 380 (26.5) | 207 (29.7) | 1.2 [1.0–1.6] | 185 (30.5) | 1.2 [1.0–1.6] | 18 (23.7) | 1.3 [0.7–2.4] |
| 2or1 | 266 (18.6) | 121 (17.4) | 1.1 [0.9–1.5] | 97 (16.0) | 1.0 [0.8–1.3] | 21 (27.6) | 2.3 [1.2–4.2] |
| 0 | 73 (5.1) | 34 (4.9) | 1.1 [0.7–1.7] | 27 (4.5) | 1.0 [0.6–1.6] | 6 (7.9) | 2.2 [0.9–5.7] |
| Missing data | 31 (2.2) | 24 (3.5) | 19 (3.1) | 5 (6.6) | |||
| Total number of vaccine doses before age 6 months | |||||||
| ≥10 | 1052 (73.4) | 538 (77.3) | ref | 475 (78.4) | ref | 51 (67.1) | ref |
| 1 to 9 | 278 (19.4) | 100 (14.4) | 0.9 [0.7–1.1] | 85 (14.0) | 0.8 [0.6–1.1] | 14 (18.4) | 1.1 [0.6–2.1] |
| 0 | 73 (5.1) | 34 (4.9) | 1.0 [0.6–1.5] | 27 (4.5) | 0.9 [0.6–1.4] | 6 (7.9) | 1.7 [0.7–4.2] |
| Missing data | 31 (2.2) | 24 (3.5) | 19 (3.1) | 5 (6.6) | |||
Non conditional logistic regression adjusted on age and gender
Adjustment for birth order, maternal and paternal educational level and degree of urbanization, which were related to variables such as the number of injections received and age of vaccination, did not modify any of the results for AL, ALL or AML.
Discussion
ESCALE was the first national study to include all the cases of frequent childhood neoplastic diseases (leukaemia, lymphoma, neuroblastoma and brain tumor) diagnosed in France over a two-year period (2003 – 2004). With a 5 % alpha error, the statistical power of the study was greater than 80% for most results.
The study showed no evidence of a relationship between vaccination and the risk of childhood acute leukaemia (ALL or AML), irrespective of the vaccine (including hepatitis B vaccine), total number of injections received or total number of vaccine doses received.
There was no evidence for a relationship between unvaccinated or less-vaccinated status at age 6 months and the risk of acute leukaemia or ALL.
The French National Registry of Childhood Hematopoietic Malignancies includes all the cases of childhood leukaemia diagnosed in the population of mainland France aged less than 15 years since January 1990. The exhaustiveness of the registry has been estimated to be 99.2% for leukaemia1. It is therefore probable that almost all the cases diagnosed in 2003 and 2004 were registered. Since there is no evidence for a relationship between vaccination history and the prognosis of leukaemia, the exclusion of early deaths for ethical reasons is unlikely to have induced a selection bias.
The controls were randomly selected from the overall population. The national telephone directory was used as the basis for random selection, given that the subpopulation of telephone owners is comparable to the overall population as regards most sociodemographic characteristics and lifestyle. Unlisted numbers were computer generated prior to the random selection in order to avoid selection bias. The cases and controls did not differ with respect to age (considering all types of cancer) or gender. There was no difference between the controls and overall population with regard to birth order 2–4, indicating that the quota recruitment process was successful. The controls were representative of the national population in terms of the number of children (0–14) living in the household, which was used as a proxy of birth order, in order to prevent selection bias. Birth order may be, indeed, highly sensitive to the sampling process and may also be related to a number of other variables such as early daycare, early common infections5 and compliance with the recommended vaccination program6. The control mothers’ educational level was very similar to that of the French population 2–4 but the control fathers’ educational level was higher than that of the overall population. However, the results were unchanged after adjusting for those two variables. A report on childhood vaccinal coverage 7 showed that, in France (1998), 97% of 2-year-old children had been immunized against diphtheria, poliomyelitis and tetanus, 81% against tuberculosis, 82% against measles and rubella, and 96% against mumps_compared to 95%, 90%, 88% and 88%, respectively, in the 2-year-old controls in 2003 and 2004.. The immunization status of the controls was thus very similar to that of the overall population.
The vaccination data were as objective as possible since each mother was asked to read out her child’s vaccination records line by line. Moreover, in order to prevent recall bias, responses given by mothers unable to consult the vaccination records were considered missing data. Some vaccination records may have been incompletely filled out by the children’s physicians. However, this should not have induced differential bias since record completion was independent of case/control status. A recent study has shown that children’s personal health record are usually complete 8. It is therefore likely that vaccination records are also complete. It was difficult to exclude interviewer error in data recording. In order to minimize the risk of error, particularly that associated with the emotional stress of interviewing cases’ mothers, the same standardized questionnaire was used for both the cases and controls and each interviewer interviewed both case and control mothers.
The data on vaccinations 6 months prior to the reference date were censored. However, the results remained unchanged when none of those data were censored.
Most of the cases and controls had been immunized against tuberculosis, diphtheria, tetanus, poliomyelitis, pertussis and Hib at the reference date and very few children had never been vaccinated. There were no differences between the cases and controls. The results with regard to immunization prevalence rates and the lack of association with the risk of childhood leukaemia are consistent with those of most studies9–13.
An association between hepatitis B vaccination and the risk of childhood leukaemia has been suggested in a preliminary report from the Northern California Childhood Leukaemia Study 14. The present study provided no evidence of such an association irrespective of the type of acute leukaemia, age or number of vaccine doses received. These results are consistent with the final results of the Northern California Childhood Leukaemia Study11 and with the two studies that have investigated hepatitis B vaccination and the risk of acute leukaemia 9, 11. This study is the first to include the mother’s hepatitis B status in the analysis. Maternal hepatitis B status could not, therefore, have constituted a confounding factor.
Several studies have reported an increasing negative association between the number of vaccine or combined-vaccine injections received and the risk of childhood acute leukaemia9, 11–13. Only one study of the relationship between the overall number of injections and the risk of ALL has been published 13. It showed the same increasing negative association. None of those studies addressed the relationship between the number of doses received and the risk of acute leukaemia other than ALL. In this study, there was no obvious association between the number of injections received or the number of vaccine doses received and the risk of ALL or AML.
Given that a relationship between a low number of early common infections and the risk of acute leukaemia has been suggested in several articles 5, 15–19, it was considered pertinent to address the relationship between delayed early vaccinations and the risk of childhood leukaemia. No relationship between delayed early vaccinations and the risk of ALL was detected. An association was found for AML with fewer injections before the age of 6 months, but it appeared unstable and without any trend as regards the age of injections, suggesting that this association was most probably observed by chance.
Despite the statistical power of the study (80%), no evidence of a relationship between vaccination and the risk of acute lymphoblastic or myeloblastic leukaemia was evidenced irrespective of the type of vaccine, number of doses or number of early vaccinations.
Acknowledgments
We are grateful to: Marie-Hélène Da Silva and Christophe Steffen (Inserm U170), who coordinated the recruitment of the cases; Aurélie Goubin and the staff of the French National Registry of Childhood Hematopoietic Malignancies, who contributed to case detection and verification; Sabine Mélèze and Marie-Anne Noel (Institut CSA), who coordinated the selection of the controls and the interviews; and Catherine Tricoche (Callson) and the team of interviewers, who interviewed the cases and the controls.
This work was supported by grants from Inserm, the Fondation de France, the Association pour la Recherche centre le Cancer, the Agence Française de Sécurité Sanitaire et des Produits de Santé (AFSSAPS), the Agence Française de Securité pour la Santé et l’Environnement et du travail (AFSSET) and the association, Cent pour sang la vie.
Appendix (SFCE Investigators of the ESCALE study)
| Principal Investigator | Hospital | City (France) |
|---|---|---|
| André Baruchel | Hôpital Saint-Louis | Paris |
| Hôpital Robert Debré | ||
| Catherine Behar | American Memorial Hospital | Reims |
| Claire Berger | Centre Hospitalier Universitaire | Saint-Etienne |
| Christophe Bergeron | Centre Léon Bérard | Lyon |
| Jean-Louis Bernard | Hôpital La Timone | Marseille |
| Pierre Bordigoni | Centre Hospitalier Universitaire | Nancy |
| Patrick Boutard | Centre Hospitalier Régional Universitaire | Caen |
| Gérard Couillault | Hôpital d’Enfants | Dijon |
| Lionel De Lumley | Centre Hospitalier Régional Universitaire | Limoges |
| Anne-Sophie Defachelles | Centre Oscar Lambrais | Lille |
| François Demeocq | Hôpital Hôtel-Dieu | Clermont-Ferrand |
| Alain Fisher | Hôpital des Enfants Malades | Paris |
| Virginie Gandemer | Hôpital Sud | Rennes |
| Olivier Hartmann | Institut Gustave Roussy | Villejuif |
| Jean-Pierre Lamagnere | Centre Gatien de Clocheville | Tours |
| Françoise Lapierre | Centre Hospitalier Universitaire Jean Bernard | Poitiers |
| Guy Leverger | Hôpital Armand Trousseau | Paris |
| Patrick Lutz | Hôpital de Hautepierre | Strasbourg |
| Geneviève Marguerite | Arnaud de Villeneuve | Montpellier |
| Françoise Mechinaud | Hôpital Mère et Enfants | Nantes |
| Gérard Michel | Hôpital La Timone | Marseille |
| Jean Michon | Institut Curie | Paris |
| Frédéric Millot | Centre Hospitalier Universitaire Jean Bernard | Poitiers |
| Brigitte Nelken | Hôpital Jeanne de Flandre | Lille |
| Brigitte Pautard | Centre Hospitalier Universitaire | Amiens |
| Yves Perel | Hôpital Pellegrin - le Tripode | Bordeaux |
| Yves Bertrand | Hôpital Debrousse | Lyon |
| Alain Pierre-Kahn | Hôpital des Enfants Malades | Paris |
| Emmanuel Plouvier | Centre Hospitalier Régional | Besançon |
| Xavier Rialland | Centre Hospitalier Universitaire | Angers |
| Alain Robert | Hôpital des Enfants | Toulouse |
| Hervé Rubie | Hôpital des Enfants | Toulouse |
| Nicolas Sirvent | Hôpital L’Archet | Nice |
| Christine Soler | Fondation Lenval | Nice |
| Danièle Sommelet | Centre Hospitalier Universitaire | Nancy |
| Jean-Pierre Vannier | Hôpital Charles Nicolle | Rouen |
Footnotes
* SFCE: Société Française de lutte contre les Cancers de l’Enfant et de l’Adolescent
1 Ma X, Does M, Buffler PA, Wiencke JK. Hepatitis B vaccination and the risk of childhood leukaemia. Poster session abstract 3801, American Association for Cancer Research annual meeting, San Francisco, April, 2002
References
- 1.Clavel J, Goubin A, Auclerc MF, Auvrignon A, Waterkeyn C, Patte C, Baruchel A, Leverger G, Nelken B, Philippe N, Sommelet D, Vilmer E, et al. Incidence of childhood leukaemia and non-Hodgkin’s lymphoma in France: National Registry of Childhood Leukaemia and Lymphoma, 1990–1999. Eur J Cancer Prev. 2004;13(2):97–103. doi: 10.1097/00008469-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Blondel B, Norton J, Du Mazaubrun C, Breart G. Enquête nationale périnatale 1995. 1996 [Google Scholar]
- 3.Blondel B, Norton J, Du Mazaubrun C, Breart G. Enquête nationale périnatale 1998. 2000 [Google Scholar]
- 4.Blondel B, Supernant K, Du Mazaubrun C, Breart G. Enquête nationale périnatale 2003. 2004 doi: 10.1016/s0368-2315(06)76409-2. [DOI] [PubMed] [Google Scholar]
- 5.Perrillat F, Clavel J, Auclerc MF, Baruchel A, Leverger G, Nelken B, Philippe N, Schaison G, Sommelet D, Vilmer E, Hemon D. Day-care, early common infections and childhood acute leukaemia: a multicentre French case-control study. Br J Cancer. 2002;86(7):1064–9. doi: 10.1038/sj.bjc.6600091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angelillo IF, Ricciardi G, Rossi P, Pantisano P, Langiano E, Pavia M. Mothers and vaccination: knowledge, attitudes, and behaviour in Italy. Bull World Health Organ. 1999;77(3):224–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Antona D, Badeyan G, Bussiere E, Grassullo V, Guerin N, Guignon N, Janvrin M-P, Lefur P, Levy-Bruhl D, Manigat R, Perrocheau A, Romano M-C, et al. Mesure de la couverture vaccinale en France. Bilan des outils et des méthodes en l’an 2000. InVS. 2000 [Google Scholar]
- 8.Vincelet C, Tabone MD, Berthier M, Bonnefoi MC, Chevallier B, Lemaire JP, Dommergues JP. How are personal child health records completed? A multicentric evaluation study. Arch Pediatr. 2003;10(5):403–9. doi: 10.1016/s0929-693x(03)00086-1. [DOI] [PubMed] [Google Scholar]
- 9.Dockerty JD, Skegg DC, Elwood JM, Herbison GP, Becroft DM, Lewis ME. Infections, vaccinations, and the risk of childhood leukaemia. Br J Cancer. 1999;80(9):1483–9. doi: 10.1038/sj.bjc.6690548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groves FD, Gridley G, Wacholder S, Shu XO, Robison LL, Neglia JP, Linet MS. Infant vaccinations and risk of childhood acute lymphoblastic leukaemia in the USA. Br J Cancer. 1999;81(1):175–8. doi: 10.1038/sj.bjc.6690668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma X, Does MB, Metayer C, Russo C, Wong A, Buffler PA. Vaccination history and risk of childhood leukaemia. Int J Epidemiol. 2005 doi: 10.1093/ije/dyi113. [DOI] [PubMed] [Google Scholar]
- 12.Petridou E, Trichopoulos D, Kalapothaki V, Pourtsidis A, Kogevinas M, Kalmanti M, Koliouskas D, Kosmidis H, Panagiotou JP, Piperopoulou F, Tzortzatou F. The risk profile of childhood leukaemia in Greece: a nationwide case-control study. Br J Cancer. 1997;76(9):1241–7. doi: 10.1038/bjc.1997.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuz J, Kaletsch U, Meinert R, Kaatsch P, Michaelis J. Association of childhood leukaemia with factors related to the immune system. Br J Cancer. 1999;80(3–4):585–90. doi: 10.1038/sj.bjc.6690395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma X, Does M, Buffler O, Wiencke J. Hepatitis B vaccination and risk of childhood leukemia . Poster presented at the 93rd Annual Conference of American Association for Cancer Research; 2002. [Google Scholar]
- 15.van Steensel-Moll HA, Valkenburg HA, van Zanen GE. Childhood leukemia and infectious diseases in the first year of life: a register-based case-control study. Am J Epidemiol. 1986;124(4):590–4. doi: 10.1093/oxfordjournals.aje.a114431. [DOI] [PubMed] [Google Scholar]
- 16.Neglia JP, Linet MS, Shu XO, Severson RK, Potter JD, Mertens AC, Wen W, Kersey JH, Robison LL. Patterns of infection and day care utilization and risk of childhood acute lymphoblastic leukaemia. Br J Cancer. 2000;82(1):234–40. doi: 10.1054/bjoc.1999.0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan LC, Lam TH, Li CK, Lau YL, Yuen HL, Lee CW, Ha SY, Yuen PM, Leung NK, Patheal SL, Greaves MF, Alexander FE. Is the timing of exposure to infection a major determinant of acute lymphoblastic leukaemia in Hong Kong? Paediatr Perinat Epidemiol. 2002;16(2):154–65. doi: 10.1046/j.1365-3016.2002.00406.x. [DOI] [PubMed] [Google Scholar]
- 18.Jourdan-Da Silva N, Perel Y, Mechinaud F, Plouvier E, Gandemer V, Lutz P, Vannier JP, Lamagnere JL, Margueritte G, Boutard P, Robert A, Armari C, et al. Infectious diseases in the first year of life, perinatal characteristics and childhood acute leukaemia. Br J Cancer. 2004;90(1):139–45. doi: 10.1038/sj.bjc.6601384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilham C, Peto J, Simpson J, Roman E, Eden TO, Greaves MF, Alexander FE. Day care in infancy and risk of childhood acute lymphoblastic leukaemia: findings from UK case-control study. Bmj. 2005 doi: 10.1136/bmj.38428.521042.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]


