Abstract
The ability to predict drug disposition involves concurrent consideration of many chemical and physiological variables and the effect of food on the rate and extent of availability adds further complexity due to postprandial changes in the gastrointestinal (GI) tract. A system that allows for the assessment of the multivariate interplay occurring following administration of an oral dose, in the presence or absence of meal, would greatly benefit the early stages of drug development. This is particularly true in an era when the majority of new molecular entities are highly permeable, poorly soluble, extensively metabolized compounds (BDDCS Class 2), which present the most complicated relationship in defining the impact of transporters due to the marked effects of transporter-enzyme interplay. This review evaluates the GI luminal environment by taking into account the absorption / transport / elimination interplay and evaluates the physiochemical property issues by taking into account the importance of solubility, permeability and metabolism. We concentrate on the BDDCS and its utility in predicting drug disposition. Furthermore, we focus on the effect of food on the extent of drug availability (F), which appears to follow closely what might be expected if a significant effect of high fat meals is inhibition of transporters. That is, high fat meals and lipidic excipients would be expected to have little effect on F for Class 1 drugs; they would increase F of Class 2 drugs, while decreasing F for Class 3 drugs.
Keywords: absorption, BCS, BDDCS, disposition, elimination, food effects, interplay, transporter
1. INTRODUCTION
Food-drug interactions have been widely associated with alterations of pharmacokinetic and pharmacodynamic parameters and proven to have significant clinical implications.1,2 The influence of concomitant food intake prompted the Food and Drug Administration (FDA) to issue a guidance for industry entitled, “Food-Effect Bioavailability and Fed Bioequivalence Studies.”3 As a result, it is common to find medication labeling containing language denoting that maximum effect is achieved if the drug is administered with a meal. Conversely, some drug products show a decrease in the extent of availability and decreased efficacy with meal coadministration. Of course there are very many drugs for which food-drug interactions are non-existent or negligible.
The effect of food on the extent of availability is a significant concern during drug development. Ideally, it is most advantageous if a recommendation of oral drug administration can be provided independent of meal considerations. A food-drug interaction model would be beneficial in the early stages of development when preclinical predictions could be of particular use and service to the industry. Although various in vitro and in vivo models can be found,4,5,6,7 to date no standard system exists to predict drug absorption and the effect of food.
The role of food, and its subsequent digestion, in oral drug absorption may be attributed to a myriad of variables ranging from the chemical characteristics of the drug itself to the post-prandial changes in the gastrointestinal (GI) tract. Therefore, when attempting to predict variations in pharmacokinetics it is imperative to consider not only the physiochemical properties of the drug, but the GI luminal environment as well. In this review, we evaluate the GI luminal environment by taking into account the absorption / transport / elimination interplay and we evaluate the physiochemical property issues by taking into account the importance of solubility, permeability and metabolism. Here, we concentrate on combining these aspects into a comprehensive system to predict disposition and the role of food on drug absorption.
2. THE BIOPHARMACEUTICS CLASSIFICATION SYSTEM
2.1. The BCS and the FDA
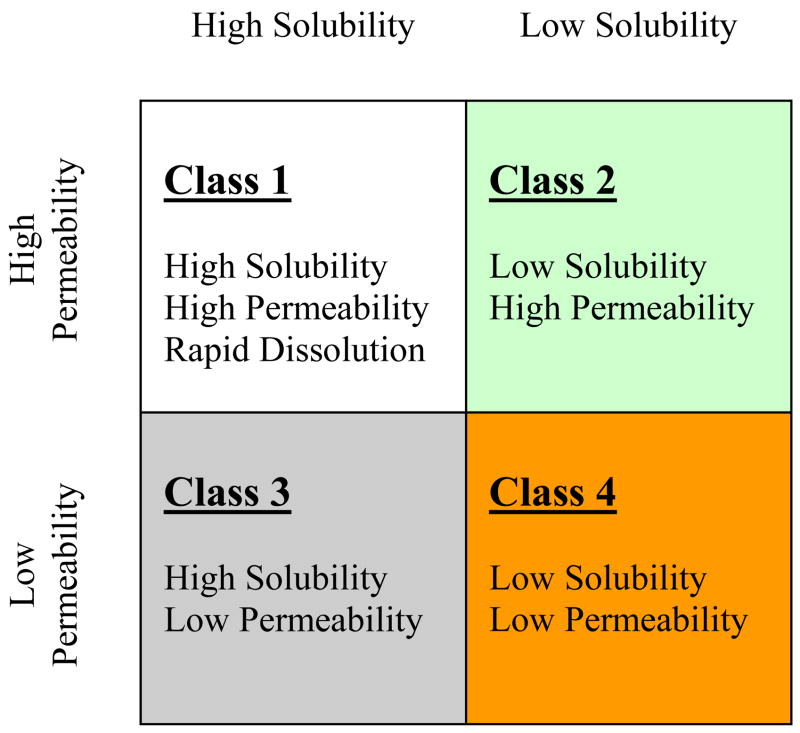
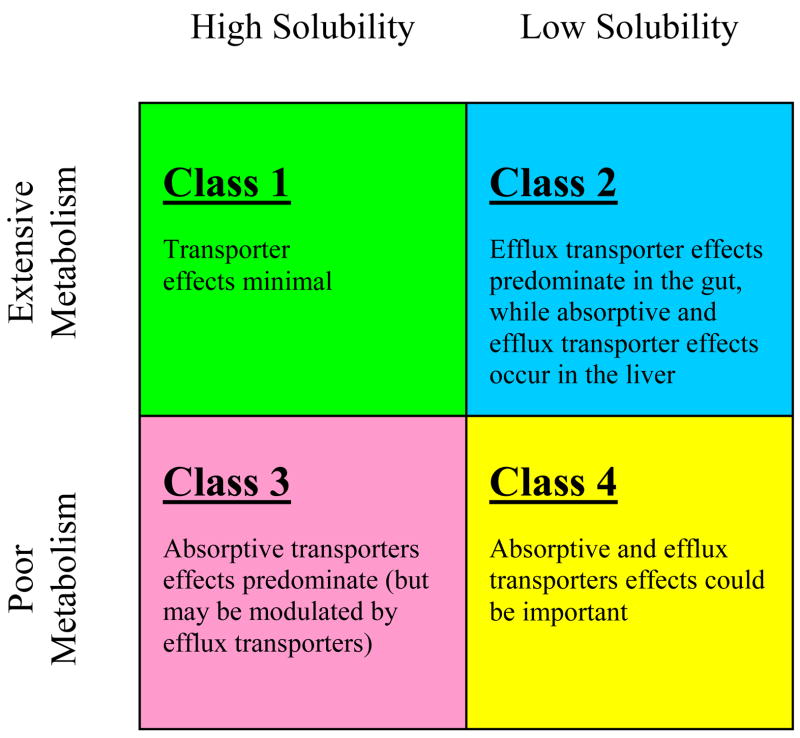
The oral absorption of a drug is fundamentally dependent on that drug’s aqueous solubility and gastrointestinal permeability. Extensive research into these fundamental parameters by Amidon et al.8 led to the Biopharmaceutics Classification System (BCS) that categorizes drugs into four groups, Class 1 – Class 4 (Fig. 1). The BCS classifies compounds based on the critical components related to oral absorption. Centrally embracing permeability and solubility, the objective of the BCS is to allow prediction of in vivo pharmacokinetic performance of drug products from in vitro measurements of permeability and solubility.
Figure 1.
The Biopharmaceutics Classification System (BCS) as defined by the FDA9 after Amidon et al.8
In 2000, the FDA promulgated the BCS as a science based approach to allow waiver of in vivo bioavailability and bioequivalence testing for immediate release solid dosage forms for Class 1 compounds, highly soluble and highly permeable drugs, when such drug products also exhibit rapid dissolution.9 In brief, bioequivalence is achieved if the generic product shows the same rate and extent of bioavailability with rate evaluated in terms of Cmax and extent measured in terms of AUC. The criteria include a 90% confidence interval around point estimates of the ratios of Cmax and AUC, test/reference, falling within 0.8–1.25 on a log normal distribution.
2.2. The Framework of the BCS
As depicted in Figure 1, the BCS sorts drugs on a scale in terms of solubility versus permeability. A drug substance is considered “highly soluble” when the highest marketed dose strength is soluble in 250 ml of aqueous media over a pH range of 1–7.5 at 37°C.9 A drug substance in considered to be “highly permeable” when the extent of absorption in humans is determined to be greater or equal to 90% of an administered dose based on a mass balance determination or in comparison to an intravenous reference dose.9 Accordingly, Class 1 compounds possess high properties of both solubility and permeability, while Class 4 compound possess low properties; Class 2 are highly permeable and poorly soluble, while Class 3 possess the opposite characteristics of poor permeability and high solubility.
The classification framework of the BCS is believed to be useful in the earliest stages of drug discovery research. Its applications improve the prediction of oral absorption and disposition of new molecular entities. In addition, the BCS has proven to be an asset to the FDA by creating a framework for which to allow a waiver of in vivo bioequivalence studies for a suitable Class 1 compounds, albeit a limited number. In 2005, Wu and Benet10 constructed a Table of 130 compounds in the four BCS classes (Table I). These compounds were predominantly gathered from available literature but judiciously edited.8,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26 Extensive research into the assembled list of compounds in the four categories has demonstrated that the BCS provides further predictive utility. Here, we focus on its application with respect to the food-drug effect.
Table I.
130 compounds categorized by BCS Class.
| High Solubility | Low Solubility | |||
|---|---|---|---|---|
| High Permeability | Class 1 | Class 2 | ||
| Abacavir | Ketoprofen | Amiodarone | Itraconazole | |
| Acetaminophen | Ketorolac | Atorvastatin | Ketoconazole | |
| Acyclovir | Labetolol | Azithromycin | Lansoprazole | |
| Amiloride | Levodopa | Carbamazepine | Lovastatin | |
| Amitryptyline | Levofloxacin | Carvedilol | Mebendazole | |
| Antipyrine | Lidocaine | Chlorpromazine | Naproxen | |
| Atropine | Lomefloxacin | Ciprofloxacin | Nelfinavir | |
| Buspirone | Meperidine | Cisapride | Ofloxacin | |
| Caffeine | Metoprolol | Cyclosporine | Oxaprozin | |
| Captopril | Metronidazole | Danazol | Phenazopyridine | |
| Chloroquine | Midazolam | Dapsone | Phenytoin | |
| Chlorpheniramine | Minocycline | Diclofenac | Piroxicam | |
| Cyclophosphamide | Misoprostol | Diflunisal | Raloxifene | |
| Desipramine | Nifedipine | Digoxin | Ritonavir | |
| Diazepam | Phenobarbital | Erythromycin | Saquinavir | |
| Diltiazem | Phenylalanine | Flurbiprofen | Sirolimus | |
| Diphenhydramine | Prednisolone | Glipizide | Spironolactone | |
| Disopyramide | Primaquine | Glyburide | Tacrolimus | |
| Doxepin | Promazine | Griseofulvin | Talinolol | |
| Doxycycline | Propranolol | Ibuprofen | Tamoxifen | |
| Enalapril | Quinidine | Indinavir | Terfenadine | |
| Ephedrine | Rosiglitazone | Indomethacin | Warfarin | |
| Ergonovine | Salicylic acid | |||
| Ethambutol | Theophylline | |||
| Ethinyl estradiol | Valproic acid | |||
| Fluoxetine | Verapamil | |||
| Glucose | Zidovudine | |||
| Imipramine | ||||
| Low Permeability | Class 3 | Class 4 | ||
| Acyclovir | Fexofenadine | Amphotericin B | ||
| Amiloride | Folinic acid | Chlorothiazide | ||
| Amoxicillin | Furosemide | Chlorthalidone | ||
| Atenolol | Ganciclovir | Ciprofloxacin | ||
| Atropine | Hydrochlorothiazide | Colistin | ||
| Bidisomide | Lisinopril | Furosemide | ||
| Bisphosphonates | Metformin | Hydrochlorothiazide | ||
| Captopril | Methotrexate | Mebendazole | ||
| Cefazolin | Nadolol | Methotrexate | ||
| Cetirizine | Penicillins | Neomycin | ||
| Cimetidine | Pravastatin | |||
| Ciprofloxacin | Ranitidine | |||
| Cloxacillin | Tetracycline | |||
| Dicloxacillin | Trimethoprim | |||
| Erythromycin | Valsartan | |||
| Famotidine | Zalcitabine | |||
3. PREDICTING DRUG DISPOSITION
3.1. Oral absorption and the gastrointestinal tract
A majority of drugs marketed worldwide are administered orally. The efficacy of these drugs is dependent on the oral bioavailability, which in turn, is dependent on extent of absorption. Oral absorption, in basic terms, is dependent on the intestinal drug solubilization and the intestinal drug permeability. The amount of drug that goes into solution is affected by factors such as volume, pH, temperature and the compound’s octanol/water partition coefficient, log Po/w. Thermodynamically, the solubility of a drug is determined by the intermolecular forces in the solid state versus the intermolecular forces of the solute-solvent state.27 The amount of drug that crosses the membrane is affected by factors such as concentration, temperature, time, surface area, viscosity, and affinity to a membrane transporter. Mechanistically, the permeability of a drug is additively determined by a number of parallel processes such as a passive component and one or more active components.28,29,30,31,32
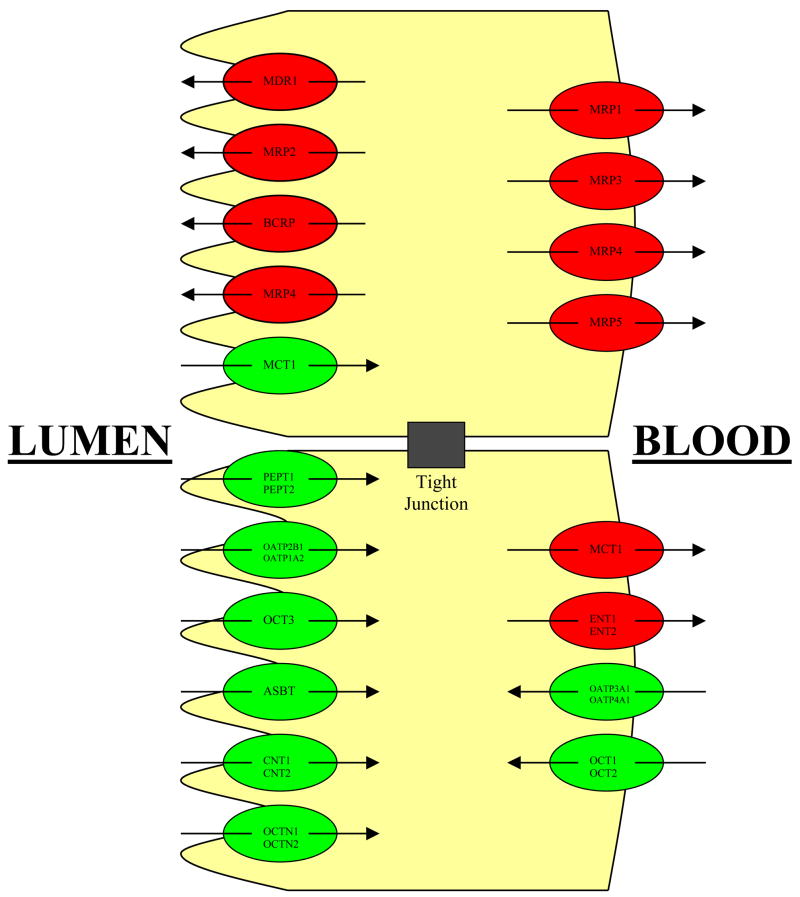
As an oral dose transits through the gastrointestinal (GI) tract, not only can it decompose but it may also undergo gut wall metabolism.33,34 Moreover, the drug is exposed to intestinal transporters, both efflux and absorptive (Fig. 2).35,36,37,38 The environment of the GI tract varies markedly following meal ingestion.39,40 Effects on absorption have been characterized as reduced, delayed, increased and accelerated, and those causing no change in absorption.41 The volume of intestinal fluids may increase 2–3 fold following a meal and the levels of phospholipids and bile salts in the gut also may increase 4–5 fold.42,43
Figure 2.
Various transporters, both efflux and absorptive, that are expressed in the gastrointestinal tract.
It is well documented that food can influence the pharmacokinetics of a drug by affecting one or more of the factors (e.g. bile flow, splanchnic blood flow, GI pH, gastric emptying, physical/chemical interactions with the drug) listed by the FDA.2,3 Chelation of a drug to certain food components can result in decreased bioavailability.44 Decreased solubility at higher gastrointestinal pH can occur for poorly soluble weak bases leading to a decrease in bioavailability.45 More rapid splanchnic blood flow is believed to improve the bioavailability of drugs that experience a significant first pass effect.46 Enhanced solubility in the postprandial intestine can markedly improve the bioavailability of a poorly soluble drug.47 Prolonged stomach exposure due to delayed emptying can reduce bioavailability due to drug interaction with the gastric acid secretions or by simply causing increased degradation of an unstable compound.8,48
While evidence as described above, of drug, meal, and formulation interactions, as well as their influences on intestinal absorption and elimination within the GI environment can be readily found in the current literature, much less is known about such influences and their clinical significance with respect to intestinal transporters. This work discusses the latter, the less extensively characterized effects on transporters, while focusing on the ever important absorption/elimination/transport interplay. Understanding the absorption/elimination/transport interplay is integral in predicting the role of food on drug disposition.
3.2. Predicting routes of elimination
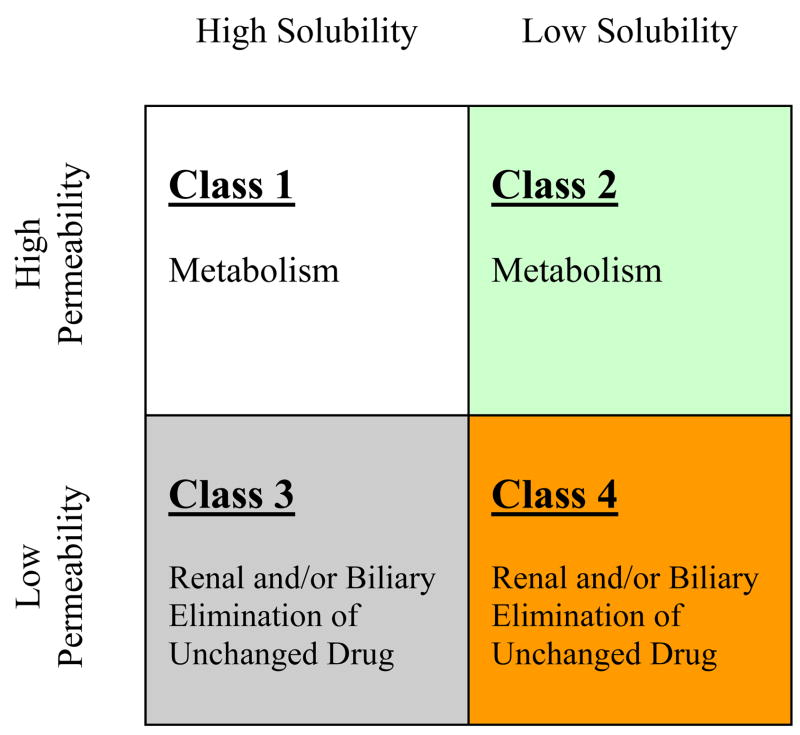
Critical evaluation of drug substances listed in the four BCS classes on Table I allows for prominent trends to become apparent specifically with respect to major routes of elimination. Class 1 and Class 2 compounds are eliminated primarily via metabolism, whereas Class 3 and Class 4 compounds are primarily eliminated unchanged into the urine and the bile (Fig. 3). This is consistent with the generally held belief that more permeable lipophilic compounds make good substrates for Cytochrome P450 (CYP) enzymes.49 While the theory that metabolism increases as lipid solubility (log P) increases was noted, it had not been previously been recognized in terms of the Biopharmaceutics Classification System.10 It is important to note that the differential permeability characteristics defined in the BCS do not necessarily reflect differences in permeability into hepatocytes. This is evident by the fact that a high number of Class 3 and Class 4 compounds are eliminated into the bile. The differential permeability characteristics denoted by the high versus low designation reflects the differential access of the compound to the metabolizing enzymes within the hepatocytes.
Figure 3.
Predominant routes of elimination by BCS class.
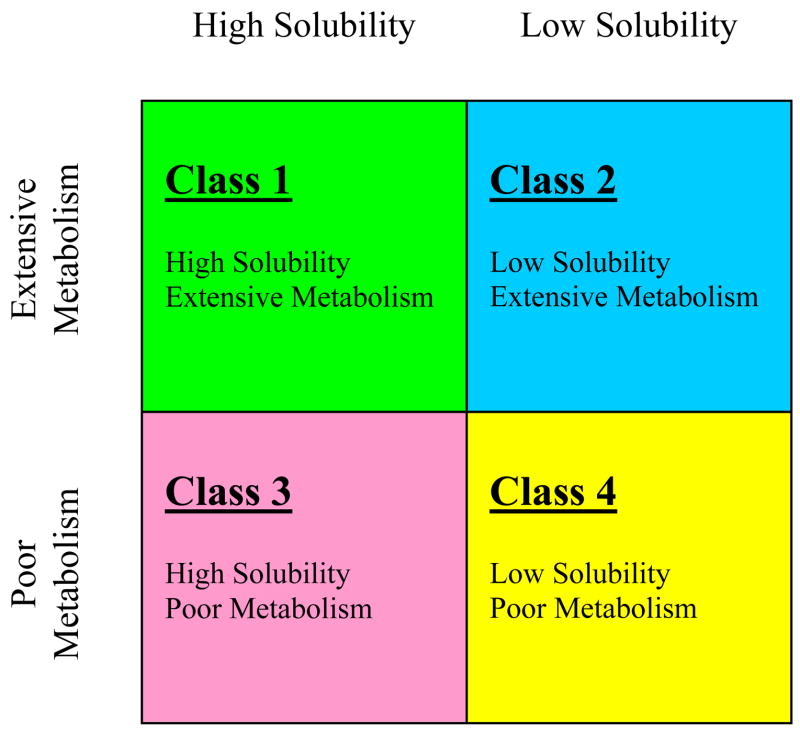
Recognition of the elimination pathway differences for Class 1 and Class 2 drugs versus Class 3 and Class 4 drugs, combined with the inherent difficulty in quantifying the percent of drug absorbed in humans at a level of 90%, prompted Wu and Benet10 to suggest a revised category scheme entitled the Biopharmaceutics Drug Disposition Classification System (BDDCS)(Fig. 4).
Figure 4.
The Biopharmaceutics Drug Disposition Classification System (BDDCS) after Wu and Benet.10
3.3 Framework of the BDDCS
The BDDCS replaces the permeability criteria with the major route of elimination because of the belief that it is easier and less ambiguous to determine the assignment of BDDCS for marketed drugs based on the extent of metabolism than using permeability (i.e. extent of absorption) in BCS assignments thereby eliminating many instances of uncertainly in human permeability leading to differences in class designation by different authors.
When initially proposed, “extensive metabolism” was defined as ≥ 50% metabolism of an oral dose in vivo in humans. Further consideration of this parameter designation led to the realization that there are very few drugs/compounds that are intermediately metabolized. It is now proposed that the definition of “extensive metabolism” be pushed to ≥ 70% metabolism of an oral dose in vivo in humans while the “poor metabolism” be defined as ≥ 50% of the dose be excreted unchanged. Use of the more stringent BDDCS metabolism criteria versus the BCS permeability characterization resulted in only 10 compounds requiring reclassification and allowed for the inclusion of an additional 38 drugs/compounds. An updated list modified from the BDDCS Table first published by Wu and Benet10 is provided in Table II.
Table II.
168 compounds categorized by BDDCS Class.
| High Solubility | Low Solubility | |||
|---|---|---|---|---|
| Extensive Metabolism | Class 1 | Class 2 | ||
| Abacavir | Ketoprofen | Albendazole | Ketoconazole | |
| Acetaminophen | Ketorolac | Amiodarone | Lansoprazole | |
| Albuterol | Labetolol | Atorvastatin | Lopinavir | |
| Allopurinol | Levamisole | Azathioprine | Lovastatin | |
| Amitryptyline | Levodopa | Azithromycin | Mebendazole | |
| Antipyrine | Lidocaine | Carbamazepine | Mefloquin | |
| Buspirone | Meperidine | Carvedilol | Nalidixic acid | |
| Caffeine | Metoprolol | Chlorpromazine | Naproxen | |
| Chloramphenicol | Metronidazole | Cisapride | Nelfinavir | |
| Chlorpheniramine | Midazolam | Clofazamine | Nevirapine | |
| Codeine | Minocycline | Cyclosporine | Nifedipine | |
| Colchicine | Misoprostol | Danazol | Oxaprozin | |
| Cyclophosphamide | Morphine | Dapsone | Phenytoin | |
| Desipramine | Phenobarbital | Diclofenac | Piroxicam | |
| Dexamethasone | Phenylalanine | Diflunisal | Praziquantel | |
| Diazepam | Prednisolone | Efavirenz | Raloxifene | |
| Diltiazem | Primaquine | Flurbiprofen | Rifampin | |
| Diphenhydramine | Promazine | Glipizide | Ritonavir | |
| Disopyramide | Promethazine | Glyburide | Saquinavir | |
| Doxepin | Propranolol | Griseofulvin | Sirolimus | |
| Enalapril | Pyranzinamide | Haloperidol | Spironolactone | |
| Ergonovine | Quinidine | Ibuprofen | Sulfamethoxazole | |
| Ergotamine | Quinine | Indinavir | Tacrolimus | |
| Ethinyl estradiol | Rosiglitazone | Indomethacin | Tamoxifen | |
| Fluoxetine | Salicyclic acid | Itraconazole | Terfenadine | |
| Glucose | Theophylline | Ivermectin | Warfarin | |
| Hydralazine | Valproic acid | |||
| Imipramine | Verapamil | |||
| Isoniazid | Zidovudine | |||
| Isosorbid dinitrate | ||||
| Poor Metabolism | Class 3 | Class 4 | ||
| Acyclovir | Ganciclovir | Acetazolamide | ||
| Amiloride | Hydrochlorothiazide | Aluminum hydroxide | ||
| Amoxicillin | Lamivudine | Amphotericin | ||
| Atenolol | Levofloxacin | Chlorothiazide | ||
| Atropine | Lisinopril | Chlorthalidone | ||
| Bidisomide | Lithium | Ciprofloxacin | ||
| Bisphosphonates | Lomefloxacin | Colistin | ||
| Captopril | Metformin | Digoxin | ||
| Cefazolin | Methotrexate | Furosemide | ||
| Cetirizine | Metoclopramide | Neomycin | ||
| Chloroquine | Nadolol | Nystatin | ||
| Cimetidine | Neostigmine | Ofloxacin | ||
| Cloxacillin | Penicillins | Phenazopyridine | ||
| Dicloxacillin | Pravastatin | Talinolol | ||
| Doxycycline | Pyridostigmine | |||
| Ephedrine | Ranitidine | |||
| Erythromycin | Riboflavin | |||
| Ethambutol | Tetracycline | |||
| Famotidine | Trimethoprim | |||
| Fexofenadine | Valsartan | |||
| Fluconazole | Zalcitabine | |||
| Folinic acid | ||||
3.4 Predicting the effects of transporters
The investigation of the effects of drug transporters on drug disposition has been ongoing for many years.50 The well characterized Madin Darby Canine Kidney (MDCK) cell line has been widely utilized throughout industry and academia with the most frequent application being the MDCK-MDR1 cell line incorporating the stable expression of the MDR1 gene encoding for the P-glycoprotein (P-gp) efflux transporter.51,52,53 Compared to various alternative cell monolayers, the MDCK-MDR1 cell system possesses very tight junctions as reflected by their high transepithelial electrical resistance (TEER) values. TEER values are measured by the Millicell electrical resistance system utilizing “chopstick” electrodes (Millipore Corporation, Bedford, MA) and are expressed in units of resistance and surface area. Our laboratory, similar to other research groups, employs semi-porous membranes with a pore size of 0.4μm and surface area of 4.2 cm2 upon which a cell monolayer is grown and, therefore, the ohm (Ω) resistance measured is then multiplied by 4.2 cm2. The MDCK-MDR1 cells, mentioned above as having very high TEER, possess values of approximately 5000–6000 Ω·cm2. The untransfected parental MDCK cell system possesses TEER values of approximately 200–300 Ω·cm2. The commonly used human colonic adenocarcinoma cell system (Caco-2) possesses TEER values of approximately 800–1000 Ω·cm2. It should be noted that reported TEER values for the same cell line can vary significantly from laboratory to laboratory. This may be attributed to variables such as the growth media and the frequency of media replacement as well as the passage of the cells at the time of experimentation. For example, Lu et al.54 reported Caco-2 cells, passage 44, to have TEER values of 664 ± 42 Ω·cm2 while the same cells, but of passage 98, possessed TEER values of 1423 ± 12 Ω·cm2.
Measurement of the bidirectional transport, apical to basolateral (A→B) and basolateral to apical (B→A), across the MDCK-MDR1 monolayer allows for the calculation of apparent permeability (Papp) values as well as net flux ratios (B→A/A→B). Transport studies by Polli and coworkers52 with various drugs in the MDCKII-MDR1 cell system demonstrate that P-gp substrates display a net flux ratio of 2 or higher. It has also been shown using studies in Caco-2 cells that drugs with Papp values less than 1 × 10−6 cm/sec are poorly (0–20%) absorbed; drugs with Papp values between 1–10 × 10−6 cm/sec are moderately (20–70%) absorbed; and drugs with Papp values greater than 10 × 10−6 cm/sec are well (70–100%) absorbed.55
Recent advances in the pharmaceutical sciences have provided new and improved tools for discovering and investigating transporter substrates. Although numerous substrates of the P-gp efflux transporter have been identified, this has not been the case for intestinal uptake transporters. Until now studies have not been able to show which drugs and compounds gain access into the cell by means of carrier mediated uptake transporters. Various studies have documented the utility of Caco-2 cells and that these cells do endogenously express a multitude of uptake transporters.56,57,58,59,60 Unfortunately, the sensitivity of assays run in Caco-2 cells have proved unsuccessful in consistently identifying uptake transporter substrates. However, isolation and stable transfection of uptake transporter proteins has lead to new lines that allow investigation of substrates for uptake transporters.61,62 The MDCK-PepT1 cell system has been used to for permeability screening of classic peptide substrates.63 The HEK/OATP2B1 cells have been used to demonstrate the effects of fruit juices on glyburide uptake.64
It is important to note that unless a drug molecule can passively gain intracellular access, it is not possible to simply investigate whether the molecule is a substrate for efflux transporters. To alleviate this complexity many researchers have employed a double transfection approach. A fine example is represented by recent work by Matsushima et al.65 with the MDCKII line; one line containing both the OATP1B1 and MDRl genes and another line containing both OATP1B1 and BCRP. With such tools, it is now feasible to evaluate vectorial transport of drugs and compounds that are substrates for both uptake and efflux transporters.66,67,68
It has been the belief of our laboratory that almost all drugs are substrates for some transporter. However, this is not to say that transporter effects will always be clinically relevant. One of the reasons researchers employ the MDCK-MDR1 cell line is because of its superior TEER values. This exceedingly tight cell system allows for increased sensitivity in identifying P-gp substrates. However, a transporter effect observed in the MDCK-MDR1 cell system may not necessarily be observed in the Caco-2 cell system. The lower TEER values of the Caco-2 cells makes for a leakier system that we believe is far more representative of the human intestine. Consequently, for certain drugs considered to be “model” P-gp substrates it may be difficult to predict their transporter interactions in the gut. In fact, Sahin et al. (2007 AAPS Annual Meeting, Abstract #1733) have shown that verapamil, which is designated as a model P-gp substrate by the FDA,9 has a net flux ratio close to one in the Caco-2 cell system. Furthermore, Sahin et al. (2007 AAPS Annual Meeting, Abstract #1733) demonstrated that this ratio remained unchanged in the presence of a P-gp inhibitor suggesting that verapamil absorption is controlled by its passive diffusion and not P-gp. Work such as this has been ongoing in our laboratory for several years and has led us to a number of generalizations regarding transporter effects following oral dosing (Fig. 5).
Figure 5.
Transporter effects, following oral dosing, by BDDCS class.
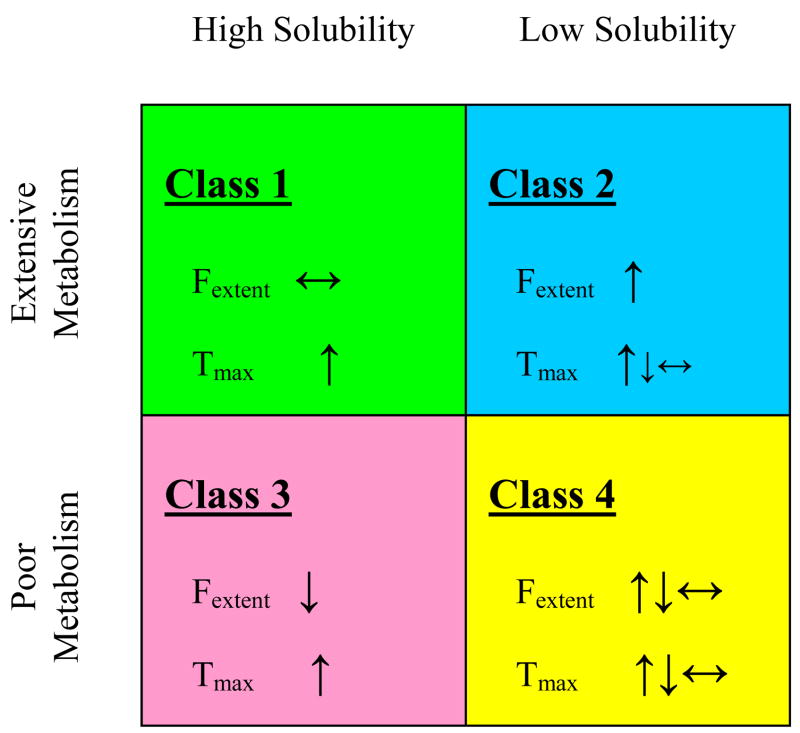
3.4.1. Class 1 compounds
The gut lumen of the gastrointestinal tract is sufficiently leaky so that small molecular weight, soluble, non-polar compounds (i.e. Class 1 compounds) readily pass through the membrane. Alternatively, the high permeability/high solubility of Class 1 drugs allows high concentrations in the gut to saturate any transporter, both efflux and absorptive. That is, Class 1 compounds may be substrates for both uptake and efflux transporters in vitro in cellular systems under the right conditions (for example, midazolam69 and verapamil9 are substrates for P-glycoprotein), but transporter effects will not be important clinically.
3.4.2. Class 2 compounds
Similarly, for Class 2 drugs, the high permeability will allow ready access into the gut membranes making intestinal uptake transporters generally unimportant due to the rapid permeation of the drug molecule into the enterocytes. That is, absorption of Class 2 compounds is primarily passive and a function of lipophilicity. However, the low solubility of these compounds will limit the concentrations coming into the enterocytes, thereby preventing saturation of the efflux transporters. Consequently, efflux transporters will affect the extent of oral bioavailability and the rate of absorption of Class 2 drugs. Moreover, there will be little opportunity to saturate intestinal enzymes such as CYP3A4 and UDP-glucuronosyltransferases (UGTs) due to the low solubility. Thus, changes in transporter expression, and inhibition or induction of efflux transporters will cause changes in intestinal metabolism of drugs that are substrates for the intestinal enzymes. Many Class 2 compounds are primarily substrates for CYP3A as well as substrates or inhibitors of the efflux transporter P-glycoprotein. Work in our laboratory has characterized this interplay in the absorption process for the investigational cysteine protease inhibitor K7770,71 and the immunosuppressive sirolimus,72 substrates for CYP3A and P-glycoprotein and for raloxifene,73 a substrate for UGTs and P-glycoprotein. However, as noted in Figure 5, Class 2 compounds may be affected by both uptake and efflux transporters in the liver.74
3.4.3. Class 3 and Class 4 compounds
For Class 3 compounds, sufficient drug will be available in the gut lumen due to good solubility, but an absorptive transporter will be necessary to overcome the poor permeability characteristics of these compounds. However, intestinal apical efflux transporters may also be important for the absorption of such compounds when sufficient enterocyte penetration is achieved via an uptake transporter. That is, since influx of Class 3 compounds will generally be rate limited by an absorptive transporter, the counter effects of efflux transporters will not be saturated and can also be important. In general, these principles also hold for Class 4 compounds, although Class 4 drugs may achieve sufficient solubility in the natural surfactant containing gut contents so that they act like Class 3 compounds.
4. PREDICTING THE EFFECTS OF FOOD ON ABSORPTION
4.1. Fleisher et al. and the trends in the BCS
Fleisher and coworkers14 documented several cases of food effects while taking into account the drug and its formulation classifications as established by the Biopharmaceutics Classification System (BCS).9 The review of Fleisher14 showed that in general high fat meals have no effect on the extent of oral drug availability for BCS Class 1 compounds, generally showed increased availability for Class 2 compounds, and generally showed decreased availability for Class 3 compounds. For example, in vivo studies such as that reported by Gupta et al.75 for cyclosporine, a Class 2 drug, demonstrated that when given with a high fat meal to healthy volunteers, bioavailability markedly increased as compared to individuals receiving the drug with a low fat meal. More recently, Ratain et al.47 showed that the bioavailability of the new anti-cancer agent, lapatinib, increased 325% when coadministered with a high fat meal when compared with fasting conditions. Lapatinib is a Class 2 drug. In contrast, the angiotensin converting enzyme (ACE) inhibitor captopril, a BCS Class 3 compound, shows a significant decrease in serum drug levels when administered with a meal.76 These observations are consistent with what might be predicted, in terms of our group’s Biopharmaceutics Drug Disposition Classification System (BDDCS), if high fat meal ingestion results in an inhibitory effect on intestinal transporter function.
In the BDDCS, we predict that high fat meals will have no significant effect on Class 1 drugs, despite the fact that many Class 1 drugs are transporter substrates, because the high gut permeability and intestinal fluid high solubility possessed by this class of drugs dictate that transporter effects will be minimal. For Class 2 compounds, which are highly metabolized and therefore are often dual substrates of enzymes and transporters, we predict an increase in bioavailability due in part to transporter inhibition resulting in decreased extraction of the drug. For Class 3 compounds, which are poorly metabolized and poorly permeable and therefore are often reliant on uptake transporters, we predict a decrease in bioavailability due in part to transporter inhibition resulting in the reduction of AUC.
4.2. FDA’s Guidance to the Industry
It is well known that food can influence drug bioavailability, both increasing and decreasing the extent of availability and the rate of availability. In December 2002, the FDA issued a guidance entitled, “Food-Effect Bioavailability and Fed Bioequivalence Studies.”3 High fat meals are recommended by the FDA for food-effect studies, as such meal conditions (800–1000 calories; 50–65% from fat, 25–30% from carbohydrates, and 15–20% protein) are expected to provide the greatest effects on gastrointestinal physiology so that systemic drug availability is maximally affected. An example of a test meal that would be suitable for a food effect bioavailability and fed bioequivalence study would include the following: two eggs fried in butter, two strips of bacon, two slices of toast with butter, four ounces of hash browns potatoes and eight ounces of whole milk. 3,77
It is generally believed that food effects result from changes in drug solubility and other factors as listed by the FDA,3 such as food may: “delay gastric emptying; stimulate bile flow; change gastrointestinal pH; increase splanchnic blood flow; change luminal metabolism of a drug substance; and physically or chemically interact with a dosage form or a drug substance.” However, it is also necessary to consider the fluidic environment and the resulting components present in the GI tract following a meal high in fat.
4.3. The postprandial intestine
A myriad of variables are introduced into the gastrointestinal tract upon ingestion and subsequent digestion of a meal. In addition to the actual constituents of meal, the resulting changes in the volume of intestinal fluids and the fluidic environment is significant. While the total volume may vary by individual, estimated volumes have been reported as follows: 300–500 ml for the fasted state stomach; 800–900 ml for the fed state stomach; 500 ml for the fasted state small intestine; 900–1000 ml for the fed state small intestine.1,41 The basal level gastric secretions are estimated to be about 300 ml and may increase up to five times following a meal. The increased gastric secretions introduce bile and alter intestinal pH, which may influence the bioavailability of a given compound.78 The larger volume GI environment may influence dissolution by simply presenting more liquid to the drug as well as creating a solvent drag effect. For poorly soluble compounds, the higher levels of bile may allow for enhanced wetting of the drug as well as increased micellar solubilization, both of which can increase the dissolution rate.79
Food constituents have been widely studied with the caloric make-up of various meals being evaluated in humans to determine the pharmacokinetic variations in response to ingestion of meals high in fiber, high in protein, high in carbohydrates, and high in fat. (e.g., studies with indinavir following high calorie protein, fat and carbohydrate meals.45) These examples, save for the high fat meal, are outside the scope of this review. Our predictions focus on the influences of high fat meals because, as recommended by the FDA in food-effect studies,3 such meals are believed to produce maximum effect if any is to be observed.
Triglycerides are predominant in our fatty foods, and their digestion and absorption have been widely examined.80 When fats reach the small intestines, fluid secretion increases with peak bile flow occurring about 30 minutes post meal. The fed state bile concentrations may increase to 10–20 mM from levels of the fasted state of 4–6 mM. Bile acids, their salts and lipases break down fats into monoglycerides and fatty acids that are capable of traversing the intestinal mucosal cell membrane. In addition, bile salts undergo aggregation into polymolecular micelles that, when emulsified with the breakdown products of lipid hydrolysis, increase in size, which results in improved solubilization capacity. Both bile salts and lipids have been reported to reduce the function of transporters.81,82,83 Konishi and coworkers81,82 have documented the increased cellular accumulation of P-gp substrates in the presence of various monoglycerides. Moreover, studies have reported the inhibitory effect of taurocholate on additional transporters.84
The fact that monoglycerides are a product of dietary triglyceride hydrolysis in the digestive tract, combined with the upregulation of bile salts during fatty meal digestion, suggests a trend in the observed variations in drug disposition. Examining the directional changes for the extent of availability in light of the predictions concerning the effects of transporters on drug bioavailability presented in the BDDCS, we hypothesize that drug-transporter interactions could be an important mechanism for the food effect in addition to the other mechanisms listed above in the FDA guidance.
4.4. Predicting effects by BDDCS Class
The predicted effects of food on absorption by BDDCS class is summarized in Figure 6.
Figure 6.
Predicted high fat meal effects by BDDCS class.
4.4.1. Class 1 compounds
High fat meals will have no significant effect on the extent of bioavailability for Class 1 compounds. Complete absorption would be expected for high solubility/high permeability compounds. Because the absorption process would be dominated by passive diffusion for Class 1 compounds, no significant transporter drug interactions would be expected. However, high fat meals may delay stomach emptying and therefore cause an increase in peak time.
4.4.2. Class 2 compounds
High fat meals will increase the extent of bioavailability for Class 2 compounds. This increased bioavailability with a concomitant high fat meal could be due to inhibition of efflux transporters, such a P-gp, in the intestine. Benet et al.85,86 have reported that a dynamic interplay exists between P-gp and CYP3A4 demonstrating that P-gp efflux transport prolongs the exposure of drug to CYP3A4 by “cycling” the drug in and out of the cell. Therefore, inhibition of P-gp limits the “cycling,” reducing access of drug to the metabolizing enzyme. This mechanism is specifically relevant to compounds that are dual substrates of both enzyme and transporters, as is the case for Class 2 drugs. Hence, blocking the transporter decreases the amount of drug metabolized thereby increasing the bioavailability. Another mechanism that could be acting in concert with the transporter inhibition is increased solubilization of drug in the intestinal lumen (e.g. micelle formation). In addition to change in exposure, peak time could decrease due to inhibition of efflux cycling or increase due to slowing of stomach emptying; a combination of the two will usually be dominated by the delayed emptying.
Formulation changes that markedly increase the solubility of Class 2 compounds will decrease or eliminate the high fat meal effects for these drugs. We believe that this is the reason that the newer cyclosporine microemulsion formulation (Neoral®) eliminates the food effects associated with the older olive oil formulation (Sandimmune®). In practice, drug formulators attempt to enable a Class 2 compound to function in the body as a Class 1 compound, thereby eliminating food effects on the extent of availability and other transporter-drug interactions.
4.4.3. Class 3 and Class 4 compounds
High fat meals will decrease the extent of availability for Class 3 compounds. Class 3 compounds could show lower extent of availability with high fat meals due to inhibition of uptake transporters in the intestine. Recent evidence suggests that intestinal drug uptake can be decreased by inhibiting organic anion transporting polypeptides (OATPs), as shown by the effect of fruit juices on fexofenadine87 and glyburide.64 As noted in Figure 5, Class 3 compounds will also be substrates for intestinal efflux transporters. Depending upon whether the meal effects are more pronounced on efflux or influx transporters for a Class 3 drug that is a substrate for both, an unexpected increase in the extent of bioavailability or no meal effect can be observed. Wu and Benet10 hypothesized that this may be the explanation for the lack of a high fat meal effect on acyclovir and the slight increase observed with ganciclovir.88 For Class 3 drugs, peak time would be expected to be increased by a high fat meal due to the combination of delayed stomach emptying and slower absorption.
For Class 4 compounds, it is difficult to predict what will occur, as all of the interacting effects mentioned for Class 2 and Class 3 compounds can be seen here. However, although not shown in Figure 6, if high fat meal effects are to occur, an increase of the extent of bioavailability is more likely, resulting from the combination of increased solubilization of drug in the intestine and inhibition of efflux transporters.
4.5. Predicting effects by statistical modeling regression analysis
In addition to the Fleisher et al.14 examination of food-drug effects based on the BCS and the Wu and Benet10 examination based on the BDDCS, other groups have utilized a statistical modeling approach to predict the effect of food on drug absorption. Singh89 applied Pearson’s correlation test on a diverse set of drugs and concluded that partition coefficient, solubility and dose/solubility ratio are the most useful parameters in determining the dependence and correlation of the effect of food on absorption. Gu et al.90 employed a logistic regression model to evaluate the relationship between the physiochemical properties of a given drug and the effect of food on AUC of that drug. Focusing on the physiochemical parameters of solubility and log D at pH 7, the polar and total surface areas, number of hydrogen bond donors and acceptors, and the clinical dose used in the published food effect study, the authors categorized 92 marketed drugs based on dose number and maximum allowable dose (MAD). These 92 drugs sufficiently cover all response groups (positive effect, negative effect and no effect) and were selected because of their previously assigned BCS Class and their known and documented response to high fat meals during clinical studies. Further subcategorizing the drugs into either MAD < clinical dose or MAD ≥ clinical dose, Gu et al.90 were able to apply their logistic regression model and concluded that the critical parameters for food effect prediction are solubility, permeability and dose, reinforcing the aforementioned beliefs of those such as Fleisher et al.14 and Wu and Benet.10 However, Gu et al.90 also concluded that their statistical method provided a better food-effect prediction than that the BCS classification (80% vs. 67%). The authors successfully predicted 74 out of 92 drugs into the correct response category with 97% accuracy for positive effect, 79% accuracy for negative effect and 68% accuracy for no effect.
As is the case with any modeling, limitations exist. Lipinski’s Rule of 5 was developed to set “drugability” guidelines for NMEs.91,92 In the drug discovery setting, the Rule of 5 predicts that poor absorption or permeation is most likely when there are more than 5 H-bond donors, 10 H-bond acceptors, the molecular weight is greater than 500 and the calculated Log P (Clog P) is greater than 5. However, Lipinski91,92 specifically states that the Rule of 5 only holds for compounds that are not substrates for active transporters. Likewise, in reference to their statistical approach, Gu et al.90 state, “The current model also cannot predict food effect on the activity of a specific transporter responsible for the absorption of a particular compound as the current understanding of food effect on transporter is limited.”
The inability of a logistic regression analysis to accurately predict a negative food effect for fexofenadine (model predicted no food effect)90 is an example of the limitations inherent in statistical modeling. As suggested, the misprediction could be attributed to the high log D value of fexofenadine that is listed as 2.68 and is calculated using ACD/PhysChem Batch software. (It should be noted that a search through the literature reveals different calculated values reported by different investigators for log D and log P for the same compound.) However, as acknowledged above, transporter effects are not accounted for in such models and may be a cause for the erroneous prediction of fexofenadine, which is a known uptake transporter substrate. Fexofenadine is a BDDCS Class 3 drug, a class of compounds for which we believe transporters will play a significant role in oral drug absorption. Furthermore, for Class 3 drugs we predict a decrease in bioavailability due in part to transporter inhibition by high fat meals, which fits with the effect observed for fexofenadine in vivo.
Much work has been done to develop models to closely represent the upper GI tract that would mimic physiological media conditions of a fasted and a fed state as well as various other gastric and intestinal fluids.42 These studies have been primarily applied to evaluating food effects on dissolution properties of various classes of drugs as well as various dosage forms (e.g. soft-gels versus hard gelatin capsules) and have demonstrated that simulated physiological fluids mimicking the fed state can increase the solubility of certain compounds.42,93,94 Such studies have led to the potential explanation that changes in bioavailability are attributed to increased solubility.90 While this finding may provide a reasonable explanation for the increase in bioavailability for many poorly soluble drugs, it does not explain the decrease in bioavailability frequently observed in BCS and BDDCS Class 3 drugs or the variability in bioavailability in food-effects for Class 4 drugs. Therefore, it is an inadequate and inappropriate generalization for the effect of food on absorption. It has also been postulated that components of high fat meals cause changes in bioavailability by impeding the access of drug to the epithelium surface of the intestinal enterocytes.1,90 While this finding may provide a reasonable explanation for the decrease in bioavailability for Class 3 drugs, it does not explain the increase observed in Class 2 or the variability observed in Class 4 drugs. Taking efflux and influx transporters into account, however, may help elucidate the behavioral trends currently observed in the literature.
5. JUICE/FOOD/HERBAL EXTRACT EFFECTS
Considerable effort has been put into elucidating the effects of food on drug absorption and disposition. With very few exceptions, the mechanistic research representative of this effort has been retrospective in that first, an in vivo food effect is observed in a patient, followed by in vitro studies to the determine the cause of that effect. Such is the scenario that led to the discovery of grapefruit juice’s potent inhibition of metabolic function in the intestine.95
Regarding intestinal metabolism, metabolic food-drug interactions have been widely reported with Cytochrome P450 3A4 (CYP3A4) receiving considerable attention. This is not surprising as numerous drug marketed worldwide are metabolized by CYP3A4. Documented examples include, but are not limited to, modulations in metabolism of drugs such as cyclosporine, felodipine, and indinavir affected by interactions with garlic, grapefruit juice, St John’s wort, and red wine.2 Except for the inducing effect of St. John’s wort, these food constituents act as direct inhibitors or inactivators of CYP3A4.
The breadth of reports on food-enzyme interactions is in stark contrast to the more limited literature on food-transporter interactions. As mentioned earlier, however, new and improved tools are becoming available for discovering and investigating transporter substrates, which has facilitated the identification of transporter inhibitors and inducers. Thus, the publication rate on food-transporter interactions is increasing as a result.
5.1. Studies with fexofenadine
Fexofenadine, a Class 3 drug, has been shown to be a substrate for OATPs and P-glycoprotein. Dresser et al.87 first described a decrease in the oral bioavailability of fexofenadine due to the inhibition of OATPs by fruit (orange, grapefruit and apple) juices. This research group noted the counterposing effects for Class 3 compounds such as fexofenadine.96,97 That is, fexofenadine is a substrate for a gut uptake transporter, OATP, and a gut efflux transporter, P-glycoprotein,98 but the fruit juice effects on uptake were more pronounced than they were on efflux,96,97 therefore oral bioavailability is decreased. Kamath et al.99 reported the counteractive effects of fruit juices on uptake and efflux with respect to the oral bioavailability of fexofenadine in rats.
5.2. Grapefruit juice effects
It is well known that grapefruit juice inhibits intestinal CYP3A4 and increases the bioavailability of many Class 1 and Class 2 compounds. However, with the recognition that grapefruit juice can also inhibit OATPs and P-glycoprotein, a number of studies have been carried out to investigate this potential interaction. No effect on the bioavailability of digoxin, a Class 4 BDDCS drug that is not a substrate for CYP3A4 in humans, was observed with concomitant grapefruit juice.100 In contrast, grapefruit juice ingestion significantly reduced the bioavailability of talinolol, another Class 4 BDDCS drug, where the authors concluded that the effect of grapefruit juice on inhibition of uptake was more significant than for efflux.101
5.3. The effects of herbal extracts
More recently, attention has focused on the potential effects of constituents of herbal extracts in terms of transporter inhibition that could potentially affect bioavailability. A number of diverse substances including curcumin,102 flavonoids,103 extracts from bitter melon,81,82 tea catechins,104 cnidiadin105 and alkyl gallates106 have all been shown to be effective inhibitors of P-glycoprotein and thus could lead to increased bioavailability.
Most recently, Fuchikami et al.107 demonstrated that many herbal extracts can inhibit human OATP2B1. They suggested that these inhibitory effects may be primarily attributable to flavonoids. Less work has been carried out with other transporters, but Tseng et al.108 have shown that mustard oils can inhibit MRP1 mediated transport, while Wu and coworkers109 have shown that plant phenols can inhibit MRPs 1, 4 and 5.
Thus, as seen above, it may be expected that food and herbal extracts can inhibit both uptake and efflux transporters in the intestine and the final result on drug bioavailability will be dependent on which is the more important component of absorption.
6. EFFECT OF LIPIDS
6.1. Influence of lipids
High fat meals, as recommended by the FDA in Food-Effect Bioavailability and Fed Bioequivalence Studies,3 result in significantly higher levels of lipids than compared to a fasting state.110,111 Persson et al.112 employed the in vivo single-pass perfusion model in the proximal human jejunum to evaluate the GI response to meal and showed that the tri-, di-, monoglyceride and free fatty acid levels rapidly and markedly increased to 9.5 mM in response to food. Considerable attention has been focused not only on lipids resulting from food ingestion but also on lipids introduced to the GI tract from lipidic excipients, since enhanced bioavailability has been demonstrated for poorly water-soluble drugs when coadministered with a high fat meal or a lipid-based formulation to deliver the drug.113 Increased lipid levels has also been shown to influence the pharmacokinetics of lipid soluble compounds that bind extensively to triglyceride-rich lipoproteins with effects being greater for more lipophilic compounds.114,115
Lipid based delivery systems116 are an ever-growing segment of pharmaceutics that primarily involves BDDCS Class 2 compounds, as the benefits provided by lipid formulations for BDDCS Class 4 drug are insufficient to overcome their poor permeability characteristics. With the goal of the lipid systems being to improve drug solubilization behavior, the formulation utilized depends on the aqueous solubility of the drug as well as the drug’s solubility in lipids. This also holds true when high fat meals are coadministered with the goal of solubility enhancement, as exemplified in a study in which Mueller et al.117,118 demonstrated that fat-rich meal effects are drug formulation dependent. Moreover, Kaukonen et al.119 demonstrated that triglyceride (TG) lipid suspension formulations differentially affected various model, poorly soluble compounds, including griseofulvin, danazol and halofantrine, which had previously been shown to exhibit a significant high fat meal effect. The authors examined the effects of various TG formulations on griseofulvin, danazol, halofantrine and diazepam and cinnarizine observing that simple TG solutions offer better solubilization for the more lipophilic compounds while the TG suspensions better enhance the solubility of the less lipid-soluble drugs by increasing the solubilization capacity of the colloidal phase.118 The colloidal phase is one example of the various phases that occur in the gastrointestinal tract resulting from the digestion of lipids that are ingested in formulations or food.79,120 Accordingly, the role of these intermediate digestion phases, including the lamellar (Lα) liquid crystalline phase and the viscous cubic (C) phase in addition to the aqueous colloidal liquid (L1) phases, must also be taken into account.121,122 Besides the influences on solubilization capacity and intermediate digestion phases, lipids have also been widely reported to influence lymphatic transport of drugs.116 Again, however, these influences are only mentioned and cannot be adequately covered in this review, as our emphasis is lipidic effects on transporters, both efflux and influx, and predicting when such effects may play a significant role in altering drug availability.
6.2. Lipid effects on transporters
The fact that triglycerides are predominant in high fat meals is of particular relevance when considering the effect of lipids on the intestinal transporter function. As discussed in Section 4.3., triglycerides are hydrolyzed in the gut into monoglycerides and fatty acids that are capable of traversing the intestinal mucosa cell membrane. Konishi et al.81,82 previously documented the inhibitory effect of monoglycerides on P-gp activity. The authors employed Caco-2 cells and the known P-gp substrates, rhodamine-123 and daunomycin and demonstrated that various monoglycerides and fatty acids were capable of inhibiting P-gp mediated efflux, thereby increasing the cellular accumulation of rhodamine-123 and daunomycin. 81,82
The inhibitory effect of monoglycerides reported by Konishi et al.81,82 is highlighted here since monoglycerides are found in lipid excipients. For example, Peceol®, an excipient used to enhance absorption of poorly water soluble compounds, is in fact, 2,3-dihydroxypropyl (Z)-octadec-9-enoate, also known as monoolein. Subramanian and Wasan123 have suggested that Peceol® possesses inhibitory properties of P-gp. Following one week treatment of Caco-2 cells with Peceol® (0.25% v/v), the authors reported decreased MDR1 mRNA levels and decreased P-gp protein expression levels. 124,125
An increased level of monoglycerides following high fat meal ingestion coincides with the upregulation of bile secretions from the gallbladder. As a result, increased levels of bile salts accompany high monoglycerides levels. In addition to the inhibitory effect of bile salt on uptake transport mentioned earlier, studies have shown effects on efflux transport as well. Ingels and coworkers83,126 employed the bile salt, sodium taurocholate, and the known P-gp substrate, cyclosporine. The authors studied the changes in the apical to basolateral (A→B) flux and the basolateral to apical (B→A) flux across Caco-2 cell monolayers demonstrating that an increase in bile salts results in a decreased B→A flux and an increased A→B flux, amounting to a decrease in net flux ratio of ~16 (control buffer) to ~1 (control buffer plus 3 mM sodium taurocholate). A reduction of the net flux ratio to a level where A→B and B→A fluxes are nearly equivalent (i.e. ratio = 1) is consistent with inhibition of an efflux transporter such a P-gp.
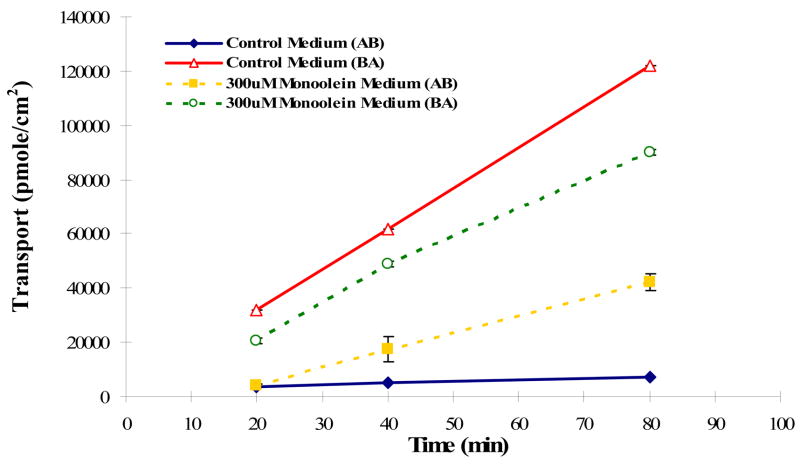
To further assess the effects of lipids on transporter function, we evaluated the bidirectional transport of vinblastine, a Class 2 drug, in MDCKI-MDR1 and control MDCKI cells grown on permeable filter supports evaluating basolateral to apical (B→A) and apical to basolateral (A→B) transport. Figure 7A depicts the bidirectional flux in MDCKI cells transfected with human MDR1. The B→A transport of vinblastine in this transfected cell is 26-fold higher than the A→B transport. When 300 μM monoolein was added to the transport buffer, B→A transport decreased and A→B transport increased so that the ratio in the presence of monoolein was only 2; a 92% decrease. In control MDCKI cells it can be seen that monoolein has no effect on the B→A and A→B transport fluxes (Fig. 7B). These results suggest a potentially important inhibitory effect of monoglycerides on drug bioavailability. This can be interpreted as suggesting that the monoglycerides, which appear as breakdown products from a high fat meal, may be responsible for the increased bioavailability of Class 2 compounds.
Figure 7.
Bidirectional transport of vinblastine in (A) MDCKI-MDR1 cells and (B) MDCKI cells in the presence and absence of 300 and 500 μM monoolein added to both apical and basolateral compartments.
7. SUMMARY AND FUTURE PROSPECTS
The BCS, as developed by Amidon et al.,8 has proven to be an asset to the FDA by creating a framework by which waivers of in vivo bioequivalence studies for suitable Class 1 compounds can be approved. However, the full predictive utility of a classification system may be realized and facilitated by the BDDCS, as developed by Wu and Benet,10 as this system allows for concurrent consideration of the absorption / transport / elimination interplay thereby extending predictive application to food effects, specifically high fat meals. The majority of new molecular entities are highly permeable, poorly soluble, extensively metabolized compounds (BDDCS Class 2), and are vulnerable to food effects, as are the poorly permeable, poorly metabolized Class 3 and 4 compounds. Isolation and stable transfection of uptake transporter proteins has led to new tools that allow investigation of substrates for uptake transporters. This will allow for improved predictions of drug disposition as the various intestinal transporters responsible for the uptake of Class 3 and 4 drugs are identified and characterized. Advances in statistical regression modeling have reinforced the critical importance of solubility and permeability in predicting food effects. Yet we believe food effects cannot be predicated based on solubility and permeability alone. Knowledge and inclusion of a wider database of compounds into the BDDCS will assist in confirming current trends as well as identifying new trends. Benet and Oprea will soon publish such a compilation for more than 500 drugs. Incorporating the vast information on the structural similarities of the drugs within a given class will also improve predictability. In combination with the knowledge of when transporters may play a significant role, we may be progressing towards an accepted system to predict drug absorption and the effect of food.
Acknowledgments
We greatly appreciate and thank Dr. Selma Sahin for her careful review and helpful editing of this manuscript. This work was supported in part by NIGMS grants GM56847 and GM75900 as well an unrestricted grant from Amgen Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Singh BN. Effects of food on clinical pharmacokinetics. Clin Pharmacokinet. 1999;37:213–255. doi: 10.2165/00003088-199937030-00003. [DOI] [PubMed] [Google Scholar]
- 2.Harris RZ, Jang GR, Tsunoda S. Dietary effects on drug metabolism and transport. Clin Pharmacokinet. 2003;42:1071–1088. doi: 10.2165/00003088-200342130-00001. [DOI] [PubMed] [Google Scholar]
- 3.Food and Drug Administration. Guidance for Industry: Food-effect bioavailability and fed bioequivalence studies. Food and Drug Administration; Rockville, MD: 2002. Available at http://www.fda.gov/cder/guidance/index.htm. [Google Scholar]
- 4.Jones HM, Parrott N, Ohlenbusch G, Lavé T. Predicting pharmacokinetic food effects using biorelevant solubility media and physiologically based modeling. Clin Pharmacokinet. 2006;45:1213–1226. doi: 10.2165/00003088-200645120-00006. [DOI] [PubMed] [Google Scholar]
- 5.Lentz KA, Quitko M, Morgan DG, Grace JE, Gleason C, Marathe PH. Development and validation of a preclinical food effect model. J Pharm Sci. 2007;96:459–472. doi: 10.1002/jps.20767. [DOI] [PubMed] [Google Scholar]
- 6.Wei H, Löbenberg R. Biorelevant dissolution media as a predictive tool for glyburide a class II drug. Eur J Pharm Sci. 2006;29:45–52. doi: 10.1016/j.ejps.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Kataoka M, Masaoka Y, Sakuma S, Yamashita S. Effect of food intake on the oral absorption of poorly water-soluble drugs: in vitro assessment of drug dissolution and permeation assay system. J Pharm Sci. 2006;95:2051–2061. doi: 10.1002/jps.20691. [DOI] [PubMed] [Google Scholar]
- 8.Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutics drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413–420. doi: 10.1023/a:1016212804288. [DOI] [PubMed] [Google Scholar]
- 9.Food and Drug Administration, Guidance for Industry. Food and Drug Administration; Rockville, MD: 2000. Waiver of in vivo bioavailability and bioequivalence studies for immediate release solid oral dosage forms based on a biopharmaceutics classification system. Retrieved from www.fda.gov/cder/guidance/index.htm. [Google Scholar]
- 10.Wu CY, Benet LZ. Predicting drug disposition via application of BCS: transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22:11–23. doi: 10.1007/s11095-004-9004-4. [DOI] [PubMed] [Google Scholar]
- 11.Lennernas H. Human jejunal effective permeability and its correlation with preclinical drug absorption models. J Pharm Pharmacol. 1997;49:627–638. doi: 10.1111/j.2042-7158.1997.tb06084.x. [DOI] [PubMed] [Google Scholar]
- 12.van de Waterbeemd H. The fundamental variables of the biopharmaceutics classification system (BCS): a commentary. Eur J Pharm Sci. 1998;7:1–3. doi: 10.1016/s0928-0987(98)00051-7. [DOI] [PubMed] [Google Scholar]
- 13.Blume HH, Schug BS. The biopharmaceutics classification system (BCS): class III drugs—better candidates for BA/BE waiver? Eur J Pharm Sci. 1999;9:117–121. doi: 10.1016/s0928-0987(99)00076-7. [DOI] [PubMed] [Google Scholar]
- 14.Fleisher D, Li C, Zhou Y, Pao LH, Karim A. Drug, meal and formulation interactions influencing drug absorption after oral administration, Clinical implications. Clin Pharmacokinet. 1999;36:233–254. doi: 10.2165/00003088-199936030-00004. [DOI] [PubMed] [Google Scholar]
- 15.Löbenberg R, Amidon GL. Modern bioavailability, bioequivalence and biopharmaceutics classification system. New scientific approaches to international regulatory standards. Eur J Pharm Biopharm. 2000;50:3–12. doi: 10.1016/s0939-6411(00)00091-6. [DOI] [PubMed] [Google Scholar]
- 16.Avdeef A. Physicochemical profiling (solubility, permeability and charge state) Curr Top Med Chem. 2001;1:277–351. doi: 10.2174/1568026013395100. [DOI] [PubMed] [Google Scholar]
- 17.Rege BD, Yu LX, Hussain AS, Polli JE. Effect of common excipients on Caco-2 transport of low-permeability drugs. J Pharm Sci. 2001;90:1776–1786. doi: 10.1002/jps.1127. [DOI] [PubMed] [Google Scholar]
- 18.Tannergren C, Langguth P, Hoffmann KJ. Compound mixtures in Caco-2 cell permeability screens as a means to increase screening capacity. Pharmazie. 2001;56:337–342. [PubMed] [Google Scholar]
- 19.Kanfer I. Report on the International Workshop on the Biopharmaceutics Classification System (BCS): scientific and regulatory aspects in practice. J Pharm Pharm Sci. 2002;5:1–4. [PubMed] [Google Scholar]
- 20.Lennernas H, Knutson L, Knutson T, Hussain A, Lesko L, Salmonson T, Amidon GL. The effect of amiloride on the in vivo effective permeability of amoxicillin in human jejunum: experience from a regional perfusion technique. Eur J Pharm Sci. 2002;15:271–277. doi: 10.1016/s0928-0987(02)00005-2. [DOI] [PubMed] [Google Scholar]
- 21.Martinez MN, Amidon GL. A mechanistic approach to understanding the factors affecting drug absorption: a review of fundamentals. J Clin Pharmacol. 2002;42:620–643. doi: 10.1177/00970002042006005. [DOI] [PubMed] [Google Scholar]
- 22.Taub ME, Kristensen L, Frokjaer S. Optimized conditions for MDCK permeability and turbidimetric solubility studies using compounds representative of BCS classes I-IV. Eur J Pharm Sci. 2002;15:331–340. doi: 10.1016/s0928-0987(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 23.Yu LX, Amidon GL, Polli JE, Zhao H, Mehta MU, Conner DP, Shah VP, Lesko LJ, Chen ML, Lee VH, Hussain AS. Biopharmaceutics classification system: the scientific basis for biowaiver extensions. Pharm Res. 2002;19:921–925. doi: 10.1023/a:1016473601633. [DOI] [PubMed] [Google Scholar]
- 24.Bergström CA, Strafford M, Lazorova L, Avdeef A, Luthman K, Artursson P. Absorption classification of oral drugs based on molecular surface properties. J Med Chem. 2003;46:558–570. doi: 10.1021/jm020986i. [DOI] [PubMed] [Google Scholar]
- 25.Tannergren C, Knutson T, Knutson L, Lennernas H. The effect of ketoconazole on the in vivo intestinal permeability of fexofenadine using a regional perfusion technique. Br J Clin Pharmacol. 2003;55:182–190. doi: 10.1046/j.1365-2125.2003.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindenberg M, Kopp S, Dressman JB. Classification of orally administered drugs on the World Health Organization Model of Essential Medicines according to the biopharmaceutics classification system. Eur J Pharm Biopharm. 2004;58:265–278. doi: 10.1016/j.ejpb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Jorgensen WL, Duffy EM. Prediction of drug solubility from structure. Adv Drug Deliv Rev. 2002;54:355–366. doi: 10.1016/s0169-409x(02)00008-x. [DOI] [PubMed] [Google Scholar]
- 28.Bergström CA. In silico predictions of drug solubility and permeability: two rate-limiting barriers to oral drug absorption. Basic Clin Pharmacol Toxicol. 2005;96:156–161. doi: 10.1111/j.1742-7843.2005.pto960303.x. [DOI] [PubMed] [Google Scholar]
- 29.Bergström CA. Computational models to predict aqueous drug solubility, permeability and intestinal absorption. Expert Opin Drug Metab Toxicol. 2005;1:613–627. doi: 10.1517/17425255.1.4.613. [DOI] [PubMed] [Google Scholar]
- 30.Amidon GL, Sinko PJ, Fleisher D. Estimating human oral fraction dose absorbed: a correlation using rat intestinal membrane permeability for passive and carrier-mediated compounds. Pharm Res. 1988;5:651–654. doi: 10.1023/a:1015927004752. [DOI] [PubMed] [Google Scholar]
- 31.Kunta JR, Sinko PJ. Intestinal Drug Transporters: in vivo function and clinical importance. Curr Drug Met. 2004;5:109–124. doi: 10.2174/1389200043489144. [DOI] [PubMed] [Google Scholar]
- 32.Fagerholm U. Prediction of human pharmacokinetics - gastrointestinal absorption. J Pharm Pharmacol. 2007;59:905–916. doi: 10.1211/jpp.59.7.0001. [DOI] [PubMed] [Google Scholar]
- 33.Paine MF, Shen DD, Kunze KL, Perkins JD, Marsh CL, McVicar JP, Barr DM, Gillies BS, Thummel KE. First-pass metabolism of midazolam by the human intestine. Clin Pharmacol Ther. 1996;60:14–24. doi: 10.1016/S0009-9236(96)90162-9. [DOI] [PubMed] [Google Scholar]
- 34.DeSesso JM, Jacobson CF. Anatomical and physiological parameters affecting gastrointestinal absorption in humans and rats. Food Chem Toxicol. 2001;39:209–228. doi: 10.1016/s0278-6915(00)00136-8. [DOI] [PubMed] [Google Scholar]
- 35.Shitara Y, Horie T, Sugiyama Y. Transporters as a determinant of drug clearance and tissue distribution. Eur J Pharm Sci. 2006;27:425–446. doi: 10.1016/j.ejps.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Kim RB. Transporters and drug discovery: why, when, and how. Mol Pharm. 2005;3:26–32. doi: 10.1021/mp050084o. [DOI] [PubMed] [Google Scholar]
- 37.Seithel A, Karlsson J, Hilgendorf C, Björquist A, Ungell AL. Variability in mRNA expression of ABC- and SLC-transporters in human intestinal cells: comparison between human segments and Caco-2 cells. Eur J Pharm Sci. 2006;28:291–299. doi: 10.1016/j.ejps.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Englund G, Rorsman F, Rönnblom A, Karlbom U, Lazorova L, Gråsjö J, Kindmark A, Artursson P. Regional levels of drug transporters along the human intestinal tract: co-expression of ABC and SLC transporters and comparison with Caco-2 cells. Eur J Pharm Sci. 2006;29:269–277. doi: 10.1016/j.ejps.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Kalantzi L, Goumas K, Kalioras V, Abrahamsson B, Dressman JB, Reppas C. Characterization of the human upper gastrointestinal contents under conditions simulating bioavailability/bioequivalence studies. Pharm Res. 2006;23:165–176. doi: 10.1007/s11095-005-8476-1. [DOI] [PubMed] [Google Scholar]
- 40.Brouwers J, Tack J, Lammert F, Augustijns P. Parallel monitoring of plasma and intraluminal drug concentrations in man after oral administration of fosamprenavir in the fasted and fed state. Pharm Res. 2007 April 19; doi: 10.1007/s11095-007-9307-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 41.Welling PG. Effects of food on drug absorption. Annu Rev Nutr. 1996;16:383–415. doi: 10.1146/annurev.nu.16.070196.002123. [DOI] [PubMed] [Google Scholar]
- 42.Galia E, Nicolaides E, Hörter D, Löbenberg R, Reppas C, Dressman JB. Evaluation of various dissolution media for predicting in vivo performance of class I and II drugs. Pharm Res. 1998;15:698–705. doi: 10.1023/a:1011910801212. [DOI] [PubMed] [Google Scholar]
- 43.Dressman JB, Reppas C. In vitro-in vivo correlations for lipophilic, poorly water-soluble drugs. Eur J Pharm Sci. 2000;11:S73–80. doi: 10.1016/s0928-0987(00)00181-0. [DOI] [PubMed] [Google Scholar]
- 44.Leyden JJ. Absorption of minocycline hydrochloride and tetracycline hydrochloride, Effect of food, milk, and iron. J Am Acad Dermatol. 1985;12:308–312. doi: 10.1016/s0190-9622(85)80041-4. [DOI] [PubMed] [Google Scholar]
- 45.Carver PL, Fleisher D, Zhou SY, Kaul D, Kazanjian P, Li C. Meal composition effects on the oral bioavailability of indinavir in HIV-infected patients. Pharm Res. 1999;16:718–724. doi: 10.1023/a:1018880726035. [DOI] [PubMed] [Google Scholar]
- 46.Liedholm H, Melander A. Concomitant food intake can increase the bioavailability of propranolol by transient inhibition of its presystemic primary conjugation. Clin Pharmacol Ther. 1986;40:29–36. doi: 10.1038/clpt.1986.135. [DOI] [PubMed] [Google Scholar]
- 47.Ratain MJ, Cohen EE. The value meal: how to save $1,700 per month or more on lapatinib. J Clin Oncol. 2007 July 16; doi: 10.1200/JCO.2007.12.0758. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt LE, Dalhoff K. Food-drug interactions. Drugs. 2002;62:1481–1502. doi: 10.2165/00003495-200262100-00005. [DOI] [PubMed] [Google Scholar]
- 49.Smith DA. Design of drugs through a consideration of drug metabolism and pharmacokinetics. Eur J Drug Metab Pharmacokinet. 1994;3:193–199. doi: 10.1007/BF03188921. [DOI] [PubMed] [Google Scholar]
- 50.Ito K, Suzuki H, Horie T, Sugiyama Y. Apical/basolateral surface expression of drug transporters and its role in vectorial drug transport. Pharm Res. 2005;22:1559–1577. doi: 10.1007/s11095-005-6810-2. [DOI] [PubMed] [Google Scholar]
- 51.Kim RB, Fromm MF, Wandel C, Leake B, Wood AJ, Roden DM, Wilkinson GR. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Invest. 1998;101:289–294. doi: 10.1172/JCI1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Polli JW, Wring SA, Humphreys JE, Huang L, Morgan JB, Webster LO, Serabjit-Singh CS. Rational use of in vitro P-glycoprotein assays in drug discovery. J Pharmacol Exp Ther. 2001;299:620–628. [PubMed] [Google Scholar]
- 53.Taub ME, Kristensen L, Frokjaer S. Optimized conditions for MDCK permeability and turbidimetric solubility studies using compounds representative of BCS classes I-IV. Eur J Pharm Sci. 2002;15:331–340. doi: 10.1016/s0928-0987(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 54.Lu S, Gough AW, Bobrowski WF, Stewart BH. Transport properties are not altered across Caco-2 cells with heightened TEER despite underlying physiological and ultrastructural changes. J Pharm Sci. 1996;85:270–273. doi: 10.1021/js950269u. [DOI] [PubMed] [Google Scholar]
- 55.Yee S. In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man--fact or myth. Pharm Res. 1997;14:763–766. doi: 10.1023/a:1012102522787. [DOI] [PubMed] [Google Scholar]
- 56.Watanabe K, Sawano T, Terada K, Endo T, Sakata M, Sato J. Studies on intestinal absorption of sulpiride (1): carrier-mediated uptake of sulpiride in the human intestinal cell line Caco-2. Biol Pharm Bull. 2002;25:885–890. doi: 10.1248/bpb.25.885. [DOI] [PubMed] [Google Scholar]
- 57.Watanabe K, Sawano T, Endo T, Sakata M, Sato J. Studies on intestinal absorption of sulpiride (2): transepithelial transport of sulpiride across the human intestinal cell line Caco-2. Biol Pharm Bull. 2002;25:1345–1350. doi: 10.1248/bpb.25.1345. [DOI] [PubMed] [Google Scholar]
- 58.Sun D, Lennernas H, Welage LS, Barnett JL, Landowski CP, Foster D, Fleisher D, Lee KD, Amidon GL. Comparison of human duodenum and Caco-2 gene expression profiles for 12,000 gene sequences tags and correlation with permeability of 26 drugs. Pharm Res. 2002;19:1400–1416. doi: 10.1023/a:1020483911355. [DOI] [PubMed] [Google Scholar]
- 59.Bourdet DL, Pollack GM, Thakker DR. Intestinal absorptive transport of the hydrophilic cation ranitidine: a kinetic modeling approach to elucidate the role of uptake and efflux transporters and paracellular vs. transcellular transport in Caco-2 cells. Pharm Res. 2006;23:1178–1187. doi: 10.1007/s11095-006-0204-y. [DOI] [PubMed] [Google Scholar]
- 60.Hilgendorf C, Ahlin G, Seithel A, Artursson P, Ungell AL, Karlsson J. Expression of thirty-six drug transporter genes in human intestine, liver, kidney, and organotypic cell lines. Drug Metab Dispos. 2007;35:1333–1340. doi: 10.1124/dmd.107.014902. [DOI] [PubMed] [Google Scholar]
- 61.Balakrishnan A, Sussman DJ, Polli JE. Development of stably transfected monolayer overexpressing the human apical sodium-dependent bile acid transporter (hASBT) Pharm Res. 2005;22:1269–1280. doi: 10.1007/s11095-005-5274-8. [DOI] [PubMed] [Google Scholar]
- 62.Balakrishnan A, Polli JE. Apical sodium dependent bile acid transporter (ASBT, SLC10A2): a potential prodrug target. Mol Pharm. 2006;3:223–230. doi: 10.1021/mp060022d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Balimane PV, Chong S, Patel K, Quan Y, Timoszyk J, Han YH, Wang B, Vig B, Faria TN. Peptide transporter substrate identification during permeability screening in drug discovery: comparison of transfected MDCK-hPepT1 cells to Caco-2 cells. Arch Pharm Res. 2007;30:507–518. doi: 10.1007/BF02980227. [DOI] [PubMed] [Google Scholar]
- 64.Satoh H, Yamashita F, Tsujimoto M, Murakami H, Koyabu N, Ohtani H, Sawada Y. Citrus juices inhibit the function of human organic anion-transporting polypeptide OATP-B. Drug Metab Dispos. 2005;33:518–523. doi: 10.1124/dmd.104.002337. [DOI] [PubMed] [Google Scholar]
- 65.Matsushima S, Maeda K, Kondo C, Hirano M, Sasaki M, Suzuki H, Sugiyama Y. Identification of the hepatic efflux transporters of organic anions using double-transfected Madin-Darby canine kidney II cells expressing human organic anion-transporting polypeptide 1B1 (OATP1B1)/multidrug resistance-associated protein 2, OATP1B1/multidrug resistance 1, and OATP1B1/breast cancer resistance protein. J Pharmacol Exp Ther. 2005;314:1059–1067. doi: 10.1124/jpet.105.085589. [DOI] [PubMed] [Google Scholar]
- 66.Sasaki M, Suzuki H, Ito K, Abe T, Sugiyama Y. Transcellular transport of organic anions across a double-transfected Madin-Darby canine kidney II cell monolayer expressing both human organic anion-transporting polypeptide (OATP2/SLC21A6) and Multidrug resistance-associated protein 2 (MRP2/ABCC2) J Biol Chem. 2002;277:6497–6503. doi: 10.1074/jbc.M109081200. [DOI] [PubMed] [Google Scholar]
- 67.Letschert K, Komatsu M, Hummel-Eisenbeiss J, Keppler D. Vectorial transport of the peptide CCK-8 by double-transfected MDCKII cells stably expressing the organic anion transporter OATP1B3 (OATP8) and the export pump ABCC2. J Pharmacol Exp Ther. 2005;313:549–556. doi: 10.1124/jpet.104.081224. [DOI] [PubMed] [Google Scholar]
- 68.Mita S, Suzuki H, Akita H, Hayashi H, Onuki R, Hofmann AF, Sugiyama Y. Inhibition of bile acid transport across Na+/taurocholate cotransporting polypeptide (SLC10A1) and bile salt export pump (ABCB 11)-coexpressing LLC-PK1 cells by cholestasis-inducing drugs. Drug Metab Dispos. 2006;34:1575–1581. doi: 10.1124/dmd.105.008748. [DOI] [PubMed] [Google Scholar]
- 69.Tolle-Sander S, Rautio J, Wring S, Polli JW, Polli JE. Midazolam exhibits characteristics of a highly permeable P-glycoprotein substrate. Pharm Res. 2003;20:757–764. doi: 10.1023/a:1023433502647. [DOI] [PubMed] [Google Scholar]
- 70.Cummins CL, Jacobsen W, Benet LZ. Unmasking the dynamic interplay between intestinal P-glycoprotein and CYP3A4. J Pharmacol Exp Ther. 2002;300:1036–1045. doi: 10.1124/jpet.300.3.1036. [DOI] [PubMed] [Google Scholar]
- 71.Cummins CL, Salphati L, Reid MJ, Benet LZ. In vivo modulation of intestinal CYP3A metabolism by P-glycoprotein: studies using the rat single-pass intestinal perfusion model. J Pharmacol Exp Ther. 2003;305:306–314. doi: 10.1124/jpet.102.044719. [DOI] [PubMed] [Google Scholar]
- 72.Cummins CL, Jacobsen W, Christians U, Benet LZ. CYP3A4-transfected Caco-2 cells as a tool for understanding biochemical absorption barriers: studies with sirolimus and midazolam. J Pharmacol Exp Ther. 2004;308:143–155. doi: 10.1124/jpet.103.058065. [DOI] [PubMed] [Google Scholar]
- 73.Chang JH, Benet LZ. Glucuronidation and the transport of the glucuronide metabolites in LLC-PK1 cells. Mol Pharm. 2005;2:428–434. doi: 10.1021/mp050018m. [DOI] [PubMed] [Google Scholar]
- 74.Lau YY, Huang Y, Frassetto L, Benet LZ. Effect of OATP1B transporter inhibition on the pharmacokinetics of atorvastatin in healthy volunteers. Clin Pharmacol Ther. 2007;81:194–204. doi: 10.1038/sj.clpt.6100038. [DOI] [PubMed] [Google Scholar]
- 75.Gupta SK, Benet LZ. Effect of food on the pharmacokinetics of cyclosporine in healthy subjects following oral and intravenous administration. J Clin Pharmacol. 1990;30:643–653. doi: 10.1002/j.1552-4604.1990.tb01868.x. [DOI] [PubMed] [Google Scholar]
- 76.Physicians’ Desk Reference. 61. Montvale, NJ: Thomson PDR; 2007. pp. 2149–2153. [Google Scholar]
- 77.Klein S, Butler J, Hempenstall JM, Reppas C, Dressman JB. Media to simulate the postprandial stomach I. Matching the physiochemical characteristics of standard breakfasts. J Pharm Pharmacol. 2004;56:605–610. doi: 10.1211/0022357023367. [DOI] [PubMed] [Google Scholar]
- 78.Lui CY, Amidon GL, Berardi RR, Fleisher D, Youngberg C, Dressman JB. Comparison of gastrointestinal pH in dogs and humans: implications on the use of the beagle dog as a model for oral absorption in humans. J Pharm Sci. 1986;75:271–274. doi: 10.1002/jps.2600750313. [DOI] [PubMed] [Google Scholar]
- 79.Charman WN, Porter CJH, Mithani S, Dressman JB. Physiochemical and physiological mechanisms for the effects of food on drug absorption: the role of lipids and pH. J Pharm Sci. 1997;86:269–282. doi: 10.1021/js960085v. [DOI] [PubMed] [Google Scholar]
- 80.Tso P. Intestinal lipid absorption. In: Johnson R, editor. Physiology of the Gastrointestinal Tract. Raven Press; New York: 1994. pp. 1867–1907. [Google Scholar]
- 81.Konishi T, Satsu H, Hatsugai Y, Aizawa K, Inakuma T, Nagata S, Sakuda SH, Nagasawa H, Shimizu M. Inhibitory effect of a bitter melon extract on the P-glycoprotein activity in intestinal Caco-2 cells. Br J Pharmacol. 2004;143:379–387. doi: 10.1038/sj.bjp.0705804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Konishi T, Satsu H, Hatsugai Y, Aizawa K, Inakuma T, Nagata S, Sakuda SH, Nagasawa H, Shimizu M. A bitter melon extract inhibits the P-glycoprotein activity in intestinal Caco-2 cells: monoglyceride as an active compound. Biofactors. 2004;22:71–74. doi: 10.1002/biof.5520220113. [DOI] [PubMed] [Google Scholar]
- 83.Ingels F, Deferme S, Destexhe E, Oth M, Van den Mooter G, Augustijns P. Simulated intestinal fluid as transport medium in the Caco-2 cell culture model. Int J Pharm. 2002;232:183–192. doi: 10.1016/s0378-5173(01)00897-3. [DOI] [PubMed] [Google Scholar]
- 84.Balakrishnan A, Polli JE. Apical sodium dependent bile acid transporter (ASBT, SLC10A2): a potential prodrug target. Mol Pharm. 2006;3:223–230. doi: 10.1021/mp060022d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Benet LZ, Cummins CL, Wu CY. Unmasking the dynamic interplay between efflux transporters and metabolic enzymes. Int J Pharm. 2004;277:3–9. doi: 10.1016/j.ijpharm.2002.12.002. [DOI] [PubMed] [Google Scholar]
- 86.Benet LZ, Cummins CL, Wu CY. Transporter-enzyme interactions: implications for predicting drug-drug interactions from in vitro data. Curr Drug Metab. 2003;4:393–398. doi: 10.2174/1389200033489389. [DOI] [PubMed] [Google Scholar]
- 87.Dresser GK, Bailey DG, Leake BF, Schwarz UI, Dawson PA, Freeman DJ, Kim RB. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther. 2002;71:11–20. doi: 10.1067/mcp.2002.121152. [DOI] [PubMed] [Google Scholar]
- 88.Lavelle J, Follansbee S, Trapnell CB, Buhles WC, Griffy KG, Jung D, Dorr A, Connor J. Effect of food on the relative bioavailability of oral ganciclovir. J Clin Pharmacol. 1996;36:238–241. doi: 10.1002/j.1552-4604.1996.tb04193.x. [DOI] [PubMed] [Google Scholar]
- 89.Singh BN. A quantitative approach to probe the dependence and correlation of food-effect with aqueous solubility, dose/solubility ratio, and partition coefficient (Log P) for orally active drugs administered as immediate-release formulations. Drug Dev Res. 2005;65:55–75. [Google Scholar]
- 90.Gu CH, Li H, Levons J, Lentz K, Gandhi RB, Raghavan K, Smith RL. Predicting effect of food on extent of drug absorption based on physicochemical properties. Pharm Res. 2007;24:1118–1130. doi: 10.1007/s11095-007-9236-1. [DOI] [PubMed] [Google Scholar]
- 91.Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods. 2000;44:235–249. doi: 10.1016/s1056-8719(00)00107-6. [DOI] [PubMed] [Google Scholar]
- 92.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 93.Azarmi S, Roa W, Löbenberg R. Current perspectives in dissolution testing of conventional and novel dosage forms. Int J Pharm. 2007;28:12–21. doi: 10.1016/j.ijpharm.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 94.Al-Behaisi S, Antal I, Morovján G, Szúnyog J, Drabant S, Marton S, Klebovich I. In vitro simulation of food effect on dissolution of deramciclane film-coated tablets and correlation with in vivo data in healthy volunteers. Eur J Pharm Sci. 2002;15:157–162. doi: 10.1016/s0928-0987(01)00195-6. [DOI] [PubMed] [Google Scholar]
- 95.Bailey DG, Spence JD, Edgar B, Bayliff CD, Arnold JM. Ethanol enhances the hemodynamic effects of felodipine. Clin Invest Med. 1989;12:357–362. [PubMed] [Google Scholar]
- 96.Dresser GK, Bailey DG. The effects of fruit juices on drug disposition: a new model for drug interactions. Eur J Clin Invest. 2003;33:10–16. doi: 10.1046/j.1365-2362.33.s2.2.x. [DOI] [PubMed] [Google Scholar]
- 97.Dresser GK, Kim RB, Bailey DG. Effect of grapefruit juice volume on the reduction of fexofenadine bioavailability: possible role of organic anion transporting polypeptides. Clin Pharmacol Ther. 2005;77:170–177. doi: 10.1016/j.clpt.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 98.Petri N, Tannergren C, Rungstad D, Lennernas H. Transport characteristics of fexofenadine in the Caco-2 cell model. Pharm Res. 2004;21:1398–1404. doi: 10.1023/b:pham.0000036913.90332.b1. [DOI] [PubMed] [Google Scholar]
- 99.Kamath AV, Yao M, Zhang Y, Chong S. Effect of fruit juices on the oral bioavailability of fexofenadine in rats. J Pharm Sci. 2005;94:233–239. doi: 10.1002/jps.20231. [DOI] [PubMed] [Google Scholar]
- 100.Parker RB, Yates CR, Soberman JE, Laizure SC. Effects of grapefruit juice on intestinal P-glycoprotein evaluation using digoxin in humans. Pharmacotherapy. 2003;23:979–987. doi: 10.1592/phco.23.8.979.32881. [DOI] [PubMed] [Google Scholar]
- 101.Schwarz UI, Seemann D, Oertel R, Miehlke S, Kuhlish E, Fromm MF, Kim RB, Bailey DG, Kirch W. Grapefruit juice ingestion significantly reduces talinolol bioavailability. Clin Pharmacol Ther. 2005;77:291–301. doi: 10.1016/j.clpt.2004.11.111. [DOI] [PubMed] [Google Scholar]
- 102.Anuchapreeda S, Leechanachai P, Smith MM, Ambudkar SV, Limtrakul P. Modulation of P-glycoprotein expression and function by curcumin in multidrug-resistant human KB cells. Biochem Pharmacol. 2002;64:573–582. doi: 10.1016/s0006-2952(02)01224-8. [DOI] [PubMed] [Google Scholar]
- 103.Zhang S, Morris ME. Effects of the flavonoids biochanin A, morin, phloretin, and silymarin on P-glycoprotein-mediated transport. J Pharmacol Exp Ther. 2003;304:1258–1267. doi: 10.1124/jpet.102.044412. [DOI] [PubMed] [Google Scholar]
- 104.Kitagawa S, Nabekura T, Kamiyama S. Inhibition of Pglycoprotein function by tea catechins in KB-C2 cells. J Pharm Pharmacol. 2004;56:1001–1005. doi: 10.1211/0022357044003. [DOI] [PubMed] [Google Scholar]
- 105.Barthomeuf C, Grassi J, Demeule M, Fournier C, Boivin D, Béliveau R. Inhibition of P-glycoprotein transport function and reversion of MDR1 multidrug resistance by cnidiadin. Cancer Chemother Pharmacol. 2005;56:173–181. doi: 10.1007/s00280-004-0914-y. [DOI] [PubMed] [Google Scholar]
- 106.Kitagawa S, Nabekura T, Kamiyama S, Takahashi T, Nakamura Y, Kashiwada Y, Ikeshiro Y. Effects of alkyl gallates on Pglycoprotein function. Biochem Pharmacol. 2005;70:1262–1266. doi: 10.1016/j.bcp.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 107.Fuchikami H, Satoh H, Tsujimoto M, Ohdo S, Ohtani H, Sawada Y. Effects of herbal extracts on the function of human organic anion-transporting polypeptide OATP-B. Drug Metab Dispos. 2006;34:577–582. doi: 10.1124/dmd.105.007872. [DOI] [PubMed] [Google Scholar]
- 108.Tseng E, Kamath A, Morris ME. Effect of organic isothiocyanates on the P-glycoprotein-and MRP1-mediated transport of daunomycin and vinblastine. Pharm Res. 2002:1509–1515. doi: 10.1023/a:1020460700877. [DOI] [PubMed] [Google Scholar]
- 109.Wu CP, Calcagno AM, Hladky SB, Ambudkar SV, Barrand MA. Modulatory effects of plant phenols on human multidrugresistance proteins 1, 4, and 5 (ABCC1, 4 and 5) FEBS J. 2005;272:4725–4740. doi: 10.1111/j.1742-4658.2005.04888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brocks DR, Wasan KM. The influence of lipids on stereoselective pharmacokinetics of halofantrine: Important implications in food-effect studies involving drugs that bind to lipoproteins. J Pharm Sci. 2002;91:1817–1826. doi: 10.1002/jps.10182. [DOI] [PubMed] [Google Scholar]
- 111.Humberstone AJ, Porter CJ, Edwards GA, Charman WN. Association of halofantrine with postprandially derived plasma lipoproteins decreases its clearance relative to administration in the fasted state. J Pharm Sci. 1998;87:936–942. doi: 10.1021/js9704846. [DOI] [PubMed] [Google Scholar]
- 112.Persson EM, Nilsson RG, Hansson GI, Löfgren LJ, Libäck F, Knutson L, Abrahamsson B, Lennernäs H. A clinical single-pass perfusion investigation of the dynamic in vivo secretory response to a dietary meal in human proximal small intestine. Pharm Res. 2006;23:742–751. doi: 10.1007/s11095-006-9607-z. [DOI] [PubMed] [Google Scholar]
- 113.Humberstone AJ, Charman WN. Lipid-based vehicles for the oral delivery of poorly water soluble drugs. Adv Drug Deliv Rev. 1997;25:103–128. [Google Scholar]
- 114.Gershkovich P, Shtainer D, Hoffman A. The effect of a high-fat meal on the pharmacodynamics of a model lipophilic compound that binds extensively to triglyceride-rich lipoproteins. Int J Pharm. 2007;333:1–4. doi: 10.1016/j.ijpharm.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 115.Gershkovich P, Hoffman A. Effect of a high-fat meal on absorption and disposition of lipophilic compounds: The importance of degree of association with triglyceride-rich lipoproteins. Eur J Pharm Sci. 2007 May 18; doi: 10.1016/j.ejps.2007.05.109. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 116.Porter CJ, Trevaskis NL, Charman WN. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov. 2007;6:231–248. doi: 10.1038/nrd2197. [DOI] [PubMed] [Google Scholar]
- 117.Mueller EA, Kovarik JM, van Bree JB, Grevel J, Lücker PW, Kutz K. Influence of a fat-rich meal on the pharmacokinetics of a new oral formulation of cyclosporine in a crossover comparison with the market formulation. Pharm Res. 1994;11:151–155. doi: 10.1023/a:1018922517162. [DOI] [PubMed] [Google Scholar]
- 118.Mueller EA, Kovarik JM, Kutz K. Minor influence of a fat-rich meal on the pharmacokinetics of a new oral formulation of cyclosporine. Transplant Proc. 1994;26:2957–2958. [PubMed] [Google Scholar]
- 119.Kaukonen AM, Boyd BJ, Charman WN, Porter CJ. Drug solubilization behavior during in vitro digestion of suspension formulations of poorly water-soluble drugs in triglyceride lipids. Pharm Res. 2004;21:254–260. doi: 10.1023/b:pham.0000016283.87709.a9. [DOI] [PubMed] [Google Scholar]
- 120.Carey MC, Small DM, Bliss CM. Lipid digestion and absorption. Annu Rev Physiol. 1983;45:651–677. doi: 10.1146/annurev.ph.45.030183.003251. [DOI] [PubMed] [Google Scholar]
- 121.Kossena GA, Charman WN, Boyd BJ, Dunstan DE, Porter CJ. Probing drug solubilization patterns in the gastrointestinal tract after administration of lipid-based delivery systems: a phase diagram approach. J Pharm Sci. 2004;93:332–348. doi: 10.1002/jps.10554. [DOI] [PubMed] [Google Scholar]
- 122.Kossena GA, Charman WN, Boyd BJ, Porter CJ. Influence of the intermediate digestion phases of common formulation lipids on the absorption of a poorly water-soluble drug. J Pharm Sci. 2005;94:481–492. doi: 10.1002/jps.20260. [DOI] [PubMed] [Google Scholar]
- 123.Subramanian R, Wasan KM. Effect of lipid excipients on in vitro pancreatic lipase activity. Drug Dev Ind Pharm. 2003;29:885–890. doi: 10.1081/ddc-120024184. [DOI] [PubMed] [Google Scholar]
- 124.Risovic V, Sachs-Barrable K, Boyd M, Wasan KM. Potential mechanisms by which Peceol increases the gastrointestinal absorption of amphotericin B. Drug Dev Ind Pharm. 2004;30:767–774. doi: 10.1081/ddc-120039793. [DOI] [PubMed] [Google Scholar]
- 125.Wasan KM, Sachs-Barrable K, Lee SD. Potential mechanisms by which lipid excipients increase the oral gastrointestinal absorption of drugs: a case study using amphotericin B. Bulletin Technique Gattefossé. 2006:31–42. [Google Scholar]
- 126.Ingels FM, Augustijns PF. Biological, pharmaceutical, and analytical considerations with respect to the transport media used in the absorption screening system, Caco-2. J Pharm Sci. 2003;92:1545–1558. doi: 10.1002/jps.10408. [DOI] [PubMed] [Google Scholar]