Abstract
Reality monitoring refers to the process of discriminating between internally- and externally-generated information. Two different tasks have often been used to assess this ability: memory for perceived vs. imagined stimuli; and memory for participant- vs. experimenter-performed operations, but it is not known whether these two reality monitoring tasks share neural substrates. The present study involved use of a within-subjects fMRI design to examine common and distinct brain mechanisms associated with the two reality monitoring conditions. The sole difference between the two lay in greater activation in medial anterior prefrontal cortex when recollecting whether the participant or the experimenter had carried out an operation during prior encoding as compared to recollecting whether an item had been perceived or imagined. This region has previously been linked with attending to mental states. Task differences were also reflected in the nature of functional connectivity relationships between medial anterior and right lateral prefrontal cortex: there was a stronger correlation in activity between the two regions during recollection of self/experimenter context. This indicates a role for medial anterior prefrontal cortex in the monitoring of retrieved information relating to internal or external aspects of context. Finally, given the importance of reality monitoring to understanding psychotic symptoms, brain activity was related to measures of proneness to psychosis and schizotypal traits. The observation of significant correlations between reduced medial anterior prefrontal signal and scores on such measures corroborates these theoretical links.
INTRODUCTION
Reality monitoring refers to the ability to discriminate information that was generated by internal cognitive functions, such as thought and imagination, from information that was derived from the outside world by perceptual processes (Johnson & Raye, 1981; Johnson, Hashtroudi, & Lindsay, 1993). In the laboratory, reality monitoring ability can be tested by asking participants to recollect whether stimuli were previously presented on a monitor screen or whether the participants imagined the stimuli for themselves. Alternatively, participants may be asked to remember whether they, or another person, performed a particular operation on the stimuli (e.g., a semantic judgment). Both of these forms of reality monitoring require the consideration of internally-generated and externally-derived information in order to achieve success and have thus tended to be used interchangeably. However, the two tasks would appear to differ in that the former relates to the self only (“Did I perceive or imagine that stimulus?”) whereas the latter relates to two different agents (“Did I or the other person perform the task?”).
Previous neuroimaging studies have focused on the former kind of reality monitoring, contrasting memory for self-generated versus perceptual information (Simons, Owen, Fletcher, & Burgess, 2005; Simons, Gilbert, Owen, Fletcher, & Burgess, 2005; Dobbins & Wagner, 2005; Simons, Davis, Gilbert, Frith, & Burgess, 2006; Vinogradov et al., 2006). Typically, these studies have highlighted the role of a region of medial anterior prefrontal cortex (PFC) in discriminating between internally-generated and perceptually-derived contextual details. For example, this region is sensitive to remembering the cognitive operations carried out at initial presentation of stimuli, rather than to remembering where (Simons, Owen et al., 2005) or when (Simons, Gilbert et al., 2005) the stimuli were presented, or remembering their size on the screen (Dobbins & Wagner, 2005). The same region is involved in remembering whether word pairs or sentences were previously presented in their entirety on the screen (e.g., “Laurel and Hardy”), or whether a word was missing which participants had to imagine (e.g., “Laurel and ?”) in order to complete the word pair or sentence themselves (Simons et al., 2006; Vinogradov et al., 2006). This medial anterior PFC region involved in reality monitoring has been differentiated from a more lateral anterior region that plays a general role in recollection, regardless of the internal or external nature of the relevant context (Rugg, Fletcher, Chua, & Dolan, 1999; Ranganath, Johnson, & D’Esposito, 2000; Dobbins, Foley, Schacter, & Wagner, 2002; Simons, Owen et al., 2005; Simons, Gilbert et al., 2005; Dobbins & Wagner, 2005).
The questions motivating this study are: is the same region of medial anterior PFC involved in other forms of reality monitoring, such as remembering whether oneself or the experimenter performed a particular operation on stimuli? How does activity in this region relate to other prefrontal areas concerned with different stages of the retrieval process? Finally, can individual variability in reality monitoring ability and in associated neural responses be predicted by individuals’ proneness to psychosis and schizotypal traits? To answer these questions, a within-subjects experiment was conducted in which healthy volunteers, prior to completing a series of psychosis-proneness scales, underwent fMRI scanning while making recollection decisions relating to the self/other or perceived/imagined status of previously-encountered stimuli.
The first question was whether common areas of medial anterior PFC might be engaged during recollection of perceived/imagined and self/other status. As far as we are aware, no published data exist concerning the brain regions involved in remembering context details relating to the self or another agent. In the absence of relevant data from recollection studies, clues can be gleaned from outside the field of memory. A number of neuroimaging studies have investigated the brain regions associated with mentalizing, or attending to one’s own mental states and the mental states of others (Frith & Frith, 2003). These studies have typically focused on an area of medial PFC that is more caudal to the region identified in the abovementioned reality monitoring experiments, with a mean y-coordinate of 53 according to a recent meta-analysis (Gilbert et al., 2006)1, as opposed to a mean value of 60 for the reality monitoring studies, t (30) = 3.58, P < 0.01. It may be the case that the processes involved in mentalizing might be recruited during recollection of whether oneself or another agent performed a particular task. If so, contrasting this form of reality monitoring with a form that does not require an agency discrimination (in this study, recollection of perceived versus imagined stimuli) may elicit differential activation in the more caudal region of medial anterior PFC. Alternatively, if the difference in activation location highlighted above is attributable to the fact that the reality monitoring studies involved contextual recollection whereas the mentalizing studies reviewed by Gilbert et al. did not, both forms of reality monitoring should be associated with activity in the more rostral region of medial anterior PFC.
The second question relates to which of the different processing stages of retrieval might be supported by medial anterior PFC: pre-retrieval processes such as cue specification / retrieval orientation (Rugg & Wilding, 2000), or post-retrieval monitoring of recovered information (Burgess & Shallice, 1996). Previous evidence suggests that lateral anterior PFC may be associated with pre-retrieval processes and that medial anterior PFC may contribute to a later stage of retrieval (Simons, Gilbert et al., 2005), but it was not possible in that study to determine whether the later stage of retrieval related specifically to post-retrieval monitoring. In the present study, evidence was sought for functional connections between medial anterior PFC and regions known to play a role in monitoring, such as right lateral PFC (Fletcher, Shallice, Frith, Frackowiak, & Dolan, 1998; Henson, Shallice, & Dolan, 1999; Henson, Rugg, Shallice, Josephs, & Dolan, 1999; Rugg et al., 1999).
The third question relates to previous suggestions of a link between medial anterior PFC function during reality monitoring and some of the symptoms associated with psychotic disorders such as schizophrenia. Simons et al. (2006) reported that reduced engagement of medial anterior PFC was associated with the same kind of misattribution errors often observed in schizophrenia (Frith & Done, 1989; Frith, 1992; Danion, Rizzo, & Bruant, 1999; Keefe, Arnold, Bayen, McEvoy, & Wilson, 2002). Further evidence of such a link was sought by administering to healthy participants in the present study questionnaires assessing proneness to psychosis and schizotypy. On the basis of the previous finding, it would be expected that reduced activation in medial anterior PFC associated with reality monitoring might correlate with scores on these questionnaires.
METHOD
Participants
Sixteen right-handed native speakers of English (7 male, 9 female), with normal or corrected-to-normal vision, took part in the experiment. The volunteers (mean age = 24.3 years, range 19-36) were screened using a comprehensive medical questionnaire and, after complete description of the study, written informed consent was obtained in a manner approved by Cambridge Local Research Ethics Committee.
Design and Procedure
The stimuli consisted of 160 well-known word-pairs (e.g., “Laurel and Hardy”, “bacon and eggs”, “rock and roll”), adapted from those used by Simons et al. (2006) and pilot tested to ensure their familiarity in the target population recruited for the fMRI experiment. These word-pairs were used as target items in the study and test phases. In addition, 80 naturally-occurring and 80 manmade object words were used as baseline items in the test phase. The words were matched for Kucera-Francis frequency.
Participants each undertook 5 study and 5 test phases while lying in the scanner, although only the test phases were scanned. Study phases involved 32 trials, each of which began with a cue at the top of the screen (either “SUBJECT” or “EXPERIMENTER”) indicating who was going to be undertaking that trial (see Fig. 1). After 500 ms, either a word-pair (e.g., “Laurel and Hardy”; perceive condition) or the first word in a word-pair and a question mark (e.g., “Laurel and ?”; imagine condition) were presented on the screen. If the trial had begun with the cue “SUBJECT”, the participant was instructed to view the presented stimulus and, in the perceive condition, read the whole word-pair out aloud. In the imagine condition, the participant was instructed to imagine the second word of the word-pair and read the whole word-pair out aloud. If the cue had been “EXPERIMENTER”, the experimenter undertook the perceive or imagine tasks and read the word-pair out aloud over the scanner intercom. The subject/experimenter and perceive/imagine conditions were crossed as experimental factors, with trial order pseudo-randomized such that no more than three consecutive trials were of the same condition.
Figure 1.
Examples of the cues and stimuli used in the study and test phases. See text for details.
Test phases consisted of 64 trials, divided into blocks of 4 trials each, with each block preceded by an instruction, presented for a varying period of 2-8 s, indicating the type of judgment that would be required during that block (see Fig. 1). In all trials, participants were then presented with a single word in the center of the screen and a reminder instruction cue at the bottom of the screen. Two of the conditions tested memory for details of the context in which word-pairs had been encountered in the preceding study phase. The first word of a previously-studied word-pair was presented and participants judged either whether the accompanying word in the word-pair had been perceived or imagined in the study phase, or whether the participant or the experimenter had read the word-pair out aloud. In a third baseline condition, a non-studied word was presented and participants judged whether the word referred to a naturally-occurring or man-made object. In all conditions, participants indicated their response by pressing one of two buttons on a button box, holding down the button to indicate how confident they were in their judgment. A confidence bar at the bottom of the screen increased in size to illustrate to participants the confidence rating they were making. Participants had 4.5 s to make their response.
Blocks alternated between context memory and baseline conditions, with context memory blocks ordered in an AABBAABB sequence between perceived/imagined and self/experimenter conditions. In one perceived/imagined block of each pair, all trials had been read aloud by the participant, whereas in the other block the experimenter had read aloud the trials; similarly, paired self/experimenter blocks alternated between trials all having been perceived or all having been imagined during the study phase. Perceive/imagine and self/experimenter status in the study phase was systematically counterbalanced between subjects, as was the type of recollection cued in the test phase and the ordering of test conditions. The inter-trial interval was jittered according to an exponential distribution between 500 ms and 1400 ms. Participants were familiarized with the paradigm during practice sessions both prior to the experiment and inside the scanner.
Following participation in the experiment, participants were asked to complete three pencil and paper questionnaires assessing proneness to psychosis and schizotypy: the Oxford-Liverpool Inventory of Feelings and Experiences (O-LIFE; Mason, Claridge, & Jackson, 1995), measuring unusual experiences, cognitive disorganization, introvertive anhedonia, and impulsive nonconformity; the Peters et al. Delusions Inventory (PDI-21; Peters, Joseph, Day, & Garety, 2004), assessing delusions of control, delusions of reference, persecution, depersonalization, etc.; and the Chapman et al. Psychosis Proneness Scales (Chapman, Chapman, & Raulin, 1976, 1978; Eckblad & Chapman, 1983), comprising revised physical and social anhedonia scales (measuring negative symptoms) and magical ideation and revised perceptual aberration scales (measuring positive symptoms). Questionnaire data could not be obtained from one participant.
Imaging Acquisition and Data Analysis
A 3T Siemens TIM Trio system was used to acquire structural and echo-planar functional images (TR=2.25 s, TE=30 ms, 36 sequential axial slices oriented approximately 10-20° to the AC-PC transverse plane, 2 mm thickness, 1 mm inter-slice skip, 5 sessions [functional runs] each of 200 volume acquisitions). The first 5 volumes from each session were discarded to allow for T1 equilibration.
Data were preprocessed and analyzed using SPM5 (Wellcome Department of Imaging Neuroscience, London). Functional images were first corrected for motion by realigning all images with respect to the first, and for differences in slice timing by re-sampling all slices in time to match the middle slice. The participant’s structural image was coregistered to the mean of the realigned functional images and then segmented to separate out gray matter, which was normalized to the gray matter in a template image in MNI stereotactic space (Cocosco, Kollokian, Kwan, & Evans, 1997). The realigned EPI images were then spatially normalized using the structural normalization parameters, re-sampled into 3 mm cubic voxels and spatially smoothed with an 8 mm FWHM isotropic Gaussian kernel. A high-pass filter of 1/128 Hz was used to remove low-frequency noise, and an AR(1) model corrected for temporal autocorrelation.
Random effects statistical analysis was undertaken in two stages. In the first stage, event types for each session were modeled by convolving onset times of trials associated with correct responses with a canonical hemodynamic response function. An additional model included reaction times as a parametric modulator across both types of recollection. Parameters for each regressor were estimated using a subject-specific model, with movement parameters in the 3 directions of motion and 3 degrees of rotation included as confounds, and covariates representing the mean session effects. Linear contrasts were used to obtain subject-specific estimates for each of the effects of interest. These estimates were entered into the second stage of analysis treating subjects as a random effect, using one-sample t-tests across subjects. A priori regions of interest for lateral and medial anterior PFC were defined as 10 mm spheres centered on mean coordinates from the previous studies of reality monitoring described in the introduction (Simons, Owen et al., 2005; Simons, Gilbert et al., 2005; Dobbins & Wagner, 2005; Simons et al., 2006; Vinogradov et al., 2006)2. Activations that occurred within lateral and medial anterior PFC were reported if they exceeded the family-wise error threshold of P < 0.05 corrected for voxels within the regions of interest. Activations occurring outside the regions of interest were reported if they exceeded the threshold of P < 0.05 corrected for multiple comparisons across the entire brain and were greater than 10 voxels in extent. The peak locations of significant activations were localized on an average of the participants’ structural scans, with approximate Brodmann areas estimated from the Talairach and Tournoux (1988) atlas, after adjusting coordinates to allow for differences between the MNI and Talairach templates (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach).
RESULTS
Behavioral Results
Accuracy and reaction time data are displayed in Table 1. It can be seen that recollection of perceived/imagined status was associated with lower accuracy, t (15) = 6.59, P < 0.001, and longer reaction times, t (15) = 7.27, P < 0.001, than recollection of self/experimenter status. The possibility that any observed fMRI activation might be attributable to differences in task difficulty was addressed by restricting the fMRI analysis model to correct recollection trials only and including reaction times as a parametric modulator in the statistical model (see below).
TABLE 1.
Accuracy (proportion correct) and reaction time (ms) data
| Accuracy | Reaction Time | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Perceived/Imagined | 0.80 | 0.06 | 1859 | 244 | |
| Self/Experimenter | 0.87 | 0.05 | 1602 | 196 | |
| Semantic Baseline | 0.99 | 0.01 | 1090 | 167 | |
The link between proneness to psychosis and reality monitoring was investigated by examining correlations between scores on the administered O-LIFE, PDI-21, and Chapman scales and performance in the two reality monitoring conditions. It should be noted that the sample size, although typical of fMRI experiments, is smaller than that often used in studies of personality traits; any null results could be attributable to lack of power. There was variability between participants in scores obtained from the scales, but mean scores were within the normal range (O-LIFE: M = 31.7, SD = 13.3; PDI-21: M = 32.1, SD = 18.7; Chapman: M = 33.2, SD = 18.1). There was no significant correlation between total score on the questionnaires and accuracy in either of the reality monitoring conditions, r = 0.19 and 0.24, n.s. There appeared to be more of an association with reaction times, however, with a trend towards participants who scored more highly on the questionnaires tending to take longer to make accurate reality monitoring responses both in the self/experimenter, r = 0.49, P = 0.06, and perceived/imagined, r = 0.38, P = 0.16, conditions. Looking at the individual scales, this putative association with reaction time seemed to be most evident with the PDI-21, scores on which correlated significantly with reaction time in the self/experimenter condition particularly, r = 0.54, P < 0.05. The effect of psychosis score on reaction time seemed to be specific to reality monitoring as there was no correlation with reaction time in the semantic baseline condition, r = 0.21, n.s.
Neuroimaging Results
The brain regions associated with each type of reality monitoring were examined first by contrasting the two recollection conditions against the semantic baseline. As might be expected, given the highly similar nature of the two types of recollection, there was considerable overlap in patterns of activation between conditions, with a number of regions appearing to play a general role in reality monitoring. As can be seen in Tables 2 and 3, these regions included bilateral lateral anterior PFC, dorsolateral PFC, insula / ventrolateral PFC, anterior cingulate, and lateral parietal cortex. As noted in the introduction, previous studies have consistently documented these regions to be involved in recollecting the context in which events were experienced, irrespective of the kind of contextual detail being tested (Rugg et al., 1999; Simons, Owen et al., 2005; Simons, Gilbert et al., 2005; Dobbins & Wagner, 2005; Simons et al., 2006; Vinogradov et al., 2006).
TABLE 2.
Regions exhibiting significantly greater activation during correct recollection of perceived/imagined status than the baseline condition
| Coordinates | ||||||
|---|---|---|---|---|---|---|
| Brain Region | x | y | z | Z | ||
| Left anterior PFC (BA 10) | -33 | 57 | 6 | 5.0 | ||
| Right anterior PFC (BA 10) | 39 | 60 | 6 | 4.9 | ||
| Left dorsolateral PFC (BA 9/46) | -51 | 27 | 30 | 5.4 | ||
| Right dorsolateral PFC (BA 9/46) | 51 | 24 | 30 | 6.3 | ||
| Anterior cingulate (BA 8) | 0 | 24 | 48 | 6.1 | ||
| Right insula / ventrolateral PFC (BA 47) | 30 | 24 | -6 | 5.8 | ||
| Left ventrolateral PFC (BA 44) | -57 | 15 | 18 | 6.1 | ||
| Right caudate (BA 25) | 12 | 12 | 3 | 6.3 | ||
| Left caudate (BA 25) | -15 | 9 | 9 | 7.1 | ||
| Right superior PFC (BA 8) | 36 | 9 | 60 | 5.6 | ||
| Left superior PFC (BA 6) | -30 | 0 | 60 | 5.7 | ||
| Left cingulate cortex (BA 23) | -6 | -18 | 30 | 5.8 | ||
| Left lateral parietal cortex (BA 40) | -45 | -42 | 42 | 6.2 | ||
| Right lateral parietal cortex (BA 40) | 39 | -51 | 48 | 6.7 | ||
| Right cerebellum | 9 | -75 | -21 | 5.5 | ||
| Left occipital cortex (BA 19) | -27 | -78 | -15 | 5.2 | ||
Note: Coordinates are in MNI atlas space (Cocosco et al., 1997), with brain regions and Brodmann areas (BA) estimated from the Talairach and Tournoux (1988) atlas.
TABLE 3.
Regions exhibiting significantly greater activation during correct recollection of self/experimenter status than the baseline condition
| Coordinates | ||||||
|---|---|---|---|---|---|---|
| Brain Region | x | y | z | Z | ||
| Left anterior PFC (BA 10) | -33 | 48 | 6 | 4.9 | ||
| Right anterior PFC (BA 10) | 33 | 57 | 3 | 4.3 | ||
| Left dorsolateral PFC (BA 46) | -42 | 30 | 24 | 5.7 | ||
| Right dorsolateral PFC (BA 9) | 48 | 30 | 33 | 5.6 | ||
| Anterior cingulate cortex (BA 32) | 3 | 24 | 42 | 6.0 | ||
| Left Insula / ventrolateral PFC (BA 47) | -30 | 24 | -3 | 5.5 | ||
| Right Insula / ventrolateral PFC (BA 47) | 30 | 24 | -3 | 5.3 | ||
| Right superior PFC (BA 8) | 36 | 12 | 57 | 5.6 | ||
| Left caudate (BA 25) | -12 | 9 | 6 | 5.4 | ||
| Right globus pallidus | 12 | -3 | -3 | 5.3 | ||
| Left reticular formation | -6 | -18 | -12 | 5.1 | ||
| Posterior cingulate cortex (BA 23) | -6 | -30 | 27 | 5.8 | ||
| Right lateral parietal cortex (BA 40) | 39 | -48 | 54 | 6.5 | ||
| Left lateral parietal cortex (BA 7) | -33 | -54 | 54 | 5.8 | ||
| Right precuneus (BA 7) | 6 | -72 | 48 | 6.0 | ||
| Right cerebellum | 12 | -75 | -21 | 5.6 | ||
| Left occipital cortex (BA 18) | -27 | -90 | -3 | 5.1 | ||
Note: Coordinates are in MNI atlas space (Cocosco et al., 1997), with brain regions and Brodmann areas (BA) estimated from the Talairach and Tournoux (1988) atlas.
When the two forms of reality monitoring were contrasted against one another, the only area of the brain to show significant activation was in medial anterior PFC (centered on -18, 51, 9; BA 10; Z = 3.5), in which signal was higher during recollection of self/experimenter status than perceived/imagined status (see Fig. 2). The peak of this activation was located significantly caudally to the region (mean y-coordinate = 60) identified as contributing to reality monitoring in five previous studies, t (5) = 2.08, P < 0.05 (Simons, Owen et al., 2005; Simons, Gilbert et al., 2005; Dobbins & Wagner, 2005; Simons et al., 2006; Vinogradov et al., 2006), and was within half a standard deviation of the mean coordinate (y = 53) previously associated with mentalizing (Gilbert et al., 2006). Activation in medial anterior PFC did not correlate with reaction time across participants, r = -0.03, n.s., and including reaction time as a parametric modulator in each participant’s statistical model failed to reveal any significant correlation in this area between activation and reaction time within participants, even with the threshold as low as P < 0.05 uncorrected. These findings are consistent with previous observations that, even when behavioral differences exist between tasks, activation in medial anterior PFC during recollection does not vary as a function of task difficulty (Simons, Owen et al., 2005; Simons, Gilbert et al., 2005; Simons et al., 2006). No region exceeded the threshold for significance in the perceived/imagined status vs. self/experimenter status contrast.
Figure 2.
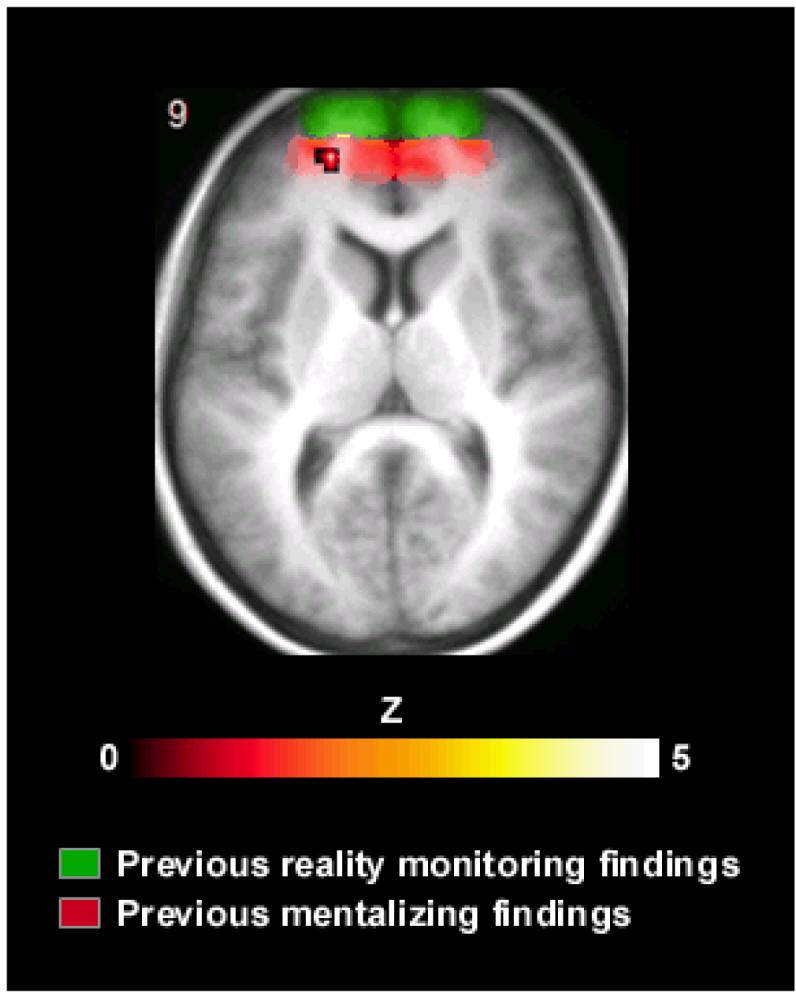
Significantly greater activation during recollection of self/experimenter status than perceived/imagined status, displayed on the participants’ mean normalized structural image. The distribution of activations identified in previous studies of reality monitoring that used the perceived/imagined task is illustrated by the green shading, and the distribution of previous mentalizing activations indicated in red.
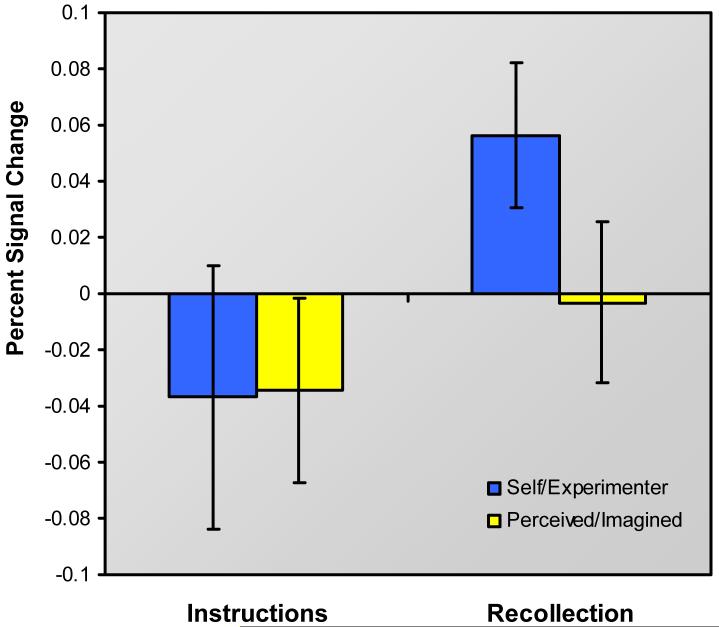
To try to understand which stage of the retrieval process might be supported by the identified medial anterior PFC region, analysis first sought evidence for a role in pre-retrieval processes such as retrieval orientation and/or cue specification. Such processes might be expected to be engaged when retrieval instructions are presented, prior to the onset of target stimuli, and continue to be engaged when target stimuli are presented and retrieval searches ensue. Analysis thus targeted baseline-corrected activity associated with retrieval instructions for the two reality monitoring conditions. As shown in Fig. 3, signal in medial anterior PFC associated with self/experimenter retrieval instructions did not differ from that associated with perceived/imagined instructions, t (15) = 0.08, n.s. This was true regardless of whether the instruction periods were modeled as events or as epochs with variable durations. Although the inferences that can be drawn from a null result are limited, this result is consistent with previous findings suggesting that medial anterior PFC is not sensitive to retrieval orientation, and may indeed contribute to a later stage in the retrieval processing stream (Simons, Gilbert et al., 2005). When the statistical threshold was lowered to P < 0.005 uncorrected, greater activation associated with self/experimenter vs. perceived/imagined retrieval instructions emerged in left lateral anterior PFC (-33, 48, 15; BA 10; Z = 2.8) among other areas (replicating a similar finding from Simons, Gilbert et al., 2005), whereas medial anterior PFC did not exhibit instruction-related activation even when the threshold was dropped to P < 0.05 uncorrected.
Figure 3.
Plot of signal in medial anterior prefrontal cortex during presentation of different reality monitoring retrieval instructions, and during recollection of self/experimenter and perceived/imagined context. Error bars represent standard errors of the mean.
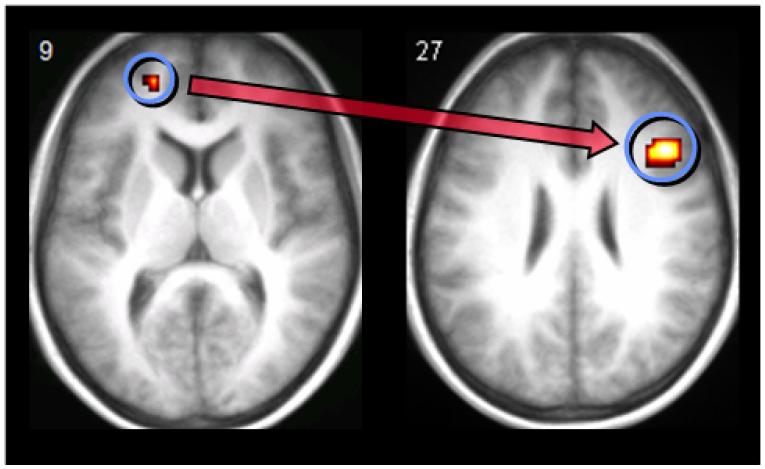
If medial anterior PFC is involved in a relatively late stage of retrieval, then evidence might be expected of connectivity with a key region of right lateral PFC, which has been linked in many previous studies with post-retrieval monitoring processes that operate on the products of retrieval (Fletcher et al., 1998; Henson, Shallice et al., 1999; Henson, Rugg et al., 1999; Rugg et al., 1999). To address this prediction, a psychophysiological interaction analysis was undertaken (Friston et al., 1997). In essence, this analysis identifies task-specific inter-regional covariance relationships, which are considered a measure of functional connectivity (Friston et al., 1997; Gitelman, Penny, Ashburner, & Friston, 2003). In the present study, of particular interest was whether there was evidence of connectivity between medial anterior PFC and right lateral PFC (which might be taken as indirect evidence that the former participates, with the latter, in post-retrieval monitoring) and whether this connectivity was significantly greater during recollection of self/other than perceived/imagined status. Following standard procedures, this involved extracting signal on a subject-by-subject basis from within a 6 mm sphere centered on the nearest significant voxel to the medial anterior PFC group maximum. Data were adjusted for effects of interest and entered into a psychophysiological interaction analysis to reveal areas in which, when activation was regressed onto activity in medial anterior PFC, a significant difference was observed between the regression slopes associated with the self/experimenter vs. perceived/imagined conditions. These subject-specific estimates were analysed at the random effects level using one-sample t-tests across subjects, thresholded at P < 0.001 uncorrected. Consistent with the suggestion that medial anterior PFC plays a role in monitoring, this analysis revealed a significant psychophysiological interaction between medial anterior PFC and right lateral PFC (centered on 45, 18, 27; BA 46; Z = 4.5) (see Fig. 4). The observation of a task-dependent functional connection indicates that, during the self/experimenter condition in particular, medial anterior PFC may modulate activity in right lateral PFC during the monitoring of retrieved contextual information.
Figure 4.
Results of the effective connectivity analysis which identified a psychophysiological interaction between medial anterior prefrontal cortex and right lateral prefrontal cortex, displayed on the participants’ mean normalized structural image.
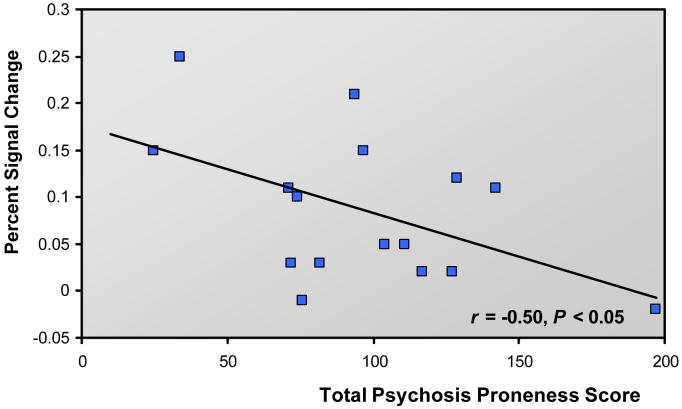
The link between activation in medial anterior PFC and proneness to psychosis was investigated by examining correlations between signal in the two reality monitoring conditions and total score on the psychosis scales. As shown in Fig. 5, there was a significant negative correlation between total questionnaire score and medial anterior PFC signal in the self/experimenter vs. perceived/imagined contrast, r = -0.50, P < 0.05. Looking at the different scales individually, the association between questionnaire score and medial anterior PFC activity could be mostly explained by the PDI-21, which was the only scale for which scores exhibited a significant negative correlation with medial anterior PFC signal, r = -0.56, P < 0.05 (although null results for other scales may of course be attributable to lack of power). The observed correlations with proneness to psychosis did not generalize to other relevant areas of the brain: for example, there was no correlation between questionnaire score and activity in lateral anterior PFC, r = -0.04, n.s., or right lateral PFC, r = -0.21, n.s. These results indicate that participants who exhibited greater proneness to psychosis were less likely to show activity in medial anterior PFC that was specific to the self/other distinction.
Figure 5.
Scatter plot illustrating the significant correlation between reduced activation in medial anterior prefrontal cortex and total score on scales assessing proneness to psychosis and schizotypal traits.
DISCUSSION
With respect to the three key questions motivating this study, the data show that remembering context details relating to the self or another agent differs, in terms of the response of medial anterior PFC, from remembering whether stimuli were perceived or imagined. Moreover, recollection of self/other status was associated with increased connectivity between medial anterior PFC and right lateral PFC, suggesting that the former region might contribute to post-retrieval monitoring operations. Finally, consistent with the proposed relationship between reality monitoring performance and the aberrant cognitive processing that may engender psychotic phenomena, heterogeneity of self/other related activation in this region was linked to participants’ scores on standard scales estimating proneness to psychosis.
The discriminations involved in the self/other and perceived/imagined recollection conditions have both been characterized in the literature as reality monitoring (Johnson & Raye, 1981; Johnson et al., 1993), in that they require participants to differentiate between context details that were internally-generated and externally-derived when the memory was formed. Accordingly, a number of brain regions were commonly active across both conditions, including bilateral lateral anterior PFC, dorsolateral PFC, insula / ventrolateral PFC, anterior cingulate, and lateral parietal cortex. Activation in all these regions has been reported in numerous previous studies of recollection (see Fletcher & Henson, 2001; Simons & Spiers, 2003; Wagner, Shannon, Kahn, & Buckner, 2005, for recent reviews). However, our results are also consistent with an important distinction between the two reality monitoring conditions. Specifically, they differ in the extent to which they involve consideration of information relating to the participant’s own experience versus the experience of different agents. This distinction was reflected in the differential response of medial anterior PFC during the recollection of self/other and perceived/imagined status.
The medial anterior prefrontal region identified in the present study was significantly more caudally located than the region that has consistently been observed during reality monitoring tasks that have contrasted memory for perceived versus imagined stimuli (Simons, Owen et al., 2005; Simons, Gilbert et al., 2005; Dobbins & Wagner, 2005; Simons et al., 2006; Vinogradov et al., 2006). Whereas the medial anterior region isolated in those studies had a mean y-coordinate of 60, the present activation (centered at a y-coordinate of 51) lay instead near the center of the distribution of previous activations associated with mentalizing (Gilbert et al., 2006; see also Gilbert et al., 2007). This result suggests that when a recollection decision requires discrimination between whether oneself or another person performed a task, the brain regions recruited include those involved in attending to one’s own mental states and the mental states of others (Frith & Frith, 2003). This would accord with the view expressed by Johnson and colleagues (1993) that when making such a discrimination, an individual may judge whether the information retrieved from memory includes traces of internal cognitive functions such as thought and imagination that might indicate that the individual themselves read aloud the word-pair. If such information cannot be retrieved but, for example, an auditory representation associated with the memory is recovered that matches the individual’s idea of the experimenter’s voice, then the individual may attribute the reading aloud of the word-pair to the experimenter (Johnson et al., 1993).
The apparently separable medial anterior PFC regions involved in reality monitoring (a rostral locus more sensitive to internal/perceived discriminations and a caudal locus more sensitive to self/other discriminations) can be further distinguished from lateral anterior PFC, which has been shown to be involved not only in reality monitoring, but in context discriminations generally, irrespective of the kind of context being retrieved. So, for example, lateral anterior PFC has been reported as exhibiting significant activation (relative to item recognition or some other baseline condition) during recollection of which encoding task was carried out with stimuli (Rugg et al., 1999; Dobbins et al., 2002; Kahn, Davachi, & Wagner, 2004; Simons, Owen et al., 2005; Simons, Gilbert et al., 2005; Dobbins & Wagner, 2005), where (Simons, Owen et al., 2005; Simons et al., 2006) and when (Simons, Gilbert et al., 2005) stimuli were presented, their size on the screen (Ranganath et al., 2000; Dobbins & Wagner, 2005), as well as whether stimuli were perceived or imagined (Simons et al., 2006) and by whom (present data).
The ubiquity of source-related activity in lateral anterior PFC suggests that the region plays an important role in recollection of context. This role may lie in the specification of retrieval cues prior to the instigation of a search of memory (Simons, Gilbert et al., 2005), a processing stage that has been termed retrieval orientation (Rugg & Wilding, 2000). In the study by Simons, Gilbert, et al. (2005), left lateral anterior PFC showed significant activation both when retrieval instructions were presented and a retrieval search undertaken, and when instructions were presented but no search initiated. As such, the activation was attributed to pre-retrieval processes operating on the presented instructions, such as cue specification. Consistent with this view, in the present data, activation was seen in left lateral anterior PFC (when a liberal threshold was used) that was associated with the presentation of reality monitoring retrieval instructions. Taken together, these fMRI results echo observations from recent event-related potential studies that have reported left anterior frontal scalp distributions exhibiting recollection effects that were locked to presentation of the retrieval cue, prior to the onset of the target stimulus (Herron & Wilding, 2004, 2006).
In contrast to the pre-retrieval role ascribed to lateral anterior PFC, the second main finding of the present experiment was that the medial anterior PFC region identified as showing greater activity associated with self/experimenter distinctions than perceived/imagined distinctions appeared to contribute to the post-retrieval monitoring stage of retrieval processing. There was a significant task-specific covariation in activity between medial anterior PFC and right lateral PFC, a region previously associated with post-retrieval monitoring operations (Fletcher et al., 1998; Henson, Shallice et al., 1999; Henson, Rugg et al., 1999; Rugg et al., 1999; for reviews, see Fletcher & Henson, 2001; Simons & Spiers, 2003). These data are consistent with previous evidence that medial anterior PFC is involved in a relatively late stage of the retrieval process, with activation peaking significantly later than in the abovementioned lateral anterior region linked with pre-retrieval processes (Simons, Gilbert et al., 2005). It was not possible in the previous study to determine whether the later stage of retrieval related specifically to post-retrieval monitoring, but the observation of condition-specific connectivity with right lateral PFC in the present data is compatible with this view.
If the role of the pre-retrieval left lateral anterior region can be conceived in terms of specifying retrieval cues and criteria for success, then the evidence suggests that medial anterior PFC, along with right lateral PFC, may contribute to the evaluation of retrieved information against the specified verification criteria. The fact that medial anterior PFC is consistently sensitive to reality monitoring manipulations indicates that it makes a contribution to post-retrieval monitoring particularly when discriminating context details that were internally-generated during encoding versus those that were derived from the outside world. Thus, it may be that recollective monitoring operations in general are primarily supported by right lateral PFC (Fletcher et al., 1998; Henson, Shallice et al., 1999; Henson, Rugg et al., 1999; Rugg et al., 1999), but that during reality monitoring, additional cognitive control processes that modulate attention between internally-generated and externally-derived representations (Burgess, Simons, Dumontheil, & Gilbert, 2005) are recruited to assist in achieving successful verification of retrieved information. The evidence suggests that the more rostral area of medial anterior PFC may be utilized if the monitoring operation requires discrimination between thoughts and perceptions (Simons et al., 2006) and that the more caudal area may be brought to bear when discrimination between different agents is necessary (as in the present data). This distinction fits with recent conceptions of the rostrocaudal separation of general information processing functions in medial anterior PFC (Gilbert et al., 2007).
A consequence of dysfunction affecting the processes involved in reality monitoring may include reduced ability to distinguish imagined stimuli from those occurring in the outside world. Misattributing internal thoughts to external sources is considered to be a possible account for the hallucinations and delusions often observed in psychotic disorders such as schizophrenia (Frith & Done, 1989; Frith, 1992; Johnson & Raye, 2000). A number of observations are compatible with this link between reality monitoring and psychosis: first, individuals with hallucinations and/or delusions show some impairment on reality monitoring tasks (Bentall, Baker, & Havers, 1991; Vinogradov et al., 1997; Danion et al., 1999; Keefe et al., 2002), including those individuals in whom the symptoms have been pharmacologically induced (Honey et al., 2006). Second, there is evidence of overlap between the brain regions activated during reality monitoring in healthy individuals and those areas that are dysfunctional in psychosis; moreover, a significant correlation was observed between likelihood of misattributing imagined information as having been perceived in the outside world and reduced signal in the key brain region sensitive to reality monitoring, medial anterior PFC (Simons et al., 2006). The current study adds a third important piece of evidence for this link: reduced activation in medial anterior PFC associated with self/experimenter distinctions correlated with scores on measures of proneness to psychosis and schizotypal traits in healthy individuals.
The questionnaires administered were the O-LIFE (Mason et al., 1995), measuring unusual experiences, cognitive disorganization, introvertive anhedonia, and impulsive nonconformity; the PDI-21 (Peters et al., 2004), assessing delusions of control, delusions of reference, persecution, depersonalization, etc.; and the Chapman Scales (Chapman et al., 1976, 1978; Eckblad & Chapman, 1983), which measure negative symptoms (revised physical and social anhedonia scales) and positive symptoms (magical ideation and revised perceptual aberration scales). A significant correlation was observed between reduced activation in medial anterior PFC and total score on the administered questionnaires. Examination of each of the scales individually revealed this association to be driven primarily by scores on the PDI-21, suggesting the possibility that dysfunction of the more caudal region of medial anterior PFC involved in self/experimenter discriminations, at least, might be bound up in the delusional thinking found in psychotic illness.
To conclude, this study has uncovered three main findings. A region of medial anterior PFC was identified in which activation was associated with the reality monitoring task of recollecting whether oneself or the experimenter read aloud a word-pair. This region was significantly more caudally located than the area that previous studies have associated with reality monitoring tasks involving the discrimination between perceived and imagined information, suggesting that the processes supporting these different kinds of reality monitoring may be separable neurally. The identified medial anterior PFC region was shown by connectivity analysis to be linked with right lateral PFC, previously associated with post-retrieval monitoring, and was thus interpreted as being recruited to assist in the monitoring of retrieved information when discrimination between internally-generated and externally-derived information is required. Finally, previous evidence implicating medial anterior PFC in psychosis was corroborated by the observation of a significant correlation between reduced activation in medial anterior PFC and scores on scales measuring proneness to psychosis and schizotypal traits in healthy individuals.
Acknowledgements
We are grateful to James Rowe, Garry Honey, and Phil Corlett for advice and discussion, and to Marieke Schölvinck and Simon Davis for help with stimulus development. We also thank the staff of the MRC Cognition and Brain Sciences Unit MRI Facility for scanning assistance. This work was supported by the UK Medical Research Council and the Wellcome Trust.
Footnotes
The meta-analysis by Gilbert et al. (2006) was restricted to studies reporting activation in anterior PFC.
Medial BA 10 activations reported in the previous studies differed in the hemisphere in which they were located, so absolute x-values were used to calculate the mean coordinate and analogous regions of interest were defined in both hemispheres.
REFERENCE LIST
- Bentall RP, Baker GA, Havers S. Reality monitoring and psychotic hallucinations. British Journal of Clinical Psychology. 1991;30:213–222. doi: 10.1111/j.2044-8260.1991.tb00939.x. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Shallice T. Confabulation and the control of recollection. Memory. 1996;4:359–411. doi: 10.1080/096582196388906. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Simons JS, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. In: Duncan J, Phillips L, McLeod P, editors. Measuring the Mind: Speed, Control, and Age. Oxford: Oxford University Press; 2005. pp. 217–248. [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. Journal of Abnormal Psychology. 1976;85:374–382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Body-image aberration in schizophrenia. Journal of Abnormal Psychology. 1978;87:399–407. doi: 10.1037//0021-843x.87.4.399. [DOI] [PubMed] [Google Scholar]
- Cocosco CA, Kollokian V, Kwan RKS, Evans AC. Brainweb: Online interface to a 3D MRI simulated brain database. NeuroImage. 1997;5:425. [Google Scholar]
- Danion JM, Rizzo L, Bruant A. Functional mechanisms underlying impaired recognition memory and conscious awareness in patients with schizophrenia. Archives of General Psychiatry. 1999;56:639–644. doi: 10.1001/archpsyc.56.7.639. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: Multiple prefrontal processes subserve source memory. Neuron. 2002;35:989–996. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Wagner AD. Domain-general and domain-sensitive prefrontal mechanisms for recollecting events and detecting novelty. Cerebral Cortex. 2005;15:1768–1778. doi: 10.1093/cercor/bhi054. [DOI] [PubMed] [Google Scholar]
- Eckblad M, Chapman LJ. Magical ideation as an indicator of schizotypy. Journal of Consulting and Clinical Psychology. 1983;51:215–225. doi: 10.1037//0022-006x.51.2.215. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Henson RNA. Frontal lobes and human memory: Insights from functional neuroimaging. Brain. 2001;124:849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Shallice T, Frith CD, Frackowiak RSJ, Dolan RJ. The functional roles of prefrontal cortex in episodic memory: II. Retrieval. Brain. 1998;121:1249–1256. doi: 10.1093/brain/121.7.1249. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Frith CD. The Cognitive Neuropsychology of Schizophrenia. Hove: Lawrence Erlbaum; 1992. [Google Scholar]
- Frith CD, Done DJ. Experiences of alien control in schizophrenia reflect a disorder in the central monitoring of action. Psychological Medicine. 1989;19:359–363. doi: 10.1017/s003329170001240x. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, et al. Functional specialization within rostral prefrontal cortex (area 10): A meta-analysis. Journal of Cognitive Neuroscience. 2006;18:932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Williamson IDM, Dumontheil I, Simons JS, Frith CD, Burgess PW. Distinct regions of medial rostral prefrontal cortex supporting social and nonsocial functions. Social Cognitive and Affective Neuroscience. 2007;2:217–226. doi: 10.1093/scan/nsm014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: The importance of hemodynamic deconvolution. NeuroImage. 2003;19:200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: An event-related functional magnetic resonance imaging study. Journal of Neuroscience. 1999;19:3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RNA, Shallice T, Dolan RJ. Right prefrontal cortex and episodic memory retrieval: A functional MRI test of the monitoring hypothesis. Brain. 1999;122:1367–1381. doi: 10.1093/brain/122.7.1367. [DOI] [PubMed] [Google Scholar]
- Herron JE, Wilding EL. An electrophysiological dissociation of retrieval mode and retrieval orientation. NeuroImage. 2004;22:1554–1562. doi: 10.1016/j.neuroimage.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Herron JE, Wilding EL. Neural correlates of control processes engaged before and during recovery of information from episodic memory. NeuroImage. 2006;30:634–644. doi: 10.1016/j.neuroimage.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Honey GD, O’Loughlin C, Turner DC, Pomarol-Clotet E, Corlett PR, Fletcher PC. The effects of a subpsychotic dose of ketamine on recognition and source memory for agency: Implications for pharmacological modelling of core symptoms of schizophrenia. Neuropsychopharmacology. 2006;31:413–423. doi: 10.1038/sj.npp.1300846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Hashtroudi S, Lindsay DS. Source monitoring. Psychological Bulletin. 1993;114:3–28. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Raye CL. Reality monitoring. Psychological Review. 1981;88:67–85. [Google Scholar]
- Johnson MK, Raye CL. Cognitive and brain mechanisms of false memories and beliefs. In: Schacter DL, Scarry E, editors. Memory, Brain, and Belief. Cambridge, MA: Harvard University Press; 2000. pp. 35–86. [Google Scholar]
- Kahn I, Davachi L, Wagner AD. Functional-neuroanatomic correlates of recollection: Implications for models of recognition memory. Journal of Neuroscience. 2004;24:4172–4180. doi: 10.1523/JNEUROSCI.0624-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RSE, Arnold MC, Bayen UJ, McEvoy JP, Wilson WH. Source-monitoring deficits for self-generated stimuli in schizophrenia: Multinomial modeling of data from three sources. Schizophrenia Research. 2002;57:51–67. doi: 10.1016/s0920-9964(01)00306-1. [DOI] [PubMed] [Google Scholar]
- Mason O, Claridge G, Jackson M. New scales for the assessment of schizotypy. Personality and Individual Differences. 1995;18:7–13. [Google Scholar]
- Peters E, Joseph S, Day S, Garety P. Measuring delusional ideation: The 21-item Peters et al. Delusions Inventory (PDI) Schizophrenia Bulletin. 2004;30:1005–1022. doi: 10.1093/oxfordjournals.schbul.a007116. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Johnson MK, D’Esposito M. Left anterior prefrontal activation increases with demands to recall specific perceptual information. Journal of Neuroscience. 2000;20:RC108:1–5. doi: 10.1523/JNEUROSCI.20-22-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Fletcher PC, Chua PML, Dolan RJ. The role of the prefrontal cortex in recognition memory and memory for source: An fMRI study. NeuroImage. 1999;10:520–529. doi: 10.1006/nimg.1999.0488. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Wilding EL. Retrieval processing and episodic memory. Trends in Cognitive Sciences. 2000;4:108–115. doi: 10.1016/s1364-6613(00)01445-5. [DOI] [PubMed] [Google Scholar]
- Simons JS, Davis SW, Gilbert SJ, Frith CD, Burgess PW. Discriminating imagined from perceived information engages brain areas implicated in schizophrenia. NeuroImage. 2006;32:696–703. doi: 10.1016/j.neuroimage.2006.04.209. [DOI] [PubMed] [Google Scholar]
- Simons JS, Gilbert SJ, Owen AM, Fletcher PC, Burgess PW. Distinct roles for lateral and medial anterior prefrontal cortex in contextual recollection. Journal of Neurophysiology. 2005;94:813–820. doi: 10.1152/jn.01200.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Owen AM, Fletcher PC, Burgess PW. Anterior prefrontal cortex and the recollection of contextual information. Neuropsychologia. 2005;43:1774–1783. doi: 10.1016/j.neuropsychologia.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nature Reviews Neuroscience. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Stuttgart: Thieme; 1988. [Google Scholar]
- Vinogradov S, Luks TL, Simpson GV, Schulman BJ, Glenn S, Wong AE. Brain activation patterns during memory of cognitive agency. NeuroImage. 2006 doi: 10.1016/j.neuroimage.2005.12.058. [DOI] [PubMed] [Google Scholar]
- Vinogradov S, Willis-Shore J, Poole JH, Marten E, Ober BA, Shenaut GK. Clinical and neurocognitive aspects of source monitoring errors in schizophrenia. American Journal of Psychiatry. 1997;154:1530–1537. doi: 10.1176/ajp.154.11.1530. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Sciences. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]